Abstract
Previous studies have shown that Akita mice bearing the Ins2+/C96Y mutation have significant advantages as a type I diabetes platform for developing models of diabetic nephropathy (DN; Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Am J Physiol Renal Physiol 290: F214–F222, 2006). In view of the critical role for genetic factors in determining susceptibility to DN in humans, we investigated the role of genetic background on kidney injury in Akita mice. To generate a series of inbred Akita mouse lines, we back-crossed the Ins2C96Y mutation more than six generations onto the 129/SvEv and DBA/2 backgrounds and compared the extent of hyperglycemia and renal disease with the standard C57BL/6-Ins2+/C96Y line. Male mice from all three Akita strains developed marked and equivalent hyperglycemia. However, there were significant differences in the level of albuminuria among the lines with a hierarchy of DBA/2 > 129/SvEv > C57BL/6. Renal and glomerular hypertrophy was seen in all of the lines, but significant increases in mesangial matrix compared with baseline nondiabetic controls were observed only in the 129 and C57BL/6 backgrounds. In F1(DBA/2 x C57BL/6)-Ins2+/C96Y mice, the extent of albuminuria was similar to the parental DBA/2-Ins2+/C96Y line; they also developed marked hyperfiltration. These studies identify strong effects of genetic background to modify the renal phenotype associated with the Ins2C96Y mutation. Identification of these naturally occurring strain differences should prove useful for nephropathy modeling and may be exploited to allow identification of novel susceptibility alleles for albuminuria in diabetes.
Keywords: mouse model of type I diabetes, albuminuria, strain comparison
diabetic nephropathy is the most common cause of end-stage renal disease (ESRD) in the United States (26) with >40% of incident ESRD patients having diabetes (26) . Animal models of diabetic nephropathy that more closely recapitulate the features of human disease would be useful tools in understanding the pathogenesis and developing new treatment strategies for this devastating disorder. Because of the potential for genetic manipulation, a model of diabetic nephropathy in the mouse would be particularly attractive. Yet, while progress has been made, there are still no mouse models that reproduce the pathological and functional features of human diabetic nephropathy (2b). As a platform for developing models of diabetic complications, the Akita mouse line shows promise (5). These mice have a single nucleotide substitution in the Ins2 gene (Ins2C96Y), originally identified as a spontaneous mutation in a colony of C57BL/6 mice in Akita, Japan (30). The mutation leads to improper folding of the insulin protein, causing proteotoxicity to the pancreatic β cell. Heterozygosity for the mutation in male mice is associated with marked hyperglycemia, and homozygosity leads to perinatal lethality.
In previous studies, we compared the characteristics of C57BL/6-Ins2+/C96Y mice with C57BL/6 mice treated with streptozotocin (STZ) (5). Relative to STZ-treated animals, the C57BL/6-Ins2+/C96Y mice developed more marked hyperglycemia and elevated blood pressure. C57BL/6-Ins2+/C96Y mice also had higher levels of albuminuria and more consistent renal pathological changes. Thus the C57BL/6-Ins2Akita model appeared to have some advantages over the STZ model of chemically induced diabetes as a platform for developing models of diabetic nephropathy (5). Nonetheless, even the C57BL/6-Ins2+/C96Y mice develop only modest levels of proteinuria, and renal pathological changes are limited to mesangial expansion.
A key contribution of genetic factors to the development of diabetic nephropathy in humans is well established. For example, within the population of patients with diabetes followed longitudinally for as long as 30 years, only a subset develops nephropathy (12). Within this group, the incidence of clinical nephropathy peaks between 10 and 20 years of diabetes and then declines with time, consistent with a defined population of susceptible subjects. Furthermore, the risk of overt nephropathy is significantly greater in certain ethnic groups including African-Americans and Native Americans and in patients with siblings or parents with diabetic nephropathy (17, 23, 25). Thus it seems very likely that genetic factors play a major role in determining susceptibility, as well as resistance, to kidney disease in diabetes. Specific genes determining susceptibility to diabetic nephropathy in humans have not been identified, but several large-scale human studies have been established with the goal of identifying genes associated with the development of diabetic nephropathy (16, 31).
Genetic determinants also seem to modulate the susceptibility to kidney injury from diabetes in mice. For example, we and others have identified significant strain differences affecting the severity of proteinuria and renal pathological changes in mice with chemically induced (STZ) diabetes (5, 18). Accordingly, we reasoned that genetic background might also influence the development of kidney injury in Akita mice. Identification of strains with enhanced susceptibility to kidney injury in diabetes should be useful for developing better mouse models of nephropathy. Moreover, these strains could be used to identify specific gene variants accelerating proteinuria and kidney injury in this setting.
Here, we provide evidence for genetic modifiers of albuminuria and other renal manifestations of diabetes in Akita mice. The C57BL/6 background confers resistance whereas the 129/SvEv and DBA/2 backgrounds are associated with enhanced susceptibility to kidney injury. Animals from an F1 intercross generated between the resistant strain and one of the susceptible strains (DBA/2) predominantly expressed the susceptible phenotype, consistent with dominant inheritance of genetic modifiers that increase the propensity for albuminuria in this model of type I diabetes.
