Abstract
Phosphorylation at serine 68 of phospholemman (PLM) in response to β-adrenergic stimulation results in simultaneous inhibition of cardiac Na+/Ca2+ exchanger NCX1 and relief of inhibition of Na+-K+-ATPase. The role of PLM in mediating β-adrenergic effects on in vivo cardiac function was investigated with congenic PLM-knockout (KO) mice. Echocardiography showed similar ejection fraction between wild-type (WT) and PLM-KO hearts. Cardiac catheterization demonstrated higher baseline contractility (+dP/dt) but similar relaxation (−dP/dt) in PLM-KO mice. In response to isoproterenol (Iso), maximal +dP/dt was similar but maximal −dP/dt was reduced in PLM-KO mice. Dose-response curves to Iso (0.5–25 ng) for WT and PLM-KO hearts were superimposable. Maximal +dP/dt was reached 1–2 min after Iso addition and declined with time in WT but not PLM-KO hearts. In isolated myocytes paced at 2 Hz. contraction and intracellular Ca2+ concentration ([Ca2+]i) transient amplitudes and [Na+]i reached maximum 2–4 min after Iso addition, followed by decline in WT but not PLM-KO myocytes. Reducing pacing frequency to 0.5 Hz resulted in much smaller increases in [Na+]i and no decline in contraction and [Ca2+]i transient amplitudes with time in Iso-stimulated WT and PLM-KO myocytes. Although baseline Na+-K+-ATPase current was 41% higher in PLM-KO myocytes because of increased α1- but not α2-subunit activity, resting [Na+]i was similar between quiescent WT and PLM-KO myocytes. Iso increased α1-subunit current (Iα1) by 73% in WT but had no effect in PLM-KO myocytes. Iso did not affect α2-subunit current (Iα2) in WT and PLM-KO myocytes. In both WT and NCX1-KO hearts, PLM coimmunoprecipitated with Na+-K+-ATPase α1- and α2-subunits, indicating that association of PLM with Na+-K+-ATPase did not require NCX1. We conclude that under stressful conditions in which [Na+]i was high, β-adrenergic agonist-mediated phosphorylation of PLM resulted in time-dependent reduction in inotropy due to relief of inhibition of Na+-K+-ATPase.
Keywords: FXYD1, excitation-contraction coupling, contractile function, intracellular Na+ and Ca2+ regulation, fura-2, sodium-binding benzofuran isophthalate
fxyd proteins, a family of small single-transmembrane domain proteins (26), are involved in regulation of ion transport (8, 34) and cell volume (9). Phospholemman (PLM), the first cloned member of the FXYD family (19), is highly expressed in the heart (21). It is phosphorylated by protein kinase (PK)A at serine 68 and by PKC at both serine 63 and serine 68 (30). Recent data, however, suggest that additional sites are phosphorylated by PKA (serine 63) and PKC (threonine 69) (13). When phosphorylated at serine 68, PLM disinhibits Na+-K+-ATPase (10, 22) while actively inhibiting cardiac Na+/Ca2+ exchanger NCX1 (24, 32).
In 2005, Tucker and colleagues (18) characterized a PLM-knockout (KO) mouse that exhibits increased cardiac mass, larger cardiac myocyte cross-sectional area, and higher ejection fraction compared with wild type (WT). Follow-up studies demonstrated absence of myocyte hypertrophy (10, 29), higher Na+-K+-ATPase activity (2) and increased Na+-K+-ATPase current (Ipump) (10, 25), higher NCX1 current (32), but little to no change in total Na+-K+-ATPase or its α1- and α2-subunits (2, 29) and NCX1 protein levels (29) in PLM-KO myocytes. Despite slight cardiac hypertrophy (∼14%) in PLM-KO hearts, Bell et al. (2) reported no significant differences in measured parameters of cardiac performance between WT and PLM-KO mice. Only in isolated hearts perfused with a blood- and albumin-free solution did PLM-KO hearts exhibit significantly lower left ventricular (LV) developed pressure compared with WT hearts.
Since both Na+-K+-ATPase and NCX1 regulate intracellular Ca2+ concentration ([Ca2+]i) on a beat-to-beat basis (4), the effects of PLM on cardiac contractility are rather complex and difficult to predict. Depending on experimental conditions, PLM has been shown to regulate myocyte contractility by modulating either NCX1 (25) or Na+-K+-ATPase activity (12).
The present study was undertaken to characterize, by echocardiography and in vivo catheterization, performance parameters in WT and PLM-KO hearts in the absence and presence of β-adrenergic stimulation. In addition, the effects of isoproterenol (Iso) on contraction and [Ca2+]i transient amplitudes, intracellular Na+ concentration ([Na+]i), and Ipump [separated into currents due to α1 (Iα1)- and α2 (Iα2)-subunits] were evaluated in WT and PLM-KO myocytes. Our results suggest that under conditions in which [Na+]i was high, β-adrenergic agonist-mediated phosphorylation of PLM resulted in time-dependent reduction in inotropy due to relief of inhibition of Na+-K+-ATPase.
METHODS
Generation of PLM-deficient mice and animal care.
PLM-KO mice backcrossed to a pure congenic C57BL/6 background were generated as described previously (18, 29). Homozygous adult littermates ∼3 mo old were used. Mice were housed and fed on a 12:12-h light-dark cycle in the Thomas Jefferson University Animal Facility supervised by veterinary staff members. Standard care was provided to all mice used for experiments. All protocols applied to the mice in this study were approved and supervised by the Institutional Animal Care and Use Committees at Thomas Jefferson University, the University of Virginia, and UCLA.
Myocardial histopathology.
Hearts were harvested from PLM-KO and WT mice (n = 3 for each group), fixed in freshly prepared formalin in PBS, processed for paraffin sectioning (6-μm thickness), and stained with Masson trichrome. Ten to fifteen sections were obtained from the LV free wall of each mouse. Quantitation of fibrous areas was performed with NIS-Elements D software. The ratio of area affected by fibrosis (blue color) to total cardiac area in each section was calculated and expressed as percent fibrosis (15).
Echocardiographic and hemodynamic analysis of cardiac function.
Transthoracic two-dimensional echocardiography (TTE) was performed in anesthetized (2% inhaled isoflurane) WT and PLM-KO mice with a 12-MHz probe (31). TTE in M mode was carried out in the parasternal short axis to assess LV diameter and function. For in vivo hemodynamic measurements, a 1.4-Fr micromanometer-tipped catheter (SPR-671, Millar Instruments) was inserted into the right carotid artery and then advanced into the LV of lightly anesthetized (tribromoethanol-amylene hydrate, Avertin; 2.5% wt/vol, 8 μl/g ip) mice with spontaneous respirations placed on a heated (37°C) pad (31). Hemodynamic parameters including heart rate (beats/min), LV end-diastolic pressure (LVEDP), and maximal first time derivative of LV pressure rise (+dP/dt) and fall (−dP/dt) were recorded in closed-chest mode, both at baseline and in response to increasing doses of Iso (0.1, 0.5, 1, 5, 10, and 25 ng) (31).
