Abstract
ANG II AT2 receptor (AT2R)-deficient mice exhibit abnormal ureteric bud (UB) budding, increased incidence of double ureters, and vesicoureteral reflux. However, the role of the AT2R during UB morphogenesis and the mechanisms by which aberrant AT2R signaling disrupts renal collecting system development have not been fully defined. In this study, we mapped the expression of the AT2R during mouse metanephric development, examined the impact of disrupted AT2R signaling on UB branching, cell proliferation, and survival, and investigated the cross talk of the AT2R with the glial-derived neurotrophic factor (GDNF)/c-Ret/Wnt11 signaling pathway. Embryonic mouse kidneys express AT2R in the branching UB and the mesenchyme. Treatment of embryonic day 12.5 (E12.5) metanephroi with the AT2R antagonist PD123319 or genetic inactivation of the AT2R in mice inhibits UB branching, decreasing the number of UB tips compared with control (34 ± 1.0 vs. 43 ± 0.6, P < 0.01; 36 ± 1.8 vs. 48 ± 1.3, P < 0.01, respectively). In contrast, treatment of metanephroi with the AT2R agonist CGP42112 increases the number of UB tips compared with control (48 ± 1.8 vs. 39 ± 12.3, P < 0.05). Using real-time quantitative RT-PCR and whole mount in situ hybridization, we demonstrate that PD123319 downregulates the expression of GDNF, c-Ret, Wnt11, and Spry1 mRNA levels in E12.5 metanephroi grown ex vivo. AT2R blockade or genetic inactivation of AT2R stimulates apoptosis and inhibits proliferation of the UB cells in vivo. We conclude that AT2R performs essential functions during UB branching morphogenesis via control of the GDNF/c-Ret/Wnt11 signaling pathway, UB cell proliferation, and survival.
Keywords: angiotensin, GDNF, c-Ret
in the classic renin-angiotensin system (RAS), renin cleaves angiotensinogen (AGT) to generate angiotensin (ANG) I, which is then converted to ANG II by the angiotensin-converting enzyme (ACE). ANG II is the principal effector peptide growth factor of the RAS which acts via two major G protein-coupled receptors: AT1R and AT2R (4). Convincing evidence indicates that the RAS is critical for proper kidney development. Use of ACE inhibitors or AT1R antagonists during fetal life, as well as mutations in the genes encoding AGT, renin, ACE, or AT1R in humans, are associated with renal tubular dysgenesis (18). Genetic inactivation of AGT, renin, ACE, or AT1R in mice causes pelvic dilation (hydronephrosis) and hypoplastic papilla (15, 34, 36, 46, 47). Mutations in the AT2R gene in mice are associated with increased incidence of double ureters and vesicoureteral reflux (35). Collectively, these findings indicate that the ureteric bud (UB) and its derivatives, the collecting ducts, are principal targets for the RAS.
UB outgrowth from the nephric duct is followed by repetitive branching to form the renal collecting system (ureters, pelvis, calyces, and collecting ducts) (1, 14, 19, 43). Concurrently, emerging UB tips induce surrounding mesenchymal cells to undergo mesenchymal-to-epithelial transition (MET) and form proximal epithelial elements of the nephrons (from the glomerulus to the distal tubule). Even subtle decreases in the efficiency of UB branching result in a profound decrease in nephron endowment (42). Together, aberrant UB branching and nephrogenesis cause renal hypodysplasia and lead to eventual progression to chronic renal failure (6, 28).
We have recently reported that ANG II induces the expression of glial-derived neurotrophic factor (GDNF)/Ret/Wnt11 pathway genes, promotes cell proliferation preferentially in UB tip cells and stimulates UB branching (52). Here, we demonstrate an essential role for ANG II AT2R in UB morphogenesis. AT2R is expressed in the UB epithelia during metanephric development. Pharmacological antagonism of the AT2R during early stages of UB development or genetic inactivation of the AT2R in mice result in impaired UB branching. Finally, inhibition of AT2R signaling downregulates GDNF/Ret/Wnt11 pathway gene expression, decreases proliferation, and induces apoptosis of the UB cells.
MATERIALS AND METHODS
Immunohistochemistry for AT2R.
Immunolocalization of AT2R protein was performed in C57BL/6J mouse embryos (E) from day E11.5 to E16 (n = 4/age group; Hybrid-Ready Tissue, Novagen). Immunostaining was performed by the immunoperoxidase technique using the Vectastain Elite kit (Vector Laboratories, Burlingame, CA). A polyclonal rabbit AT2R antibody directed against the NH2-terminal domain of the AT2R (N-19, sc-7421, Santa Cruz) was utilized at concentrations of 1/100 to 1/400. Specificity of immunostaining was documented by the elimination of immunoreactivity after preabsorption of the primary antibody with its immunogen or omission of the primary antibody.
Western blot analysis.
Mouse kidneys and UB cells (generously provided by Dr. Jonathan Barash, Columbia University) were homogenized in cold lysis buffer containing a cocktail of enzyme inhibitors. The samples were centrifuged, and the supernatants containing proteins (20 μg/lane) were resolved on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. After blocking nonspecific binding, the membranes were incubated with the AT2R antibody (1:500, N-19, Santa Cruz Biotechnology) at room temperature for 1 h. After washes in PBS/Tween, the nitrocellulose membrane was exposed for 1 h at room temperature to the secondary antibody. Immunoreactive bands were visualized using the enhanced chemiluminescence detection system (ECL, Amersham) as previously described (25).
RT-PCR.
Semiquantitative RT-PCR was utilized to determine whether cultured UB cells and mesenchymal (MK4) cells express AT2R mRNA. The MK4 cell line represents embryonic metanephric mesenchyme undergoing epithelial conversion. MK4 cells are epithelial in shape and express genes typical of late mesenchyme, including paired box 2 (Pax-2), Pax-8, Wnt-4, and cadherin-6 (51). UB, MK4 cells, and total kidney RNA was extracted using the TRIzol reagent (Invitrogen). RNA (3 μg) was reverse-transcribed. cDNA was amplified using the PerkinElmer Gene Amplification system 2400 (Cetus Instruments, Norwalk, CT) from 25% of the RT mixture using gene-specific primers: sense 5′-ATTCCTGTTCTCTACTAC-3′; antisense 5′-GTAACACGTTGCTATGAA-3′.
