Abstract
Studies have shown that certain cells of the glomerular tuft begin to express proteins considered unique to other cell types upon injury. Little is known about the response of parietal epithelial cells (PEC) to injury. To determine whether PECs change their phenotype upon injury to also express proteins traditionally considered podocyte specific, the following four models of glomerular disease were studied: the transforming growth factor (TGF)-β1 transgenic mouse model of global glomerulosclerosis, the adriamycin model of focal segmental glomerulosclerosis (FSGS), the anti-glomerular basement membrane (GBM) model of crescentic glomerulonephritis, and the passive Heymann nephritis model of membranous nephropathy. Double immunostaining was performed with antibodies to podocyte-specific proteins (synaptopodin and Wilms' tumor 1) and antibodies to PEC specific proteins (paired box gene 8 and claudin-1). No double staining was detected in normal mice. In contrast, the results showed a statistical increase in the number of cells attached to Bowman basement membrane that were double-positive for both podocyte/PEC proteins in TGF-β;1 transgenic, anti-GBM, and membranous animals. Double-positive cells for both podocyte and PEC proteins were also statistically increased in the glomerular tuft in TGF-β1 transgenic, anti-GBM, and FSGS mice. These results are consistent with glomerular cells coexpressing podocyte and PEC proteins in experimental glomerular disease, but not under normal circumstances.
Keywords: glomerulonephritis, differentiation, phenotype, FSGS, membranous nephropathy
a large body of evidence shows that the terminally differentiated epithelial cells called podocytes have a vey low proliferative capacity (15, 25, 32, 34). This is one of the major reasons underlying the decline in overall podocyte number in experimental and human glomerular diseases that are characterized by podocyte detachment and/or apoptosis. Reduced podocyte number leads to proteinuria and glomerular scarring (33). Similarly, in many disease states, podocytes are unable to maintain their highly specialized phenotype and begin to lose expression of the proteins that define their function (5, 12, 14, 18, 20, 27, 31, 35, 37, 38). These observations raise several questions: are there conditions under which podocyte replenishment occurs when podocytes are lost, and if so, what is the cellular precursor of these regenerating podocytes? When podocytes lose their specialized phenotype, are they able to regain it?
Parietal epithelial cells (PECs) have received recent attention as they and podocytes share a common lineage until the S-shaped stage of glomerulogenesis. Between the S-shaped body and capillary loop stages, PECs and podocytes begin to express unique genes specific to each cell's function. In podocytes, the transcription factor Wilms' tumor 1 (WT-1) and the actin cytoskeleton linking the protein synaptopodin are expressed exclusively in the podocyte at this stage. In PECs, the transcription factor paired box gene 8 (PAX8) and the tight junction protein claudin 1 are expressed during this stage (21, 22). Thereafter. these proteins are constitutively expressed in each cell type in mature glomeruli and contribute to functional characteristics of each cell type.
Both the shared common lineage between PECs and podocytes and the close proximity of PECs to podocytes make PECs good potential candidates as podocyte precursors. Moreover, some have suggested the existence of unique cells called parietal podocytes (4, 8). In contrast to podocytes, PECs readily proliferate in certain forms of glomerular disease. More recently, studies by Appel et al. (2) shed new light on the notion of a local podocyte precursor function for PECs in the developing kidney. In the current studies, we sought to determine whether following injury, PECs express podocyte-specific proteins and/or whether following injury, podocytes express PEC proteins. Four models of experimental glomerular diseases were analyzed.
METHODS
Animal studies.
Four types of animal models were examined in this study. First, the transforming growth factor (TGF)-β1 transgenic mouse has been previously described (30). The alb/TGF-β1 mice carry a construct with constitutively activated TGF-β1 (protein sequence identical between mouse and human) under the control of the murine albumin promoter, resulting in elevated circulating plasma levels of active TGF-β1 as early as 2 wk of age, podocyte injury at 2 wk of age, and glomerulosclerosis at 3 wk of age. Alb/TGF-β1 mice and control C57Bl6 X CBA mice were maintained at the animal care facilities at the National Institutes of Health under a protocol approved by the Animal Care and Use Committee, according to Guide for the Care and Use of Laboratory Animals.
The second model utilized was the adriamycin (ADR)-induced experimental model of focal segmental glomerulosclerosis (FSGS) in mice. ADR nephropathy was induced in male Balb/c mice, aged 12 wk, by tail vein injection of ADR (12 mg/kg body wt × 2; n = 8/group), following a protocol that was a modification of methods previously described (6, 7). For predictable and reproducible induction of sustained proteinuria and progressive glomerulosclerosis, two doses, separated by 4 wk, were utilized in our studies. Control animals were injected with equal volumes of vehicle only (0.9% NaCl). Successful induction of proteinuria was confirmed by measuring the protein concentration of the collected urine as previously described (19). Mice were killed 8 wk after disease induction.
The third model used was the anti-glomerular basement membrane nephritis model. Sheep anti-rabbit glomeruli antibody-induced experimental crescentic glomerulonephritis was produced as previously published (24). Briefly, polyclonal antibody was produced by immunizing sheep with whole rabbit glomeruli. Antiserum was heat inactivated, and IgG was isolated using caprylic acid precipitation of serum proteins. Experimental crescentic glomerulonephritis was induced in Balb/c mice (Charles River Laboratories, Wilmington, MA) by intraperitoneal injection of sheep anti-rabbit GBM IgG (15 mg/20 g body wt). Mice were killed at day 9.
The final model was the rat model of membranous nephropathy, the passive Heymann nephritis (PHN) model. PHN was induced in male Sprague-Dawley rats (Charles River,) weighing 300–350 g, with a single intraperitoneal injection of sheep anti-rat tubular fraction 1a (Fx1a) antibody (5 ml/kg body wt) as previously described (26). Rats were housed in the animal care facility of the University of Washington under standardized pathogen-free conditions (25°C, 50% humidity, 12:12-h light-dark cycle) with food and water available ad libitum. The experimental protocols were approved by the University of Washington Animal Care Committee (9). All animal procedures were conducted in accordance with the Institutional Animal Care and Use Committee.
