Abstract
The role of 5-hydroxytryptamine (5-HT) 1A (5-HT1A) receptors in lower urinary tract function was examined in urethane-anesthetized female Sprague-Dawley rats. Bladder pressure and the external urethral sphincter electromyogram (EUS EMG) activity were recorded during continuous-infusion transvesical cystometrograms (TV-CMGs) to allow voiding and during transurethral-CMGs (TU-CMGs) which prevented voiding and allowed recording of isovolumetric bladder contractions. 8-Hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT), a 5-HT1A receptor agonist, decreased volume threshold (VT) for initiating voiding and increased contraction amplitude (CA) during TU-CMGs but decreased CA during TV-CMGs. 8-OH-DPAT prolonged EUS bursting as well as the intrabursting silent periods (SP) during voiding. N-[2-[4-(2-methoxyphenyl)-1- piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamine trihydrochloride (WAY-100635), a 5-HT1A antagonist, increased VT, increased residual volume, markedly decreased voiding efficiency, decreased the amplitude of micturition contractions recorded under isovolumetric conditions, and decreased the SP of EUS bursting. These results indicate that activation of 5-HT1A receptors by endogenous 5-HT lowers the threshold for initiating reflex voiding and promotes voiding function by enhancing the duration of EUS relaxation, which should reduce urethral outlet resistance.
Keywords: micturition, external urethral sphincter, urinary bladder, electromyogram, serotonin
the localization of nerve terminals containing serotonin (5-hydroxytryptamine; 5-HT) in sympathetic, parasympathetic (6), and urethral sphincter motor nuclei (27) in the lumbosacral spinal cord has focused attention on the role of 5-HT as a neurotransmitter in the central neural pathways controlling lower urinary tract function (9, 11, 25, 28). Pharmacological studies in animals have revealed that administration of 5-HT receptor agonists or antagonists modulates reflex bladder and urethral sphincter activity (1, 2, 12, 14, 15, 17, 19, 20, 30–32, 34, 36). These effects are mediated by activation of multiple 5-HT receptors, which are distributed at various sites in the central pathways controlling the lower urinary tract (9, 12, 16, 21, 28, 29, 37).
The effect of 5-HT on bladder and sphincter activity varies in different species. For example, in the cat, activation of 5-HT1A receptors inhibits the parasympathetic excitatory input to the urinary bladder (14, 15, 27, 31, 36). On the other hand, in the rat, activation of 5-HT1A receptors enhances reflex activity of the bladder and sphincter (1, 2, 12, 20), whereas block of 5-HT1A receptors suppresses bladder and sphincter activity, suggesting that endogenous 5-HT exerts a tonic influence on the neural pathways controlling the lower urinary tract (1, 9, 17, 19, 26, 32).
In rats, 5-HT1A receptors may regulate urine storage as well as voiding by facilitating two types of external urethral sphincter (EUS) activity (1, 2, 12). Administration of a 5-HT1A agonist, 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT), enhances tonic EUS activity that occurs during bladder filling. This would be expected to promote urinary continence. 8-OH-DPAT also enhances bursting activity, which should promote efficient voiding. In rats with an intact neuraxis, the facilitation of tonic EUS activity is due to activation of 5-HT1A receptors in the spinal cord, whereas the facilitation of bursting activity is dependent on supraspinal pathways involving the pontine micturition center (2). The facilitation of EUS bursting is eliminated by acute transection of the thoracic spinal cord but recovers in chronic spinal cord-injured rats presumably due to reorganization of voiding reflexes in spinal segments caudal to the injury (2).
The present experiments, which were conducted in rats with an intact neuraxis, utilized simultaneous recordings of intravesical pressure and EUS electromyographic (EMG) activity to evaluate the contribution of 5-HT1A receptors to bladder-EUS coordination and voiding efficiency. Receptors were activated or blocked, respectively, by systemic administration of 8-OH-DPAT, a receptor agonist, or WAY-100635, a 5-HT1A receptor antagonist. The results indicate that activation of 5-HT1A receptors by endogenous 5-HT decreases the micturition volume threshold and produces changes in EUS EMG activity that facilitate voiding,
MATERIALS AND METHODS
General preparation.
Fifty-three female Sprague-Dawley rats (weight 230–300 g) were used in this study. The animals were anesthetized with a subcutaneous injection of urethane (1.2 g/kg). The trachea was cannulated to facilitate respiration. The femoral vein was catheterized for fluid and drug administration. Body temperature was maintained between 36 and 38°C by a heat lamp. Most animals breathed spontaneously; however, a few were artificially ventilated after intravenous injection of pancuronium bromide (Organon), a neuromuscular blocking agent, which was used to block EUS activity during the experiment.
Transvesical cystometrograms.