Isolation of adult murine cardiac myocytes.
Cardiac myocytes were isolated from the septum and LV free wall of WT and PLM-KO mice (∼27 g) according to the protocol of Zhou et al. (35) as modified by us (25, 29, 31). In all experiments, myocytes were used within 2–8 h of isolation, except for [Na+]i measurements, in which myocytes cultured for 18 h were used (25).
Myocyte shortening measurements.
Myocytes adherent to coverslips were bathed in 0.6 ml of air- and temperature-equilibrated (37°C), HEPES-buffered (20 mM, pH 7.4) medium 199 with a Ca2+ concentration ([Ca2+]o) of 1.8 mM. Measurements of myocyte contraction (0.5 or 2 Hz) were performed as previously described (25, 29, 31).
[Ca2+]i transient measurements.
Fura-2-loaded (0.67 μM fura-2 AM, 15 min, 37°C) myocytes were field stimulated to contract (0.5 or 2 Hz, 37°C) in medium 199 containing 1.8 mM [Ca2+]o. [Ca2+]i transient measurements, daily calibration of fura-2 fluorescent signals, and [Ca2+]i transient analyses were performed as previously described (25, 29, 31).
[Na+]i measurements.
The protocol of Despa et al. (11) was followed with few modifications. Briefly, myocytes cultured overnight were exposed to 10 μM sodium-binding benzofuran isophthalate (SBFI) AM in the presence of Pluronic F127 (0.05% wt/vol) for 2 h at 37°C. Medium was changed, and myocytes were incubated for a further 30 min to allow deesterification of the AM ester. Measurements of [Na+]i were performed in myocytes incubated in Tyrode solution containing (in mM) 140 NaCl, 4 KCl, 1 CaCl2, 10 glucose, and 5 HEPES, pH 7.4. Dual excitation wavelengths (340 ± 15 and 380 ± 15 nm; alternating at 5 Hz) were directed to a single myocyte via a Nikon ×40/1.30 numerical aperture UV oil objective situated in a Nikon TE200U inverted microscope. Emission from a small area of the myocyte was recorded at 510 ± 40 nm (Ionoptix, Milton, MA). The myocyte was also continuously illuminated with red light (650-nm long-pass filter) to track contractions simultaneously with a charge-coupled device camera (Myocam, Ionoptix). In preliminary experiments, illumination with red light did not interfere with SBFI fluorescence measurements. All measurements on paced myocytes (0.5 or 2 Hz) were performed at 37°C. To minimize SBFI photobleaching and myocyte photodamage, emission data were collected at 60-s intervals. Under our measurement conditions, the average autofluorescences from 10 myocytes collected at both excitation wavelengths were <10% of SBFI signals and were ignored.
At the end of each [Na+]i measurement, in vivo calibration of SBFI signals of the same myocyte was performed by exposing the myocyte to different extracellular Na+ concentrations ([Na+]o; 0, 10, and 20 mM) in the presence of gramicidin D (10 μM) and strophanthidin (100 μM). Myocytes were exposed to each Na+ calibrating solution for ≥10 min to allow equilibration before SBFI signals were measured. The composition of calibration solutions was identical to those described by Despa et al. (11) with the exception that 2,3-butanedione monoximine (BDM; 10 mM) was included to minimize myocyte hypercontracture.
Ipump measurements.
Whole cell patch-clamp recordings were performed at 30°C as described previously (25, 28, 29, 31, 33). Pipette diameter was 4–6 μm, and pipette resistance was 0.8–1.4 MΩ when filled with standard pipette solution, which contained (in mM) 70 Na+-aspartate, 20 K+-aspartate, 8 CsOH, 7 MgSO4, 11 EGTA, 10 TEA-Cl, 1 CaCl2, 5 HEPES, 5 Na2ATP, and 0.2 GTP (pH 7.2). To decrease pipette Na+ concentration ([Na+]pip) to 10 mM, Cs+ was substituted for Na+. External solution contained (in mM) 137.7 NaCl, 18 KCl, 2.3 NaOH, 1 MgCl2, 2 BaCl2, 1 CdCl2, 5 HEPES, and 10 glucose (pH 7.4) (25, 33). Holding potential was 0 mV. After steady-state current was obtained, dihydroouabain (DHO, 5 μM) was added and the difference current was defined as Iα2. A higher concentration of DHO (1 mM) was then added, and the additional reduction in current was defined as Iα1. After washout of DHO and return of pump current to baseline, Iso (1 μM) was added and increases in Iα1 and Iα2 were measured.
Coimmunoprecipitation of PLM and α1- and α2-subunits of Na+-K+-ATPase.
Coimmunoprecipitation experiments were performed as previously described (1). Briefly, LV homogenates prepared from WT and cardiac-specific NCX1-KO mice (16) were precleared with protein A agarose for 1 h at 4°C. Precleared supernatants (1.1 mg protein) were incubated with either 5 μg of preimmune rabbit IgG (polyclonal antibody control) or 5 μg of polyclonal PLM antibody (C2) (23) overnight at 4°C. The next day, 40 μl (50% slurry) of washed, suspended protein A agarose beads were added to each sample and incubated for a further 2 h at 4°C. Beads were pelleted, washed four to six times with 1.5 ml of buffer III (in mM: 140 NaCl, 25 imidazole, and 1 EDTA, with a combination of complete protease inhibitor and phosphatase inhibitor cocktails) containing 0.05% C12E8, and resuspended in 40 μl of 2× Laemmli sample buffer (+dithiothreitol). Beads were boiled for 5 min at 95°C, and immunoblotting (7.5% SDS-PAGE) was performed.
Primary antibodies against α1- and α2-subunits of Na+-K+-ATPase (1:1,000 each) were obtained from Upstate. The secondary antibody used was donkey anti-rabbit IgG conjugated horseradish peroxidase (1:2,000; Amersham Biosciences). Immunoreactivity was detected by enhanced chemiluminescence.
Statistics.
All results are expressed as means ± SE. For analysis of Ipump as a function of group (WT vs. PLM-KO) and [Na+]pip and in vivo hemodynamic parameters as a function of group and Iso, two-way ANOVA was used. For analysis of percent fibrosis and in vivo hemodynamic and echocardiographic parameters, one-way ANOVA was used. A commercial software package (JMP version 7, SAS Institute, Cary, NC) was used. In all analyses, P < 0.05 was taken to be statistically significant.
RESULTS
In vivo cardiac performance.