In vitro tubulogenesis assay.
The in vitro tubulogenesis assay was performed as previously described (17). UB cells were initially obtained from microdissected UB of an E11.5 mouse transgenic for simian virus 40 (SV40) large T antigen (Immorto-mouse, Charles River Laboratories, New York, NY) (2). UB cells were used at passage numbers 10–15. The cells were trypsinized, and 150 × 103 cells/well were suspended in type I rat-tail collagen (Upstate Biotechnology), 10 × DMEM, 200 mM HEPES (pH 8.0) in an 8:1:1 ratio at 150 × 103 cells/ml. After gelation, DMEM/F12 medium with 0.5% FBS or medium with 0.5% FBS containing ANG II (10−5 M, Sigma, St. Louis, MO) alone or ANG II and AT2R antagonist PD123319 (10−6 M, Sigma) was added to each well on top of the collagen gel. After a 48-h incubation period at 37°C and 5% CO2, 40 individual cells/well were scored for the presence of cell processes directly from the plates utilizing an Olympus IX70 inverted phase-contrast microscope, and the average number of processes per cell was calculated. Each condition was set up in triplicate (n = 4 separate experiments). Images were acquired directly from the plates via an Olympus MagnaFire FW camera and processed with Adobe PhotoShop 7.0.
Metanephric organ culture.
Wild-type CD1 mice embryos (Charles River) were dissected aseptically from the surrounding tissues on E12.5. Embryos from AT2R mutant mice were dissected on E14.5. The day when the vaginal plug was observed was considered to be E0.5. CD1 mice metanephroi were grown on an air-fluid interface on polycarbonate Transwell filters (0.5 μm, Corning Costar) inserted into six-well plates containing DMEM/F12 medium (GIBCO BRL) alone, in the presence of ANG II AT2R antagonist PD123319 (10−6 M, Sigma) or AT2R agonist CGP42112 (10−9 M, Sigma) for 48 h at 37°C and 5% CO2 as previously described (51) and then processed for the whole mount immunohistochemistry or in situ hybridization. AT2R mutant mice in C57/BL6 background, originally generated by Dr. Tadashi Inagami (Vanderbilt University, Nashville, TN) (24), were obtained from Dr. Alvin Schmaier (Case Western Reserve University). AT2R mutant metanephroi were immediately processed for the whole mount immunofluorescence using an anti-cytokeratin antibody (Sigma). The effect of drug treatment was studied in paired kidneys obtained from the same fetus (i.e., the left kidney was incubated with media and the right kidney with PD123319, or the left kidney with media and the right kidney with CGP42112).
In situ hybridization.
AT2R mRNA expression and the effect of PD123319 (10−6 M) on the GDNF/c-Ret/Wnt11 pathway and Sprouty (Spry) 1 gene expression was examined by whole mount in situ hybridization. Mouse full-length AT2R cDNA was from Open Biosystems (Rockford, IL). c-Ret, GDNF, Wnt11, and Spry1 cDNAs were kind gifts from Drs. F. Costantini, A. McMahon, and J. Licht. Preparation of RNA probes and whole mount in situ hybridization were performed according to protocols (http://www.hhmi.ucla.edu/derobertis) established in the De Robertis laboratory. Five embryonic kidneys per treatment group per probe were examined. All experiments were done at least twice. The metanephroi were photographed using an Olympus model SC35 camera mounted on an Olympus model BH-2 microscope, and digital images were captured using Adobe Photoshop software.
Quantitative real-time RT-PCR.
Quantitative real-time RT-PCR was utilized to determine whether AT2R antagonist PD123319 alters GDNF, c-Ret, Wnt11, and Spry1 mRNA expression in the whole metanephroi grown ex vivo. E12.5 CD1 mice metanephroi were grown on an air-fluid interface in the presence of media (control) or AT2R antagonist PD123319 (10−6 M, Sigma) for 24 h at 37°C and 5% CO2. In addition, we examined the effect of genetic inactivation of AT2R in mice on GDNF, c-Ret, Wnt11, Spry1, and bone morphogenetic protein 4 (BMP4) mRNA levels in the kidney on E13.5. SYBR Green quantitative real-time RT-PCR was conducted using Mx3000P equipment (Stratagene, La Jolla, CA) using MxPro QPCR software (Stratagene) as described previously (16). The quantity of each target mRNA expression was normalized by that of GAPDH mRNA expression. Three RNA samples per treatment group were analyzed in triplicate in each run. PCR was performed three times.
Cell proliferation and apoptosis assays.
To investigate the role of endogenous ANG II and AT2R in UB cell proliferation and survival, E12.5 CD1 mice metanephroi were grown on filters in the presence of DMEM/F12 medium alone (n = 10) or with AT2R antagonist PD123319 (10−6 M, n = 10) for 24 h at 37°C. In addition, cell proliferation and apoptosis were examined in E13.5 metanephroi from AT2R+/+ and AT2R−/− mice (n = 4/genotype). The kidneys were fixed in 10% neutral buffered formalin overnight at 4°C, processed for paraffin embedding, and 4-μm-thick sections were cut. Cell proliferation was examined by incubating the sections in PBS+3% BSA with anti-phospho-histone H3 (pH3) antibody (1:50, Cell Signaling, Danvers, MA) overnight at 4°C. Following washings in PBS/Tween and blocking with IgG, the sections were incubated with anti-cytokeratin antibody (1:200, Sigma). For the negative controls, the primary antibody was replaced with a PBS/3% BSA solution. After the final wash in PBS, 4′-6-diamidino-2-phenylindole (DAPI; Invitrogen, Eugene, OR) was added to the mounting media to mark the nuclei. The number of pH3-positive (red) and cytokeratin-positive (green) cells was determined in a blinded fashion in four randomly selected UBs of each kidney section by fluorescent microscopy. A cell proliferation index (percentage of pH3-positive cells) was calculated from the ratio of pH3-positive to total nuclei. Apoptosis was assessed by terminal uridine triphosphate (UTP) nick-end labeling (TUNEL; TACS TdT Kit, R&D Systems, Minneapolis, MN) as previously described (52) or with an anti-cleaved caspase 3 antibody (Cell Signaling). The number of TUNEL-positive cells per UB tip or stalk was determined in each kidney section (n = 10 kidneys/group; 3 sections/kidney), and the mean number of TUNEL-positive cells per UB tip or stalk was calculated. In addition, the number of pH3- and caspase 3-positive cells per UB or mesenchyme was determined in three sections/kidney in AT2R+/+ and AT2R−/− mice (n = 4 kidneys/genotype). All experiments involving mice were approved by Tulane Institutional Animal Care and Use Committee.
