Abstract
AVP resistance of the medullary collecting duct (mCD) in postobstructive uropathy (POU) has been attributed to increased production of PGE2. P2Y2 receptor activation causes production of PGE2 by the mCD. We hypothesize that increased P2Y2 receptor expression and/or activity may contribute to the diuresis of POU. Sprague-Dawley rats were subjected to bilateral ureteral obstruction for 24 h followed by release (BUO/R, n = 17) or sham operation (SHM/O, n = 15) and euthanized after 1 wk or 12 days. BUO/R rats developed significant polydipsia, polyuria, urinary concentration defect, and increased urinary PGE2 and decreased aquaporin-2 protein abundance in the inner medulla compared with SHM/O rats. After BUO/R, the relative mRNA expression of P2Y2 and P2Y6 receptors was increased by 2.7- and 4.9-fold, respectively, without significant changes in mRNA expression of P2Y1 or P2Y4 receptor. This was associated with a significant 3.5-fold higher protein abundance of the P2Y2 receptor in BUO/R than SHM/O rats. When freshly isolated mCD fractions were challenged with different types of nucleotides (ATPγS, ADP, UTP, or UDP), BUO/R and SHM/O rats responded to only ATPγS and UTP and released PGE2, consistent with involvement of the P2Y2, but not P2Y6, receptor. ATPγS- or UTP-stimulated increases in PGE2 were much higher in BUO/R (3.20- and 2.28-fold, respectively, vs. vehicle controls) than SHM/O (1.68- and 1.30-fold, respectively, vs. vehicle controls) rats. In addition, there were significant 2.4- and 2.1-fold increases in relative mRNA expression of prostanoid EP1 and EP3 receptors, respectively, in the inner medulla of BUO/R vs. SHM/O rats. Taken together, these data suggest that increased production of PGE2 by the mCD in POU may be due to increased expression and activity of the P2Y2 receptor. Increased mRNA expression of EP1 and EP3 receptors in POU may also help accentuate PGE2-induced signaling in the mCD.
Keywords: collecting duct, P2 receptors, extracellular nucleotides, prostaglandin E2, cyclooxygenases, ureteral obstruction
obstructive uropathy due to benign prostatic hyperplasia or prostatic or gynecological cancers is a common condition. Benign prostatic hyperplasia affects 50% of men between 51 and 60 yr of age (3). Release of obstruction is often associated with natriuresis or diuresis, leading to postobstructive uropathy, which is a form of acquired nephrogenic diabetes insipidus (NDI). Although it is a self-resolving condition, postobstructive uropathy can cause severe diuresis and natriuresis, with accompanying morbidity (3, 18, 39–41). In addition, because of age-related impairment of the thirst mechanism elderly patients even with mild-to-moderate diuresis are at higher risk of dehydration (25, 33).
The medullary collecting duct appears to be the critical nephron segment affected by ureteral/urethral obstruction, since postobstructive diuresis occurs despite reduced delivery of fluid from the more proximal nephron (47). Studies in animal models of diuresis induced by bilateral ureteral obstruction and release (BUO/R) documented decreased protein abundances of major renal aquaporin (AQP) water channels and sodium and urea transporters in the kidney (10, 19, 20, 29, 31). Most of these channels and transporters, which are involved in the urinary concentration mechanism, are regulated by AVP, which suggests a generalized AVP-resistant state during the diuretic phase of postobstructive uropathy. One of the mechanisms of AVP resistance in postobstructive diuresis is increased production of renal PGE2 (8, 9), which is a potent antagonist of AVP action on urinary concentration (11, 36, 44, 45). Other mechanisms, including enhanced PGE2-mediated medullary blood flow, structural and functional tubular damage with decreased AVP-resistant sodium reabsorption, and activation of natriuretic factors (13, 28), have been proposed.
Accordingly, currently used methods aimed at direct inhibition of cyclooxygenases (COX), such as administration of indomethacin or COX-2-specific inhibitors, are effective in the treatment of diuresis of postobstructive uropathy. Studies in animal models of BUO/R also documented that suppression of PGE2 biosynthesis significantly relieves AVP resistance, apparently by preventing downregulation of key renal water and sodium transport proteins (6, 37, 38). However, the approach of direct inhibition of the activities of COX enzymes is often associated with adverse effects in the clinical setting (42). Hence, identification of upstream signals for increased production of PGE2 and/or other methods to overcome AVP resistance in postobstructive uropathy may help us develop novel and safer therapies.
In this context, we previously reported that agonist (ATP/UTP) activation of purinergic P2Y2 receptor in the rat medullary collecting duct induces production and release of PGE2 (51). Subsequently, we documented that purinergic receptor-stimulated PGE2 production in medullary collecting ducts is markedly enhanced in sucrose-water-induced polyuria and is blunted in the dehydrated condition (48). Recently, using mice genetically lacking the P2Y2 receptor, we documented that purinergic signaling exerts an overarching effect on the urinary concentration mechanism by balancing the action of AVP and, thus, is potentially involved in regulation of protein abundances of AQP-2, urea transporter isoform A, and bumetanide-sensitive cotransporter (BSCI or sodium-potassium-chloride cotransporter isoform 2) of the medullary thick ascending limb (32, 58).
Hence, we hypothesized that the P2Y2 receptor or related purinergic receptors may play a key role in the pathogenesis of diuresis of postobstructive uropathy. In this study, we document that, in a rat model of postobstructive uropathy, mRNA expression and protein abundance of the P2Y2 receptor are significantly increased in the inner medulla during the diuretic phase of BUO/R and are associated with enhanced extracellular nucleotide-stimulated PGE2 production by medullary collecting ducts. In parallel, we show that mRNA expression of the prostanoid E receptors EP1 and EP3 is markedly increased in the inner medulla during the diuretic phase of BUO/R, which likely accentuates the action of PGE2 on medullary collecting duct.
METHODS
Experimental animals.
The animal protocols were approved by the Institutional Animal Care and Use Committees of the Department of Veterans Affairs Salt Lake City Health Care System and the University of Utah. Specific pathogen-free male Sprague-Dawley rats, weighing ∼150 g at the time of arrival, were purchased from Charles River Laboratories (Wilmington, MA). Rats were acclimated to the housing conditions for ≥1 wk, with free access to standard rat chow and drinking water and a 12:12-h light-dark cycle.
BUO/R.