Immunostaining of kidney sections.
For indirect immunoperoxidase staining, renal biopsies from mice and rats were fixed in neutralized formalin and embedded in paraffin. Indirect immunoperoxidase staining was performed on 4-μm sections as previously reported (23) with minor modifications.
In brief, paraffin was removed using Histoclear (National Diagnostics, Atlanta, GA), and sections were rehydrated in ethanol. Antigen retrieval was performed by boiling sections in the microwave in 1 mM EDTA, pH 8.0 (for double staining of WT-1 and claudin-1) or 1 mM EDTA, pH 6.0 (for PAX8 and double staining of PAX8 and synaptopodin). Endogenous peroxidase activity was quenched with 3% hydrogen peroxidase, and nonspecific protein binding was blocked with Background Buster (Accurate Chemical & Scientific, Westbury, NY) and endogenous biotin activity was quenched with a Avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). After blocking, tissue sections were incubated overnight at 4°C with the primary antibodies listed in Table 1. The appropriate biotinylated secondary antibodies anti-rabbit IgG, anti-mouse IgG, or anti-guinea pig IgG (Vector Laboratories) were applied followed by an R.T.U. Vectastain kit (Vector Laboratories).
Table 1.
Summary of changes in cell numbers
| PEC Number | BBM (PEC+/podo+) | Tuft (PEC+/podo+) | |
|---|---|---|---|
| TGF-β1 transgenic | ↑ | ↑ | ↑ |
| ADR | ↔ | ↔ | ↑ |
| Anti-GBM | ↑ | ↑ | ↑ |
| PHN | ↑ | ↑ | ↔ |
PEC, parietal epithelial cells; BBM, Bowman's basement membrane; TGF, transforming growth factor; podo, podocytes; ADR, adriamycin; GBM, glomerular basement membrane; PHN, passive Heymann nephritis.
For synaptopodin staining, a Promark mouse on mouse kit (Biocare Medical) was used for additional blocking and substitutive secondary antibody according to the manufacturer's protocol. Staining was visualized by precipitation of diaminobenzidine (Sigma).
For double staining of PAX8 and synaptopodin, synaptopodin was stained first as described above, and then PAX8 Ab was applied and color was developed using Vector SG (Vector).
For double staining of claudin-1 and WT-1, because both of these antibodies were developed in rabbit, additional blocking was performed between the first (claudin-1) and second (WT-1) set of antibodies. After diaminobenzidine color development of claudin-1 staining, the activity of the secondary antibody used for claudin-1 staining was blocked with rabbit nonreactive IgG (Abcam) followed by an anti-rabbit IgG antibody Fab fragment (Jackson ImmunoResearch). In addition, peroxidase activity and biotin activity derived from first set of staining were blocked using 3% hydrogen peroxidase and the Avidin/Biotin blocking system (Vector).
Quantification of positively stained cells was performed with more than 30 glomeruli/animal. To evaluate preliminary changes following injury, crescentic glomeruli were excluded from quantification in the anti-GBM nephritis model.
Statistical analysis.
Statistical comparisons were analyzed by Student's t-test. GraphPad Prism version 5 for Windows (GraphPad Software, San Diego, CA; www.graphpad.com) was used for data analysis.
RESULTS
PEC cell number increases in TGF-β1 transgenic mice.
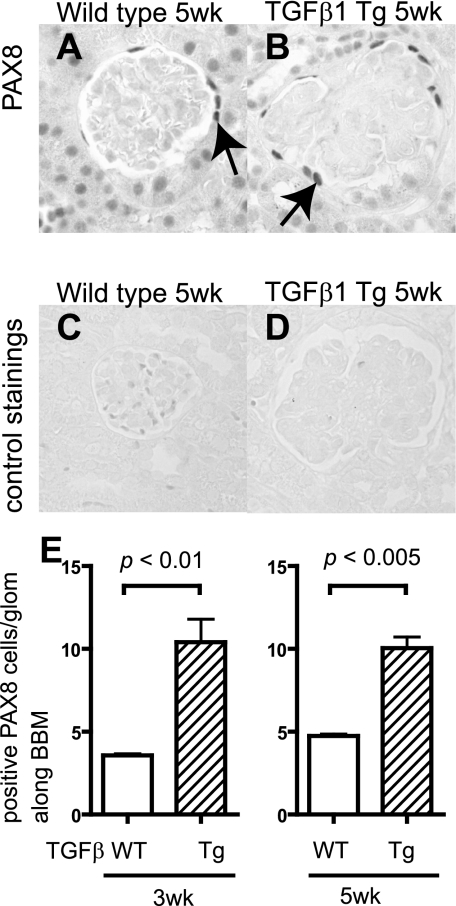
Reports show that within the glomerulus, PAX8 staining is normally restricted to PECs (36). Our findings in this study validate these observations. Accordingly, staining was performed for PAX8 in both wild-type and transgenic mice at 3 and 5 wk of age (Fig. 1, A–D), and the number of PAX8-positive cell was quantified. Figure 1E shows a significant increase in the number of cells with staining for PAX8 along Bowman's capsule in TGF-β1 mice at 3 wk (P < 0.01 vs. wild-type) and 5 wk (P < 0.005 vs. wild-type).
Fig. 1.
Paired box gene 8 (PAX8) staining in transforming growth factor (TGF)-β1 transgenic (Tg) mice. PAX8 staining was performed to evaluate the change in the number of parietal epithelial cells (PECs) in wild-type (WT; A and C) and TGF-β1 Tg mice (B and D). C and D: the primary antibody was omitted as a control. E: statistical analysis showed a significant increase in the number of PECs in TGF-β1 Tg mice at both 3 and 5 wk of age.
Coexpression of podocyte and PEC proteins in TGF-β1 transgenic mice.