MATERIALS AND METHODS
Animals.
C57BL/6-Ins2+/C96Y (Akita) and wild-type DBA/2(J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and wild-type 129/SvEv mice from Taconic Farms (Germantown, NY). Inbred DBA/2-Ins2Akita and 129SvEv-Ins2Akita mice were generated by successive backcrossing of the Akita mutation onto wild-type DBA/2 and 129/SvEv mice, respectively. For each of the strains, experiments were performed using mice generated after six successive backcrosses. Transmission of the C96Y point mutation was determined by PCR analysis of genomic DNA obtained from weanling mice (27). Mice were housed in an Association for the Assessment and Accreditation of Laboratory Animal Care-accredited animal facility at the Durham Veterans Affairs Medical Center under National Institutes of Health guidelines. Throughout the period of study, animals were provided free access to water and standard rodent chow (Lab Diet, Purina Mills, St. Louis, MO). Because of the limited hyperglycemia observed in diabetic, female mice, only male mice were studied.
Blood glucose measurements.
Beginning at 10 wk of age, blood glucose was measured at monthly intervals using a Glucometer Elite testing system (Ascensia Bayer). After the tarsal area of the left leg was shaved, blood samples were obtained by puncturing the left lateral saphenous vein with a 25-gauge needle (8). Approximately 2 μl of blood was collected directly onto the testing strip for measurement.
Blood pressure measurements.
During the last month of the study, systolic blood pressures were measured in conscious mice using a computerized tail-cuff system (Hatteras Instruments, Cary, NC) that determines systolic blood pressure using a photoelectric sensor as described (10, 11). This system allows measurements in four mice simultaneously and minimizes the potential for observer bias. Before measurements were initiated, mice were adapted to the apparatus for at least 10 days. Blood pressures were then measured for at least 15 days. The validity of this system has been established previously (10, 11).
Urinary albumin measurements.
At the end of the study, 24-h urine collections were obtained from mice housed in individual metabolic cages with free access to water and food. Water intake, urine volume, and body weight were monitored to ensure basal conditions. Urinary creatinine concentration was measured using a picric acid assay according to the manufacturer's instructions (The Creatinine Companion, Exocell, Philadelphia, PA). Urinary albumin excretion was measured using an indirect competitive ELISA according to the manufacturer's instructions (Albuwell M, Exocell).
Measurement of glomerular filtration rate.
FITC-inulin clearance was measured in conscious mice as previously described (19). FITC-inulin (5%) was injected into the penile vein of mice that were briefly anesthetized with isoflurane. At 3, 7, 10, 15, 35, 55, and 75 min after the FITC-inulin injection, blood samples were obtained from the lateral saphenous vein (8). Fluorescence intensity was measured at 485 excitation/520 emission using a FLUOstar Omega plate reader (BMG Labtech). Plasma fluorescence was fitted to a two-phase exponential decay using nonlinear regression (GraphPad Prism), and glomerular filtration rate (GFR) was calculated as using standard formulas (18) and is reported as microliters per minute per gram body weight (7).
Renal pathological examination.
At 6 mo of age, kidneys were harvested for pathological examination. Before harvest, a cannula was placed in the left ventricle and the kidneys were perfused in situ with freshly prepared 4% paraformaldehyde (PFA) using a peristaltic pump (Peristaltic Pump P-1, Amersham Biosciences) at a rate of 7 ml/min. The right kidney was then removed, decapsulated, and weighed. Perfusion-fixed tissue was embedded in paraffin, and sections were stained with periodic acid-Schiff for examination by light microscopy. Glomerular volume was calculated from the mean cross-sectional area of 20 glomerular profiles of each animal using the method of Weibel (29). Glomerular mesangial area was scored from 0 to 3 on 25–40 glomerular profiles from a single section, and the mean was entered as the score for the animal.
Statistical analysis.
The values for each parameter within a group are expressed as means ± SE. For comparisons between two experimental groups, an unpaired t-test was used to assess statistical significance, and a paired t-test was used for comparisons within a group. For comparisons among multiple groups, ANOVA with Tukey's multiple comparisons test was used.
RESULTS
Equivalent hyperglycemia in Akita mouse lines.
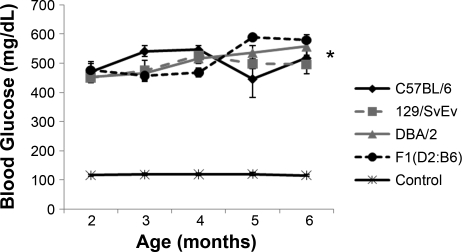
As shown in Fig. 1, blood glucose levels were significantly elevated in Ins2+/C96Y mice on all three genetic backgrounds compared with their respective wild-type (Ins2+/+) controls. The marked levels of hyperglycemia were sustained from the beginning of the experiment when the animals were 8 wk old, through the entire 16-wk observation period. During the study period, there were no statistically significant differences in blood glucose levels among the inbred diabetic strains (P = 0.59 ANOVA+Tukey's).
Fig. 1.
