Abstract
Large-conductance Ca2+-activated K+ channels (BKCa) in the inner mitochondrial membrane may play a role in protecting against cardiac ischemia-reperfusion injury. NS1619 (30 μM), an activator of BKCa channels, was shown to increase respiration and to stimulate reactive oxygen species generation in isolated cardiac mitochondria energized with succinate. Here, we tested effects of NS1619 to alter matrix K+, H+, and swelling in mitochondria isolated from guinea pig hearts. We found that 30 μM NS1619 did not change matrix K+, H+, and swelling, but that 50 and 100 μM NS1619 caused a concentration-dependent increase in matrix K+ influx (PBFI fluorescence) only when quinine was present to block K+/H+ exchange (KHE); this was accompanied by increased mitochondrial matrix volume (light scattering). Matrix pH (BCECF fluorescence) was decreased slightly by 50 and 100 μM NS1619 but markedly more so when quinine was present. NS1619 (100 μM) caused a significant leak in lipid bilayers, and this was enhanced in the presence of quinine. The K+ ionophore valinomycin (0.25 nM), which like NS1619 increased matrix volume and increased K+ influx in the presence of quinine, caused matrix alkalinization followed by acidification when quinine was absent, and only alkalinization when quinine was present. If K+ is exchanged instantly by H+ through activated KHE, then matrix K+ influx should stimulate H+ influx through KHE and cause matrix acidification. Our results indicate that KHE is not activated immediately by NS1619-induced K+ influx, that NS1619 induces matrix K+ and H+ influx through a nonspecific transport mechanism, and that enhancement with quinine is not due to the blocking of KHE, but to a nonspecific effect of quinine to enhance current leak by NS1619.
Keywords: NS1619, quinine, Ca2+-sensitive K+ channels, K+ influx
the mitochondrial k+ cycle consists of influx and efflux of K+ between the mitochondrial matrix and the inter membrane space (26). It was proposed that K+ transport across the inner mitochondrial membrane (IMM) has distinctive roles in mitochondria such as volume homeostasis to prevent excess matrix swelling (41) or contraction (23), as well as a role in cell signaling (20, 29, 37). In the absence of ADP there exists a very high electrochemical potential gradient (ΔμH) and membrane potential gradient (ΔΨm) across the IMM, which creates a powerful driving force that favors K+ leak into the matrix (26). K+ entry may also occur via opening of putative mitochondrial K+ channels such as the ATP-regulated K+ channel (mKATP) or through the adenine nucleotide translocase (6). Regardless of the way K+ enters into the matrix, many studies suggest that matrix K+ influx and the physiological consequences it has on mitochondrial bioenergetics may play a key role in cardiac protection against ischemia and reperfusion (IR) injury (27, 51, 52).
There is some evidence for the existence of large-conductance voltage and Ca2+-activated K+ channels (BKCa) on the IMM of cardiac myocytes (mBKCa) (52). Similar to the putative mKATP channels, putative mBKCa channels, or their agonists, may be involved in ischemic preconditioning or pharmacologic preconditioning. The latter was demonstrated by using the BKCa channel activator 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one (NS1619) (14), although a recent study has questioned the specificity of this drug in activating putative mBKCa channels (13). Nonetheless, NS1619 was shown to produce cardiac protection and paxilline, which inhibits sarcolemmal BKCa channels, to block this protection (44, 45, 51).
We demonstrated recently that 30 μM NS1619 protected against IR injury in the isolated heart (51). We also showed that at this concentration, mitochondrial respiration was accelerated during resting states (states 2, 4), and that it caused a small increase in reactive oxygen species generation (ROS) when the FADH2-linked substrate succinate was given to energize mitochondria (29). We hypothesized that as matrix K+ influx increased through the mBKCa channel, an equivalent K+ efflux was balanced, due to the high trans-matrix H+ gradient, by a small inward proton leak through a K+/H+ exchanger (KHE); this caused respiration to accelerate and ROS production to increase while ΔΨm was maintained.
In the present study our aim was to further test the above hypothesis by examining the effects of opening the putative mBKCa channel with NS1619 on mitochondrial matrix K+, pH, as well as on matrix volume. We theorized that if the thermodynamically more favorable H+ influx by KHE were allowed when K+ influx is increased, then opening of mBKCa channels by NS1619 should cause a transient increase in matrix K+ and consequently a decrease in matrix pH. To explore this, we monitored, in real time, changes in matrix K+, volume, and pH by spectrophotometry. To eliminate the possibility of an active KHE that rapidly replaces matrix K+ with H+, quinine was administered in many experiments to inhibit KHE, which was intended to help expose effects of NS1619 to increase matrix K+. A large surge in K+ influx into the matrix is coupled to inward water movement, as demonstrated by matrix swelling (16). To test this, we measured mitochondrial light scattering as an index of mitochondrial matrix volume. Other drugs such as valinomycin, a K+ ionophore, were given to support or disprove our hypothesis. Our results indicate that NS1619 induces an increase in matrix K+ that is detectable only in the presence of quinine. Moreover, this increase in matrix K+ is not solely a result of blocking KHE and is not likely mediated through putative mBKCa channels, but rather through another nonspecific cation transport mechanism across the IMM.
MATERIALS AND METHODS
All experiments were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, Revised 1996) and were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
Mitochondrial isolation.
Heart mitochondria were isolated from 53 ketamine-anesthetized (50 mg/kg ip) guinea pigs (250–350 g) by differential centrifugation as described previously (28, 29, 47). Briefly, ventricles were excised, placed in an isolation buffer (200 mM mannitol, 50 mM sucrose, 5 mM KH2PO4, 5 mM MOPS, 1 mM EGTA, and 0.1% BSA, with pH adjusted to 7.15 with KOH), and minced into 1-mm3 pieces. The suspension was homogenized for 15 s in 2.5 ml isolation buffer containing 5 U/ml protease (Bacillus licheniformis) and for another 15 s after addition of 17 ml isolation buffer. The suspension was centrifuged at 8,000 g for 10 min, and the pellet was resuspended in 25 ml isolation buffer and centrifuged at 750 g for 10 min. Next the supernatant was centrifuged again at 8,000 g for 10 min, and the final pellet was suspended in 0.5 ml of isolation buffer and kept on ice. The protein content was determined by the Bradford method (10). All isolation procedures were conducted at 4°C and all experiments were conducted at room temperature.
In random studies, the respiratory control index (state 3/state 4 respiration) was determined to be 11.6 ± 0.3 for the complex I substrate pyruvate, and 2.4 ± 0.2 for the complex II substrate succinate with rotenone. This demonstrated a strong coupling between mitochondrial respiration and phosphorylation in our mitochondrial preparation.
Mitochondrial matrix volume measurement.
Matrix volume was measured following changes in 90° light scattering (5, 13) inside a cuvette-based spectrophotometer (model QM-8, Photon Technology International) with a beam of light filtered at excitation and emission wavelengths of 520 nm.
Mitochondrial matrix pH measurement.
Matrix pH was measured as previously described (16, 35). Briefly, isolated guinea pig heart mitochondria were incubated with 5 μM BCECF-AM in the same isolation buffer described above for 20 min at room temperature with continuous stirring. The suspension was then diluted with isolation buffer and centrifuged at 8,000 g for 10 min to remove excess dye. Mitochondria were then resuspended in isolation buffer and stored on ice. Assays were carried out at room temperature inside a cuvette-based spectrophotometer (model QM-8, Photon Technology International) with the light beam filtered at the excitation wavelength of 504 nm and photon activity measured at the emission wavelength of 530 nm.