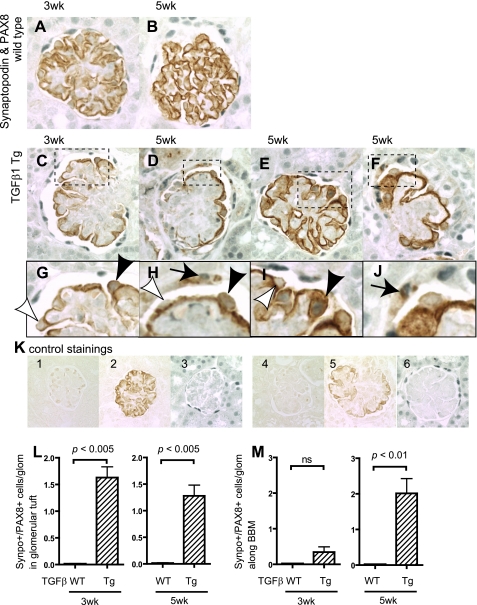
To examine the expression of podocyte and PEC proteins in cells along Bowman's basement membrane (BBM), double immunohistochemical staining was performed with the combination of primary antibodies to one podocyte protein and one PEC protein. Figure 2 shows the staining results using antibodies to synaptopodin and PAX8 representing podocyte and PEC proteins, respectively. No cells in the glomerular tuft or along BBM stained positive for both synaptopodin and PAX8 in wild-type mice (Fig. 2, A and B). This was not a false negative, because single staining was present when either of the primary antibodies was omitted. Two interesting observations were made in the TGF-β1 transgenic mice. First, there was a statistically significant increase in the number of double-positive synaptopodin/PAX8-staining cells along the glomerular tuft at 3 wk (P < 0.005 vs. wild-type) and 5 wk (P < 0.005 vs. wild-type) of age (Fig. 2L). These cells were limited to the periphery of the glomerular tuft (Fig. 2, G–I). Second, there was a progressive increase in the number of double-positive synaptopodin/PAX8-staining cells lining BBM in TGF-β1 transgenic mice by 5 wk of age (P < 0.01 vs. wild-type) (Fig. 2M). Appropriate controls for the immunostaining showed that these results were not false positives (Fig. 2, K1–6).
Fig. 2.
Double-positive cells for synaptopodin and PAX8 in TGF-β1 Tg mice. Double-positive cells for synaptopodin and PAX8 were evaluated in WT mice at 3 (A) and 5 wk (B) as well as in TGF-β1 Tg mice at 3 (C) and 5 wk (D–F). Higher magnification images are also shown (G–J). Arrows indicate double-positive cells along Bowman's basement membrane (BBM; G–J). Black arrowheads show double-positive cells in tuft, while white arrowheads show single-positive (synaptopodin-positive, PAX8-negative) cells (G–J). K: images of controls in which primary antibodies were omitted. Images from WT mice with no primary antibody (K1), with synaptopodin only (K2), and PAX8 only (K3) and from TGF-β1 Tg mice with no primary antibody (K4), with synaptopodin only (K5), and PAX8 only (K6) are shown. Double-positive cells were quantified, and results are shown in L and M. Double-positive cells for synaptopodin and PAX8 in the glomerular tuft significantly increased (L, 3 and 5 wk) as well as along BBM (M, 5 wk). Synpo, synaptopodin.
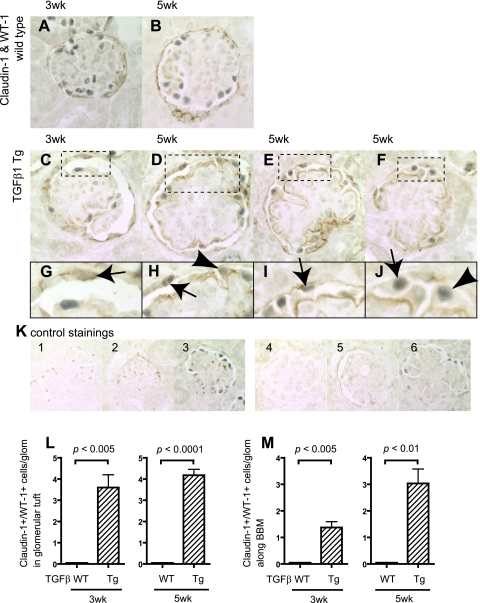
Double immunostaining was also performed for the podocyte protein WT-1 (Wilms' tumor antigen) and the PEC protein claudin-1 in wild-type and TGF-β1 transgenic mice, and these results are shown in Fig. 3. Cells positive for both claudin-1 and WT-1 were not detected in the glomerular tuft, nor along BBM in wild-type mice (Fig. 3, A and B). In contrast, cells positive for both claudin-1 and WT-1 were detected in glomerular tuft of both 3-wk (P < 0.005 vs. wild-type)- and 5-wk (P < 0.001 vs. wild-type)-old TGF-β1 transgenic mice (Fig. 3L).
Fig. 3.
Double-positive cells for claudin-1 and Wilms' tumor 1 (WT-1) in TGF-β1 Tg mice. Double-positive cells for claudin-1 and WT-1 were evaluated in WT mice at 3 (A) and 5 wk (B) as well as in TGF-β1 Tg mice at 3 (C) and 5 wk (D–F). Higher magnification images are also shown (G–J). Arrows indicate the double-positive cells along BBM (G–J), and arrowheads show the double-positive cells in tuft (G–J). K: images of controls in which primary antibodies were omitted. Images from WT mice with no primary antibody (K1), with claudin-1 only (K2), and WT-1 only (K3) and from TGF-β1 Tg mice with no primary antibody (K4), with claudin-1 only (K5), and WT-1 only (K6) are shown. Double-positive cells were quantified, and results are shown in L and M. Double-positive cells for claudin-1 and WT-1 in tuft significantly increased (L, 3 and 5 wk) as well as along BBM (M, 3 and 5 wk).
