Abstract
Phosphorylation of adenine nucleotide translocator 1 (ANT1) at residue Y194, which is part of the aromatic ladder located within the lumen of the carrier, critically regulates mitochondrial metabolism. Recent data support the concept that members of the Src family of nonreceptor tyrosine kinases are constitutively present in mitochondria and key to regulation of mitochondrial function. Herein, we demonstrate that site mutations of ANT1 (Y190→F190, Y194→F194) mimicking dephosphorylation of the aromatic ladder resulted in loss of oxidative growth and ADP/ATP exchange activity in respiration-incompetent yeast expressing mutant chimeric yN-hANT1. ANT1 is phosphorylated at Y194 by the Src family kinase members Src and Lck, and increased phosphorylation is tightly linked to reduced cell injury in preconditioned protected vs. unprotected cardiac mitochondria. Molecular dynamics simulations find the overall structure of the phosphorylated ANT1 stable, but with an increased steric flexibility in the region of the aromatic ladder, matrix loop m2, and four helix-linking regions. Combined with an analysis of the putative cytosolic salt bridge network, we reason that the effect of phosphorylation on transport is likely due to an accelerated transition between the main two conformational states (c↔m) of the carrier during the transport cycle. Since “aromatic signatures” are typical for other mitochondrial carrier proteins with important biological functions, our results may be more general and applicable to these carriers.
Keywords: mitochondrial adenosine 5′-diphosphate/adenosine 5′-triphosphate translocases, Src family tyrosine kinase, mitochondria, molecular dynamics simulations, preconditioning
adenine nucleotide translocase (ANT), also called ADP/ATP carrier (AAC), is a model member of the mitochondrial carrier family and has the shape of a basket built of six tilted transmembrane (TM) helices (22, 25, 28). In addition to the mitochondrial carrier family motif PX(D/E)XX(K/R), ANT has the RRRMMM signature, characteristic for nucleotide transporters. ANT takes center stage in cellular energy gating, since it is the principal carrier located in the inner mitochondrial membrane transporting ATP in exchange for ADP from the mitochondrial matrix to the cytosol. Triggered by substrate binding, ANT undergoes drastic conformational changes between a cytosolic-open state (c-state) and a matrix-open state (m-state), both of which are required for the successful progression of the nucleotides through the protein funnel (27). A current transport model suggests that basic residues at the entrance of the cavity help attracting negatively charged nucleotides and three highly conserved tyrosine residues [“tyrosine ladder” (22)] on TM helix 4 (Y186, Y190, Y194) with side chains oriented toward the cavity would subsequently steer the ADP coming from the intermembrane space—by means of π-π interactions between the adenine ring and the tyrosine residues—into the binding site (7, 39).
Our laboratory and others recently observed that ANT, isolated from brain and heart mitochondria, is phosphorylated at two of the residues (Y190, Y194) within the tyrosine ladder (2, 3, 11, 14, 19). We further reported that mutation of Y194→F194 abolished oxidative growth capacity in a ANT/AAC-disrupted yeast model. Tyrosine phosphorylation of ANT1 was markedly reduced in unprotected mitochondria from rat hearts exposed to ischemia-reperfusion injury, as opposed to protected pre- and postconditioned mitochondria, implying that preservation of tyrosine phosphorylation in ANT1 may constitute an essential cardioprotective mechanism (11). Recent data support the concept that members of the Src family of nonreceptor tyrosine kinases (Src-FKs) are constitutively present in mitochondria and key to regulation of mitochondrial function (15, 23, 24, 33, 37). Src-FKs comprise nine highly homologous proteins (Src, Fyn, Yes, Fgr, Lyn, Hck, Lck, Blk, Yrk) that are critically involved in a variety of signal transduction pathways related to cell division, motility, adhesion, angiogenesis, and cell survival (36). Miyazaki et al. (18) first demonstrated in 2003 that Src activity in mitochondria affects energy metabolism by phosphorylating and increasing the activity of cytochrome-c oxidase subunit II. Examining the sequences of mammalian ANTs, we noticed that several species isoforms of ANT1 (human, bovine, rat, and mouse) contain the Src-FK SH2 domain binding motif YDEI between residues 290 and 293 (located on TM helix 6), suggesting that Src-FKs could indeed mediate phosphorylation of the tyrosine ladder.
The aim of this study was to provide experimental evidence that Src-FKs are responsible for the phosphorylation of the tyrosine ladder in ANT1, and to explore the effects of the tyrosine ladder phosphorylation on AAC activity and its significance in the context of ischemia-reperfusion injury of the heart. Using molecular dynamics (MD) simulations, we further aimed at investigating the structural stability of the phosphorylated form and the mechanisms at the atomic level as to how tyrosine ladder phosphorylation might affect the function of ANT1. We here report a novel fundamental downstream mechanism of cell protection established through Src-FK-mediated phosphorylation of the tyrosine ladder in ANT, the key protein responsible for mitochondrial energy gating.
MATERIAL AND METHODS
Experimental protocols used in this investigation were approved by the Animal Care and Use Committee of the University of Zurich, Switzerland, and all procedures conformed to the Guiding Principles in the Care and Use of Animals of the American Physiological Society and were in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH publication 85–23, revised 1996). Reagents were purchased from Sigma (St. Louis, MO), and yeast media from Brunschwig (Basel, Switzerland). [8-14C]adenosine 5′-diphosphate was purchased from PerkinElmer (Schwerzenbach, Switzerland).
Preparation of plasmids.
A pair of complementary oligonucleotides containing yeast AAC2 NH2-terminal 75-bp nucleotides and Nde I and Aat II overhang sequences (bold underlined) were synthesized (forward: 5′-ATACATATGTCTTCTAACGCCCAAGTCAAAACCCCACTACCTCCAGCCCCAGCTCCAAAGAAGGAATCTAACTTTTTGATTGACGTCGGT-3′, and reverse: 5′-ACCGACGTCAATCAAAAAGTTAGATTCCTTCTTTGGAGCTGGGGCTGGAGGTAGTGGGGTTTTGACTTGGGCGTTAGAAGACATATGTAT-3′). This pair of complementary oligonucleotides was annealed and digested with Nde I and Aat II to obtain a Nde I-Aat II fragment of yeast AAC2 NH2-terminal region. The pRS314-YA2P vectors encoding hANT1 were generated, as described previously. A hANT1T31G mutant in pRS314-YA2P was generated using the QuickChange kit (Stratagene Europe, Amsterdam, the Netherlands), according to the instruction manual, to create an artificial restriction site of Aat II. The chimeric yN-hANT1 construct (termed pRS314-YA2P-yN-hANT1) was then obtained by replacement of the first 30 bp of hANT1 cDNA with a Nde I-Aat II fragment of yeast AAC2 NH2-terminal region. pRS314-YA2P-yN-hANT1Y190F, yN-hANT1Y194F, and yN-hANT1Y190F/Y194F were generated using the QuickChange kit. pcDNA3.HA vector and pcDNA3.HA-Src constructs (wild-type Src-WT, kinase-dead/deficient Src-KD K295M, and constitutively active Src-CA Y527F) were kind gifts from Dr. J. Park (Dept. of Pharmacology, College of Medicine, Chungnam National University, Taejeon, South Korea) (40). The Lck mammalian expression vector pdKCR-Lck was kindly provided by Dr. T. Taniguchi (Dept. of Immunology, Faculty of Medicine, University of Tokyo, Tokyo, Japan) (35). The BamH I site in Lck cDNA was deleted by silencing site-mutagenesis using the QuikChange kit. The BamH I-EcoR I fragment of full length Lck cDNA was amplified by PCR and cloned into pcDNA3.HA vector to obtained pcDNA3.HA-Lck. The mutants at K273 (kinase-deficient Lck-KD K273R) and Y505 (constitutively active Lck-CA Y505F) were created by using the QuikChange kit. All constructs were verified by DNA sequencing.
