Abstract
Renin-angiotensin system (RAS) activation contributes to kidney injury through oxidative stress. Renin is the rate-limiting step in angiotensin (ANG II) generation. Recent work suggests renin inhibition improves proteinuria comparable to ANG type 1 receptor (AT1R) blockade (ARB). Thereby, we investigated the relative impact of treatment with a renin inhibitor vs. an ARB on renal oxidative stress and associated glomerular structural and functional changes in the transgenic Ren2 rat, which manifests hypertension, albuminuria, and increased tissue RAS activity. Young Ren2 and age-matched Sprague-Dawley (SD) controls (age 6–9 wk) were treated with a renin inhibitor (aliskiren), an ARB (irbesartan), or vehicle for 21 days. Ren2 rats exhibited increases in systolic pressure (SBP), albuminuria, and renal 3-nitrotyrosine content as well as ultrastructural podocyte foot-process effacement and diminution of the podocyte-specific protein nephrin. Structural and functional alterations were accompanied by increased renal cortical ANG II, AT1R, as well as NADPH oxidase subunit (Nox2) expression compared with SD controls. Abnormalities were attenuated to a similar extent with both aliskiren and irbesartan treatment. Despite the fact the dose of irbesartan used caused a greater reduction in SBP than aliskerin treatment (P < 0.05), the effects on proteinuria, nephrin, and oxidative stress were similar between the two treatments. Our results highlight both the importance of pressor-related reductions on podocyte integrity and albuminuria as well as RAS-mediated oxidant stress largely comparable between ARB and renin inhibition treatment.
Keywords: podocyte, NADPH oxidase, oxidative stress
the renin-angiotensin system (RAS) plays a central role in regulating normal cardiovascular and kidney function (6, 37). However, excessive RAS activity contributes to renal maladaptive remodeling through promotion of inflammation and oxidative stress, processes that promote glomerular filtration barrier injury and ensuing albuminuria (29, 32, 37, 45, 48–50). Antihypertensive agents that inhibit the actions of angiotensin II (ANG II), either through inhibition of ANG-converting enzyme (ACE) or blockade of the ANG type 1 receptor (AT1R), have been shown to reduce the progression of albuminuria as well as slow the decline in glomerular filtration rate (43) and progression to end-stage renal disease (9).
While inhibition of the RAS with blockade of the AT1R attenuates the progression of increasing albuminuria and kidney damage, the burden of kidney disease remains high and there exists an unmet need for treatment strategies that further reduce progression of proteinuria and renal disease (14, 39). Recently, both (pro)renin (13, 20) and renin (18, 51) receptors have been identified and characterized in the kidney. Binding to these receptors is specific for renin and prorenin and induces phosphorylation of tyrosine and serine residues, thereby promoting fibronectin and collagen gene expression and associated fibrosis (35). The observation that animals deficient in ANG II, ACE, or ANG II receptors have high renin levels and develop glomerulosclerosis (12, 36, 46) suggests renin may act through a receptor-mediated ANG II-independent mechanism to promote glomerular disease.
As renin is the rate-limiting step in the generation of ANG II, blockade of renin activation is an attractive therapeutic target to prevent glomerular injury and associated proteinuria. Aliskiren is a potent direct renin inhibitor, with high specificity for human and mouse renin (2, 28, 33). Recent studies conducted in vitro and in animals indicate that treatment with this direct renin inhibitor reduces the number of (pro)renin receptors (35) and attenuates fibrosis in the kidney (13, 24). Furthermore, this drug reduces proteinuria (13, 33) in animals and humans (14, 39). However, the precise protective effects of treatment with this direct renin inhibitor on glomerular filtration barrier integrity have not been investigated. This is important as loss of this barrier is the seminal abnormality underlying the onset of albuminuria (22, 27, 38, 41).
Due to a high species specificity for only human and mouse renin, aliskiren cannot be studied effectively in conventional rat models. To circumvent this issue, we utilized the transgenic TG(mRen2)27 rat (Ren2), which harbors both the native Ren1 and the murine renin transgene and is a model of excessive tissue RAS activity and develops hypertension, systemic insulin resistance, and proteinuria (16, 26, 48, 49). Use of the Ren2 rat allows for interrogation of the specific role of direct renin inhibition compared with AT1R blockade (ARB) as it contributes to glomerular filtration barrier injury (48–50). Accordingly, we hypothesized that in vivo direct renin inhibition with aliskiren would attenuate the loss of glomerular filtration barrier/integrity and associated albuminuria comparable to that of an ARB, in part, through attenuation of renal oxidative stress.