Staining for both claudin-1 and WT-1 were also detected in cells along BBM (Fig. 3, C–J), and this increase was statistically significant in transgenic mice at 3 wk (P < 0.001 vs. wild-type) and 5 wk (P < 0.001 vs. wild-type) of age (Fig. 3M). Taken together, these results show that there was a significant increase in the number of cells expressing both podocyte and PEC proteins within the glomerular tuft and along BBM in TGF-β1 transgenic mice at 3 and 5 wk of age.
Coexpression of podocyte and PEC proteins in ADR mice.
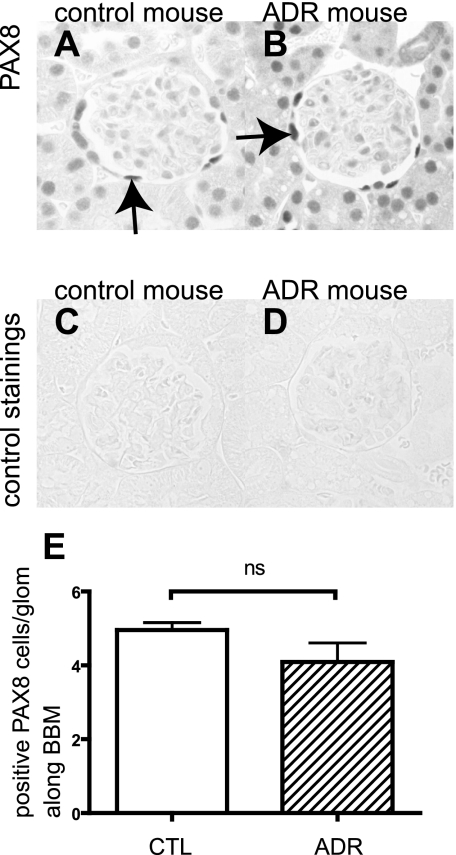
The ADR mouse model of minimal change/FSGS was used to determine whether PECs also stain positive for podocyte proteins in a second model of glomerular injury characterized by podocyte injury. Proteinuric mice were studied 8 wk after disease induction, and the results are shown in Fig. 4. PAX8 was stained to evaluate the change in the number of PAX8-positive PECs along BBM following injury. In contrast to the TGF-β1 transgenic mice, there was no significant difference in the number of PAX8-positive cells between ADR mice and control mice (Fig. 4E).
Fig. 4.
PAX8 staining in adriamycin (ADR) nephropathy mice. PAX8 was stained to evaluate the change in the number of PECs in control (A and C) and ADR mice (B and D). C and D: primary antibody-omitted staining control. E: no statistical difference was observed between ADR nephropathy mice and control mice.
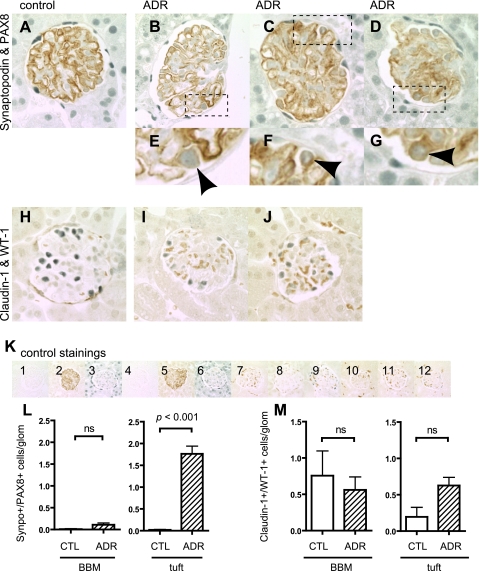
To determine whether cells expressed both podocyte and PEC proteins, double immunostaining was performed for synaptopodin and PAX8, and for WT-1 and claudin-1, and the results are shown in Fig. 5. Double staining for synaptopodin/PAX8 was barely detected in cells lining BBM in control or ADR mice (Fig. 5L). In contrast, there was a significant increase in cells double stained for synaptopodin/PAX8 in the glomerular tuft of ADR mice (P < 0.001 vs. control mice) (Fig. 5L).
Fig. 5.
Double-positive cells in ADR nephropathy mice. Double-positive cells for synaptopodin and PAX8 as well as claudin-1 and WT-1 were evaluated in ADR mice 8 wk after disease induction. Double-staining images of control (A) and ADR mice (B–D: full images of glomeruli; E–G: high magnification) for synaptopodin and PAX8 are shown. Arrowheads indicate the double-positive cells in tuft (E–G). Double-staining images are shown for control (H) and ADR mice (I and J) for claudin-1 and WT-1. Most of cells did not show double-positive signals. K: images of controls in which primary antibodies were omitted for synaptopodin and PAX8 (K1–6) and for claudin-1 and WT-1 (K7–12). Images from control mice with no primary antibody (K1), with synaptopodin only (K2), and PAX8 only (K3); from ADR mice with no primary antibody (K4), with synaptopodin only (K5), and PAX8 only (K6); from control mice with no primary antibody (K7), with claudin-1 only (K8), and WT-1 only (K9); and from ADR mice with no primary antibody (K10), with claudin-1 only (K11), and WT-1 only (K12) are shown. Double-positive cells were quantified, and results are shown in L and M. Double-positive cells for synaptopodin and PAX8 in the tuft significantly increased (L), but no statistical difference was observed in cells along BBM (L). No difference was observed in double staining of claudin-1 and WT-1 in either cells along BBM or in tuft.
Claudin-1 and WT-1 double staining was performed as a second method to examine the coexpression of podocyte and PEC proteins. Occasional double-positive cells were detected in both the glomerular tuft and lining BBM (Fig. 5M). However, there were no differences between diseased and control animals. Taken together, these results show that although there (21, 22) few cells with double-positive staining for podocyte-PEC proteins along BBM, there was an increase in cells that expressed synaptopodin/PAX8 within the glomerular tuft in the ADR mouse model of minimal change/FSGS disease.