Blood glucose levels in male mice from ages 2 to 6 mo. The control curve represents a composite control of all nondiabetic animals. The Ins2C96Y mutation caused sustained and robust hyperglycemia throughout the 4-mo experimental period in each of the strains tested (*P < 0.0001, unpaired t-test for all strains). There was no difference in the level of hyperglycemia among the 3 inbred Ins2+/C96Y lines (P = 0.591, ANOVA).
Throughout the entire study period, both C57BL/6-Ins2+/C96Y and 129/SvEv-Ins2+/C96Y mice appeared healthy and gained weight normally such that their body weights remained similar to nondiabetic wild-type littermate controls. In contrast, the DBA/2- Ins2+/C96Y animals failed to gain weight and their mean body weights at the end of the study (22.9 ± 1.5 g) were not significantly different from the values at 2 mo of age when the study was initiated (23.9 ± 0.5 gm; P = 0.8). At the end of the study, body weights in the DBA/2-Ins2+/C96Y mice (22.9 ± 1.5 g) were significantly lower than DBA/2-Ins2+/+ nondiabetic controls (30.1 ± 0.8 g; P = 0.0003). As the study progressed, many of the DBA/2-Ins2+/C96Y animals appeared ill and >50% had to be euthanized before completion of the study because of failure to thrive.
Blood pressures in inbred Akita mice.
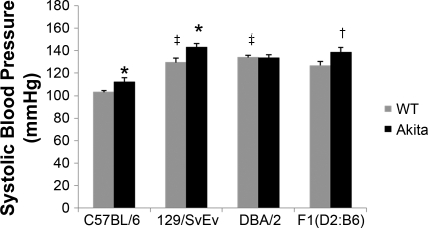
Since hypertension is a typical clinical feature associated with diabetic nephropathy in humans, we monitored systolic blood pressure in all of the groups of mice in this study. In the nondiabetic controls, we found significant strain differences in baseline blood pressures (Table 1). As shown in Fig. 2, systolic blood pressures were higher in both the nondiabetic 129/SvEv-Ins2+/+ (130 ± 4 mmHg) and DBA/2-Ins2+/+ animals (134 ± 2 mmHg) compared with the C57BL/6-Ins2+/+ group (103 ± 2 mmHg; P < 0.01, ANOVA). The blood pressure responses to diabetes also varied among strains. Systolic blood pressures were significantly higher in the C57BL/6-Ins2+/C96Y (112 ± 4 vs. 103 ± 2 mmHg; P = 0.04) and 129/SvEv-Ins2+/C96Y groups (143 ± 3 vs. 130 ± 4 mmHg; P = 0.03) compared with their respective Ins2+/+ wild-type littermates. In contrast, blood pressures were virtually identical in 6-mo-old DBA/2-Ins2+/C96Y and DBA/2-Ins2+/+ wild-type controls (134 ± 3 vs. 134 ± 2 mmHg; P = 0.89).
Table 1.
Systolic blood pressure in inbred mouse lines
| Strain | Systolic Blood Pressure, mmHg |
|
|---|---|---|
| Control | Diabetic | |
| C57BL/6 | 103 ± 2‡ (n = 7) | 112 ± 4* (n = 6) |
| 129/SvEv | 130 ± 4 (n = 7) | 143 ± 3* (n = 5) |
| DBA/2 | 134 ± 2 (n = 10) | 134 ± 3 (n = 6) |
| F1 (D2:B6) | 127 ± 34 (n = 20) | 138 ± 5† (n = 20) |
Values are means ± SE. n, No. of mice.
P < 0.04 between diabetic and wild-type.
P = 0.06 between diabetic and wild-type.
P < 0.01 compared with 129/SvEv and DBA/2 wild-type.
Fig. 2.
Systolic blood pressure (SBP) measured by tail-cuff manometry in 6-mo-old male mice. There were significant differences in blood pressures among the strains of nondiabetic (Ins2+/+) mice with similar levels of elevated SBP in the 129/SvEv-Ins2+/+ and DBA/2-Ins2+/+ animals compared with the C57BL/6-Ins2+/+ group (‡P < 0.01, ANOVA). Compared with the respective nondiabetic controls, the Ins2+/C96Y mutation was associated with an increase in blood pressure (*P < 0.04) in the C57BL/6, 129/SvEv, and F1 (DBA/2 x C57BL/6) (†P = 0.06) strains, but not the DBA/2 strain.
Differences in albuminuria among strains of inbred Akita mice.