Yeast experiments and culture conditions.
The haploid strain of S. cerevisiae W303–1B (MATα ade2–1 leu2–3,112 his3–22,15 trp1–1 ura3–1 can1–100) and the triple AAC-disrupted yeast strain of JL-1–3 (MATα ade2–1 trp1–1 ura3–1 can1–100 aac1::LEU2 aac2::HIS3 aac3::URA) were gifts from Dr. J. Kolarov (Dept. of Biochemistry, Comenius University, Bratislava, Slovakia). The pRS314-YA2P vectors encoding the chimeric yN-hANT1 and its various mutants were transformed into JL-1–3 yeast strain by the lithium chloride method and grown using selective media (0.67% yeast nitrogen base plus the indicated carbon source) supplemented with yeast synthetic drop-out medium without histidine, leucine, tryptophan, and uracil. Yeast cells were cultivated aerobically at 30°C on YP-agar plates (1% yeast extract, 2% BactoPeptone), supplemented with either 2% glucose (YPD), 3% glycerol (YPGly), 2% lactate (YPLact), or 3% galactose (YPGal), as indicated (11).
Preparation of yeast mitochondria.
Yeast mitochondria were prepared essentially as previously described (21). The final mitochondria pellet was suspended in STE buffer, consisting of 250 mM sucrose, 10 mM Tris·HCl, pH 7.4, and 0.2 mM ethylenediaminetetraacetic acid (EDTA). Mitochondrial protein concentration was determined by the Bio-Rad DC Protein Assay (Bio-Rad Laboratories, Reinach BL, Switzerland).
Assay of ADP transport activity.
The activity of ANT was measured under deenergized conditions by a slight modification of the inhibitor-stop method, previously described by Hashimoto et al. (12). The transport of ADP in this assay is related to the exchange of ATP that is endogenously present in mitochondria (for more details see supplemental material; the online version of this article contains supplemental data). Briefly, yeast mitochondria (200 μg) were suspended in 180 μl of ice-cold STE buffer containing 2 μg/ml oligomycin and 1 μM rotenone for 5 min on ice. The reaction was initiated by addition of 100 μM of [14C]ADP (specific activity 37 kBq/μmol ADP) and terminated by addition of 20 μM carboxyatractyloside and 5 μM bongkrekic acid after 10-s incubation on ice. Mitochondria were washed with ice-cold STE buffer containing 20 μM carboxyatractyloside and 5 μM bongkrekic acid four times and suspended in 2% SDS. 14C incorporation was determined by Cerenkov counting.
Flow cytometry and Rh123 uptake of isolated yeast mitochondria.
Mitochondrial size was determined by measuring forward light scatter using a FACScalibur flow cytometer (Becton Dickinson, San Jose, CA). Mitochondrial membrane potential (ΔΨm) was evaluated according to Emaus et al. (8) using the fluorescent dye rhodamine 123 (Rh123), which accumulates into energized mitochondria, depending on the negativity of the inner mitochondrial membrane, where its fluorescence is quenched. Briefly, isolated yeast mitochondria (0.5 mg protein/ml) were prepared in 200 μl of STE buffer containing 0.1% BSA, 1 mM NADH, 1 mM K2PO4, and 1 μM rotenone. ΔΨm was assessed by measuring the uptake of Rh123 using a Synergy HT Multi-Detection Microplate Reader (BioTek Instruments, Winooski, VT) with excitation at 507 nm and emission at 529 nm after addition of 2.6 μM of Rh123 to the mitochondrial suspension at 25°C. Rh123 was taken up by mitochondria in a potential dependent manner, resulting in the decrease of measured Rh123 fluorescence.
Expression and purification of glutathione S-transferase-ANT1.
BL21(DE3) cells were used to transform and express pGEX2T-ANT1 plasmid. The transformed bacteria strain was initially grown in LB media at 37°C until absorbance at 600 nm reached 0.4–0.5, and subsequently induced with 0.5 mM isopropyl-thio-β-d-galactopyranoside (Promega) for 4 h at 30°C. Cells were washed and lysed in lysis buffer (1% Triton X-100, 50 mM Tris·HCl, pH 7.5, 120 mM NaCl, 5 mM EDTA, 1 mM dithiothreitol, 1 mM benzamidine, and 0.1 mM phenylmethylsulfonyl fluoride), and then centrifuged at 100,000 g for 30 min. The glutathione S-transferase (GST)-ANT1 was purified on glutathione-agarose beads (Amersham Biosciences, Otelfingen, Switzerland), according to the instruction manual.
Production of site- and phosphorylation state-specific antibody.
The peptide Ala189-Tyr-Phe-Gly-Val-Tyr194-Asp-Thr-Ala-Lys-Gly199-Cys and phosphopeptide Ala189-Tyr-Phe-Gly-Val-pTyr194-Asp-Thr-Ala-Lys-Gly199-Cys were synthesized, and the phosphopeptides were coupled with keyhole limpet hemocyanine and immunoinjected into rabbits by PolyPeptide Laboratories (Strasbourg, France). The rabbit polyclonal anti-phosphopeptide antibody was prepared and affinity-purified using a non-phosphopeptide affinity column, followed by a phosphopeptide affinity column, as previously described (10).
Cell culture, transfection, and stimulation.
Human embryonic kidney (HEK)-293 and HeLa cells were maintained in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Basel, Switzerland). Hemagglutinin (HA)-tagged Src and Lck constructs were transfected into HEK-293 cells using jetPEI (Polyplus-transfection, Illkirch, France), following the instructions provided by the manufacturer. For treatment of cells, subconfluent cells were serum starved for 24 h, pretreated with 10 μM PP2, a Src-family kinase inhibitor (Calbiochem, San Diego, CA) for 1 h, and then the starved cells were treated with either 0.1 mM pervanadate prepared with 0.1 mM H2O2 (40), or with 1 mM H2O2 for the indicated time.
Immunoprecipitation and in vitro Src and Lck kinase assay.