Coexpression of podocyte and PEC proteins in anti-GBM nephritis mice.
The anti-GBM model of glomerulonephritis is characterized by injury to both podocytes and PECs, with marked proliferation of PECs and, less commonly, podocytes, leading to crescent formation. To investigate whether PECs express podocyte proteins in anti-GBM nephritis mice, we examined animals 9 days after disease induction.
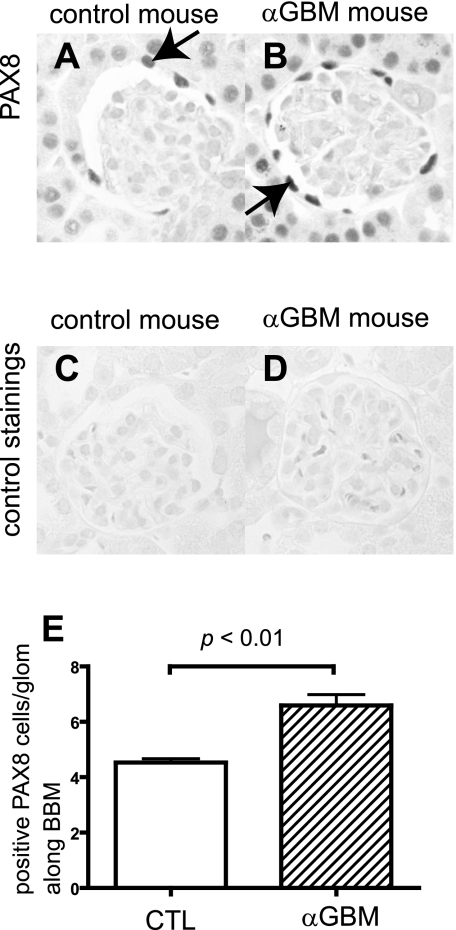
Staining for PAX8 was performed to determine changes in the number of PAX8-positive cells (PECs) along BBM during disease. There was a significant increase in PAX8-positive cells at day 9 in anti-GBM nephritis, even in glomeruli without crescents (P < 0.001 vs. normal) (Fig. 6E).
Fig. 6.
PAX8 staining in anti-glomerular basement membrane (GBM) nephritis mice. PAX8 was stained to evaluate the change in the number of PECs in control mice (A and C) and anti-GBM mice (B and D) 9 days after disease induction. C and D: primary antibody-omitted staining control. E: statistical analysis showed a significant increase in the number of PECs in anti-GBM mice.
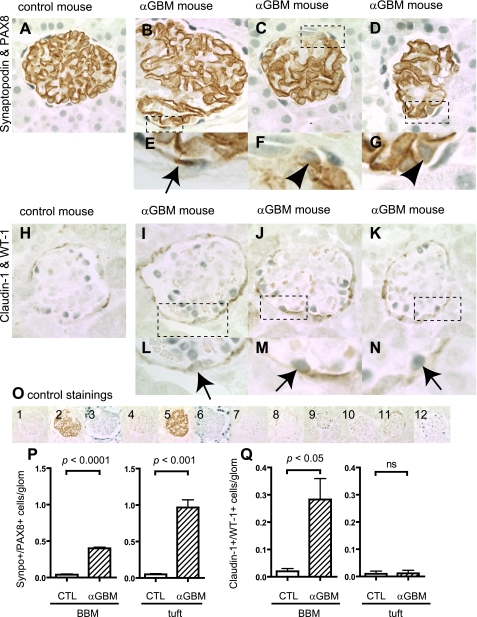
Double staining was performed for synaptopdin and PAX8, as well as claudin-1 and WT-1, and the results are shown in Fig. 7. As expected, cells staining positive for both synaptopodin and PAX8 were not detected in the glomerular tuft nor along BBM in control animals. In contrast, there was a significant increase in the number of cells staining positive for both synaptopodin and PAX8 in the glomerular tuft (P < 0.001 vs. control) and in cells along BBM (P < 0.01 vs. control) in mice with anti-GBM disease (Fig. 7P). Staining for synaptopodin did not significantly decrease in the glomerular tuft in glomeruli without crescents.
Fig. 7.
Double positive cells in anti-GBM nephritis mice. Double-positive cells for synaptopodin and PAX8 as well as claudin-1 and WT-1 were evaluated in anti-GBM nephritis mice 9 days after disease induction. Double-staining images of control (A) and anti-GBM mice (B–D: full images of glomeruli, E–G: high magnification) for synaptopodin and PAX8 are shown. Arrow indicates the double-positive cells along BBM (E), and arrowheads indicate the double-positive cells in tuft (F and G). Double-staining images of control (H) and anti-GBM mice (I–K: full images of glomeruli, L–N: high magnification) for claudin-1 and WT-1 are shown. Arrows indicate the double-positive cells along BBM (L–N). O: images of controls in which primary antibodies were omitted for synaptopodin and PAX8 (O1–6), and for claudin-1 and WT-1 (O7–12). Images from control mice with no primary antibody (O1), with synaptopodin only (O2), and PAX8 only (O3); from anti-GBM mice with no primary antibody (O4), with synaptopodin only (O5), PAX8 only (O6); from control mice with no primary antibody (O7), with claudin-1 only (O8), and WT-1 only (O9); and from anti-GBM mice with no primary antibody (O10), with claudin-1 only (O11), and WT-1 only (O12) are shown. Double-positive cells were quantified, and results are shown in P and Q. Double-positive cells for synaptopodin and PAX8 significantly increased in tuft and along BBM (P). Double-positive cells for claudin-1 and WT-1 also increased significantly along BBM, but an increase was not observed in tuft (Q).
As expected, claudin-1 and WT-1 double staining was not detected in control animals. Similarly, there was no increase in cells staining double-positive within the glomerular tuft in mice with anti-GBM disease (Fig. 7Q). In contrast, there was a significant increase in cells staining double-positive for claudin-1 and WT-1 along the lining of BBM in anti-GBM disease (P < 0.05 vs. control) (Fig. 7Q).