Mitochondrial matrix K+ measurement.
Matrix K+ was measured as previously described (16). Briefly, isolated guinea pig heart mitochondria were incubated with 5 μM of the K+ binding fluorescent indicator (PBFI-AM) in the same isolation buffer described above for 10 min at room temperature with continuous stirring. Mitochondria were then incubated for an additional 2 min in isolation buffer containing 50 mM tetraethylammonium chloride (TEA+ Cl) to remove K+ from inside the matrix and to bring K+ concentration ([K+]) into the sensitivity range of PBFI (16). The suspension was then diluted with isolation buffer and twice centrifuged at 8,000 g for 3 min. Mitochondria were resuspended again in isolation buffer and kept on ice for further measurements. K+ measurement was carried out at room temperature inside the same cuvette-based spectrophotometer with the light beam filtered at dual excitation wavelengths of 340/380 nm and photon activity measured at the emission of wavelength of 500 nm.
Mitochondrial protocol.
Mitochondria (adjusted to 0.5 mg protein/ml) were suspended in experimental buffer (130 mM KCl, 5 mM K2HPO4, 20 mM MOPS, 2.5 mM EGTA, 1 μM Na4P2O7, and 0.1% BSA, with pH adjusted to 7.15 with KOH) with either 10 mM pyruvate or 10 mM succinate + 10 μM rotenone added to the suspension. Some experiments in the K+ measurement group were carried out in the same experimental buffer but with 130 mM choline chloride (ChCl) to substitute for K+ after adjusting the pH using CsOH. Buffer [Ca2+] was calculated as 100 nM by using indo-1 AM (unpublished observations). This ensured that mBKCa channels were not opened in our preparation due to high buffer Ca2+. ATP, 200 μM, was added to the suspension to prevent mKATP channel opening. After a stabilization period, energized mitochondria were treated either with NS1619 (30, 50, 100 μM) or with their respective vehicles (0.3%, 0.5%, 1% DMSO) and measurements were carried out for an additional 2–3 min. In some experiments, we added 500 μM quinine or 50 nM N,N′-dicyclohexylcarbodiimide (DCCD) to the mitochondrial suspension to inhibit KHE (24, 43) or 5 μM paxilline to block mBKCa channels at the beginning of experiments. It was reported that quinine blocks K+ efflux by up to 90% at 500 μM but is less effective at blocking KHE when given at lower concentrations (19). We used DCCD (50 nM) (18) as an alternative KHE inhibitor to compare with quinine.
In addition to its effect on KHE, quinine has been reported to have other effects on mitochondria (16), which make interpretation of the data somewhat complicated. To test these effects, another set of experiments was carried out using a protocol similar to that above but with quinine (500 μM) added later to the mitochondrial suspension after the stabilization period to determine its effects on mitochondrial K+ flux, matrix pH, and swelling.
Lipid bilayer technique and protocol.
The lipid bilayer was used to ascertain the nonspecific effects (ion/current leak) of NS1619 and quinine. Our methods for this technique have been described previously (32, 42). Lipid bilayer experiments were conducted at room temperature. Phospholipids were prepared by mixing l-α-phosphatidylethanolamine, l-α-phosphatidylserine, and l-α-phosphatidylcholine in a 4:4:2 (vol/vol) ratio with 3% tetramyristoyl cardiolipin. The resulting phospholipids were dried under nitrogen and resuspended in n-decane to a final concentration of 25 mg/ml. Recordings were obtained in symmetrical solution containing 150 mM KCl, 1 mM CaCl2, 2 mM MgCl2, and 10 mM HEPES. Ag/AgCl electrodes were placed into each chamber of the bilayer setup via agar salt (3 M KCl) bridges. The trans chamber was connected to the head stage of the bilayer amplifier (Axopatch 200B, Molecular Devices, Sunnyvale, CA) and the cis chamber was held at virtual ground. Current recordings were obtained using a voltage-ramp protocol from −80 to +80 mV from a holding potential of 0 mV, digitized at 5 kHz and low pass filtered at 1 kHz. Data acquisition and analysis were conducted using pClamp 9 software (Molecular Devices), and additional analyses were performed with Origin 7 software (Origin Lab, Northampton, MA). DMSO, NS1619, or quinine was added to the cis chamber.
Chemicals and reagents.
PBFI-AM and BCECF-AM were purchased from Invitrogen (Carlsbad, CA), and KCl (Ca2+ < 0.00001%) from EMD Chemicals (Gibbstown, NJ); all other chemicals were purchased from Sigma Chemical. NS1619, paxilline, valinomycin, and DCCD were dissolved in dilute DMSO. All other chemicals were dissolved in water.
Statistical analysis.
The effect of each treatment was calculated by taking the difference in fluorescence measured before and after treatment and subtracting the effect of the proper control (DMSO or H2O) on the fluorescence. Data are presented as percent change from the baseline. All data were compiled using Microsoft Excel and compared statistically using analysis of variance. If F values (P < 0.05) were significant, post hoc comparisons of means tests (Student-Newman-Keuls) were considered statistically significant at P < 0.05 (2-tailed). Values are means ± SE.
RESULTS
Changes in matrix K+.
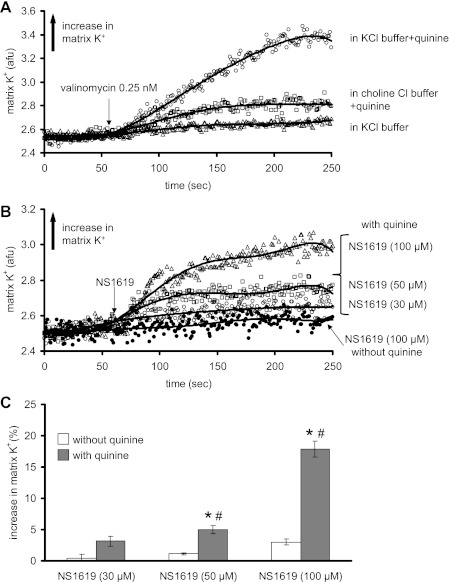
Figure 1A shows representative traces of changes in matrix K+ over time after adding the K+ ionophore valinomycin in energized mitochondria. Valinomycin caused a very small increase in matrix K+ in the KCl buffer. When quinine was added to the suspension to inhibit KHE, later addition of valinomycin caused a marked and sustained increase in matrix K+. This increase in matrix K+ by valinomycin in the presence of quinine was mostly absent in ChCl buffer. Figure 1B shows representative traces of matrix K+ measurement over time with 30, 50, and 100 μM NS1619 in the presence of quinine and with 100 μM NS1619 in the absence of quinine. NS1619 caused a concentration-dependent increase in matrix K+ when quinine was present. NS1619 at any concentration did not induce a significant increase in matrix K+ when quinine was absent as shown in Fig. 1, B and C.
Fig. 1.