HEK-293 cells were harvested 24 h after transfection and lysed with lysis buffer containing 50 mM Tris·HCl, pH 7.5, 1% (vol/vol) Nonidet P-40, 120 mM NaCl, 25 mM sodium fluoride, 40 mM β-glycerol phosphate, 0.1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 nM okadaic acid, and 1 μM microcystin-LR. Lysates were centrifuged for 15 min at 12,000 g, and HA-tagged proteins were immunoprecipitated from 200 μg of lysates with anti-HA (12CA5) monoclonal antibody immobilized on protein G-Sepharose (Amersham Biosciences). The immune complexes were washed once with lysis buffer containing 0.5 M NaCl, followed by lysis buffer, and finally with kinase buffer (30 mM HEPES, pH 7.4, 1 mM MgCl2, 5 mM MnCl2, 0.1 mM dithiothreitol, 0.1 mM sodium orthovanadate). In vitro kinase assays were performed for 30 min at 30°C in a 30-μl reaction volume containing 0.5 μg GST-ANT1, and 0.2 mM ATP as substrates. Reactions were stopped by adding EDTA to a final concentration of 50 mM and were centrifuged at 3,000 g for 2 min to spin down the protein G-sepharose. Aliquots of the supernatant and the protein G-sepharose pellets were subjected to Western blotting analysis.
Preparation of mitochondria from HeLa cells.
HeLa cells were treated as described and then placed on ice. After washing once in ice-cold PBS, cells were scraped in 300 μl of ice-cold MSH buffer (220 mM mannitol, 70 mM sucrose, 10 mM HEPES, pH 7.4) containing 25 mM sodium fluoride, 40 mM β-glycerol phosphate, 0.1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 nM okadaic acid, and 1 μM microcystin-LR, and then homogenized using a Polytron PT 1600E (Kinematica, Luzern, Switzerland). Homogenates were centrifuged at 600 g for 10 min to remove the nuclear fraction and intact cells. The supernatant was centrifuged at 10,000 g for 20 min to separate cytoplasmic fraction and crude mitochondrial fraction. The mitochondrial pellet was washed in MSH buffer three times and resuspended in 62.5 mM Tris·HCl, pH 6.8, 5 mM EDTA, 7% sucrose, and 2% SDS, and the protein concentration was determined.
Western blot analysis.
Western blot analysis was carried out as previously described (11). The following antibodies were used: rabbit polyclonal anti-phospho-Src family (Tyr416; 2101) and mouse monoclonal anti-Src (L4A1; 2110) (Cell Signaling Technology, Danvers, MA), goat polyclonal anti-ANT (N-19 and Q-18) (Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-NDUFS4 (MS104) (MitoSciences, Eugene, OR), rabbit polyclonal anti-sMtCK3 (a kind gift from Dr. Theo Wallimann, Institute of Cell Biology, ETH Zurich, Zurich, Switzerland), monoclonal anti-α-tubulin and rabbit polyclonal anti-GST (Sigma, St. Louis, MO), rabbit polyclonal anti-Lck (BioVision, Mountain View, CA), mouse monoclonal anti-yeast cytochrome oxidase subunit III (DA5) (Invitrogen, Eugene, OR), monoclonal anti-HA (12CA5) (Roche Applied Science, Indianapolis, IN).
Perfused rat hearts and isolation of mitochondria from rat heart tissue.
Hearts from Wistar rats were perfused in a noncirculating Langendorff apparatus with Krebs-Henseleit buffer gassed with 95% O2 and 5% CO2 at pH 7.4 and 37°C, and subsequently exposed to 40 min of global no-flow ischemia, followed by 15 min of reperfusion (Supplemental Fig. S1) (11). Hearts were assigned to each of the following three groups: 1) ischemia followed by 15 min of reperfusion; 2) PreC (pharmacological preconditioning) induced by isoflurane administered for 15 min at 1.5 minimum alveolar concentration (corresponding to 2.1 vol%) and washed out for 10 min before test ischemia (11, 38); 3) PreC by isoflurane in the presence of 5 μM PP2 blocker, administered 2 min before until 2 min after isoflurane application. The buffer solution was equilibrated with isoflurane using an Isotec 3 vaporizer (Datex-Ohmeda, Tewksbury, MA) with an air bubbler. Isoflurane concentrations were also measured in the buffer solution using a gas chromatograph (Perkin-Elmer, Norwalk, CT): 0.53 + 0.04 mM. Left ventricular developed pressures were recorded as previously described. Rat heart mitochondria were isolated at 0–4°C, as previously described (11).
MD simulations.
A model of ANT embedded in a palmitoyl-oleyl-phosphatidylcholine bilayer was built and simulated (10 ns), as previously described (39). To investigate the effect of phosphorylation and mutation of Y190 and Y194, nine additional ANT variants were prepared by modifying the appropriate residues and simulated for 10–20 ns each: Y190F ∼10 ns, Y194F ∼10 ns, Y190F/Y194F ∼10 ns, pY190− ∼20 ns, pY1902− ∼10 ns, pY194− ∼20 ns, pY1942− ∼10 ns, pY190−/pY194− ∼10 ns, pY1902−/pY1942− ∼10 ns. All simulations were performed using the program NAMD 2.6 (29) and the CHARMM27 parameter set with the CMAP correction (16, 34). The temperature was kept constant at 310 K using Langevin dynamics, with a damping coefficient of 1 ps−1. The lipid surface area was fixed (NPnT), and 1 atm constant pressure was maintained with Nosé-Hoover Langevin piston (9). Short-range interaction cutoff was set to 12 Å, and the Particle Mesh Ewald method (5) with a grid density of at least 1 Å−3 was used to compute long-range electrostatic interactions. Bonded, nonbonded, and Particle Mesh Ewald were calculated at 2-, 2-, and 4-fs intervals, respectively.
Statistics.
Data are presented as means ± SD. Comparisons between groups were made using t-tests. P values were multiplied by the number of comparisons (Bonferroni correction). P < 0.05 was considered as significant. SigmaStat (version 2.0; SPSS Science, Chicago, IL) was used for the analyses.
RESULTS
ANT transport activity is abolished in JL-1–3 yeast strains expressing chimeric ANT (yN-hAN1T), with site mutations mimicking the dephosphorylated state in the tyrosine ladder.