Statistical analysis.
Data are presented as means ± SE. Differences among the treatment groups in mRNA levels, the number of UB tips, and the number of TUNEL-positive cells in media vs. PD123319 were analyzed by Student's t-test. Differences among the treatment groups for cell proliferation index were analyzed by the Mann-Whitney rank sum test. A P value of <0.05 was considered statistically significant.
RESULTS
Expression of the AT2R protein in developing metanephros in vivo.
We previously demonstrated that AT1R protein is expressed in the UB as early as E12 of murine development (26). In this study, we examined the expression of the AT2R protein in mouse kidneys on E11.5–E16. On E11.5 (T-stage UB), AT2R immunostaining was localized on both luminal and basolateral aspects of the UB followed by the induced mesenchyme (Fig. 1). On E12, distinct but modest AT2R immunostaining was present in the medullary stroma, vesicles, and UB tips (Fig. 1). On E13, strong expression was observed in UB branches and tips, followed by the stroma and maturing glomeruli, which expressed AT2R in the tuft but not in podocytes. On E14, AT2R remained prominent on both luminal and basolateral membranes of UB branches followed by nephron progenitors and medullary stroma, whereas the cortical stroma was mostly AT2R negative (Fig. 2). On E16, AT2 was expressed diffusely in inner cortical tubules, which resembled morphologically proximal tubules (Fig. 2).
Fig. 1.
Immunolocalization of ANG II A2 receptor (AT2R) protein in the fetal kidney on embryonic (E) day E11.5 and E12. A and B: on E11.5, AT2R is detectable using antibody concentrations of 1/100. In a T-stage ureteric bud (UB), AT2R immunostaining (brown) is present in both luminal and basolateral aspects of the UB followed by the induced mesenchyme (arrows). C: on E12, AT2R is detectable using antibody concentrations of 1/200. In the E12 metanephros, distinct but modest staining is present in the stromal mesenchyme, vesicles, and UB tips. D: the adrenal medulla exhibits the strongest immunostaining. E and F: control consecutive sections demonstrate marked attenuation in AT2R immunostaining after preadsorbtion of the primary antibody with its immunogen. Ad, adrenal gland.
Fig. 2.
Immunolocalization of AT2R protein in the fetal kidney on E14 and E16. A and B: AT2R is detectable using antibody concentrations of 1/400. On E14, AT2R immunoreactivity is present in both luminal and basolateral membranes of UB branches followed by nephron progenitors and medullary stroma. C: on E16, AT2R is expressed diffusely in inner cortical tubules, which resemble morphologically proximal tubules, followed by glomeruli and medullary stroma. D: control section where the addition of the primary antibody was omitted demonstrates no staining. UB, ureteric bud; G, glomerulus; S, stroma; T, tubules.
Effect of ANG II and AT2R antagonist PD123319 on UB cell morphogenesis in vitro.
UB cells cultured in gels form processes and branching tubular structures when exposed to various growth factors, providing a convenient experimental system for analyzing mechanisms of epithelial branching morphogenesis (25). A principal advantage of this approach is the ability to examine the direct effect of a specific factor on UB cell growth that is independent from confounding soluble factors released by the mesenchyme. Importantly, cultured UB cells maintain expression of AT2R mRNA and protein (Fig. 3, A and B). ANG II increased the number of UB cells with processes per well compared with control cells cultured in the presence of DMEM/F12 media with 0.5% FBS alone (16.9 ± 0.9 vs. 9.1 ± 2.6, P < 0.05; n = 4 experiments). The effect of ANG II on UB cell branching was abrogated by pretreatment with the AT2R antagonist PD123319 (10−6 M, 6.9 ± 1.6 vs. 9.1 ± 2.6, P < 0.01; n = 4 experiments) (Fig. 3, C–G). The results demonstrate that AT2R is expressed during UB cell morphogenesis and is capable of stimulating branching of UB cells in vitro. This observation is consistent with the ability of AT2R to induce neurite outgrowth and elongation (17).
Fig. 3.
Effect of ANG II and PD123319 on UB cell branching. Top: RT-PCR and Western blot analysis of AT2R. A: ethidium bromide-stained gel showing RT-PCR analysis of AT2R mRNA expression. B: Western blot (0.02 mg total protein/lane) showing AT2R protein expression. KRNK cells (positive control); P1, postnatal day 1 mouse kidney. C–F: phase-contrast micrographs of UB cells grown for 48 h in collagen gels in the presence of ANG II (10−5 M) alone or combined with PD123319 (10−6 M). G: bar graph shows the effect of treatments on the number of UB cells with processes after 48 h of culture.
Effect of AT2R antagonist PD123319 or genetic inactivation of AT2R in mice on UB morphogenesis in whole metanephric kidney.
To examine the role of endogenous ANG II and its AT2R in UB branching in the intact metanephros, we utilized the AT2R antagonist PD123319. Treatment of E12.5 metanephroi grown ex vivo with PD123319 (10−6 M) decreased the number of UB tips compared with control after 48 h (34 ± 1.0 vs. 43 ± 0.8, P < 0.01; n = 5/treatment group) (Fig. 4, A, B, and E). In contrast, treatment of metanephroi with the AT2R agonist CGP42112 (10−9 M) increased the number of UB tips compared with control (48 ± 1.8 vs. 39 ± 12.3, P < 0.05; n = 5/treatment group) (Fig. 4, C–E). Importantly, at 10−9 M concentration, CGP41221 acts as a selective agonist of the AT2R (5). These findings are consistent with the results obtained in UB cells demonstrating that ANG II-induced effects on UB cell branching are mediated in part via AT2R. Moreover, these findings indicate that the inhibitory effect of PD123319 on branching of UB cells cultured in three-dimensional gels is a physiologically relevant event since it can be recapitulated in the intact organ culture.
