Abstract
Na+/H+ exchanger 3 (NHE3) is phosphorylated and regulated by multiple kinases, including PKA, SGK1, and CK2; however, the role of phosphatases in the dephosphorylation and regulation of NHE3 remains unknown. The purpose of this study was to determine whether serine/threonine phosphatases alter NHE3 activity and phosphorylation and, if so, at which sites. To this end, we first examined the effects of calyculin A [a combined protein phosphatase 1 (PP1) and PP2A inhibitor] and okadaic acid (a PP2A inhibitor) on general and site-specific NHE3 phosphorylation. Calyculin A induced a phosphorylation-dependent NHE3 gel mobility shift and increased NHE3 phosphorylation at serines 552 and 605. No change in NHE3 phosphorylation was detected after okadaic acid treatment. An NHE3 gel mobility shift was also evident in calyculin A-treated COS-7 cells transfected with either wild-type or mutant (S552A, S605G, S661A, S716A) rat NHE3. Since the NHE3 gel mobility shift occurred despite mutation of known phosphorylation sites, novel sites of phosphorylation must also exist. Next, we assayed NHE3 activity in response to calyculin A and okadaic acid and found that calyculin A induced a 24% inhibition of NHE3 activity, whereas okadaic acid had no effect. When all known NHE3 phosphorylation sites were mutated, calyculin A induced a stimulation of NHE3 activity, demonstrating a functional significance for the novel phosphorylation sites. Finally, we established that the PP1 catalytic subunit can directly dephosphorylate immunopurified NHE3 in vitro. In conclusion, our data demonstrate that a calyculin A-sensitive phosphatase, most likely PP1, is involved in the regulation and dephosphorylation of NHE3 at known and novel sites.
Keywords: Na+/H+ exchanger, protein phosphatase 1, protein phosphatase 2A
a na+/h+ exchanger found on the apical membrane of proximal tubule cells, NHE3, is responsible for the majority of sodium bicarbonate reabsorption by the proximal tubule of the kidney. As such, it is essential for the maintenance of normal volume and acid-base status under widely varying physiological conditions, and its activity can be modulated by many physiological and hormonal factors (25, 28, 30, 38, 43). On a molecular and cellular level, the following mechanisms have been shown to modulate the activity of NHE3: direct protein phosphorylation, subcellular trafficking, and interaction with accessory proteins (21, 30, 31, 44, 47). On the basis of our studies and those of others, it is clear that NHE3 phosphorylation occurs in a controlled and directed fashion and is an important mechanism for the regulation of NHE3 (21, 23, 42, 47). On the other hand, the mechanics of NHE3 dephosphorylation have only been minimally investigated (9, 26). Therefore, the purpose of this study was to determine the role of phosphatases in the dephosphorylation and regulation of NHE3.
Phosphatases are widely expressed enzymes that mediate the dephosphorylation and functional regulation of many proteins, including some renal channels and transporters (2, 5, 8, 17, 22, 27, 41). Phosphatases typically function antagonistically with kinases to achieve fine control over the phosphorylation state of proteins. Generally, phosphatases can be divided into two main groups: serine/threonine phosphatases and phosphotyrosine phosphatases. Although tyrosine phosphorylation of NHE3 has been predicted, it has not been successfully demonstrated (9). Therefore, we have focused our studies of NHE3 dephosphorylation on serine/threonine phosphatases. The primary serine/threonine phosphatases in eukaryotes are protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A), members of the PPP subfamily. These two enzymes are among the most highly conserved proteins in all of evolution, and it has been said that they are responsible for more than 90% of all phosphatase activity in mammalian cells (5, 17, 33). They are ubiquitously expressed in virtually all cells and tissues, including the kidney (5, 17, 18, 27, 46). Other members of the PPP subfamily are relatively low in abundance and include PP2B, PP4, PP5, PP6, and PP7. PP2B is also ubiquitously expressed but can be easily distinguished from the remainder of the PPP family on the basis of inhibitor profiles (40). PP4 and PP6 are remarkably similar to PP2A in their structure and inhibitor sensitivities and are therefore collectively referred to as type 2A phosphatases (19, 34). PP5 shares a similar inhibitor profile with PP1 and is reported to have weak expression in the kidney and very low levels of basal activity (3, 13). PP7 was not considered in this study because it has been detected only the retina (15). In summary, based on the expression patterns, relative abundances, and basal activities of the PPP phosphatases, PP1 and PP2A are the best candidates to mediate dephosphorylation of NHE3.
Both PP1 and PP2A exist as multimeric holoenzymes composed of catalytic and regulatory subunits. For both enzymes, there are a small number of genetically distinct, but homologous, catalytic subunits; however, the regulatory subunits are numerous and nonhomologous (6, 17). Although the roles of PP1 and PP2A have been most extensively studied in the context of cell cycle regulation and glycogen metabolism, they also have been shown to regulate, either directly or indirectly, multiple channels and transporters such as the inwardly rectifying K+ channel, Na+-K+-Cl− cotransporter (NKCC1), CFTR, epithelial Na+ channel (ENaC), and aquaporin-2 (AQP2) (2, 5, 8, 17, 22, 27, 41). To our knowledge, there is limited published data on the relationship between phosphatases and NHE3. The one published article by Levine et al. (26) demonstrated a stimulation of NHE3 activity by okadaic acid (an inhibitor of PP2A, PP4, and PP6).
The goal of this study was to determine the effect of serine/threonine phosphatases on NHE3 activity and phosphorylation. We report that a calyculin A-sensitive phosphatase, most likely PP1, catalyzes the dephosphorylation of NHE3 at both known and novel phosphorylation sites and regulates NHE3 activity.
MATERIALS AND METHODS
Materials.
We purchased adult male Sprague-Dawley rats from Charles River Laboratories, goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody from Zymed, and goat anti-mouse HRP-conjugated secondary antibody from Molecular Probes. Dulbecco's modified Eagle's medium (DMEM), PBS, fetal calf serum, penicillin-streptomycin, and Na+-pyruvate were obtained from GIBCO. Polyvinylidene difluoride (PVDF) microporous membrane, enhanced chemiluminescence system, and 22Na+ were obtained from Perkin-Elmer Life Sciences, and 24- and 6-well plates for cell culture were acquired from BD Falcon. The polyclonal antibodies to phospho-eukaryotic translation elongation factor 2 (p-eEF2) and phospho-NKCC1 (pNKCC1) (R5) were kind gifts from Drs. A. C. Nairn (29) and B. Forbush (10), respectively, from Yale School of Medicine. GAPDH antibody was purchased from Novus Biologicals. PP1α, PP1β, PP1γ, PP2A–C, and PP5 antibodies were obtained from Millipore, as were calyculin A, PP1 enzyme isoforms, PP1 catalytic subunit, and PP2A. Calf intestinal phosphatase (CIP), KpnI, PmlI, and EcoRV were obtained from New England Biolabs. Complete mini EDTA-free protease inhibitor cocktail tablets were acquired from Roche Diagnostics. Protein G-Sepharose 4 Fast Flow was acquired from GE Healthcare, and Lipofectamine 2000 and pcDNA3.1 from Invitrogen. Wild-type rat NHE3 plasmid in a pCMV vector was kindly provided by Dr. John Orlowski at McGill University. Anti-NHE3 (3H3), monoclonal anti-PS552 (14D5), monoclonal anti-PS605 (10A8), and polyclonal anti-PS605 antibodies were all produced and characterized by our laboratory as previously described (21). All other reagents and chemicals were obtained from Sigma-Aldrich or American Bioanlytical. The NIH densitometry program (Scion Image) was provided by Scion.