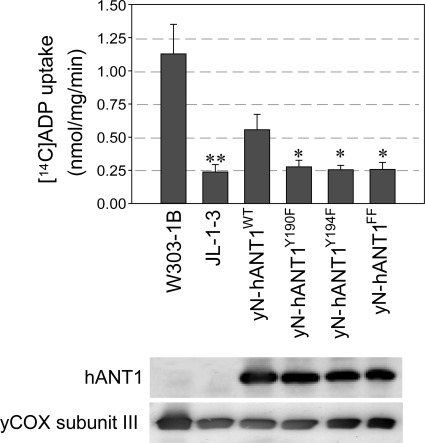
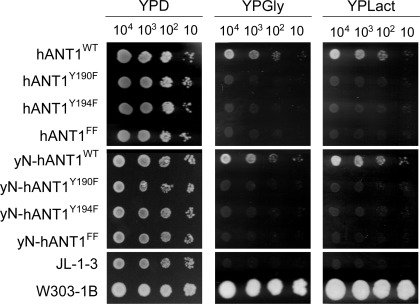
Expression of hANT1 in ANT-deficient yeast is low and does not allow measuring ADP uptake into mitochondria. However, replacing the NH2-terminal sequence of human ANT1 by the yeast sequence results in a markedly higher expression of chimeric ANT1 (yN-hANT1) and enabled us to determine the ANT transport activity of mitochondria expressing wild-type and site-mutated chimeric ANT1 (Fig. 1) (12). Our experiments clearly show a loss of ADP uptake into mitochondria in yeast strains expressing Y190F, Y194F, or double site-mutated (Y190F/Y194F) yN-hANT1. Our laboratory previously reported that mutation of Y194 to phenylalanine (F) in human ANT resulted in cessation of yeast growth on nonfermentable media (11). We here tested whether the mutation of Y190 located in close vicinity of Y194 and part of the tyrosine ladder would have the same effect. Our study shows that replacement of either tyrosine residue in this aromatic ladder (Y190F, Y194F, or the double-mutated Y190F/Y194F) entailed a loss of oxidative growth capacity in yeast (Fig. 2), confirming and complementing the results from ANT transport activity. Importantly, the consequences of site mutations on the oxidative growth phenotype were similar and independent of whether nonchimeric human or chimeric ANT1 (yN-hANT1) containing the yeast NH2-terminal amino acid sequence of ANT1 was used (Fig. 2). Flow cytometry analysis of yeast mitochondria expressing wild-type and site-mutated human ANT1 further showed larger mitochondria in wild-type respiring compared with site-mutated nonrespiring yeast (Supplemental Fig. S2). Also, Rh123 uptake into mitochondria expressing wild-type vs. mutant human ANT1 was higher, indicating a more negative mitochondrial potential in these strains (Supplemental Fig. S2). Together, these experiments in the yeast model provide evidence that phosphorylation of the tyrosine residues in the aromatic ladder in ANT1 is required for an efficient cellular respiration by oxidative phosphorylation.
Fig. 1.
ADP transport measurements. ADP transport activity is shown in mitochondria from Saccharomyces cerevisiae strains JL-1–3 (yANT/AAC-deficient), W303–1B (respiration-competent parent strain of JL-1–3), and JL-1–3 expressing chimeric yN-hANT1WT, yN-hANT1Y190F, yN-hANT1Y194F, and yN-hANT1FF. The expression of chimeric human adenine nucleotide translocase (ANT) 1 was determined by Western blot analysis with anti-ANT antibody (N-19), and equal loading was controlled by anti-yeast cytochrome-c oxidase subunit antibody (DA5). Values are means ± SD (n = 10). *P < 0.001 vs. yN-hANT1WT. **P < 0.001 vs. W303–1B.
Fig. 2.
Growth characteristics of transformed yeast strains. Growth characteristics are shown of Saccharomyces cerevisiae strains transformed with various human ANT1 (hANT1) and chimeric human ANT1 (yN-hANT1) constructs on glucose (YPD), glycerol (YPGly), and lactate (YPLact) plates. JL-1–3, ANT/AAC (ADP/ATP carrier)-deficient strain; W303–1B, respiration-competent parent strain of JL-1–3.
Src family kinases (Src/Lck) phosphorylate ANT1 at Y194 in vitro and in vivo in HeLa cells upon stimulation with pervanadate and H2O2.
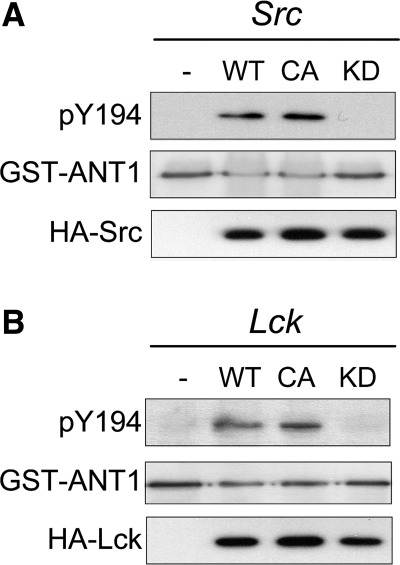
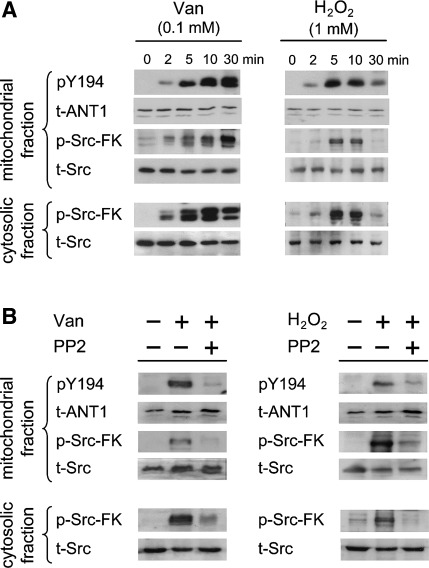
Next, we wanted to know which kinases would be involved in the phosphorylation of the ANT1 tyrosine ladder. For this purpose, we generated a polyclonal antibody against phosphorylated Y194 in ANT1. Due to previously reported interaction between the SH2 domain of Src and motifs containing aromatic amino acids (26) and the fact that Src-FKs have been repeatedly identified in mitochondria (15, 31, 33, 37), we focused on Src-FK members in our experiments. GST-tagged ANT1 and HA-tagged wild type, constitutively active, and kinase-dead Src and Lck were used for in vitro kinase assays, together with the pY194 site-specific antibody. These experiments show phosphorylation exclusively in wild-type and constitutively active kinases, providing in vitro evidence that Src and Lck are indeed capable of phosphorylating Y194 in ANT1 (Fig. 3). Using pervanadate and H2O2 stimulation in HeLa cells, two established protein-tyrosine phosphatase inhibitors, we tested whether Y194 phosphorylation also occurs in vivo in mitochondria of human cells. Our experiments show activation of Src-FKs, recognized by an antibody targeting the identical phosphorylation and activation site of all Src-FK members (anti-phospho-Src-FK), in the cytosolic and mitochondrial fraction upon stimulation, which is accompanied with Y194 phosphorylation in ANT1 (Fig. 4A). Phosphorylation of Src-FKs and ANT1 was abolished by PP2, a specific inhibitor of Src-FKs (Fig. 4B). The findings confirm the in vitro kinase assays and further provide evidence that the tyrosine ladder is phosphorylated in vivo in mammalian cells in a Src-FK-dependent manner.
Fig. 3.
Src and Lck phosphorylate ANT1 at Y194 in vitro. Wild-type (WT), constitutively active form (CA), and kinase-dead form (KD) of hemagglutinin (HA)-tagged human Src (A) and mouse Lck (B), or pcDNA3 empty vector (−) were transfected into human embryonic kidney-293 cells and immunoprecipitated with anti-HA antibody. The kinase assay was carried out using 0.2 mM ATP and 0.5 μg glutathione S-transferase (GST)-ANT1 as substrates. Samples were analyzed by Western blotting with antibodies against pY194, GST, and HA.
Fig. 4.
