Abstract
The bladder urothelium is currently believed to be a sensory structure, contributing to mechano- and chemosensation in the bladder. Transient receptor potential (TRP) cation channels act as polymodal sensors and may underlie some of the receptive properties of urothelial cells. However, the exact TRP channel expression profile of urothelial cells is unclear. In this study, we have performed a systematic analysis of the molecular and functional expression of various TRP channels in mouse urothelium. Urothelial cells from control and trpv4−/− mice were isolated, cultured (12–48 h), and used for quantitative real-time PCR, immunocytochemistry, calcium imaging, and whole cell patch-clamp experiments. At the mRNA level, TRPV4, TRPV2, and TRPM7 were the most abundantly expressed TRP genes. Immunohistochemistry showed a clear expression of TRPV4 in the plasma membrane, whereas TRPV2 was more prominent in the cytoplasm. TRPM7 was detected in the plasma membrane as well as cytoplasmic vesicles. Calcium imaging and patch-clamp experiments using TRP channel agonists and antagonists provided evidence for the functional expression of TRPV4, TRPV2, and TRPM7 but not of TRPA1, TRPV1, and TRPM8. In conclusion, we have demonstrated functional expression of TRPV4, TRPV2, and TRPM7 in mouse urothelial cells. These channels may contribute to the (mechano)sensory function of the urothelial layer and represent potential targets for the treatment of bladder dysfunction.
Keywords: vanilloid receptor, bladder, ion channel
the urothelium is a complex epithelial cell layer lining the inside of the urinary bladder wall. This specialized epithelium is able to maintain a highly impermeable barrier for water, solutes, and pathogens despite large variations in mucosal surface area during urine storage and voiding (20, 31). Moreover, it is currently believed that the urothelial cells have a sensory role, contributing to mechano- and chemosensation in the bladder. Urothelial cells express a large variety of ion channels, including transient receptor potential (TRP) channels (14), as well as purinergic (8), muscarinic (22), and nicotinic receptors (1). In addition, urothelial cells are able to release a number of signaling molecules, such as ATP (13), nitric oxide (NO) (2), and acetylcholine (ACh) (16). During bladder filling, urothelial cells release ATP (13), thereby promoting apical membrane trafficking in neighboring cells (39) and activating nearby sensory nerve fibers (8, 20). Under pathological conditions, the urothelium may contribute to the development of functional bladder disorders such as interstitial cystitis (IC) or overactive bladder (OAB).
TRP channels are unique cellular sensors characterized by highly variable mechanisms of activation. Based on sequence homology, the 28 mammalian TRPs are classified into 6 subfamilies: TRPC (canonical), TRPM (melastatin), TRPV (vanilloid), TRPA (ankyrin), TRPP (polycystin), and TRPML (mucolipin). Importantly, mutations in different TRP genes are linked to human diseases (32, 33).
Recent studies have indicated that several TRP channels, namely, TRPV1, TRPV2, TRPV4, TRPM8, and TRPA1, are expressed in the bladder, where they may act as sensors of stretch and/or chemical irritation (10). As such, TRP channels represent potential pharmacological targets for the treatment of bladder dysfunction. The exact distribution of these channels in urothelial cells and their particular role in the generation of afferent signals is still a matter of debate. In this report we therefore focus on the functional expression of TRP receptors in freshly isolated mouse urothelial cells by using a combination of imaging and electrophysiological techniques.
MATERIALS AND METHODS
Animals.
Wild-type (C57Bl/6) and trpv4−/− mice, backcrossed on a C57Bl/6 background, were used. Experiments were performed on bladders from 10- to 12-wk-old mice. All animal experiments were carried out in accordance with the European Union Community Council guidelines and were approved by the local ethics committee.
Primary urothelial cell culture.
Isolation and culture of urothelial cells was performed as previously described by others (4, 37). After euthanasia, bladders were quickly removed, cut open, and stretched out on a Sylgard-coated dish containing MEM (Invitrogen) with 2.5 mg/ml dispase (Invitrogen) for 2 h at room temperature. After incubation, the urothelium was gently scraped from the underlying tissue, treated with trypsin-EDTA (Invitrogen) for 15 min, and resuspended in keratinocyte medium (Invitrogen). The cell suspension was plated on collagen (type IV; Sigma)-coated coverslips. Cells were used for experiments 12–48 h after isolation.
Quantitative real-time PCR.
For quantitative real-time PCR (qPCR) experiments, RNA was isolated from cultured urothelial cells and freshly dissected urothelial tissue. Total RNA was extracted using the RNeasy mini kit (Qiagen). Subsequently, cDNA was synthesized using Ready-To-Go You-Prime first-strand beads (GE Healthcare), and qPCR was performed with the 7500 Fast Real-Time PCR system (Applied Biosystems) using specific TaqMan gene expression assays for TRPA1, TRPM6, TRPM7, TRPM8, TRPV1, TRPV2, and TRPV4 (Applied Biosystems). GAPDH and β-actin were used as endogenous controls (Applied Biosystems). The protocol consisted of 40 replication cycles. Data represent relative expression of detected mRNAs normalized to TRPV4 mRNA, which was used as a calibrator for comparative analysis.
Immunocytochemistry.