Preparation of cortical whole homogenate and brush-border membrane vesicles.
All animal work was conducted in accordance with an Institutional Animal Care and Use-approved protocol at Yale School of Medicine. Male Sprague-Dawley rats were anesthetized, the kidneys were removed, and the rats were then euthanized. The kidneys were immediately placed in ice-cold K-HEPES buffer with protease and phosphatase inhibitors (200 mM mannitol, 80 mM HEPES, 41 mM KOH, 40 μg/ml PMSF, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin, 50 mM NaF, and 15 mM sodium pyrophosphate, pH 7.5.) The isolated renal cortices were homogenized in the K-HEPES buffer to make cortical whole homogenate. Brush-border membrane vesicles were made from the cortical whole homogenate by the technique of differential centrifugation and magnesium precipitation as previously described by our laboratory and others (1, 21). Protein concentrations were determined by the method of Lowry, and all samples were stored at −80°C.
Opossum kidney and COS-7 cell culture.
Opossum kidney (OKP) and COS-7 cells were grown in DMEM with 10% fetal calf serum, 50 U/ml penicillin, 50 mg/ml streptomycin, and 1 mM sodium pyruvate. Cells were transferred to 24- or 6-well plates, serum-starved for 24 h, and then used at 90–100% confluency for Western blotting, immunoprecipitation, or radioactive sodium uptake experiments.
SDS-PAGE and immunoblotting.
Cortical whole homogenate, brush-border membrane vesicles, OKP cells, or COS-7 cells were solubilized in sample buffer (2% SDS, 20% glycerol, 2% β-mercaptoethanol, and 2.9 mM Tris, pH 6.8.) and then subjected to SDS-PAGE using 7.5% polyacrylamide gels. Proteins were subsequently transferred to a PVDF membrane and used for immunoblotting. The PVDF membrane was incubated for 1 h at room temperature with a blocking solution (5% nonfat dry milk and 0.1% Tween in PBS, pH 7.4), followed by overnight incubation with the primary antibody. The primary antibody concentrations were as follows: anti-PP1α, 1:1,000; anti-PP1β, 1:1,000; anti-PP1γ, 1:500; anti-PP2A, 1:500; anti-NHE3, 1:3,000; anti-PS552, 1:1,000; monoclonal anti-PS605, 1:50; polyclonal anti-PS605, 1:500; anti-GAPDH, 1:50,000; p-eEF2, 1:500; and pNKCC1 (R5), 1:2,000. The membrane was then washed thoroughly with blocking solution, followed by incubation with an HRP-conjugated secondary antibody at 1:2,000. The secondary antibody was allowed to incubate with the PVDF membrane for 1 h at room temperature. Goat anti-mouse secondary antibody was used for anti-NHE3, anti-PS552, anti-PS605, and GAPDH antibodies. Goat anti-rabbit secondary antibody was used for the anti-PP1α, anti-PP1β, anti-PP1γ, anti-PP2A, p-eEF2, and pNKCC1 antibodies. Membranes were again washed with blocking solution and then rinsed with PBS. Finally, antibody was visualized using an enhanced chemiluminescence system.
Inhibitor treatment of OKP cells.
OKP cells were treated with vehicle, forskolin-IBMX (F/I), calyculin A, or okadaic acid for 30 min at room temperature at the following concentrations: forskolin, 10−4 M; IBMX, 10−3 M; calyculin A, 10−6 M; and okadaic acid, 10−6 M. COS-7 cells were treated with either vehicle or calyculin A at 10−6 M for 30 min at 37°C. The inhibitors were diluted in an isotonic NH4Cl solution (30 mM NH4Cl, 90 mM choline chloride, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose, and 20 mM HEPES-Tris, pH 7.4). At the end of the treatment period, the cells were solubilized in SDS sample buffer for Western blotting or a Triton-based buffer (see below) for immunoprecipitation or subjected to 22Na+ uptake as described below.
CIP treatment of OKP cells.
OKP cells were grown as described above and used at 90–100% confluency in a 24-well plate. Cells were incubated for 30 min at room temperature in an isotonic NH4Cl solution (30 mM NH4Cl, 90 mM choline chloride, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose, and 20 mM HEPES-Tris, pH 7.4) with or without 1 μM calyculin A. Cells were lysed in cold RIPA buffer without phosphatase inhibitors (150 mM NaCl, 50 mM Tris · HCl, 5 mM EDTA, 0.5% sodium deoxycholate, 1% Triton X, 0.1% SDS, and 1 Complete mini EDTA-free tablet per 10 ml) for 30 min on ice at 4°C. After centrifugation at 20,000 g and 4°C for 10 min, cleared lysates were divided into two aliquots each. Vehicle or 100 units of CIP were added to the aliquots and incubated at 37°C for 1 h. SDS sample buffer was added, boiled, and used for SDS-PAGE and immunoblotting.
Site-directed mutagenesis.
Wild-type rat NHE3 plasmid (24) was mutated using the Stratagene QuikChange Site-Directed (or Multi Site-Directed) Mutagenesis kit following the manufacturer's protocol (42). Primers were designed using the QuikChange Primer Design program available at www.stratagene.com. Mutations were verified by direct sequencing.
Transient transfection of COS-7 cells.
COS-7 cells were transiently transfected with either wild-type or mutant rat NHE3 cDNA. The mutant NHE3 had four serines replace by alanine or glycine (S552A, S605G, S661A, and S716A). The transfections were performed using Lipofectamine 2000 according to the manufacturer's protocol. The cells were used for SDS-PAGE and Western blotting 24 h after transfection.
Radioactive sodium uptake in OKP and PS120 cells.
OKP cells were grown as described above and used at 90–100% confluency in a 24-well plate for 22Na+ uptake assays. After aspiration of the cell culture medium, the cells were acid-loaded by using the NH4Cl prepulse technique, in which the cells were incubated in an isotonic NH4Cl solution (30 mM NH4Cl, 90 mM choline chloride, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose, and 20 mM HEPES-Tris, pH 7.4) with or without inhibitors at room temperature for 25 min. This solution was then aspirated, and an isotonic choline chloride solution containing 22Na+ (1 μCi/ml 22Na+, 1 mM NaCl, 120 mM choline chloride, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose, and 20 mM HEPES-Tris, pH 7.4) was added with or without inhibitors for 5 min. At the end of the 5 min, the influx of radioactive 22Na+ was terminated by three rapid washes with ice-cold isotonic choline chloride solution. The cells were solubilized with 0.2 M NaOH and then neutralized with the addition of an equal amount of 0.2 N HCl. Protein concentrations were determined using the method of Lowry. Results were normalized for the amount of protein per well.