Phosphorylation of ANT at Y194 in HeLa cells. A: serum-starved HeLa cells stimulated with 0.1 mM pervanadate (Van) or 1 mM H2O2 for the indicated times before harvest. B: serum-starved HeLa cells were untreated (−) or pretreated with 10 μM Src-FK inhibitor PP2 (+) for 60 min and then stimulated with 0.1 mM pervanadate for 30 min or 1 mM H2O2 for 5 min before harvest. Cytosolic and mitochondrial fractions were separated and analyzed by Western blotting with anti-pY194, anti-phospho-Src family kinase (pSrc-FKY416), anti-ANT (Q-18), and anti-Src antibodies, as indicated.
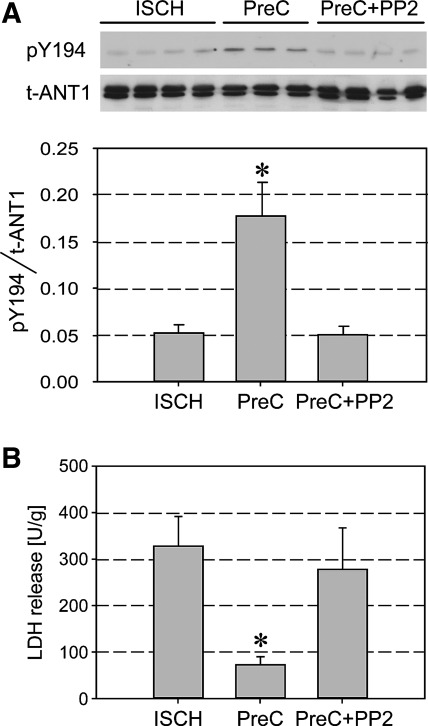
Prevention of Y194 dephosphorylation in ANT1 of preconditioned hearts is Src family kinase dependent and correlates with improved myocardial recovery and decreased cell injury.
Previous work in the field of cardiac preconditioning suggests the presence of Src-FKs in cardiac mitochondria (31). Using Src- and Lck-specific antibodies, we were able to confirm the presence of Src and Lck in the cytosolic and mitochondrial fraction of untreated virgin rat hearts (Supplemental Fig. S3). Our laboratory previously showed, with the aid of a phospho-tyrosine-specific antibody, that phosphorylation of ANT1 is preserved after ischemia-reperfusion injury in (isoflurane-preconditioned) protected, as opposed to unprotected, hearts (11). We now wanted to know whether this is also true for the Y194 site of the tyrosine ladder, for which our yeast experiments prove a pivotal role in regulating ANT1 transport activity. For this purpose, isolated rat hearts were exposed to 40 min of ischemia and reperfused, as previously described (11). Some hearts were preconditioned in the presence and absence of the Src-FK inhibitor PP2. Our experiments show an increased Y194 phosphorylation in ANT1 of PreC hearts, as opposed to unprotected hearts (Fig. 5A), which was closely correlated with an improved functional recovery (developed pressure at 15 min of reperfusion: PreC 47 ± 5 mmHg vs. ischemic 9 ± 4 mmHg, P < 0.001) and reduced release of the necrosis marker LDH (Fig. 5B). The protection was abolished in the presence of PP2 (PreC + PP2 13 ± 5 mmHg). Hence, our data reveal that phosphorylation of ANT1 at Y194 is Src-FK dependent and that prevention of dephosphorylation of the tyrosine ladder may represent an important mechanism of cell protection.
Fig. 5.
Protection by pharmacological preconditioning leads to increased ANT1 phosphorylation at residue Y194 in cardiac mitochondria. A: in Langendorff-perfused, isolated rat hearts, pharmacological preconditioning was induced with 2.1 vol% isoflurane for 15 min, followed by 10 min of washout before 40 min of test ischemia [preconditioning (PreC)]. Non-PreC hearts were exposed to 40 min of ischemia alone (ISCH). Some hearts were concomitantly treated with the Src-FK inhibitor PP2 (5 μM) during pharmacological PreC (PreC + PP2). Purified rat heart mitochondrial fractions were subjected to Western blot analysis with anti-pY194 and anti-ANT (N-19) antibodies. Representative Western blots and averaged density data of pY194/t-ANT for each group are expressed as arbitrary units. *P < 0.05 vs. ISCH. B: release of the cardiac injury marker lactate dehydrogenase (LDH). *P < 0.01 vs. ISCH. Values are mean ± SD (n = 3 or 4).
Molecular insights from in silico MD simulations: how could phosphorylation of the ANT1 tyrosine ladder affect AAC activity?
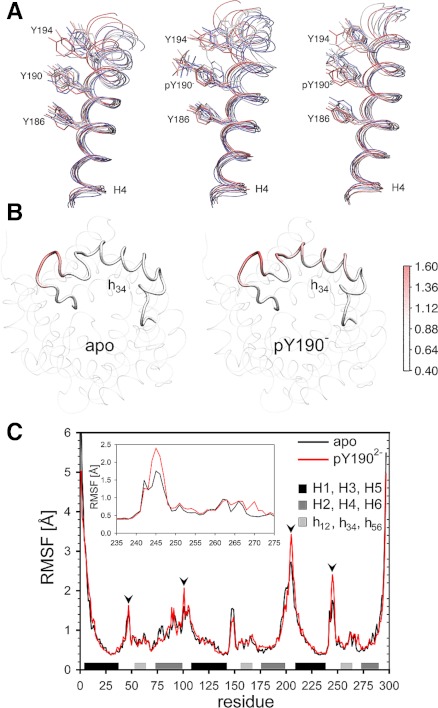
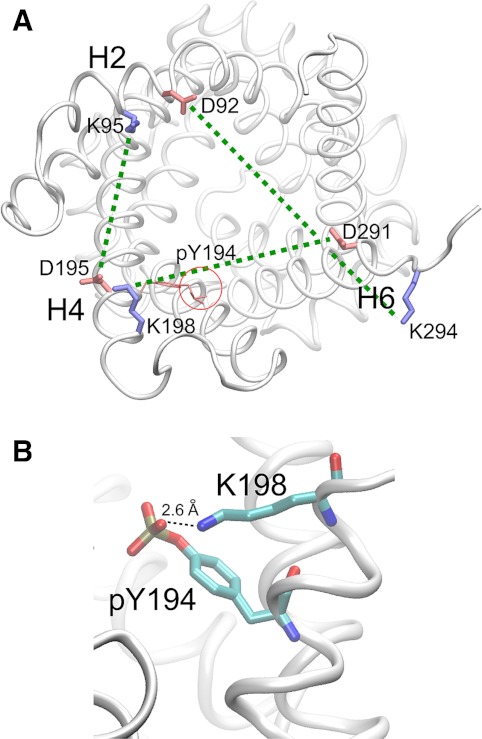
To address this question, we first clarified whether the introduced supplementary negative charge from phosphate groups (Supplemental Fig. S4) located at the entrance of ANT1 would affect the attraction and progression of large, negatively charged chloride ions. Our in silico experiments show that chloride ions readily enter the lumen, providing evidence that the phosphorylation events do not result in significant reduction of the attractive electrostatic potential of the lumen for a negative substrate (not shown). Subsequent simulations served to detect conformational alterations in the three-dimensional structure of ANT1 due to phosphorylation of the tyrosine ladder and due to mutation of the tyrosines to phenylalanines, as performed in our experiments. These simulations reveal two prominent findings. First, phosphorylation of Y190 disturbs the “stacking” of the aromatic ladder because of increased fluctuations of the phosphorylated phenol ring (Fig. 6A). Second, phosphorylation of Y190 also increases fluctuations of matrix loop m2 containing h34 (Fig. 6B). Since movements in this region are known to take place when ANT1 changes from the c- to the m-state (6), it is likely that this conformational flexibility could facilitate the transition. Moreover, the di-anionic (-2) pY190 exhibited increased flexibility at four strategic helix-linking regions (Fig. 6C). Additional MD simulations also showed that the Y190F mutation does not alter the conformation of ANT1, although it eliminates two hydrogen bonds (between Y190 and T220 and between Y190 and A216). In fact, the root mean square deviation differences between the dephosphorylated ANT1 (apo) and various mutated/phosphorylated species stay clearly <0.5 Å, suggesting that these hydrogen bonds do not play an essential role in the stability of the protein structure. Finally, our structural analyses show that pY194 is placed in close vicinity of the salt bridge K198-D291 of the putative cytosolic salt bridge network (Fig. 7) that is suggested to form in the m-state of ANT (32). This network is broken when ANT changes from the m- to the c-state. pY194 potentially destabilizes the salt bridges and facilitates the transition. In fact, our simulations of the phosphorylated tyrosine ladder show that the phosphate group of Y194 interacts with K198, potentially annihilating its interaction with D291 (Supplemental Movie and Supplemental Fig. S5). Together, our analyses show that important structural changes of key elements, intricately involved in nucleotide transport through the pore, do occur upon phosphorylation of the tyrosine ladder, providing potential explanations at the atomic level as to why phosphorylation of the tyrosine ladder may markedly affect mitochondrial bioenergetics and cell protection.