On the basis of a previously published method (10, 31), we established and characterized a surgical model of bilateral ureteral obstruction for 24 h followed by release (BUO/R) or sham operation (sham). Briefly, rats were anesthetized with isoflurane (3–5% on a rodent anesthesia machine) and placed on a heated pad with a circulating water jacket to maintain rectal temperature close to 37°C, as monitored with an electronic rectal probe. Hair on the abdomen was clipped, and skin was cleaned using a preventive antiseptic. The following procedure was conducted under aseptic conditions. A midline abdominal incision was made, and ureters were exposed one at a time. A 5-mm-long piece of bisected polyethylene (PE) tubing (PE-50, Clay Adams) was placed around the mid portion of each ureter. The ureter was then occluded by gentle tightening of the tubing with 5-0 silk ligature. Animals recovered within 5 min after the isoflurane flow was shut off. Sham rats underwent a similar procedure (laparotomy and handling of ureters), but their ureters were not occluded with PE tubing and silk. After 24 h, the abdomen was opened again under isoflurane anesthesia, and the obstructed ureters, which were clearly enlarged proximal to the PE tubing ligature, were decompressed by gentle cutting of the silk ligature with a fine Iris forceps followed by removal of the PE tubing. With this technique, the ureters were completely occluded for 24 h and released, without evidence of residual obstruction. BUO/R rats voided watery urine within a few minutes after recovery from anesthesia, thus confirming release of the ureters. Sham rats were also subjected to a second laparotomy and handling of ureters 24 h later. During both surgical procedures, ∼3–5 ml of warm sterile normal saline (0.9% NaCl, USP) were instilled into the abdominal cavity before closure to compensate for surgical losses of body fluids. On both occasions, a morphine-based pain reliever (buprenorphine, 0.05 mg/kg im) was administered before the animals recovered from anesthesia. Buprenorphine was administered at 12-h intervals during the first 2 postoperative days. Animals were monitored for signs of pain, distress, and significant weight loss or gain. Postoperatively, all rats had free access to standard chow and drinking water. Rats were euthanized by pentobarbital sodium overdose 7 or 8 days (n = 11 sham, n = 13 BUO/R) or 12 days (n = 4 each group) after the second surgical operation. Kidneys were collected, and inner medullas were dissected out for laboratory analysis. Thus 32 rats (n = 15 sham, n = 17 BUO/R) were used in this study.
Analysis of urine samples.
Rats were placed in individual plastic metabolic cages with free access to chow and drinking water for periodic monitoring of 24-h urine output. Clear supernatants were obtained by centrifugation of aliquots of urine samples. Osmolality of clear urine samples was measured by the vapor pressure method on an osmometer (Wescor, Logan, UT). Urinary excretion of PGE2 was determined using a PGE metabolite enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI), as described by us previously (48, 49, 57). Briefly, PGE2 in the urine was converted to a single stable derivative, 13,14-dihydro-15-keto metabolite, and quantified by EIA.
Quantitative real-time RT-PCR assays of inner medullary samples.
Quantitative real-time RT-PCR was carried out according to methods established in our laboratory (24, 49, 56). Briefly, total RNA from the inner medulla of sham or BUO/R rats was isolated by the TRIzol method (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The extracted RNA was further purified by passage through RNeasy Mini Columns and treated with DNase I (Qiagen, Valencia, CA) for removal of traces of genomic DNA in the samples. cDNA was made by reverse transcription of RNA samples using SuperScript Reverse Transcriptase II or SuperScript First-Strand Synthesis SuperMix (both from Invitrogen). cDNA was quantified by real-time amplifications in the Smart Cycler II system (Cepheid, Sunnyvale, CA) or Applied Biosystems 7500 Real-Time PCR system (Foster City, CA), with platinum Taq polymerase or AmpliTaq gold and SYBR Green used for detection. Table 1 shows the nucleotide sequences of the primer pairs used in the PCR. cDNAs were amplified for 40 cycles. In our hands, with the given primer pairs, PCR amplifications were linear, with r2 ≥ 0.98, and amplification efficiency was ≥95%, as determined by serial dilutions of cDNA samples. Specificity of amplifications was determined by sequencing PCR products in the DNA core facility and then blasting the sequences in the National Center for Biotechnology Information nucleotide database. Positive controls were generated by PCR amplifications on cDNA prepared from different organs or tissues from rats. Expression of target genes was computed relative to expression of the housekeeping gene β-actin. The reliability of β-actin as a housekeeping gene in postobstructive uropathy was verified by comparison with three other unrelated housekeeping genes, GAPDH, peptidylprolyl isomerase A (Ppia), and ribosomal protein 13a (Rp113a), with use of commercially available primer pairs (Real Time Primers, Elkins Park, PA). When the expression levels of the target genes, such as P2Y or EP receptors, were normalized to the different housekeeping genes, GAPDH, Ppia, and β-actin gave comparable values, which are very close to each other, whereas Rpl13a gave very different values (data not shown). This validated the use of β-actin for this study.
Table 1.
Nucleotide sequences of primer pairs used in PCR
| Gene | Accession No. | Primer Position | Primer Sequence | Amplicon Size, bp | Reference No. |
|---|---|---|---|---|---|
| P2Y1 | NM_012800 | 1235–1254 | ACGTCAGATGAGTACCTGCG | 289 | 52 |
| 1504–1523 | CCCTGTCGTTGAAATCACAC | ||||
| P2Y2 | NM_017255 | 1270–1293 | ACCCGCACCCTCTATTACTCCTTC | 130 | 24, 49 |
| 1376–1399 | AGTAGAGCACAGGGTCAAGGCAAC | ||||
| P2Y4 | NM_031680 | 263–284 | TGTTCCACCTGGCATTGTCAG | 294 | 52 |
| 537–556 | AAAGATTGGGCACGAGGCAG | ||||
| P2Y6 | NM_057124 | 644–665 | TGCTTGGGTGGTATGTGGAGTC | 339 | 52 |
| 960–982 | TGGAAAGGCAGGAAGCTGATAAC | ||||
| COX-1 | NM_017043.3 | 814–834 | TAAGTACCAGGTGCTGGATGG | 265 | 16 |
| 1058–1078 | GGTTTCCCCTATAAGGATGAG | ||||
| COX-2 | NM_017232.3 | 1200–1222 | TACAACCAGTGGCAAAGGCC | 283 | 16 |
| 1310–1333 | CAGTATTGAGGAGAACAGATGGG | ||||
| EP1 | NM_013100.1 | 1395–1414 | TCCATCACTTCAACCACAGC | 300 | 1 |
| 1675–1694 | GGGTAGGAGGCGAAGAAGTT | ||||
| EP3 | NM_012704.1 | 886–903 | ACTGTCCGTCTGCTGGTC | 101 | 46 |
| 968–986 | CCTTCTCCTTTCCCATCTG | ||||
| β-Actin | NM_031144.2 | 18–37 | CACCCGCGAGTACAACCTTC | 207 | 56 |
| 205–224 | CCCATACCCACCATCACACC |
P2Y1, P2Y2, P2Y4, and P2Y6, purinergic P2Y receptors; EP1 and EP3, prostanoid E receptors.
Western blot analysis of tissue samples.
Inner medullary tissue samples were prepared and immunoblotted for semiquantitative determination of protein abundances of AQP-2, P2Y2 receptor, and β-actin by the methods established in our laboratory (24, 49, 56, 58). Briefly, proteins in whole inner medullary tissue homogenates were size fractionated by electrophoresis under denaturing condition. Quality of tissue sample preparation and equality of protein loading were verified by staining loading gels with Coomassie blue (Gelcode Blue, Pierce Endogen, Rockford, IL) followed by visualization of the sharpness of the bands and differences in density of the bands among different samples, respectively. The size-fractionated proteins were electrotransferred to nitrocellulose membranes, which were incubated with rabbit polyclonal antibodies to AQP-2 (GN-762) or P2Y2 receptor (L246) or β-actin (Biolegend, San Diego, CA). Generation and characterization of the primary antibodies to AQP-2 and P2Y2 receptor have been described by us previously (22, 23). After the primary antibodies were washed off, the nitrocellulose membranes were incubated with a peroxidase-conjugated secondary antibody to rabbit IgG (Dako North America, Carpintaria, CA). A chemiluminescence reaction was used to detect sites of antigen-antibody reactions (SuperSignal Substrate, Pierce Endogen) and captured on Kodak X-Omat film. Images were digitized, and band densities were quantified using Un-Scan-It software (Silk Scientific, Orem, UT). The band densities of AQP-2 and P2Y2 receptor were normalized to the band density of β-actin. Mean values of relative band densities thus obtained for AQP-2 or P2Y2 receptor in BUO/R rats are expressed as a percentage of respective mean values obtained in the sham group.