Fig. 4.
Effect of media, PD123319, CGP42112, or genetic inactivation of the AT2R on UB branching in mouse metanephroi. A and B: after 48 h in culture, kidney explants were costained with anti-pancytokeratin antibody to label the UB (red) and Lotus tetragonolobus agglutinin (LTA) to label proximal tubule epithelia (green). C and D: UB is visualized with anti-pancytokeratin antibody (green). The number of UB tips was compared between the treatment groups. A–D: representative images of branching UBs in paired metanephroi. A: left kidney, media; B: right kidney, PD123319 (PD). C: left kidney, media. D: right kidney, CGP42112 (10−9 M; CGP). E: bar graph showing the effect of media, PD123319, or CGP42112 on the number of UB tips. F–H: effect of genetic inactivation of the AT2R on UB branching on E13.5. Metanephroi were costained with anti-pancytokeratin antibody to label the UB (green) and anti-WT1 antibody to label the mesenchyme and podocytes (red). F: AT2R+/+ kidney. G: AT2R−/− kidney. H: bar graph showing the effect of AT2R genotype on the number of UB tips. Numbers on A–D and F and G indicate the number of UB tips.
To address the concerns of the specificity of the AT2R antagonist in UB development, we examined UB branching in AT2R null mice on E13.5. The number of UB tips was decreased in metanephroi from AT2R−/− compared with AT2R+/+ littermates (36 ± 1.8 vs. 48 ± 1, P < 0.01; n = 6/group) (Fig. 4, F–H). In summary, our results indicate that AT2R signaling stimulates early UB branching and are consistent with previous reports which implicate AT2R in renal branching morphogenesis (8).
AT2R gene is expressed in mesenchyme and UB in vivo.
To determine the spatial expression of the AT2R gene, we mapped localization of AT2R mRNA in the metanephros by in situ hybridization on E11.5–E13.5. On E11.5, AT2R mRNA is expressed predominantly in the ureter and mesenchyme adjacent to the UB tips (Fig. 5A). On E12.5, AT2R mRNA is expressed throughout branching UB and mesenchyme (Fig. 5C). On E13.5, AT2R mRNA is present in the UB, mesenchyme, and maturing glomeruli (Fig. 5E). These data are in concert with our findings demonstrating that cultured UB and MK4 cells express AT2R mRNA (Fig. 3A). Localization of AT2R expression in the kidney mesenchyme is consistent with earlier studies in rodents using in situ hybridization (26). Given that AT2R null mice demonstrate a decreased rate of apoptosis of mesenchymal cells around the UB (35), it is conceivable that an absence of timely apoptosis of mesenchymal cells in AT2R-deficient mice may hinder mesenchyme-UB cross talk to affect ureteric branching. This possibility is supported by the findings that activation of AT2R induces Pax-2 gene expression in renal mesenchymal cells (54). Together, it is conceivable that both UB and mesenchymal AT2R stimulate UB branching.
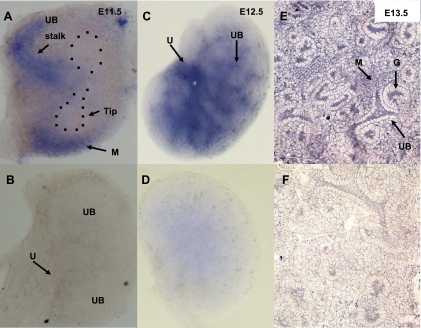
Fig. 5.
AT2R mRNA expression in the mouse metanephros on E11.5–E13.5 as determined by in situ hybridization (ISH). A–D: whole mount ISH. E and F: ISH on kidney sections. A: on E11.5, AT2R mRNA is expressed predominantly in the UB stalk and mesenchyme (M) adjacent to the T-stage UB tip (blue staining). AT2R mRNA is not present in the UB tips (outlined by dotted lines). C: on E12.5, AT2R mRNA is expressed in the ureter, UB branches, and mesenchyme. E: on E13.5, AT2R mRNA is present in the UB, mesenchyme, and maturing glomeruli (G). B, D, and F: control kidneys hybridized with the sense AT2R probe show remarkably attenuated staining.
Effect of AT2R antagonist PD123319 or genetic inactivation of AT2R in mice on GDNF, c-Ret, Wnt11, Spry1, and BMP4 gene expression in metanephric kidney.
The GDNF/c-Ret/Wnt11 signaling pathway is a major positive regulator of the UB branching morphogenesis program (10, 33, 44). In a previous study, we demonstrated that ANG II upregulates GDNF/c-Ret/Wnt11 gene expression in embryonic metanephric kidneys grown ex vivo (52). In the present study, we examined the impact of endogenous ANG II and its AT2R on GDNF, c-Ret, Wnt11, and Spry1 gene expression during early stages of UB morphogenesis. Whole mount in situ hybridization showed a decrease in Spry1, GDNF, c-Ret, and Wnt11 gene expression in metanephroi treated with PD123319 (10−6 M) for 24 h (Fig. 6A). To allow a more quantitative analysis of changes in GDNF, c-Ret, Wnt11, and Spry1 gene expression, we examined the effect of PD123319 on GDNF, c-Ret, Wnt11, and Spry1 mRNA levels in cultured metanephroi by quantitative real-time RT-PCR. Treatment with PD123319 resulted in a significant decrease in GDNF (0.59 ± 0.06 vs. 1.0 ± 0, P < 0.01), c-Ret (0.57 ± 0.03 vs. 1.0 ± 0, P < 0.01), Wnt11 (0.62 ± 0.11 vs. 1.0 ± 0, P < 0.05), and Spry1 (0.52 ± 0.04 vs. 1.0 ± 0, P < 0.01) mRNA levels compared with control (Fig. 6B). In accordance with these findings, GDNF (0.73 ± 0.05 vs. 1.0 ± 0, P < 0.01), c-Ret (0.76 ± 0.05 vs. 1.0 ± 0, P < 0.01), Wnt11 (0.53 ± 0.05 vs. 1.0 ± 0, P < 0.01), and Spry1 (0.74 ± 0.06 vs. 1.0 ± 0, P < 0.01) mRNA levels were lower in E13.5 metanephroi of AT2R−/− compared with AT2R+/+ mice (Fig. 6B). Therefore, the stimulatory effects of endogenous ANG II on GDNF/c-Ret/Wnt11 gene expression are mediated, in part, via the AT2R. Since genetic deficiency or pharmacological antagonism of AT2R decreases UB branching, the AT2R-mediated effect on GDNF/c-Ret/Wnt11 is physiologically important. An observed decrease in Spry1 mRNA levels in PD123319-treated and AT2R−/− metanephroi suggests that AT2R-dependent stimulation of GDNF/c-Ret/Wnt11 gene expression cannot be attributed to downregulation of Spry1, an antagonist of c-Ret signaling.