Fig. 6.
Molecular dynamics simulations of ANT1 phosphorylated at Y190 and Y194. A: fluctuations of the “tyrosine ladder” in the dephosphorylated ANT1 (left), mono-anionic (middle), and di-anionic pY190-ANT1 (right). The order of colors, red-white-blue, corresponds to the time course (red: time t = 0 ns, white: t = 5 ns, blue: t = 10 ns). B: increased fluctuations of the matrix loop m2 containing h34 in mono-anionic pY190-ANT1. Red color intensity indicates Cα-atom root mean square fluctuations (RMSF) in pY190-ANT1 normalized to Cα-atom fluctuations in dephosphorylated ANT1 (apo). C: comparison of RMSF in dephosphorylated ANT1 (apo) and pY190-ANT1 (di-anionic form) exhibits increased flexibility at strategic links between ANT helices (arrowheads). H1–H6, transmembrane helices. h12, h34, h56, Short helical stretches in matrix loops m1, m2, and m3, respectively.
Fig. 7.
Perturbation of the putative cytosolic salt bridge network by pY194. A: the putative cytosolic salt bridge network consisting of K95-D195, K198-D291, and K294-D92 ion pairs is marked by dashed green lines. Blue, positively charged residues; red, negatively charged residues. pY194 places a negative phosphate group (red circle) in the jaws of the K198-D291 salt bridge, decreasing the activation energy for the interconversion from the m- to the c-state. B: the phosphate group of pY194 interacts with K198, potentially annihilating its interaction with D291.
DISCUSSION
Here we show the following salient findings. First, using ANT1 site mutations in a yeast model, mimicking dephosphorylation of the aromatic ladder (Y190/Y194), we provide evidence that effective cellular respiration and ATP generation in mitochondria, i.e., oxidative growth on nonfermentable media, depends on phosphorylation of residues Y190 and Y194. These results are supported by the fact that ADP/ATP exchange activity is virtually lost in ANT1 mutants, mimicking the dephosphorylated state. Interestingly, dephosphorylation of only one of the tyrosine residues in the aromatic ladder is sufficient to abrogate nucleotide transport. Second, our studies further show that Src and Lck, two members of the Src-FKs, are capable of mediating phosphorylation of the aromatic ladder in ANT1, and that Y194 phosphorylation strongly correlates with improved functional recovery and decreased cell injury after ischemia-reperfusion injury of the heart. Finally, our MD simulations and structural analyses of phosphorylated vs. dephosphorylated ANT1 reveal increased fluctuations of pY190 of the aromatic ladder, accompanied by fluctuations of loop m2, and a possible salt bridge breaking effect by pY194 facilitating interconversions between m- and c-state upon ligand binding. These observations provide potential mechanisms as to why phosphorylation of the tyrosine ladder may be critical in regulating mitochondrial energy transfer.
ANT is the principal carrier, which shuttles cytosolic ADP for ATP across the inner mitochondrial membrane. Crystallization with carboxyatractyloside, blocking ANT in the stable c-state conformation, enabled the X-ray structure of ANT to be determined at 2.2 Å resolution (28). ANT is built of six TM α-helices, with proline-induced kinks in the odd-numbered helices, and three matrix-facing repeat domains (h12, h34, h56). In the c-state, the protein forms a basket with a wide positively charged opening toward the intermembrane space and a bottom closed by the three kinked helices. During the ΔΨm-driven electrogenic 1:1 ADP/ATP exchange, ANT changes its three-dimensional structure between two extreme conformations, namely the c (cytosolic)-state and the m (matrix)-state (25). A salt bridge network on the matrix side (PXDEXXRK motifs on helices H1, H3, H5) tightening together the odd-numbered helices is disrupted upon ADP binding from the cytosolic side to the nucleotide binding site (39), which is located in the central region of the cavity, allowing the release of ADP into the mitochondrial matrix (20, 28). Symmetry analyses propose a second salt bridge network consisting of conserved residues located on the cytosolic side (FYDEXXRK motifs on helices H2, H4, H6), which may be disrupted when ATP from the matrix binds to the nucleotide binding site (32). Hence, nucleotide transport is thought to be the result of breaking and reestablishing two salt bridge networks located at opposite sides of the carrier and securing the strict impermeability of the inner mitochondrial membrane. In a recent phosphoproteome analysis, our laboratory reported phosphorylation of Y194 in ANT1 isolated from heart mitochondria (11). Using a phospho-tyrosine-specific antibody, we were further able to link increased tyrosine phosphorylation of ANT1 to cardioprotection elicited by pre- and postconditioning, two of the most protective anti-ischemic therapies. Phosphorylation of Y190 in ANT1 was more recently also reported by others (2, 14, 19). The three closely neighboring tyrosine residues Y186/Y190/Y194 on TM helix 4 are facing the carrier lumen and form an aromatic ladder, also called tyrosine ladder. Based on recent MD simulations (7, 39), it has been suggested that this tyrosine ladder, which is highly conserved over a wide phylogenetic range, may have an important role in steering the nucleotides into their binding site. In this process, an intricate network of noncovalent bonds, including van der Waals contacts and π-π stacking interactions between tyrosines and the heterocyclic adenine ring of the nucleotides, appears to be important for both substrate specificity and transport efficiency of ANT1. Using site mutations of ANT1 in a yeast model, mimicking dephosphorylation of Y190 and Y194, we here demonstrate that dephosphorylation of either residue annihilates oxidative growth, i.e., cell respiration, and ANT carrier function. Since the mitochondrial ADP/ATP exchange drives the rate of oxidative phosphorylation in aerobic cells, disruption of ANT function inevitably leads to loss of mitochondrial function and cell death.