Time-dependent changes in mitochondrial matrix K+ (arbitrary fluorescence units, AFU) assessed using the K+ binding fluorescent indicator PBFI-AM. A: K+ influx induced by valinomycin (0.25 nM) in KCl buffer without quinine (▵), in KCl buffer with quinine present at time 0 (○), and in ChCl buffer to substitute for K+ with quinine present at time 0 (□). B: K+ influx induced by different concentrations of NS1619 in KCl buffer with quinine present at time 0 and with 100 μM NS1619 without quinine present. C: summary (n = 12) of the effects of different concentrations of NS1619 on matrix K+ influx with and without quinine present. Note that a concentration-dependent increase in K+ by NS1619 occurred only in the presence of quinine. The effect of the appropriate control (DMSO) on signals was subtracted from the background signal with NS1619 present, which makes control equal to 0. *P < 0.05, NS1619 (30, 50, 100 μM) ± quinine vs. control. #P < 0.05, NS1619 (30, 50, 100 μM) with quinine vs. NS1619 alone.
The effects of NS1619 are summarized in Fig. 1C. NS1619 increased matrix K+ in a concentration-dependent manner, but only in the presence of quinine. Blockers of mBKCa [paxilline (Table 1), iberiotoxin, or charybdotoxin (data not shown)] given before NS1619 did not alter the effect of NS1619 to increase matrix K+. The effects of NS1619 to increase matrix K+ were attenuated when experiments were done in ChCl buffer (Table 1).
Table 1.
Effects of different concentrations of NS1619 on matrix K+ influx in K+ -containing and K+ -free buffers in the absence or presence of 5 μM paxilline to block mBKCa
| NS1619 (30 μM) | NS1619 (50 μM) | NS1619 (100 μM) | |
|---|---|---|---|
| In KCl | 3.1 ± 0.8 | 5.0 ± 0.6* | 17.9 ± 1.2* |
| In KCl + 5 μM paxilline | 3.0 ± 1.1 | 5.1 ± 0.9* | 16.4 ± 1.3* |
| In choline Cl | 1.7 ± 0.9 | 1.5 ± 0.7† | 1.7 ± 1.3† |
Values are means ± SE of % increase in K+ influx with any treatment after subtracting control (DMSO) value. All mitochondrial suspensions contain quinine (500 μM) to block K+/H+ exchange. mBKCa, large-conductance Ca2+-activated K+ channels on the inner mitochondrial membrane of cardiac myocytes.
P < 0.05 NS1619 vs. control;
P < 0.05 NS1619 + paxilline or NS1619 in choline Cl vs. NS1619 alone in KCl.
Mitochondrial matrix volume.
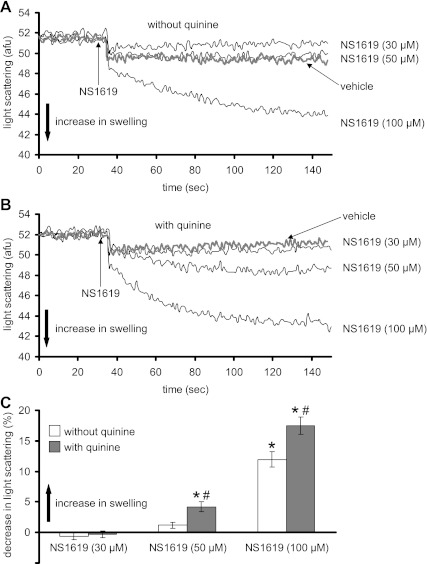
Figure 2, A and B, shows representative traces of matrix volume changes over time at the three concentrations of NS1619 in the absence (Fig. 2A) or presence (Fig. 2B) of quinine. NS1619, in the absence of quinine, caused a steady increase in matrix swelling over ∼100 s only at 100 μM; in the presence of quinine, NS1619 (50, 100 μM) augmented matrix volume. Figure 2C summarizes the maximal steady-state effects of NS1619 on matrix volume. NS1619 alone had no significant effect on matrix volume at lower concentrations (30, 50 μM), whereas it increased volume at higher concentrations (100 μM). Moreover, the swelling was accentuated in the presence of quinine by 50 and 100 μM NS1619.
Fig. 2.
Changes in mitochondrial matrix volume (swelling) (AFU) assessed using the 90° light scattering method. A and B: matrix volume changes induced by different concentrations of NS1619 in the absence (A) or presence (B) of quinine at time 0. C: summary (n = 12) of the effects of NS1619 with and without quinine on matrix volume. Note that the concentration-dependent increase in matrix volume by NS1619 was greater in the presence of quinine. The effect of the appropriate control (DMSO) on signal was subtracted from the background signal with NS1619 present, which makes control equal to 0. *P < 0.05, NS1619 (30, 50, 100 μM) ± quinine vs. control. #P < 0.05, NS1619 (30, 50, 100 μM) with quinine vs. NS1619 alone.
Matrix pH.
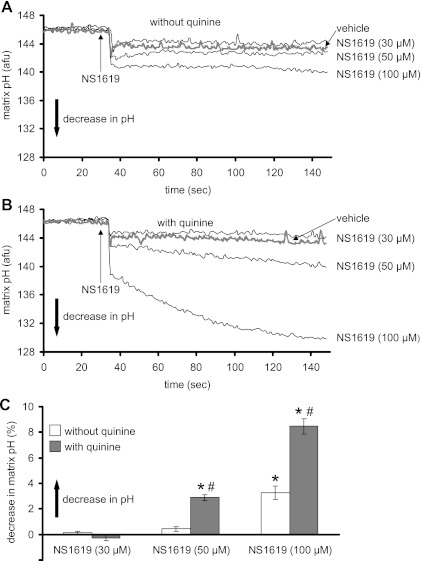
Figure 3 shows representative traces of matrix pH over time after adding 30, 50, or 100 μM NS1619 to the mitochondrial suspension in the absence (Fig. 3A) or presence (Fig. 3B) of quinine. NS1619 at 100 μM caused a significant matrix acidification in the absence of quinine (Fig. 3A); when quinine was present, NS1619 led to greater acidification of the matrix. Figure 3C summarizes the steady-state effects of NS1619 on matrix pH in the presence and absence of quinine. NS1619, at 100 μM, but not at 30 or 50 μM, decreased matrix pH. These effects on matrix pH were accentuated in the presence of quinine.
Fig. 3.
Changes in matrix pH (AFU) assessed using BCECF-AM fluorescence. A and B: matrix pH changes induced by different concentrations of NS1619 in the absence (A) or presence (B) of quinine at time 0. C: summary (n = 12) of the effects of NS1619 with and without quinine on matrix pH. Note that the concentration-dependent increase in matrix acidity by NS1619 was greater in the presence of quinine. The effect of the appropriate control (DMSO) on signal was subtracted from the background signal with NS1619 present, which makes control equal to 0. *P < 0.05, NS1619 (30, 50, 100 μM) ± quinine vs. control. #P < 0.05, NS1619 (30, 50, 100 μM) with quinine vs. NS1619 alone.