Wild-type and mutant rat NHE3 constructs in pcDNA3.1.
Wild-type rat NHE3 in pCMV (24) was digested with KpnI and PmlI, followed by insertion of the entire NHE3 sequence into pcDNA3.1 that had been digested with KpnI and EcoRV. Successful insertion was confirmed by direct sequencing. Mutations in NHE3-pcDNA3.1 were made using the Stratagene QuikChange Multi Site-Directed Mutagenesis kit according to the manufacturer's protocol.
Generation PS120 mixed stable transfectants.
Cells were grown in DMEM with 10% fetal calf serum, 50 U/ml penicillin, and 50 mg/ml streptomycin at 37°C in 5% CO2-95% air (26). Cells were grown to confluence in a six-well plate and then transiently transfected with an NHE3-pcDNA3.1 construct using the Lipofectamine 2000 system according to the manufacturer's protocol. Neomycin was added to the cell culture after 24 h, and 1 wk after transfection, the cells were exposed to an acid load. Acid loading was performed twice a week until more than 80% of cells consistently survived the acid loading procedure. At this point, the cells were either used for 22Na+ uptake or maintained in neomycin-containing culture medium with acid loading every other week (26).
Immunoprecipitation of NHE3 and dephosphorylation by PP1 and CIP.
OKP cells were solubilized in a Triton-based buffer (30 mM Tris, 20 mM MES, 100 mM NaCl, 15 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 μM pepstatin, 1 μM leupeptin, 230 μM PMSF, 1% Triton X, and 1 Complete mini EDTA-free tablet per 25 ml) for 45 min at 4°C. The insoluble material was cleared by centrifugation in a tabletop centrifuge at 20,000 g for 10 min at 4°C. The solubilized fraction was incubated with the primary antibody for 1 h at 4°C. Immune complexes were incubated with protein G-Sepharase (50 μl of beads for each 1 ml of lysate) for 1 h at 4°C. The beads were thoroughly washed with the solubilization buffer and then placed with or without calyculin A at 10−6 M in a phosphatase reaction buffer (50 mM HEPES, pH 7.2, 10 mM MgCl2, 0.1% BSA, and 1 mM DTT) containing vehicle, 7 units of PP1 catalytic subunit, or 50 units of CIP for 30 min at 37°C. Finally, immune complexes were eluted with boiling SDS sample buffer and then used for SDS-PAGE and immunoblotting with anti-NHE3, anti-PS552, or polyclonal anti-PS605 antibody.
RESULTS
PP1 and PP2A are expressed in the brush-border membrane of the rat renal proximal tubule and in OKP cells, a proximal tubule cell line.
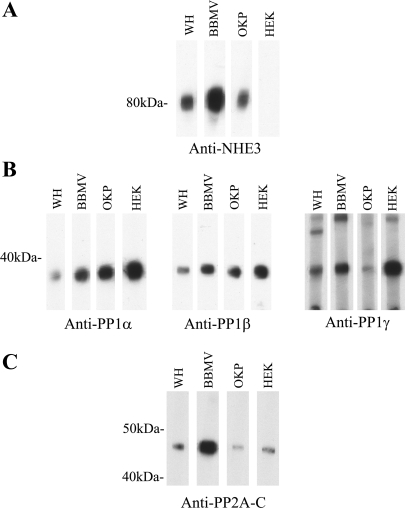
The subcellular localization of NHE3 is well described, and it is located primarily in the brush-border membrane of the proximal tubule (4, 21). We sought to determine whether the primary eukaryotic serine/threonine phosphatases, PP1 and PP2A, are present in the same subcellular region of the proximal tubule as NHE3. To this end, we prepared whole cortical homogenates and purified brush-border membrane vesicles from Sprague-Dawley rats and loaded equal protein amounts of each onto a gel. The samples were subjected to SDS-PAGE and then immunoblotted with anti-NHE3, anti-PP1α, anti-PP1β, anti-PP1γ, or anti-PP2A antibody (20, 21). In addition, we evaluated the presence of PP1 and PP2A catalytic subunits in OKP and human embryonic kidney (HEK) cells. OKP cells are a well-established cell culture model of the proximal tubule with excellent endogenous expression of NHE3 (20, 21). The HEK cells served as a positive control for endogenous PP1 and PP2A expression (7, 14). Of note, anti-PP1α does weakly recognize the β- and γ catalytic subunits, whereas anti-PP1β and anti-PP1γ are specific for their respective catalytic subunits (39). Anti-PP2A recognizes both α and β catalytic subunits of PP2A. Figure 1A demonstrates that NHE3 expression is enriched in the brush-border membrane region of the renal proximal tubule. NHE3 expression was also seen in OKP cells, but not HEK cells. Both PP1 and PP2A were present in the brush-border membrane of the renal proximal tubule and also were present in OKP and HEK cells (Fig. 1, B and C). There were no dramatic differences in the expression patterns of the different catalytic subunits of PP1 (Fig. 1B).
Fig. 1.
Protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) are present in rat brush-border membrane vesicles and expressed in OKP cells, a proximal tubule cell line. Thirty micrograms of rat cortical whole homogenate (WH), 30 μg of rat brush-border membrane vesicles (BBMV), 15 μg of OKP cells, or 15 μg of human embryonic kidney (HEK) cells were solubilized, separated by SDS-PAGE, and prepared for immunoblotting with anti-NHE3 antibody (A), anti-PP1α, anti-PP1β, or anti-PP1γ antibody (B), or anti-PP2A–C antibody (C).
NHE3 is dephosphorylated by one or more calyculin A-sensitive phosphatases.
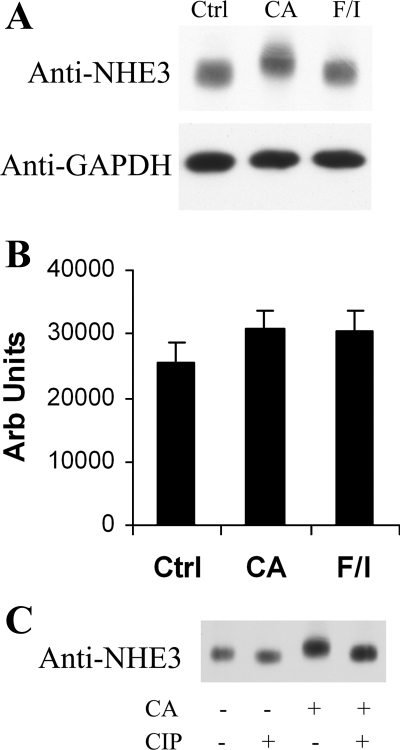
Since OKP cells have endogenous NHE3 as well as endogenous PP1 and PP2A, we selected OKP cells as a proximal tubule cell model for our NHE3 phosphorylation and dephosphorylation studies. Use of this cell line provided the opportunity to study these proteins in their natural environment, maximizing the likelihood of overall physiological relevance. To determine whether serine/threonine phosphatases catalyze the dephosphorylation of endogenous OKP NHE3, we exposed the cells to calyculin A, a potent inhibitor of all members of the PPP family except PP2B and PP7. Specifically, OKP cells were treated with vehicle, calyculin A, or F/I for 30 min at room temperature. Calyculin A inhibits both PP1 and PP2A, whereas forskolin and IBMX increase cAMP levels and served as a positive control for changes in NHE3 phosphorylation and activity in these experiments (8, 12, 20, 21). Lysates of these samples were subjected to SDS-PAGE, followed by Western blotting with anti-NHE3 and anti-GAPDH (loading control). As shown in Fig. 2, treatment of OKP cells with calyculin A induced a marked shift in NHE3 mobility by SDS-PAGE (Fig. 2A), and this mobility shift was almost completely reversed by CIP treatment (Fig. 2C). Densitometric analysis of multiple similar experiments revealed no significant change in total NHE3 content in samples treated with calyculin A or F/I compared with control (Fig. 2B). It should be noted that CIP altered NHE3 gel mobility even in cells in which there was no pretreatment with calyculin A (Fig. 2C, lanes 1 and 2), suggesting that NHE3 is phosphorylated at baseline.