METHODS
Animals and treatments.
All animal procedures were approved by the University of Missouri animal care and use committees and housed in accordance with National Institutes of Health guidelines. Ren2 rats (6–9 wk of age) and age-matched Sprague-Dawley (SD) littermates were randomly assigned to sham treatment (Ren2-C and SD-C, respectively; n = 5 each), aliskiren treatment (Ren2-A and SD-A; n = 6 each) at 50 mg·kg−1·day−1, or irbesartan treatment (Ren2-I; n = 5) at 30 mg·kg−1·day−1 in saline via intraperitoneal injection for 21 days. Aliskiren was provided by Novartis research laboratories and prepared fresh daily in sterile 0.9% normal saline. Dosing was based on previous studies in Ren2 rats (13, 50).
Systolic blood pressure and albuminuria.
Systolic blood pressure (SBP) was determined after acclimatization, and urine albumin and creatinine were performed as previously described (48, 49).
Ultrastructural observations with transmission electron microscopy.
Kidney cortical tissue was thinly sliced, placed immediately in primary transmission electron microscopy (TEM) fixative, and prepared as previously described (49). A JOEL 1200-EX TEM microscope (JOEL, Tokyo, Japan) was used to view all samples.
Western blot analysis.
Standard Western blot analysis was performed as previously described in detail with some modifications (49). Briefly, kidney cortical tissue was taken from 9-wk-old animals, weighed, and then transferred to sucrose homogenization buffer. Four fractions were prepared by differential centrifugation (Sorvall), and the whole tissue lysate fraction was used for Western blots. Each lane was loaded with 50 μg of protein and equal loading was ensured by both Ponceau staining and the housekeeping protein, β-actin. Anti-nephrin (Santa Cruz Biotechnology, Santa Cruz, CA) was diluted 1:200 in 1× TBS with 0.1% Tween 20 (TBST). Following an overnight incubation, the membranes were washed in TBST (4 × 4 min), incubated with horseradish peroxidase (HRP)-conjugated anti-goat IgG at 1:10,000 for 1 h at room temperature, washed again in TBST (5 × 5 min), treated with SuperSignal West Dura Chemiluminescent Substrate (Pierce, Rockford, IL), and digitally imaged with a Bio-Rad Chemi-Doc XRS. Protein bands were quantified using Bio-Rad's Quantity One software.
Immunohistochemistry.
Harvested kidney tissue was prepared as previously described (48–50). Briefly, rehydrated paraffin-embedded sections were blocked in 5% BSA, 5% donkey serum, and 0.01% sodium azide in HEPES buffer for 4 h in a humidity chamber. Following a brief rinse, sections were incubated with 1:100 goat polyclonal ANG II (Santa Cruz Biotechnology), 1:50 rabbit polyclonal AT1R (Santa Cruz Biotechnology), 1:100 goat polyclonal Nox2 (Santa Cruz Biotechnology), and 1:200 mouse monoclonal Rac1 (Upstate Cell Signaling) antibodies in 10-fold diluted blocking agent overnight. After being washed, sections were incubated for 4 h with 1:300 Alexa fluor donkey anti-goat 647 (Invitrogen) for ANG II and Nox2, donkey anti-rabbit for AT1R, and donkey anti-mouse for Rac1. The slides were examined under a bi-photon confocal microscope (Zeiss LSM, 510 MLO, Thornwood, NY), and the images were captured with LSM imaging system. Signal intensities were analyzed with MetaVue.
3-Nitrotyrosine immunostaining.
3-Nitrotyrosine (3-NT) was quantified as previously described (48, 49). Briefly, samples were incubated overnight with 1:200 primary rabbit polyclonal anti-nitrotyrosine antibody (DakoCytomation, Carpinteria, CA). Sections were then washed and incubated 30 min each with secondary antibodies, biotinylated link, and streptavidin-HRP. After several rinses with distilled water, diaminobenzidine was applied for 12 min, and sections were again rinsed and stained with hematoxylin for 45 s, rehydrated, and mounted with a permanent media. The slides were checked under a bright field (Nikon 50i) microscope and the ×40 images were captured with a cool snapcf camera.