Urothelial cells from wild-type and trpv4−/− mice were fixed with 3.7% formaldehyde for 10 min, permeabilized with 0.2% Triton X-100 for 10 min, and blocked with 3% BSA for 3 h. Primary antibodies for TRPV4 [1:200, rabbit anti-rat TRPV4 (14)], TRPV2 (2 μg/ml, PC421; Calbiochem), TRPM7 (1:400, ab85016; Abcam), and cytokeratin 7 (CK7; 1:50, clone OV-TL 12/3; Dako) were incubated overnight. The secondary antibodies (1:1,000, Alexa594-conjugated anti-mouse IgG and AlexaFluor488-conjugated anti-rabbit IgG) were applied for 1 h at room temperature. Triple washing with PBS was performed between each step. Finally, the coverslips were mounted in a medium containing 4′,6-diamidino-2-phenylindole. Since we had no access to trpv2−/− mice for negative controls, we used a blocking peptide against the TRPV2 primary antibody (COOH terminal, KNSASEEDHLPLQVLQSP; Calbiochem) to check primary antibody specificity. The primary antibody and a 10× excess of the blocking peptide were incubated for 2 h at 4°C before the above-described immunohistochemistry was performed. Images were taken using a confocal microscope imaging system (Zeiss).
Measurement of intracellular Ca2+ concentration.
Cells were loaded with 2 μM fura-2 acetoxymethyl ester for 30 min at 37°C. Intracellular Ca2+ concentration ([Ca2+]i) was monitored through the ratio of fluorescence measured by alternating illumination at 340 and 380 nm, as described in detail elsewhere (38), using an MT-10 illumination system and cellm software (Olympus). Calibration was performed as described previously (38). The temperature of the perfusate was controlled using an SC-20 dual in-line heater/cooler (Warner Instruments).
Patch-clamp experiments.
Membrane currents were measured in the whole cell mode of the patch-clamp technique using an EPC-10 amplifier (HEKA Elektronik, Lambrecht, Germany). Patch electrodes had a direct current (DC) resistance between 2 and 4 MΩ when filled with intracellular solution. An AgCl wire was used as a reference electrode. Currents were sampled at 20 kHz and digitally filtered at 2.9 kHz. Capacitance and access resistance were monitored continuously. Between 50 and 70% of the series resistance was electronically compensated to minimize voltage errors. A ramp protocol was applied consisting of a voltage step from the holding potential of 0 mV to −150 mV, followed by a 400-ms linear ramp to +150 mV applied every 2 s. The voltage-step protocol consisted of 40-ms steps from −80 to +200 mV. The standard extracellular solution for electrophysiological measurements contained (in mM) 150 NaCl, 6 KCl, 1 MgCl2, 1.5 CaCl2, 10 glucose, and 10 HEPES, buffered at pH 7.4 with NaOH. The osmolality of this solution, measured with a vapor pressure osmometer (Wescor 5500; Schlag, Gladbach, Germany), was 320 mosmol/kgH2O. The pipette solution was composed of (in mM) 20 CsCl, 100 Cs-aspartate, 1 MgCl2, 10 HEPES, 4 Na2ATP, 10 BAPTA, and 2.93 CaCl2, adjusted to pH 7.2 with CsOH. For TRPM7 measurements, the pipette solution contained either 0 or 8 mM MgCl2. The free Ca2+ concentration of this solution was 50 nM. This Cs+-containing, K+-free intracellular solution efficiently prevented contamination of outward TRPM7 currents by K+ currents, such as the recently described ATP-sensitive K+ channels (46). For measuring TRPV1 and TRPM8 currents, the extracellular solution contained (in mM) 150 NaCl, 1 MgCl2, and 10 HEPES, buffered at pH 7.4 with NaOH. All measurements were carried out at room temperature, 22–25°C. Cell membrane capacitance values were used to calculate current densities.
Reagents.
Capsaicin, icilin, ruthenium red (RR), and mustard oil (MO; allyl isothiocyanate) were purchased from Sigma-Aldrich (Bornem, Belgium). Menthol was acquired from Merck (Darmstadt, Germany), AM251 and AM630 from Cayman Chemical (Ann Arbor, MI), and 4α-phorbol 12,13-didecanoate (4α-PDD) from Alexis Biochemicals (Lausen, Switzerland). Tetrahydrocannabinol (THC) was provided by G. Appendino, and 4α-phorbol 12,13-dihexanoate (4α-PDH) was synthesized as described previously (21). HC067047 was a kind gift from Hydra (Cambridge, MA). Stock solutions were dissolved in DMSO or ethanol.
Data analyses.
Electrophysiological data were analyzed using PATCHMASTER and FITMASTER programs (HEKA Elektronik, Lambrecht, Germany). For statistical analysis and data display, the Origin 7.1 software package was used (OriginLab, Northampton, MA). Data are means ± SE. The Student's unpaired, two-tailed t-test was used for statistical comparison, and P < 0.05 was considered statistically significant.
RESULTS
TRP expression in urothelial cells.
Using the qPCR technique, we tested the expression of seven different TRP genes and compared their expression level relative to the TRPV4 gene. We tested the expression of TRPA1, TRPM8, TRPV1, TRPV2, and TRPV4, which are known to be expressed in the bladder. In addition, we tested the expression of TRPM7, an ubiquitously expressed TRP that was recently suggested to have mechanosensitive properties (35). Finally, TRPM6 was tested to differentiate whether the Mg2+-inhibited currents, observed in our cultured urothelial cells (described below), were mediated by TRPM7 or TRPM6.