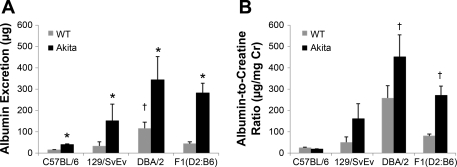
We measured 24-h albumin excretion and albumin-to-creatinine ratio as a marker of renal injury associated with diabetes. Among the nondiabetic wild-type (Ins2+/+) mice, there were strain differences in albumin excretion. Wild-type DBA/2 mice had significantly higher levels of albumin excretion (116 ± 28 μg/day) than wild-type C57BL/6 animals (14 ± 2 μg/day; P = 0.009) (Fig. 3), whereas albumin excretion rates in wild-type C57BL/6 and 129/SvEv mice (33 ± 20 μg/day; P = NS) were not significantly different. On each of the three genetic backgrounds, the presence of the Ins2+/C96Y mutation was associated with significant increases in albumin excretion. Compared with their respective nondiabetic controls, albuminuria was increased by ≈2.8-fold in C57BL/6-Ins2+/C96Y mice (P < 0.0001), 3-fold the DBA/2-Ins2+/C96Y group (P = 0.048), and ∼5-fold in 129/SvEv-Ins2+/C96Y animals (P = 0.03). Among the three Akita strains, absolute levels of albumin excretion were highest in the DBA/2-Ins2+/C96Y mice (345 ± 110 μg/day), intermediate in 129/SvEv-Ins2+/C96Y animals (151 ± 77 μg/day), and lowest in the C57BL/6-Ins2+/C96Y group (40 ± 3 μg/day). These general patterns were also observed with albumin-to-creatinine ratios (Fig. 3B). Thus, while the presence of the Akita mutation was associated with increased albumin excretion in each of the lines, strain-specific genetic modifiers influence the magnitude of albuminuria in this model of diabetes.
Fig. 3.
A: urinary albumin excretion in 6-mo-old male mice. Wild-type (Ins2+/+) DBA/2 mice had significantly higher levels of albumin excretion than wild-type C57BL/6 animals (†P = 0.009), whereas albumin excretion rates in wild-type C57BL/6 and 129/SvEv mice were similar. On each of the 3 genetic backgrounds, the presence of the InsC96Y mutation was associated with a significant increase in albumin excretion (*P < 0.05 unpaired t-test for all strains). Among the three Akita strains, absolute levels of albumin excretion were highest in the DBA/2 line, intermediate in 129/SvEv group, and lowest in the C57BL/6 line. B: albumin-to-creatinine ratios were numerically increased in all strains except C57BL/6. †P < 0.0001 for DBA/2 WT vs. DBA/2-Ins2Akita and F1(DBA/2 x C57BL/6) wild-type vs. F1(DBA/2 x C57BL/6) -Ins2Akita.
GFR in Akita strains.
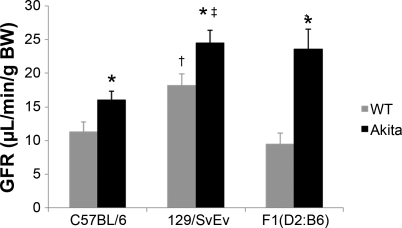
To determine whether there were strain differences in the effect of diabetes on renal function, we measured GFR in the C57BL/6 and 129/SvEv strains as described (19). Compared with the nondiabetic, wild-type mice, GFR was significantly higher in the 129/SvEv (18.2 ± 1.7 μl·min−1·g body wt−1) than the C57BL/6 mice (11.3 ± 1.4 μl·min−1·g body wt−1; P = 0.04). The levels of GFR increased significantly in Akita mice on both backgrounds compared with their respective nondiabetic, wild-type controls (Fig. 4, Table 2). Between the two strains of Akita mice, GFR was significantly higher in the 129-Ins2+/C96Y compared with the C57BL/6-Ins2+/C96Y mice (P = 0.0004). Thus, at similar levels of hyperglycemia, the magnitude of hyperfiltration differs between strains of Akita mice and higher levels of GFR are associated with more severe albuminuria.
Fig. 4.
Glomerular filtration rate (GFR) in 6-mo-old male mice. GFR was measured in C57BL/6, 129/SvEv, and F1(DBA/2 x C57BL/6) wild-type and Akita (Ins2+/C96Y) mice. GFR was higher in wild-type 129/SvEv group compared with wild-type C57BL/6 and wild-type F1(DBA/2 x C57BL/6) mice (†P < 0.04). Also, diabetic 129/SvEv-Ins2+/C96Y mice had higher GFR levels compared with C57BL/6-Ins2+/C96Y mice (‡P = 0.0004). The levels of GFR were significantly higher in all groups of diabetic Akita mice compared with their respective wild-type controls (*P < 0.05).
Table 2.
Urine studies and glomerular filtration rate measurements
| Strain | Albumin Excretion, μg/day |
Urine Volume, ml/day |
Glomerular Filtration Rate, μl/min/g body wt |
|||
|---|---|---|---|---|---|---|
| Control | Diabetic | Control | Diabetic | Control | Diabetic | |
| C57BL/6 | 14.3 ± 2.2 | 39.9 ± 3.2* | 1.2 ± 0.2 | 21.2 ± 1.9† | 11.3 ± 1.4 | 16.0 ± 1.3* |
| 129/SvEv | 33.1 ± 20.4 | 151.3 ± 77.9* | 1.1 ± 0.1 | 11.8 ± 1.8† | 18.2 ± 1.7† | 24.5 ± 1.9*‡ |
| DBA/2 | 116.2 ± 27 | 344.7 ± 109* | 1.3 ± 0.2 | 15.5 ± 2.8† | NA | NA |
| F1(D2:B6) | 43.1 ± 9.7 | 239.7 ± 54.0* | 1.2 ± 0.1 | 23.9 ± 1.4† | 9.5 ± 1.6 | 23.6 ± 2.96* |
Values are means ± SE.