Fig. 2.
The combined PP1/PP2A inhibitor calyculin A induces a phosphorylation-dependent mobility shift of NHE3 by SDS-PAGE. A: OKP cells were treated with vehicle (Ctrl), calyculin A at 10−6 M (CA), or forskolin at 10−4 M with IBMX at 10−3 M (F/I) for 30 min and then solubilized. Lysates were subjected to SDS-PAGE and then immunoblotted with anti-NHE3 or anti-GAPDH antibody. B: densitometric analysis of multiple Western blotting experiments similar to that shown in A. Data are means + SE; n = 5 for Ctrl and CA, n = 3 for F/I. P values were calculated using a paired t-test. *P < 0.05 compared with control. Arb units, arbitrary units. C: OKP cells were treated with either vehicle or CA and then, after solubilization, treated with either vehicle or calf intestinal phosphatase (CIP). Samples were then subjected to SDS-PAGE and Western blotting with anti-NHE3 antibody.
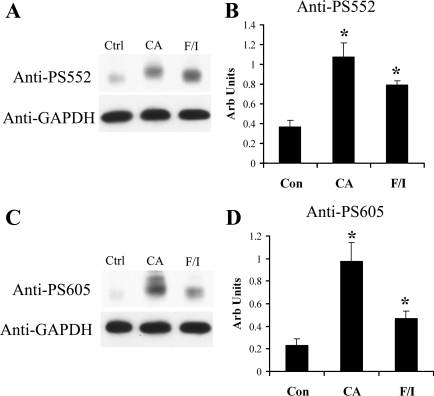
We have previously published the generation and characterization of the NHE3 phosphospecific antibodies anti-PS552 and anti-PS605, which recognize NHE3 only when phosphorylated at their respective serines, 552 and 605 (21). Of note, these same serines are numbered 560 and 613 in OKP NHE3 but for simplicity are referred to as serines 552 and 605 (S552 and S605) for the remainder of this report. These antibodies are especially valuable in their ability to study site-specific changes in endogenous NHE3 phosphorylation and have been used previously to demonstrate that PKA activation increases NHE3 phosphorylation at these sites in OKP cells and Sprague-Dawley rats (20, 21). In the following experiments, they were used to determine the relative amounts of NHE3 phosphorylated at serines 552 and 605 after calyculin A or F/I treatment compared with control. OKP cells were treated with vehicle, calyculin A, or F/I and then subjected to SDS-PAGE, followed by Western blotting with anti-PS552, anti-PS605, or anti-GAPDH (loading control). The representative Western blot illustrates the increased NHE3 phosphorylation seen at serine 552 (Fig. 3A) and serine 605 (Fig. 3C) with calyculin A and F/I treatments. A second band is often seen with anti-PS605 in OKP cells after calyculin A treatment, but the significance of this additional band is not known. It is possible that the upper band represents a pool of NHE3 that is more heavily phosphorylated than the lower band. Figure 2A represents the Western blot for total NHE3 for this same experiment. The increases in NHE3 phosphorylation at serines 552 and 605 seen in the representative experiment were confirmed by densitometric analysis of multiple independent experiments (Fig. 3, B and D) and were statistically significant. Of note, anti-PS552 and anti-PS605 signals were normalized to anti-NHE3 signals. In summary, inhibition of the PPP family of phosphatases leads to a dramatic increase in NHE3 phosphorylation at serines 552 and 605.
Fig. 3.
The combined PP1/PP2A inhibitor CA increases NHE3 phosphorylation at serines 552 and 605. OKP cells were treated with vehicle, CA, or F/I for 30 min, solubilized, subjected to SDS-PAGE, and then immunoblotted with anti-PS552 (A), monoclonal anti-PS605 (C), or anti-GAPDH antibody (A and C). Densitometric analysis is shown for multiple similar experiments for anti-PS552 (B) and anti-PS605 (D) normalized to the total NHE3 signal for the same experiment. Data are means + SE; n = 5–6 for Ctrl, n = 5–6 for CA, and n = 3 for F/I. P values were calculated using a paired t-test. *P < 0.05 compared with control.
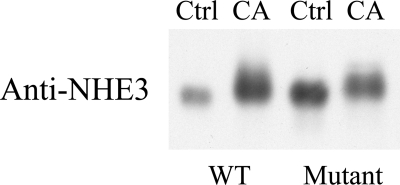
Both calyculin A and F/I treatment increased NHE3 phosphorylation at serines 552 and 605; however, only calyculin A induced an NHE3 gel mobility shift under the conditions used for our experiments. In addition, simultaneous treatment of OKP cells with both calyculin A and F/I yielded a greater NHE3 mobility shift and increase in NHE3 phosphorylation at serines 552 and 605 than detected with either alone (results not shown). These findings strongly suggest that calyculin A also increases phosphorylation of NHE3 at non-PKA sites. To date, four NHE3 phosphorylation sites have been identified (serines 552, 605, 661, and 716), but none have been reported to produce an NHE3 gel mobility shift (21, 24, 37, 42, 47). Therefore, phosphatase inhibition seems to have uncovered the presence of previously unidentified phosphorylation sites. To further substantiate this hypothesis, we transfected COS-7 cells with either wild-type NHE3 or a mutant NHE3 in which the known phosphorylation sites (serines 552, 605, 661, and 716) were replaced with alanine or glycine (21, 24, 37, 42, 47). The transfected cells were then treated with either vehicle or calyculin A, solubilized in SDS sample buffer, subjected to SDS-PAGE, and used for immunoblotting with anti-NHE3. A gel mobility shift after calyculin A treatment was seen for both wild-type and mutant forms of NHE3 (Fig. 4). The continued presence of a gel mobility shift despite these mutations provides further evidence for the presence of additional phosphorylation sites.
Fig. 4.
Known inducible sites of NHE3 phosphorylation do not fully account for the CA-induced gel mobility shift of NHE3. COS-7 cells were transfected with either wild-type (WT) or mutant NHE3 (S552A/S605G/S661A/S716A) and then treated with either vehicle or CA for 30 min, solubilized, subjected to SDS-PAGE, and then immunoblotted with anti-NHE3 antibody.
