Abstract
The goal of this study was to determine whether caveolin-2 (Cav-2) is capable of controlling endothelial cell (EC) proliferation in vitro. To realize this goal, we have directly compared proliferation rates and cell cycle-associated signaling proteins between lung ECs isolated from wild-type (WT) and Cav-2 knockout (KO) mice. Using three independent proliferation assays, we have determined that Cav-2 KO ECs proliferate by ca. 2-fold faster than their WT counterparts. Cell cycle analysis by flow cytometry of propidium iodide-stained cells showed a relatively higher percentage of Cav-2 KO ECs in S and G2/M and lower percentage in Go/G1 phases of cell cycle relative to their WT counterparts. Furthermore, an over 2-fold increase in the percentage of S phase-associated Cav-2 KO relative to WT ECs was independently determined with bromodeoxyuridine incorporation assay. Mechanistically, the increase in proliferation/cell cycle progression of Cav-2 KO ECs correlated well with elevated expression levels of predominantly S phase- and G2/M phase-associated cyclin A and B1, respectively. Further mechanistic analysis of molecular events controlling cell cycle progression revealed increased level of hyperphosphorylated (inactive) form of G1 to S phase transition inhibitor, the retinoblastoma protein in hyperproliferating Cav-2 KO ECs. Conversely, the expression level of the two cyclin-dependent kinase inhibitors p16INK4 and p27Kip1 was reduced in Cav-2 KO ECs. Finally, increased phosphorylation (activation) of proproliferative extracellular signal-regulated kinase 1/2 was observed in hyperproliferating Cav-2 KO ECs. Overall, our data suggest that Cav-2 negatively regulates lung EC proliferation and cell cycle progression.
Keywords: phospho-Rb, phospho-ERK1/2, p16INK4, p27Kip1
caveolin proteins are key components of detergent-resistant and cholesterol lipid-rich membranes including lipid rafts and caveolae. There are three members within the caveolin protein family: caveolin-1 (Cav-1), Cav-2, and Cav-3 (51). Cav-1 and -2 are coexpressed in most cell types and tissues, while Cav-3 is muscle specific (51). Cav-2 interacts with Cav-1 to form a hetero-oligomeric complex within caveolae (7, 45). This interaction with Cav-1 is required to transport Cav-2 to the cell surface (28, 33). In the absence of Cav-1, Cav-2 is degraded, and its expression is markedly decreased (8, 35). Caveolins play many important roles. In addition to being key structural proteins that organize caveolae, caveolin proteins are important in regulating endocytosis and various aspects of cellular signaling (51),(17). Although relative to Cav-1, the functional role of Cav-2 is less well defined, most recent studies have started to provide a growing body of evidence suggesting a tissue/cell-specific role for Cav-2. For example, original observations involving Cav-2 knockout (KO) mice revealed hyperplasia in the lung (36), suggesting a role of Cav-2 in regulating lung cell proliferation and/or differentiation. More recent studies revealed skeletal muscle abnormalities in Cav-2 KO mice, including mitochondrial aggregation and increased numbers of M-cadherin-positive satellite cells (39). Studies on the functional role of Cav-2 using overexpression and/or small interfering/short hairpin RNA approaches suggest that Cav-2 is involved in caveolae formation in epithelial cells (12, 20, 45). Furthermore, Cav-2 appears to facilitate infection of mammalian cells with Pseudomonas aeruginosa (53, 54) and Rickettsia conorii (5). Cav-2 has also been shown to regulate endocytosis and trafficking of the M1 muscarinic receptor in MDCK cells (43) and apical lipid trafficking in the intestine of Caenorhabditis elegans (32). There is also evidence for a role of Cav-2 in regulating proliferation and STAT3 signaling in rat fibroblast cell line Hirc-B (16, 18, 19).
Endothelial cell (EC) proliferation is essential for the process of new blood vessel formation called angiogenesis (4, 10, 11). Angiogenesis is required for successful tumor growth, wound healing, as well as normal growth and development (4, 9, 10). The possibility for the involvement of Cav-2 in regulating physiological angiogenesis in the lung is suggested by the observation that Cav-2 KO mice develop a hyperproliferative phenotype in the lungs involving VEGF receptor 2 (Flk-1)-positive cells (36).
Because Flk-1 is widely believed to be predominantly expressed in mouse ECs, this observation suggests that Cav-2 may negatively regulate EC proliferation in the lung. However, because of the overall complexity of the in vivo system, it is impossible to unequivocally conclude whether Cav-2 directly regulates EC proliferation. Therefore, the goal of the present study was to determine whether Cav-2 expression in ECs regulates proliferation of these cells in a homogenous culture system. To realize this goal, we immunoisolated and characterized pure populations of lung ECs from Cav-2 KO and wild-type (WT) mice, followed by comparing their proliferation potential and cell cycle-associated signaling proteins. Our data suggest that Cav-2 directly suppresses lung EC proliferation possibly via inhibition of extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation, increased expression of cyclin-dependent kinase (cdk) inhibitors p16INK4 and p27Kip1, and activation (hypophosphorylation) of the retinoblastoma (Rb) protein, resulting in a decreased cell cycle progression.
MATERIALS AND METHODS
Antibodies.
Antibodies against Cav-2, Cav-1, platelet endothelial cell adhesion molecule-1 (PECAM-1), and Hsp-90 were from BD Transduction Labs. Antibodies to alpha-smooth actin, cyclin A, B1, D1, cdk inhibitors: p16INK4, p27Kip1, EC marker proteins: endothelial nitric oxide synthase (eNOS), Flk-1, and VE-cadherin were from Santa Cruz Biotech. Phospho (serine 780)-Rb, phospho (threonine 202/tyrosine 204)-ERK1/2, and total ERK1/2 were from Cell Signaling Biotech. Antibody against EC marker protein von Willebrand factor (vWF) was from Abcam.
Cells.
Mouse lung endothelial cells (MLECs) were isolated from 2- to 3-wk-old WT and Cav-2 KO mice as previously described (24) with minor modifications. Use of animals for this study was approved by the University of Missouri and the Thomas Jefferson University Animal Care and Use Committees. Briefly, mice were euthanized with an overdose of ketamine/xylazine, and the lungs were excised, minced, and digested with 0.1% collagenase in RPMI medium. The digest was homogenized by passing multiple times through a 14-gauge needle, filtered through 70-μm cell strainers, and the cell suspension plated on 0.1% gelatin-coated dishes. After 2 to 3 days, cells were immortalized by two rounds of infection with retrovirus encoding the polyoma middle T antigen. Cells were allowed to recover for 24 h, and then MLECs were isolated by immunoselection with PECAM-1-conjugated magnetic beads. When cells reached confluence, a second round of immunoisolation was performed. Cells were propagated in 40% DMEM (low glucose)/40% Ham's F-12 medium, 20% FBS, heparin (100 μg/ml), l-glutamine, penicillin-streptomycin, and endothelial cell growth supplement (50 μg/ml). Only low passages (3–5) of MLECs were used in these studies.
