Abstract
The lipid mediator sphingosine 1-phosphate (S1P) confers survival benefits in cardiomyocytes and isolated hearts subjected to oxidative stress. High-density lipoprotein (HDL) is a major carrier of S1P in the serum, but whether HDL-associated S1P directly mediates survival in a preparation composed exclusively of cardiomyocytes has not been demonstrated. Accordingly, we tested the hypothesis that signal activation and survival during simulated ischemia-reperfusion injury in response to HDL require lipoprotein-associated S1P. As a model, we used adult mouse cardiomyocytes subjected to hypoxia-reoxygenation. Cells were treated or not with autologous mouse HDL, which significantly increased myocyte viability as measured by trypan blue exclusion. This survival effect was abrogated by the S1P1 and SIP3 receptor antagonist VPC 23019. The selective S1P3 antagonist CAY10444, the Gi antagonist pertussis toxin, the MEK (MAPK/ERK) kinase inhibitor PD-98059, and the phosphoinositide-3 kinase inhibitor wortmannin also inhibited the prosurvival effect of HDL. We observed that HDL activated both Akt (protein kinase B) and the MEK1/2-ERK1/2 pathway and also stimulated phosphorylation of glycogen synthase kinase-3β. ERK1/2 activation was through an S1P1 subtype receptor-Gi protein-dependent pathway, whereas the activation of Akt was inhibited by CAY10444, indicating mediation by S1P3 subtype receptors. We conclude that HDL, via its cargo of S1P, can directly protect cardiomyocytes against simulated oxidative injury in the absence of vascular effects and that prosurvival signal activation is dependent on both S1P1 and S1P3 subtype receptors.
sphingosine-1-phosphate (S1P) is a potent lipid mediator involved in a variety of cell activities, including growth, survival, differentiation, cytoskeletal rearrangements, motility, angiogenesis, and calcium mobilization (20, 32). Five subtypes of S1P-specific receptors, termed S1P1-SIP5, have been identified (4, 12). We have observed that S1P1 and S1P3 receptors are the most abundant subtypes in adult mouse cardiomyocytes (44). We also have reported that S1P reduces infarct size in ex vivo murine hearts subjected to ischemia-reperfusion injury (13) and enhances survival of hypoxic neonatal rat and adult mouse cardiomyocytes in vitro (15, 44).
Yatomi et al. (43) were the first to show that S1P is a component of human plasma, present at a concentration of ∼200 nM. Subsequent evidence demonstrated that lipoproteins serve as major carriers for extracellular S1P in circulating blood. Thus ∼65% of the S1P in blood is associated with the lipoproteins LDL, VLDL, and HDL, and the bulk of lipoprotein-associated S1P (∼54%) is bound to HDL (1, 26).
The plasma level of HDL is inversely correlated with the risk of atherosclerosis and associated cardiovascular disease (2, 29). In vascular endothelial cells, HDL mediates cell survival, cell migration, inhibition of adhesion molecule expression, and enhancement of endothelial nitric oxide (NO) production via its “cargo” of S1P (11, 16–18, 27, 28). HDL also affects functions of other cell types, such as smooth muscle cells and neural cells (22, 30, 34, 37). Kimura et al. (16, 17) recently reported that HDL-induced endothelial cell migration and survival in response to the absence of serum and growth factors are mediated by the S1P receptor subtypes S1P1 and S1P3, which couple to ERK and Akt (protein kinase B), respectively. It also has been demonstrated that the S1P3 receptor mediates HDL protection of the heart against ischemia-reperfusion injury in vivo (40).
However, it has not been fully established that S1P associated with HDL directly mediates cardiomyocyte survival produced by this lipoprotein in the absence of effects on the vasculature. In the present study, we tested the hypothesis that the effects of HDL on cultured adult mouse cardiomyocytes, including survival under hypoxic conditions and signal activation, require lipoprotein-associated S1P.
MATERIALS AND METHODS
Materials.
S1P, PD-98059, wortmannin, and pertussis toxin (PTX) were obtained from Biomol International (Plymouth Meeting, PA). VPC 23019 was obtained from Avanti Polar Lipids (Alabaster, AL). CAY10444 was acquired from Cayman Chemical (Ann Arbor, MI). Antibodies directed against phosphorylated and total endogenous forms of ERK, MEK (MAPK/ERK), Akt, and glycogen synthase kinase-3β (GSK-3β) were purchased from Cell Signaling Technology (Danvers, MA).