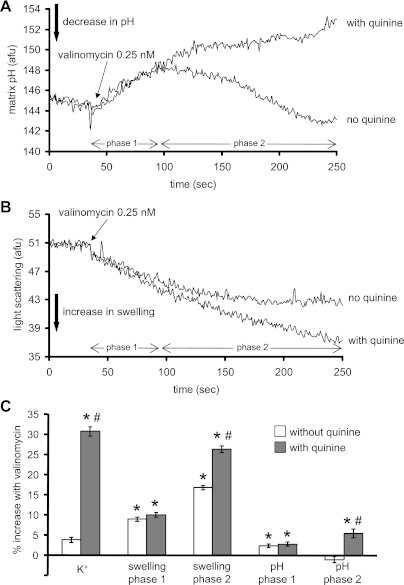
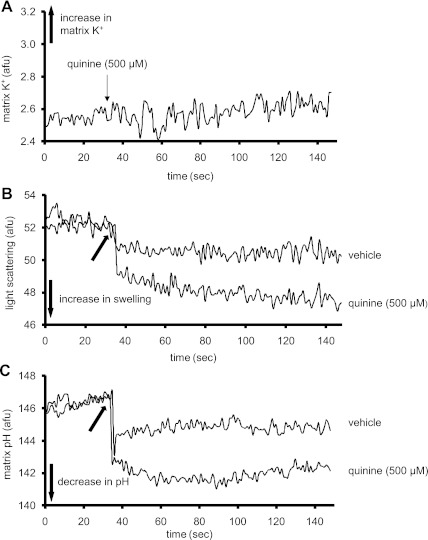
Figure 4A shows effects of K+ influx induced by 0.25 nM valinomycin on matrix pH in the presence and absence of quinine. In the absence of quinine, valinomycin induced a biphasic change in matrix pH as shown initially by matrix alkalinization with a peak pH after about 50 s followed by a decrease in matrix pH over the next 150 s. When quinine was present, valinomycin caused only a sustained matrix alkalinization. In additional experiments conducted, DCCD did not mimic the effects of quinine shown in Fig. 4A (data not displayed).
Fig. 4.
Effects of valinomycin on matrix pH (A) and matrix swelling (B) in the absence or presence of quinine at time 0. C: summary (n = 5) of the effects of valinomycin on matrix K+, pH, and swelling. Note that a valinomycin-induced increase in K+ occurred only in the presence of quinine. Also note the time-dependent differential effects of valinomycin on matrix pH and volume. The effect of the appropriate control (DMSO) on signal was subtracted from the background signal with valinomycin present, which makes control equal to 0. *P < 0.05, valinomycin ± quinine vs. control. #P < 0.05, valinomycin with quinine vs. valinomycin alone.
Changes in matrix volume were also monitored after adding valinomycin with and without quinine (Fig. 4B). Valinomycin, in the absence of quinine, induced an increase in mitochondrial volume to a steady-state level after ∼50 s. In the presence of quinine, the change in mitochondrial volume was analogous to the change in pH; i.e., mitochondrial volume continued to increase beyond the period observed in the absence of quinine. Figure 4C summarizes the effects of valinomycin on matrix K+, pH, and swelling in the absence or presence of quinine.
As noted above, in some experiments, quinine was given to block mitochondrial KHE. However, it has been reported that quinine has side effects on mitochondria (16). Consequently, we tested the effects of quinine alone on mitochondrial matrix K+, pH, swelling (n = 5), ΔΨm, and on mitochondrial respiration (n = 7). Figure 5 shows that quinine alone (500 μM) did not appreciably change matrix K+ influx (Fig. 5A), while it caused a small significant increase (6.34 ± 0.41%, P < 0.05) in matrix volume (Fig. 5B) and a small significant increase (1.31 ± 0.35%, P < 0.05) in matrix acidity (Fig. 5C). Moreover, quinine enhanced respiration during resting states 2 and 4 by 56 ± 8% and 48 ± 10% (P < 0.05), respectively, and caused a small but significant depolarization (1.33 ± 0.4%, P < 0.05) of ΔΨm.
Fig. 5.
Representative traces showing the effects of quinine (500 μM) on matrix K+ (A), matrix swelling (B), and matrix pH (C). Note that quinine caused a very small, gradual increase in matrix K+. Also quinine caused a small increase in matrix volume and a small decrease in matrix pH. These traces were reproducible in other experiments (n = 5). The arrow in B and C at 35 s indicates when vehicle or quinine was administered.
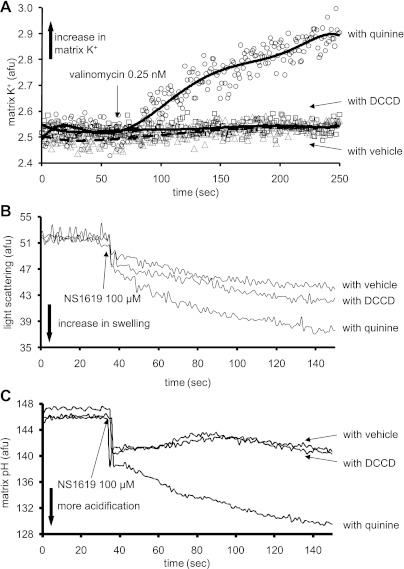
To test whether the results obtained with NS1619 in the presence of quinine to inhibit KHE are due to other effects of quinine on mitochondria, we used DCCD as an alternative KHE blocker. Figure 6 displays the effects of valinomycin and NS1619 on matrix K+, pH, and swelling in the presence of either quinine or DCCD. Valinomycin did not induce any detectable K+ influx in the presence of DCCD when compared with quinine (Fig. 6A). In the presence of quinine, NS1619 (100 μM) induced more matrix swelling (Fig. 6B) and acidification (Fig. 6C) than in the presence of DCCD.
Fig. 6.
Effects of valinomycin (0.25 nM) on matrix K+ influx (A), and effects of NS1619 (100 μM) on matrix swelling (B) and matrix pH (C) in the presence of either quinine or N,N′-dicyclohexylcarbodiimide (DCCD) to inhibit K+/H+ exchange (KHE). Note that valinomycin caused a very small increase in matrix K+ influx in the presence of DCCD relative to quinine. Also, NS1619 caused more matrix swelling and more matrix acidification when quinine was used to inhibit KHE relative to DCCD. Also note that changes in matrix K+, pH, and swelling were not different when DCCD was used to block KHE relative to vehicle. These data may indicate another role for quinine in potentiating an ionic leak promoted by either valinomycin or by NS1619 at a higher concentration.
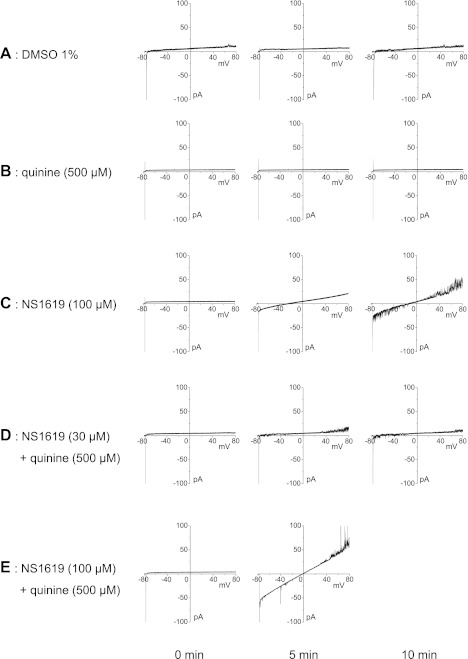
To test for any possible ionophoretic properties (current leak), we investigated the effects of NS1619, quinine, or NS1619 + quinine on lipid bilayer conductance as shown in Fig. 7. Control recordings with 1% DMSO, vehicle for 100 μM NS1619, showed minimal current leak during the 10-min recording period at 5-min intervals (Fig. 7A). Quinine alone (500 μM) did not produce any current leak (Fig. 7B). In contrast, NS1619 (100 μM) induced a significant leakage current across the lipid bilayer and the amplitude of the current leak increased during the 10-min period (Fig. 7C). NS1619 (30 μM) alone did not cause significant leakage, but when it was combined with quinine (Fig. 7D), a small current leak was detected. The leak was much more evident when NS1619 (100 μM) was combined with quinine (Fig. 7E) after which a substantial current leak developed within 5 min. This current leak was greater than that caused by 100 μM NS1619 alone at the 5-min mark, and it caused a loss in the integrity of the lipid bilayer before the recording at the 10-min mark.