Determination of nucleotide-stimulated release of PGE2 by medullary collecting ducts.
Nucleotide-stimulated release of PGE2 by medullary collecting ducts was determined using the methods established in our laboratory (48, 49, 51, 57). Briefly, collagenase and hyaluronidase digestion was used to prepare fractions enriched in medullary collecting ducts from freshly obtained and pooled inner medullas from BUO/R or sham rats. Inner medullary collecting ducts (IMCD) were separated from the non-IMCD elements by low-speed centrifugation and washing of the digested material. Aliquots of the IMCD fractions thus obtained were suspended in a physiological solution and challenged with nucleotides at 37°C. Reactions were stopped by addition of cold buffer, and the incubation medium and IMCD fractions were separated by centrifugation. PGE2 released into the medium was assayed with an EIA kit (Cayman Chemical). Protein content of IMCD pellets was determined using Coomassie Plus protein assay reagent kit (Pierce Biotechnology, Rockford, IL). The PGE2 contents of the incubations were normalized to the protein contents of the respective pellets. Because of the labor-intensive and time-consuming nature of the double surgical operations, it was necessary to evaluate the nucleotide-stimulated PGE2 release in BUO/R and sham rats on separate days. In addition, because of changes in the physical consistency of inner medullary tissue, the duration of digestion needed to obtain near-homogeneous suspensions of medullary collecting ducts, as seen in the sham group, was different in the BUO/R group. Because of these unusual experimental conditions, we did not directly compare absolute amounts of PGE2 released in each group. Instead, we present a comparative pharmacological profile of nucleotide-stimulated PGE2 release.
Statistical analysis.
All quantitative data are expressed as means ± SE. Comparisons among several groups were made by ANOVA, followed by assessment of differences by the Tukey-Kramer multiple comparison test. Differences between the means of two groups were assessed by unpaired t-test; where applicable, Welch's correction was applied, or the Mann-Whitney nonparametric test was used. P < 0.05 was considered significant.
RESULTS
Characterization of postobstructive uropathy in rats.
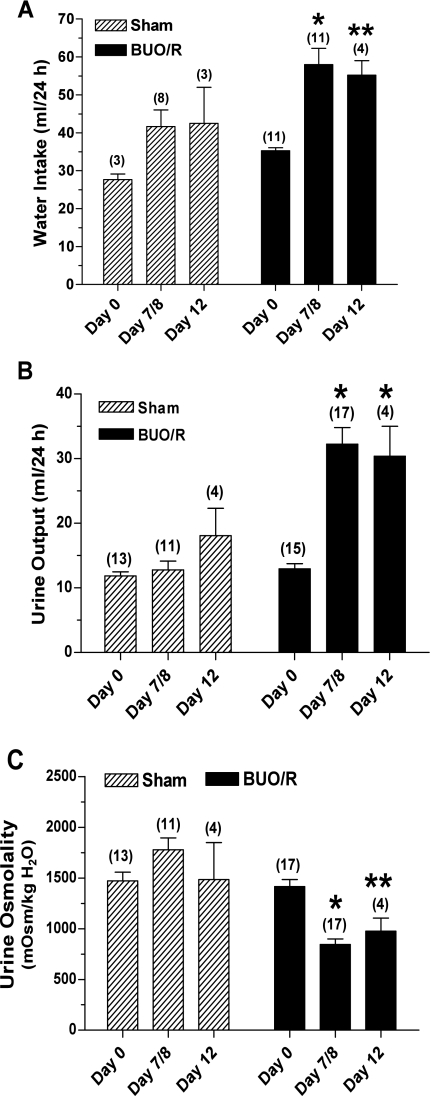
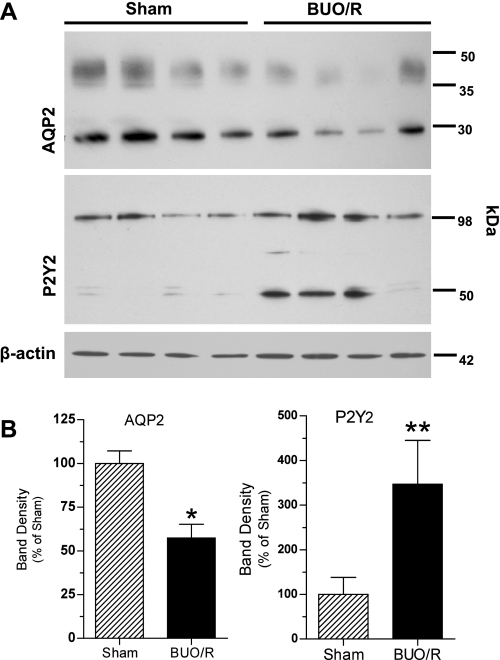
We used an established method to induce postobstructive uropathy (10, 31). Figure 1 shows water intake, urine output, and urine osmolalities in BUO/R and sham (control) rats. Since the results of these parameters on day 7 or 8 were essentially similar, we combined data from days 7 and 8 and plotted them as day 7/8. As shown in Fig. 1, BUO/R rats developed significant polydipsia and polyuria, associated with decreased urine concentration ability. Furthermore, mean body weight was 26% greater in sham rats euthanized on day 7/8 than in sham rats on day 0; however, the mean weight gain in BUO/R rats on day 7/8 was only 7% (data not shown). On the contrary, at the time of euthanasia, the kidneys of BUO/R rats were ∼2- to 2.5-fold larger than the kidneys of sham rats (data not shown). Western blot analysis of whole inner medullary tissue homogenates showed decreased intensity of 29-kDa native and 35- to 50-kDa glycosylated forms of AQP-2 protein in the BUO/R rats compared with sham rats on day 12 (Fig. 2). Densitometry revealed a significant decrease in the combined band densities of 29-kDa native and 30- to 35-kDa glycosylated forms of AQP-2 protein in BUO/R rats (57% of sham, P < 0.01; Fig. 2B).
Fig. 1.
Characterization of postobstructive uropathy in rats. Sprague-Dawley rats were subjected to bilateral ureteral obstruction for 24 h followed by release (BUO/R) or sham operation (sham). Water intake (A), urine output (B), and urine osmolality (C) were monitored before surgical procedures (day 0) and 7 or 8 days and 12 days after release of obstruction. Since there were no significant differences in these parameters 7 or 8 days after release, data were combined and are presented as day 7/8. Values are means ± SE of number of rats shown in parentheses. *P < 0.001; **P < 0.05 vs. corresponding day 0 value (by ANOVA).
Fig. 2.