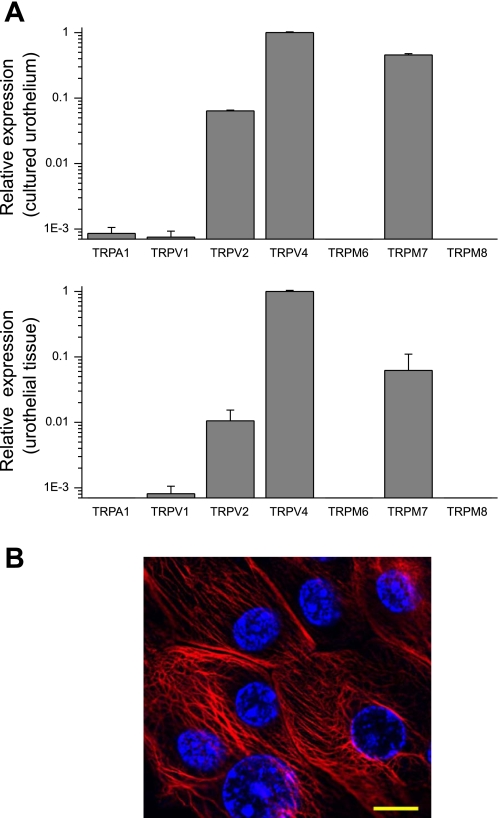
The use of cDNA generated from total RNAs isolated from urothelial cells in culture for 48 h revealed that TRPV4 is the most abundantly expressed TRP gene, followed by TRPM7 and TRPV2 (Fig. 1A). Messenger RNAs of three other TRPs were detected (TRPA1, TRPV1, and TRPM6), but their expression levels were more than 1,000 times lower than that of TRPV4 mRNA. TRPM8 mRNA was below the detection level. Similar expression patterns of TRP genes were obtained in samples prepared from freshly dissected urothelial tissue (Fig. 1A), indicating that the cultured urothelial cells are a relevant model to study urothelial TRP channel function. Cultured cells were stained for CK7, an intermediate filament protein that is used as a marker for urothelial cells. The majority of cells (∼90%) in our culture showed a positive staining for CK7, confirming their urothelial nature (Fig. 1B).
Fig. 1.
Quantitative RT-PCR comparing the expression level of transient receptor potential (TRP) channel genes in samples from cultured mouse urothelial cells (n = 4) and freshly isolated urothelial tissue (n = 4). A: mRNA levels are quantified relative to TRPV4. At the mRNA level, TRPV4, TRPM7, and TRPV2 are the most prominent TRP channels. B: the cultured cells were positive for the intermediate filament cytokeratin 7 (CK7; red), confirming their epithelial nature. Blue, 4′,6-diamidino-2-phenylindole (DAPI). Scale bars, 10 μM.
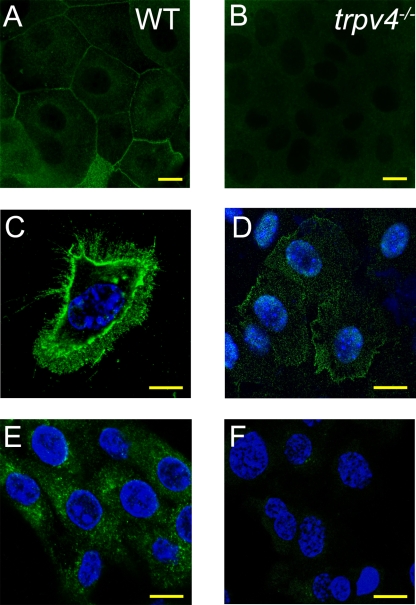
Immunocytochemistry revealed a clear staining of TRPV4 in the membrane of the urothelial cells, with only a weak staining of TRPV4 in the cytoplasm (Fig. 2, A and C). In single urothelial cells, expression of TRPV4 could be detected at the plasma membrane and in the filopodia of the migrating cells (Fig. 2C). These stainings were absent in cells obtained from trpv4−/− mice (Fig. 2B).
Fig. 2.
Immunocytochemistry on cultured urothelial cells. A and C: TRPV4 (green) is stained in the plasma membrane of confluent (A) and single urothelial cells (C). B: no TRPV4 immunoreactivity was detected in cells from trpv4−/− mice. D: TRPM7 (green) is present in the plasma membrane as well as cytoplasmic vesicles. E: TRPV2 (green) staining shows the presence of TRPV2 in the cytoplasm. F: no TRPV2 staining was detected after incubation of the blocking peptide. Blue, DAPI. Scale bars, 10 μM.
TRPM7 expression was detected in the plasma membrane, as well as cytoplasmic vesicles (Fig. 2D), as previously described by others (5, 36). Urothelial cells also stained positive for TRPV2, showing a predominant expression in cytoplasmic compartments (Fig. 2E). The cytoplasmic staining was no longer observed after preincubation of the antibody with a blocking peptide (Fig. 2F). Unfortunately, trpm7−/− and trpv2−/− mice were not available to confirm the specificity of these stainings.
Ca2+ imaging.
To address the functional expression of TRP channels in urothelial cells, we tested whether these cells responded to known TRP agonists. ATP (10 μM) was used as a positive control.