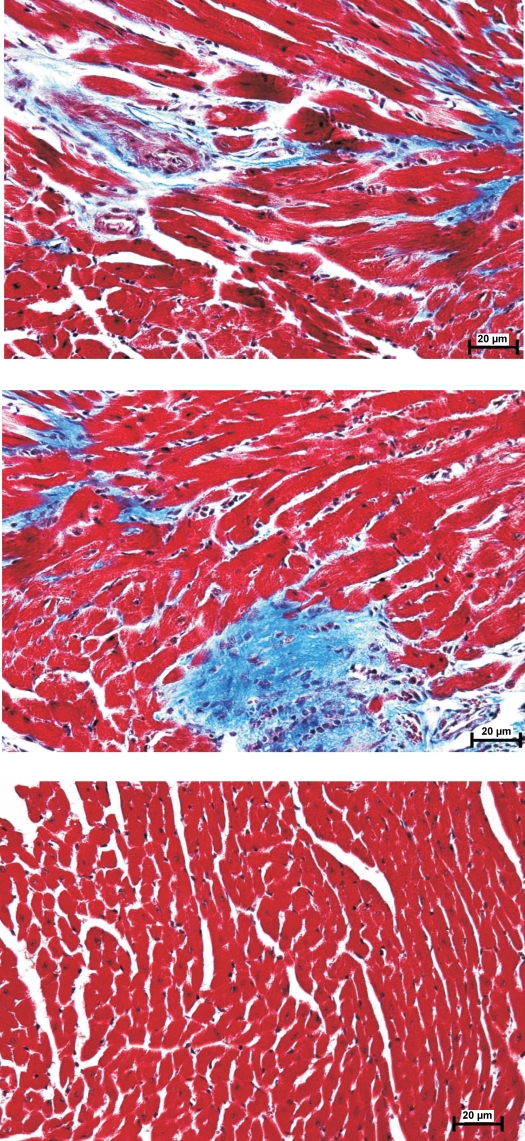
In the present study we used two independent methods to detect potential differences in cardiac function between WT and PLM-KO mice at 3 mo of age. There were no differences in body weight between WT and PLM-KO mice (Table 1). By echocardiography, LV anterior and posterior wall thicknesses were significantly higher in PLM-KO hearts. As a consequence, calculated LV mass was ∼45% higher in PLM-KO mice. Myocardial histology (Fig. 1) demonstrated increased fibrosis in PLM-KO hearts (1.03 ± 0.29%) compared with WT hearts (0.15 ± 0.08%) (P < 0.008). There were no differences in heart rate, ejection fraction, fractional shortening, stroke volume, and cardiac output between WT and PLM-KO mice.
Table 1.
In vivo cardiac performance of WT and PLM-KO mice
| WT | PLM-KO | |
|---|---|---|
| Echocardiography | ||
| Body wt, g | 25.3 ± 0.4 (5) | 24.4 ± 0.4 (5) |
| LVIDD, mm | 3.27 ± 0.02 | 3.55 ± 0.06* |
| LVIDS, mm | 1.73 ± 0.13 | 2.03 ± 0.04 |
| ANT, mm | 0.51 ± 0.00 | 0.60 ± 0.00* |
| PWT, mm | 0.54 ± 0.02 | 0.65 ± 0.01* |
| LV mass, mg | 38.5 ± 2.0 | 55.8 ± 1.1* |
| Heart rate, beats/min | 401 ± 18 | 406 ± 13 |
| Ejection fraction, % | 78.9 ± 4.1 | 74.8 ± 1.2 |
| Fractional shortening, % | 47.1 ± 3.7 | 42.8 ± 1.1 |
| Stroke volume, μl | 34.2 ± 1.7 | 39.5 ± 1.8 |
| Cardiac output, ml/min | 13.8 ± 1.0 | 16.0 ± 0.9 |
| In vivo catheterization | ||
| +dP/dt, mmHg/s | 6,728 ± 304 (9) | 7,920 ± 338* (14) |
| Max +dP/dt, mmHg/s | 13,559 ± 896 | 13,642 ± 363 |
| −dP/dt, mmHg/s | 7,189 ± 341 | 8,163 ± 365 |
| Max −dP/dt, mmHg/s | 13,903 ± 1278 | 11,750 ± 933* |
Values are means ± SE for no. of mice in parentheses. WT, wild type; PLM-KO, phospholemman knockout; LV, left ventricular; LVIDD, LV internal dimension at end diastole; LVIDS, LV internal dimension at end systole; ANT, anterior wall thickness; PWT, posterior wall thickness. LV mass is given by the formula: LV mass = 1.04[(ANT + LVIDD + PWT)3 − (LVIDD)3]. Maximal +dP/dt and maximal −dP/dt are peak hemodynamic responses after 25-ng isoproterenol infusion.
P < 0.003, PLM-KO vs. WT.
Fig. 1.
Myocardial histology of wild-type (WT) and phospholemman (PLM)-knockout (KO) hearts. Hearts from 10- to 12-wk-old mice were sectioned and stained with Masson trichrome. Representative images from PLM-KO (top and middle) and WT (bottom) hearts are shown.
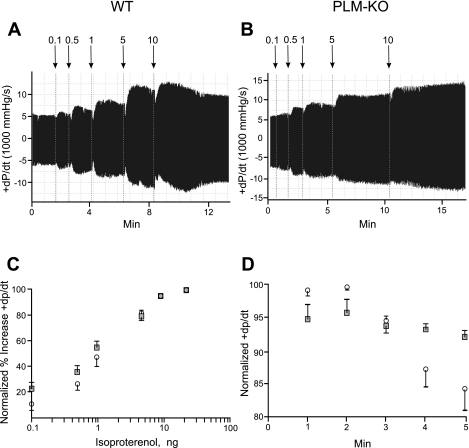
In vivo catheterization in closed-chest mice demonstrated significantly higher +dP/dt both at baseline and when stimulated with low to moderate doses (0.1–1 ng) of Iso in PLM-KO hearts (Fig. 2 and Table 1; group effect, P < 0.01). The differences in +dP/dt between WT and PLM-KO hearts disappeared as the dose of Iso was progressively increased to a maximum of 25 ng (Fig. 2C and Table 1). Sensitivity to β-adrenergic agonists was similar in both groups as demonstrated by no shift in dose-response curves (Fig. 2C). There were no differences in −dP/dt at baseline, although maximal relaxation rate in PLM-KO hearts was slower at the highest dose of Iso (Table 1; P < 0.015).
Fig. 2.
In vivo hemodynamics and β-adrenergic responsiveness of WT and PLM-KO hearts. In vivo catheterization was performed in anesthetized mice, and 1st time derivatives of left ventricular (LV) pressure rise (+dP/dt) and fall (−dP/dt) were continuously measured, both at baseline and with increasing doses of isoproterenol (Iso). A and B: representative original tracings of dP/dt in WT (A) and PLM-KO (B) mice. Arrows indicate addition of increasing doses of Iso (ng). C: to construct the dose-response curve for Iso, +dP/dt was normalized to the maximal value measured in each mouse. There are 9 WT (○) and 14 PLM-KO (■) mice. Error bars are not shown if they fall within the boundaries of the symbol. Composite data are shown in Table 1. D: time course of normalized +dP/dt following addition of maximal doses (10 or 25 ng) of Iso to WT (○; n = 5) and PLM-KO (■; n = 8) mice.