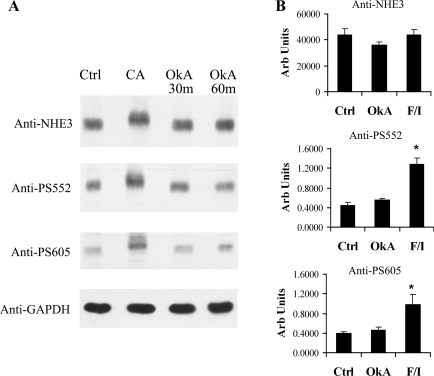
Experiments similar to those shown in Figs. 2 and 3 were conducted with okadaic acid, which is a potent inhibitor of the type 2A phosphatases (PP2A, PP4, and PP6) (34, 40). Okadaic acid was used at a dose previously shown to affect NaPi-IIa degradation in OKP cells (16). OKP cells were treated with vehicle, okadaic acid, calyculin A, or F/I and then subjected to SDS-PAGE, followed by Western blotting with anti-NHE3, anti-PS552, anti-PS605, or anti-GAPDH (loading control). As shown in the representative Western blot in Fig. 5A, no shift in NHE3 mobility or increase in NHE3 phosphorylation at serine 552 or 605 was detected after okadaic acid treatment. Relative changes in the amount of NHE3 phosphorylation at serines 552 and 605 were quantified by densitometry on multiple similar experiments, with F/I as the positive control and with a 30-min exposure to okadaic acid. As before, anti-PS552 and anti-PS605 signals were normalized to total NHE3 (anti-NHE3 signal). The results of densitometric analysis are depicted in Fig. 5B and confirm no change in NHE3 phosphorylation at serine 552 or 605 with okadaic acid treatment. As expected, F/I did increase NHE3 phosphorylation at both serines but did not change total NHE3 expression.
Fig. 5.
Okadaic acid, a potent PP2A-type inhibitor, does not alter NHE3 gel mobility or NHE3 phosphorylation at serine 552 or 605. A: OKP cells were treated with vehicle, CA for 30 min, or okadaic acid at 10−6 M (OkA) for 30 or 60 min and then solubilized, subjected to SDS-PAGE, and immunoblotted with anti-NHE3, anti-PS552, monoclonal anti-PS605, or anti-GAPDH antibody. B: densitometric analysis of multiple similar experiments in which OKP cells were treated with vehicle, OkA, or F/I for 30 min and then solubilized, subjected to SDS-PAGE, and immunoblotted with anti-NHE3, anti-PS552, or anti-PS605 antibody. Anti-PS552 and anti-PS605 signals were normalized to total NHE3 signals for the same experiment. Data are means + SE; n = 5–6 for Ctrl, n = 5–6 for OkA, and n = 4–6 for F/I. P values were calculated using a paired t-test. *P < 0.05 compared with control.
Calyculin A inhibits both PP1 and PP2A, whereas okadaic acid inhibits PP2A but not PP1, in OKP cells.
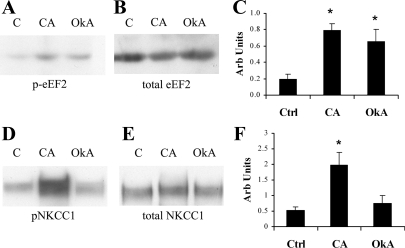
To verify the effects of calyculin A and okadaic acid on PP1 and PP2A in intact OKP cells, we tested the effect of these drugs on the phosphorylation of eEF2 and NKCC1. These proteins were chosen for the following reasons: 1) they are substrates for PP2A and PP1, respectively; 2) they are endogenously expressed in OKP cells; and 3) phosphospecific antibodies were available for both (8, 10, 29, 32, 35, 36). Based on previously published inhibitor sensitivities, it is reasonable to assume that a drug dose that inhibits PP1 will also inhibit PP5; likewise, a dose that inhibits PP2A will also inhibit PP4 and PP6 (40). OKP cells were treated with either calyculin A or okadaic acid, solubilized in SDS sample buffer, subjected to SDS-PAGE, and then immunoblotted with antibody to either p-eEF2, total eEF2, pNKCC1, or total NKCC1. Anti-GAPDH was again used as a loading control. As shown in Fig. 6A, eEF2 phosphorylation was increased by both calyculin A and okadaic acid treatments, whereas total eEF2 amounts were not affected by either (Fig. 6B). In contrast, NKCC1 phosphorylation was increased by calyculin A treatment only (Fig. 6D). Total NKCC1 expression remained unchanged after calyculin A or okadaic acid treatment (Fig. 6E). Changes in eEF2 and NKCC1 phosphorylation were confirmed by densitometric analyses of multiple independent experiments in which p-eEF2 and pNKCC1 signals were normalized to total eEF2 and NKCC1 signals, respectively (Fig. 6, C and F). Specifically, changes in eEF2 phosphorylation were statistically significant after both calyculin A and okadaic acid treatment compared with control, whereas changes in NKCC1 phosphorylation were statistically significant only after calyculin A treatment. Overall, Fig. 6 confirms the expected inhibitor profiles for calyculin A and okadaic acid in OKP cells: calyculin A inhibited both PP1 and PP2A, whereas okadaic acid inhibited PP2A but not PP1.
Fig. 6.
CA inhibits PP1 and PP2A, whereas OkA inhibits only PP2A. OKP cells were treated with vehicle (C), CA for 30 min, or OkA for 30 or 60 min and then solubilized, subjected to SDS-PAGE, and immunoblotted with an antibody to either phospho-eukaryotic translation elongation factor 2 (p-eEF2; A), total eEF2 (B), phospho-Na+-K+-Cl− cotransporter (p-NKCC1; D), or total NKCC1 (E). Densitometric analysis is shown for multiple similar experiments in which p-eEF2 (C) and p-NKCC1 signals (F) were quantitated and normalized to total eEF2 and total NKCC1, respectively. Data are means + SE; n = 4. P values were calculated using a paired t-test. *P < 0.05 compared with control.
PP5 is expressed in OKP and HEK cells but is not enriched in the brush-border membrane of proximal tubule cells.
On the basis of the inhibitor studies presented thus far, only PP1 and PP5 remain candidates for our observed dephosphorylation of NHE3. Therefore, we sought to determine whether PP5 is also present in the same subcellular region of the proximal tubule as NHE3. Equal protein amounts of whole cortical homogenates and purified brush-border membrane vesicles from Sprague-Dawley rats were loaded onto a gel, separated by SDS-PAGE, and then subjected to Western blotting with anti-PP5. As shown in Fig. 7, PP5 is found in whole cell lysates of OKP and HEK cells and in rat cortical whole homogenate, but very little is present in the brush-border membrane of proximal tubule cells.
Fig. 7.
PP5 is expressed in OKP cells, a proximal tubule cell line, but minimally present in BBMV. Thirty micrograms of rat cortical WH, 30 μg of rat BBMV, 15 μg of OKP cells, or 15 μg of HEK cells were solubilized, separated by SDS-PAGE, and prepared for immunoblotting with anti-PP5 antibody.