P < 0.05 between diabetic and wild-type.
P < 0.03 between 129 wild-type vs. C57BL/6 wild-type and F1(D2:B6) wild-type.
P < 0.0001 ANOVA between 129/SvEv-Ins2+/C96Y and C57BL/6-Ins2+/C96Y.
Effects of Ins2+/C96Y mutation on kidney size and pathology.
Renal hypertrophy is a characteristic feature of human diabetes. Thus we compared the kidney sizes among the different strains of Akita mice. Interestingly, there were strain differences in kidney size among the 6-mo-old nondiabetic mice. The mean kidney weight-to-body weight ratio in the DBA/2-Ins2+/+ mice (9.63 ± 0.16) was significantly greater than that of the C57BL/6-Ins2+/+ (5.95 ± 0.1) or 129/SvEv animals (6.06 ± 0.1; P < 0.001 ANOVA). Compared with the kidney sizes in their respective wild-type control strains, all of the Ins2+/C96Y lines manifested significant renal hypertrophy by 6 mo of age, and among the diabetic animals, the kidney weight-to-body weight ratio was also highest in the DBA/2-Ins2+/C96Y group (P < 0.001, ANOVA, Table 3).
Table 3.
Kidney weights
| Strain | Left Kidney Weight, mg |
Kidney Weight-to-Body Weight Ratio, mg/g |
||
|---|---|---|---|---|
| Control | Diabetic | Control | Diabetic | |
| C57BL/6 | 162.2 ± 3.5 (n = 7) | 217.4 ± 4.3* (n = 6) | 5.95 ± 0.11 | 8.23 ± 0.4* |
| 129/SvEv | 150.7 ± 5.1 (n = 7) | 258.4 ± 12.7* (n = 5) | 6.06 ± 0.09 | 9.63 ± 0.25* |
| DBA/2 | 292.5 ± 9.9† (n = 10) | 297.8 ± 13.2‡ (n = 6) | 9.63 ± 0.16† | 12.8 ± 0.97*† |
| F1(D2:B6) | 267 ± 9.8 (n = 20) | 378 ± 12.9* (n = 22) | 6.9 ± 0.2 | 12.4 ± 0.3* |
Values are means ± SE. n, No. of mice.
P < 0.001 between diabetic and wild-type.
P < 0.001 vs. other strains.
P = 0.002 vs. C57BL/6
Along with the generalized increased kidney size seen with diabetes, increased glomerular volume is also typical of humans with diabetic nephropathy (3, 13). Among the Ins2+/+ controls, the glomerular volumes were significantly increased in the DBA/2 line compared with the other two strains (P < 0.001, ANOVA), and glomerular volumes were significantly higher in C57BL/6 wild-type mice compared with 129/SvEv (P < 0.001, ANOVA). The glomerular volumes were significantly higher in both the C57BL/6- and 129/SvEv-Ins2+/C96Y groups with diabetes compared with their respective nondiabetic controls (P < 0.0002). However, there was no difference in the glomerular volume between the DBA/2-Ins2+/C96Y and DBA/2-Ins2+/+ groups and, thus, despite the strain differences at baseline, glomerular volumes were very similar among the three diabetic lines at 6 mo of age (Table 4).
Table 4.
Glomerular profiles
| Strain | Mesangial Score |
Glomerular Volume |
||
|---|---|---|---|---|
| Control | Diabetic | Control | Diabetic | |
| C57BL/6 | 0.37 ± 0.04 | 0.94 ± 0.06* | 0.26 ± 0.01 | 0.36 ± 0.1* |
| 129/SvEv | 0.48 ± 0.07 | 1.05 ± 0.06* | 0.18 ± 0.01 | 0.30 ± 0.02* |
| DBA/2 | 0.56 ± 0.09 | 0.56 ± 0.06 | 0.32 ± 0.02 | 0.36 ± 0.03* |
| F1(D2:B6) | 0.51 ± 0.05 | 0.91 ± 0.06* | 0.27 ± 0.01 | 0.38 ± 0.01* |
Values are means ± SE.