Time course of isoproterenol-induced increase in inotropy in vivo.
Addition of Iso resulted in increases in +dP/dt that reached maximum in <2 min in both WT and PLM-KO hearts (Fig. 2). After 2 min, the inotropic response to Iso started to decline significantly (P < 0.0001) in WT (from 99.1 ± 0.5% to 84.6 ± 3.1% at 5 min) but not in PLM-KO (from 95.4 ± 1.9% to 91.9 ± 1.0% at 5 min) hearts (Fig. 2D).
Effects of isoproterenol on isolated myocyte contractility, [Ca2+]i transients and [Na+]i.
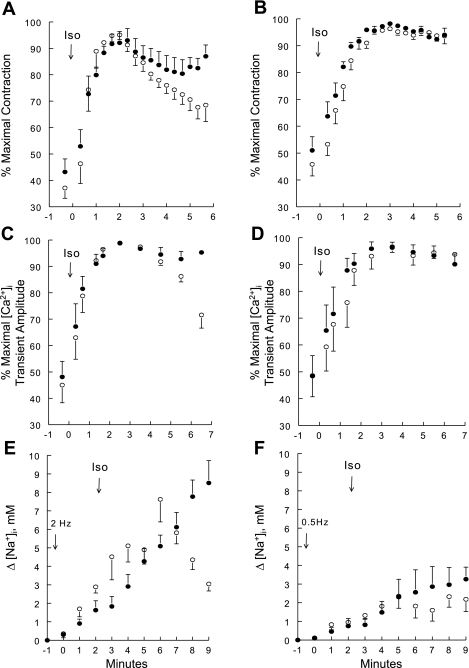
To evaluate the cellular mechanisms responsible for the decline in inotropy in Iso-stimulated WT hearts, we measured contraction, [Ca2+]i transients, and [Na+]i in single myocytes. When paced at 2 Hz, contraction amplitude reached maximum at ∼2 min after Iso (1 μM) addition but declined significantly (P < 0.002) with time in WT (from 95.1 ± 1.7% to 68.5 ± 6.2%) but not in PLM-KO (from 92.1 ± 4.3% to 87.0 ± 4.3%) (Fig. 3A) myocytes. Similarly, [Ca2+]i transient amplitude reached maximum at ∼2–3 min after Iso addition and started to decline in WT (from 98.9 ± 0.5% to 71.6 ± 5.0%) but not in PLM-KO (from 98.8 ± 0.7% to 92.8 ± 2.9%) myocytes (Fig. 3C). In WT myocytes, baseline [Na+]i was 7.8 ± 2.0 mM (n = 10) and was not significantly different from that measured in PLM-KO myocytes (5.5 ± 1.6 mM; n = 5). Pacing at 2 Hz and 37°C for 2 min resulted in increases in [Na+]i over baseline (Δ[Na+]i) by 2.9 ± 0.3 mM in WT and 1.6 ± 0.5 mM in PLM-KO myocytes (Fig. 3E). Addition of Iso (1 μM) to WT myocytes paced at 2 Hz resulted in biphasic response of Δ[Na+]i: initial increase followed by progressive decline (Δ[Na+]i decreased from peak of 7.6 ± 1.2 mM at 4 min to 3.0 ± 0.4 mM at 7 min after Iso addition) (Fig. 3E). By contrast, in PLM-KO myocytes paced at 2 Hz, Δ[Na+]i continued to increase during the course of the experiment to 8.5 ± 1.2 mM at 7 min after Iso addition (Fig. 3E).
Fig. 3.
Contractility, intracellular Ca2+ concentration ([Ca2+]i) transient amplitudes, and increases in intracellular Na+ concentration (Δ[Na+]i) in Iso-stimulated WT and PLM-KO myocytes paced at 0.5 or 2 Hz. Contraction (A and B), [Ca2+]i transients (C and D), and Δ[Na+]i (E and F) were measured in WT (○) and PLM-KO (●) myocytes at 37°C. Myocytes were paced at either 2 (A, C, and E) or 0.5 (B, D, and F) Hz. To facilitate comparison between myocytes, contraction and [Ca2+]i transient amplitudes are normalized to the maximum values observed for each myocyte. There are 6 WT (2 mice) and 6 PLM-KO (3 mice) myocytes in A; 10 WT (4 mice) and 6 PLM-KO (2 mice) in B; 8 WT (3 mice) and 6 PLM-KO (2 mice) myocytes in C; 7 WT (3 mice) and 5 PLM-KO (2 mice) in D; 5 WT (2 mice) and 7 PLM-KO (3 mice) myocytes in E; and 6 WT (2 mice) and 5 PLM-KO (2 mice) myocytes in F. In A–D, Iso (1 μM) was added at “time 0” (arrows) after steady-state contraction was achieved (usually 1–2 min after initiation of pacing). In E and F, after resting [Na+]i was obtained (at −1 min) pacing was initiated at “time 0” and Iso (1 μM) was added at ∼2 min after initiation of pacing. Error bars are not shown if they fall within the boundaries of the symbol.
When myocytes were paced at a slower rate of 0.5 Hz in an attempt to slow the rise of [Na+]i, maximum contraction amplitude was reached at ∼3 min after addition of Iso. However, there was no decline in contraction amplitude with time in either WT (from 96.3 ± 0.8% to 93.6 ± 2.9%) or PLM-KO (from 98.2 ± 0.7% to 94.0 ± 2.5%) myocytes (Fig. 3B). Similarly, at 0.5 Hz, [Ca2+]i transient amplitude reached maximum at ∼3 min after Iso addition and did not decline with time in either WT (from 96.3 ± 1.8% to 93.8 ± 3.4%) or PLM-KO (from 96.6 ± 1.6% to 90.1 ± 3.8%) myocytes (Fig. 3D). Pacing myocytes at 0.5 Hz for 2 min resulted in much smaller Δ[Na+]i (WT: 0.9 ± 0.2 mM; PLM-KO: 0.8 ± 0.4 mM) (Fig. 3F) compared with Δ[Na+]i measured at 2 Hz (Fig. 3E). Iso stimulation of myocytes paced at 0.5 Hz also resulted in smaller peak Δ[Na+]i (WT: 2.4 ± 0.6 mM; PLM-KO: 3.2 ± 0.6 mM) (Fig. 3F) compared with peak Δ[Na+]i measured at 2 Hz. More importantly, there was no decline in [Na+]i in WT myocytes paced at 0.5 Hz and stimulated with Iso (Fig. 3F). The time course of Δ[Na+]i of PLM-KO myocytes paced at 0.5 Hz and stimulated with Iso was similar to that of WT myocytes (Fig. 3F).
Effects of isoproterenol on Ipump.