Fig. 7.
Representative traces showing the effects of quinine and NS1619 on an artificial lipid bilayer. Currents across the bilayer were recorded during a voltage ramp from −80 to +80 mV from a holding potential of 0 mV. The capacitive current is denoted by the transient inward current at the start of the ramp protocol. Recordings were obtained at 5-min intervals under the following conditions: 1% DMSO (A), 500 μM quinine (B), 100 μM NS1619 (C), 500 μM quinine + 30 μM NS1619 (D), and 500 μM quinine + 100 μM NS1619 (E). These results were reproduced in 3 separate runs.
DISCUSSION
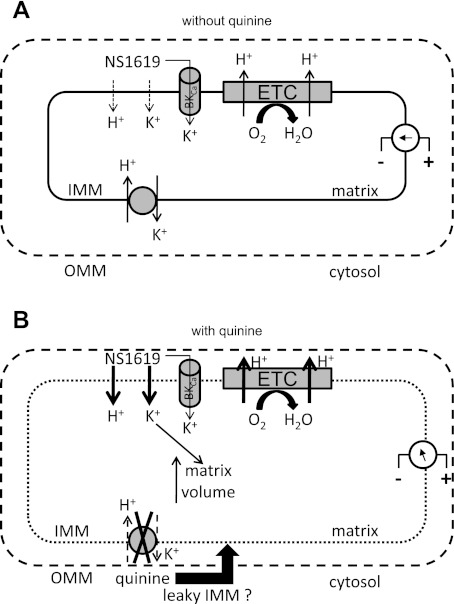
Our major objective was to ascertain whether NS1619, a known activator of sarcolemmal BKCa channels, induces matrix K+ influx through a putative BKCa channel in cardiac cell mitochondria. We found no direct evidence for K+ influx at lower concentrations of NS1619 after incubating mitochondria with the K+ binding fluorescent indicator PBFI-AM. Only by using a high concentration of NS1619 and blocking KHE with quinine were we able to observe significant K+ influx. However, the NS1619-induced increase in K+ influx in the presence of quinine was not blocked by known BKCa channel blockers. Moreover, we found several other effects of quinine not related to blocked KHE that complicated our interpretation of the results. We conclude that NS1619 could indeed have an underlying effect to open a K+ channel or to otherwise promote K+ uptake, but that K+ influx is masked by a direct or indirect (i.e., via KHE) protonophoretic effect of this drug to induce partial mitochondrial uncoupling. Figure 8 shows a schematic representation of the plausible effects of NS1619 on mitochondrial bioenergetics in the absence (Fig. 8A) or presence (Fig. 8B) of quinine.
Fig. 8.
Proposed effect of NS1619 on mitochondrial ion fluxes in the absence (A) or presence (B) of quinine to block KHE. A: low concentrations of NS1619 may induce only small K+ and H+ influxes across the inner mitochondrial membrane (IMM), which would accelerate electron flow across the electron transport chain (ETC) but maintain membrane potential gradient (ΔΨm). B: quinine blocks KHE but also induces matrix swelling which may cause the IMM to become more permeable to NS1619-induced ions fluxes (represented by thicker arrows), which will lead to even more swelling. The ΔΨm will become more depolarized and respiration will be accelerated (uncoupling). OMM, outer mitochondrial membrane.
K+ influx and KHE.
The K+ electrochemical gradient favors continuous matrix K+ accumulation, which would cause unfavorable matrix swelling. To counter K+ entry into the matrix, Mitchell (41) long ago postulated exchange-diffusion carrier systems that allow the reversible exchange of cations with H+ to regulate the osmotic difference across the IMM. Thus K+ entry is believed to be matched by K+ exit in exchange for H+ entry, i.e., KHE. However, this enhanced H+ entry has a cost in dissipating ΔΨm and increasing respiration not coupled to ATP synthesis. This implied that the permeability to cations must be kept very low to maintain mitochondrial energy efficiency. Biophysically, the existence of a distinct KHE seems assured. An 82-kDa protein represents the best candidate for the mitochondrial KHE (38, 39).
NS1619 and ion fluxes.
Previously, we showed that low concentrations of NS1619 (up to 30 μM) mildly accelerated mitochondrial respiration during resting states (states 2, 4 respiration), without changing ΔΨm, and induced a small increase in ROS generation (29). However, NS1619, at a higher concentration (50 μM), accelerated respiration during resting states, partially depolarized ΔΨm, and did not increase ROS (29). We hypothesized that as matrix K+ influx increased through the mBKCa channel, an equivalent K+ efflux was balanced by inward H+ leak through KHE, which would accelerate respiration while maintaining ΔΨm and, thus, allow greater electron leak and O2·− generation. However, greater K+ influx (such as with a higher concentration of NS1619) would saturate KHE, causing a decline in ΔΨm and a decline in O2·− generation (29).
In the work reported here we sought to further test the above hypothesis by directly measuring any changes in matrix K+, pH, and matrix volume induced by NS1619. Unexpectedly, NS1619 alone, at the concentrations used, did not cause a detectable increase in matrix K+, although 100 μM NS1619 caused matrix swelling and appeared to cause a small, but nonsignificant increase in matrix K+. We stated that a possibility for these results was a rapid 1:1 exchange of K+ with H+ through KHE, which would lead to matrix acidification and, as we reported earlier, to a fall in ΔΨm and increased respiration (29). Indeed, 100 μM NS1619 caused matrix acidification, which may indicate an undetectable K+ influx by NS1619 because matrix K+ is quickly replaced by H+. To further explore this possibility, we used quinine, a known reversible blocker of KHE (36), to prevent the entering K+ from exiting by exchange with H+. When quinine was present, we observed a significant concentration-dependent increase in matrix K+ induced by NS1619 (Fig. 1, B and C). This increase in matrix K+ was coupled with matrix swelling (due to inward water movement), which was significant only at the higher concentrations of NS1619 (50, 100 μM) (Fig. 2, B and C).