P < 0.0002 between diabetic and wild-type
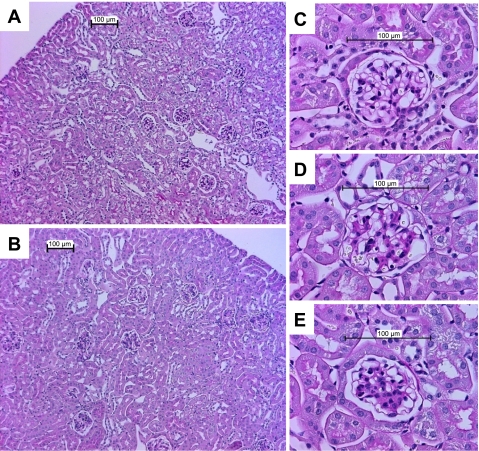
In general, the Ins2+/C96Y mutation caused only modest abnormalities of renal structure, largely confined to expansion of mesangial matrix, and the patterns of mesangial pathology were similar between the groups. As shown in Fig. 5, pathological changes were largely confined to the glomerulus in diabetic animals with preservation of normal morphology in the tubular and interstitial regions. To compare the severity of glomerular pathology between the Akita lines, the extent of mesangial expansion was scored on a scale of 0–3 as described previously (5). Representative glomeruli with severity scores of 0 (normal), 1 (mild), and 2 (moderate) are depicted in Fig. 5. Severely affected glomeruli (score of 3) were rarely observed. There were no significant differences in the mesangial pathology scores among the wild-type Ins2+/+ animals from the three strains (Table 4, Fig. 5, A and C). However, mesangial pathology scores were significantly higher in diabetic C57BL/6-Ins2+/C96Y and 129/SvEv-Ins2+/C96Y mice compared with their respective nondiabetic littermate controls (Table 4). The magnitude of increase was very similar between these two strains (2- to 2.5-fold). On the other hand, mesangial scores were virtually identical in 6-mo-old wild-type DBA/2 (Ins2+/+) and DBA/2-Ins2+/C96Y mice. Accordingly, Akita mice on the C57BL/6 and 129/SvEv backgrounds had more severe mesangial expansion than the DBA/2-Ins2+/C96Y animals (P < 0.001, ANOVA). Of note, several of the DBA/2-Ins2+/C96Y animals developed focal area interstitial inflammation consisting of substantial numbers of polymorphonuclear neutrophils along with other mononuclear inflammatory cells with an overall appearance suggestive of pyelonephritis and were excluded from analysis.
Fig. 5.
Photomicrographs of periodic acid-Schiff-stained sections of mouse kidneys. Low-power views of kidneys from a wild-type 129/SvEv-Ins2+/+ mouse (A) and a diabetic 129/SvEv-Ins2+/C96Y animal (B). As seen in the representative section (B), abnormalities in the Akita mice were largely confined to the glomerulus without evidence of chronic tubulointerstitial disease. C–E: high-power views of glomeruli illustrating the scoring system used to quantitate mesangial pathology. C: representative normal glomerulus from a wild-type mouse, score “0.” D: glomerulus from an Akita mouse with minimal mesangial pathology, score “1.” E: moderate mesangial pathology in a glomerulus from and Akita mouse, score “2.”
Intercross of strains with differential susceptibilities to diabetic kidney injury.
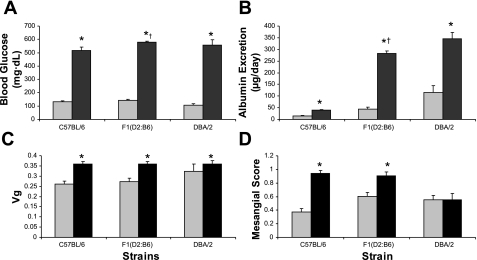
Our initial studies suggested the presence of strain-specific modifiers affecting susceptibility to albuminuria and kidney injury associated with the Ins2+/C96Y mutation. To assess the heritability of these traits, we carried out an intercross between a strain that was relatively resistant to albuminuria (C57BL/6) and a more susceptible strain (DBA/2). We then analyzed diabetes-related phenotypes of a cohort of F1(DBA/2 x C57BL/6)Ins2+/C96Y mice. As shown in Figs. 1 and 6, these F1 animals developed levels of sustained and robust hyperglycemia that were similar to the other lines throughout the study period and were not different from the parental DBA/2-Ins2+/C96Y lines at 6 mo of age. At the end of the study period, F1-Ins2+/C96Y mice, like the parental DBA/2-Ins2+/C96Y group, weighed significantly less than their wild-type littermates (30.3 ± 0.4 vs. 38 ± 1.0 g; P < 0.0001). Compared with the F1-Ins2+/+ controls, the diabetic F1- Ins2+/C96Y animals tended to have elevated blood pressure (138 ± 5 vs. 127 ± 4 mmHg; P = 0.06) (Fig. 2). Moreover, the level of blood pressure in the F1-Ins2+/C96Y animals was significantly higher than the parental C57BL/6-Ins2+/C96Y line (127 ± 4 vs. 112 ± 4 mmHg; P < 0.001, ANOVA) and similar to the DBA/2-Ins2+/C96Y group (134 ± 3 mmHg). The F1(DBA/2 x C57BL/6)-Ins2+/C96Y mice also developed a more than sixfold increase in albuminuria compared with their littermate Ins2+/+ controls (283 ± 48 vs. 43 ± 10 μg/day; P < 0.0001, Fig. 6B). This level of albuminuria was significantly higher than the C57BL/6-Ins2+/C96Y animals (P = 0.02), but was not different from the DBA/2-Ins2+/ C96Y mice. Interestingly, in a comparison of nondiabetic controls, the level of albuminuria in the nondiabetic F1(DBA/2 x C57BL/6)-Ins2+/+ mice was intermediate (43 ± 10 μg/day) between that of the two parental lines, which had significantly different levels of baseline albumin excretion: 14 ± 2 μg day for C57BL/6-Ins2+/+ and 116 ± 27 μg/day for DBA/2-Ins2+/+ (P = 0.002 ANOVA).