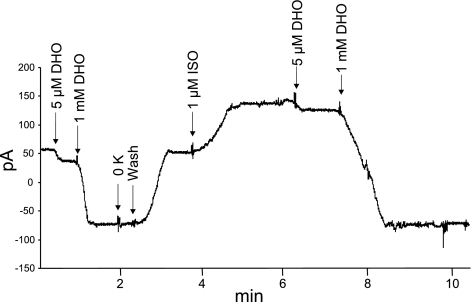
Ionic current due to Na+-K+-ATPase activity was separated into two components by taking advantage of the differential sensitivity of α1- and α2-subunits to digitalis glycosides. In resting WT myocytes, ∼82% of Ipump was contributed by the α1-subunit of Na+-K+-ATPase (Fig. 4 and Table 2). It should be noted that after addition of 1 mM DHO removal of extracellular K+ did not result in any further decreases in current (Fig. 4), indicating effective blockade of Ipump by DHO. At 80 mM [Na+]pip (Vmax conditions), Ipump was ∼41% higher in resting PLM-KO myocytes, and this was entirely due to larger Iα1 (Table 2). Addition of Iso to WT myocytes resulted in ∼73% increase in Iα1 but not Iα2 (Fig. 4 and Table 2). By contrast, addition of Iso resulted in little to no increase in either Iα1 or Iα2 in PLM-KO myocytes. At 10 mM [Na+]pip (near the apparent Km for Na+ of Na+-K+-ATPase), there were no appreciable differences in Ipump between WT and PLM-KO myocytes, with the α1-subunit contributing ∼85% of Ipump (Table 2). Iso addition increased Iα1 by ∼47% in WT myocytes but had no effect on PLM-KO myocytes.
Fig. 4.
Iso increases pump current due to α1 (Iα1)- but not α2 (Iα2)-subunit of Na+-K+-ATPase in WT cardiac myocytes. Whole cell patch-clamp recordings were performed on WT (shown) and PLM-KO myocytes held at 0 mV, 30°C, and 18 mM extracellular K+ concentration, with pipette Na+ concentration at either 80 (shown) or 10 mM. After baseline pump current was recorded dihydroouabain (DHO; 5 μM) was added, and the difference current is taken to be Iα2. DHO (1 mM) was then added, and the additional decrease in current is taken to be Iα1. Note that removing K+ from extracellular solution did not result in further reduction in current, indicating the efficacy of 1 mM DHO to block pump currents due to both α1- and α2-subunits of Na+-K+-ATPase. After DHO washout and recovery of pump current to baseline, Iso (1 μM) was added and pump currents due to α1- and α2-subunits of Na+-K+-ATPase were separated as before. Composite results for both WT and PLM-KO myocytes are presented in Table 2.
Table 2.
Effects of isoproterenol on Ipump in WT and PLM-KO myocytes
| WT | PLM-KO | |
|---|---|---|
| [Na+]pip = 80 mM | ||
| Ipump | 0.90 ± 0.08 (5) | 1.27 ± 0.07* (6) |
| Iα1 | 0.74 ± 0.08 | 1.10 ± 0.09* |
| Iα2 | 0.16 ± 0.01 | 0.16 ± 0.03 |
| ΔIα1 | 0.54 ± 0.20 | 0.11 ± 0.06* |
| ΔIα2 | 0.05 ± 0.03 | 0.07 ± 0.05 |
| [Na+]pip = 10 mM | ||
| Ipump | 0.40 ± 0.05 (5) | 0.40 ± 0.05 (5) |
| Iα1 | 0.34 ± 0.04 | 0.34 ± 0.04 |
| Iα2 | 0.06 ± 0.02 | 0.06 ± 0.01 |
| ΔIα1 | 0.16 ± 0.02 | 0.03 ± 0.02* |
| ΔIα2 | 0.03 ± 0.04 | 0.00 ± 0.03 |
Values are means ± SE for no. of myocytes in parentheses. [Na+]pip, pipette Na+ concentration; Iα1 and Iα2, current densities (pA/pF) of α1- and α2-subunits of Na+-K+-ATPase, respectively; Ipump, total current density of Na+-K+-ATPase; ΔIα1 and ΔIα2, steady-state changes in Iα1 and Iα2, respectively, in response to isoproterenol (1 μM).
P < 0.02, WT vs. PLM-KO.
Association of PLM with subunits of Na+-K+-ATPase.
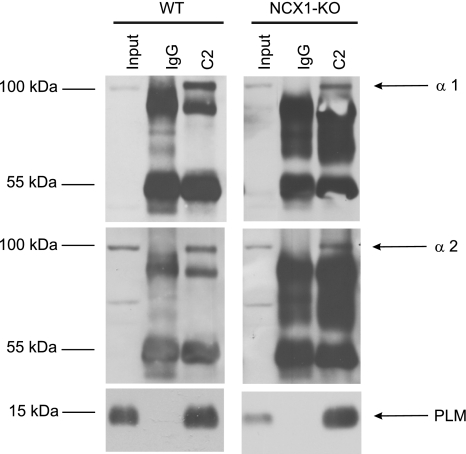
The results of Fig. 4 and Table 2 suggest that, quantitatively, the effect of PLM on the α1-subunit was much larger than that on the α2-subunit of Na+-K+-ATPase. We therefore examined whether PLM associated with both subunits of Na+-K+-ATPase. In WT myocytes, PLM coimmunoprecipitated with α1- and α2-subunits of Na+-K+-ATPase (Fig. 5). Since the α2-subunit of Na+-K+-ATPase is known to regulate NCX1 activity (17, 27), and since PLM physically associates with NCX1 in cardiac myocytes (1), we tested the hypothesis that PLM indirectly associates with the α2-subunit of Na+-K+-ATPase via NCX1. In LV homogenates prepared from cardiac-specific NCX1-KO hearts (16, 20), PLM coimmunoprecipitated with α1- and α2-subunits of Na+-K+-ATPase (Fig. 5), indicating that PLM directly associated with the α2-subunit of Na+-K+-ATPase. Under our experimental conditions and using commercially available antibodies, we could not detect β1- and β2-subunits of Na+-K+-ATPase in the PLM immunoprecipitates.
Fig. 5.
Coimmunoprecipitation of PLM with α1- and α2-subunits of Na+-K+-ATPase is independent of Na+/Ca2+ exchanger (NCX1). Immunoprecipitates from precleared LV homogenates (Input) from either WT or NCX1-KO mice with 5 μg of anti-PLM antibody (C2) or control IgG were obtained. PLM and α1- and α2-subunits of Na+-K+-ATPase were identified by immunoblotting with anti-PLM, anti-α1, and anti-α2 antibodies, respectively. Antibodies used for immunoblots are indicated on right, and molecular mass markers (in kDa) are shown on left. This experiment was performed 5 times with similar results.