Western blot analysis.
Cells were lysed in Laemmli SDS loading buffer, followed by boiling for 5 min. An equal protein amount was loaded on SDS-PAGE, and proteins were electro-transferred onto nitrocellulose membranes. The membranes were washed in Tris-buffered saline with 0.1% Tween, blocked in 5% milk, and incubated with the appropriate primary antibodies at 4°C overnight, followed by incubation with horseradish peroxidase-labeled secondary antibodies, and developed by enhanced chemiluminescence.
Densitometric assessment of cell cycle-associated protein expression and phosphorylation.
The densitometric values for cell cycle associated proteins were determined using Scion Image software (National Institutes of Health, Bethesda, MD). The data are expressed as mean densitometric ratios ± SD of cyclin proteins and cell cycle inhibitors to Hsp-90 or phosphorylated ERK1/2 to total ERK1/2 from three immunoblots.
Immunofluorescence microscopy.
Cells were fixed with 3% paraformaldehyde in Dulbecco's phosphate-buffered saline, pH 7.4 (DPBS), for 30 min, and washed three times with DPBS. Cells were then incubated sequentially with 0.1% Triton X-100 (vol/vol) in DPBS for 10 min, DPBS plus 5% goat serum for 30 min, and thereafter with appropriate antibodies in 0.2% BSA for 2 h, washed three times, and incubated with fluorescein isothiocyanate- or Texas Red-labeled secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA) diluted 1:500, followed by staining with 4′-6-diamidino-2-phenylindole (DAPI). Slides were mounted with Slowfade (Molecular Probes, Eugene, OR), and cells were observed and analyzed with a Zeiss Axiovert epifluorescence microscope.
Cell counts.
MLECs were plated onto 60-mm dishes at 4 × 104 cells per dish. On days 1, 2, 3, and 4, cells were trypsinized and counted with a hemocytometer under inverted microscope. Cell numbers are expressed as means ± SD (n = 9) from one representative out of a total of three experiments.
MTT colorimetric proliferation assay.
MLECs were seeded onto 96-well plates at 2.5 × 103/well in 100 μl complete medium. After 1–4 days of incubation, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] stock solution was added to each well at a final concentration of 0.5 mg/ml and incubated at 37°C for 4 h, followed by lysis with 100 μl of 10% SDS-HCl lysis buffer to solubilize the final product of MTT metabolism, formazan precipitate. After overnight incubation at 37°C, the optical density at 550 nm minus 690 nm was determined using a microplate reader, followed by blank subtraction. The data are expressed as means ± SD (n = 3) from one representative out of a total of four experiments.
Methylene blue colorimetric proliferation assay.
MLECs were plated onto 96-well plates at 5 × 103 cells/well in 100 μl complete medium. Two to six days later, cells were washed with PBS, followed by staining with 1% Methylene Blue solution in 50% ethanol-water for 30 min. Cells were then washed three times with water, dried overnight, followed by lysis with 5% SDS in water for 2 h and colorimetric measurement of optical density at 655 nm using a microplate reader, followed by blank subtraction. The data are expressed as means ± SD (n = 3) from one representative out of a total of four experiments.
Analysis of cell cycle by flow cytometry.
MLECs were plated at 3 × 105 per 150-mm dish and 2 days later were fixed with ice-cold ethanol and stained with 25 μg/ml propidium iodide (PI; Molecular Probes), followed by analytic flow cytometry using a FACScan (BD Biosciences, Mountain View, CA). DNA content cell cycle distribution was assessed on the basis of DNA content determined in linear scale.
Bromodeoxyuridine labeling.
To determine the S phase fraction (DNA synthesizing cells), proliferating MLECs were incubated with 5-bromo-2-deoxyuridine (BrdU; 50 μM) for 2 h, followed by fixing and denaturing DNA with 2 N HCl, and costaining with fluorescein isothiocyanate-conjugated anti-BrdU antibody and DAPI. BrdU-positive and total cells (stained with DAPI) were examined in five random fields using a fluorescent microscope, and the data are expressed as mean percentages ± SD of BrdU-positive cells (n = 5) from one representative out of a total of three experiments.
RESULTS
Newly isolated WT and Cav-2 KO MLECs express EC-specific marker proteins and are devoid of mural cell marker protein α-actin.
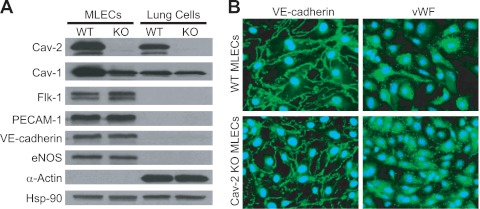
To determine the physiological role of Cav-2 in ECs, we used a genetic approach and isolated MLECs from WT and Cav-2 KO C57Bl6 mice. As seen in Fig. 1A, immunoselection with PECAM-1 (EC-specific marker) antibody coated to Dynabeads (2 rounds) yields populations of WT and Cav-2 KO MLECs expressing EC-specific markers, i.e., Flk-1, PECAM-1, VE-cadherin, and eNOS, but not α-actin (a marker of mural cells, such as vascular smooth muscle cells and pericytes) (Fig. 1A, lanes 1 and 2). Conversely, remaining after the first immunoselection are “lung cells” (mostly fibroblasts, myofibroblasts, smooth muscle cells, and pericytes) that are negative in EC markers, but positive for α-actin (Fig. 1A, lanes 3 and 4). Importantly, WT MLECs express more Cav-2 than the remaining lung cell population of non-EC origin (Fig. 1A, lane 1 vs. lane 3), suggesting a particular abundance of Cav-2 in lung ECs. Consistent with original data involving total lung homogenates from Cav-2 KO mice (36), Cav-2 KO MLECs display ca. 50% reduction in Cav-1 expression (Fig. 1A, lane 1 vs. lane 2). Interestingly, no obvious reduction of Cav-1 could be seen in the lung cells remaining after immunoisolation (Fig. 1A, lane 3 vs. lane 4).
Fig. 1.
Isolation and initial characterization of mouse lung endothelial cells (MLECs). A: Western blot characterization of the purity of isolated wild-type (WT) and caveolin-2 (Cav-2) knockout (KO) MLECs relative to “lung cells” remaining after first immunoisolation with anti-platelet endothelial cell adhesion molecule-1 (PECAM-1) antibody. Note that, unlike WT, Cav-2 KO MLECs and lung cells are completely devoid of Cav-2. Both WT and Cav-2 KO MLEC protein lysates are positive for endothelial cell (EC)-specific marker proteins, i.e., Flk-1, PECAM-1, VE-cadherin, and endothelial nitric oxide synthase (eNOS), but negative for the mural cell marker protein α-actin. Conversely, protein lysates from lung cells are negative for EC marker proteins but positive for α-actin. B: immunofluorescent microscopy images of MLECs colabeled with 4′-6-diamidino-2-phenylindole (DAPI; blue nuclear staining) and with antibodies against EC marker proteins: VE-cadherin (green plasma membrane staining) or von Willebrand factor (vWF; green perinuclear staining). Note that all WT and Cav-2 KO cells are VE-cadherin or vWF positive. Hsp-90, heat shock protein 90.