Animals.
Male C57Bl/6 mice (20–25 g) were purchased from Charles River Laboratories (Hollister, CA). All studies were approved by the Institutional Animal Care and Use Committee of the San Francisco Veterans Affairs Medical Center. This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Isolation of HDL from plasma.
C57Bl/6 mice were anesthetized with pentobarbital sodium and bled by retro-orbital puncture. HDLs (density 1.063–1.21 g/ml) were isolated from fresh plasma by preparative ultracentrifugation, as described previously (10), and dialyzed against phosphate-buffered saline. HDLs were stored on ice and used for experiments within 2 wk.
Adult mouse cardiomyocyte isolation and culture.
Adult mouse cardiomyocytes were isolated and cultured using a modification of the collagenase dissociation method described by Zhou et al. (45) as previously described in our laboratory (38, 44). Mice were treated with heparin (50 units) and anesthetized by intraperitoneal injection with pentobarbital sodium (200 mg/kg). The heart was quickly excised, and the aorta was cannulated for retrograde perfusion in a Langendorff apparatus at a constant flow rate of 3 ml/min at 37°C. The heart was perfused for 9–10 min with isolation buffer [120 mM NaCl, 5.4 mM KCl, 1.2 mM MgSO4, 1.2 mM NaH2PO4, 5.6 mM glucose, 5 mM NaHCO3, 10 mM HEPES, 50 μM CaCl2, 10 mM 2, 3-butanedione monoxime (BDM) and 5 mM taurine], followed by digestion for 9 min with collagenase II (1.5 mg/ml; Worthington, Lakewood, NJ) in isolation buffer. After digestion, the soft and flaccid heart was removed, and myocytes were suspended in isolation buffer. A series of four centrifugations (40 g, 1 min) and resuspensions were used for stepwise Ca2+ reintroduction from 50 μM to 1.0 mM, which was the final medium Ca2+ concentration.
Isolated cardiomyocytes were plated for 2 h on 35- and 60-mm tissue culture dishes coated with 10 μg/ml laminin. The cells were suspended in minimum essential medium (MEM) with Hanks' buffered salt solution (HBSS), 10 μg/ml penicillin, 1.5 μM vitamin B12, and 10 mM BDM. After this period of attachment, the medium was changed to MEM-HBSS containing 10 μg/ml penicillin, 1.5 μM vitamin B12, and 1 mM BDM and was incubated overnight at 37°C in a humidified atmosphere of 1% CO2 and air. The culture protocol yielded an average of 80% rod-shaped myocytes at a plating density of 50 cells/mm2 that were viable at pH 7.2 for 48 h. Experiments were performed the day following isolation and culture.
Hypoxia-reoxygenation protocol.
On the day after isolation and culture, cardiomyocytes were rinsed and subsequently incubated in a Bactron I anaerobic chamber containing a humidified atmosphere of 1% CO2 and 99% N2 for 3 h. This procedure produced a Po2 of <4 Torr. Experimental medium was changed to serum-free, glucose-free MEM with HBSS that did not contain BDM. This medium was preequilibrated for 16 h in an anaerobic chamber containing 1% CO2 and 99% N2. Normoxic experimental medium also was preequilibrated for 16 h in a water-jacketed incubator in a humidified atmosphere of 1% CO2 and air.
Measurement of cell survival.
Cardiomyocyte survival was measured by staining cells in tissue culture dishes with trypan blue solution (Sigma Chemical, St. Louis, MO) diluted to a final concentration of 0.04% (wt/vol). Myocytes were visualized using bright-field microscopy at ×100 magnification. The number of viable (unstained) and nonviable (blue stained) cardiac myocytes in 10 random microscopic fields was recorded, and at least 300 cells were counted in each dish. Percent survival was defined as the number of unstained myocytes counted in each hypoxic treatment dish divided by the number of unstained myocytes counted in each corresponding normoxic control dish. This calculation accounted for the detachment and loss of nonviable cells during the experimental hypoxia protocols.
Western blot analysis.