We here provide for the first time evidence that ANT1 is phosphorylated at Y194 by Src-FK members Src and Lck, and that increased phosphorylation at this particular site is tightly linked to cell protection in PreC hearts. However, since the catalytic subdomains of all Src-FK members are highly conserved and their activation loops are identical among all Src-FKs, we cannot exclude from our experiments that other Src-FKs may have also contributed to phosphorylation of the tyrosine ladder. Reversible phosphorylation of tyrosine residues is an important means of regulating mitochondrial function (1, 4, 15, 18, 23, 24, 33, 37). Salvi et al. (33) confirmed the presence of the Src-FK members Fyn, Lyn, and Src in highly purified rat brain mitochondria and showed that tyrosine-phosphorylated proteins are membrane bound and located on the inner surface of the outer mitochondrial membrane or the outer surface of the inner membrane, while the kinases were located in the intermembrane space, as evidenced by immunogold labeling. Using mass spectrometry, this group also reported phosphorylation at Y194 and Y190 in ANT isolated from rat brain mitochondria (14). Ping et al. identified seven from a total of nine previously described Src-FK members expressed in rabbit hearts (31). In their study, Src and Lck were activated upon ischemic preconditioning trigger protocols and mediated the cardioprotective phenotype. This is consistent with results by Hattori et al. (13) using ischemic preconditioning in a rat heart model. Interestingly, ischemic preconditioning promotes the formation of signaling modules between PKC-ε and Lck, which are a prerequisite for effective cardioprotection (30).
How could phosphorylation of the tyrosine ladder affect ANT function? To address this question, we have performed MD simulations of ANT1 embedded in a lipid bilayer, which enabled us to characterize the nanosecond time scale conformational changes of phosphorylated and dephosphorylated Y190/Y194 in ANT1. Although the overall protein conformation was stable over the course of the simulations, and deviations from the reported crystal structure were relatively small, our data support a transport model in which pY190 increases flexibility of ANT1 subunits facilitating ligand progression through the pore. Wang and Tajkhorshid (39) and Dehez et al. (7) demonstrated how the initial orientation of ADP may hamper ligand transfer, with ADP being trapped in “congealed” conformations captured by sustained molecular interactions with neighboring residues. Our simulations suggest that such abortive conformations of the ligand may be prevented by fluctuating pY190, which keeps the carrier in more conducive conformations. Obviously, the unusually strong positive electrostatic potential at the entrance and in the lumen of ANT (∼1.4 V) is an advantage for attracting and binding the negatively charged nucleotides, but, on the other side, it increases the probability of stable ligand-receptor interactions (39). Phosphorylation of Y190 and Y194 may further tighten π-π stacking between tyrosine residues and the adenosine moiety of nucleotides by changing the quadrupole moment of the phenol ring and thus increase carrier selectivity (17). Interestingly, pY190 also increases fluctuations on matrix loop m2, which connects the straight TM helix 4 to kinked TM helix 3. Movements in this region typically occur upon ADP binding, when ANT changes from the c-state to the m-state, and are a prerequisite for successful nucleotide progression (6). Accordingly, mutations of Y186 and F191 to A were reported to inhibit matrix loop m2 movements and annihilate ANT function (6). This opens the possibility that pY190 may facilitate transition from the c- to the m-state by helix H3 movements, which participate in opening the basket to the matrix side. Our MD simulations did not unveil a direct rationale for the observed effect of Y194 phosphorylation on ANT carrier activity. Previous MD simulations on ATP binding to the c-state of ANT (39) revealed only weak interaction [mainly π-π interactions between the adenine ring and the (unphosphorylated) tyrosine residues], arguing against the possibility that elimination of phosphorylation by Y194F mutation could prevent the release of ATP from ANT. However, based on the importance of disruption and reestablishment of salt bridge networks during the carrier cycle, we propose the following mechanism. The supplementary negative charges of pY194, a residue of the cytosolic salt bridge network motif on TM helix 4 (VYDTAK) and a direct neighbor to the putative K198-D291 salt bridge (32), may facilitate the salt bridge breaking step induced by binding of the negatively charged ATP4− from the matrix side (Fig. 7). pY194 places a large negatively charged destabilizing phosphate group in the jaws of the K198-D291 salt bridge, decreasing the activation energy (ΔG#) for the interconversion from the m- to the c-state. Together, the observed overall increased flexibility of ANT1 in the presence of phosphorylated tyrosines in the aromatic ladder appears to be consistent with an accelerated transition between the main two conformational states (c↔m) during the transport cycle. Since “aromatic signatures” are typical for other carrier proteins (32), our results may be more general and applicable to these carriers.
GRANTS
This study was supported by Grants 3200B0–103980/1 and 3200B0-116110/1 from the Swiss National Science Foundation (M. Zaugg); a grant from Heart and Stroke Foundation of Canada (M. Zaugg); a grant from the Mazankowski Alberta Heart Institute (M. Zaugg); the 5th Frontiers in Anesthesia Research Award from the International Anesthesia Research Society (M Zaugg); Swiss Cancer League (K. Zaugg); Swiss Federation against Cancer (Oncosuisse) (K. Zaugg); National Institute of General Medical Sciences Grants R01-GM086749 and R01-GM067887 (E. Tajkhorshid); and TeraGrid allocation Grant MCA06N060 (E. Tajkhorshid).
DISCLOSURES
The authors are not aware of financial conflict(s) with the subject matter or materials discussed in this manuscript with any of the authors, or any of the authors– academic institutions or employers.