The urinary bladder was exposed via a midline abdominal incision. Two fine insulated silver wire electrodes (0.05-mm diameter) with exposed tips were inserted into lateral sides of the midurethra, where muscle fibers of the EUS were identified. The recorded EUS EMG was attributed to striated muscle because the activity was eliminated after neuromuscular blockade with pancuronium bromide (3). A polyethylene (PE) tube (1.0-mm inner diameter and 1.5-mm outer diameter) was inserted into the bladder lumen. The bladder end of the PE tube was heated to create a collar and passed through a small incision at the apex of the bladder dome, and a suture was tightened around the collar of the tube. Then, the abdominal wall was closed with nylon suture. The PE tube was in turn connected via a three-way stopcock to an infusion pump and a pressure transducer, and the system was filled with physiological saline. Urodynamic examination usually began 3–4 h after the induction of anesthesia. After the bladder was emptied, transvesical cystometrograms (CMG) were performed at an infusion rate of 0.123 ml/min with physiological saline at room temperature. The urethra was open, and fluid could be evacuated during micturition. The infusion pump was turned off after two to three voiding contractions, and residual volume was measured. Residual saline was withdrawn through the intravesical tube, and then the bladder was expressed manually by pressure on the abdominal wall. Various parameters were measured: 1) volume threshold, the volume of saline sufficient to induce bladder contractions exceeding a pressure of 15 cmH2O; 2) contraction amplitude, the maximal intravesical pressure during voiding; 3) contraction duration, duration of voiding contraction; 4) residual volume, the volume of saline withdrawn through the intravesical catheter after voiding; and 5) voiding efficiency, the ratio between voiding volume (volume threshold minus residual volume) and volume threshold. The EUS EMG activity analysis was blinded to the status of the rat. As described in an earlier paper (4), various EUS EMG parameters were measured including average interval of the bursting duration, silent period, active period, total silent period in each voiding, and the ratio of bursting duration to contraction duration. All parameters were calculated with the aid of Acknowledge software (Biopac Systems). Computed data were compiled in spreadsheets using Excel (Microsoft). EUS EMG activity was displayed on a storage oscilloscope and recorded on VCR tape and a paper recorder along with bladder pressure.
Transurethral CMG.
A PE tube (0.76-mm inner diameter, 1.22-mm outer diameter) was inserted into the bladder through the urethra and tied in place by a ligature around the urethral orifice. By means of a three-way stopcock, the catheter was connected to a pressure transducer to record the bladder pressure isovolumetrically and to a syringe for bladder infusion. The catheter system was filled with 0.9% saline. After emptying of the bladder, a CMG was performed by filling with a constant infusion (0.123 ml/min) of saline. The infusion pump was turned off after the onset of rhythmic bladder contractions. For isovolumetric recording, ureters were tied distally, cut, and the proximal stumps were cannulated and drained externally. The volume of saline sufficient to induce bladder contractions exceeding a pressure of 15 cmH2O was defined as the micturition volume threshold. Three or four CMGs were performed in each animal after vehicle or each drug.
Drugs.
8-OH-DPAT and WAY-100635 (both from Sigma, St. Louis, MO) were dissolved in saline. Drug solutions were administered after control recordings and vehicle administration just before the start of a CMG. Two different experimental protocols were employed in the course of this study. Protocol 1 was conducted with drugs administered in the following sequence: vehicle, 8-OH-DPAT (0.3 mg/kg iv), and WAY-100635 (0.1 mg/kg iv) at intervals of at least 1 h. Based on the short half-life of 8-OH-DPAT in the rat (12, 20), it is assumed that the effect of this drug had worn off before the administration of WAY-100635. However, this was confirmed by testing WAY-100635 (0.1 mg/kg iv) in the absence of 8-OH-DPAT in protocol 2, where WAY-100635 was administered 1 h after vehicle. Doses of drugs were selected based on results of previous experiments (5, 19). The dose of 8-OH-DPAT was selected to produce a consistent enhancement of bladder activity, while the dose of WAY-100635 has been shown to affect bladder activity but not blood pressure in the rat (5).
The Institutional Animal Care and Use Committee, Taichung Veterans General Hospital, approved the protocol used in this study.
Statistical analysis.
The results are given as means ± SE. For comparisons between values obtained before and after drug administration, one-way ANOVA was used for comparisons between vehicle and 5-HT1A agonist or antagonist treatment and was followed by a post hoc least significance difference test. P < 0.05 was considered statistically significant.
RESULTS
Effect of 8-OH-DPAT and WAY-100635 on bladder activity during transvesical CMGs.
With the urethra outlet open, to allow intravesical fluid to be evacuated during voiding, various parameters of bladder activity were assessed, including volume threshold, contraction amplitude, contraction duration, postvoid residual volume, and voiding efficiency before and after serial injection of vehicle, 8-OH-DPAT (0.3 mg/kg iv), and WAY-100635 (0.1 mg/kg iv). Vehicle did not significantly change any parameters. In protocol 1, volume threshold and contraction amplitude were significantly decreased (P < 0.05) by 20–30% after administration of 8-OH-DPAT (Fig. 1B), while contraction duration, residual volume and voiding efficiency were not significantly changed. WAY-100635 administered after 8-OH-DPAT significantly increased volume threshold (Fig. 1C), residual volume, and decreased voiding efficiency beyond the vehicle control levels (Fig. 1, Table 1). In protocol 2, WAY-100635 was administered following vehicle administration. WAY-100635 significantly increased volume threshold and residual volume, and decreased voiding efficiency as noted in experiments performed using protocol 1 (Table 2).
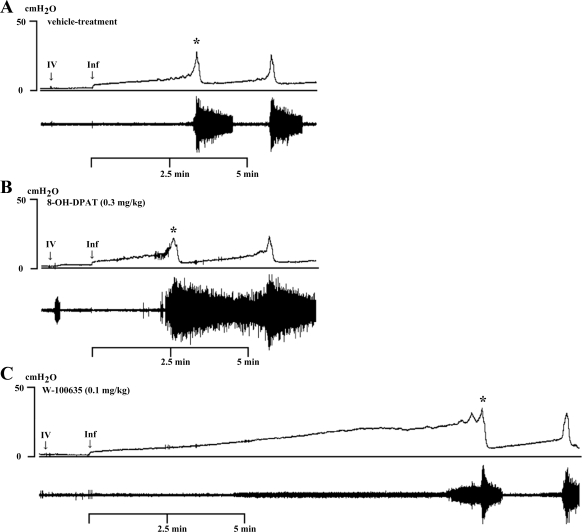
Fig. 1.