Fig. 6.
Blood glucose, urinary albumin excretion, glomerular volumes, and mesangial scores for F1(DBA/2 x C57BL/6)Ins2+/C96Y mice compared with the parental C57BL/6-Ins2+/C96Y and DBA/2-Ins2+/C96Y lines. A: F1(DBA/2 x C57BL/6)-Ins2+/C96Y mice developed sustained and robust hyperglycemia at levels that were similar to the parental DBA/2 line but modestly increased compared with the C57BL/6 line (†P = 0.003, ANOVA). B: F1 animals developed a >6-fold increase in albuminuria compared with their littermate Ins2+/+ controls. This level of albuminuria was significantly higher than in the C57BL/6-Ins2+/C96Y animals (†P = 0.02), but was not different from the DBA/2-Ins2+/ C96Y line. C: F1(DBA/2 x C57BL/6)Ins2+/C96Y mice developed significant glomerular hypertrophy compared with nondiabetic F1 controls. The extent of their hypertrophy was similar to the DBA/2-Ins2+/C96Y parental line and greater than the C57BL/6-Ins2+/C96Y line. D: like the C57BL/6-Ins2+/C96Y line, F1(DBA/2 x C57BL/6)Ins2+/C96Y mice also developed significant mesangial expansion compared with wild-type littermates whereas the parental DBA/2-Ins2+/ C96Y mice did not. *P < 0.05 between Akita (black bars) and wild-type (gray bars).
We also measured GFR in wild-type and Akita mice on the F1(DBA/2 x C57BL/6) background. In the wild-type F1 mice, levels of GFR were similar to the parental C57BL/6 strain but significantly lower than wild-type 129/SvEv mice (Fig. 4, Table 2). Like the other Ins2+/C96Y strains, F1(DBA/2 x C57BL/6)Ins2+/C96Y mice also developed significant hyperfiltration with diabetes and the absolute levels of GFR were similar to the 129-Ins2+/C96Y group and higher than the C57BL/6-Ins2+/C96Y mice. Moreover, the relative change in GFR in the F1(DBA/2 x C57BL/6)Ins2+/C96Y mice compared with their wild-type controls (250%) was greater than the 129 (130%) and C57BL/6 (140%) groups. Among the three strains, the relative increases in GFR parallel their susceptibilities to proteinuria (F1>129>C57BL/6).
Similar to the other Ins2+/C96Y strains, the F1(DBA/2 x C57BL/6)Ins2+/C96Y mice developed significant renal hypertrophy compared with nondiabetic F1(DBA/2 x C57BL/6)Ins2+/+ controls (kidney weight-to-body weight ratio: 12.4 ± 0.3 vs. 6.9 ± 0.2; P < 0.0001). The extent of their hypertrophy was similar to the DBA/2-Ins2+/C96Y parental line and greater than the C57BL/Ins2+/C96Y line (Table 3). Among the nondiabetic Ins2+/+ lines, glomerular volumes in the F1-Ins2+/+ group were similar to the levels seen in the two parental lines, C57BL/6 and DBA/2 (Table 4, Fig. 6C). With diabetes, the glomerular volumes increased further in the F1(DBA/2 x C57BL/6)-Ins2+/C96Y animals to 0.38 ± 0.01 (Table 4), also similar to increases in the two parental lines.
Mesangial scores in the nondiabetic F1(DBA/2 x C57BL/6)-Ins2+/+ animals (Table 4) were virtually identical to the relatively high basal levels seen in wild-type DBA/2 mice (0.51 ± 0.05 vs. 0.56 ± 0.09; P = 0.142, ANOVA). As discussed above, there was no further increase in the severity of mesangial pathology in the DBA/2-Ins2+/C96Y mice compared with the baseline levels seen in the DBA/2-Ins2+/+ controls. By contrast, mesangial scores were significantly higher in the F1(DBA/2 x C57BL/6)Ins2+/C96Y mice than their nondiabetic littermate controls (0.91 ± 0.06 vs. 0.51 ± 0.05; P < 0.001), such that the extent of mesangial pathology in the F1 mice was very similar to that seen in the parental C57BL/6-Ins2+/C96Y line (0.94 ± 0.06) (Fig. 6D, Table 4). As with the other lines, this corresponds to a relatively modest degree of mesangial expansion. Interestingly, several of the F1(DBA/2 x C57BL/6)Ins2+/C96Y mice developed the pathological picture of pyelonephritis that was observed in the parental DBA/2-Ins2+/C96Y line.