However, since K+ cannot exit the matrix through the inhibited KHE then H+ cannot enter through KHE, so it would be reasonable to expect not to observe matrix pH acidification after adding NS1619. Surprisingly, when quinine was already present in the buffer to block KHE, addition of NS1619 caused even more acidification than did NS1619 alone (Fig. 3, B and C). This suggests that the H+ entry into the matrix observed after NS1619 with quinine is not exclusively through KHE. One possible route for H+ entry is NS1619-induced H+ transport across the IMM, i.e., akin to a protonophore (Fig. 8). Moreover, our results suggest that NS1619 induces a K+ leak across the IMM in addition to, or in lieu of, K+ flux through putative mBKCa channels for the following reasons: 1) the increases in K+ flux induced by NS1619 were not inhibited or minimized by paxilline, iberiotoxin, or charybdotoxin at any concentration of NS1619; 2) NS1619 (100 μM) caused a current leak in lipid bilayers (Fig. 7C); and 3) the enhanced K+ flux induced by NS1619 was only observed when quinine was present. Quinine is also reported to block K+ channels (7, 22, 52) and K+ transport across the plasma membrane (1). Although the molecular mechanism of quinine's action is not fully understood, it was shown (7) that quinine interacts with a mitochondrial sulfonylurea receptor and in so doing inhibits [3H]glibenclamide binding to the IMM. Furthermore, the authors observed that quinine inhibited activity of the mKATP channel reconstituted into bilayer lipid membranes. These findings led to the conclusion that the mKATP channel in cardiac mitochondria is blocked by quinine acting on the mitochondrial sulfonylurea receptor.
From our findings we suggest that NS1619 causes H+ as well as K+ flux across the IMM into the matrix. This suggestion agrees with another study (13) that showed NS1619-induced mitochondrial depolarization can occur in a K+-free media, possibly due to H+ leak across the IMM. Moreover, in that study (13) the authors suggested that NS1619 increased mitochondrial permeability to K+ and that NS1619 also promoted nonselective permeabilization of the IMM to other ions rather than acting on a specific IMM K+ channel. However, Debska et al. (17) reported that NS1619 (50 μM) did not increase bilayer membrane conductance. This finding is consistent with our results that show that NS1619 at lower concentrations did not promote any appreciable ion fluxes; only at higher concentration of NS1619 (100 μM) or in the presence of quinine were we able to detect nonspecific ion fluxes.
Valinomycin and ion fluxes.
To confirm the movement of K+ by NS1619, we also measured matrix K+ in the presence of the K+ ionophore valinomcyin (0.25 nM). Valinomycin in the absence of quinine also promoted only a very small increase in matrix K+ (Fig. 1A), again likely because of a simultaneous K+ extrusion for H+ influx via KHE. Higher concentrations of valinomycin (1, 10 nM) also did not cause any detectable significant increase in matrix K+ (data not shown). However, by incubating the mitochondrial suspension in quinine before adding valinomycin, we were able to observe significant concentration-dependent increases in matrix K+ influx. This indicated that KHE is very active in exchanging K+ for H+, and that after blocking KHE with quinine (36), K+ accumulates inside the matrix, which renders it detectable by PBFI while at the same time exacerbating matrix swelling.
Our conclusion from above that mitochondrial KHE is active under our experimental conditions appears to disagree with other reports that mitochondria possess a quiescent KHE but an active Na+/H+ exchanger (11, 21, 26, 49). To further ascertain whether K+ influx activates KHE, we examined the effects of valinomycin, not only on K+ flux, but also on changing matrix pH and swelling. We observed a biphasic effect of valinomycin on matrix pH but not on swelling. If K+ were to be exchanged immediately with H+ through an active KHE (as suggested above), then matrix acidification should be observed after adding valinomycin. However, valinomycin, in the absence of quinine, induced matrix alkalinization initially (phase 1) (Fig. 4A). It appears that K+ influx induced by valinomycin was associated with matrix swelling until matrix volume reached a certain level at which time KHE was then triggered (phase 2) (Fig. 4B). When KHE became activated, the increase in matrix volume ceased and matrix pH began to return to baseline values (Fig. 4A).
This acidification that followed matrix alkalinization after valinomycin was not observed in another set of experiments in which quinine was already present in the preparation to inhibit KHE. This suggests and supports the notion that KHE is not initially active but becomes activated later when matrix volume increases to a certain level. This theory is supported by an earlier study, which showed an increase in matrix volume may induce mitochondrial membrane stretching and conformational changes (9), and thus activate KHE (9, 12).
Quinine and ion fluxes.
Taken together, we conclude that the detection of changes in matrix K+, which was only observed in the presence of quinine when measured using PBFI-AM, is not entirely due to inhibited KHE, but rather to a possible nonspecific effect of quinine to enhance ions transport across the IMM when induced by other drugs such as NS1619 (Fig. 7E). Furthermore, we compared matrix K+ influx induced by valinomycin when KHE was inhibited with DCCD vs. inhibited with quinine (Fig. 6). DCCD is an irreversible inhibitor of KHE (39) that reacts with carboxylic groups that are buried within the hydrophobic core of the membrane (2). Surprisingly, valinomycin did not induce any detectable increase in matrix K+ when DCCD was present, and changes in matrix volume and pH after adding NS1619 (100 μM) were smaller when DCCD rather than quinine was used to inhibit KHE; this further supports the notion of a nonspecific effect of quinine on mitochondria that enhances ion leak by other drugs.
It is not clear why quinine enhances H+ and possibly K+ influx induced by NS1619 across IMM. One possibility is that quinine itself induces matrix swelling, as we observed in this study (Fig. 5). It is believed that charged mitochondria extrude K+ during respiration through KHE (39) that leads to a respiration-dependent matrix contraction. Since quinine blocks KHE, it should inhibit the respiration-dependent contraction and produce matrix swelling. This is also supported by our data showing that quinine induced matrix swelling. The swelling of mitochondria due to quinine (Fig. 5) may be a plausible reason why mitochondria become more permeable to ions such as shown with the H+ leak promoted by NS1619 (Fig. 3B).
It has been postulated that a uniporter becomes more activated by the unfolding of the IMM, such as would occur during matrix swelling. This could lead to a several-fold increase in the surface area of the IMM that is in direct contact with the surrounding medium (4). It is possible that quinine induces swelling which causes the IMM cristae to unfold and by doing so, enhances H+ leak and possibly even NS1619-induced K+ leak across the IMM. It is also possible that the unfolding of the IMM provides a larger surface area for NS1619 to interact with. Another possibility is that quinine, an amine local anesthetic, may act as an uncoupler (25), and that as a protonated cation forms a lipophilic ion pair with an appropriate anion. This neutral ion pair then diffuses across the membrane, releases the proton and then cycles back as neutral amine. It is conceivable that quinine, by this mechanism, causes a disturbance in the IMM and makes it more vulnerable to ion leak promoted by other drugs, which agrees with an earlier study (36) indicating that quinine at high concentration may induce an increase in membrane permeability. This is supported by our experiments in artificial lipid bilayers (Fig. 7) showing that the combination of quinine and NS1619, especially at high concentration, induced a nonspecific leakage current across the lipid membrane. On the other hand, quinine was reported to inhibit cation transport mediated by ionophores (3, 33, 34, 40, 46). However, this does not appear to occur in our experiments since the K+ ionophore valinomycin caused even greater K+ influx in the presence of quinine.
Mitochondrial permeability transition pore (mPTP) opening causes dissipation of ΔΨm and influx of small solutes that could lead to mitochondrial swelling and rupture (50). However, it is unlikely that quinine enhances ion fluxes, induced by NS1619 or valinomycin, by opening the mPTP. In fact, quinine was reported to promote mPTP closure possibly by dislodging Ca2+ bound to protein(s) directly involved in mPTP opening (15). Regardless, our results seem to indicate a quinine-enhancing effect on NS1619-induced ion leak, but the mechanism of this is yet to be determined.
Summary and limitations.