Statistical analysis.
This investigation was powered based on prior sensitivity and variability measurements of albuminuria to achieve a significance of P < 0.05 with a power of 0.8 (49). All values are expressed as means ± SE. Statistical analyses were performed in SPSS 13.0 (SPSS, Chicago, IL) using ANOVA with Fisher's LSD as appropriate and Student's t-test for paired analysis.
RESULTS
SBP and albuminuria.
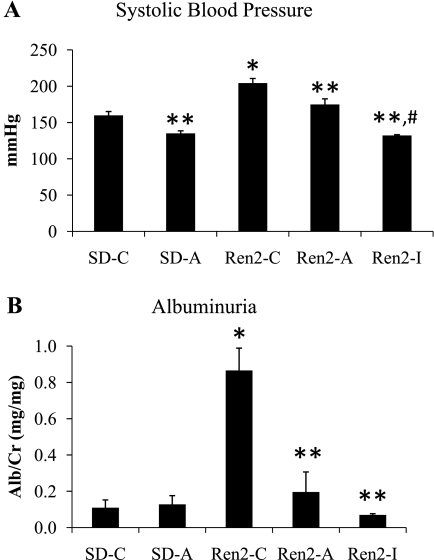
The Ren2 is a rat model of RAS activation driven by the overexpression of the mouse renin transgene with manifestation of hypertension and albuminuria. Consistent with prior observations (48–50), we observed increases in SBP in the Ren2 compared with SD controls (P < 0.05) that were improved with administration of both aliskiren and irbesartan in Ren2 animals (each P < 0.05; Fig. 1A). There were slightly greater reductions in SBP in irbesartan-treated Ren2 rats compared with aliskiren treatment (P < 0.05). A similar pattern was observed with urine albumin excretion. There were again increases in the Ren2 compared with SD controls (P < 0.05) that were improved with both aliskiren and irbesartan treatment (each P < 0.05; Fig. 1B).
Fig. 1.
Systolic blood pressure (SBP) and albuminuria in the Ren2 rat. SBP (A) as measured by tail cuff and albuminuria (B) as determined by urine albumin-to-creatinine ratio, both determined at the end of the treatment period of 21 days. Values presented as means ± SE. *P < 0.05 when Ren2 controls (Ren2-C; n = 5) are compared with age-matched Sprague-Dawley controls (SD-C; n = 5). **P < 0.05 when aliskiren- or irbesartan-treated Ren2 rats (Ren2-A and Ren2-I; n = 5) are compared with age-matched Ren2-C. #P < 0.05 when Ren2-I are compared with Ren2-A.
Glomerular filtration barrier/podocyte injury.
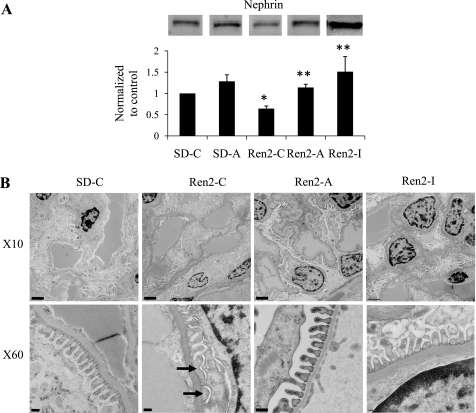
Albuminuria in this model is characterized by podocyte injury (48, 49). Nephrin is an IgG-like integral membrane protein that plays a critical role in maintaining podocyte architecture (22, 27, 38, 41). Consistent with prior observations of podocyte injury-associated increases in albuminuria, there was decreased expression of the podocyte-specific protein nephrin in Ren2 cortical tissue compared with SD controls (P < 0.05), and this reduction was significantly improved with both aliskiren and irbesartan treatment (Fig. 1C).