Changes in protein abundances of aquaporin-2 (AQP-2) and P2Y2 receptor in rat inner medulla during postobstructive uropathy. BUO/R and sham rats (n = 4/group) were euthanized on day 12. Whole inner medullary tissue samples were immunoblotted using specific antibodies to AQP-2 or P2Y2 receptor. A: immunoblots of AQP-2 and P2Y2 receptor in sham and BUO/R rats. Equal amounts of proteins from 1 rat per lane were loaded onto gels. Equality of protein loading was checked by staining loading gels with Coomassie blue or probing blots for protein abundance of β-actin. AQP-2 protein blots show 29-kDa native band and a smear of glycosylated band extending between 35 and 50 kDa. In P2Y2 receptor protein blots, 45- and 105-kDa bands are specific, as documented previously (21, 24, 49). B: densitometry of bands in immunoblots. Immunoblots were digitized, and pixel densities of the bands were determined. Combined density values (means ± SE) of 29- and 35- to 50-kDa protein bands of AQP-2 and 47- and 105-kDa protein bands of P2Y2 receptor in sham and BUO/R groups were normalized to density of respective β-actin bands. Results are plotted as percentage of mean values in the sham group. *P < 0.01; **P < 0.05 vs. sham.
Increased urinary excretion of PGE2 during postobstructive uropathy.
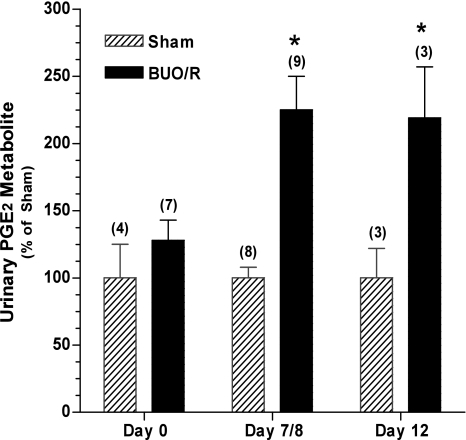
Previous studies reported increased urinary excretion of PGE2 in postobstructive uropathy (8, 9, 37, 38). Therefore, to determine whether, in our model of BUO/R, PGE2 excretion in urine is increased, we converted the unstable PGE2 in aliquots of urine samples collected over a 24-h period to a stable metabolite and assayed its content using methods established in our laboratory. Figure 3 shows that the mean PGE2 metabolite contents in urine of sham and BUO/R rats were not different from each other on day 0. However, on day 7/8 and day 12, the mean PGE2 metabolite content was 2.25- and 2.19-fold higher, respectively, in BUO/R than in the corresponding sham rats (P < 0.01).
Fig. 3.
Increased excretion of urinary PGE2 metabolites during postobstructive uropathy. BUO/R and sham rats were euthanized on day 8 or 12. Aliquots of 24-h urine samples were collected and processed for assay of PGE2 metabolite. Nanograms of urinary PGE2 metabolite per 24 h are presented as percentage of values in sham rats on the corresponding days: 17.1, 17.2, and 9.9 ng urinary PGE2/24 h on days 0, 8, and 12, respectively. Values are means ± SE of number of rats in parentheses. *P < 0.01 vs. sham on the corresponding day.
Changes in gene expression of molecules involved in prostanoid biosynthesis and signaling during postobstructive uropathy.
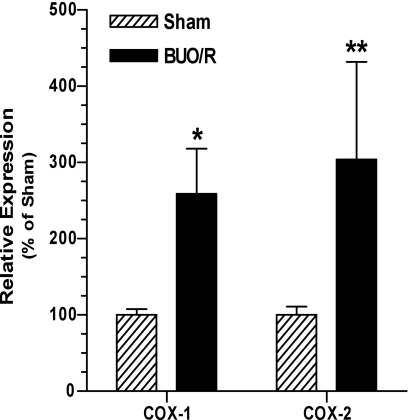
Biosynthesis of PGE2 from arachidonic acid is catalyzed by COX. Earlier studies reported increased expression of COX enzymes, especially COX-2, in the medulla of rats with postobstructive uropathy (37, 38). Hence, using real-time RT-PCR, we determined relative mRNA expression of COX-1 and COX-2 in the inner medulla of BUO/R and sham rats. Figure 4 shows that, on day 12, the relative mRNA expression of COX-1 and COX-2 enzymes was increased significantly by ∼2.5- and 3-fold, respectively, in BUO/R compared with sham rats.
Fig. 4.
Changes in relative expression of cyclooxygenases 1 and 2 (COX-1 and COX-2) in rat inner medulla during postobstructive uropathy. BUO/R and sham rats (n = 4/group) were euthanized on day 12. RNA was extracted and reverse transcribed, and cDNA samples were amplified with gene-specific primer pairs for COX-1, COX-2, or β-actin using real-time PCR. Expression of COX enzymes was normalized to that of β-actin and plotted as percentage of mean values in sham group. Values are means ± SE. *P < 0.04; (unpaired t-test); **P < 0.03 (Mann-Whitney test) vs. corresponding sham.
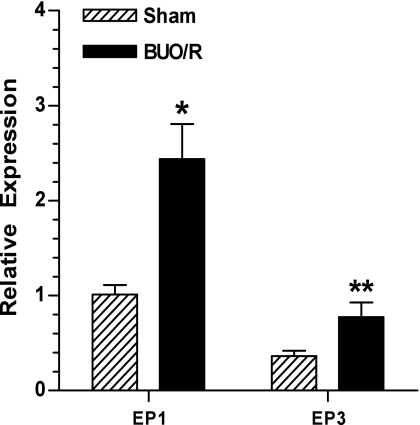
Furthermore, prostanoid signaling in different tissues is mediated through EP receptors. EP receptors that bind PGE2 are differentially expressed along the nephron and collecting duct (12). EP1 and EP3 receptors are expressed in the medullary collecting duct cells and are coupled to the phosphoinositide signaling pathway (12). Activation of these two prostanoid receptors is known to result in loss of water and sodium (12). Therefore, to understand the potential role of EP receptors in the diuresis of postobstructive uropathy, using real-time RT-PCR, we determined the relative mRNA expression of EP1 and EP3 receptors in the inner medulla of BUO/R and sham rats on day 8. Figure 5 documents that, after BUO/R, the relative mRNA expression of EP1 and EP3 receptors was significantly increased by 2.4- and 2.1-fold, respectively, compared with the sham group.
Fig. 5.
Changes in relative expression of prostanoid E (EP) receptor subtypes 1 and 3 (EP1 and EP3) in rat inner medulla during postobstructive uropathy. BUO/R (n = 4) and sham (n = 3) rats were euthanized on day 8. RNA was extracted and reverse transcribed, and cDNA samples were amplified with gene-specific primer pairs for EP1 and EP3 receptors or β-actin using real-time PCR. Expression of EP receptor subtypes was normalized to that tk;4of β-actin to obtain relative expression values. Values are means ± SE. *P < 0.03; **P < 0.05 vs. corresponding sham (by unpaired t-test).
Changes in relative mRNA expression of P2Y receptor subtypes in rat inner medulla during postobstructive uropathy.