We conclude that NS1619, a known activator of large-conductance Ca2+-activated K+ channels in the sarcolemmal membrane, does not promote matrix K+ influx solely through mBKCa, but also through a nonspecific ion transport mechanism across the IMM. In addition, NS1619 appears to promote H+ leak across the IMM. It remains possible that NS1619 induces K+ influx through specific mBKCa channels, but this influx may be negligible to detect using fluorescence or swelling techniques. Our results may seem initially to disagree with what was previously shown by Xu et al. (52), who reported an increase in mitochondrial matrix PBFI excitation ratio (F340/F380 nm) with K+ concentrations ranging from 0 to 5 mM, and that this K+ uptake was slowed by charybdotoxin, iberiotoxin, Ba2+, and quinine, indicating a role of mBKCa. Interestingly, in their study, Xu et al. reported that NS1619 also accelerated the rate of K+ uptake as in our experiments, but they did not report whether this K+ uptake induced by NS1619 was blocked by the known BKCa channel blockers.
Previously, we showed that NS1619 protected hearts against IR injury by a preconditioning mechanism, and we proposed that this was related to its induction of K+ influx and ROS generation that follows (29, 51). However, in the current study we did not find any evidence for NS1619 in inducing a detectable matrix K+ influx at the concentrations used in the previous studies (28, 29, 51). We think it could be simply that the K+ dye PBFI-AM is not sensitive enough to detect the expected small changes in matrix K+ fluxes, but even the indirect measurement of K+ influx by monitoring changes in matrix volume failed to show any significant change at the lower concentrations (30 μM) previously used.
Our results also agree with a recent study (6) that showed that pharmacological agents such as diazoxide and NS1619 do not provide any evidence for detectable activities of mKATP and mBKCa channels in heart mitochondria. Furthermore, the authors (6) suggested that the changes in matrix volume induced by NS1619 were not related to its induction of K+ influx into the matrix because matrix swelling occurred in a K+-free media.
It is possible that NS1619-induced preconditioning is due primarily to its mild uncoupling effect at higher concentrations. It was shown that uncouplers expressed in the heart of transgenic mice (30), or added as pretreatment to ventricular myocytes (48), protect against ischemia and reperfusion damage. In fact, even the mKATP channel openers diazoxide and pinacidil have been shown to act as uncoupling protonophores, which may be an important component in their mechanism of cardioprotection (31). Our experiments and those of others (13) suggest that NS1619 is either not a specific activator of guinea pig cardiac mBKCa channels, or that it has other confounding effects. Other more specific drugs such as NS11021 (8), which are possibly more specific and potent for mBKCa, could be used to further investigate if there are mitochondrial functional effects of endogenous opening of mBKCa channels in the IMM.
GRANTS
This work was supported in part by the American Heart Association (0355608Z and 0855940G to D. F. Stowe), the National Institutes of Health (K01 HL73246 to A. K. S. Camara; R01 HL089514 to D. F. Stowe; and P01 GM 066730-07 to Z. J. Bosnjak, Project Director), and the Veterans Administration (VA Merit 8204-05P to D. F. Stowe).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank James Heisner, Johan Haumann, Age Boelens, Timothy Trichler, Anita Tredeau, and Steven Contney for valuable assistance.
This work was published in part in abstract form: Aldakkak et al., Biophys J, 441-pos, 2007; Aldakkak et al., Biophys J, 436-pos, 2008; and Aldakkak et al., Biophys J, 1244-pos, 2009.
REFERENCES
- 1.Armando-Hardy M, Ellory JC, Ferreira HG, Fleminger S, Lew VL. Inhibition of the calcium-induced increase in the potassium permeability of human red blood cells by quinine. J Physiol 250: 32P–33P, 1975 [PubMed] [Google Scholar]
- 2.Azzi A, Casey RP, Nalecz MJ. The effect of N,N′-dicyclohexylcarbodiimide on enzymes of bioenergetic relevance. Biochim Biophys Acta 768: 209–226, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Azzi A, Scarpa A. Inhibition of K+ transport in liver mitochondria. Biochim Biophys Acta 135: 1087–1088, 1967 [DOI] [PubMed] [Google Scholar]
- 4.Azzone GF, Bortolotto F, Zanotti A. Induction of electroneutral exchanges of H+ with K+ in rat liver mitochondria. FEBS Lett 96: 135–140, 1978 [DOI] [PubMed] [Google Scholar]
- 5.Beavis AD, Brannan RD, Garlid KD. Swelling and contraction of the mitochondrial matrix. I. A structural interpretation of the relationship between light scattering and matrix volume. J Biol Chem 260: 13424–13433, 1985 [PubMed] [Google Scholar]
- 6.Bednarczyk P, Barker GD, Halestrap AP. Determination of the rate of K+ movement through potassium channels in isolated rat heart and liver mitochondria. Biochim Biophys Acta 1777: 540–548, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Bednarczyk P, Kicinska A, Kominkova V, Ondrias K, Dolowy K, Szewczyk A. Quinine inhibits mitochondrial ATP-regulated potassium channel from bovine heart. J Membr Biol 199: 63–72, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bentzen BH, Osadchii O, Jespersen T, Hansen RS, Olesen SP, Grunnet M. Activation of big conductance Ca2+-activated K+ channels (BK) protects the heart against ischemia-reperfusion injury. Pflügers Arch 457: 979–988, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Bernardi P, Azzone GF. Electroneutral H+-K+ exchange in liver mitochondria. Regulation by membrane potential. Biochim Biophys Acta 724: 212–223, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 11.Brierley GP. Passive permeability and energy-linked ion movements in isolated heart mitochondria. Ann NY Acad Sci 227: 398–411, 1974 [DOI] [PubMed] [Google Scholar]
- 12.Brierley GP, Jurkowitz MS, Farooqui T, Jung DW. K+/H+ antiport in heart mitochondria. J Biol Chem 259: 14672–14678, 1984 [PubMed] [Google Scholar]
- 13.Cancherini DV, Queliconi BB, Kowaltowski AJ. Pharmacological and physiological stimuli do not promote Ca2+-sensitive K+ channel activity in isolated heart mitochondria. Cardiovasc Res 73: 720–728, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Cao CM, Xia Q, Gao Q, Chen M, Wong TM. Calcium-activated potassium channel triggers cardioprotection of ischemic preconditioning. J Pharmacol Exp Ther 312: 644–650, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Catisti R, Vercesi AE. Permeability transition pore closure promoted by quinine. J Bioenerg Biomembr 31: 153–157, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Costa AD, Quinlan CL, Andrukhiv A, West IC, Jaburek M, Garlid KD. The direct physiological effects of mitoKATP opening on heart mitochondria. Am J Physiol Heart Circ Physiol 290: H406–H415, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Debska G, Kicinska A, Dobrucki J, Dworakowska B, Nurowska E, Skalska J, Dolowy K, Szewczyk A. Large-conductance K+ channel openers NS1619 and NS004 as inhibitors of mitochondrial function in glioma cells. Biochem Pharmacol 65: 1827–1834, 2003 [DOI] [PubMed] [Google Scholar]