To confirm podocyte injury, we utilized ultrastructural observations of the glomerular filtration barrier of the Ren2, which revealed podocyte foot process effacement and loss of slit pore integrity compared with SD controls (Fig. 2). Consistent with effacement, there were increases in podocyte foot process base width as well as decreases in intact slit pore diaphragm surface in the untreated Ren2 compared with control and aliskiren-treated SD rats. These observed ultrastructural abnormalities were corrected to a similar extent with both aliskiren and irbesartan treatment in the Ren2.
Fig. 2.
Podocyte foot process integrity in the Ren2. A: Western blot analysis of the podocyte-specific protein nephrin from kidney cortical tissue homogenates. Each lane was loaded with 50 μg of protein and equal loading was ensured by both Ponceau staining and the housekeeping protein, β-actin. B: ultrastructural analysis of the glomerular filtration barrier on transmission electron microscopy at ×10,000 (top) and ×60,000 (bottom). The Ren2 (Ren2-C; n = 4) rat displays loss of glomerular filtration barrier integrity with podocyte foot process effacement and loss of the slit pore diaphragm (arrows) compared with Sprague-Dawley (SD-C; n = 5) controls. Podocyte foot process effacement is improved with both aliskiren and irbesartan treatment in the Ren2 (Ren2-A and Ren2-I; n = 5) rats. *P < 0.05 when Ren2-C (n = 5) are compared with SD-C (n = 5). **P < 0.05 when Ren2-A and Ren2-I (n = 5) are compared with age-matched Ren2-C.
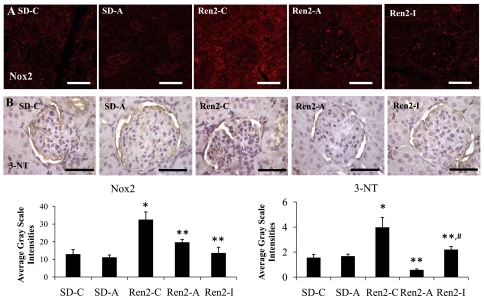
Renal cortical 3-NT and Nox2 content.
Excess RAS activation has been linked to increases in oxidative stress. 3-NT content, as a marker for peroxynitrite (ONOO−) formation, was similarly increased in the Ren2 compared with SD controls (P < 0.05; Fig. 3). 3-NT represents increased oxido- and nitroso-lipid products, not simply reactive oxygen species (ROS) formation. Indeed, we and others showed that enhanced activation of the NADPH oxidase enzyme, in addition to altered nitric oxide (NO) metabolism, contributes to RAS-mediated generation of 3-NT (48). Consistent with the notion that ANG II activates the NADPH oxidase enzyme complex, we observed increases in Nox2 subunit immunostaining in cortical tissue compared with SD controls (P < 0.05). Both aliskiren and irbesartan treatment reduced this NADPH oxidase subunit expression and 3-NT content in Ren2 cortical tissue (each P < 0.05).
Fig. 3.
Semiquantitative analysis of oxidative stress in Ren2 cortical tissue. Representative images of immunohistochemistry analysis of kidney cortical tissue NADPH oxidase subunit Nox2 (A) and 3-nitrotyrosine (3-NT; B), as a marker for lipid peroxidative and oxidative stress, with corresponding average gray scale intensities below. Values presented as means ± SE. *P < 0.05 when Ren2-C (n = 5) are compared with SD-C (n = 5). **P < 0.05 when Ren2-A and Ren2-I (n = 5) are compared with age-matched Ren2-C. #P < 0.05 when Ren2-I are compared with Ren2-A. Scale bar = 50 μm.
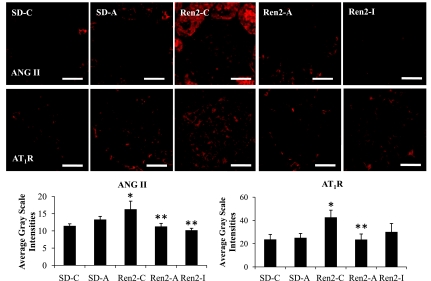
Renal cortical ANG II and AT1R levels.