Effect of 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT; 0.3 mg/kg iv) and WAY-100635 (0.1 mg/kg iv) on bladder (top traces) and external urethral sphincter electromyogram (EUS EMG) activity (bottom traces) during a continuous-infusion (0.123 ml/min) transvesical cystometrogram (CMG) in an anesthetized rat with urethral outlet open and bladder able to empty. iv, Intravenous drug administration; Inf, start of saline infusion to the bladder. A: CMG pattern after vehicle administration showing large-amplitude EUS EMG activity occurring at the onset and during reflex bladder contractions. B: 8-OH-DPAT reduced the volume threshold (VT) for inducing a reflex bladder contraction and activated EUS EMG activity during the filling phase and bladder contractions. C: in the same rat, 1 h after administration of 8-OH-DPAT, WAY-100635 increased VT and suppressed EUS EMG activity during the filling phase and bladder contractions. Vertical calibration, intravesical pressure (in cmH2O); horizontal calibration, time (in minutes). Asterisks indicate voiding responses shown on an expanded scale in Fig. 3.
Table 1.
Parameters of bladder activity in transvesical CMGs after serial administration of vehicle, 8-OH-DPAT, and WAY-100635 in urethane-anesthetized rats
| VT, ml | CA, cmH2O | CD, min | RV, ml | VE, % | |
|---|---|---|---|---|---|
| Vehicle (n = 18) | 0.73 ± 0.14 | 32.92 ± 6.38 | 0.40 ± 0.10 | 0.17 ± 0.05 | 71.6 ± 12.9 |
| Agonist (8-OH-DPAT; 0.3 mg/kg) | 0.52 ± 0.13* | 26.66 ± 4.89* | 0.47 ± 0.11 | 0.06 ± 0.03 | 86.1 ± 10.6 |
| Antagonist (WAY-100635; 0.1 mg/kg) | 1.55 ± 0.36*† | 36.74 ± 6.47† | 0.36 ± 0.10† | 1.05 ± 0.31*† | 29.3 ± 22.9*† |
Values are means ± SE. 8-OH-DPAT, 8-hydroxy-2-(di-n-propylamino)-tetralin; CMGs, cystometrograms; VT, volume threshold; CA, contraction amplitude; CD, contraction duration; RV, residual volume; VE, voiding efficiency = VT − RV/VT;
P < 0.05 indicates a statistically significant difference compared with vehicle treatment (post hoc least significance difference test).
P < 0.05 indicates a statistically significant difference compared with 8-OH-DPAT treatment (post hoc least significance difference test).
Table 2.
Parameters of bladder activity in transvesical CMGs after vehicle and WAY-100635 treatment in urethane-anesthetized rats
| VT, ml | CA, cmH2O | CD, min | RV, ml | VE, % | |
|---|---|---|---|---|---|
| Vehicle (n = 11) | 0.42 ± 0.14 | 31.45 ± 3.18 | 0.39 ± 0.10 | 0.11 ± 0.04 | 73.5 ± 7.8 |
| Antagonist (WAY-100635; 0.1 mg/kg) | 0.98 ± 0.24* | 33.40 ± 5.01 | 0.41 ± 0.08 | 0.62 ± 0.35* | 39.2 ± 22.1* |
Values are means ± SE.
P < 0.05 indicates a statistically significant difference compared with vehicle treatment (post hoc least significance difference test).
Effect of 8-OH-DPAT and WAY-100635 on EUS EMG activity during transvesical CMGs.
During continuous infusion CMGs before the onset of voiding, two types of EUS EMG activity were detected (Figs. 1 and 2). Most animals exhibited consistent, low amplitude, tonic EUS EMG activity during the filling phase; and in some rats, this tonic activity increased gradually as the infusion volume approached the micturition volume threshold. Just prior to voiding, phasic EUS EMG activity was noted in some experiments (Fig. 2C). During a bladder contraction, the EUS EMG activity markedly increased and consisted of an initial period of tonic activity followed by a bursting pattern of activity characterized by clusters of high-frequency spikes (active periods) and separated by periods of quiescence (silent periods) (Fig. 2). After vehicle treatment, the total interval of the average bursting duration was 4.60 ± 1.45 s, and the average silent period was much longer (0.14 ± 0.03 s) than the average active period (0.07 ± 0.01 s).
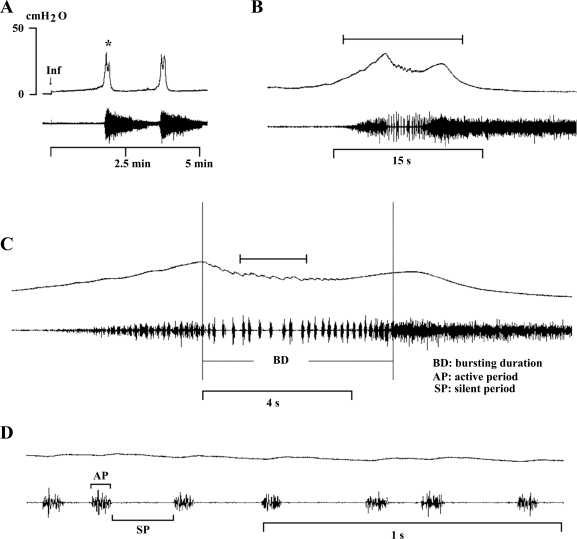
Fig. 2.