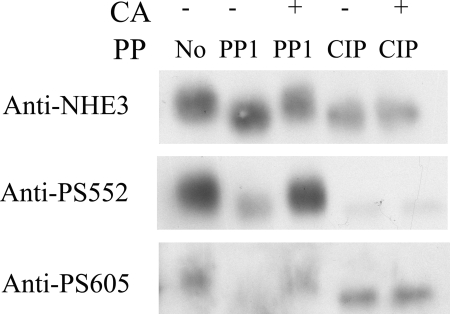
PP1 can directly dephosphorylate NHE3 in vitro.
In light of the above studies, PP1 was the best candidate for the dephosphorylation of NHE3 in OKP cells, and we sought to determine whether it is able to directly dephosphorylate NHE3 in vitro. To this end, we immunoprecipitated NHE3 from calyculin A-treated OKP cells and then incubated the immunopurified NHE3 with vehicle, PP1 catalytic subunit (PP1), PP1 plus calyculin A, CIP, or CIP plus calyculin A. The CIP treatment served as a positive control for NHE3 dephosphorylation in this experiment. Both PP1 and CIP were able to dephosphorylate NHE3, as demonstrated by a decrease in the gel mobility shift and a decrease in NHE3 phosphorylation at serines 552 and 605 (Fig. 8). Addition of calyculin A to the incubation solution largely prevented PP1-mediated NHE3 dephosphorylation but had no effect on CIP activity. Therefore, PP1 is able to directly dephosphorylate NHE3 in vitro in a calyculin A-sensitive manner.
Fig. 8.
PP1 can directly dephosphorylate NHE3 in vitro as demonstrated by a reversal in size shift and decreased phosphorylation at S552 and S605. OKP cells were treated with either vehicle or CA for 30 min. NHE3 was then immunopurified from the CA-treated solubilized cells and incubated with either vehicle (No), the catalytic fragment of PP1 (PP1), PP1 + CA, CIP, or CIP + CA. Immunopurified NHE3 was then subjected to SDS-PAGE and Western blotting with anti-NHE3, anti-PS552, or polyclonal anti-PS605 antibody.
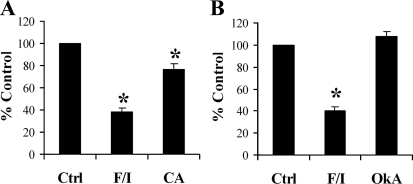
Calyculin A, but not okadaic acid, alters NHE3 activity.
To determine the functional consequences of serine/threonine phosphatase inhibition on NHE3 function, we assayed NHE3 activity in OKP cells by determining 22Na+ uptake in the presence of F/I, calyculin A, or okadaic acid. F/I has been previously demonstrated to inhibit NHE3 activity and served as a positive control for altered NHE3 activity in these experiments. As shown in Fig. 9A, F/I exerted the typical 60% inhibition of NHE3 activity, whereas calyculin A inhibited NHE3 activity by only 24%. Figure 9B demonstrates that okadaic acid did not significantly alter NHE3 activity in OKP cells.
Fig. 9.
CA, but not OkA, alters NHE3 activity. A: 22Na+ uptake was measured in OKP cells treated with vehicle, F/I, or CA (n = 9). B: 22Na+ uptake was measured in OKP cells treated with vehicle, F/I, or OkA (n = 4). Results are expressed as %control. Data are means + SE. P values were calculated using Student's t-tests. *P < 0.05 compared with control.
Novel NHE3 phosphorylation sites are functionally significant.
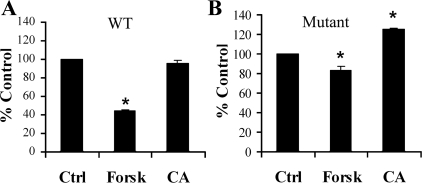
To determine the functional significance of novel phosphorylation sites on NHE3, we assayed NHE3 activity in PS120 cells stably expressing either wild-type NHE3 or NHE3 in which all known phosphorylation sites (S552, S605, S661, and S716) are mutated to alanine and glycine. To this end, wild-type or mutant NHE3 were transfected into PS120 cells, a non-NHE3-containing fibroblastic cell line, followed by generation of mixed stable lines using the acid loading technique (26). On the day of the experiment, the mixed stable transfectants were exposed to vehicle, forskolin at 10−4 M, or calyculin A at 10−6 M. Na+/H+ exchange activity was then assayed by 22Na+ uptake. Ideally, we would have conducted this experiment under the exact same conditions used in Fig. 9A for OKP cells, in which calyculin A induced a 24% inhibition of wild-type NHE3 activity. However, after a 30-min exposure to calyculin A (as done in OKP cells), PS120 cells were not able to tolerate the vigorous washes used in the 22Na+ uptake protocol. Therefore, a shorter exposure to calyculin A (6 min) was used for the 22Na+ uptake experiments in PS120 cells. In particular, PS120 cells with stable expression of wild-type or mutant NHE3 were exposed to vehicle or calyculin A for 6 min or to forskolin for 30 min. Although this short calyculin A exposure induced no observable change in wild-type NHE3 activity (Fig. 10A), mutation of all previously known phosphorylation sites uncovered a remarkably consistent and statistically significant stimulation of NHE3 activity (Fig. 10B). As expected, forskolin induced an inhibition of wild-type NHE3 activity, and this inhibition was attenuated in the mutant (Fig. 10, A and B) (24, 47). Figure 10B demonstrates that novel sites of NHE3 phosphorylation are functionally significant and that their effect was masked by the net contribution of known phosphorylation sites on NHE3 activity.
Fig. 10.
Novel sites of NHE3 phosphorylation are important in the regulation of NHE3. 22Na+ uptake was assayed in PS120 cells stably expressing either WT (A) or mutant NHE3 (B) in the setting of treatment with vehicle, CA for 6 min, or forskolin at 10−4 M (Forsk) for 30 min (n = 4). Results are expressed as %control. Data are means + SE. P values were calculated using a Student's t-test. *P < 0.05 compared with control.
DISCUSSION
We report in this article the first evidence for the dephosphorylation of NHE3 by a calyculin A-sensitive phosphatase such as PP1. NHE3 phosphorylation is a well-studied phenomenon, but until now, minimal attention has been focused on the role of phosphatases in the regulation of NHE3. PP1 and PP2A are said to be responsible for more than 90% of all mammalian serine/threonine phosphatase activity, and they are abundantly expressed in most tissues, including the kidney (5, 17, 18, 27, 46). Their expression has been previously demonstrated in the renal cortex, but further subcellular localization of these proteins has been limited (27, 45). On the other hand, the subcellular localization of NHE3 is well described, and it is located primarily in the brush-border membrane of the proximal tubule (4, 21). We determined that like NHE3, both PP1 and PP2A are present in the brush-border membrane of proximal tubule cells and also are present in OKP and HEK cells. Therefore, based on subcellular localization and coexpression in a proximal tubule cell model, PP1 and PP2A are good candidates for the dephosphorylation of NHE3.