Fig. 6.
AT2R blockade downregulates Spry1, glial-derived neurotrophic factor (GDNF), c-Ret, and Wnt11 mRNA expression in E12.5 mouse metanephroi that were grown ex vivo for 24 h. After 24 h in culture, kidney explants were processed for whole mount in situ hybridization or total RNA was extracted and Spry1, GDNF, c-Ret, and Wnt11 mRNA levels were analyzed by real-time quantitative RT-PCR. A: representative images demonstrate that treatment with PD123319 decreases the expression of c-Ret and Wnt11 in the UB tips, of GDNF in the mesenchyme, and of Spry1 in the UB tips and mesenchyme. Note that GDNF staining in the UB is nonspecific. B: bar graph showing that treatment with PD123319 decreases Spry1, GDNF, c-Ret, and Wnt11 mRNA levels as determined by RT-PCR.
To test the hypothesis that a decrease in UB branching observed in AT2R−/− mice is mediated by BMP4, a known inhibitor of UB branching (35), we examined the effect of genetic inactivation of AT2R in mice on BMP4 gene expression in E13.5 metanephroi by real-time qPCR. BMP4 mRNA levels were higher in AT2R−/− compared with AT2R+/+ mice (1.26 ± 0.05 vs. 1.0 ± 0.0, P < 0.01). These findings indicate that the stimulatory effects of endogenous AT2R on UB branching are mediated, in part, via downregulation of BMP4. Alternatively or additionally, since AT2R is expressed in the mesenchyme and GDNF mRNA levels are lower in AT2R−/− metanephroi, the observed decrease in UB branching may be secondary to a direct effect on GDNF.
Effect of AT2R antagonist PD123319 or genetic inactivation of AT2R in mice on cell proliferation in embryonic kidney.
To investigate the cellular mechanisms leading to stimulation of UB branching by endogenous ANG II and its AT2R, we examined the effect of AT2R antagonism or genetic inactivation of the AT2R on proliferation of the UB epithelium, utilizing an antibody to pH3. Treatment of intact metanephroi with PD123319 (10−6 M) for 48 h in vitro decreased cell proliferation index in the UB tip (5.5 ± 0.7 vs. 20.2 ± 2.9; P < 0.01) and stalk (5.9 ± 1.9 vs. 15.3 ± 2.8; P < 0.05) cells compared with control (Fig. 7, F–J). The number of proliferating cells was lower in the UB of AT2R−/− than AT2R+/+ mice (49 ± 8 vs. 86 ± 8; P < 0.05) (Fig. 8, F–J). In contrast, the number of pH3-positive cells in the mesenchyme did not differ (374 ± 41 vs. 383 ± 75; P = 0.9) (Fig. 8, F–J). These results demonstrate a direct stimulatory effect of ANG II AT2R on UB cell proliferation.
Fig. 7.
Effect of AT2R antagonist PD123319 on UB cell apoptosis and proliferation. A–D: apoptotic cells were identified by terminal uridine triphosphate (UTP) nick-end labeling (TUNEL; brown staining). A: media. B and C: PD123319 (10−6 M). D: kidney treated with TACS-nuclease to generate DNA breaks in every cell (positive control). E: bar graph shows the effect of media or PD123319 on the number of TUNEL-positive cells in the UB tip and stalk cells. F–I: proliferating cells are identified by anti-phospho-histone H3 (pH3) antibody (red staining). UB epithelia are visualized with anti-cytokeratin antibody (green). F and G: media. H and I: PD123319 (10−6 M). PD123319-treated metanephroi have less pH3 staining in UB (arrows) compared with control (media; marked by rectangles in G and I). J: bar graph shows the effect of media or PD123319 on cell proliferation index in the UB tip and stalk cells.
Fig. 8.
Effect of AT2R genotype on cell apoptosis and proliferation in E13.5 mouse metanephroi. A–D: apoptotic cells are identified by anti-caspase 3 antibody staining (green). B and D: merged images show cell nuclei visualized with 4′-6-diamidino-2-phenylindole (DAPI; blue) and UBs visualized with anti-cytokeratin antibody (red). E: bar graph shows the effect of AT2R genotype on the number of caspase 3-positive cells in the UB and mesenchyme. F–I: proliferating cells are identified by anti-pH3 antibody (green). Merged images show cell nuclei visualized with DAPI (blue) and UBs visualized with anti-cytokeratin antibody (red). J: bar graph shows the effect of AT2R genotype on the number of pH3-positive cells in the UB and mesenchyme. Magnification ×20.
AT2R antagonism promotes apoptosis of UB cells.