Supplementary Material
REFERENCES
- 1.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab 9: 265–276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballif BA, Carey GR, Sunyaev SR, Gygi SP. Large-scale identification and evolution indexing of tyrosine phosphorylation sites from murine brain. J Proteome Res 7: 311–318, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Boja ES, Phillips D, French SA, Harris RA, Balaban RS. Quantitative mitochondrial phosphoproteomics using iTRAQ on an LTQ-Orbitrap with high energy collision dissociation. J Proteome Res 8: 4665–4675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardone L, Carlucci A, Affaitati A, Livigni A, deCristofaro T, Garbi C, Varrone S, Ullrich A, Gottesman ME, Avvedimento EV, Feliciello A. Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of src signaling. Mol Cell Biol 24: 4613–4626, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darden T, York D, Pedersen L. Particle mesh Ewald: an Nxlog(N) method for Ewald sums in large systems. J Chem Phys 98: 10089–10092, 1993 [Google Scholar]
- 6.David C, Arnou B, Sanchez JF, Pelosi L, Brandolin G, Lauquin GJM, Trezeguet V. Two residues of a conserved aromatic ladder of the mitochondrial ADP/ATP carrier are crucial to nucleotide transport. Biochemistry 47: 13223–13231, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Dehez F, Pebay-Peyroula E, Chipot C. Binding of ADP in the mitochondrial ADP/ATP carrier is driven by an electrostatic funnel. J Am Chem Soc 130: 12725–12733, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Emaus RK, Grunwald R, Lemasters JJ. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta 850: 436–448, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Feller SE, Zhang Y, Pastor RW, Brooks BR. Constant pressure molecular dynamics simulation: the Langevin piston method. J Chem Phys 103: 4613–4621, 1995 [Google Scholar]
- 10.Feng J, Tamaskovic R, Yang Z, Brazil DP, Merlo A, Hess D, Hemmings BA. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J Biol Chem 279: 35510–35517, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Feng J, Zhu M, Schaub MC, Gehrig P, Roschitzki B, Lucchinetti E, Zaugg M. Phosphoproteome analysis of isoflurane-protected heart mitochondria: phosphorylation of adenine nucleotide translocator-1 on Tyr194 regulates mitochondrial function. Cardiovasc Res 80: 20–29, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto M, Shinohara Y, Majima E, Hatanaka T, Yamazaki N, Terada H. Expression of the bovine heart mitochondrial ADP/ATP carrier in yeast mitochondria: significantly enhanced expression by replacement of the N-terminal region of the bovine carrier by the corresponding regions of the yeast carriers. Biochim Biophys Acta 1409: 113–124, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Hattori R, Otani H, Uchiyama T, Imamura H, Cui J, Maulik N, Cordis GA, Zhu L, Das DK. Src tyrosine kinase is the trigger but not the mediator of ischemic preconditioning. Am J Physiol Heart Circ Physiol 281: H1066–H1074, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Lewandrowski U, Sickmann A, Cesaro L, Brunati AM, Toninello A, Salvi M. Identification of new tyrosine phosphorylated proteins in rat brain mitochondria. FEBS Lett 582: 1104–1110, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Livigni A, Scorziello A, Agnese S, Adornetto A, Carlucci A, Garbi C, Castaldo I, Annunziato L, Avvedimento EV, Feliciello A. Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Mol Biol Cell 17: 263–271, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B 102: 3586–3616, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Meyer EA, Castellano RK, Diederich F. Interactions with aromatic rings in chemical and biological recognition. Angew Chem Int Ed Engl 42: 1210–1250, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki T, Neff L, Tanaka S, Horne WC, Baron R. Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J Cell Biol 160: 709–718, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munton RP, Tweedie-Cullen R, Livingstone-Zatchej M, Weinandy F, Waidelich M, Longo D, Gehrig P, Potthast F, Rutishauser D, Gerrits B, Panse C, Schlapbach R, Mansuy IM. Qualitative and quantitative analyses of protein phosphorylation in naive and stimulated mouse synaptosomal preparations. Mol Cell Proteomics 6: 283–293, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Nelson DR, Felix CM, Swanson JM. Highly conserved charge-pair networks in the mitochondrial carrier family. J Mol Biol 277: 285–308, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Nelson DR, Lawson JE, Klingenberg M, Douglas MG. Site-directed mutagenesis of the yeast mitochondrial ADP/ATP translocator. Six arginines and one lysine are essential. J Mol Biol 230: 1159–1170, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Nury H, Dahout-Gonzalez C, Trezeguet V, Lauquin GJ, Brandolin G, Pebay-Peyroula E. Relations between structure and function of the mitochondrial ADP/ATP carrier. Annu Rev Biochem 75: 713–741, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Pagliarini DJ, Dixon JE. Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem Sci 31: 26–34, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Pagliarini DJ, Wiley SE, Kimple ME, Dixon JR, Kelly P, Worby CA, Casey PJ, Dixon JE. Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic beta cells. Mol Cell 19: 197–207, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflügers Arch 447: 689–709, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Pawson T, Gish GD, Nash P. SH2 domains, interaction modules and cellular wiring. Trends Cell Biol 11: 504–511, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Pebay-Peyroula E, Brandolin G. Nucleotide exchange in mitochondria: insight at a molecular level. Curr Opin Struct Biol 14: 420–425, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trezeguet V, Lauquin GJ, Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426: 39–44, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem 26: 1781–1802, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ping P, Song C, Zhang J, Guo Y, Cao X, Li RC, Wu W, Vondriska TM, Pass JM, Tang XL, Pierce WM, Bolli R. Formation of protein kinase C(epsilon)-Lck signaling modules confers cardioprotection. J Clin Invest 109: 499–507, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ping P, Zhang J, Zheng YT, Li RC, Dawn B, Tang XL, Takano H, Balafanova Z, Bolli R. Demonstration of selective protein kinase C-dependent activation of Src and Lck tyrosine kinases during ischemic preconditioning in conscious rabbits. Circ Res 85: 542–550, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Robinson AJ, Overy C, Kunji ER. The mechanism of transport by mitochondrial carriers based on analysis of symmetry. Proc Natl Acad Sci USA 105: 17766–17771, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvi M, Brunati AM, Bordin L, La Rocca N, Clari G, Toninello A. Characterization and location of Src-dependent tyrosine phosphorylation in rat brain mitochondria. Biochim Biophys Acta 1589: 181–195, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Schlenkrich M, Brickmann J, MacKerell AD, Karplus M. Empirical potential energy function for phospholipids: criteria for parameter optimization and applications. In: Biological Membranes: A Molecular Perspective from Computation and Experiment, edited by Merz KM, Roux B. Boston, MA: Birkhauser, 1996, p. 31–81 [Google Scholar]
- 35.Shibuya H, Kohu K, Yamada K, Barsoumian EL, Perlmutter RM, Taniguchi T. Functional dissection of p56lck, a protein tyrosine kinase which mediates interleukin-2-induced activation of the c-fos gene. Mol Cell Biol 14: 5812–5819, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Tibaldi E, Brunati AM, Massimino ML, Stringaro A, Colone M, Agostinelli E, Arancia G, Toninello A. Src-Tyrosine kinases are major agents in mitochondrial tyrosine phosphorylation. J Cell Biochem 104: 840–849, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Uecker M, Da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Translocation of protein kinase C isoforms to subcellular targets in ischemic and anesthetic preconditioning. Anesthesiology 99: 138–147, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Tajkhorshid E. Electrostatic funneling of substrate in mitochondrial inner membrane carriers. Proc Natl Acad Sci USA 105: 9598–9603, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang KJ, Shin S, Piao L, Shin E, Li Y, Park KA, Byun HS, Won M, Hong J, Kweon GR, Hur GM, Seok JH, Chun T, Brazil DP, Hemmings BA, Park J. Regulation of 3-phosphoinositide-dependent protein kinase-1 (PDK1) by Src involves tyrosine phosphorylation of PDK1 and Src homology 2 domain binding. J Biol Chem 283: 1480–1491, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.