Furthermore, immunofluorescent labeling with EC marker-specific antibodies against VE-cadherin (green staining of EC plasma membrane junctions) and vWF (green perinuclear staining) shown in Fig. 1B revealed homogenous populations (100% positive in VE-cadherin and vWF in all examined fields) of WT and Cav-2 KO MLECs.
Cav-2 KO MLECs proliferate faster than their WT counterparts.
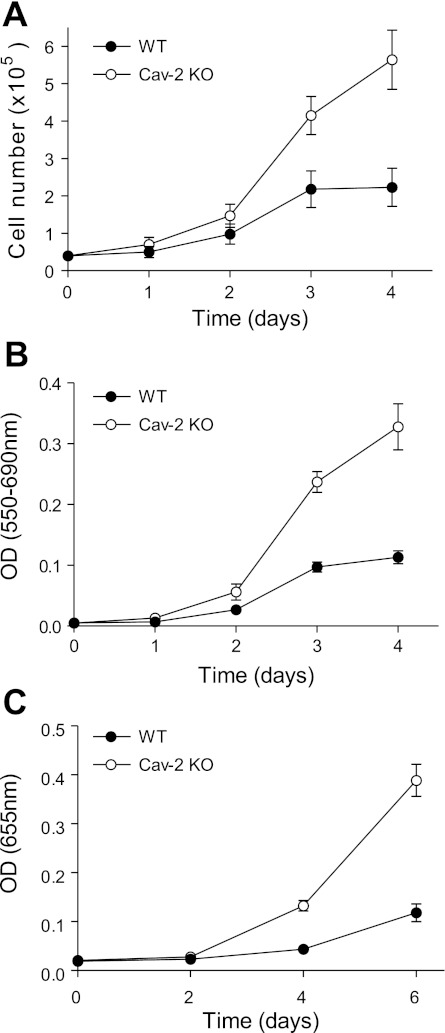
We have used the newly characterized MLECs (passage 3 shown in Fig. 1) to address the role of endogenous Cav-2 in regulating EC proliferation by three independent proliferation assays (Fig. 2).
Fig. 2.
Higher proliferation rate in Cav-2 KO relative to WT MLECs. A: direct determination of cell proliferation rate by cell count using hemocytometer. MLECs were plated at 4 × 104 cells per 60-mm dish, and cell numbers were determined at the indicated time points by cell count and are expressed as means ± SD (n = 9). Values are from one representative out of a total of 3 experiments. B: indirect determination of cell proliferation rate by colorimetric assessment of MTT to formazan conversion. MLECs were plated at 2.5 × 103 cells per well of 96-well plate, and optical density (OD) was measured at the indicated time points using a plate reader with a test wavelength of 550 nm and a reference wavelength of 690 nm to obtain a sample signal (550–690 nm). Data are expressed as means ± SD (n = 3). Values are from one representative out of a total of 4 experiments. C: indirect determination of cellular proliferation rate by colorimetric assessment of cellular DNA staining with methylene blue. MLECs were plated at 5 × 103 per well in a 96-well plate, and OD was measured at the indicated time points using a plate reader with a test wavelength of 655 nm. Data are expressed as means ± SD (n = 3). Values are from one representative out of a total of 4 experiments.
First, we have compared the time-dependent increase in cell number using cell count (Fig. 2A). This approach revealed ca. 2.5-fold higher proliferation rate for Cav-2 KO MLECs (5.64 ± 0.79 × 105) relative to WT MLECs (2.23 ± 0.51 × 105) on day 4.
Furthermore, colorimetric measurement of MTT to formazan conversion (Fig. 2B) has determined ca. 3-fold increase in Cav-2 KO MLECs (0.33 ± 0.04) vs. their WT counterparts (0.11 ± 0.01) on day 4.
Finally, an independent colorimetric proliferation assay utilizing methylene blue staining of cellular DNA and RNA (Fig. 2C) has revealed ca. 3.3-fold increase in proliferation of Cav-2 KO MLECs (0.39 ± 0.03) relative to their WT counterparts (0.12 ± 0.02) on day 6.
Hyperproliferating Cav-2 KO MLECs display enhanced cell cycle progression relative to their WT counterparts.
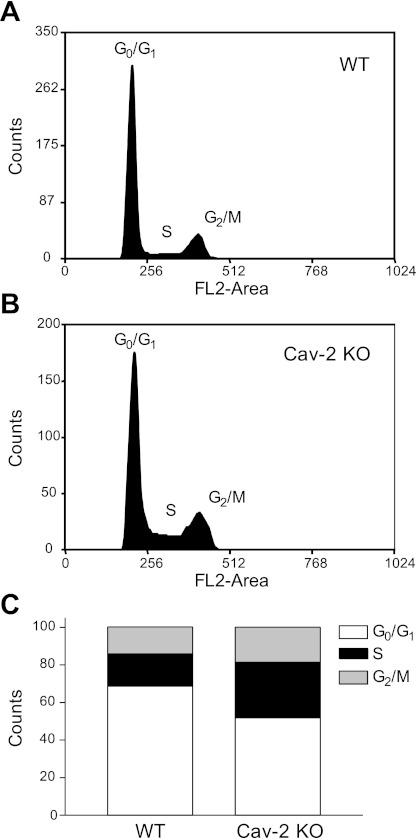
To determine how increased proliferation in Cav-2 KO MLECs is reflected by cell cycle distribution, we have performed flow cytometric analysis of DNA content in proliferating PI-stained MLECs. As seen in Fig. 3, Cav-2 KO MLECs displayed a reduced proportion of cells in the Go/G1 phase of the cell cycle. Specifically, quantitative assessment of cell fraction associated with a given phase of the cell cycle (Fig. 3C) revealed ca. 20% reduction of Go/G1 phase in Cav-2 KO (50% total cell population) relative to WT MLECs (70% of the total cell population). Conversely, there was a nearly twofold increase in the number of Cav-2 KO cells in the S phase (ca. 30% population) relative to WT MLECs (ca. 17% population).
Fig. 3.
Increased association in hyperproliferating Cav-2 KO MLECs with S and G2/M phases and the concurrent decreased association with G0/G1 phases of cell cycle determined by flow cytometry. WT and Cav-2 KO MLECs were plated at 3 × 105 per 150-mm dish, and 2 days later, harvested with trypsin, fixed in ethanol, stained with propidium iodide, and analyzed by flow cytometry using fluorescent channel 2 (FL2-area). Representative cell cycle profiles from one representative out of a total of 3 experiments are shown for WT (A) and Cav-2 KO MLECs (B). Quantitative assessment of the percentage of cellular population associated with each phase of the cell cycle is illustrated in C.