Bladder (top traces) and EUS EMG activity (bottom traces) recorded during a continuous-transvesical infusion CMG in an anesthetized rat. A: intravesical pressure and EUS EMG activity were relatively stable during the filling phase. A reflex bladder contraction, indicated by an abrupt, large increase in bladder pressure, was accompanied by large-amplitude EUS EMG activity. B: same recording indicated by asterisk in A shown at faster time scale. The bracket in B indicates the recording period in C, and the bracket in C indicates the recording period in D at a faster time scale. Note the decline in intravesical pressure during EUS EMG bursting in B and C, which indicates the period of voiding. C: tonic EUS EMG activity precedes the large rise in intravesical pressure and shifts to a bursting pattern at the peak of bladder contraction before the onset of voiding. Small oscillations in intravesical pressure coincide with each burst of EMG activity. D: recordings in C shown at very fast time scale showing individual EUS EMG bursts composed of active (AP) and silent periods (SP; brackets) and the small fluctuations in intravesical pressure accompanying each burst. Vertical calibration, intravesical pressure (in cmH2O); horizontal calibration, time (in minutes or seconds); Inf, start of saline infusion.
Although the basic pattern of EUS EMG activity during micturition was similar after either 8-OH-DPAT or WAY-100635 treatment, there were marked quantitative differences. 8-OH-DPAT enhanced tonic EUS EMG activity between voids (Figs. 1B and 3B), and WAY-100635 suppressed this activity (Figs. 1C and 3C). 8-OH-DPAT markedly increased the average silent period (0.23 ± 0.06 vs. 0.14 ± 0.03 s, P < 0.05) and total bursting duration (7.75 ± 1.54 vs. 4.60 ± 1.45 s, P < 0.05) and the total silent period (5.75 ± 1.31 vs. 3.03 ± 1.01 s, P < 0.05), but did not affect the duration of the average active period (0.08 ± 0.01 vs. 0.07 ± 0.01 s) (Table 2, Fig. 3B). WAY-100635 shortened the average silent period (0.10 ± 0.02 s) but did not significantly change other parameters (Fig. 3C). The percentage of the time during a bladder contraction (indicated by contraction duration) occupied by EUS EMG bursting (i.e., duration of the bursting duration/contraction duration) was increased (∼33%) but not significantly after 8-OH-DPAT treatment (Table 3). The effects of WAY-100635 were similar in experiments using protocols 1 and 2 where the drug was administered after 8-OH-DPAT and vehicle, respectively (Table 4).
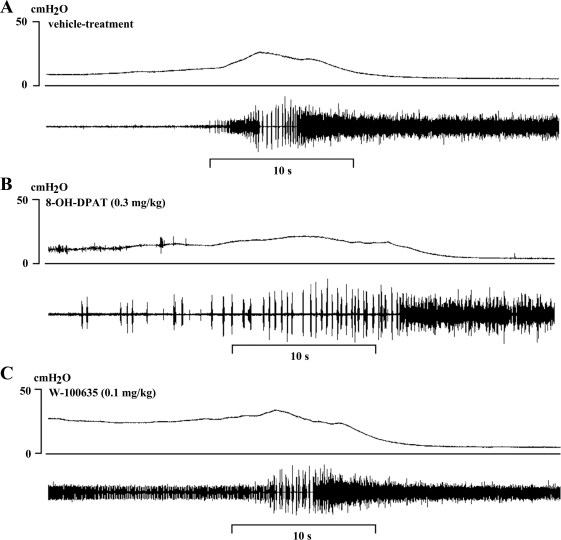
Fig. 3.
Bladder (top traces) and EUS EMG activity (bottom traces) during bladder contractions after sequential administration in the same rat shown in Fig. 1 of vehicle (A), 8-OH-DPAT (0.3 mg/kg iv; B), and WAY-100635 (0.1 mg/kg iv) 1 h after 8-OH-DPAT (C). Recordings of the intravesical pressure and EUS EMG activity depicted in Fig. 1 at increasing time scales showing the interval of silent and active period during bursting period. Vertical calibration, intravesical pressure (in cmH2O); horizontal calibration, time (in seconds).
Table 3.
Parameters of EUS EMG activity after serial administration of vehicle, 8-OH-DPAT, and WAY-100635 in urethane-anesthetized rats
| SP, s | AP, s | BD, s | TSP, s | BD/CD, % | |
|---|---|---|---|---|---|
| Vehicle (n = 18) | 0.14 ± 0.03 | 0.07 ± 0.01 | 4.60 ± 1.45 | 3.03 ± 1.01 | 21.05 ± 10.21 |
| Agonist (8-OH-DPAT; 0.3 mg/kg) | 0.23 ± 0.06* | 0.08 ± 0.01 | 7.75 ± 1.54* | 5.75 ± 1.31* | 28.74 ± 9.25 |
| Antagonist (WAY-100635; 0.1 mg/kg) | 0.10 ± 0.02*† | 0.08 ± 0.01 | 3.45 ± 2.10† | 1.90 ± 1.15† | 15.07 ± 5.87† |
Values are means ± SE. EUS EMG, external urethral sphincter electromyogram. Silent (SP) and active period (AP) denote the average duration of quiescent and tonic EUS EMG activity during the bursting period shown in Fig. 2. TSP, total duration of the silent periods during each void; BD, bursting duration; CD, contraction duration.
P < 0.05 indicates a statistically significant difference compared with vehicle treatment (post hoc least significance difference test).
P < 0.05 indicates a statistically significant difference compared with 8-OH-DPAT treatment (post hoc least significance difference test).
Table 4.
Parameters of EUS EMG activity after vehicle and WAY-100635 treatment in urethane-anesthetized rats
| SP, s | AP, s | BD, s | TSP, s | BD/CD, % | |
|---|---|---|---|---|---|
| Vehicle (n = 11) | 0.12 ± 0.02 | 0.07 ± 0.01 | 5.23 ± 1.67 | 3.39 ± 1.07 | 23.23 ± 9.25 |
| Antagonist (WAY-100635; 0.1 mg/kg) | 0.10 ± 0.01* | 0.07 ± 0.01 | 5.23 ± 1.50 | 3.09 ± 0.89 | 21.75 ± 7.09 |
Values are means ± SE.