DISCUSSION
Previous studies on PLM-KO mice indicate cardiac hypertrophy compared with WT hearts, regardless of whether the mice were of mixed C57BL/6 and 129/SvJ (18) or congenic C57BL/6 (2) background. Our ∼45% increase in estimated LV mass in PLM-KO hearts appears higher than the ∼23.5% increase in heart weight-to-tibial length ratio (18) and the ∼12.1% increase in total ventricular-to-body weight ratio (2) reported in previous studies. The varied degrees of cardiac hypertrophy are not due to different ages of the animals studied (∼3 mo of age in all 3 studies) but likely related to different measurement techniques for cardiac hypertrophy. The results from three independent laboratories strongly indicate that genetic ablation of PLM results in modest myocardial hypertrophy. A new finding is that PLM-KO hearts exhibited a higher degree of fibrosis, perhaps accounting for part of the myocardial hypertrophy and reduced maximal relaxation rate (−dP/dt). Interestingly, single myocytes isolated from PLM-KO mice (3–6 mo old) of congenic C57BL/6 background do not exhibit cellular hypertrophy, as evidenced by similar cell lengths and widths (29) and whole cell capacitance (a measure of cell surface membrane area) (10, 29) compared with WT myocytes. Although it is possible that our cell isolation procedure may preferentially select against hypertrophied myocytes, we doubt this is the case since we have been able to detect significant hypertrophy in myocytes isolated from 3-wk postinfarction mouse hearts compared with myocytes isolated from sham-operated hearts (unpublished results).
With respect to the effects of PLM on baseline myocardial contractility, early noninvasive studies on PLM-KO mice of mixed C57BL/6 and 129/SvJ genetic background indicate an ejection fraction that is 9% higher by magnetic resonance imaging (18). When in vivo hemodynamics were measured in PLM-KO mice of congenic C57BL/6 background both ejection fraction and cardiac output were slightly lower in PLM-KO hearts, but the differences did not reach statistical significance (2). Our first major findings that ejection fraction and cardiac output were not different between WT and PLM-KO hearts are in agreement with both of these studies (2, 18). Bell et al. (2), however, reported that both +dP/dt and −dP/dt are significantly depressed in PLM-KO hearts. Their results are in sharp contrast to our in vivo hemodynamic results, in which baseline +dP/dt was significantly higher and −dP/dt was not different in PLM-KO hearts. The differences between our results and those of Bell et al. (2) may be due to different anesthesia (ketamine, medetomidine, and atropine vs. tribromoethanol-amylene hydrate), mechanical ventilation vs. spontaneous respiration, different surgical techniques (opening the chest followed by LV puncture vs. catheterizing right carotid artery in a closed-chest preparation), and potentially increased heat dissipation in open-chest mice. The weight of present evidence suggests that baseline in vivo performance of PLM-KO hearts is at least as good as, if not better than, that of WT hearts.
Despite similar protein levels of α1- and α2-subunits of Na+-K+-ATPase in WT and congenic PLM-KO hearts (2, 29), Na+-K+-ATPase enzymatic activity (2) and Ipump (10, 25) are higher in PLM-KO hearts, as expected from the relief of inhibition of Na+-K+-ATPase. Higher Na+ pump activity would result in lower contractility in PLM-KO hearts, as predicted by the paradigm of Bell et al. (2). Our observation as well as those of others (2, 18), however, indicated that baseline performance of PLM-KO hearts was at least as good, if not better, than that of WT hearts. The discrepancy between theoretical prediction and experimental observations may be reconciled by the following considerations. First, there may be a distinct pool of Na+-K+-ATPase not regulated by PLM but intimately involved in regulation of cardiac contractility in the basal state. This hypothesis is given support by our present findings in C57BL/6 murine myocytes and those of Silverman et al. (22) in guinea pig myocytes that the regulatory effects of PLM were more apparent for the α1- than the α2-subunit of Na+-K+-ATPase. The α2-subunit of Na+-K+-ATPase is preferentially distributed in T tubules in mouse myocytes (3) and is involved in regulation of Ca2+ (17) and contractility (27). A second possibility is that maintenance of [Na+]i under basal conditions only requires a fraction of cardiac Na+-K+-ATPase activity. In the resting state, the fraction of PLM phosphorylated at serine 68 has been estimated to be between 25% and 46% in rodent hearts (22, 24, 33). The fact that inhibition of Na+-K+-ATPase by the ∼54–75% unphosphorylated PLM in WT myocytes did not result in any discernible differences in baseline [Na+]i compared with PLM-KO myocytes (10, 12) provides support for the second hypothesis. A third plausible explanation is that PLM regulates Na+-K+-ATPase activity by both Vmax (2, 33) and Km effects (6, 10). Since resting [Na+]i (5–11 mM) (11, 14) is close to the Km for Na+ of Na+-K+-ATPase (∼10 mM) (11, 33) in rodent myocytes, the effects of PLM on Na+-K+-ATPase activity at resting [Na+]i may be rather small. This interpretation is supported by our observation that Ipump was higher in PLM-KO myocytes under high- but not low-[Na+]pip conditions.
Another major new finding of the present study is that despite higher baseline +dP/dt in PLM-KO hearts, the maximal in vivo inotropic response to β-adrenergic agonists was similar between WT and PLM-KO hearts. This is in agreement with our previous observation (29) that maximal contraction amplitudes were not different between WT and PLM-KO myocytes stimulated with Iso, whether measured at 0.6, 1.8, or 5.0 mM [Ca2+]o. Genetic ablation of PLM also did not reduce the sensitivity of β-adrenergic receptors in the heart, as indicated by the absence of shift of the dose-response curve. The most striking difference between WT and PLM-KO hearts stimulated with Iso was that after reaching maximal +dP/dt cardiac contractility started to decline in WT but not PLM-KO hearts. Despa et al. (12) suggested that β-adrenergic agonists increase PLM phosphorylation at serine 68, thereby disinhibiting Na+-K+-ATPase, resulting in lower [Na+]i. The lower [Na+]i promotes Ca2+ efflux via NCX1, resulting in lower [Ca2+]i transient amplitudes and, by inference, myocyte contractility. Our observations not only confirm but extend the findings of Despa et al. (12) in that insights from in vitro myocyte studies are successfully applied to explain in vivo observations.
Our [Na+]i of 5.5–7.8 mM in quiescent murine myocytes was similar to the 5.1 ± 0.3 mM and 7.8 ± 0.3 mM reported for guinea pig and rat myocytes, respectively (14), but somewhat lower than the 12.5 ± 1.8 mM in WT murine myocytes reported by Despa et al. (10). In agreement with the results of Despa et al. (10), we did not detect significant differences in resting [Na+]i between WT and PLM-KO myocytes. [Na+]i increased with pacing, and the rise of [Na+]i was greater at the higher contraction rate. After 2 min of pacing, our observed increase in [Na+]i from resting values was 0.75–0.95 mM at 0.5 Hz and 1.6–2.9 mM at 2 Hz. This finding is similar to the 0.7 and 1.6 mM increase in [Na+]i when rat myocytes were paced at 0.5 and 2 Hz, respectively (14). At 2 Hz, Despa et al. (12) also showed ∼2 mM increase in [Na+]i in both WT and PLM-KO myocytes after 2 min of pacing. The agreement between our results and those of other investigators lends credence to our SBFI measurements.