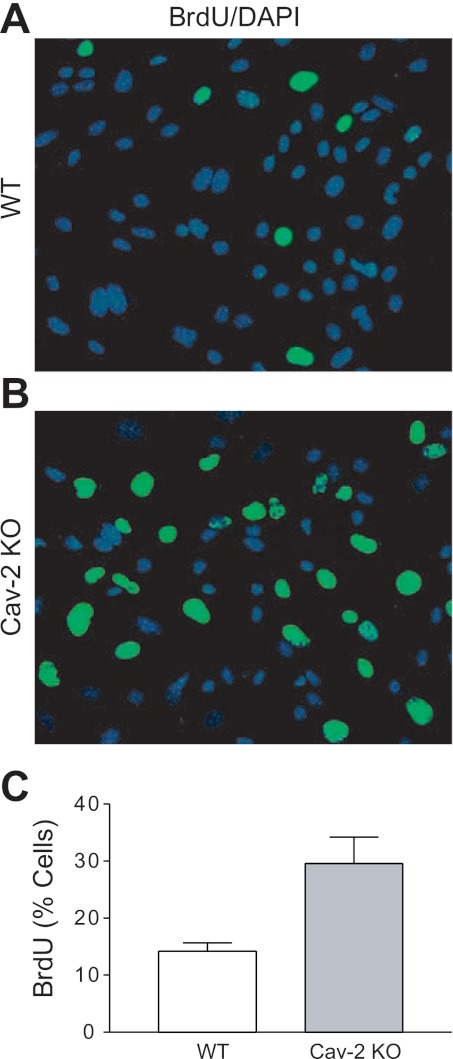
For an independent determination of the S phase fraction, we have used BrdU incorporation assay, followed by fluorescent microscopy (Fig. 4). As seen in Fig. 4, A and B, a considerably higher fraction of Cav-2 KO MLECs incorporated BrdU relative to WT MLECs. Specifically, quantitative analysis from five independent fields (Fig. 4C) revealed ca. 2-fold increase in S phase association for Cav-2 KO MLECs (29.58 ± 4.62%) relative to their WT counterparts (14.21 ± 1.43%). Overall, BrdU incorporation data correlate well with flow cytometric assessment of S phase (shown in Fig. 3) and further support the increased proliferation of Cav-2 KO MLECs.
Fig. 4.
Enhanced S phase association in hyperproliferating Cav-2 KO MLECs determined by BrdU incorporation assay. A and B: WT (A) and Cav-2 KO (B) MLECs were plated at 5 × 103 per chamber of an 8-chamber glass slide precoated with 0.2% gelatin, and 3 days later, pulsed with 5-bromo-2-deoxyuridine (BrdU) for 2 h. Fixing and immunofluorescence staining with fluorescein isothiocyanate-conjugated anti-BrdU antibody (green) and DAPI (blue) was then performed. Note the considerably higher number of BrdU-positive (green) cells in Cav-2 KO (B) relative to WT MLECs (A). C: % BrdU-positive cells was calculated and data are expressed as means ± SD (n = 5). Values are from one representative out of a total of 3 experiments.
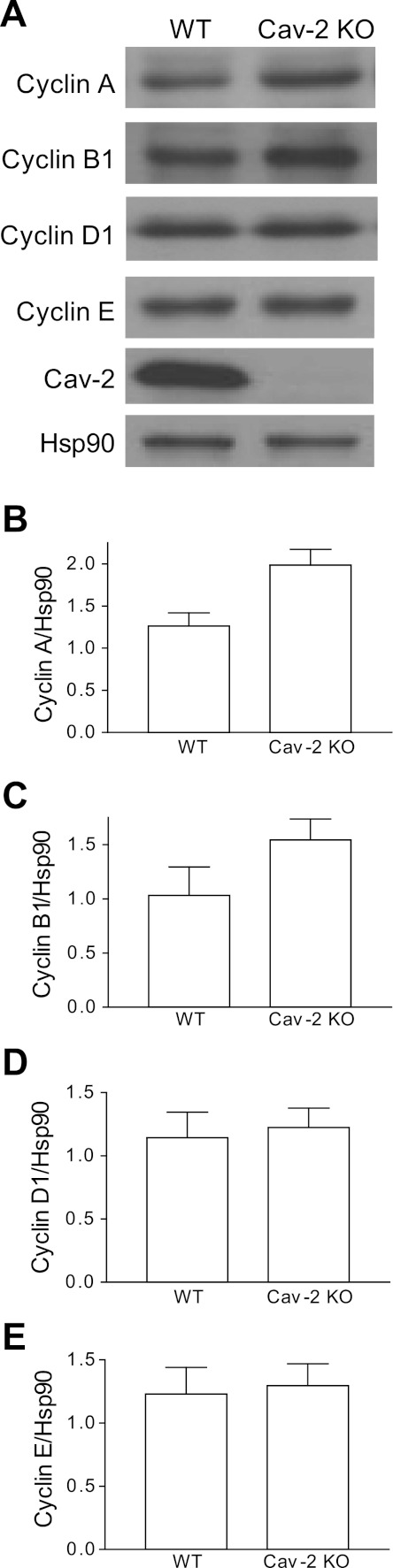
Hyperproliferating Cav-2 KO MLECs express increased levels of S and G2/M phase-associated cyclins A and B1 relative to their WT counterparts.
To gain further mechanistic insight as to how Cav-2 could control cell cycle progression in MLECs, we have examined the expression levels of the cell cycle-associated cyclin proteins in proliferating cells. As seen in Fig. 5A, hyperproliferating Cav-2 KO MLECs expressed more S phase-associated cyclin A and mitotic cyclin B1. Quantitative analysis of densitometric ratios of cyclins/Hsp-90 revealed ca. 1.6-fold increase for cyclin A in Cav-2 KO MLECs (1.99 ± 0.19) relative to their WT counterparts (1.26 ± 0.16) (Fig. 5B). Similarly, ca. 1.5-fold increase in the relative expression levels of cyclin B1 was observed in Cav-2 KO (1.54 ± 0.19) relative to WT (1.03 ± 0.26) cells (Fig. 5C). Conversely, no appreciable changes could be seen in G1 and G1/S phase-associated cyclins D1 and E, respectively (Fig. 5, A, D, and E). These data correlate very well with increased association of Cav-2 KO MLECs with S and presumably G2/M phase of the cell cycle as determined by flow cytometry (Fig. 3) and BrdU incorporation assay (Fig. 4), and further strengthen the negative role for Cav-2 in regulating cell cycle progression in MLECs.
Fig. 5.