On the day after isolation and culture, cardiomyocytes were treated with pharmacological agonists and antagonists under normoxic conditions for the indicated times. For whole cell extraction, cells were lysed in buffer containing 20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, 0.1% protease inhibitor mixture (Roche Applied Science, Indianapolis, IN), and phosphatase inhibitor cocktails (Sigma). Protein concentration was determined using the Bradford method. Equal amounts of protein were resuspended in 4× Laemmli sample buffer, boiled for 5 min, and subjected to sodium dodecyl (lauryl) sulfate-polyacrylamide gel electrophoresis. After transfer to a polyvinylidene fluoride membrane, the extract was blocked in 5% nonfat milk in Tris-buffered saline (TBS) plus 0.1% Tween 20 for 1 h. The membranes were probed overnight with primary antibodies, washed three times with TBS-Tween 20 for 5 min, and probed with secondary antibodies for 1 h. The membranes were rinsed three times, and the signal was detected using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instructions.
Statistical analysis.
Data are means ± SE. Mean values were compared using one-way analysis of variance and post hoc Student-Newman-Keuls testing. P < 0.05 was considered statistically significant.
RESULTS
HDL protects adult mouse cardiomyocytes against hypoxia-reoxygenation injury.
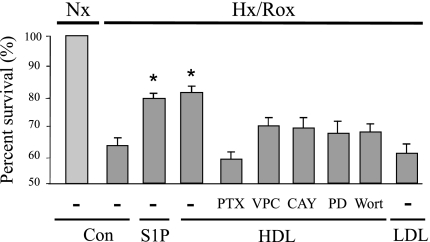
In initial studies we subjected isolated adult mouse cardiomyocytes to 3 h of hypoxia and 16 h of reoxygenation. As shown in Fig. 1, myocyte viability as determined by trypan blue exclusion was markedly diminished. Both S1P and HDL increased viability significantly and had equivalent effects. This increase was substantially but not completely inhibited by the S1P1 and SIP3 receptor antagonist VPC 23019, indicating that it is the S1P associated with HDL that enhances myocyte survival. Substantial but not complete inhibition also was achieved by the selective S1P3 receptor antagonist CAY10444, the Gi antagonist PTX, the MEK kinase inhibitor PD-98059, and the phosphoinositide-3 kinase (PI3-kinase) inhibitor wortmannin. None of the inhibitors by themselves affected viability during hypoxia-reoxygenation (n = 3–4; data not shown). Interestingly, LDL by itself did not have a significant effect on cell viability (Fig. 1).
Fig. 1.
Cell viability after exposure to hypoxia and glucose deprivation. Isolated adult mouse cardiomyocytes were exposed to hypoxia and glucose deprivation for 3 h and then reoxygenated for 16 h in serum-free medium containing glucose. Sphingosine 1-phosphate (S1P) at a concentration of 200 nM was added at the time of reoxygenation. HDL at a concentration of 100 μg/ml and the S1P1 and S1P3 antagonist VPC23019 (VPC; 1 μM), the S1P3 antagonist CAY10444 (CAY; 10 μM), the MEK kinase inhibitor PD-98059 (PD; 10 μM), the phosphoinositide-3 kinase (PI3-kinase) inhibitor wortmannin (Wort; 200 nM), or LDL (50 μg/ml) was added at the time of reoxygenation. Pertussis toxin (PTX; 100 ng/ml) was added 16 h before the initiation of the experiment. Cell viability was quantified by staining cells with trypan blue solution and counting the number of cells that excluded this agent. Survival is expressed as the percentage of the normoxic control values, which are set at 100%. Data are means ± SE of 3–8 independent experiments. *P < 0.05 vs. other conditions. Nx, normoxia; Hx/Rox, hypoxia-reoxygenation; Con, control.
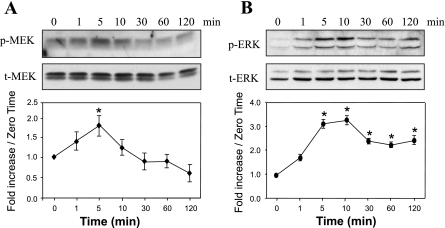
HDL activates the MEK1/2-ERK1/2 pathway.
At 100 μg/ml, HDL elicited transient phosphorylation of MEK1/2, starting at 1 min and reaching a maximum at 5 min, with no change in the level of nonphosphorylated MEK1/2 (Fig. 2A). HDL at 100 μg/ml also stimulated time-dependent ERK1/2 phosphorylation, starting at 1 min and reaching a maximum between 5 and 10 min, with no change in the level of nonphosphorylated ERK 1/2 (Fig. 2B).