P < 0.05 indicates a statistically significant difference compared with vehicle treatment (post hoc least significance difference test).
Effect of pancuronium bromide on bladder activity during transvesical CMGs.
Administration of pancuronium totally suppressed EUS EMG activity and altered CMG parameters presumably by eliminating the EUS regulation of the urethral outlet. After injection of pancuronium, the volume threshold and residual volume increased and voiding efficiency significantly decreased (60%) even though contraction amplitude and contraction duration were not changed (Table 5). In these animals, 8-OH-DPAT decreased volume threshold and residual volume but did not significantly change voiding efficiency. WAY-100635 increased volume threshold and residual volume and further impaired voiding efficiency (Table 5).
Table 5.
Parameters of bladder activity in transvesical CMGs after serial administration of vehicle, pancuronium bromide, 8-OH-DPAT, and WAY-100635 in urethane-anesthetized rats
| VT, ml | CA, cmH2O | CD, min | RV, ml | VE, % | |
|---|---|---|---|---|---|
| Vehicle (n = 12) | 0.69 ± 0.15 | 24.12 ± 4.03 | 0.37 ± 0.06 | 0.16 ± 0.07 | 77.2 ± 7.2 |
| Pancuronium bromide (1.5 mg/kg) | 0.85 ± 0.17 | 25.59 ± 5.82 | 0.31 ± 0.06 | 0.59 ± 0.17‡ | 30.4 ± 14.2‡ |
| Agonist (8-OH-DPAT; 0.3 mg/kg) | 0.57 ± 0.15* | 22.56 ± 5.39 | 0.33 ± 0.07 | 0.33 ± 0.11* | 37.1 ± 18.2 |
| Antagonist (WAY-100635; 0.1 mg/kg) | 1.58 ± 0.26*† | 27.64 ± 6.66 | 0.37 ± 0.08 | 1.26 ± 0.23*† | 19.0 ± 13.0† |
Values are means ± SE.
P < 0.05 indicates a statistically significant difference compared with pancuronium bromide administration (post hoc least significance difference test).
P < 0.05 indicates a statistically significant difference compared with 8-OH-DPAT treatment (post hoc least significance difference test).
P < 0.05 indicates a statistically significant difference compared with vehicle treatment (post hoc least significance difference test).
Effect of 8-OH-DPAT and WAY-100635 on bladder activity during transurethral CMGs.
Various parameters of the bladder activity, including basal pressure, volume threshold, contraction amplitude, contraction duration, and intercontraction interval were evaluated after infusing of saline into the bladder under isovolumetric conditions to elicit rhythmic bladder contractions (n = 12). Because the urethral outlet was ligated, voiding efficiency and postvoid residual volume were not measured in these animals. The average volume threshold for evoking a micturition reflex significantly decreased (60%) after 8-OH-DPAT injection, but increased (55%) after WAY-100635 (Table 6). 8-OH-DPAT significantly increased contraction amplitude (Fig. 4B), and WAY-100635 decreased the amplitude to a level below the vehicle control (Fig. 4C, Table 4). The intercontraction interval was slightly but not significantly reduced by 8-OH-DPAT (Fig. 4B) but significantly increased by 50% after WAY-100635 administration (Fig. 4C, Table 6). Basal pressure was unaffected by the different treatments.
Table 6.
Parameters of bladder activity in transurethral CMGs after serial administration of vehicle, 8-OH-DPAT, and WAY-100635 in urethane-anesthetized rats
| VT, ml | Base, cmH2O | CA, cmH2O | CD, min | ICI, min | |
|---|---|---|---|---|---|
| Vehicle (n = 12) | 0.80 ± 0.29 | 14.77 ± 1.95 | 50.94 ± 5.70 | 0.62 ± 0.15 | 0.81 ± 0.20 |
| Agonist (8-OH-DPAT; 0.3 mg/kg) | 0.33 ± 0.10* | 14.99 ± 3.73 | 58.03 ± 7.56* | 0.66 ± 0.23 | 0.68 ± 0.19 |
| Antagonist (WAY-100635; 0.1 mg/kg) | 1.26 ± 0.31*† | 14.10 ± 2.68 | 42.86 ± 3.15*† | 0.65 ± 0.20 | 1.02 ± 0.16*† |
Values are means ± SE. Base: basal pressure; ICI: intercontraction interval.
P < 0.05 indicates a statistically significant difference compared with vehicle treatment (post hoc least significance difference test).
P < 0.05 indicates a statistically significant difference compared with 8-OH-DPAT treatment (post hoc least significance difference test).
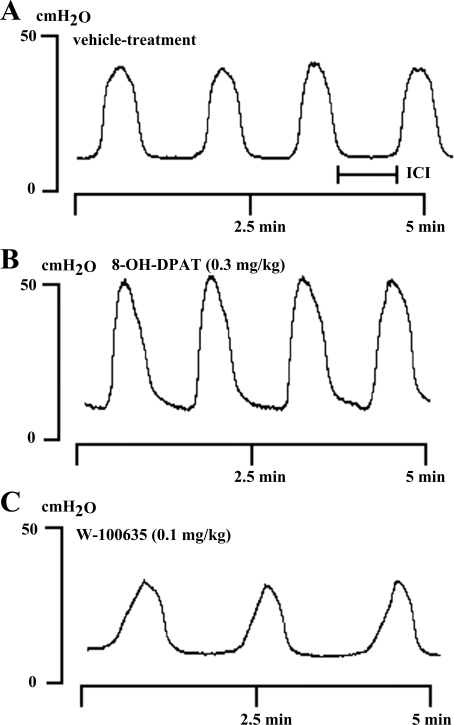
Fig. 4.