Elevated expression levels of S and G2/M phase associated cyclins A and B1 in hyperproliferating Cav-2 KO MLECs relative to their WT counterparts determined by immunoblotting. A: WT and Cav-2 MLECs were plated at 3 × 105 per 150-mm dish, and 2 days later, cells were lysed and SDS-PAGE-resolved protein lysates were immunoblotted with the indicated cyclin-specific antibodies. B–E: mean densitometric ratios ± SD (n = 3) of cyclin proteins expression levels to protein loading control Hsp-90. Note a visible increase in the expression levels of cyclins A and B1 but not D1 and E in Cav-2 KO relative to WT MLECs.
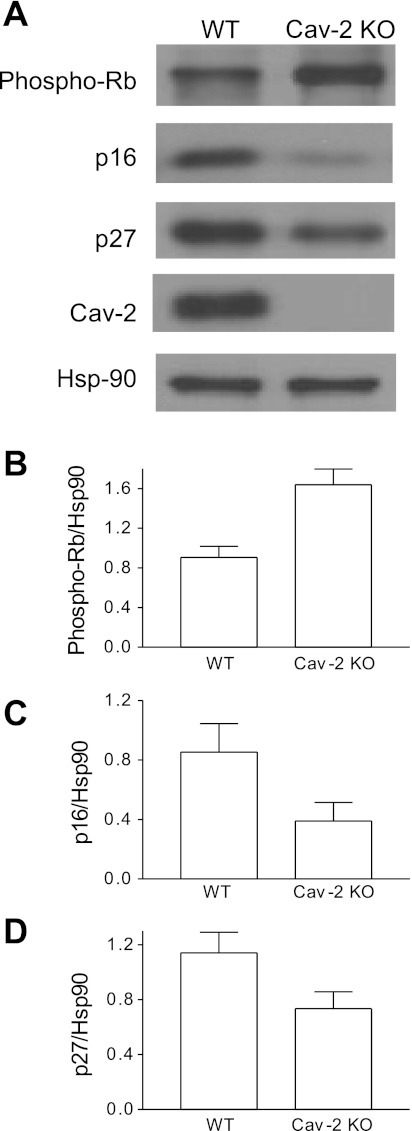
Hyperproliferating Cav-2 KO MLECs display increased phosphorylation levels of the Rb protein, a G1 to S transition inhibitor, and concurrent decrease in the expression levels of the cdk inhibitors p16INK4 and p27Kip1.
Next, we have examined how increased proliferation/cell cycle progression in Cav-2 KO MLECs correlates with the activation state or total levels of cell cycle inhibitors. Specifically, we performed immunoblotting with antibodies against the phosphorylated (inactive) form of the Rb protein and total levels of the cdk inhibitors p16INK4 and p27Kip1 (Fig. 6). There was ca. 1.8-fold increase in the “inactivating” phosphorylation level of the Rb protein in Cav-2 KO (1.64 ± 0.16) relative to WT (0.9 ± 0.11) MLECs (Fig. 6A, top, and Fig. 6B). Furthermore, the expression level of p16INK4 was reduced by ca. 2.2-fold in Cav-2 KO (0.39 ± 0.13) relative to WT (0.85 ± 0.19) MLECs (Fig. 6A, second from top, and Fig. 6C). Finally, the expression level of p27Kip1 was reduced by ca. 1.6-fold in Cav-2 KO (0.73 ± 0.12) relative to WT (1.14 ± 0.15) MLECs (Fig. 6A, bottom, and Fig. 6D). These data suggest that Cav-2 may control EC proliferation by upregulating the expression levels of cdk inhibitors p16INK4 and p27Kip1 and reducing the inactivating phosphorylation of the G1 to S transition inhibitor the Rb protein.
Fig. 6.
Increased levels of phosphorylated/inactive form of the Rb protein with concurrent decrease in the expression levels of cyclin-dependent kinase (cdk) inhibitors p27Kip1 and p16INK4 in hyperproliferating Cav-2 KO relative to WT MLECs determined by immunoblotting. A: WT and Cav-2 MLECs were plated at 3 × 105 per 150-mm dish, and 2 days later, lysed and immunoblotted with the indicated antibodies. B: mean densitometric ratios ± SD (n = 3) of phosphorylated (inactive) Rb levels to protein loading control Hsp-90. C and D: mean densitometric ratios ± SD (n = 3) of indicated cdk inhibitors p16INK4 (p16) and p27Kip1 (p27) expression levels to protein loading control Hsp-90.
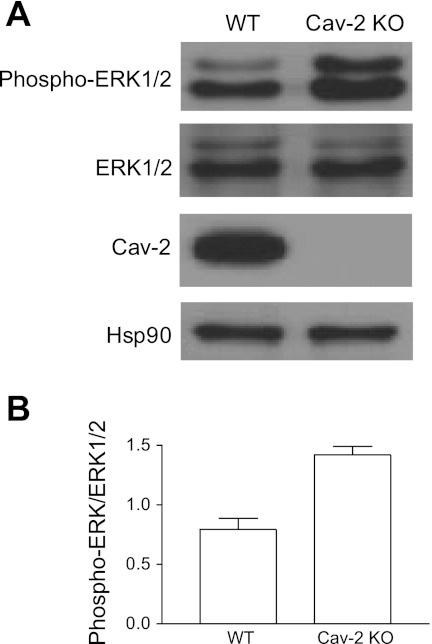
Hyperproliferating Cav-2 KO MLECs display increased phosphorylation level of ERK1/2.
Since the MAPK/ERK1/2 pathway plays an important role in cell proliferation, we have examined the phosphorylation state of ERK1/2 in proliferating MLECs (Fig. 7). We have observed an ca. 1.8-fold increase in the phosphorylation level of ERK1/2 in Cav-2 KO MLECs (1.42 ± 0.07) relative to their WT counterparts (0.8 ± 0.09) (Fig. 7, A and B). These data suggest that reducing phosphorylation of ERK1/2 is one of important mechanisms via which Cav-2 negatively regulates EC proliferation.
Fig. 7.
Augmented activation/phosphorylation state of ERK1/2 in hyperproliferating Cav-2 KO relative to WT MLECs determined by immunoblotting. A: WT and Cav-2 KO MLECs were plated at 3 × 105 per 150-mm dish, and 2 days later, were lysed and immunoblotted with the indicated antibodies. B: mean densitometric ratios ± SD (n = 3) of phosphorylated ERK1/2 (phospho-ERK1/2) to total expression level (ERK1/2).
DISCUSSION
In the current study using pure populations of ECs isolated from lungs of WT and Cav-2 KO mice, we have determined that Cav-2 can negatively regulate EC proliferation.
Assuming that the increased number of Flk-1-positive cells which was observed in the lungs of Cav-2 KO mice (36) stems from hyperproliferation of lung ECs, it is still unclear whether Cav-2 directly regulates ECs proliferation or does it via affecting other cell type(s) which in turn indirectly stimulate EC proliferation via paracrine manner. For the above mentioned reason, we have used a reductionist approach by separating WT and Cav-2 KO lung ECs from their complex multicellular environment and examined their proliferation rate along with cell cycle progression and associated signaling pathways.