The Ren2 model is characterized by increased tissue levels of ANG II (3–5). There were increases in ANG II and AT1R immunostaining in Ren2 cortical tissue compared with SD controls (each P < 0.05; Fig. 4). Aliskiren and irbesartan treatments reduced the ANG II, AT1R, and Nox2 immunostaining in Ren2 cortical tissue to a similar extent (each P < 0.05). Aliskiren treatment reduced renal cortical tissue immunostaining for 3-NT to a greater extent than observed with irbesartan treatment (P < 0.05).
Fig. 4.
Semiquantitative analysis of ANG II and AT1R in Ren2 cortical tissue. Representative images of immunohistochemistry analysis of kidney cortical tissue for ANG II and AT1R with corresponding average gray scale intensities below. Values presented as means ± SE. *P < 0.05 when Ren2-C (n = 5) are compared with SD-C (n = 5). **P < 0.05 when Ren2-A and Ren2-I (n = 5) are compared with age-matched Ren2-C. Scale bar = 50 μm.
DISCUSSION
Results from this investigation highlight the importance of SBP reductions in both glomerular injury and proteinuria with both ARB and renin inhibition treatment in the transgenic Ren2 rat. In vivo treatment with the direct renin inhibitor (aliskiren) and ARB (irbesartan) protected against tissue RAS-mediated loss of podocyte slit pore integrity to a similar extent in this transgenic rat model. These observations underscore the importance of blood pressure reduction, as well as specific effects on tissue RAS, in reducing glomerular injury and albuminuria in this model of enhanced tissue renin expression. Results of this study underscore previous work demonstrating the importance of SBP reduction as a critical determinant of abrogating proteinuria and renal injury (40). Available data suggest that RAS intervention has a renoprotective effect that may extend beyond its anti-hypertensive efficacy relative to other anti-hypertensive classes (9, 39, 40). Collectively, these observations complement and extend previous reports that renin inhibition, as well as ARB treatment, reduces renal fibrosis and proteinuria in rodents (13, 33) and reduces mesangial fibrosis and apoptosis of podocytes in cell culture systems (34, 42).
Our observations suggest that the glomerulopathy seen in the transgenic Ren2 kidney is dependent, in part, on renin activation in this tissue. Others reported that the kidney murine renin in the Ren2 rat is 60-fold more active than rat renin on rat angiotensinogen and subsequent expression of tissue ANG II (3, 4). Indeed, compared with SD animals, Ren2 rats are characterized by increased plasma total renin, prorenin, and ANG II as well as increased kidney ANG II levels (3–5). Activation of the renin/prorenin receptor has been proposed to further contribute to the observed abnormalities in the Ren2 kidney, including inflammation and interstitial fibrosis as well as albuminuria (13). In this context, a potential benefit of blocking renin activation would be to reduce renin availability to bind to renin/prorenin receptors, the binding of which has been shown to have pathological consequences in other tissues (8, 16, 21, 26, 33).
To the best of our knowledge, this is the first investigation demonstrating a beneficial effect of renin inhibition on the filtration barrier and podocyte integrity. The glomerular filtration barrier is composed of three layers: the fenestrated capillary endothelium, the glomerular basement membrane, and the terminally differentiated visceral epithelial cells termed podocytes (22, 27, 38, 41). Podocytes line the outer portion of the basement membrane and serve as the final barrier against urinary protein loss. In this regard, the slit pore membrane, a diaphragmatic structure formed at the junction of the interdigitating foot processes of podocytes, is the primary size-selective permeability barrier against protein leakage into the proximal tubule (27, 38).
Aliskiren treatment reduced 3-NT cortical content suggesting a possible role for renin in slit pore membrane integrity. In this regard, it is important to note that 3-NT in this study is a single measure and represents both lipid oxidation and ONOO−, which is a highly reactive oxidant species that can be formed endogenously by the interaction of NO and superoxide anion (O2−), and this product reacts readily with tyrosine residues of proteins to form 3-NT (37). Reductions in bioavailable NO and increases in ROS are thought to be the major contributors to oxidative damage to lipids, proteins, and DNA. Our observations are also consistent with the notion that increases in ONOO− (measured as 3-NT) are highly reactive in the kidney and can contribute to glomerular/podocyte injury (48, 49). The measurement of 3-NT in renal cortical tissue reflects not just glomerular but also tubulointerstitial immunostaining.