Effect of sequential intravenous administration of vehicle (A), 8-OH-DPAT (0.3 mg/kg iv; B), and WAY-100635 (0.1 mg/kg iv; C) on rhythmic isovolumetric contractions evoked by bladder distension in the same rat. Vertical and horizontal calibrations correspond to intravesical pressure (in cmH2O) and time (in minutes), respectively. ICI, intercontraction interval.
DISCUSSION
An analysis of the effects of a 5-HT1A receptor agonist and antagonist on urinary bladder and EUS EMG activity in the female rat revealed that serotonergic mechanisms regulate urine storage as well as voiding. 8-OH-DPAT, a 5-HT1A agonist, enhanced tonic EUS EMG activity that occurred during the period between voids, an effect that would promote urine storage. On the other hand, 8-OH-DPAT reduced the bladder volume threshold for initiating reflex micturition, increased the amplitude of reflex bladder contractions recorded under isovolumetric conditions, and enhanced EUS EMG bursting activity during reflex micturition. These effects would be expected to facilitate voiding. WAY-100635, a 5-HT1A receptor antagonist produced effects opposite of those produced by 8-OH-DPAT, indicating that it blocked physiological regulatory mechanisms that were activated by endogenously released 5-HT. Thus serotonergic mechanisms involving 5-HT1A receptors (1, 2, 12, 36) and possibly other 5-HT receptor subtypes that have been identified in previous studies (9, 16, 21, 28, 29) appear to play an important role in autonomic and somatic neural pathways controlling multiple lower urinary tract functions.
It is reasonable to attribute most of the lower urinary tract effects of 8-OH-DPAT and WAY-100635 to actions on the central nervous system because intrathecal or intracerebroventricular injections of these agents have excitatory and inhibitory effects, respectively, on reflex bladder activity (17, 19, 20, 38). In addition, intravenous administration of WAY-100635 does not alter the bladder contractions induced by peripheral nerve stimulation (19), indicating that its effects are due to an action on the central nervous system. However, a direct excitatory effect of 8-OH-DPAT on the bladder cannot be excluded because a recent study showed that this agent can enhance spontaneous contractions of isolated bladder strips prepared from rats with chronic bladder outlet obstruction (22).
A reduction in the micturition volume threshold raises the possibility that 8-OH-DPAT might act by several mechanisms, including 1) an enhancement of bladder afferent input or afferent processing in the spinal cord (2, 20) or 2) a facilitation of the micturition switching circuit in the pontine micturition center (17, 38). The 8-OH-DPAT enhancement of the amplitude of bladder contractions recorded with a transurethral catheter under isovolumetric conditions is consistent with a facilitatory action on the micturition reflex pathway. However, during transvesical CMGs with the urethral outlet open and the bladder able to empty, 8-OH-DPAT decreased contraction amplitude by >20%. This decrease could reflect an inhibitory effect on the parasympathetic outflow to the bladder but is most likely due to a facilitation of EUS bursting and a decrease in urethral outlet resistance which negates the facilitatory effect on bladder contractions.
WAY-100635 prominently increased the micturition volume threshold, increased residual volume, and reduced voiding efficiency during transvesical CMGs. These effects are attributable to an action on multiple physiological mechanisms that involve endogenously released 5-HT. The doubling of the volume threshold without changing the contraction amplitude from control levels suggests that tonic activation of 5-HT1A receptors regulates the set point for micturition in the urethane-anesthetized rat but may not influence the efferent limb of the micturition reflex. However, during transurethral CMGs with the outlet closed, WAY-100635 did decrease the amplitude of the micturition contractions, raising the possibility that the efferent parasympathetic pathway to the bladder was suppressed by the drug. This effect in combination with a suppression of EUS bursting activity most likely accounts for the decrease in voiding efficiency produced by WAY-100635. The failure of WAY-100635 to change peak voiding pressure during voiding with the outlet open is probably due to the increased outlet resistance occurring in response to the reduction in EUS EMG bursting (4). WAY-100635 reduced the average silent period, indicating that activation of 5-HT1A receptors by endogenous 5-HT is necessary for bladder-sphincter coordination and efficient voiding.
To evaluate the role of EUS activity in voiding efficiency, a neuromuscular blocking agent (pancuronium) was administered to paralyze the EUS muscle. This agent did not change the volume threshold or bladder contraction amplitude but markedly increased residual volume and reduced voiding efficiency by >50%, 8-OH-DPAT decreased the volume threshold and residual volume measured after pancuronium treatment, and WAY-100635 had the opposite effect, indicating that these effects of the two drugs are due to changes in the autonomic control of the bladder and not to changes in EUS activity.
The present experiments complement and extend previous studies which revealed that 8-OH-DPAT enhances and WAY-100635 suppresses short-latency and long-latency EUS reflex activity evoked by stimulation of afferent axons in the pelvic nerve (1). It was proposed that these two reflexes are mediated by different central pathways and have different functions. The short-latency reflex is organized in the spinal cord and is mediated by a pathway that generates tonic EUS EMG activity during bladder filling. The long-latency reflex is dependent on supraspinal mechanisms and is related to bursting EUS EMG activity occurring during micturition. Because NMDA and AMPA glutamatergic mechanisms contribute to the two pelvic-EUS reflexes (1), it is probable that a convergence of 5-HT and glutamatergic pathways is necessary for the regulation of EUS activity.