Pertinent to inherent complexity of an in vivo system, even slight contamination with cells of non-EC origin could affect the results of in vitro studies. Thus, our first priority was to obtain pure EC populations from WT and Cav-2 KO mice. This was confirmed by immunofluorescent labeling with antibodies against EC-specific marker proteins such as VE-cadherin and vWF. Immunoblotting data independently confirmed that unlike the lung cells of non-EC origin remaining after immunoisolation, WT and Cav-2 KO MLEC lysates are equally enriched in EC-specific marker proteins such as Flk-1, PECAM, VE-cadherin, or eNOS. The data suggest that WT and Cav-2 KO MLECs are of equal purity and that the loss of Cav-2 does not affect the expression levels of the aforementioned EC-specific marker proteins.
Consistent with original report involving total lung homogenates from Cav-2 KO mice (36), immunoisolated Cav-2 KO MLECs retain ca. 50% of Cav-1 expression. However, comparable reduction of Cav-1 in Cav-1 (+/−) heterozygote mice did not cause a hyperproliferative phenotype and did not coassociate with an increase in the number of Flk-1-positive cells in the lungs (36). The hyperproliferative phenotype we observed in the Cav-2 KO MLECs is thus unlikely to be related to the coincidental reduction of Cav-1 in these cells. In addition, Cav-1 is still localized to plasma membrane caveolae/lipid raft membranes in lung tissue and isolated mouse embryonic fibroblasts from Cav-2 KO mice (36). Thus unlike Cav-1 KO mice, where caveolae are absent and only 5% of Cav-2 is retained and completely mislocalized to the endoplasmic reticulum/Golgi compartment (35), Cav-2 KO MLECs are an excellent model for addressing the specific role of Cav-2 in EC function.
The observed increase in the proliferation rate of Cav-2 KO over WT MLECs, determined with three independent proliferation assays, is the first genetic evidence for a direct negative role of Cav-2 in regulating EC proliferation. Furthermore, both WT and Cav-2 KO MLECs are homogenous populations of mature/differentiated ECs (i.e., positive in VE-cadherin and vWF), implying that Cav-2 is capable of controlling proliferation of mature/differentiated ECs. This is important since no marker proteins for ECs besides Flk-1 were examined in the lungs from Cav-2 KO mice (36). Because Flk-1 can also be expressed on hematopoietic progenitor cells which can differentiate not only into ECs, but also into other mesenchymal cells such as skeletal or smooth muscle cells (25), our report is the first to provide direct evidence that the increased proliferative capacity of ECs is likely responsible for the in vivo hyperproliferative phenotype.
Our cell cycle data showing an increased fraction of Cav-2 KO cells associated with the S phase of the cell cycle, suggest enhanced S phase entry of Cav-2 KO MLECs. The conclusion is supported by fluorescent microscopic assessment of the S phase population showing an increased proportion of Cav-2 KO cells incorporating BrdU. Our data showing increased levels of cyclin A in hyperproliferating Cav-2 KO MLECs further corroborate the observed increase cell number in the S phase. Cyclin A in a complex with cdk2 plays an important role in S phase progression. A type cyclins are synthesized at the onset of the S phase and phosphorylate proteins involved in DNA replication (6, 34).
To enter the S phase, cells must pass through the G1 to S transition, which requires the hyperphosphorylation/inactivation of the tumor suppressor Rb (41, 42). The Rb protein is regarded as a negative regulator of the cell cycle progression (30). Phosphorylation-induced inactivation of Rb releases the transcription factor E2F, allowing it to activate transcription of genes required for DNA synthesis, including cyclin A (48). On the basis of our cell cycle analysis, BrdU incorporation, and cyclin A expression data, we expected a less active and thus hyperphosphorylated Rb in Cav-2 KO than in WT MLECs. Indeed, immunoblotting data with phospho-Rb antibody revealing a nearly twofold increase in phosphorylated Rb in Cav-2 KO cells confirming our predictions. Thus, Cav-2 may suppress EC cycle progression via limiting inactivating phosphorylation of Rb protein leading to reduced G1 to S transition. In addition to its role in the negative regulation of the cell cycle, Rb is also considered to be a positive regulator of cellular differentiation (30). Thus, it is possible that in addition to negative regulation of EC proliferation, Cav-2 may positively regulate EC differentiation by maintaining Rb in a more active (hypophosphorylated) state. The latter possibility will deserve to be explored in future studies. The involvement of Cav-2 in regulating other cell/tissue types differentiation has been suggested by independent studies showing upregulation of Cav-2 during differentiation of various cell/tissue types including adipocytes (38), dorsal root ganglion neurons and PC12 cells (13), chondrocytes (40), and C6 glioma cells (44).
How can Cav-2 regulate Rb phosphorylation? One possibility is by influencing the expression levels of cdk inhibitors. Cdk inhibitors are negative regulators of cell cycle (22, 29). In ECs, VEGF treatment has been shown to delay senescence through downregulation of three cdk inhibitors, p21Cip1, p16INK4A, and p27Kip1, without significant changes in p53 expression (50). Importantly, our data showing reduced expression levels of p16INK4 and p27Kip1 in hyperproliferating Cav-2 KO suggest that Cav-2 may negatively control lung EC proliferation by maintaining the relatively high expression levels of these two important cdk inhibitors. p16INK4 inhibits cell cycle progression by preventing the formation of active cyclin D/cdk4-6 complexes that would otherwise phosphorylate and inactivate the Rb protein (41). Cav-2 could, by maintaining a high level of p16INK4 in the WT MLECs, limit the formation of cyclin D/cdk4-6 complex, resulting in the reduction of Rb phosphorylation that we observed. In addition to its effect on cellular proliferation, p16INK4 is often associated with cellular senescence (14). More recently, p16INK4 has been identified as one of the genes upregulated during EC differentiation into capillary lumen in three-dimensional collagen (2). Thus, our data suggest that upregulation of p16INK4 by Cav-2 may also be an important factor contributing to EC differentiation and senescence.