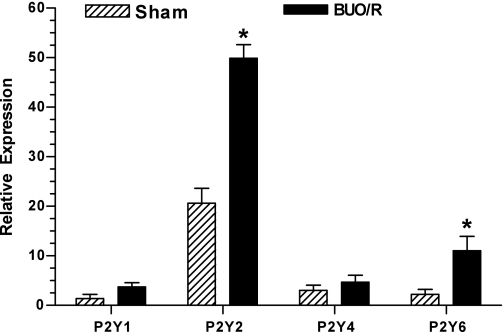
To identify the P2Y receptor subtype(s) that might be potentially involved in postobstructive uropathy, we used RNA extracted from the inner medulla to examine changes in mRNA expression of P2Y1, P2Y2, P2Y4, and P2Y6 receptors by real-time RT-PCR. These four P2Y receptor subtypes are coupled to the phosphoinositide signaling pathway and, thus, are potential candidates for nucleotide-stimulated PGE2 release (Table 2). As shown in Fig. 6, P2Y2 receptor mRNA expression is predominant in the inner medulla in sham rats, whereas mRNA expression of other P2Y receptor subtypes is very low (<15% compared with P2Y2 receptor expression). The low expression levels of P2Y1, P2Y4, and P2Y6 receptors were not due to failure of primer sets of these receptors to work properly. Under the same experimental conditions, these primer pairs gave robust amplifications with cDNA prepared from other rat organs, such as liver, ileum, spleen, and muscle (data not shown). After day 7/8, the relative mRNA expressions of P2Y2 and P2Y6 receptors increased significantly to 2.4- and 4.9-fold, respectively, in BUO/R compared with sham rats (P < 0.03, by Mann-Whitney nonparametric test).
Table 2.
Agonist potency and signal transduction mechanisms of P2Y1, P2Y2, P2Y4, and P2Y6 receptors
| Subtype | Agonist Potency | Signal Transduction Mechanism |
|---|---|---|
| P2Y1 | 2-MeS-ATP ≥ ATP >> ADP | Gq/G11; PLC-β ↑ |
| P2Y2 | UTP ≥ ATP > ATPγS >> 2-MeS-ATP | Gq/G11 [Gi/Go(?)]; PLC-β ↑ |
| P2Y4 | UDP = UTP > ATP = ADP | Gq/G11 [Gi (?)]; PLC-β ↑ |
| P2Y6 | UDP > UTP > ADP >2-MeS-ATP >>> ATP | Gq/G11 & Gs; PLC-β ↑ |
2-MeS-ATP, 2-methylthio-ATP; ATPγS, adenosine 5′-O-(3-thiotriphosphate). Adapted from Zhang et al. (56).
Fig. 6.
Changes in relative expression of P2Y receptor subtypes in rat inner medulla during postobstructive uropathy. BUO/R (n = 4) and sham (n = 3) rats were euthanized on day 8. RNA was extracted and reverse transcribed, and cDNA samples were amplified with specific primer pairs for P2Y1, P2Y2, P2Y4, or P2Y6 receptor or β-actin using real-time PCR. Expression of P2Y receptor subtypes was normalized to that of β-actin to obtain relative expression values. Values are means ± SE. *P < 0.03 vs. corresponding sham (by Mann-Whitney test).
Changes in protein abundance of the P2Y2 receptor during postobstructive uropathy.
Since P2Y2 receptor mRNA is most abundantly expressed in the inner medulla and has also been shown to increase considerably in BUO/R rats, we determined the protein abundance of the P2Y2 receptor by Western blotting. We used a P2Y2 receptor-specific antibody, which we developed and characterized previously (22, 24, 49). Figure 2 shows increased densities of 47- and 105-kDa protein bands of the P2Y2 receptor in BUO/R and sham rats. Densitometry revealed that the mean combined density of these two bands of the P2Y2 receptor was ∼3.5-fold higher in BUO/R than sham rats (P < 0.05, by unpaired t-test with Welch's correction).
Comparative pharmacological profile of nucleotide-stimulated PGE2 release by inner medullary collecting duct preparations from BUO/R and sham rats.
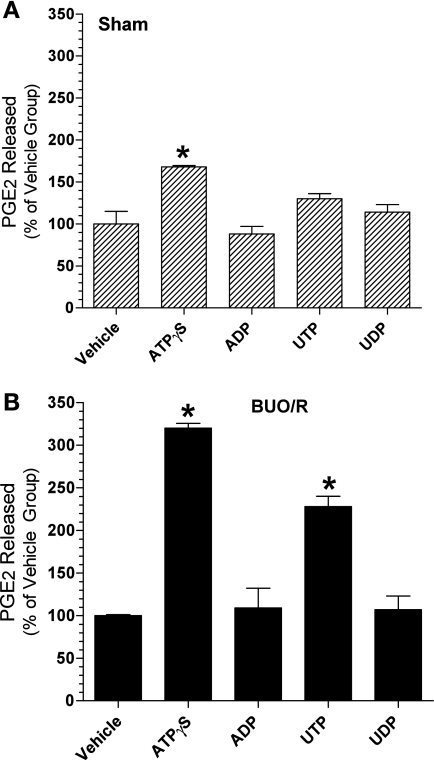
To assess the potential contribution of different P2Y receptor subtypes to PGE2 production by the medullary collecting ducts in postobstructive uropathy, we examined the pattern of ex vivo nucleotide-stimulated PGE2 release by IMCDs from BUO/R rats and compared it with that from sham rats. The results presented in Fig. 7 provide a comparative pharmacological profile of nucleotide-stimulated PGE2 release by ex vivo preparations of medullary collecting ducts in the two groups. Mean nucleotide-stimulated release of PGE2 in sham and BUO/R rats is plotted as a percentage of that in the respective vehicle-alone incubations (controls). As shown in Fig. 7A, in sham rats, only ATPγS had a significant effect (1.7-fold increase compared with vehicle incubation, P < 0.01). Although the mean PGE2 release was 1.3-fold higher in the presence of UTP than in the presence of vehicle alone, this difference did not reach statistical significance. On the contrary, in BUO/R rats, the ATPγS- and UTP-stimulated PGE2 releases were 3.2-fold (P < 0.001) and 2.3-fold (P < 0.001) higher than in the respective vehicle incubations (Fig. 7B). Thus it appeared that ATPγS and UTP had more potent stimulatory effects on medullary collecting ducts of BUO/R than sham rats. Thus the pharmacological profile of BUO/R rats is consistent with the predominant contribution from the P2Y2 receptor, but not other P2Y receptor subtypes (Table 2).
Fig. 7.
Changes in the pattern of nucleotide-stimulated PGE2 release by inner medullary collecting duct preparations during postobstructive uropathy. BUO/R and sham rats (n = 5/group) were euthanized on day 8. Inner medullas from each group were pooled separately and processed. Fractions enriched in collecting ducts were freshly prepared from pooled inner medullas. Aliquots of fractions were warmed to 37°C and then challenged with each nucleotide at 50 μM for 20 min. PGE2 released into the medium was assayed and normalized to protein contents of the corresponding incubations. Values are means ± SE of triplicate incubations. A: pattern of nucleotide-stimulated PGE2 release in sham rats plotted as percentage of mean values in vehicle incubations. *P < 0.01 vs. vehicle. B: pattern of nucleotide-stimulated PGE2 release in BUO/R rats plotted as percentage of mean values in vehicle incubations. *P < 0.001 vs. vehicle.
DISCUSSION
In this communication, we have documented that, in an established rat model of postobstructive uropathy, P2Y2 receptor mRNA expression and protein abundance are significantly increased during the diuretic phase. In parallel, we have demonstrated that ex vivo nucleotide-stimulated release of PGE2 by the IMCD is enhanced, with a pharmacological profile consistent with the exclusive contribution by the P2Y2 receptor, but not the other P2Y receptor subtypes examined. In addition, we showed that relative mRNA expression of prostanoid EP1 and EP3 receptors, which are known to mediate water and sodium excretion in the medullary collecting duct (12), is markedly increased in BUO/R rats. This, if accompanied by corresponding increases in the protein abundances of EP1 and EP3 receptors, may accentuate the action of released PGE2 on the medullary collecting duct and, thus, establish a cycle of events. These data, when considered in the light of our previously published studies (reviewed in Ref. 26) on the role of purinergic signaling in regulation of renal water transport, suggest that increased expression and activity of the P2Y2 receptor in postobstructive uropathy may contribute to the diuresis.