Taken together, these results show positive staining of two combinations of podocyte-PEC proteins in cells lining BBM in mice with anti-GBM disease, which was not detected in control animals.
Coexpression of podocyte and PEC proteins in experimental membranous nephropathy.
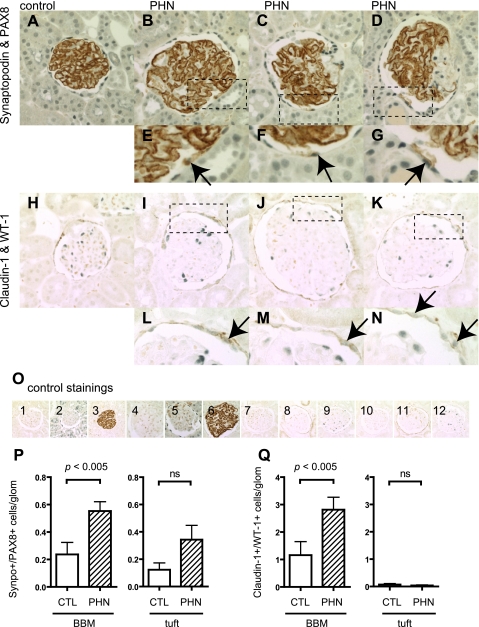
The PHN rat model of membranous nephropathy is characterized by antibody- and complement-induced podocyte injury, leading to reduced podocyte number and alterations in certain podocyte-specific proteins. To best investigate the effect of the reduced podocyte number in this model, the PHN model was studied during a more advanced disease stage where podocyte number is reduced (day 110).
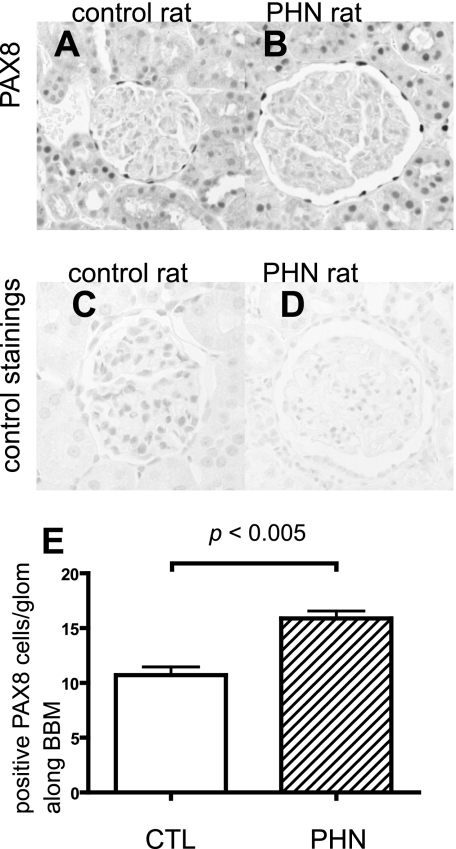
To evaluate any change in the number of PECs along BBM in control and diseased animals, staining for PAX8 was performed. There was a significant increase in the number of cells staining positive for PAX8 along BBM in PHN at day 110 (Fig. 8E).
Fig. 8.
PAX8 staining in passive Heymann nephritis (PHN) rats. PAX8 was stained to evaluate the change in the number of PECs in control rats (A and C) and PHN rats (B and D) at day 110. C and D: primary antibody-omitted staining control. E: statistical analysis showed a significant increase in the number of PECs in PHN rats.
To determine whether glomerular cells expressed both podocyte and PEC proteins, double staining was performed for synaptopodin and PAX8, as well as claudin-1 and WT-1, in control and PHN rats. As expected, cells staining positive for both synaptopodin and PAX8 were not detected in either the glomerular tuft or along BBM in control animals. There was a significant increase in the number of cells along BBM double-staining for synaptopodin and PAX8 (P < 0.05 vs. control) (Fig. 9P). In contrast, no increase was detected in the glomerular tuft of PHN rats, although a trend toward an increase was observed.
Fig. 9.
Double-positive cells in PHN rats. Double-positive cells for synaptopodin and PAX8 as well as claudin-1 and WT-1 were evaluated in PHN rats at day 110. Double-staining images of control (A) and PHN rats (B–D: full images of glomeruli, E–G: high magnification) for synaptopodin and PAX8 are shown. Arrows indicate the double-positive cells along BBM (E–G). Double-staining images of control (H) and PHN rats (I–K: full images of glomeruli, L–N: high magnification) for claudin-1 and WT-1 are shown. Arrows indicate the double-positive cells along BBM (L–N). O: images of controls in which primary antibodies were omitted for synaptopodin and PAX8 (O1–6), and for claudin-1 and WT-1 (O7–12). Images from control rats with no primary antibody (O1), with synaptopodin only (O2), and PAX8 only (O3); from PHN rats with no primary antibody (O4), with synaptopodin only (O5), and PAX8 only (O6); from control mice with no primary antibody (O7), with claudin-1 only (O8), and WT-1 only (O9); and from PHN rats with no primary antibody (O10), with claudin-1 only (O11), and WT-1 only (O12) are shown. Double-positive cells were quantified, and results are shown in P and Q. Double-positive cells for synaptopodin and PAX8 significantly increased in cells along BBM (P). Also, double-positive cells for claudin-1 and WT-1 increased significantly along BBM (Q). However, an increase was not observed in cells in tuft for either synaptopodin and PAX8 (P) or claudin-1 and WT-1 (Q).
Double staining for claudin-1 and WT-1 is shown in Fig. 9Q. As expected, claudin-1 and WT-1 double staining was not detected in control animals. Double staining was not detected in the glomerular tuft of PHN rats. In contrast, there was a significant increase in cells lining BBM that stained positive for claudin-1 and WT-1 (P < 0.05 vs. control). Taken together, these results show a significant increase in cells along BBM staining positive for both PEC and podocyte proteins.