Unlike p16INK4, p27Kip1 may negatively affect the kinase activity of multiple cdk/cyclin complexes, such as cdk2/cyclin E, cdk2/cyclin A, cdk1/cyclin A, as well as cdk1/cyclin B (1, 31, 47). Therefore, it is likely that upregulation of p27Kip1 by Cav-2 suppresses both G1 to S transition and progression through S and G2/M phases of the cell cycle. This conclusion is supported by overall increased association with S and G2/M phases in hyperproliferating Cav-2 KO MLECs along with increased levels of cyclin A and B1. How can Cav-2 upregulate p27Kip1? One potential mechanism is via ERK1/2, which has recently been shown to directly phosphorylate p27Kip1 and thereby promote its proteasomal degradation (23). Another possibility is an indirect regulation through Rb protein, which has been shown to stabilize p27Kip1 by impeding cyclin E/cdk2-mediated phosphorylation, leading to proteasomal degradation of p27Kip1 (15). Our data also show a nearly twofold increase in ERK1/2 phosphorylation in hyperproliferating Cav-2 KO MLECs. ERK1/2 plays an important role in cellular proliferation, in particular in regulating the G1 to S phase transition of the cell cycle (26). Activated (phosphorylated) ERK1/2 is known to stimulate the G1 to S phase progression by multiple mechanisms, including induction of D type cyclins and formation of complexes with cdk4, stabilization of c-Myc, downregulation of p27Kip1 expression, and downregulation of multiple antiproliferative genes (26). We can rule out the first two possibilities, since we have not observed a significant increase in cyclin D1, nor c-Myc (data not shown) expression levels in hyperproliferating Cav-2 KO MLECs. Therefore, the most likely mechanism via which phospho-ERK1/2 enhances proliferation in Cav-2 KO ECs is through downregulation of p27Kip1. Interestingly, a recent gene profiling analysis has identified a set of 173 genes that were downregulated by ERK1/2-dependent mechanisms during G1 to S phase progression (52). Thus it is likely that, in addition to p27Kip1, downregulation of some of these antiproliferative genes could contribute to ERK1/2 mediated enhancement of proliferation in Cav-2 KO MLECs.
How does Cav-2 regulate ERK1/2 phosphorylation? One potential mechanism could be through a direct interaction of the two proteins, which has previously been reported in the Hirc-B fibroblast cell line (16, 19). Alternatively, Cav-2 could affect one or more mediators involved in MAPK signaling upstream of ERK, for example GRB2, Ras, Raf, or MEK1/2, which directly phosphorylates ERK1/2. All of these signaling molecules have been shown to associate with caveolae/lipid raft microdomains or interact with caveolin proteins (37).
What could be the direct targets of Cav-2 that are responsible for its negative effect on EC proliferation? We have recently started to address this important question beginning with eNOS. eNOS has previously been shown to positively regulate EC proliferation (3, 27). Our preliminary data (not shown) indicate that there is no difference in the phosphorylation level of eNOS at serine 1179 (i.e., activated form) in Cav-2 KO MLECs relative to their WT counterparts. These data are consistent with those obtained in vivo showing that in contrast to Cav-1 KO mice, eNOS-dependent vascular response was not altered in Cav-2 KO mice (36).
In summary, Cav-2 negatively regulates proliferation and cell cycle progression in mature (VE-cadherin and vWF positive) MLECs. Mechanistically, Cav-2 may suppress proproliferative MAPK/ERK1/2 pathways and upregulates the expression levels of the cdk inhibitor p16INK4. Both of the above mentioned actions of Cav-2 may lead to reduced inactivating phosphorylation of the G1 to S transition inhibitor the Rb protein and upregulation of the cdk inhibitor p27Kip1, which would further contribute to the overall suppression of cell cycle at multiple levels such as the G1 to S transition as well as the S phase and the G2 to M progression. Further studies with primary ECs of lung and non-lung origin are needed to elucidate the detailed mechanisms regulating EC proliferation. In particular, further study of the function of previously identified serine and tyrosine phosphorylation of Cav-2 (21, 45, 46, 49) as well as their significance for physiological and pathological angiogenesis in vivo is clearly warranted. In particular, examining whether Cav-2 regulates primary EC proliferation in response to specific growth factors such as VEGF and basic FGF will be of interest as it will likely help to provide further mechanistic insights at the specific receptor and post-receptor levels.
GRANTS
This work was supported by the Grant-in-Aid from the Midwest Affiliate of the American Heart Association (0855671G to G. Sowa) and by a grant from the National Institutes of Health (1R01HL081860 to G. Sowa).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. William C. Sessa for EcoPack2 cells stably producing retrovirus expressing the polyoma middle T antigen and Dr. Robert Lim for critical reading and helpful comments regarding the manuscript.
REFERENCES
- 1.Aprelikova O, Xiong Y, Liu ET. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J Biol Chem 270: 18195–18197, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci 114: 2755–2773, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Cai J, Jiang WG, Ahmed A, Boulton M. Vascular endothelial growth factor-induced endothelial cell proliferation is regulated by interaction between VEGFR-2, SH-PTP1 and eNOS. Microvasc Res 71: 20–31, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 407: 249–257, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Chan YG, Cardwell MM, Hermanas TM, Uchiyama T, Martinez JJ. Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, c-Cbl, clathrin and caveolin 2-dependent manner. Cell Microbiol 11: 629–644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coverley D, Pelizon C, Trewick S, Laskey RA. Chromatin-bound Cdc6 persists in S and G2 phases in human cells, while soluble Cdc6 is destroyed in a cyclin A-cdk2 dependent process. J Cell Sci 113: 1929–1938, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Das K, Lewis RY, Scherer PE, Lisanti MP. The membrane-spanning domains of caveolins-1 and -2 mediate the formation of caveolin hetero-oligomers. Implications for the assembly of caveolae membranes in vivo. J Biol Chem 274: 18721–18728, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293: 2449–2452, 2001 [DOI] [PubMed] [Google Scholar]
- 9.English D, Kovala AT, Welch Z, Harvey KA, Siddiqui RA, Brindley DN, Garcia JG. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J Hematother Stem Cell Res 8: 627–634, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1: 27–31, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Folkman J, Shing Y. Angiogenesis. J Biol Chem 267: 10931–10934, 1992 [PubMed] [Google Scholar]
- 12.Fujimoto T, Kogo H, Nomura R, Une T. Isoforms of caveolin-1 and caveolar structure. J Cell Sci 113: 3509–3517, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Galbiati F, Volonte D, Gil O, Zanazzi G, Salzer JL, Sargiacomo M, Scherer PE, Engelman JA, Schlegel A, Parenti M, Okamoto T, Lisanti MP. Expression of caveolin-1 and -2 in differentiating PC12 cells and dorsal root ganglion neurons: caveolin-2 is up-regulated in response to cell injury. Proc Natl Acad Sci USA 95: 10257–10262, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol 16: 859–867, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard CM, Claudio PP, De Luca A, Stiegler P, Jori FP, Safdar NM, Caputi M, Khalili K, Giordano A. Inducible pRb2/p130 expression and growth-suppressive mechanisms: evidence of a pRb2/p130, p27Kip1, and cyclin E negative feedback regulatory loop. Cancer Res 60: 2737–2744, 2000 [PubMed] [Google Scholar]