The underlying defect in the diuresis of postobstructive uropathy is the apparent resistance of the medullary collecting duct to the action of AVP, leading to acquired NDI. One of the causes of resistance identified several years ago is increased production of renal PGE2 (8, 9) by the cortical and medullary tubules (53) in postobstructive uropathy. Increased biosynthesis of PGE2 in postobstructive uropathy, in turn, has been attributed to increased expression and/or activity of COX-2 (37, 38, 53). Although renal cortical COX-2-derived PGE2 plays critical roles in maintaining blood pressure and may participate in the pathogenesis of vascular hypertension, the medullary COX-2-derived PGE2 regulates transport of water, sodium, and urea and, thus, balances the effect of AVP on urinary concentration (12). Thus selective or nonselective COX-2 inhibition may be ameliorating diuresis of postobstructive uropathy by blocking cortical and medullary functions of COX-2. Although this approach of direct inhibition of COX enzymes is useful in the clinic, it may not be the best approach for all patients with postobstructive uropathy. Hence, there is a need for an in-depth understanding of the molecular pathophysiology of AVP resistance in postobstructive uropathy. In this context, we believe that our series of studies on purinergic regulation of water transport by the medullary collecting duct and the potential mechanisms involved in it will provide significant insights and a new direction for exploration of the AVP resistance in postobstructive uropathy (reviewed in Ref. 26).
Consistent with a previously published report on the long-term urine concentration defect following BUO/R (30), our rat model of postobstructive uropathy showed comparable polydipsia, polyuria, and urine concentration defect 7 or 8 days and 12 days after release of obstruction. In this model, as reported in published studies (37, 38), we also documented significant increases in the relative mRNA expression of COX-2 in the inner medulla and urinary excretion of PGE2. In contrast to previous studies that reported no change or a decrease in the relative expression of COX-1 immediately or 3 days after the release of obstruction (37, 38), we observed a significant increase in the relative expression of COX-1 mRNA, but this occurred 12 days after the release of obstruction.
Although the medullary collecting duct is known to be a major source of PGE2 production in the kidney (5), few studies have identified the upstream signaling molecules for the production of PGE2 by medullary collecting ducts (15, 27, 54). Increased expression of COX enzymes per se may not account for the increased production of PGE2 by medullary collecting ducts. In fact, previously, we documented that dehydration of rats, which is known to induce COX-2 expression in medullary collecting duct cells, is not associated with increased urinary excretion of PGE2 or increased nucleotide-stimulated PGE2 production by medullary collecting ducts in rats (48). Therefore, factors in addition to increased expression of COX enzymes may be responsible for the increased production of PGE2 by the medullary collecting duct in health and disease. One such factor is availability of “free” arachidonic acid, the substrate for the synthesis of PGE2 by COX enzymes. Many investigators consider that availability of free arachidonic acid, which is the initial step in PGE2 biosynthesis, is also often the rate-limiting step in PGE2 production (4, 14, 34). Cytosolic phospholipases, which release free arachidonic acid from membrane phospholipids, are under the control of signaling pathways initiated by G protein-coupled receptors, such as the P2Y2 receptor or the dopamine receptor. Hence, one can infer that increased activity of such receptors in turn increases the availability of free arachidonic acid, whereas associated increased activity of COX enzymes may only facilitate rapid formation of PGE2 after receptor stimulation. In this context, the results presented here appear to support the notion that increased P2Y2 receptor expression during the diuretic phase of postobstructive uropathy might result in increased nucleotide-stimulated PGE2 production, which is facilitated by increased COX enzyme expression.
Furthermore, in our previous studies on normal or hydrated rats, we documented that P2Y2 receptor-mediated PGE2 release by medullary collecting ducts is inhibited by COX-1-specific inhibitors, but not COX-2-specific inhibitors (48). This may be due to the fact that, in the medulla of normal or hydrated rats, COX-2 is expressed only in the interstitial cells, but not in the collecting duct principal cells, where the P2Y2 receptor is known to be localized. However, during the diuretic phase of postobstructive uropathy, COX-2 expression is induced in the medulla. For this reason, the potential involvement of COX-2 also in the nucleotide-stimulated release of PGE2 by the collecting duct in postobstructive uropathy cannot be ruled out. Future studies are needed to address this issue. Moreover, unlike our previous studies on normal rats, in the sham-operated rats, we observed a blunted response to stimulation of medullary collecting ducts by UTP that did not reach significance. Whether this is an effect of surgical procedures in otherwise-normal rats needs to be investigated.
Despite a significant increase in the relative mRNA expression of the P2Y6 receptor, another potential receptor that can increase the availability of free arachidonic acid, the pharmacological profile of nucleotide-stimulated release of PGE2 by medullary collecting ducts does not support the notion that P2Y6 receptor activity is also enhanced during the diuretic phase of postobstructive uropathy. This is because stimulation by UDP, the preferred agonist of the P2Y6 receptor, has no significant effect. Interestingly, in a recent study, we observed that enhanced nucleotide-stimulated PGE2 release by medullary collecting ducts of rats in lithium-induced NDI is associated with a 3.4-fold increase in the relative mRNA expression of the P2Y4 receptor (57). Thus it is possible that mRNA expression of different P2Y receptor subtypes may be differentially regulated in different types of acquired NDI. Their roles in these pathophysiological conditions need to be investigated.
Although prostanoids can diffuse through cell membranes, rapid release of prostanoids from cells, including the collecting duct principal cells, is facilitated via specific prostaglandin transporters in cell membranes (2). The released prostanoids interact with four types of G protein-coupled EP receptors (EP1, EP2, EP3, and EP4), which are widely distributed in the cells of the kidney (12). Of these, EP1 and EP3 receptors, which are expressed in medullary collecting duct cells, are coupled to the phosphoinositide signaling pathway. Activation of these two prostanoid receptors is known to result in loss of water and sodium (12). Our documentation that relative mRNA expression of EP1 and EP3 receptors is significantly increased in the inner medulla during the diuretic phase of postobstructive uropathy suggests that the effect of released PGE2 may be potentially amplified if the protein abundances of these two prostanoid receptors are also increased during postobstructive uropathy.
Furthermore, apart from documenting P2Y2 receptor-mediated production and release of PGE2 in the medullary collecting duct, we and others have shown that agonist stimulation of the P2Y2 receptor results in direct inhibition of AVP-stimulated water transport in the collecting duct by decreasing cAMP formation via a PKC-dependent mechanism (7, 21, 45). Thus, at cellular and molecular levels, the antagonistic effect of purinergic signaling on the AVP-mediated water transport in the collecting duct has two well-defined mechanisms. In addition, using P2Y2 receptor gene knockout mice, we recently reported that, at the whole kidney level, purinergic signaling exerts an overarching effect by balancing the action of AVP on the urinary concentration mechanism (58). Here, our study provides new insights into the potential role of the P2Y2 receptor in molecular pathophysiology of diuresis of postobstructive uropathy, which may help us develop innovative therapies for use in the clinic.