Aberrant apoptosis is a cardinal feature of renal dysplasia and hypoplasia (49). In this regard, attenuated apoptosis of mesenchymal cells during fetal metanephrogenesis has been reported in AT2R-deficient mice (35). In this study, we examined the effect of AT2R antagonism or genetic inactivation of the AT2R on cell apoptosis in intact metanephroi. Treatment of E12.5 metanephroi with PD123319 (10−6 M) for 24 h significantly increased the number of TUNEL-positive cells in the UB tips and stalks (tips: 0.76 ± 0.2 vs. 0.19 ± 0.1, P < 0.05; stalks: 0.88 ± 0.2 vs. 2.1 ± 0.4; P < 0.01) (Fig. 7, A–E). Interestingly, apoptotic cells were also detected in the nephrogenic zone and stroma (Fig. 7, A–C). Given that AT2R protein is expressed in stromal cells (Fig. 2, A and C), it is conceivable that stromal AT2R signaling is important in nephrogenesis and UB morphogenesis. The number of caspase 3-positive cells was increased in the UB of AT2R−/− compared with AT2R+/+ mice (7.8 ± 0.9 vs. 1.5 ± 0.5; P < 0.05) (Fig. 8, A–E). In contrast, the number of caspase 3-positive cells in the mesenchyme did not differ (50 ± 13 vs. 63 ± 14; P = 0.5) (Fig. 8, A–E). These findings indicate an inhibitory role of endogenous ANG II and its AT2R on apoptosis of UB cells, suggesting a role for the AT2R in epithelial cell survival during UB branching.
DISCUSSION
The present study demonstrates that ANG II AT2R is expressed in the UB epithelia, metanephric mesenchyme, and stroma and plays an essential role during early stages of UB morphogenesis. Inhibition of AT2R signaling impairs UB branching, downregulates GDNF, c-Ret, Wnt11, and Spry1 gene expression, decreases proliferation, and induces apoptosis of the UB cells.
The ANG II AT2R is a developmentally regulated G protein-coupled receptor that is highly expressed in the fetal kidney (16, 24, 26, 35). AT2R mRNA is present in undifferentiated mesenchyme at the time of UB outgrowth from the nephric duct and is detected in the UB on E13.5 in the mouse (35). Temporally, AT2R expression peaks during early fetal metanephrogenesis and rapidly declines postnatally (16). The fetal kidney expresses all the other components of the classic RAS. Angiotensinogen (AGT) and AT1R are expressed in both the UB and adjacent stromal mesenchyme as early as on E12 in the mouse (25). Renin-expressing cells are present in the stroma on E12 (29). ACE is detected in the embryonic kidney in rodents and humans (45). Collectively, the fetal metanephros has the capacity to both generate ANG II and to transmit its actions. It is intriguing that ANG II levels are higher in the fetal and newborn than adult kidney (31, 50). To this end, high ANG II levels in the embryonic kidney, coupled with upregulated AT2R expression, suggest an important role for the AT2R in metanephric development during fetal life.
Studies conducted in humans demonstrate that AT2R mutations may be linked to congenital abnormalities of the kidney and urinary tract (CAKUT). In this regard, a single polymorphism in the AT2R, the A1332G transition, is associated with ureteropelvic junction stenosis, vesicoureteral reflux, megaureter, hypoplasic, and multicystic dysplastic kidneys (20, 35, 41). Deletion of the AT2R gene in mice causes a duplicated collecting system and hydronephrosis (37). These forms of CAKUT are observed in 3.1% of AT2R null newborns. Intriguingly, aberrant UB budding is seen in up to 60% of AT2R-deficient embryos on E11, suggesting that the majority of these malformations undergo self-correction in utero. It is conceivable that initial redundant UB outgrowth in these mice is subsequently suppressed by inhibitors of UB branching such as Spry1, BMP2, or BMP4 (7, 32). Interestingly, AT2R is expressed between the nephric duct and the mesenchyme just cranial to the normal UB branching site in wild-type embryos on E11 (26). AT2R null mice demonstrate a decreased rate of apoptosis of mesenchymal cells around the developing UB (35). AT2R may mark mesenchymal cells destined to undergo apoptosis. Hence, the absence of timely apoptosis of mesenchymal cells in AT2R null mice may create a physical barrier which separates UB from the mesenchyme and hinders reciprocal interactions of genes required for proper ureteric branching.
One mechanism by which aberrant AT2R function may disrupt UB-mesenchyme cross talk is inhibition of the interactions between GDNF secreted by metanephric mesenchymal cells and its tyrosine kinase receptor c-Ret expressed in the nephric duct and subsequently in the UB tip cells (38). The GDNF/c-Ret/Wnt11 signaling pathway is a major positive regulator of UB branching in the metanephros (3). Like c-Ret, Wnt11 is expressed in the UB tip cells and interacts genetically with GDNF/c-Ret to induce UB branching (3). Recent work from our laboratory demonstrated that ANG II-induced UB branching is accompanied by activation of the GDNF/c-Ret/Wnt11 pathway (52). Our present findings that AT2R deficiency or antagonism downregulates GDNF, c-Ret, and Wnt11 expression indicate that activation of this pathway by AT2R is critical in ANG II-induced signaling to stimulate UB morphogenesis. Interestingly, ANG II, acting via AT2R, induces the expression of Pax-2, a transcription factor present in both the UB and metanephric mesenchyme (12). Genetic inactivation of Pax-2, GDNF, c-Ret, or Wnt11 in mice causes significant impairment of UB morphogenesis (12, 33, 44). Given that GDNF or c-Ret expression is activated by Pax-2 (9, 33), it is conceivable that a decrease in GDNF/c-Ret/Wnt11 gene expression observed in the present study is due to inhibition of Pax-2-stimulating effects on GDNF and c-Ret. Thus AT2R signaling controls a hierarchy of gene expression critical for UB morphogenesis. Colocalization of AGT, renin, AT2R, and wing helix transcription factor Foxd1 expression in the stroma (21, 24, 25, 27) suggests potential interactions. In this regard, genetic inactivation of the Foxd1 gene in mice reduces UB branching and alters c-Ret expression (21, 27). Furthermore, AGT and renin promoters contain a putative binding site for Foxd1 (53). Whether Foxd1 acts upstream of the RAS to regulate UB branching remains to be determined.