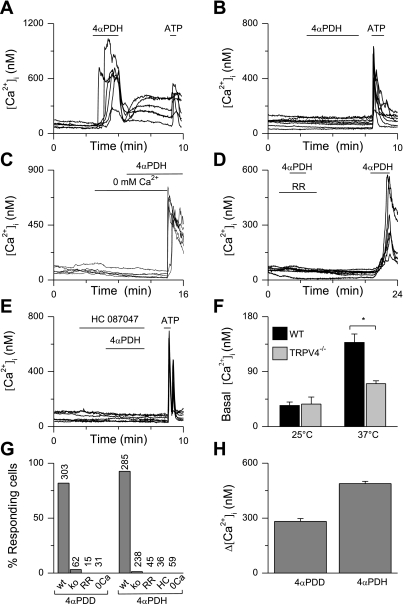
We recently described the phorbol ester 4α-PDH as a new, very potent TRPV4 agonist (21). At a concentration of 1 μM, this compound produced Ca2+ influx in most of the wild-type cells (Fig. 3, A, G, and H) but not in urothelial cells from trpv4−/− mice (Fig. 3, B and G). Responses to 4α-PDH were absent when Ca2+ was omitted from the extracellular solution (Fig. 3C). The responses were blocked by the nonspecific TRPV inhibitor RR (10 μM; Fig. 3D) and the TRPV4-specific inhibitor HC067047 (1 μM; Fig. 3E) (12). When the basal Ca2+ levels in wild-type and trpv4−/− cells were compared, no differences were observed at room temperature (25°C). However, at 37°C, basal Ca2+ levels in wild-type cells were significantly higher than in trpv4−/− cells (Fig. 3F), in accordance with the known heat activation of TRPV4 (15, 43).
Fig. 3.
Ca2+ imaging on wild-type (WT) and trpv4−/− urothelial cells. A–E: examples of 4α-phorbol 12,13-dihexanoate (4α-PDH)-induced changes in intracellular Ca2+ concentrations ([Ca2+]i), with each line representing a cell. 4α-PDH (1 μM) induced a Ca2+ influx in WT (A) but not in trpv4−/− cells (B). Responses were dependent on extracellular Ca2+ (C) and could be blocked by ruthenium red (RR; D) and HC067047 (E). F: basal Ca2+ levels in WT and trpv4−/− urothelial cells at 25 and 37°C showed significantly lower basal Ca2+ levels in trpv4−/− cells at 37°C but not at 25°C. G: percentages of cells responding to 4α-phorbol 12,13-didecanoate (4α-PDD; 1 μM) and 4α-PDH (1 μM). The total number of cells tested is indicated above the bars. H: amplitude of [Ca2+]i changes in the responding cells. Values are means ± SE. *P < 0.05.
In line with previously published data, most of the urothelial cells responded to 4α-PDD (1 μM; Fig. 3, G and H), a well-described selective agonist of TRPV4 (41). These responses were not observed in cells derived from trpv4−/− mice (Fig. 3G; see also Ref. 14).
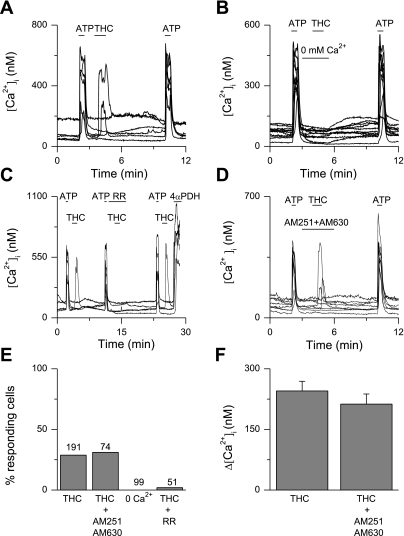
To test for the functional expression of TRPV2, we applied 30 μM THC, a known TRPV2 agonist. Single application of THC produced highly variable responses in urothelial cells from different coverslips. However, when cells were preexposed to a short pulse of ATP (10 μM), THC induced a fast Ca2+ influx in ∼30% of the cells (Fig. 4, A, E, and F). These responses were not observed in the absence of extracellular Ca2+ (Fig. 4B) and could be blocked by preapplication of RR (30 μM; Fig. 4C). The responses to THC were not influenced by application of the cannabinoid receptor blockers AM251 (80 nM) and AM630 (800 nM; Fig. 4, D–F).
Fig. 4.
Ca2+ imaging on WT urothelial cells. A–D: examples of tetrahydrocannabinol (THC)-induced changes in [Ca2+]i, with each line representing a cell. After preapplication of ATP, 30 μM THC induced a fast Ca2+ influx in ∼30% of the cells (A). Responses to THC were absent in the absence of extracellular Ca2+ (B). Responses to THC were blocked by the nonspecific TRP channel blocker RR (30 μM; C) but not by the cannabinoid receptor blockers AM251 and AM630 (D). E: percentage of cells responding to THC. The total number of cells tested is indicated above the bars. F: amplitude of [Ca2+]I changes in the responding cells. Values are means ± SE.