Fig. 2.
HDL induces a time-dependent increase in the phosphorylation of MEK1/2 and ERK1/2 in adult mouse cardiomyocytes. Myocytes were exposed or not to 100 μg/ml HDL for different times (1–120 min; A and B). Western blot analyses of phosphorylated (p-)MEK 1/2, total (t-)MEK1/2, p-ERK1/2, and t-ERK1/2 representative of 3 independent experiments are shown. The band intensities of phosphoproteins were normalized against total MEK or ERK and expressed as fold increase over the zero time point. *P < 0.05 compared with all values without an asterisk.
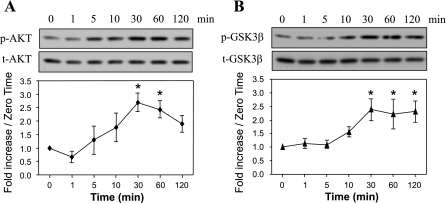
HDL activates Akt and inactivates GSK-3β.
As indicated in Fig. 3A, HDL at 100 μg/ml stimulated time-dependent Akt phosphorylation, starting at 10 min and reaching a maximum around 30 min, with no change in the level of nonphosphorylated Akt. Juhaszova et al. (14) determined that the beneficial effects of Akt activation on myocyte viability during hypoxia-reoxygenation were caused in large part by phosphorylation and inactivation of GSK-3β. In the present experiment, HDL-induced GSK-3β phosphorylation was time dependent, starting at 10 min, reaching a maximum at 30 min, and maintaining this level for at least 90 min (Fig. 3B).
Fig. 3.
HDL induces a time-dependent increase in the phosphorylation of Akt (protein kinase B) and glycogen synthase kinase-3β (GSK-3β) in adult mouse cardiomyocytes. Myocytes were exposed or not to 100 μg/ml HDL for different times (1–120 min; A and B). Western blot analyses of p-Akt, t-Akt, p-GSK-3β, and t-GSK-3β representative of 3 independent experiments are shown. The band intensities of phosphoproteins were normalized against total Akt or GSK-3β and expressed as fold increase over the zero time point. *P < 0.05 compared with all values without an asterisk.
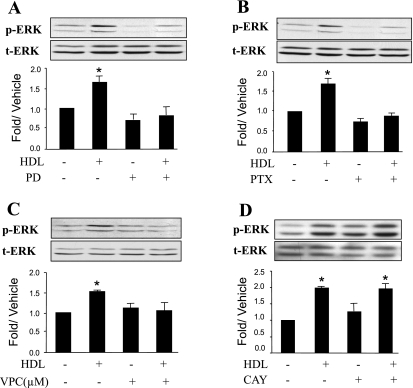
HDL activates ERK1/2 through a S1P1 receptor-Gi protein-dependent pathway in adult cardiomyocytes.
As shown in Fig. 4A, HDL-mediated ERK activation was markedly inhibited by PD-98059, an ERK kinase inhibitor. The ERK response to HDL was completely inhibited by PTX (Fig. 4B), suggesting an involvement of toxin-sensitive Gi proteins. We previously reported that adult cardiomyocytes express mRNA for the S1P1, S1P2, and S1P3 receptors, which couple to both Gi and Gq family G proteins (44). To test which S1P receptor is involved in ERK activation, we used VPC 23019, a selective S1P1 and SIP3 receptor antagonist. This antagonist has dissociation constants of 1.38 × 10−8 and 1.17 × 10−6 M for S1P1 and S1P3 receptors, respectively; thus, at 1 μM, it is expected to block >98% of S1P1 receptors and approximately one-half of S1P3 receptors (7). As shown in Fig. 4C, VPC 23019 inhibited HDL-stimulated ERK1/2 activation. In contrast, as depicted in Fig. 4D, HDL-stimulated ERK1/2 activation was insensitive to CAY10444, an S1P3-selective antagonist (19). These observations support the conclusion that HDL stimulated ERK activation through a S1P1 receptor-Gi protein-dependent pathway.
Fig. 4.