Our results at 2 Hz are in agreement with those of Despa et al. (12) in that the decline in myocyte contraction amplitude following Iso addition to WT myocytes was associated with decline in [Na+]i and [Ca2+]i transient amplitudes. By contrast, when myocytes were paced at 0.5 Hz, increases in [Na+]i were small and both [Ca2+]i transient and contraction amplitudes remained at near-maximal levels in both WT and PLM-KO myocytes. The results of our experiments suggest that the regulatory effects of PLM on Na+-K+-ATPase were manifest under stressful conditions such as high [Na+]i. When [Na+]i was not markedly elevated (e.g., at rest or slowly contracting), there were no appreciable effects of PLM on myocyte contractility or [Ca2+]i homeostasis. Thus one of the major functions of PLM is to minimize [Na+]i overload under conditions of stress.
We previously demonstrated (1, 7, 34) that PLM directly regulates NCX1, independent of its effects on Na+-K+-ATPase. In addition, by expressing PLM mutants that selectively inhibit either Na+-K+-ATPase or NCX1 in PLM-KO myocytes, we demonstrate that the effects of PLM on myocyte contractility and [Ca2+]i transients are primarily mediated via NCX1 rather than Na+-K+-ATPase (25). The results of our present study and those of Despa et al. (12) therefore appear to be in conflict with the conclusions based on our previous studies (23, 25, 29). We submit, however, that there is no conflict. Under resting conditions (1 Hz, 1.8 mM [Ca2+]o, and no Iso), there are no differences in [Ca2+]i transient and contraction amplitudes between WT and PLM-KO myocytes (25, 29). Similarly, there are no differences in baseline [Na+]i between WT and PLM-KO myocytes incubated at 1.0 mM [Ca2+]o (12). Therefore, when myocytes are not stressed, the effects of PLM on contractility mediated by either Na+-K+-ATPase or NCX1 are not apparent. However, depending on how myocytes are stressed, the effects of PLM on these two important ion transporters become manifest. Thus when either Ca2+ influx or Ca2+ efflux is favored by increasing or decreasing [Ca2+]o, the effects of PLM on myocyte contractility are mediated primarily via its actions on NCX1 (25, 29). On the other hand, when myocytes are stressed by [Na+]i loading (Iso stimulation and high pacing rate), the effects of PLM on Na+-K+-ATPase become dominant (12). Therefore, at the level of a single myocyte, the effects of PLM on contractility and [Ca2+]i homeostasis can be mediated via its actions on Na+-K+-ATPase or NCX1, depending on experimental conditions. In the intact heart, except under conditions of maximal β-adrenergic stimulation (and presumably [Na+]i loading), the mechanisms by which PLM regulates contractility (Na+-K+-ATPase vs. NCX1) are much more complex to dissect and may well require new approaches and novel genetic models to further address the issue.
There are some limitations to the present study. First, since Iα1 is the dominant pump current, determination of Iα2 relies on subtraction of two large numbers, which by its very nature is imprecise. Therefore, our measurement method may not be sensitive enough to detect small changes in Iα2 in response to Iso stimulation. Indeed, using “SWAP” mice in which the ouabain affinities of the α-subunits are reversed, Bossuyt et al. (6) demonstrated that PLM regulates the apparent affinities for Na+ of both α1- and α2-subunits of Na+-K+-ATPase. In addition, using Xenopus oocytes heterologously expressing PLM and Na+-K+-ATPase, Bibert et al. (5) showed that phosphorylated PLM increases the apparent affinities for Na+ of both α1- and α2-subunits of Na+-K+-ATPase. Therefore, PLM likely regulates both α1- and α2-subunits of Na+-K+-ATPase. Second, we did not measure [Na+]i in a beating heart in vivo. Present technology using Na+-sensitive fluorescent probes is largely limited to nonbeating cardiac preparations at room temperature (2).
In summary, baseline myocardial contractility in PLM-KO mice was equal to, if not better than, that in WT animals. Maximal β-adrenergic stimulation resulted in initial increase in inotropy followed by decline in WT but not PLM-KO hearts. Under conditions of [Na+]i loading, PLM tempered the inexorable rise in [Na+]i at the expense of decreased inotropy. PLM associated with both α1- and α2-subunits of Na+-K+-ATPase, and the association was independent of NCX1.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants RO1-HL-58672 and RO1-HL-74854 (J. Y. Cheung), RO1-HL-56205, RO1-HL-61690, RO1-HL-85503, PO1-HL-75443, and PO1-HL-91799 (W. J. Koch), RO1-HL-48509 and RO1-HL-49101 (K. D. Philipson), PO1-HL-91799 (Project 2) and the Pennsylvania Research Formulary Fund (A. M. Feldman), and American Heart Association Scientist Development Grant F64702 (T. O. Chan).
DISCLOSURES
The authors are not aware of financial conflict(s) with the subject matter or materials discussed in this manuscript with any of the authors, or any of the authors' academic institutions or employers.