An important finding of the present study is that AT2R protein is expressed on both luminal and basolateral aspects of the UB branches and in adjacent stromal mesenchyme. Since AGT and renin are expressed in the stroma, it is conceivable that ANG II can be generated in the stroma and act in a paracrine fashion on the adjacent AT2R-expressing UBs to stimulate branching. This model is supported by our present findings that AT2R antagonism abrogates ANG II-induced branching in UB cells grown in the absence of the mesenchyme. In addition, AT2R present in the stroma may be important in mediating stromal ANG II signaling to stimulate UB branching.
The balance of cell proliferation and apoptosis plays a critical role in UB branching and nephron endowment (13, 30, 39). The present study demonstrates that inhibition of endogenous AT2R signaling induces apoptosis and inhibits proliferation in UB tip and stalk cells. We speculate that AT2R plays an important role in the expansion of the ampulla, subsequent branching, and directional bud elongation. Given that BMP2 and BMP4 inhibit UB branching (32, 40), decreased UB branching in AT2R null mice may be mediated, in part, by enhanced BMP2/BMP4 signaling. Our present findings of increased BMP4 gene expression in AT2R null metanephroi suggest that the stimulatory effects of endogenous AT2R on UB branching are mediated, in part, via downregulation of BMP4. Notably, AT2R-deficient mice have smaller mean glomerular tuft volume compared with wild-type mice (8). Whether AT2R deficiency results in decreased nephron endowment remains to be determined. AT2R-dependent apoptosis may result from activation of MAP kinase phosphatase 1 or Src homology 2 domain-containing protein-tyrosine phosphatase 1, leading to inactivation of ERK1/2 (11, 22). AT2R-dependent activation of Pax-2 in whole metanephroi and mesenchymal cells is mediated by the JAK2/STAT pathway (54).
In summary, the present study demonstrates that ANG II AT2R is expressed in the UB and mesenchyme during metanephric development. Aberrant AT2R signaling downregulates GDNF, c-Ret, and Wnt11 gene expression, decreases proliferation and induces apoptosis of the UB cells, and impairs UB branching. These results support the hypothesis that abnormal collecting system development in AT2R-deficient mice is at least partly due to dysregulation of the UB branching morphogenesis program as well as aberrant UB cell proliferation and apoptosis.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants P20 RR17659 and DK-71699 (I. Yosypiv). S. El-Dahr is supported by NIH Grants DK-56264 and DK-62250.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Mercedes Schroeder (Tulane University) for assistance with the in vitro tubulogenesis assay, Drs. Frank Costantini (Columbia University Medical Center), Andrew P. McMahon (Harvard University), and Jonathan D. Licht (Northwestern University) for providing the probes for in situ hybridization, and Dr. Alvin H. Schmaier (Case Western Reserve University) for providing AT2R mutant mice originally generated by Dr. Tadashi Inagami (Vanderbilt University, Nashville, TN).
REFERENCES
- 1.Al-Awqati Q, Goldberg MR. Architectural patterns in branching morphogenesis in the kidney. Kidney Int 54: 832–1842, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Barasch J, Pressler L, Connor J, Malik A. A ureteric bud cell line induces nephrogenesis in two steps by distinct signals. Am J Physiol Renal Fluid Electrolyte Physiol 271: F50–F61, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Basson MA, Watson-Johnson J, Shakya R, Akbulut S, Hyink D, Costantini FD, Wilson PD, Mason IJ, Licht JD. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol 299: 466–77, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG. Angiotensin receptors: signaling, vascular pathophysiology, and interactions with ceramide. Am J Physiol Heart Circ Physiol 281: H2337–H2365, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Brechler V, Jones PW, Levens NR, de Gasparo M, Bottari SP. Agonistic and antagonistic properties of angiotensin analogs at the AT2 receptor in PC12W cells. Regul Pept 44: 207–213, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1: 335–347, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128: 4747–4756, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Chen YW, Tran S, Chenier I, Chan JS, Ingelfinger JR, Inagami T, Zhang SL. Deficiency of intrarenal angiotensin II type 2 receptor impairs paired homeo box-2 and N-myc expression during nephrogenesis. Pediatr Nephrol 23: 1769–1777, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Clarke JC, Patel SR, Raymond RM, Jr, Andrew S, Robinson BG, Dressler GR, Brophy PD. Regulation of c-Ret in the developing kidney is responsive to Pax2 gene dosage. Hum Mol Genet 15: 3420–3428, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Costantini F, Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays 28: 117–127, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Cui T, Nakagami H, Iwai M, Takeda Y, Shiuchi T, Daviet L, Nahmias C, Horiuchi M. Pivotal role of tyrosine phosphatase SHP-1 in AT2 receptor-mediated apoptosis in rat fetal vascular smooth muscle cell. Cardiovasc Res 49: 863–871, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Dressler GR, Wilkinson JE, Rothenpieler UW, Patterson LT, Williams-Simons L, Westphal H. Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature 362: 65–67, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Dziarmaga A, Eccles M, Goodyer P. Suppression of ureteric bud apoptosis rescues nephron endowment and adult renal function in Pax2 mutant mice. J Am Soc Nephrol 17: 1568–1575, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Ekblom P. Developmentally regulated conversion of mesenchyme to epithelium. FASEB J 3: 2141–2150, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Esther CR, Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest 7: 953–965, 1996 [PubMed] [Google Scholar]
- 16.Garcia-Villalba P, Denkers ND, Wittwer CT, Wittwer CT, Hoff C, Nelson RD, Mauch TJ. Real-time PCR quantification of AT1 and AT2 angiotensin receptor mRNA expression in the developing rat kidney. Nephron Exp Nephrol 94: e154–159, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Gendron L, Payet MD, Gallo-Payet N. The angiotensin type 2 receptor of angiotensin II and neuronal differentiation: from observations to mechanisms. J Mol Endocrinol 31: 359–372, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Gribouval O, Gonzales M, Neuhaus T, Aziza J, Bieth E, Laurent N, Bouton JM, Feuillet F, Makni S, Ben Amar H, Laube G, Delezoide AL, Bouvier R, Dijoud F, Ollagnon-Roman E, Roume J, Joubert M, Antignac C, Gubler MC. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet 37: 964–968, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Grobstein C. Inductive epithelio-mesenchymal interaction in cultured organ rudiments of the mouse metanephros. Science 118: 52–55, 1953 [DOI] [PubMed] [Google Scholar]