As described above, the qPCR assay indicated that the expression levels of TRPV1, TRPA1, and TRPM8 are low. In line with these findings, we did not measure any significant rises in [Ca2+]i in response to 1–10 μM capsaicin (0/248 cells; Fig. 5A), 100 μM-1 mM MO (0/144 cells; Fig. 5B), 100 μM menthol (0/121 cells; Fig. 5C), or 1 μM icilin (0/22 cells; Fig. 5D). From this, we conclude that TRPV1, TRPA1, and TRPM8 are not functional in cultured urothelial cells.
Fig. 5.
Ca2+ imaging on WT urothelial cells. A–D: examples of changes in intracellular calcium concentrations ([Ca2+]i), with each line representing a cell. No responses were observed in response to capsaicin (Cap; n = 248; A), mustard oil (MO; n = 144; B), menthol (n = 121; C), or icilin (n = 22; D).
Patch-clamp experiments.
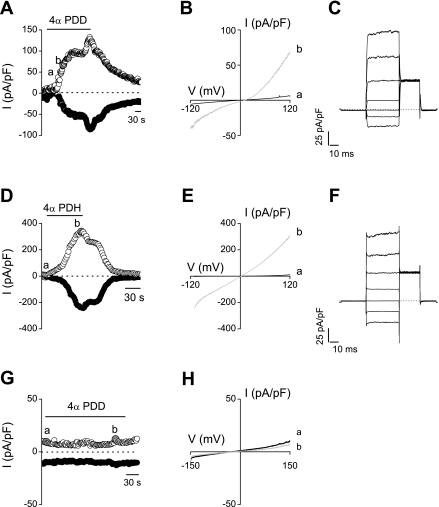
To further establish the functional expression of the different TRP channels, we directly measured channel activation using the whole cell patch-clamp technique. In urothelial cells from wild-type mice, we measured robust current activation on application of the TRPV4 agonists 4α-PDD (5 μM; −87 ± 15 pA/pF at −150 mV and +161 ± 29.3 pA/pF at +150 mV) and 4α-PDH (2 μM; −95 ± 27 pA/pF at −150 mV and +165 ± 34 pA/pF at +150 mV) (Fig. 6, A–F). In contrast, 4α-PDD or 4α-PDH did not evoke any significant current response in urothelial cells derived from the trpv4−/− mice (<2 pA/pF; n = 4; Fig. 6, G and H).
Fig. 6.
Effect of TRPV4 agonist stimulation on WT urothelial cells in patch-clamp experiments. Time courses of whole cell currents at −120 (●) and +120 mV (○) (A and D) and current (I)-voltage (V) relationships obtained at the indicated time points a and b (B and E) in WT urothelial cells show a clear response to 4α-PDD (5 μM; A) and 4α-PDH (2 μM; D). C and F: currents through WT urothelial cells after application of 4α-PDD (5 μM; C) and 4α-PDH (2 μM; F) in response to a voltage step protocol (40 mV steps from −80 to +200 mV). No response was observed in trpv4−/− urothelial cells after application of 4α-PDD (5 μM; G and H).
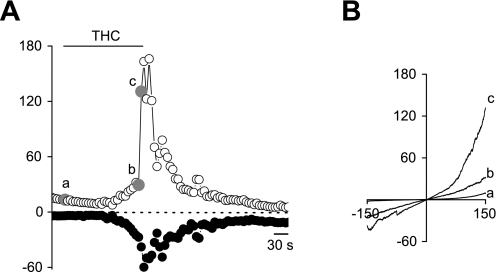
In urothelial cells from wild-type mice, application of 30 μM THC evoked an outwardly rectifying current (−17 ± 3 pA/pF at −150 mV and +30 ± 3 pA/pF at +150 mV) with an average reversal potential of +6 mV (Fig. 7, A and B). The current-voltage relationship is comparable with earlier described TRPV2 currents (30). A similar current was also observed in urothelial cells derived from trpv4−/− mice (−13 ± 4 pA/pF at −150 mV and +27 ± 6 pA/pF at +150 mV) (data not shown).
Fig. 7.
Whole cell patch-clamp recordings on WT urothelial cells. Time courses of whole cell currents at −150 and 150 mV (A) and current-voltage relationships obtained at the indicated points a, b, and c (B) are shown after stimulation of 30 μM THC.
TRPM7 is a ubiquitously expressed TRP channel that is activated when intracellular Mg2+ is lowered (29). In cultured urothelial cells, we observed a clear, gradual run-up in current density on dialyzing urothelial cells with a Mg2+-free intracellular solution (Fig. 8, A–C). These currents were partially suppressed by high external Mg2+ (10 mM; Fig. 8, A and B), indicating involvement of TRPM7 channels. No current density run-up was observed with 8 mM intracellular Mg2+ (Fig. 8, D–F). Similar TRPM7-like currents were observed in trpv4−/− cells (data not shown).
Fig. 8.
Whole cell patch-clamp recordings on WT urothelial cells. Time courses of whole cell currents at −120 and +120 mV (A) and current-voltage relationships obtained from time points indicated by shaded circles a, b, and c (B) are shown in the absence of intracellular Mg2+. Time course (D) and current-voltage relationships (E) in the presence of 8 mM intracellular Mg2+. Currents through WT urothelial cells in the absence (C) and presence (F) of intracellular Mg2+ are shown in response to a voltage step protocol.