- 18.DiResta DJ, Kutschke KP, Hottois MD, Garlid KD. K+-H+ exchange and volume homeostasis in brown adipose tissue mitochondria. Am J Physiol Regul Integr Comp Physiol 251: R787–R793, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Diwan JJ. Effect of quinine on mitochondrial K+ and Mg2+ flux. Biochem Biophys Res Commun 135: 830–836, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Dos Santos P, Kowaltowski AJ, Laclau MN, Seetharaman S, Paucek P, Boudina S, Thambo JB, Tariosse L, Garlid KD. Mechanisms by which opening the mitochondrial ATP- sensitive K+ channel protects the ischemic heart. Am J Physiol Heart Circ Physiol 283: H284–H295, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Douglas MG, Cockrell RS. Mitochondrial cation-hydrogen ion exchange. Sodium selective transport by mitochondria and submitochondrial particles. J Biol Chem 249: 5464–5471, 1974 [PubMed] [Google Scholar]
- 22.Franciolini F, Hogg R, Catacuzzeno L, Petris A, Trequattrini C, Adams DJ. Large-conductance calcium-activated potassium channels in neonatal rat intracardiac ganglion neurons. Pflügers Arch 441: 629–638, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Garlid KD. Opening mitochondrial KATP in the heart–what happens, and what does not happen. Basic Res Cardiol 95: 275–279, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Garlid KD, DiResta DJ, Beavis AD, Martin WH. On the mechanism by which dicyclohexylcarbodiimide and quinine inhibit K+ transport in rat liver mitochondria. J Biol Chem 261: 1529–1535, 1986 [PubMed] [Google Scholar]
- 25.Garlid KD, Nakashima RA. Studies on the mechanism of uncoupling by amine local anesthetics. Evidence for mitochondrial proton transport mediated by lipophilic ion pairs. J Biol Chem 258: 7974–7980, 1983 [PubMed] [Google Scholar]
- 26.Garlid KD, Paucek P. Mitochondrial potassium transport: the K+ cycle. Biochim Biophys Acta 1606: 23–41, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Grover GJ, D'Alonzo AJ, Garlid KD, Bajgar R, Lodge NJ, Sleph PG, Darbenzio RB, Hess TA, Smith MA, Paucek P, Atwal KS. Pharmacologic characterization of BMS-191095, a mitochondrial KATP opener with no peripheral vasodilator or cardiac action potential shortening activity. J Pharmacol Exp Ther 297: 1184–1192, 2001 [PubMed] [Google Scholar]
- 28.Heinen A, Aldakkak M, Stowe DF, Rhodes SS, Riess ML, Varadarajan SG, Camara AK. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am J Physiol Heart Circ Physiol 293: H1400–H1407, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Heinen A, Camara AK, Aldakkak M, Rhodes SS, Riess ML, Stowe DF. Mitochondrial Ca2+-induced K+ influx increases respiration and enhances ROS production while maintaining membrane potential. Am J Physiol Cell Physiol 292: C148–C156, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Hoerter J, Gonzalez-Barroso MD, Couplan E, Mateo P, Gelly C, Cassard-Doulcier AM, Diolez P, Bouillaud F. Mitochondrial uncoupling protein 1 expressed in the heart of transgenic mice protects against ischemic-reperfusion damage. Circulation 110: 528–533, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Holmuhamedov EL, Jahangir A, Oberlin A, Komarov A, Colombini M, Terzic A. Potassium channel openers are uncoupling protonophores: implication in cardioprotection. FEBS Lett 568: 167–170, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Jiang MT, Nakae Y, Ljubkovic M, Kwok WM, Stowe DF, Bosnjak ZJ. Isoflurane activates human cardiac mitochondrial adenosine triphosphate-sensitive K+ channels reconstituted in lipid bilayers. Anesth Analg 105: 926–932, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Johnson CL, Goldstein MA, Schwartz A. On the molecular action of local anesthetics. 1. The mitochondrion as a model membrane system for studying local anesthetic action. Mol Pharmacol 9: 360–371, 1973 [PubMed] [Google Scholar]
- 34.Johnson CL, Schwartz A. Some effects of local anesthetics on isolated mitochondria. J Pharmacol Exp Ther 167: 365–373, 1969 [PubMed] [Google Scholar]
- 35.Jung DW, Davis MH, Brierley GP. Estimation of matrix pH in isolated heart mitochondria using a fluorescent probe. Anal Biochem 178: 348–354, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Jung DW, Farooqui T, Utz E, Brierley GP. Effects of quinine on K+ transport in heart mitochondria. J Bioenerg Biomembr 16: 379–390, 1984 [DOI] [PubMed] [Google Scholar]
- 37.Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K+ channel of heart mitochondria. Am J Physiol Heart Circ Physiol 280: H649–H657, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Li XQ, Hegazy MG, Mahdi F, Jezek P, Lane RD, Garlid KD. Purification of a reconstitutively active K+/H+ antiporter from rat liver mitochondria. J Biol Chem 265: 15316–15322, 1990 [PubMed] [Google Scholar]
- 39.Martin WH, Beavis AD, Garlid KD. Identification of an 82,000-dalton protein responsible for K+/H+ antiport in rat liver mitochondria. J Biol Chem 259: 2062–2065, 1984 [PubMed] [Google Scholar]
- 40.Massari S, Azzone GF. The mechanism of ion translocation in mitochondria. 2. Active transport and proton pump. Eur J Biochem 12: 310–318, 1970 [DOI] [PubMed] [Google Scholar]
- 41.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191: 144–148, 1961 [DOI] [PubMed] [Google Scholar]
- 42.Nakae Y, Kwok WM, Bosnjak ZJ, Jiang MT. Isoflurane activates rat mitochondrial ATP-sensitive K+ channels reconstituted in lipid bilayers. Am J Physiol Heart Circ Physiol 284: H1865–H1871, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Nakashima RA, Garlid KD. Quinine inhibition of Na+ and K+ transport provides evidence for two cation/H+ exchangers in rat liver mitochondria. J Biol Chem 257: 9252–9254, 1982 [PubMed] [Google Scholar]
- 44.O'Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res 94: 420–432, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 20: 303–315, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papa S, Guerrieri F, Simone S, Lorusso M. Action of local anaesthetics on passive and energy-linked ion translocation in the inner mitochondrial membrane. J Bioenerg 3: 553–568, 1972 [DOI] [PubMed] [Google Scholar]
- 47.Riess ML, Eells JT, Kevin LG, Camara AK, Henry MM, Stowe DF. Attenuation of mitochondrial respiration by sevoflurane in isolated cardiac mitochondria is mediated in part by reactive oxygen species. Anesthesiology 100: 498–505, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Rodrigo GC, Lawrence CL, Standen NB. Dinitrophenol pretreatment of rat ventricular myocytes protects against damage by metabolic inhibition and reperfusion. J Mol Cell Cardiol 34: 555–569, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Rosen BP, Futai M. Sodium/proton antiporter of rat liver mitochondria. FEBS Lett 117: 39–43, 1980 [DOI] [PubMed] [Google Scholar]
- 50.Shalbuyeva N, Brustovetsky T, Bolshakov A, Brustovetsky N. Calcium-dependent spontaneously reversible remodeling of brain mitochondria. J Biol Chem 281: 37547–37558, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Stowe DF, Aldakkak M, Camara AK, Riess ML, Heinen A, Varadarajan SG, Jiang MT. Cardiac mitochondrial preconditioning by big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am J Physiol Heart Circ Physiol 290: H434–H440, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O'Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science 298: 1029–1033, 2002. [DOI] [PubMed] [Google Scholar]