Results from all four models used in this study are summarized in Table 1. Among these four models, TGF-β1 transgenic mice and anti-GBM nephritis mice showed the most striking findings, with increases in all three parameters examined. Meanwhile, ADR mice and PHN rats showed an increase in double-positive cells for PECs and podocyte proteins either in the tuft or along BBM, respectively. In addition, there was a correlation between an increase in the number of PECs on the BBM and double-positive cells for PECs and podocyte proteins along BBM.
DISCUSSION
The kidney glomerulus is a complex structure comprising four resident cell types, each identified by the expression of proteins specific to that cell type. These proteins typically serve functions specific to each cell type (13, 23, 36). Although the biology and function of podocytes, mesangial, and glomerular endothelial cells are quite well defined, little is known about PECs. We began by examining PEC number in normal and disease states. Studies have previously shown an increased PEC number in the anti-GBM model. Using this as a positive control, we also examined PEC number in the ADR model of FSGS, the PHN model of membranous nephropathy, and the TGF-β transgenic mouse model of glomerulosclerosis using PAX8 immunostaining. Within the glomerulus, PAX8 is recognized as being a marker of PECs.
A major finding was that the absolute number of PECs increased in TGF-β1 transgenic mice, the anti-GBM model, and also in the PHN model in noncrescentic glomeruli. While the increase in the first two models was somewhat expected, this is the first report to our knowledge showing increased PEC number in experimental membranous nephropathy.
We next asked what might be the meaning of increased PEC number in experimental glomerular disease. To this end, we performed double immunostaining with PAX8 and synaptopodin to evaluate the possibility that following injury, PECs might coexpress proteins considered PEC and podocyte specific. Indeed, the results showed that cells attached to BBM double stained for both synaptopodin and PAX8 in TGF-β transgenic mice, anti-GBM nephritis, and experimental membranous nephropathy. The increase in double-stained cells was significant as no double-stained cells were detected in normal mice.
To validate this further, double staining was then performed with claudin-1 (PEC marker) and WT-1 (podocyte marker). Claudin-1 and WT-1 double staining was not detected in normal animals. In contrast, the results showed a significant increase in the number of cells attached to BBM double-stained positive for claudin-1 and WT-1 in these three experimental glomerular injury models. Double-stained cells were not detected in the ADR-induced model of FSGS. Appropriate controls for the staining techniques confirmed that these results were not false positives. Thus the data showed that cells along BBM have phenotypic features of both PECs and podocytes, defined by costaining for proteins normally expressed by each cell type.
Two possibilities might explain these findings in disease. First, based on the location of the cells along BBM, one can speculate that the original cells (PECs) are differentiating toward becoming podocytes, as defined by the coexpression of a podocyte protein in addition to their “native” PEC proteins. A second possibility is that podocytes injured during disease begin to coexpress PEC proteins in addition to podocyte proteins, consistent with the notion that podocytes are differentiating toward becoming PECs. The latter would have to account for podocytes moving from the glomerular tuft to BBM.
One might ask why a glomerular cell would begin to express proteins considered specific to another cell type following injury. Indeed, this has been shown in other kidney cells. For example, mesangial cells express myofibroblast-like proteins (α-smooth muscle actin), tubular epithelial cells undergo epithelial-mesenchymal transition (EMT), and glomerular epithelial cells can also undergo EMT and start to express muscle-like proteins (1, 3, 10, 11, 16, 17, 28, 29, 39). The precise functional advantages or disadvantages of these phenotypic changes remain unclear. Another explanation is that cells revert to a more immature phenotype upon injury. Third, cells differentiate from one cell type to another and take on the characteristics and functions of the “new” cell. Stem cells are an example of the latter. Exciting work by Romagnani and colleagues (19a, 28a, 28b, 28c) validate the notion of PECs as a local stem cell. They elegantly describe stem/progenitor cells lining BBM in adult human kidneys. The authors can only speculate that in the studies conducted here, PECs serve to differentiate into podocytes under certain conditions, which remain poorly defined. Studies by Appel et al. (2) support, but do not prove, this notion. Definitive studies are needed.
Finally, the results in the current studies show that cells in the glomerular tuft coexpress both podocyte and PEC proteins in the anti-GBM model, TGF-β transgenic mice, and the ADR model of FSGS. One interpretation of these results is that injured podocytes begin to express PEC proteins. Another is that PECs, originally located along BBM, coexpress podocyte proteins and have moved from their original location to the edge of the glomerular tuft. The triggers and programs for these events are not known and need to be studied in more detail.
In summary, the data presented here show that cells in the glomerulus located along the BBM, and to a lesser extent cells on the periphery of the glomerular tuft, begin to coexpress proteins of both PEC and podocyte origin. Consideration should be given to specific glomerular cell differentiation upon injury in experimental disease. The functional consequences of this need to be further delineated.