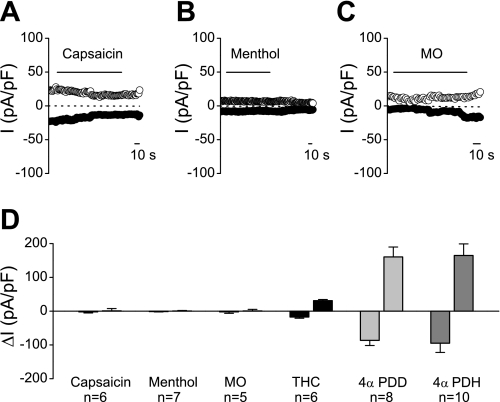
In line with the Ca2+ imaging experiments, we never observed any increase in current density after applications of capsaicin (10–100 μM; Fig. 9A), menthol (100–200 μM; Fig. 9B), and MO (1 mM; Fig. 9C). In these experiments, subsequent application of 4α-PDD or 4α-PDH consistently evoked robust TRPV4 currents.
Fig. 9.
Whole cell patch-clamp recordings in WT urothelial cells. A–C: time courses of whole cell currents at −150 and +150 mV. No response was observed after application of capsaicin (100 μM; A), menthol (200 μM; B), or MO (1 mM; C). D: comparison of the average inward and outward current, respectively, at −150 and +150 mV activated by capsaicin, menthol, MO, THC, 4α-PDD, and 4α-PDH.
DISCUSSION
The urothelium is the epithelial layer that lines the urinary tract from the renal pelvis to the urethra. Urothelium is a very specialized epithelium that acts as a permeability barrier, protecting underlying tissues against noxious urine components (20, 25, 31). Recently, an important sensory role was attributed to the urothelium of the bladder. The urothelial cells have mechano- and chemosensory properties and are able to communicate to the underlying afferent nerve fibers (20). In the present report, we provide evidence for the functional expression of three important TRP channels, TRPV4, TRPV2, and TRPM7, in mouse urothelial cells.
TRPV4 was described in detail as a channel activated by hypotonic cell swelling (34) but can be activated by other physical stimuli (shear stress and innocuous warmth, ∼27–35°C) and chemical ligands [endogenous (e.g., anandamide and arachidonic acid metabolites; Ref. 42) and synthetic ligands (e.g., phorbol esters; Ref. 33)]. Quantitative RT-PCR experiments revealed that TRPV4 is highly expressed in cultured urothelial cells as well as freshly dissected urothelial tissue. Immunohistochemistry demonstrated a clear expression of TRPV4 in the plasma membrane. In addition, Ca2+ imaging in isolated urothelial cells showed robust Ca2+ influxes in response to the TRPV4 agonists 4α-PDD and 4α-PDH, but only in the presence of external calcium. At higher temperatures (37°C), a lower basal Ca2+ level was observed in trpv4−/− urothelial cells compared with wild type. The abundance of TRPV4 was confirmed in patch-clamp experiments, in which robust increases in current densities were observed after application of TRPV4 activators, whereas no responses were measured in trpv4−/− urothelial cells. These data are consistent with our previous report showing the expression of TRPV4 in the urothelium (14). A functional role of TRPV4 in the bladder was previously demonstrated, based on the observation that trpv4−/− mice exhibit an increased micturition threshold and reduced ATP release in response to bladder stretch (14). These data suggest that TRPV4 can function as an important urothelial mechanosensor, mediating stretch-induced Ca2+ influx and subsequent ATP release.
Our present data provide evidence for the functional expression of TRPV2 in urothelial cells. TRPV2 can be activated by noxious heat (>52°C) (7), 2-aminoethoxydiphenyl borate (2-APB) (30), and THC (30). In serum free conditions, TRPV2 resides in the cytoplasm and is translocated to the plasma membrane by the addition of growth factors such as IGF-1 (19). The exact activation mechanisms and physiological roles of TRPV2 are still controversial and hampered by the lack of specific pharmacology or trpv2−/− mice. RT-PCR and immunocytochemistry revealed the expression of TRPV2 in urothelial cells. During Ca2+ imaging experiments, the onset of responses to THC was very slow and highly variable between cells. Moreover, Ca2+ influx was often observed on washout of the drug. However, when the cells were pretreated with ATP, fast and consistent responses to THC were observed. These responses were dependent on extracellular Ca2+ and could be blocked by preapplication of RR (30 μM), in line with the properties of TRPV2. Possibly, stimulation of urothelial cells with ATP facilitates translocation of TRPV2 from the cytoplasm to the plasma membrane, thereby priming the cells for subsequent THC-induced TRPV2 activation. Similarly, we could measure THC-induced currents in urothelium cells, with properties reminiscent of TRPV2. The Ca2+ influxes observed in response to THC were not influenced by coapplication of AM251 (24) and AM360 (17), antagonists of cannabinoid receptors CB1 and CB2, respectively, indicating these responses are not mediated by urothelial cannabinoid receptors. Although THC can also activate another TRP channel, TRPA1 (18), we could exclude a contribution of TRPA1 to the THC responses in urothelial cells, because no TRPA1 expression was detected and stimulation by MO did not evoke a measurable response. Expression of TRPV2 in the urothelium has already been described at the mRNA and protein level in human (6) and rat (3), but no functional data about the expression of TRPV2 in the urothelium have been published yet. Importantly, a role for TRPV2 as a mechanosensor in vascular smooth muscle cells has been described (28), suggesting that TRPV2 may contribute to the mechanosensitive properties of the urothelium.