Calyculin A is a potent inhibitor of all members of the PPP family except PP2B and PP7. Calyculin A induced a phosphorylation-dependent gel mobility shift of NHE3 as well as increased NHE3 phosphorylation at serines 552 and 605 in our proximal tubule cell culture model. These findings demonstrate that under baseline conditions, phosphorylation of NHE3 is constitutively regulated by one or more members of the PPP family of phosphatases. In addition to changes in NHE3 phosphorylation, calyculin A induced a 24% inhibition of NHE3 activity in OKP cells.
Okadaic acid is a potent inhibitor of the PP2A-type phosphatases, PP2A, PP4, and PP6. In contrast to calyculin A, okadaic acid treatment did not alter NHE3 phosphorylation or activity in OKP cells. Our findings of NHE3 activity with okadaic acid are in contrast to those presented by Levine et al. (26), who reported a 26% stimulation of NHE3 activity. These varied results may be attributable to differences between fibroblastic and epithelial cell lines. We conducted our studies in a proximal tubule cell line with endogenous NHE3 expression to ensure the greatest physiological relevance. Furthermore, in our cell culture system, we verified the effects of calyculin A and okadaic acid on endogenous PP1 and PP2A-type phosphatases.
Although the literature is replete with evidence that calyculin A inhibits most PPP phosphatases (PP1, PP2A, PP4, PP5, and PP6), whereas okadaic acid inhibits primarily the PP2A-type phosphatases (PP2A, PP4, and PP6), there are limited published studies in which these drugs are used in intact OKP cells (16). Therefore, we considered it critical to confirm the inhibitor profiles of calyculin A and okadaic acid in our cell culture model. To measure relative changes in PP1 and PP2A activities in OKP cells, we used the phosphorylation status of NKCC1 and eEF2, because they are substrates for PP1 and PP2A, respectively. Darman et al. (8) demonstrated that a region on the NH2 terminus of NKCC1 directly binds PP1 and that mutation of this binding sequence alters the phosphorylation and activity of NKCC1. Nairn and Palfrey (32) first demonstrated that eEF2 is directly and specifically dephosphorylated by PP2A. These findings have been confirmed in multiple subsequent studies (35, 36), and phosphorylation of eEF2 has since been used as an indirect measure of PP2A activity (11, 29). We confirmed that under the conditions used for our experiments in OKP cells, calyculin A inhibited both PP1 and PP2A, whereas okadaic acid inhibited only PP2A. Therefore, PP1 is likely responsible for the changes in NHE3 phosphorylation seen in our studies. Although PP5 activity cannot be completely excluded, it is reported to have very low basal activity (3, 13), and we have demonstrated that it is minimally present in the brush-border membrane of the proximal tubule.
Since multiple kinases phosphorylate NHE3 at distinct sites, it is highly possible that multiple phosphatases also dephosphorylate NHE3. Therefore, our results should not be taken to imply that other phosphatases may not be involved in the regulation of NHE3 or that indirect effects of phosphatases may not also play a role. The involvement of additional, and as yet unidentified, kinases in the phosphorylation of NHE3 is also highly likely, given that activation of kinases known to phosphorylate NHE3 (PKA, SGK1, and CK2) have not been shown to produce the striking NHE3 gel mobility shift seen in the present study.
Finally, we find it particularly interesting that phosphorylation of NHE3 at distinct sites can impact NHE3 activity differently. For example, SGK1 phosphorylates NHE3 at serine 661 and leads to an increase in NHE3 activity (42); in contrast, PKA phosphorylates NHE3 at serines 552 and 605 but is associated with an inhibition of NHE3 activity (21, 24, 47). We speculate that the modest effect of calyculin A treatment on NHE3 activity is due to the cumulative effect of multiple phosphorylation sites with differing downstream effects. Future studies are necessary to determine the novel phosphorylation/dephosphorylation sites and to identify additional kinases that may be involved in the regulation of NHE3. Nonetheless, our studies have already demonstrated that novel phosphorylation sites do exist, are dephosphorylated by a calyculin A-sensitive phosphatase, and are important in the regulation of NHE3.
In summary, we have demonstrated that a calyculin A-sensitive phosphatase, most likely PP1, dephosphorylates NHE3 at multiple sites and alters NHE3 activity in OKP cells. Accordingly, we have confirmed the presence of PP1 in the same subcellular region of the proximal tubule as NHE3 and established its ability to directly dephosphorylate NHE3 in vitro. Finally, we have established the presence of novel NHE3 phosphorylation sites that are functionally significant.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK072075 and DK17433 as well as a Charles H. Hood Foundation Child Health Research Grant.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Aronson PS. Energy-dependence of phlorizin binding to isolated renal microvillus membranes. Evidence concerning the mechanism of coupling between the electrochemical Na+ gradient the sugar transport. J Membr Biol 42: 81–98, 1978 [DOI] [PubMed] [Google Scholar]
- 2.Becchetti A, Malik B, Yue G, Duchatelle P, Al-Khalili O, Kleyman TR, Eaton DC. Phosphatase inhibitors increase the open probability of ENaC in A6 cells. Am J Physiol Renal Physiol 283: F1030–F1045, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Becker W, Kentrup H, Klumpp S, Schultz JE, Joost HG. Molecular cloning of a protein serine/threonine phosphatase containing a putative regulatory tetratricopeptide repeat domain. J Biol Chem 269: 22586–22592, 1994 [PubMed] [Google Scholar]
- 4.Biemesderfer D, Rutherford PA, Nagy T, Pizzonia JH, Abu-Alfa AK, Aronson PS. Monoclonal antibodies for high-resolution localization of NHE3 in adult and neonatal rat kidney. Am J Physiol Renal Physiol 273: F289–F299, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev 84: 1–39, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Ceulemans H, Stalmans W, Bollen M. Regulator-driven functional diversification of protein phosphatase-1 in eukaryotic evolution. Bioessays 24: 371–381, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Possemato R, Campbell KT, Plattner CA, Pallas DC, Hahn WC. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5: 127–136, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Darman RB, Flemmer A, Forbush B. Modulation of ion transport by direct targeting of protein phosphatase type 1 to the Na-K-Cl cotransporter. J Biol Chem 276: 34359–34362, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Donowitz M, Li X. Regulatory binding partners and complexes of NHE3. Physiol Rev 87: 825–872, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B. Activation of the Na-K-Cl cotransporter NKCC1 detected with a phospho-specific antibody. J Biol Chem 277: 37551–37558, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Gergs U, Boknik P, Buchwalow I, Fabritz L, Matus M, Justus I, Hanske G, Schmitz W, Neumann J. Overexpression of the catalytic subunit of protein phosphatase 2A impairs cardiac function. J Biol Chem 279: 40827–40834, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Gupta V, Ogawa AK, Du X, Houk KN, Armstrong RW. A model for binding of structurally diverse natural product inhibitors of protein phosphatases PP1 and PP2A. J Med Chem 40: 3199–3206, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Hinds TD, Jr, Sanchez ER. Protein phosphatase 5. Int J