REFERENCES
- 1.Ahlers BA, Zhang XQ, Moorman JR, Rothblum LI, Carl LL, Song J, Wang J, Geddis LM, Tucker AL, Mounsey JP, Cheung JY. Identification of an endogenous inhibitor of the cardiac Na+/Ca2+ exchanger, phospholemman. J Biol Chem 280: 19875–19882, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bell JR, Kennington E, Fuller W, Dighe K, Donoghue P, Clark JE, Jia LG, Tucker AL, Moorman JR, Marber MS, Eaton P, Dunn MJ, Shattock MJ. Characterization of the phospholemman knockout mouse heart: depressed left ventricular function with increased Na-K-ATPase activity. Am J Physiol Heart Circ Physiol 294: H613–H621, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Berry RG, Despa S, Fuller W, Bers DM, Shattock MJ. Differential distribution and regulation of mouse cardiac Na+/K+-ATPase alpha1 and alpha2 subunits in T-tubule and surface sarcolemmal membranes. Cardiovasc Res 73: 92–100, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Bibert S, Roy S, Schaer D, Horisberger JD, Geering K. Phosphorylation of phospholemman (FXYD1) by protein kinases A and C modulates distinct Na,K-ATPase isozymes. J Biol Chem 283: 476–486, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bossuyt J, Despa S, Han F, Hou Z, Robia SL, Lingrel JB, Bers DM. Isoform-specificity of the Na/K-ATPase association and regulation by phospholemman. J Biol Chem 284: 26749–26757, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung JY, Rothblum LI, Moorman JR, Tucker AL, Song J, Ahlers BA, Carl LL, Wang J, Zhang XQ. Regulation of cardiac Na+/Ca2+ exchanger by phospholemman. Ann NY Acad Sci 1099: 119–134, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Crambert G, Fuzesi M, Garty H, Karlish S, Geering K. Phospholemman (FXYD1) associates with Na,K-ATPase and regulates its transport properties. Proc Natl Acad Sci USA 99: 11476–11481, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis CE, Patel MK, Miller JR, John JE, 3rd, Jones LR, Tucker AL, Mounsey JP, Moorman JR. Effects of phospholemman expression on swelling-activated ion currents and volume regulation in embryonic kidney cells. Neurochem Res 29: 177–187, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Despa S, Bossuyt J, Han F, Ginsburg KS, Jia LG, Kutchai H, Tucker AL, Bers DM. Phospholemman-phosphorylation mediates the beta-adrenergic effects on Na/K pump function in cardiac myocytes. Circ Res 97: 252–259, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Despa S, Islam MA, Pogwizd SM, Bers DM. Intracellular [Na+] and Na+ pump rate in rat and rabbit ventricular myocytes. J Physiol 539: 133–143, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Despa S, Tucker AL, Bers DM. PLM-mediated activation of Na/K-ATPase limits [Na]i and inotropic state during beta-adrenergic stimulation in mouse ventricular myocytes. Circulation 117: 1849–1855, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller W, Howie J, McLatchie L, Weber R, Hastie CJ, Burness K, Pavlovic D, Shattock MJ. FXYD1 phosphorylation in vitro and in adult rat cardiac myocytes: threonine 69 is a novel substrate for protein kinase C. Am J Physiol Cell Physiol 296: C1346–C1355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison SM, McCall E, Boyett MR. The relationship between contraction and intracellular sodium in rat and guinea-pig ventricular myocytes. J Physiol 449: 517–550, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heineke J, Wollert KC, Osinska H, Sargent MA, York AJ, Robbins J, Molkentin JD. Calcineurin protects the heart in a murine model of dilated cardiomyopathy. J Mol Cell Cardiol ( October22, 2009). doi: 10.1016/j.yjmcc.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res 95: 604–611, 2004 [DOI] [PubMed] [Google Scholar]
- 17.James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA, Lingrel JB. Identification of a specific role for the Na,K-ATPase alpha2 isoform as a regulator of calcium in the heart. Mol Cell 3: 555–563, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Jia LG, Donnet C, Bogaev RC, Blatt RJ, McKinney CE, Day KH, Berr SS, Jones LR, Moorman JR, Sweadner KJ, Tucker AL. Hypertrophy, increased ejection fraction, and reduced Na-K-ATPase activity in phospholemman-deficient mice. Am J Physiol Heart Circ Physiol 288: H1982–H1988, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Palmer CJ, Scott BT, Jones LR. Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J Biol Chem 266: 11126–11130, 1991 [PubMed] [Google Scholar]
- 20.Pott C, Philipson KD, Goldhaber JI. Excitation-contraction coupling in Na+-Ca2+ exchanger knockout mice: reduced transsarcolemmal Ca2+ flux. Circ Res 97: 1288–1295, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Presti CF, Jones LR, Lindemann JP. Isoproterenol-induced phosphorylation of a 15-kilodalton sarcolemmal protein in intact myocardium. J Biol Chem 260: 3860–3867, 1985 [PubMed] [Google Scholar]
- 22.Silverman BZ, Fuller W, Eaton P, Deng J, Moorman JR, Cheung JY, James AF, Shattock MJ. Serine 68 phosphorylation of phospholemman: acute isoform-specific activation of cardiac Na/K ATPase. Cardiovasc Res 65: 93–103, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Song J, Zhang XQ, Carl LL, Qureshi A, Rothblum LI, Cheung JY. Overexpression of phospholemman alters contractility and [Ca2+]i transients in adult rat myocytes. Am J Physiol Heart Circ Physiol 283: H576–H583, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Song J, Zhang XQ, Ahlers BA, Carl LL, Wang J, Rothblum LI, Stahl RC, Mounsey JP, Tucker AL, Moorman JR, Cheung JY. Serine 68 of phospholemman is critical in modulation of contractility, [Ca2+]i transients, and Na+/Ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 288: H2342–H2354, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Song J, Zhang XQ, Wang J, Cheskis E, Chan TO, Feldman AM, Tucker AL, Cheung JY. Regulation of cardiac myocyte contractility by phospholemman: Na+/Ca2+ exchange vs. Na+-K+-ATPase. Am J Physiol Heart Circ Physiol 295: H1615–H1625, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweadner KJ, Rael E. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics 68: 41–56, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Swift F, Tovsrud N, Enger UH, Sjaastad I, Sejersted OM. The Na+/K+-ATPase alpha2-isoform regulates cardiac contractility in rat cardiomyocytes. Cardiovasc Res 75: 109–117, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Tadros GM, Zhang XQ, Song J, Carl LL, Rothblum LI, Tian Q, Dunn J, Lytton J, Cheung JY. Effects of Na+/Ca2+ exchanger downregulation on contractility and [Ca2+]i transients in adult rat myocytes. Am J Physiol Heart Circ Physiol 283: H1616–H1626, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Tucker AL, Song J, Zhang XQ, Wang J, Ahlers BA, Carl LL, Mounsey JP, Moorman JR, Rothblum LI, Cheung JY. Altered contractility and [Ca2+]i homeostasis in phospholemman-deficient murine myocytes: role of Na+/Ca2+ exchange. Am J Physiol Heart Circ Physiol 291: H2199–H2209, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waalas SI, Czernik AJ, Olstad OK, Sletten K, Walaas O. Protein kinase C and cyclic AMP-dependent protein kinase phosphorylate phospholemman, an insulin and adrenaline-regulated membrane phosphoprotein, at specific sites in the carboxy terminal domain. Biochem J 304: 635–640, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Chan TO, Zhang XQ, Gao E, Song J, Koch WJ, Feldman AM, Cheung JY. Induced overexpression of Na+/Ca2+ exchanger transgene: altered myocyte contractility, [Ca2+]i transients, SR Ca2+ contents, and action potential duration. Am J Physiol Heart Circ Physiol 297: H590–H601, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang XQ, Ahlers BA, Tucker AL, Song J, Wang J, Moorman JR, Mounsey JP, Carl LL, Rothblum LI, Cheung JY. Phospholemman inhibition of the cardiac Na+/Ca2+ exchanger. Role of phosphorylation. J Biol Chem 281: 7784–7792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang XQ, Moorman JR, Ahlers BA, Carl LL, Lake DE, Song J, Mounsey JP, Tucker AL, Chan YM, Rothblum LI, Stahl RC, Carey DJ, Cheung JY. Phospholemman overexpression inhibits Na+-K+-ATPase in adult rat cardiac myocytes: relevance to decreased Na+ pump activity in postinfarction myocytes. J Appl Physiol 100: 212–220, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang XQ, Qureshi A, Song J, Carl LL, Tian Q, Stahl RC, Carey DJ, Rothblum LI, Cheung JY. Phospholemman modulates Na+/Ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 284: H225–H233, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol 279: H429–H436, 2000. [DOI] [PubMed] [Google Scholar]