Furthermore, we have described the functional expression of TRPM7. TRPM7 is ubiquitously expressed and essential for cellular viability. The channel is constitutively open, permeable to Ca2+, Mg2+, and other divalent cations, and can be inhibited by intracellular Mg2+ and Mg2+ nucleotides (29). Our expression experiments showed a high expression of TRPM7 in urothelial cells, whereas almost no mRNA was detected of TRPM6, a close homolog with similar functional properties. TRPM7 was detected in the plasma membrane as well as cytoplasmic vesicles. Patch-clamp experiments revealed the presence of TRPM7-like, outwardly rectifying currents, with a reversal potential around 0 mV. The currents developed gradually on dialysis of the cell with a pipette solution containing low Mg2+ and were rapidly inhibited by the application of extracellular Mg2+. Interestingly, some reports have reported TRPM7 activation by shear stress and other mechanostimuli (35), raising the possibility that the channel could contribute to sensing urothelial stretch.
Finally, we were unable to detect any functional expression of TRPV1, TRPA1, and TRPM8 in isolated mouse urothelial cells. Our qPCR experiments detected mRNA for TRPV1 and TRPA1, but these amounts were extremely low, suggesting these channels have limited functional significance. TRPM8 mRNA was, at least in our hands, not detectable at all. Moreover, we were unable to detect any responses to agonists of TRPV1 (capsaicin), TRPA1 (MO), or TRPM8 (menthol) during Ca2+ imaging or patch-clamp experiments.
With the use of trpv1−/− mice, it has been shown that TRPV1 plays a major role in regulation of the micturition reflex (4) and mechanical hypersensitivity during cystitis (40). Moreover, as the receptor for intravesical vanilloid therapy, TRPV1 is an important therapeutical target to treat detrusor overactivity (9). It is generally accepted that the therapeutic effect of vanilloid compounds is the result of TRPV1-mediated desensitization of afferent C-fibers (9). However, in addition to its expression on afferent nerve fibers, some authors suggested that TRPV1 is functionally expressed in urothelial cells in human (26), rat (3, 23), and mouse bladder (3), introducing the idea of TRPV1 as a urothelial chemosensor. In our expression and functional data, however, we were unable to confirm a functional role for TRPV1 in urothelial cells. At the mRNA level, TRPV1 could hardly be detected in both cultured urothelial cells and freshly dissected urothelial tissue, and no functional responses could be detected in Ca2+ imaging and electrophysiological experiments. These data are in agreement with recent work of other groups that failed to detect TRPV1 expression in urothelial cells at the mRNA, protein or functional level (27, 44, 45). Similarly, TRPM8 and TRPA1 have been described in urothelial cells (23), but we (this work) and others (27) were unable to confirm this.
Several factors may contribute to the discrepancies in the literature concerning the urothelial TRP channel expression profile. First, the urothelial cell isolation and culture technique itself may introduce essential differences. The urothelium is a complex epithelium that consists of three different cell layers: a basal layer, an intermediate layer, and a layer of highly differentiated umbrella cells. Many urothelial cell culture systems fail to induce terminal differentiation of the umbrella cells (37). Moreover, culturing native cells can significantly alter their gene expression. We worked with cells that were only cultured for a short period (12–48 h) and found similar TRP gene expression profiles in cultured cells and freshly dissected urothelial tissue. In both types of tissue, TRPV4, TRPM7, and TRPV2 were most abundantly expressed, whereas TRPV1, TRPA1, TRPM6, and TRPM8 were expressed at very low levels. This suggests that the urothelial culture model we used is suitable to test the functional expression of these TRP channels. Second, nonspecificity of TRP channel antibodies can account for some of the observed differences. For example, it has been well documented that commercially available anti-TRPV1 antibodies show clear immunoreactivity in bladder tissue from trpv1−/− animals, causing nonspecific signals in immunohistochemistry (11, 45) and Western blot analysis (45). Finally, it has to be noted that studies have been conducted on urothelial cells from various mammals, including mice, rats, guinea pigs, and humans. Species differences and even the genetic background of model animals may potentially influence the TRP gene expression profile.
In conclusion, we have analyzed the functional expression of TRP channels in freshly cultured urothelial cells and found evidence for the functional expression of TRPV4, TRPV2, and TRPM7. These channels may act as mechano- and/or chemosensors in the bladder urothelium. In addition, our work provides evidence that TRPV1, TRPA1, and TRPM8 are not functionally expressed in the urothelial cell layer, suggesting that their contribution to the sensory properties of the bladder is mainly confined to the afferent nerve fibers.
GRANTS
This work was supported by Belgian Federal Government Grant IUAP P5/05, Research Foundation-Flanders (FWO) Grants G.0172.03, G.0149.03, G.0565.07, and G.0686.09, Astellas European Foundation Award 2009, and Research Council of the KULeuven Grants GOA 2004/07 and EF/95/010. W. Everaerts is a doctoral fellow, J. Vriens is a postdoctoral fellow of the FWO, and D. De Ridder is a fundamental clinical fellow of the FWO.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank all members of the Laboratory of Ion Channel Research for helpful discussions. We thank Fenqin Xue for technical assistance. We thank the Cell Imaging Core facility of the Katholieke Universiteit Leuven (KULeuven) for the use of the confocal microscope. We kindly thank Hydra for supplying the HC067047.