Other studies showed that reflex EUS activity and urethral closure mechanisms in the rat can be evoked during sneezing (18, 23, 24). This response, which is presumably involved in the maintenance of urinary continence during increases in intra-abdominal pressure, is mediated by supraspinal inputs to EUS motoneurons. Pharmacological experiments revealed that the sneeze-evoked responses were enhanced by duloxetine, a serotonin-norepinephrine reuptake inhibitor and suppressed by an NMDA glutamatergic antagonist or by an α2-adrenoceptor agonist (13, 23). The effect of duloxetine was also suppressed by an α1-adrenoceptor antagonist but not by a nonselective 5-HT receptor antagonist (13, 23). It was concluded that serotoninergic, noradrenergic, and glutamatergic excitatory pathways as well as noradrenergic inhibitory pathways are important contributors to the supraspinal sneeze-evoked EUS response. These studies indicate that the function of the EUS is dependent on a complex interaction between multiple neurotransmitters at spinal and supraspinal levels. Because the 8-OH-DPAT-evoked facilitation of EUS bursting activity also occurs in rats after chronic transection of the spinal cord at the midthoracic level, it is clearly dependent on activation of 5-HT1A receptors in the lumbosacral spinal cord (2, 12).
On the other hand, the modulation of bladder activity by 5-HT1A agonists and antagonists could be mediated by actions at supraspinal as well as spinal sites because these drugs are effective when injected intrathecally (19, 26) or intracerebroventricularly (17, 20, 26, 38) as well as intravenously (2, 9, 19, 26, 30). Bladder activity is regulated by multiple excitatory central neurotransmitter mechanisms (glutamatergic, noradrenergic, dopaminergic, and serotonergic) as well as GABAergic and glycinergic inhibitory mechanisms (10, 11). It is believed that glutamate is the essential excitatory transmitter and that other neurotransmitters such as 5-HT and norepinephrine exert their effects by modulating glutamatergic transmission (9, 11).
A similar interaction between glutamatergic transmission and monoaminergic modulatory mechanisms has been proposed to explain the reflex control of the EUS motoneurons and the action of duloxetine in the cat spinal cord (7, 33, 35), although in this species 5-HT2 rather than 5-HT1A receptors are thought to be involved (8, 33). The clinical efficacy of duloxetine to enhance EUS function and reduce stress urinary incontinence (33) suggests that monoaminergic-glutamatergic interactions may be important for promoting urinary continence in humans.
In summary, the present results indicate that activation of 5-HT1A receptors by exogenous administration of a selective receptor agonist or by endogenously released 5-HT facilitates reflex voiding by lowering the volume threshold for initiating the micturition reflex and by enhancing the relaxation of the external urethral sphincter, as evidenced by the increased silent period during external urethral sphincter bursting.
GRANTS
This study was supported by grants from the National Science Foundation, Taiwan, ROC (NSC94-2314-B-075A-017) and from the National Institutes of Health (DK49430).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Chang HY, Cheng CL, Chen JJ, de Groat WC. Roles of glutamatergic and serotonergic mechanisms in reflex control of the external urethral sphincter in urethane-anesthetized female rats. Am J Physiol Regul Integr Comp Physiol 291: R224–R234, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am J Physiol Renal Physiol 292: F1044–F1053, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng CL, Chai CY, de Groat WC. Detrusor-sphincter dyssynergia induced by cold stimulation of the urinary bladder of rats. Am J Physiol Regul Integr Comp Physiol 272: R1271–R1282, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol 187: 445–454, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Conley RK, Williams TJ, Ford AP, Ramage AG. The role of alpha(1)-adrenoceptors and 5-HT(1A) receptors in the control of the micturition reflex in male anaesthetized rats. Br J Pharmacol 133: 61–72, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlstrom A, Fuxe K. Evidence of the existence of monoamine neurons in the central nervous system. II. Experimentally induced changes in the intraneuronal amine levels of bulbospinal neuron systems. Acta Physiol Scand Suppl 247: 1–36, 1965 [PubMed] [Google Scholar]
- 7.Danuser H, Bemis K, Thor KB. Pharmacological analysis of the noradrenergic control of central sympathetic and somatic reflexes controlling the lower urinary tract in the anesthetized cat. J Pharmacol Exp Ther 274: 820–825, 1995 [PubMed] [Google Scholar]
- 8.Danuser H, Thor KB. Spinal 5-HT2 receptor-mediated facilitation of pudendal nerve reflexes in the anaesthetized cat. Br J Pharmacol 118: 150–154, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groat WC. Influence of central serotonergic mechanisms on lower urinary tract function. Urology 59: 30–36, 2002 [DOI] [PubMed] [Google Scholar]