HDL-stimulated ERK activation is PTX sensitive, requires MEK phosphorylation, and is mediated by S1P receptors. Quiescent cardiomyocytes were treated or not for 1 h with the MEK inhibitor PD (10 μM; A) or for 16 h with the Gi/o inhibitor PTX (100 ng/ml; B) before incubation with 100 μg/ml HDL for 10 min. The phosphorylation of ERK1/2 induced by HDL was significantly reduced by PTX or PD. Quiescent cardiomyocytes were also pretreated for 1 h with the S1P1 and SIP3 receptor antagonist VPC (1 μM; C) or the selective S1P3 receptor antagonist CAY (10 μM; D) before stimulation with 100 μg/ml HDL for 10 min. Equal gel loading was assessed using an antibody against total ERK1/2. Specific bands corresponding to phosphorylated forms of ERK1/2 were quantified by densitometry and expressed as a fold change relative to the corresponding vehicle control. Data are means ± SE of 3–5 independent experiments. *P < 0.05, HDL vs. all other values. Note that in D, where n = 5, HDL doubled p-ERK, and there was no effect of CAY.
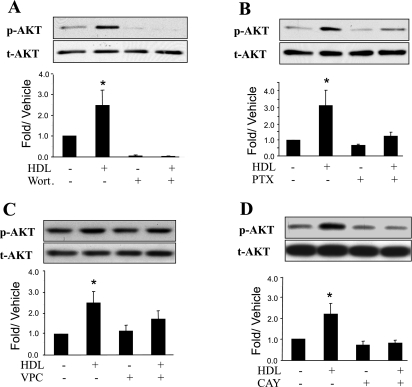
HDL activates Akt through a S1P3 receptor-dependent pathway in adult cardiomyocytes.
As shown in Fig. 5A, HDL-mediated Akt activation was markedly inhibited by wortmannin, a PI3-kinase inhibitor. Similarly, Akt activation in PTX-treated cardiomyocytes was markedly reduced compared with that in untreated cells (Fig. 5B). The subtype of S1P receptor responsible for the S1P-induced Akt activation was examined using S1P receptor subtype-selective antagonists. As shown in Fig. 5, C and D, activation of Akt was completely antagonized by the S1P3-selective antagonist CAY10444. In contrast, VPC 23019, which completely inhibits S1P1 receptors but only partially blocks S1P3 receptors at a concentration of 1 μM (7), did not significantly inhibit Akt activation. These results suggest that S1P3 receptor is the predominant receptor that mediates HDL-induced Akt activation.
Fig. 5.
HDL-mediated Akt activation is CAY-sensitive and is inhibited by wortmannin. Quiescent cardiomyocytes were treated or not for 1 h with the Akt inhibitor Wort (200 nM; A), for 16 h with the Gi/o inhibitor PTX (100 ng/ml; B), for 1 h with the S1P1 and SIP3 receptor antagonist VPC (1 μM; C), or for 1 h with the S1P3 receptor antagonist CAY (10 μM; D) before incubation with 100 μg/ml HDL for 30 min. Equal gel loading was assessed using an antibody against total Akt. Specific bands corresponding to phosphorylated forms of Akt were quantified by densitometry and expressed as a fold change relative to the corresponding vehicle control. Data are means ± SE of 3–5 independent experiments. *P < 0.05, HDL vs. all other values.
DISCUSSION
The major findings of the present study are that HDL applied directly to isolated adult mouse cardiomyocytes enhances cell survival during hypoxia-reoxygenation. We also have shown that HDL acting via Gi stimulates the prosurvival signals ERK1/2 and Akt. These prosurvival signals are mediated by S1P1 and S1P3 receptors located on the myocytes and are markedly attenuated by inhibitors of these receptors. We also observed that HDL causes phosphorylation and hence inactivation of GSK-3β, a molecule that acts as a key regulator of proapoptotic pathways (14). Although prior studies have implicated S1P-associated HDL in acute cardioprotection (36), the intact models used focused on vascular responses and precluded assessment of direct myocyte effects. This is the first study to demonstrate the S1P-mediated prosurvival mechanisms by which HDL, as a major carrier of S1P in the serum, is able to directly protect cardiomyocytes against simulated ischemic injury.