REFERENCES
- 1.Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol 290: F103–F110, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birder LA, Apodaca G, De Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol Renal Physiol 275: F226–F229, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA 98: 13396–13401, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Brauchi S, Krapivinsky G, Krapivinsky L, Clapham DE. TRPM7 facilitates cholinergic vesicle fusion with the plasma membrane. Proc Natl Acad Sci USA 105: 8304–8308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caprodossi S, Lucciarini R, Amantini C, Nabissi M, Canesin G, Ballarini P, Di Spilimbergo A, Cardarelli MA, Servi L, Mammana G, Santoni G. Transient receptor potential vanilloid type 2 (TRPV2) expression in normal urothelium and in urothelial carcinoma of human bladder: correlation with the pathologic stage. Eur Urol 54: 612–620, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398: 436–441, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407: 1011–1015, 2000 [DOI] [PubMed] [Google Scholar]
- 9.de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol: 91–138, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everaerts W, Gevaert T, Nilius B, De Ridder D. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol Urodyn 27: 264–273, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Everaerts W, Sepulveda MR, Gevaert T, Roskams T, Nilius B, De Ridder D. Where is TRPV1 expressed in the bladder, do we see the real channel? Naunyn Schmiedebergs Arch Pharmacol 379: 421–425, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Fanger CM, Mc, Namar CR, Strassmaier T, Witek J, Agueev V, Moran MM, Zhen X. Identification of novel TRPV4 channel modulators. Program No. 628.18. 2008 Neuroscience Meeting Planner Washington, DC: Society for Neuroscience, 2008 [Google Scholar]
- 13.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes–a possible sensory mechanism? J Physiol 505: 503–511, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117: 3453–3462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22: 6408–6414, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci 80: 2298–2302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosohata K, Quock RM, Hosohata Y, Burkey TH, Makriyannis A, Consroe P, Roeske WR, Yamamura HI. AM630 is a competitive cannabinoid receptor antagonist in the guinea pig brain. Life Sci 61: PL115–PL118, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol 1: 165–170, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297: F1477–F1501, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klausen TK, Pagani A, Minassi A, Ech-Chahad A, Prenen J, Owsianik G, Hoffmann EK, Pedersen SF, Appendino G, Nilius B. Modulation of the transient receptor potential vanilloid channel TRPV4 by 4alpha-phorbol esters: a structure-activity study. J Med Chem 52: 2933–2939, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Kullmann FA, Artim D, Beckel J, Barrick S, de Groat WC, Birder LA. Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol Renal Physiol 294: F971–F981, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kullmann FA, Shah MA, Birder LA, de Groat WC. Functional TRP and ASIC-like channels in cultured urothelial cells from the rat. Am J Physiol Renal Physiol 296: F892–F901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, Pertwee R, Makriyannis A. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem 42: 769–776, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, Apodaca G, Zeidel ML. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol 283: F242–F253, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Lazzeri M, Vannucchi MG, Zardo C, Spinelli M, Beneforti P, Turini D, Faussone-Pellegrini MS. Immunohistochemical evidence of vanilloid receptor 1 in normal human urinary bladder. Eur Urol 46: 792–798, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, Naruse K, Koizumi S, Takeda M, Tominaga M. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284: 21257–21264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93: 829–838, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411: 590–595, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Neeper MP, Liu Y, Hutchinson TL, Wang Y, Flores CM, Qin N. Activation properties of heterologously expressed mammalian TRPV2: evidence for species dependence. J Biol Chem 282: 15894–15902, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol Renal Fluid Electrolyte Physiol 271: F886–F894, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Nilius B. TRP channels in disease. Biochim Biophys Acta 1772: 805–812, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Nilius B, Prenen J, Wissenbach U, Bödding M, Droogmans G. Differential activation of the volume-sensitive cation channel TRP12 (OTRPC4) and volume-regulated anion currents in HEK-293 cells. Pflügers Arch 443: 227–233, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol 292: C460–C467, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res 98: 245–253, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Truschel ST, Ruiz WG, Shulman T, Pilewski J, Sun TT, Zeidel ML, Apodaca G. Primary uroepithelial cultures. A model system to analyze umbrella cell barrier function. J Biol Chem 274: 15020–15029, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Vriens J, Janssens A, Prenen J, Nilius B, Wondergem R. TRPV channels and modulation by hepatocyte growth factor/scatter factor in human hepatoblastoma (HepG2) cells. Cell Calcium 36: 19–28, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest 115: 2412–2422, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain 139: 158–167, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277: 13569–13577, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277: 47044–47051, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Xu X, Gordon E, Lin Z, Lozinskaya IM, Chen Y, Thorneloe KS. Functional TRPV4 channels and an absence of capsaicin-evoked currents in freshly-isolated, guinea-pig urothelial cells. Channels (Austin) 3: 156–160, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Yamada T, Ugawa S, Ueda T, Ishida Y, Kajita K, Shimada S. Differential localizations of the transient receptor potential channels TRPV4 and TRPV1 in the mouse urinary bladder. J Histochem Cytochem 57: 277–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu W, Khandelwal P, Apodaca G. Distinct apical and basolateral membrane requirements for stretch-induced membrane traffic at the apical surface of bladder umbrella cells. Mol Biol Cell 20: 282–295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]