GRANTS
This work was supported by a National Institutes of Health to S. J. Shankland. J. B. Kopp is supported by the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program (ZO1 DK043308).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Alpers CE, Hudkins KL, Gown AM, Johnson RJ. Enhanced expression of “muscle-specific” actin in glomerulonephritis. Kidney Int 41: 1134–1142, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bariety J, Hill GS, Mandet C, Irinopoulou T, Jacquot C, Meyrier A, Bruneval P. Glomerular epithelial-mesenchymal transdifferentiation in pauci-immune crescentic glomerulonephritis. Nephrol Dial Transplant 18: 1777–1784, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bariety J, Mandet C, Hill GS, Bruneval P. Parietal podocytes in normal human glomeruli. J Am Soc Nephrol 17: 2770–2780, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Barisoni L, Kriz W, Mundel P, D'Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis, and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Chen A, Sheu LF, Ho YS, Lin YF, Chou WY, Chou TC, Lee WH. Experimental focal segmental glomerulosclerosis in mice. Nephron 78: 440–452, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Chen A, Wei CH, Sheu LF, Ding SL, Lee WH. Induction of proteinuria by adriamycin or bovine serum albumin in the mouse. Nephron 69: 293–300, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Gibson IW, Downie I, Downie TT, Han SW, More IA, Lindop GB. The parietal podocyte: a study of the vascular pole of the human glomerulus. Kidney Int 41: 211–214, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Griffin SV, Krofft RD, Pippin JW, Shankland SJ. Limitation of podocyte proliferation improves renal function in experimental crescentic glomerulonephritis. Kidney Int 67: 977–986, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Johnson RJ, Floege J, Yoshimura A, Iida H, Couser WG, Alpers CE. The activated mesangial cell: a glomerular “myofibroblast”? J Am Soc Nephrol 2: S190–S197, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest 87: 847–858, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly DJ, Aaltonen P, Cox AJ, Rumble JR, Langham R, Panagiotopoulos S, Jerums G, Holthofer H, Gilbert RE. Expression of the slit-diaphragm protein, nephrin, in experimental diabetic nephropathy: differing effects of anti-proteinuric therapies. Nephrol Dial Transplant 17: 1327–1332, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Koop K, Eikmans M, Baelde HJ, Kawachi H, De Heer E, Paul LC, Bruijn JA. Expression of podocyte-associated molecules in acquired human kidney diseases. J Am Soc Nephrol 14: 2063–2071, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Kriz W. Progressive renal failure—inability of podocytes to replicate, and the consequences for development of glomerulosclerosis. Nephrol Dial Transplant 11: 1738–1742, 1996 [PubMed] [Google Scholar]
- 16.Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction, and proteinuria. Am J Pathol 172: 299–308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Luimula P, Ahola H, Wang SX, Solin ML, Aaltonen P, Tikkanen I, Kerjaschki D, Holthofer H. Nephrin in experimental glomerular disease. Kidney Int 58: 1461–1468, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Mancini G, Carbonara AO, Heremans JF. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry 2: 235–254, 1965 [DOI] [PubMed] [Google Scholar]
- 19a.Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, Parente E, Mancina R, Netti GS, Becherucci F, Gacci M, Carini M, Gesualdo L, Rotondi M, Maggi E, Lasagni L, Serio M, Romagnani S, Romagnani P. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med 205: 479–490, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakatsue T, Koike H, Han GD, Suzuki K, Miyauchi N, Yuan H, Salant DJ, Gejyo F, Shimizu F, Kawachi H. Nephrin, and podocin dissociate at the onset of proteinuria in experimental membranous nephropathy. Kidney Int 67: 2239–2253, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M. Pax2, and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol 18: 1121–1129, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Ohse T, Chang AM, Pippin JW, Jarad G, Hudkins KL, Alpers CE, Miner JH. Shankland SJ: A new function for parietal epithelial cells: a second glomerular barrier. Am J Physiol Renal Physiol 297: F1566–F1574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohse T, Pippin JW, Vaughan MR, Brinkkoetter PT, Krofft RD, Shankland SJ. Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture. J Am Soc Nephrol 19: 1879–1890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ophascharoensuk V, Pippin JW, Gordon KL, Shankland SJ, Couser WG, Johnson RJ. Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int 54: 416–425, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Pabst R, Sterzel RB. Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int 24: 626–631, 1983 [DOI] [PubMed] [Google Scholar]
- 26.Petermann AT, Krofft R, Blonski M, Hiromura K, Vaughn M, Pichler R, Griffin S, Wada T, Pippin J, Durvasula R, Shankland SJ. Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int 64: 1222–1231, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Pippin J, Kumar V, Stein A, Jablonski P, Shankland SJ, Davis CL. The contribution of podocytes to chronic allograft nephropathy. Nephron Exp Nephrol 111: e1–e10, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Rastaldi MP, Ferrario F, Giardino L, Dell'Antonio G, Grillo C, Grillo P, Strutz F, Muller GA, Colasanti G, D'Amico G. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int 62: 137–146, 2002 [DOI] [PubMed] [Google Scholar]
- 28a.Romagnani P. Toward the identification of a “renopoietic system”? Stem Cells. 27: 2247–2253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28b.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28c.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol. 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Sam R, Wanna L, Gudehithlu KP, Garber SL, Dunea G, Arruda JA, Singh AK. Glomerular epithelial cells transform to myofibroblasts: early but not late removal of TGF-beta1 reverses transformation. Transl Res 148: 142–148, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts AB, Sporn MB, Thorgeirsson SS. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci USA 92: 2572–2576, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saran AM, Yuan H, Takeuchi E, McLaughlin M, Salant DJ. Complement mediates nephrin redistribution, and actin dissociation in experimental membranous nephropathy. Kidney Int 64: 2072–2078, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Shankland SJ. Cell cycle regulatory proteins in glomerular disease. Kidney Int 56: 1208–1215, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Shankland SJ. The podocyte's response to injury: role in proteinuria, and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Shankland SJ, Eitner F, Hudkins KL, Goodpaster T, D'Agati V, Alpers CE. Differential expression of cyclin-dependent kinase inhibitors in human glomerular disease: role in podocyte proliferation, and maturation. Kidney Int 58: 674–683, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 108: 289–301, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong GX, Yu WM, Beaubier NT, Weeden EM, Hamele-Bena D, Mansukhani MM, O'Toole KM. Expression of PAX8 in normal, and neoplastic renal tissues: an immunohistochemical study. Mod Pathol 22: 1218–1287, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Vaughan MR, Pippin JW, Griffin SV, Krofft R, Fleet M, Haseley L, Shankland SJ. ATRA induces podocyte differentiation, and alters nephrin and podocin expression in vitro and in vivo. Kidney Int 68: 133–144, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Yuan H, Takeuchi E, Taylor GA, McLaughlin M, Brown D, Salant DJ. Nephrin dissociates from actin, and its expression is reduced in early experimental membranous nephropathy. J Am Soc Nephrol 13: 946–956, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med 82: 175–181, 2004 [DOI] [PubMed] [Google Scholar]