- 20.Hahn H, Ku SE, Kim KS, Park YS, Yoon CH, Cheong HI. Implication of genetic variations in congenital obstructive nephropathy. Pediatr Nephrol 20: 1541–1544, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hatini A, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes and Dev 10: 1467–1478, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Horiuchi M, Akishita M, Dzau VJ. Molecular and cellular mechanism of angiotensin II-mediated apoptosis. Endocr Res 24: 307–314, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo Niimura AF, Ichikawa I, Hogan BLM, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature 377: 748–750, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Inagami T, Iwai N, Sasaki K, et al. Angiotensin II receptors: cloning and regulation. Arzneimittelforschung 43: 226–228, 1993 [PubMed] [Google Scholar]
- 25.Iosipiv IV, Schroeder M. A role for angiotensin II AT1 receptors in ureteric bud cell branching. Am J Physiol Renal Physiol 285: F199–F207, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kakuchi J, Ichiki T, Kiyama S, Hogan BL, Fogo A, Inagami T, Ichikawa I. Developmental expression of renal angiotensin I.I. receptor genes in the mouse. Kidney Int 47: 140–147, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Levinson RS, Batourina E, Choi C, Vorotichina M, Kitajewski J, Mendelsohn CL. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development 132: 529–539, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lisle SJ, Lewis RM, Petry CJ, Ozanne SE, Hales CN, Forhead AJ. Effect of maternal iron restriction during pregnancy on renal morphology in the adult rat offspring. Br J Nutr 90: 33–39, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281: F345–F356, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Michael L, Davies JA. Pattern and regulation of cell proliferation during murine ureteric bud development. J Anat 204: 241–255, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazaki Y, Tsuchida S, Nishimura H, Pope 4th JC, Harris RC, McKanna JM, Inagami T, Hogan BL, Fogo A, Ichikawa I. Angiotensin induces the urinary peristaltic machinery during the perinatal period. J Clin Invest 102: 1489–1497, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105: 863–873, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature 382: 76–79, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Nagata M, Tanimoto K, Fukamizu A, Kon Y, Sugiyama F, Yagami K, Murakami K, Watanabe T. Nephrogenesis, and renovascular development in angiotensinogen-deficient mice. Lab Invest 75: 745–753, 1996 [PubMed] [Google Scholar]
- 35.Nishimura H, Yerkes E, Hohenfellner K, Miyazaki Y, Ma J, Hunley TE, Yoshida H, Ichiki T, Threadgill D, Phillips JA, 3rd, Hogan BM, Fogo A, Brock JW, 3rd, Inagami T, Ichikawa I. Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell 3: 1–10, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci USA 95: 15496–15501, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oshima K, Miyazaki Y, Brock JW, Adams MC, Ichikawa I, Pope JC., 4th Angiotensin type II receptor expression and ureteral budding. J Urol 166: 1848–1852, 2001 [PubMed] [Google Scholar]
- 38.Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119: 1005–1017, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Pepicelli CV, Kispert A, Rowitch DH, McMahon AP. GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev Biol 192: 193–198, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Piscione TD, Yager TD, Gupta IR, Grinfeld B, Pei Y, Attisano L, Wrana JL, Rosenblum ND. BMP-2 and OP-1 exert direct and opposite effects on renal branching morphogenesis. Am J Physiol Renal Physiol 273: F961–F975, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Rigoli L, Chimenz R, di Bella C, Cavallaro E, Caruso R, Briuglia S, Fede C, Salpietro CD. Angiotensin-converting enzyme and angiotensin type 2 receptor gene genotype distributions in Italian children with congenital uropathies. Pediatr Res 56: 988–993, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Sakurai H, Nigam S. In vitro branching tubulogenesis: implications for developmental and cystic disorders, nephron number, renal repair, and nephron engineering. Kidney Int 54: 14–26, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Saxen L. Organogenesis of the Kidney Cambridge, UK: Cambridge University Press, 1987 [Google Scholar]
- 44.Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367: 380–383, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Schutz S, Le Moullec JM, Corvol P, Gasc JM. Early expression of all the components of the renin-angiotensin system in human development. Am J Pathol 149: 2067–2079, 1996 [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi N, Lopez ML, Cowhig JE, Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive, and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol 16: 125–132, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, Fogo A, Utsunomiya H, Inagami T, Ichikawa I. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest 101: 755–760, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valerius MT, Patterson LT, Witte DP, Potter SS. Microarray analysis of novel cell lines representing two stages of metanephric mesenchyme differentiation. Mech Dev 112: 219–232, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Woolf AS, Price KL, Scambler PJ, Winyard PJ. Evolving concepts in human renal dysplasia. J Am Soc Nephrol 15: 998–1007, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Yosipiv IV, El-Dahr SS. Activation of angiotensin-generating systems in the developing rat kidney. Hypertension 27: 281–286, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Yosypiv IV, Schroeder M, El-Dahr SS. Angiotensin II type 1 receptor-EGF receptor cross-talk regulates ureteric bud branching morphogenesis. J Am Soc Nephrol 17: 1005–1014, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Yosypiv IV, Boh MK, Spera M, El-Dahr SS. Downregulation of Spry-1, an inhibitor of GDNF/Ret, as a mechanism for angiotensin II-induced ureteric bud branching. Kidney Int 74: 1287–1293, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Palmer R, Gao X, Kreidberg J, Gerald W, Hsiao L, Jensen RV, Gullans SR, Haber DA. Transcriptional activation of placental growth factor by the forkhead/winged helix transcription factor Foxd1. Cur Biol 13: 1625–1629, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Zhang SL, Moini B, Ingelfinger JR. Angiotensin II increases Pax-2 expression in fetal kidney cells via the AT2 receptor. J Am Soc Nephrol 15: 1452–1465, 2004 [DOI] [PubMed] [Google Scholar]