Biochem Cell Biol 40: 2358–2362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang HS, Pozarowski P, Gao Y, Darzynkiewicz Z, Lee EY. Protein phosphatase-1 inhibitor-3 is co-localized to the nucleoli and centrosomes with PP1gamma1 and PP1alpha, respectively. Arch Biochem Biophys 443: 33–44, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Honkanen RE. Molecular cloning, expression, and characterization of a novel human serine/threonine protein phosphatase, PP7, that is homologous to Drosophila retinal degeneration C gene product (rdgC). J Biol Chem 273: 1462–1468, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Jankowski M, Hilfiker H, Biber J, Murer H. The opossum kidney cell type IIa Na/Pi cotransporter is a phosphoprotein. Kidney Blood Press Res 24: 1–4, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 353: 417–439, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khew-Goodall Y, Hemmings BA. Tissue-specific expression of mRNAs encoding alpha- and beta-catalytic subunits of protein phosphatase 2A. FEBS Lett 238: 265–268, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Kloeker S, Reed R, McConnell JL, Chang D, Tran K, Westphal RS, Law BK, Colbran RJ, Kamoun M, Campbell KS, Wadzinski BE. Parallel purification of three catalytic subunits of the protein serine/threonine phosphatase 2A family (PP2AC, PP4C, and PP6C) and analysis of the interaction of PP2AC with alpha4 protein. Protein Expr Purif 31: 19–33, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Kocinsky HS, Dynia DW, Wang T, Aronson PS. NHE3 phosphorylation at serines 552 and 605 does not directly affect NHE3 activity. Am J Physiol Renal Physiol 293: F212–F218, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol 289: F249–F258, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Kubokawa M, Nakamura K, Hirano J, Yoshioka Y, Nakaya S, Mori Y, Kubota T. Regulation of inwardly rectifying K+ channel in cultured opossum proximal tubule cells by protein phosphatases 1 and 2A. Jpn J Physiol 50: 249–256, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Kurashima K, Szabo EZ, Lukacs G, Orlowski J, Grinstein S. Endosomal recycling of the Na+/H+ exchanger NHE3 isoform is regulated by the phosphatidylinositol 3-kinase pathway. J Biol Chem 273: 20828–20836, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Kurashima K, Yu FH, Cabado AG, Szabo EZ, Grinstein S, Orlowski J. Identification of sites required for down-regulation of Na+/H+ exchanger NHE3 activity by cAMP-dependent protein kinase phosphorylation-dependent and -independent mechanisms. J Biol Chem 272: 28672–28679, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Ledoussal C, Lorenz JN, Nieman ML, Soleimani M, Schultheis PJ, Shull GE. Renal salt wasting in mice lacking NHE3 Na+/H+ exchanger but not in mice lacking NHE2. Am J Physiol Renal Physiol 281: F718–F727, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Levine SA, Nath SK, Yun CH, Yip JW, Montrose M, Donowitz M, Tse CM. Separate C-terminal domains of the epithelial specific brush border Na+/H+ exchanger isoform NHE3 are involved in stimulation and inhibition by protein kinases/growth factors. J Biol Chem 270: 13716–13725, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Li D, Aperia A, Celsi G, da Cruz e Silva EF, Greengard P, Meister B. Protein phosphatase-1 in the kidney: evidence for a role in the regulation of medullary Na+-K+-ATPase. Am J Physiol Renal Fluid Electrolyte Physiol 269: F673–F680, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol Renal Physiol 277: F447–F453, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Marin P, Nastiuk KL, Daniel N, Girault JA, Czernik AJ, Glowinski J, Nairn AC, Premont J. Glutamate-dependent phosphorylation of elongation factor-2 and inhibition of protein synthesis in neurons. J Neurosci 17: 3445–3454, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moe OW. Acute regulation of proximal tubule apical membrane Na/H exchanger NHE-3: role of phosphorylation, protein trafficking, and regulatory factors. J Am Soc Nephrol 10: 2412–2425, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Moe OW, Amemiya M, Yamaji Y. Activation of protein kinase A acutely inhibits and phosphorylates Na/H exchanger NHE-3. J Clin Invest 96: 2187–2194, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nairn AC, Palfrey HC. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem 262: 17299–17303, 1987 [PubMed] [Google Scholar]
- 33.Oliver CJ, Shenolikar S. Physiologic importance of protein phosphatase inhibitors. Front Biosci 3: D961–D972, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Prickett TD, Brautigan DL. The alpha4 regulatory subunit exerts opposing allosteric effects on protein phosphatases PP6 and PP2A. J Biol Chem 281: 30503–30511, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Redpath NT, Proud CG. Activity of protein phosphatases against initiation factor-2 and elongation factor-2. Biochem J 272: 175–180, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redpath NT, Proud CG. Differing effects of the protein phosphatase inhibitors okadaic acid and microcystin on translation in reticulocyte lysates. Biochim Biophys Acta 1093: 36–41, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Sarker R, Gronborg M, Cha B, Mohan S, Chen Y, Pandey A, Litchfield D, Donowitz M, Li X. Casein kinase 2 binds to the C terminus of Na+/H+ exchanger 3 (NHE3) and stimulates NHE3 basal activity by phosphorylating a separate site in NHE3. Mol Biol Cell 19: 3859–3870, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Strack S, Kini S, Ebner FF, Wadzinski BE, Colbran RJ. Differential cellular and subcellular localization of protein phosphatase 1 isoforms in brain. J Comp Neurol 413: 373–384, 1999 [PubMed] [Google Scholar]
- 40.Swingle M, Ni L, Honkanen RE. Small-molecule inhibitors of Ser/Thr protein phosphatases: specificity, use and common forms of abuse. Methods Mol Biol 365: 23–38, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valenti G, Procino G, Carmosino M, Frigeri A, Mannucci R, Nicoletti I, Svelto M. The phosphatase inhibitor okadaic acid induces AQP2 translocation independently from AQP2 phosphorylation in renal collecting duct cells. J Cell Sci 113: 1985–1992, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Sun H, Lang F, Yun CC. Activation of NHE3 by dexamethasone requires phosphorylation of NHE3 at Ser663 by SGK1. Am J Physiol Cell Physiol 289: C802–C810, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang T, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Giebisch G, Aronson PS. Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol Renal Physiol 277: F298–F302, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Wiederkehr MR, Zhao H, Moe OW. Acute regulation of Na/H exchanger NHE3 activity by protein kinase C: role of NHE3 phosphorylation. Am J Physiol Cell Physiol 276: C1205–C1217, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Yu P, Asico LD, Eisner GM, Hopfer U, Felder RA, Jose PA. Renal protein phosphatase 2A activity and spontaneous hypertension in rats. Hypertension 36: 1053–1058, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Lin DH, Wang ZJ, Jin Y, Yang B, Wang WH. K restriction inhibits protein phosphatase 2B (PP2B) and suppression of PP2B decreases ROMK channel activity in the CCD. Am J Physiol Cell Physiol 294: C765–C773, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao H, Wiederkehr MR, Fan L, Collazo RL, Crowder LA, Moe OW. Acute inhibition of Na/H exchanger NHE-3 by cAMP. Role of protein kinase a and NHE-3 phosphoserines 552 and 605. J Biol Chem 274: 3978–3987, 1999 [DOI] [PubMed] [Google Scholar]