- 10.de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol 147, Suppl 2: S25–S40, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol 41: 691–721, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Dolber PC, Gu B, Zhang X, Fraser MO, Thor KB, Reiter JP. Activation of the external urethral sphincter central pattern generator by a 5-HT1A receptor agonist in rats with chronic spinal cord injury. Am J Physiol Regul Integr Comp Physiol 292: R1699–R1706, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Furuta A, Asano K, Egawa S, de Groat WC, Chancellor MB, Yoshimura N. Role of alpha2-adrenoceptors and glutamate mechanisms in the external urethral sphincter continence reflex in rats. J Urol 181: 1467–1473, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu B, Olejar KJ, Reiter JP, Thor KB, Dolber PC. Inhibition of bladder activity by 5-hydroxytryptamine1 serotonin receptor agonists in cats with chronic spinal cord injury. J Pharmacol Exp Ther 310: 1266–1272, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Gu B, Thor KB, Reiter JP, Dolber PC. Effect of 5-hydroxytryptamine1 serotonin receptor agonists on noxiously stimulated micturition in cats with chronic spinal cord injury. J Urol 177: 2381–2385, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Helton LA, Thor KB, Baez M. 5-Hydroxytryptamine2a, 5-hydroxytryptamine2B, and 5-hydroxytryptamine2C receptor mRNA expression in the spinal cord of rat, cat, monkey and human. Neuroreport 5: 2617–2620, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Ishizuka O, Gu B, Igawa Y, Nishizawa O, Pehrson R, Andersson KE. Role of supraspinal serotonin receptors for micturition in normal conscious rats. Neurourol Urodyn 21: 225–230, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Kaiho Y, Kamo I, Chancellor MB, Arai Y, de Groat WC, Yoshimura N. Role of noradrenergic pathways in sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol 292: F639–F646, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kakizaki H, Yoshiyama M, Koyanagi T, de Groat WC. Effects of WAY100635, a selective 5-HT1A-receptor antagonist on the micturition-reflex pathway in the rat. Am J Physiol Regul Integr Comp Physiol 280: R1407–R1413, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Lecci A, Giuliani S, Santicioli P, Maggi CA. Involvement of 5-hydroxytryptamine1A receptors in the modulation of micturition reflexes in the anesthetized rat. J Pharmacol Exp Ther 262: 181–189, 1992 [PubMed] [Google Scholar]
- 21.Mbaki Y, Ramage AG. Investigation of the role of 5-HT2 receptor subtypes in the control of the bladder and the urethra in the anaesthetized female rat. Br J Pharmacol 155: 343–356, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittra S, Malhotra S, Naruganahalli KS, Chugh A. Role of peripheral 5-HT1A receptors in detrusor over activity associated with partial bladder outlet obstruction in female rats. Eur J Pharmacol 561: 189–193, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Miyazato M, Kaiho Y, Kamo I, Chancellor MB, Sugaya K, de Groat WC, Yoshimura N. Effect of duloxetine, a norepinephrine and serotonin reuptake inhibitor, on sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol 295: F264–F271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazato M, Kaiho Y, Kamo I, Kitta T, Chancellor MB, Sugaya K, Arai Y, de Groat WC, Yoshimura N. Role of spinal serotonergic pathways in sneeze-induced urethral continence reflex in rats. Am J Physiol Renal Physiol 297: F1024–F1031, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison J, Birder L, Craggs M, de Groat WC, Downie J, Drake M, Fowler C, Thor K. Neural control. In: Incontinence, 3rd International Consultation on Incontinence, edited by Khoury S, Wein A. Plymouth, UK: Health Publications, 2005, p. 363–422 [Google Scholar]
- 26.Pehrson R, Ojteg G, Ishizuka O, Andersson KE. Effects of NAD-299, a new, highly selective 5-HT1A receptor antagonist, on bladder function in rats. Naunyn Schmiedebergs Arch Pharmacol 366: 528–536, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Rajaofetra N, Passagia JG, Marlier L, Poulat P, Pellas F, Sandillon F, Verschuere B, Gouy D, Geffard M, Privat A. Serotoninergic, noradrenergic, and peptidergic innervation of Onuf's nucleus of normal and transected spinal cords of baboons (Papio papio). J Comp Neurol 318: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Ramage AG. The role of central 5-hydroxytryptamine (5-HT, serotonin) receptors in the control of micturition. Br J Pharmacol 147, Suppl 2: S120–S131, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Read KE, Sanger GJ, Ramage AG. Evidence of the involvement of central 5-HT7 receptors in the micturition reflex in anaesthetized female rats. Br J Pharmacol 140: 53–60, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steers WD, de Groat WC. Effects of m-chlorophenylpiperazine on penile and bladder function in rats. Am J Physiol Regul Integr Comp Physiol 257: R1441–R1449, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Tai C, Miscik CL, Ungerer TD, Roppolo JR, de Groat WC. Suppression of bladder reflex activity in chronic spinal cord injured cats by activation of serotonin 5-HT1A receptors. Exp Neurol 199: 427–437, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Testa R, Guarneri L, Poggesi E, Angelico P, Velasco C, Ibba M, Cilia A, Motta G, Riva C, Leonardi A. Effect of several 5-hydroxytryptamine1A receptor ligands on the micturition reflex in rats: comparison with WAY 100635. J Pharmacol Exp Ther 290: 1258–1269, 1999 [PubMed] [Google Scholar]
- 33.Thor KB. Serotonin and norepinephrine involvement in efferent pathways to the urethral rhabdosphincter: implications for treating stress urinary incontinence. Urology 62: 3–9, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Thor KB, Hisamitsu T, de Groat WC. Unmasking of a neonatal somatovesical reflex in adult cats by the serotonin autoreceptor agonist 5-methoxy-N,N-dimethyltryptamine. Brain Res Dev Brain Res 54: 35–42, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther 274: 1014–1024, 1995 [PubMed] [Google Scholar]
- 36.Thor KB, Katofiasc MA, Danuser H, Springer J, Schaus JM. The role of 5-HT1A receptors in control of lower urinary tract function in cats. Brain Res 946: 290–297, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Thor KB, Nickolaus S, Helke CJ. Autoradiographic localization of 5-hydroxytryptamine1A, 5-hydroxytryptamine1B and 5-hydroxytryptamine1C/2 binding sites in the rat spinal cord. Neuroscience 55: 235–252, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Yoshiyama M, Kakizaki H, de Groat WC. Suppression of the micturition reflex in urethane-anesthetized rats by intracerebroventricular injection of WAY100635, a 5-HT1A receptor antagonist. Brain Res 980: 281–287, 2003 [DOI] [PubMed] [Google Scholar]