Relevant to our observations, cultured podocytes exposed to ROS demonstrate enhanced expression of proinflammatory cytokines and AT1R as well as podocyte apoptosis and cytoskeletal rearrangement (10, 15). The resulting increase in ROS can contribute to reduced expression of podocyte-specific proteins and albuminuria by several mechanisms including decreased expression, increased degradation, and altered signaling capabilities of these highly susceptible cells. Results of the current investigation employing a renin inhibitor extend prior work demonstrating that in vivo treatment of young Ren2 rats with a ROS scavenger, AT1R blockade, and MR antagonist not only decreased kidney NADPH oxidase activity and ROS generation, but also improved podocyte effacement/damage, kidney filtration barrier fibrosis, and albuminuria (48, 49). It should also be noted that Nox2 staining represents a subunit of an oxidant-generating complex, but it is not, itself, a marker of oxidative stress.
The cloning of the receptor for renin and prorenin, the (P)RR (34), led to the additional observation that prorenin and renin may exert direct renal inflammatory effects (18). Furthermore, on binding to the (P)RR prorenin undergoes nonproteolytic activation and generation of cell surface ANG I (31, 34). Additionally, the (P)RR may amplify renin-dependent actions; renin bound to(P)RR greatly enhances the catalytic actions of soluble renin (34). It has been suggested that aliskiren may exert some of its renal protective effects by reducing the activation of a functional (P)RR, the activation of which increases the activation of profibrotic pathways (13, 18, 34). Recent work demonstrated that aliskiren has an extensive and sustained renal distribution (13, 24, 51), and this drug likely has beneficial effects on renal tubule function (optimal reabsorption of filtered protein) as well as salutary effects on the glomerular slit pore diaphragm.
Investigation of glomerular disease and associated proteinuria in hypertension has generally focused on mesangial proliferation and expansion, matrix accumulation, and basement membrane abnormalities (42). However, with recent availability of sensitive markers for podocyte injury, such as nephrin, podocyte and slit pore damage can be assessed with greater reliability (1, 7, 23). The availability of TEM further advanced our understanding of podocyte and slit pore membrane damage in relation to proteinuria (48, 50). Here, consistent with our previous TEM work on Ren2 kidney tissue (48, 50), we observed podocyte foot process effacement, widening of the bases of the foot processes, and loss of slit pore diaphragm integrity. These TEM abnormalities occurred contemporaneous with decreases in nephrin protein levels and were largely corrected to a similar extent in animals treated with either aliskiren or irbesartan.
Podocytes express ANG II receptors and have been shown to be target cells for ANG II action (11, 17, 44, 49). Our current data showing that Ren2 renal cortical nephrin levels are decreased and glomerular ANG II and AT1R levels are increased are consistent with the prior reports that ANG II reduces nephrin gene expression (11) as well as protein levels (14). Furthermore, our observations are consistent with a prior report that the degree of nephrin immunohistochemical expression is related to the extent of foot process effacement on TEM (50). Nephrin exerts an important role as an adhesion molecule in cell-cell adhesion through homophilic interactions; thus, loss of this protein is thought to cause loss of cytoskeletal integrity and thus disorganization of the slit pore membrane integrity (11, 25).
In summary, results from this investigation highlight the importance of pressor-related reductions on RAS-mediated oxidant stress and improvements in glomerular/podocyte integrity (nephrin) with both ARB and renin inhibition treatment.
GRANTS
This research was supported by the National Institutes of Health (Grant R01-HL-73101-01A1) to J. R. Sowers, Grant HL-51952 to C. M. Ferrario, Veterans Affairs Merit System (0018) for J. R. Sowers as well as CDA-2 and VISN 15 for A. Whaley-Connell, and Novartis Pharmaceutical.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors acknowledge B. Hunter and E. Rehmer for editing this manuscript. The authors also acknowledge the Electron Microscopic Core Center at the University of Missouri, Columbia, MO for excellent help and tissue preparation of animal samples for viewing. Lastly, the authors acknowledge Dr. J. Paik for work on electron microscopy and Dr. C. E. Wiedmeyer for performing the albuminuria measurements.