As noted above, previous acute studies of the cardioprotective effect of HDL have explored the vascular mechanisms involved. Results have shown that human HDL stimulated murine myocardial perfusion in vivo, an effect that was abolished in S1P3 receptor null mice (20). Nofer et al. (28) reported that HDL induced NO release in human endothelial cells and caused NO-dependent vasorelaxation via the S1P3 receptor. In mice lacking this receptor, these effects could be suppressed. Theilmeier et al. (40) demonstrated that HDL and its constituent S1P acutely protected the murine heart against ischemia-reperfusion injury via an S1P3-mediated and NO-dependent pathway. In contrast, Means et al. (23) reported that deletion of neither the S1P2 nor the S1P3 receptor alone affected infarct size or Akt activation. However, in double knockout mice, infarct size following ischemia-reperfusion was increased by 50% and Akt activation was markedly attenuated (23). It also has been shown that HDL impairs recruitment of polymorphonuclear cells to the infarcted area and reduces macrophage adhesion to activated endothelium in vitro (40).
Our prior in vitro work using an agonist antibody selective for the S1P1 receptor or the S1P1 receptor-specific agonist SEW2871 identified the importance of S1P1 receptor-mediated transduction of prosurvival signals in preserving myocyte viability during prolonged oxidative stress (39, 44). Using SEW2871 in desensitization studies, we also reported that ERK was activated by S1P1 receptor agonism (39). In isolated rat hearts, Tsukada et al. (42) also noted that SEW2871 protected against ischemia-reperfusion injury. In addition, the present study employing HDL implicates the S1P3 receptor in the activation of prosurvival signaling. Our findings are in agreement with a recent report by Means et al. (24), who demonstrated in adult mouse ventricular myocytes that S1P activated Akt via the S1P3 receptor. Thus our data provide evidence that both S1P1 and S1P3 receptors are required for cardioprotection by the S1P cargo of HDL and that both are necessary but each is not completely sufficient when they are separately inhibited.
Previous studies using HDL employed human preparations (16, 20, 27, 40), whereas we used autologous mouse HDL, which may exhibit different S1P-binding properties compared with the human lipoprotein. In the only prior study of cardiomyocytes, Theilmeier et al. (40) reported that 1 mg/mg of human HDL [a concentration 10-fold higher than we used] protected neonatal rat cardiomyocytes from apoptosis induced by glucose and growth factor withdrawal as shown by inhibition of caspase-3 processing and poly(ADP-ribose) polymerase cleavage.
Other reports also have suggested that HDL might have direct effects on the myocardium. In an early study, Mochizuki et al. (25) observed that HDL decreased the incidence of ventricular arrhythmias associated with ischemia-reperfusion injury by a mechanism involving PGI2. Using reconstituted HDLs containing apolipoprotein A-I and phosphatidylcholine, Calabresi et al. (5) reported improved hemodynamic recovery and reduced creatine kinase release in isolated rat hearts. The same group (3) also noted that HDL inhibited the activation of matrix metalloproteinase-2, which was associated with reduced myocardial injury. Theilmeier et al. (40) demonstrated that HDL and S1P equally inhibited apoptosis in the infarct area 24 h after ischemia-reperfusion injury. The mechanism by which HDL might gain access to cardiomyocytes has not been elucidated. However, it is well recognized that during reperfusion injury, vascular permeability increases markedly and large molecules such as albumin (mol wt ≈69,000, equivalent to the mol wt of small HDL species) can leak into the perivascular space and come into direct contact with cardiomyocytes (8, 33, 35, 41).
There also is evidence that synthetic HDLs, which initially are devoid of S1P, are cardioprotective in acute rodent ischemia-reperfusion models (5, 6, 9, 31). Possible mechanisms include a dose-dependent reduction of ischemia-induced tumor necrosis factor-α expression and content and enhanced prostaglandin release (5, 31) and reduced adhesion molecule (VCAM-1) expression (9). None of these studies measured prosurvival signals, and in none were there any experiments in which S1P action or its downstream consequences subjected to inhibition. Although these synthetic HDLs are prepared in the absence of S1P, the possibility has not been excluded that synthetic HDL in the perfusate of these experiments could scavenge S1P that is exported from cardiomyocytes (38) and redeliver it to these cells to mediate signaling through S1P receptors on the cell surface (9).
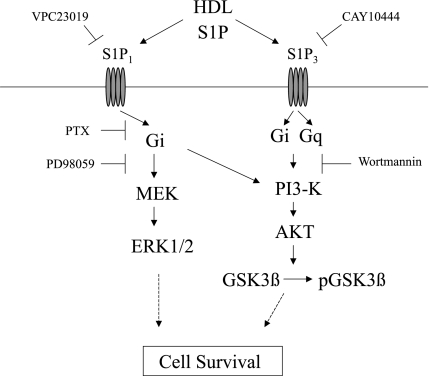
In summary, we have provided evidence that native murine HDL induces cytoprotection against hypoxia-reoxygenation injury through the G protein-coupled sphingolipid receptors S1P1 and S1P3. As shown in Fig. 6, results of the experiments using specific inhibitors suggest that HDL-induced cell survival in cardiomyocytes involves two major pathways: MEK1/2-ERK1/2 and PI3-kinase-Akt. The S1P1 receptor transduces signals via the former pathway, and the S1P3 receptor utilizes the latter pathway.