Finally, we must acknowledge the strengths and limitations of our study. Here, we convincingly documented significantly increased mRNA expression and protein abundance of the P2Y2 receptor in the inner medulla of BUO/R vs. sham rats. We also showed significantly increased nucleotide-stimulated PGE2 release by the medullary collecting ducts of BUO/R vs. sham rats. However, we did not document where the increased P2Y2 receptor expression is localized in the medulla or collecting ducts. Unlike the transporter or channel proteins, which are abundantly expressed, expression of G protein-coupled receptors, such as the P2Y2 receptor, is very low in the cells. Hence, to document minor differences in subcellular localization of the P2Y2 receptor in relation to its increased expression in the BUO/R model, one needs to use confocal immunofluorescence microscopy on in situ perfusion-fixed kidney sections, which is beyond the scope of this work. Furthermore, except for the P2Y2 receptor, for which we have a reliable antibody, the increased expression of P2Y6, EP1, and EP3 receptors was documented at the mRNA level only. Although one can assume that corresponding increases in protein abundances of these receptors are possible, such changes in protein abundances need to be documented in future studies using reliable antibodies. Pending characterization of commercial antibodies to P2Y6, EP1, and EP3 receptors in our laboratory, at this stage we suggest that purinergic signaling mediated by the P2Y2 receptor may have a potential role in the diuresis of postobstructive uropathy.
GRANTS
This work was supported by grants from the Department of Veterans Affairs Merit Review Program (to B. K. Kishore), National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-61183 (to B. K. Kishore), the National Kidney Foundation of Utah and Idaho (B. K. Kishore), and the resources and facilities at the Department of Veterans Affairs Salt Lake City Health Care System.
DISCLOSURES
The proprietary information presented here is protected by an international patent under the Patent Cooperation Treaty (PCT/US2005/038231) and was published by the World Intellectual Property Organization (WO/2006/066679).
ACKNOWLEDGMENTS
The authors thank Rujia Sun, Huihui Shi, and Andrew Hemmert for technical assistance in the initial stages of this work.
Part of this work was presented in preliminary form at the Experimental Biology 2005 Meeting (San Diego, CA) and the American Society of Nephrology Annual Meeting (October-November 2009, San Diego, CA) and appeared as printed abstracts in the proceedings of those meetings (50, 56).
REFERENCES
- 1.Aoudjit L, Potapov A, Takano T. Prostaglandin E2 promotes cell survival of glomerular epithelial cells via the EP4 receptor. Am J Physiol Renal Physiol 290: F1534–F1542, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bao Y, Pucci ML, Chan BS, Lu R, Ito S, Schuster VL. Prostaglandin transporter PGT is expressed in cell types that synthesize and release prostanoids. Am J Physiol Renal Physiol 282: F1103–F1110, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Berry SJ, Coffey DS, Walsch PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 132: 474–479, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Bomalaski JS, Clark MA. Phospholipase A2 and arthritis. Arthritis Rheum 36: 190–198, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Bonvalet JP, Pradelles P, Farman N. Segmental synthesis and actions of prostaglandins along the nephron. Am J Physiol Renal Fluid Electrolyte Physiol 253: F377–F387, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Zhang H, Lee HY, Park JM. Cyclooxygenase-2 inhibitor preserves medullary aquaporin-2 expression and prevents polyuria after ureteral obstruction. J Urol 172: 2387–2390, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Edwards RM. Basolateral, but not apical, ATP inhibits vasopressin action in rat inner medullary collecting duct. Eur J Pharmacol 438: 179–181, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Fradet Y, Label M, Grose JH, Talbot J, Charrois R. Renal prostaglandins in obstructive diuresis. Comparative study of unilateral and bilateral ureteral obstruction in conscious dogs. Prostaglandins Leukot Essent Fatty Acids 31: 123–129, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Fradet Y, Simard J, Grose JH, Lebel M. Enhanced urinary prostaglandin E2 in postobstructive diuresis in humans. Postaglandin Med 5: 29–30, 1980 [DOI] [PubMed] [Google Scholar]
- 10.Frøkiær J, Marples D, Knepper MA, Nielsen S. Bilateral ureteral obstruction downregulates expression of vasopressin-sensitive AQP-2 water channel in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 270: F657–F668, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Han JS, Maeda Y, Ecelbarger C, Knepper MA. Vasopressin-independent regulation of collecting duct water permeability. Am J Physiol Renal Fluid Electrolyte Physiol 266: F139–F146, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol 70: 357–377, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Harris RH, Yarger WE. The pathogenesis of post-obstructive diuresis: the role of circulating natriuretic and diuretic factors, including urea. J Clin Invest 56: 880–887, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirabayashi T, Shimizu T. Localization and regulation of cytosolic phospholipase A2. Biochim Biophys Acta 1488: 124–138, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Huo TH, Healy DP. Prostaglandin E2 production in rat IMCD cells. I. Stimulation by dopamine. Am J Physiol Renal Fluid Electrolyte Physiol 261: F647–F654, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Ivanov AI, Pero RS, Scheck AC, Romanovsky AA. Prostaglandin E2-synthesizing enzymes in fever: differential transcriptional regulation. Am J Physiol Regul Integr Comp Physiol 283: R1104–R1117, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Jensen BL, Mann B, Skøtt OL, Kurtz A. Differential regulation of renal prostaglandin receptor mRNAs by dietary salt intake in the rat. Kidney Int 56: 528–537, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Jones BF, Nanra RS. Post-obstructive diuresis. Aust NZ J Med 13: 519–521, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Kim SW, Cho SH, Oh BS, Yeum CH, Choi KC, Ahn KY, Lee J. Diminished renal expression of aquaporin water channels in rats with experimental bilateral ureteral obstruction. J Am Soc Nephrol 12: 2019–2028, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Kim SW, Lee J, Jung K, Ma SK, Oh Y, Kim WY, Choi KC, Kim J. Diminished expression of sodium transporters in the ureteral obstructed kidney in rats. Nephron Exp Nephrol 96: e67–e76, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kishore BK, Chou CL, Knepper MA. Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 269: F863–F869, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Kishore BK, Ginns SM, Krane CM, Nielsen S, Knepper MA. Cellular localization of P2Y2 purinoceptor in rat inner medulla and lung. Am J Physiol Renal Physiol 278: F43–F51, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Kishore BK, Krane CM, DiIulio D, Menon AG, Cacini W. Expression of renal aquaporins 1, 2, and 3 in a rat model of cisplatin-induced polyuria. Kidney Int 58: 701–711, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Kishore BK, Krane CM, Miller RL, Shi H, Zhang P, Hemmert A, Sun R, Nelson RD. P2Y2 receptor mRNA and protein expression is altered in inner medullas of hydrated and dehydrated rat: relevance to AVP-independent regulation of IMCD function. Am J Physiol Renal Physiol 288: F1164–F1172, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kishore BK, Krane CM, Reif M, Menon AG. Molecular physiology of urinary concentration defect in elderly population. Int Urol Nephrol 33: 235–248, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE. P2Y2 receptors and water transport in the kidney. Purinergic Signal 5: 491–499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohan DE. The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr Opin Nephrol Dial 15: 34–40, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kramer HJ. Mechanisms of postobstructive polyuria. Klin Wochenschr 63: 934–1043, 1985 [DOI] [PubMed] [Google Scholar]