- 16.Kim S, Pak Y. Caveolin-2 regulation of the cell cycle in response to insulin in Hirc-B fibroblast cells. Biochem Biophys Res Commun 330: 88–96, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Krajewska WM, Maslowska I. Caveolins: structure and function in signal transduction. Cell Mol Biol Lett 9: 195–220, 2004 [PubMed] [Google Scholar]
- 18.Kwon H, Jeong K, Hwang EM, Park JY, Hong SG, Choi WS, Pak Y. Caveolin-2 regulation of STAT3 transcriptional activation in response to insulin. Biochim Biophys Acta 1793: 1325–1333, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Kwon H, Jeong K, Pak Y. Identification of pY19-caveolin-2 as a positive regulator of insulin-stimulated actin cytoskeleton-dependent mitogenesis. J Cell Mol Med 13: 1549–1564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahtinen U, Honsho M, Parton RG, Simons K, Verkade P. Involvement of caveolin-2 in caveolar biogenesis in MDCK cells. FEBS Lett 538: 85–88, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Park DS, Wang XB, Scherer PE, Schwartz PE, Lisanti MP. Src-induced phosphorylation of caveolin-2 on tyrosine 19. Phospho-caveolin-2 (Tyr(P)19) is localized near focal adhesions, remains associated with lipid rafts/caveolae, but no longer forms a high molecular mass hetero-oligomer with caveolin-1. J Biol Chem 277: 34556–34567, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Lees E. Cyclin dependent kinase regulation. Curr Opin Cell Biol 7: 773–780, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Li N, Wang C, Wu Y, Liu X, Cao X. Ca(2+)/calmodulin-dependent protein kinase II promotes cell cycle progression by directly activating MEK1 and subsequently modulating p27 phosphorylation. J Biol Chem 284: 3021–3027, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Lim YC, Luscinskas FW. Isolation and culture of murine heart and lung endothelial cells for in vitro model systems. Methods Mol Biol 341: 141–154, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lugus JJ, Park C, Choi K. Developmental relationship between hematopoietic and endothelial cells. Immunol Res 32: 57–74, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 26: 3227–3239, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Metaxa E, Meng H, Kaluvala SR, Szymanski MP, Paluch RA, Kolega J. Nitric oxide-dependent stimulation of endothelial cell proliferation by sustained high flow. Am J Physiol Heart Circ Physiol 295: H736–H742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mora R, Bonilha VL, Marmorstein A, Scherer PE, Brown D, Lisanti MP, Rodriguez-Boulan E. Caveolin-2 localizes to the golgi complex but redistributes to plasma membrane, caveolae, and rafts when co-expressed with caveolin-1. J Biol Chem 274: 25708–25717, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Morgan DO. Principles of CDK regulation. Nature 374: 131–134, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Neganova I, Lako M. G1 to S phase cell cycle transition in somatic and embryonic stem cells. J Anat 213: 30–44, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O′Connor PM. Mammalian G1 and G2 phase checkpoints. Cancer Surv 29: 151–182, 1997 [PubMed] [Google Scholar]
- 32.Parker S, Walker DS, Ly S, Baylis HA. Caveolin-2 is required for apical lipid trafficking and suppresses basolateral recycling defects in the intestine of Caenorhabditis elegans. Mol Biol Cell 20: 1763–1771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parolini I, Sargiacomo M, Galbiati F, Rizzo G, Grignani F, Engelman JA, Okamoto T, Ikezu T, Scherer PE, Mora R, Rodriguez-Boulan E, Peschle C, Lisanti MP. Expression of caveolin-1 is required for the transport of caveolin-2 to the plasma membrane. Retention of caveolin-2 at the level of the golgi complex. J Biol Chem 274: 25718–25725, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Petersen BO, Lukas J, Sorensen CS, Bartek J, Helin K. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J 18: 396–410, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 276: 38121–38138, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H, Jr, Christ GJ, Edelmann W, Lisanti MP. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol 22: 2329–2344, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev 54: 431–467, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA 93: 131–135, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schubert W, Sotgia F, Cohen AW, Capozza F, Bonuccelli G, Bruno C, Minetti C, Bonilla E, Dimauro S, Lisanti MP. Caveolin-1(−/−)- and caveolin-2(−/−)-deficient mice both display numerous skeletal muscle abnormalities, with tubular aggregate formation. Am J Pathol 170: 316–333, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwab W, Galbiati F, Volonte D, Hempel U, Wenzel KW, Funk RH, Lisanti MP, Kasper M. Characterisation of caveolins from cartilage: expression of caveolin-1, -2 and -3 in chondrocytes and in alginate cell culture of the rat tibia. Histochem Cell Biol 112: 41–49, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13: 1501–1512, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 9: 1149–1163, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Shmuel M, Nodel-Berner E, Hyman T, Rouvinski A, Altschuler Y. Caveolin 2 regulates endocytosis and trafficking of the M1 muscarinic receptor in MDCK epithelial cells. Mol Biol Cell 18: 1570–1585, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva WI, Maldonado HM, Velazquez G, Rubio-Davila M, Miranda JD, Aquino E, Mayol N, Cruz-Torres A, Jardon J, Salgado-Villanueva IK. Caveolin isoform expression during differentiation of C6 glioma cells. Int J Dev Neurosci 23: 599–612, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Sowa G, Pypaert M, Fulton D, Sessa WC. The phosphorylation of caveolin-2 on serines 23 and 36 modulates caveolin-1-dependent caveolae formation. Proc Natl Acad Sci USA 100: 6511–6516, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sowa G, Xie L, Xu L, Sessa WC. Serine 23 and 36 phosphorylation of caveolin-2 is differentially regulated by targeting to lipid raft/caveolae and in mitotic endothelial cells. Biochemistry 47: 101–111, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78: 67–74, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol 3: 11–20, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Wang XB, Lee H, Capozza F, Marmon S, Sotgia F, Brooks JW, Campos-Gonzalez R, Lisanti MP. Tyrosine phosphorylation of caveolin-2 at residue 27: differences in the spatial and temporal behavior of phospho-Cav-2 (pY19 and pY27). Biochemistry 43: 13694–13706, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Watanabe Y, Lee SW, Detmar M, Ajioka I, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) delays and induces escape from senescence in human dermal microvascular endothelial cells. Oncogene 14: 2025–2032, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Williams TM, Lisanti MP. The caveolin genes: from cell biology to medicine. Ann Med 36: 584–595, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto T, Ebisuya M, Ashida F, Okamoto K, Yonehara S, Nishida E. Continuous ERK activation downregulates antiproliferative genes throughout G1 phase to allow cell-cycle progression. Curr Biol 16: 1171–1182, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Zaas DW, Duncan MJ, Li G, Wright JR, Abraham SN. Pseudomonas invasion of type I pneumocytes is dependent on the expression and phosphorylation of caveolin-2. J Biol Chem 280: 4864–4872, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Zaas DW, Swan ZD, Brown BJ, Li G, Randell SH, Degan S, Sunday ME, Wright JR, Abraham SN. Counteracting signaling activities in lipid rafts associated with the invasion of lung epithelial cells by Pseudomonas aeruginosa. J Biol Chem 284: 9955–9964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]