Fig. 6.
Proposed mechanisms by which HDL activates prosurvival signals in cardiomyocytes. See text for details.
GRANTS
Support was received from National Heart, Lung, and Blood Institute Grants 1P01 HL068738 and 1 R01 HL090606 (to J. S. Karliner) and 1R01 HL 089871 (to R. Raffai).
DISCLOSURES
None of the authors declares a conflict of interest.
REFERENCES
- 1.Argraves KM, Argraves WS. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J Lipid Res 48: 2325–2333, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Assmann G, Gotto AM., Jr HDL cholesterol and protective factors in atherosclerosis. Circulation 109, 23Suppl 1: III8–III14, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bellosta S, Gomaraschi M, Canavesi M, Rossoni G, Monetti M, Franceschini G, Calabresi L. Inhibition of MMP-2 activation and release as a novel mechanism for HDL induced cardioprotection. FEBS Lett 580: 5974–5978, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 115: 84–105, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Calabresi L, Rossoni G, Gomaraschi M, Sisto F, Berti F, Franceschini G. High density lipoproteins protect isolated rat hearts from ischemia-reperfusion injury by reducing cardiac tumor necrosis factor-α content and enhancing prostaglandin release. Circ Res 92: 330–337, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Chiesa G, Parolini C, Sirtori CR. Acute effects of high-density lipoproteins: biochemical basis and clinical findings. Curr Opinion Cardiol 23: 379–385, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem 280: 9833–9841, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Di Napoli P, Di Muzio M, Contegiacomo G, Tiloca P, Spoletini L, Di Crecchio A, Gallina S, Barsotti A. Ischemic preconditioning of the myocardium: the role of changes in the permeability of the coronary microcirculation. Cardiologica 42: 59–67, 1997 [PubMed] [Google Scholar]
- 9.Gomaraschi M, Calabresi L, Rossoni G, Lametti S, Franceschini G, Stonik JA, Remaley AT. Anti-inflammatory and cardioprotective activities of synthetic high-density lipoprotein containing apolipoprotein A-1 mimetic peptides. J Pharmacol Exp Ther 324: 776–783, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest 34: 1345–1353, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igarashi J, Miyoshi M, Hashimoto T, Kubota Y, Kosaka H. Statins induce S1P1 receptors and enhance endothelial nitric oxide production in response to high-density lipoproteins. Br J Pharmacol 150: 470–479, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem 73: 321–354, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, Goetzl EJ, Karliner JS, Gray MO. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKCε knockout mouse hearts. Am J Physiol Heart Circ Physiol 282: H1970–H1977, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3 mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113: 1535–1549, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol 33: 1713–1717, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, Kobayashi I, Ui M, Okajima F. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem 276: 31780–31785, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Kimura T, Sato K, Malchinkhuu E, Tomura H, Tamama K, Kuwabara A, Murakami M, Okajima F. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler Thromb Vasc Biol 23: 1283–1288, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kimura T, Tomura H, Mogi C, Kuwabara A, Admiring A, Ishizuka T, Sekiguchi A, Ishiwara M, Im DS, Sato K, Murakami M, Okajima F. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high-density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem 281: 37457–37467, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Koide Y, Hasegawa T, Takahashi A, Endo A, Mochizuki N, Nakagawa M, Nishida A. Development of novel EDG3 antagonists using a 3D database search and their structure-activity relationships. J Med Chem 45: 4629–4638, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Levkau B, Hermann S, Theilmeier G, van der Diet M, Chun J, Schober O, Schafers M. High-density lipoprotein stimulates myocardial perfusion in vivo. Circulation 110: 3355–3359, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, apoptosis. Biochim Biophys Acta 1585: 193–201, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Malchinkhuu E, Sato K, Muraki T, Ishikawa K, Kuwabara A, Okajima F. Assessment of the role of sphingosine 1-phosphate and its receptors in high-density lipoprotein-induced stimulation of astroglial cell function. Biochem J 370: 817–827, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 292: H2944–H2951, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Means CK, Miyamoto S, Chun J, Brown JH. S1P1 receptor localization confers selectivity for Gi-mediated cAMP and contractile responses. J Biol Chem 283: 11954–11963, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mochizuki S, Okumura M, Tanaka F, Sato T, Kagami A, Tada N, Nagano M. Ischemia-reperfusion arrhythmias and lipids: effect of human high and low-density lipoproteins on reperfusion arrhythmias. Cardiovasc Drug Ther 5, Suppl 2: 269–276, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, UIM , Okajima F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J 352: 809–815, 2000 [PMC free article] [PubMed] [Google Scholar]