- 29.Li C, Klein JD, Wang W, Knepper MA, Nielsen S, Sands JM, Frøkiær J. Altered expression of urea transporters in response to ureteral obstruction. Am J Physiol Renal Physiol 286: F1154–F1162, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Li C, Wang W, Kwon TH, Isikay L, Wen JG, Marples D, Djurhuus JC, Stockwell A, Knepper MA, Nielsen S, Frøkiær J. Downregulation of AQP1, -2, and -3 after ureteral obstruction is associated with a long-term urine concentration defect. Am J Physiol Renal Physiol 281: F163–F171, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Li C, Wang W, Kwon TH, Knepper MA, Nielsen S, Frøkiæer J. Altered expression of major renal Na transporters in rats with bilateral ureteral obstruction and release of obstruction. Am J Physiol Renal Physiol 285: F889–F901, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Listhrop R, Nelson RD, Ecelbarger CA, Kohan DE, Kishore BK. Genetic deletion of P2Y2 receptor alters the protein abundances of renal sodium transporters and channels (Abstract). FASEB J 21: A1328, 2007 [Google Scholar]
- 33.Luckey AE, Parsa CJ. Fluid and electrolytes in the aged. Arch Surg 138: 1055–1060, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Meyer MC, Rastogi P, Beckett CS, McHowat J. Phospholipase A2 inhibitors as potential anti-inflammatory agents. Curr Pharm Des 11: 1301–1312, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Murray MD, Brater DC. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu Rev Pharmacol Toxicol 32: 435–465, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Nadler SP, Zimplemann JA, Hebert RL. PGE2 inhibits water permeability at a post-cAMP site in rat terminal inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 262: F229–F235, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Nørregaard R, Jensen BL, Li C, Wang W, Knepper MA, Nielsen S, FrøkiæR J. COX-2 inhibition prevents downregulation of key renal water and sodium transport proteins in response to bilateral ureteral obstruction. Am J Physiol Renal Physiol 289: F322–F333, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Nørregaard R, Jensen BL, Topcu SO, Diget M, Schweer H, Knepper MA, Nielsen S, FrøkiæR J. COX-2 activity transiently contributes to increased water and NaCl excretion in the polyuric phase after release of ureteral obstruction. Am J Physiol Renal Physiol 292: F1322–F1333, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Nyman MA, Schwenk NM, Silverstein MD. Management of urinary retention: rapid versus gradual decompression and risk of complications. Mayo Clin Proc 72: 951–956, 1997 [DOI] [PubMed] [Google Scholar]
- 40.O'Reilly PH, Brooman PJ, Farah NB, Mason GC. High pressure chronic retention. Incidence, aetiology and sinister implications. Br J Urol 58: 644–646, 1986 [DOI] [PubMed] [Google Scholar]
- 41.Peterson LJ, Yarger WE, Schocken DD, Glenn JF. Post-obstructive diuresis: a varied syndrome. J Urol 113: 190–194, 1975 [DOI] [PubMed] [Google Scholar]
- 42.Rao PNP, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharmaceut Sci 11: 81s–110s, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Roman RJ, Lechene C. Prostaglandin E2 and F2α reduced urea absorption from the rat collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 241: F53–F60, 1981 [DOI] [PubMed] [Google Scholar]
- 44.Rouch AJ, Kudo LH. Role of PGE2 in -F2α-induced inhibition of AVP- and cAMP-stimulated H2O, Na+, and urea transport in rat inner medullary collecting duct. Am J Physiol Renal Physiol 279: F294–F301, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Rouse D, Leit M, Suki WN. ATP inhibits the hydroosmotic effect of AVP in rabbit CCR: evidence for a nucleotide P2u receptor. Am J Physiol Renal Fluid Electrolyte Physiol 267: F289–F295, 1994 [DOI] [PubMed] [Google Scholar]
- 46.Shoji Y, Takahashi M, Kitamura T, Watanabe K, Kawamori T, Maruyama T, Sugimoto Y, Negishi M, Narumiya S, Sugimura T, Wakabayashi K. Downregulation of prostaglandin E receptor subtype EP3 during colon cancer development. Gut 53: 1151–1158, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonneneberg H, Wilson DR. The role of medullary collecting ducts in postobstructive diuresis. J Clin Invest 57: 1564–1574, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun R, Carlson NG, Hemmert AC, Kishore BK. P2Y2 receptor-mediated release of prostaglandin E2 by medullary collecting duct is altered in hydrated and dehydrated rats: relevance to AVP-independent regulation of IMCD function. Am J Physiol Renal Physiol 289: F585–F592, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Sun R, Miller RL, Hemmert AC, Zhang P, Shi H, Nelson RD, Kishore BK. Chronic dDAVP infusion in rats decreases the expression of P2Y2 receptor in inner medulla and P2Y2 receptor-mediated PGE2 by IMCD. Am J Physiol Renal Physiol 289: F769–F776, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Sun R, Nelson RD, Carlson NG, Miller RL, Shi H, Zhang P, Hemmert AC, Kohan DE, Kishore BK. Enhanced purinergic-mediated PGE2 release from the medullary collecting duct of rats with acquired nephrogenic diabetes insipidus. Pathophysiological and therapeutic implications (Abstract). FASEB J 19: A583, 2005 [Google Scholar]
- 51.Welch BD, Carlson NG, Shi H, Myatt L, Kishore BK. P2Y2 receptor-stimulated release of prostaglandin E2 by rat inner medullary collecting duct preparations. Am J Physiol Renal Physiol 285: F711–F721, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Wildman SSP, Marks J, Turner CM, Yew-Booth L, Peppiate-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ. Sodium-dependent regulation of amiloride sensitive currents by apical P2 receptors. J Am Soc Nephrol 19: 731–742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanagisawa H, Moridaira K, Nodera M, Wada O. Ureteral obstruction enhances eicosanoid production in cortical and medullary tubules of rat kidneys. Kidney Blood Press Res 20: 398–405, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Zeidel ML, Brady HR, Kohan DE. Interleukin-1 inhibition of Na+-K+-ATPase in inner medullary collecting duct cells: role of PGE2. Am J Physiol Renal Fluid Electrolyte Physiol 262: F1013–F1016, 1992 [DOI] [PubMed] [Google Scholar]
- 55.Zelenina M, Christensen BM, Palmer J, Narin AC, Nielsen S, Aperia A. Prostaglandin E2 interaction with AVP: effect of AQP2 phosphorylation and distribution. Am J Physiol Renal Physiol 278: F338–F394, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Kohan DE, Nelson RD, Carlson NG, Kishore BK. Potential involvement of P2Y2 receptor in the diuresis of post-obstructive uropathy in rats (Abstract). J Am Soc Nephrol 20: 827A, 2009 [Google Scholar]
- 57.Zhang Y, Nelson RD, Carlson NG, Kamerath CD, Kohan DE, Kishore BK. Potential role of purinergic signaling in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 296: F1194–F1201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Sands JM, Kohan DE, Nelson RD, Martin CF, Carlson NG, Kamerath CD, Ge Y, Klein JD, Kishore BK. Potential role of purinergic signaling in urinary concentration in inner medulla: insights from P2Y2 receptor gene knockout mice. Am J Physiol Renal Physiol 295: F1715–F1724, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]