REFERENCES
- 1.Bianchi S, Bigazzi R, Campese VM. Microalbuminuria in essential hypertension: significance, pathophysiology, and therapeutic implications. Am J Kidney Dis 34: 973–995, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Blundell T, Sibanda BL, Pearl L. Three-dimensional structure, specificity and catalytic mechanism of renin. Nature 304: 273–275, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Bohlender J, Menard J, Edling O, Ganten D, Luft FC. Mouse and rat plasma renin concentration and gene expression in (mRen2)27 transgenic rats. Am J Physiol Heart Circ Physiol 274: H1450–H1456, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Campbell DJ, Kelly DJ, Wilkinson-Berka JL, Cooper ME, Skinner SL. Increased bradykinin and “normal” angiotensin peptide levels in diabetic Sprague-Dawley and transgenic (mRen-2)27 rats. Kidney Int 56: 211–221, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Campbell DJ, Rong P, Kladis A, Rees B, Ganten D, Skinner SL. Angiotensin and bradykinin peptides in the TGR(mRen-2)27 rat. Hypertension 25: 1014–1020, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 293: H2009–H2023, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Coward RJ, Foster RR, Patton D, Ni L, Lennon R, Bates DO, Harper SJ, Mathieson PW, Saleem MA. Nephrotic plasma alters slit diaphragm-dependent signaling and translocates nephrin, podocin, and CD2 associated protein in cultured human podocytes. J Am Soc Nephrol 16: 629–637, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Danser JAH. Prorenin: back into the arena. Hypertension 47: 824–826, 2006 [DOI] [PubMed] [Google Scholar]
- 9.De Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS, Singhal PC. Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol 283: F173–F180, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G. Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes 52: 1023–1030, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Esther CR, Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest 74: 953–965, 1996 [PubMed] [Google Scholar]
- 13.Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W, Persohn E, Schuetz H, Jan Danser AH, Nguyen G. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension 52: 130–136, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Fisher ND, Jan Danser AH, Nussberger J, Dole WP, Hollenberg NK. Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation 117: 3199–3205, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Greiber S, Müller B, Daemisch P, Pavenstädt H. Reactive oxygen species alter gene expression in podocytes: induction of granulocyte macrophage-colony-stimulating factor. J Am Soc Nephrol 13: 86–95, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Habibi J, Whaley-Connell A, Hayden MR, DeMarco VG, Schneider R, Sowers SD, Karuparthi P, Ferrario CM, Sowers JR. Renin inhibition attenuates insulin resistance, oxidative stress, and pancreatic remodeling in the transgenic Ren2 rat. Endocrinology 149: 5643–5653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann S, Podlich D, Hähnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 15: 1475–1487, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W. Renin increases mesangial cell transforming growth factor-B and matrix proteins trough receptor-mediated, angiotensin II-independent mechanisms. Kidney Int 69: 105–113, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Ibsen H, Olsen MH, Wachtell K, Borch-Johnsen K, Lindholm LH, Mogensen CE, Dahlöf B, Devereux RB, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wan Y. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 45: 198–202, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the handle region for nonproteolytic activation of prorenin. J Clin Invest 114: 1128–1135, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imanishi T, Tsujioka H, Ikejima H, Kuroi A, Takarada S, Kitabata H, Tanimoto T, Muragaki Y, Mochizuki S, Goto M, Yoshida K, Akasaka T. Renin inhibitor aliskiren improves impaired nitric oxide bioavailability and protects against atherosclerotic changes. Hypertension 52: 467–469, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Johnstone DB, Holtzman LB. Clinical impact of research on the podocyte slit diaphragm. Nat Clin Pract Nephrol 2: 271–282, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, Sakai T, Yamamoto T, Salant DJ, Shimizu F. Cloning of rat nephrin: expression in developing glomeruli and in proteinuric states. Kidney Int 57: 1949–1961, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Kelly DJ, Zhang Y, Moe G, Naik G, Gilbert RE. Aliskiren, a novel renin inhibitor, is renoprotective in a model of advanced diabetic nephropathy in rats. Diabetologia 50: 2398–2404, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Khoshnoodi J, Sigmundsson K, Ofverstedt LG, Skoglund U, Obrink B, Wartiovaara J, Tryggvason K. Nephrin promotes cell-cell adhesion through homophilic interactions. Am J Pathol 163: 2337–2346, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lastra G, Habibi J, Whaley-Connell AT, Manrique C, Hayden MR, Rehmer J, Patel K, Ferrario C, Sowers JR. Direct renin inhibition improves systemic insulin resistance and skeletal muscle glucose transport in a transgenic rodent model of tissue renin overexpression. Endocrinology 150: 561–568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehtonen S. Connecting the interpodocyte slit diaphragm and actin dynamics: emerging role for the nephrin signaling complex. Kidney Int 73: 903–905, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Lu H, Rateri DL, Feldman DL, RJ, Fukamizu A, Ishida J, Oesterling EG, Cassis LA, Daugherty A. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest 118: 984–993, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, Kimura S, Yukimura T, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol 16: 2906–2912, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Morris EM, Whaley-Connell A, Thyfault JP, Britton SL, Lock LG, Wei Y, Ibdah JI, Sowers JR. Low aerobic capacity and high fat diet contributes to oxidative stress and IRS-1 degradation in the kidney. Am J Nephrol 30: 112–119, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nabi AH, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F. Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med 18: 483–488, 2006 [PubMed] [Google Scholar]
- 32.Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt sensitive rats and is reversed by aldosterone blocker. Hypertension 471: 1084–1093, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Nguyen G, Danser AHJ. Prorenin and (pro)renin receptor: a review of available data from in vitro studies and experimental models in rodents. Exp Physiol 93: 557–563, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer JD. Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int 50: 1897–1903, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niimura F, Labosky PA, Kakuchi J, Okubo S, Yoshida H, Oikawa T, Ichiki T, Naftilan AJ, Fogo A, Inagami T. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest 96: 2947–2954, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nistala R, Whaley-Connell A, Sowers JR. Redox control of renal function and hypertension. Antioxid Redox Signal 10: 2047–2089, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. AVOID Study Investigators. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 358: 2433–2446, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Parving HH, Andersen AR, Smidt UM, Hommel E, Mathiesen ER, Svendsen PA. Effect of antihypertensive treatment on kidney function in diabetic nephropathy. BMJ 294: 1443–1447, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 83: 253–258, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Phillips DJ, Dai T, Feldman DL. Aliskerin attenuates high glucose induced extracellular matrix and protects against cell death in cultured podocytes. J Am Soc Nephrol 18: 169A, 2007 [Google Scholar]
- 43.Rossing P, Hommel E, Smidt UM, Parving HH. Reduction in albuminuria predicts a beneficial effect on diminishing the progression of human diabetic nephropathy during antihypertensive treatment. Diabetologia 37: 511–516, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Sharma M, Sharma R, Greene AS, McCarthy ET, Savin VJ. Documentation of angiotensin II receptors in glomerular epithelial cells. Am J Physiol Renal Physiol 274: F623–F627, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension 49: 355–364, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest 101: 755–760, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wartiovaara J, Ofverstedt LG, Khoshnoodi J, Zhang J, Mäkelä E, Sandin S, Ruotsalainen V, Cheng RH, Jalanko H, Skoglund U, Tryggvason K. Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest 114: 1475–1483, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whaley-Connell A, Rehmer N, Habibi J, Rehmer J, Ferrario CM, Sowers JR. Nebivolol reduces proteinuria and renal NADPH oxidase generated reactive oxygen species in the transgenic Ren2 rat. Am J Nephrol 30: 354–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whaley-Connell A, Habibi J, Nistala R, Cooper SA, Karuparthi PR, Hayden MR, Rehmer N, DeMarco VG, Andresen BT, Wei Y, Ferrario C, Sowers JR. Attenuation of Angiotensin II-mediated NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension 51: 474–480, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whaley-Connell A, Habibi J, Cooper SA, DeMarco VG, Link CD, Stump CS, Hayden MR, Ferrario C, Sowers JR. Effect of renin inhibition and AT1R blockade on myocardial remodeling in the transgenic Ren2 rat. Am J Physiol Endocrinol Metab 295: E103–E109, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiggins KJ, Kelly DJ. Aliskiren: a novel renoprotective agent or simply an alternative to ACE inhibitors? Kidney Int 76: 23–31, 2009 [DOI] [PubMed] [Google Scholar]