- 27.Nofer JR, Levkau B, Wolinska I, Junker R, Fobker M, von Eckardstein A, Seedorf U, Assmann G. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J Biol Chem 276: 34480–34485, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Nofer JR, van der Giet M, Tölle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Gödecke A, Ishii I, Kleuser B, Schäfers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest 113: 569–581, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rader DJ. Regulation of reverse cholesterol transport and clinical implications. Am J Cardiol 92: 42J–49J, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Robbesyn F, Garcia V, Auge N, Vieira O, Frisach MF, Salvayre R, Negre-Salvayre A. HDL counterbalances the proinflammatory effect of oxidized LDL by inhibiting intracellular reactive oxygen species rise, proteasome activation, and subsequent NF-kappaB activation in smooth muscle cells. FASEB J 17: 743–745, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Rossoni G, Gomaraschi M, Verti F, Sirtori CR, Franceschini G, Calabresi L. Synthetic high-density lipoproteins exert cardioprotective effects in myocardial ischemia/reperfusion injury. J Pharmacol Exp Ther 308: 79–84, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res 94: 724–734, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Saeed M, van Dijke CF, Mann JS, Wendland MF, Rosenau W, Higgins CB, Brasch RC. Histologic confirmation of microvascular hyperpermeability to macromolecular MR contrast medium in reperfused myocardial infarction. J Magn Reson Imaging 8: 561–567, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Sato K, Malchinkhuu E, Horiuchi Y, Mogi C, Tomura H, Tosaka M, Yoshimoto Y, Kuwabara A, Okajima F. HDL-like lipoproteins in cerebrospinal fluid affect neural cell activity through lipoprotein-associated sphingosine 1-phosphate. Biochem Biophys Res Commun 359: 649–654, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Skepper JN, Pierson RN, Young VK, Rees JA, Powell JM, Navaratnam V, Cary NR, Tew DN, Bacon PJ, Wallwork J, White DJ, Menon DK. Cytochemical demonstration of sites of hydrogen peroxide generation and increased vascular permeability in isolated pig hearts after ischaemia and reperfusion. Microsc Res Tech 42: 369–385, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Sattler K, Levkau B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc Res 82: 201–211, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Tamama K, Tomura H, Sato K, Malchinkhuu E, Damirin A, Kimura T, Kuwabara A, Murakami M, Okajima F. High-density lipoprotein inhibits migration of vascular smooth muscle cells through its sphingosine 1-phosphate component. Atherosclerosis 178: 19–23, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Tao R, Zhang J, Vessey DA, Honbo N, Karliner JS. Deletion of the sphingosine kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc Res 74: 56–63, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Tao R, Hoover HE, Zhang J, Honbo N, Alano CC, Karliner JS. Cardiomyocyte S1P1 receptor-mediated extracellular signal-related kinase signaling and desensitization. J Cardiovasc Pharmacol 53: 486–494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schäfers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 114: 1403–1409, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Tilton RG, Daugherty A, Sutera SP, Larson KB, Land MP, Rateri DL, Kilo C, Williamson JR. Myocyte contracture, vascular resistance, and vascular permeability after global ischemia in isolated hearts from alloxan-induced diabetic rabbits. Diabetes 38: 1484–1491, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Tsukada YT, Sanna MG, Rosen H, Gottlieb RA. S1P1-selective agonist SEW2871 exacerbates reperfusion arrhythmias. J Cardiovasc Pharmacol 50: 660–669, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem (Tokyo) 121: 969–973, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol 293: H3150–H3158, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol 279: H429–H436, 2000. [DOI] [PubMed] [Google Scholar]