DISCUSSION
In humans with diabetic nephropathy, control of blood pressure and blockade of the renin-angiotensin system can slow progression of renal disease. However, these maneuvers only delay inexorable progression of kidney injury, and new approaches to therapy are needed (6). Development of animal models recapitulating the features of diabetic nephropathy in humans could be very useful for identifying new therapeutic targets in this area. Such models might also have value for understanding the early phases of diabetic kidney disease where relatively modest increases in albuminuria predict poor cardiovascular outcomes (1, 28). The mouse is particularly attractive for this sort of human disease modeling because of its tractability for genetic manipulation. While current mouse models of diabetes recapitulate features of the early phases of diabetic kidney disease including microalbuminuria and mesangial expansion, progress toward mouse models with pathological and functional characteristics of later stages of human diabetic nephropathy has been limited (2).
In previous studies comparing type I diabetes models, we found that the Akita (Ins2+/C96Y) mouse has some advantages as a platform for complication modeling compared with chemically induced diabetes with STZ (5). These included sustained and robust hyperglycemia, enhanced levels of albuminuria, more consistent mesangial pathology, and simple genetics wherein a single copy of the mutant allele generates profound diabetes. However, in our studies carried out with the original line of C57BL/6-Ins2+/C96Y mice from Jackson Laboratories, the extent of proteinuria and mesangial pathology was relatively modest (5). As we and others have shown that genetic background has a strong influence on the characteristics of kidney injury associated with STZ-induced diabetes (5, 18), we considered the possibility that genetic background might have similar effects in the Ins2+/C96Y model. Accordingly, we generated novel lines of inbred Ins2+/C96Y mice by successive generations of back-crossing. Since our objective was to produce Akita mouse lines with enhanced renal injury, we chose the DBA/2 strain, based on its enhanced susceptibility for albuminuria in the STZ model (5, 18), and the 129/SvEv strain, which has been demonstrated to have enhanced propensity for kidney injury in nondiabetic models (9, 21).
We carried out extensive phenotyping of the inbred Ins2+/C96Y lines with a primary focus on albuminuria, an early clinical indicator of renal involvement in humans with diabetes (15) where it is also a potent marker of increased cardiovascular risk (4, 22). Even among the nondiabetic wild-type control groups, we found significant strain differences in albuminuria, with the highest levels observed in the DBA/2 strain. This is consistent with a recent study that also found increased albuminuria in wild-type DBA/2 mice and mapped several, interacting quantitative trait loci (QTL) influencing this trait to mouse chromosome 2 (24). One of these loci overlaps a QTL for albuminuria previously identified in rats (33) and humans (34). We find that exaggerated albuminuria in the wild-type, nondiabetic DBA/2 mice was associated with increased kidney size and higher glomerular volumes, but no evidence of structural changes in the kidney. Because of the loss of some the DBA/2 Akita cohort before the study was completed, it is possible that our data might reflect selection against more severely affected diabetic mice. However, we were able to collect urine for albumin measurements in all but one mouse in this group. Furthermore, even with this possible skewing, the levels of albuminuria in the DBA/2-Ins2+/C96Y animals were significantly greater than in the C57BL/6 or 129 strains. Moreover, a similar phenotype was observed in the F1(DBA/2 x C57BL/6)-Ins2+/C96Y animals, which were hardier and survived the entire study period.
The Ins2+/C96Y mutation caused robust and equivalent levels of hyperglycemia in each of the strains. However, the manifestations of diabetes in the kidney were quite variable, suggesting differences in genetic susceptibilities to diabetes-induced renal injury. While albumin excretion was increased in each of the three Ins2+/C96Y strains compared with their respective wild-type controls, levels of albuminuria were significantly higher in the 129/SvEv-Ins2+/C96Y and DBA/2-Ins2+/C96Y lines compared with the C57BL/6 Ins2+/C96Y animals. Similar to our previous study using the STZ model of diabetes, the highest levels of albumin excretion were observed with Ins2+/C96Y mutation on the DBA/2 background. However, because of the high baseline level of albuminuria in the DBA/2-Ins2+/+ controls, the relative change in albuminuria was only about threefold in the DBA/2-Ins2+/C96Y line compared with almost fivefold in the 129/SvEv-Ins2+/C96Y group.
We also observed strain-related differences in GFR, both in the wild-type animals and with diabetes. Wild-type 129 mice had a higher GFR than wild-type C57BL/6 male mice of the same age (Fig. 4). In the presence of diabetes, hyperfiltration developed in all strains tested, with the largest increase occurring in F1(DBA/2 x C57BL/6)-Ins2+/C96Y mice, the strain with the highest albuminuria. Increased GFR is an almost universal finding in the early phases of diabetes. Moreover, glomerular hyperfiltration has been suggested to play an important role in the pathogenesis of progression to overt nephropathy (13, 14, 20, 32). Compared with their respective wild-type controls, each of the Akita strains developed hyperfiltration and the extent of the GFR increase roughly correlated with the severity of albuminuria.
Across the strains, there was a variable level of correlation between levels of albuminuria and alterations in GFR or extent of mesangial pathology. The C57BL/6-Ins2+/C96Y mice had the lowest levels of proteinuria, but among the highest mesangial pathology scores. By contrast, the DBA/2-Ins2+/C96Y line with the most albuminuria had the least mesangial pathology. Accordingly, the 129/SvEv-Ins2+/C96Y line may have advantages for modeling kidney injury based on their combination of robust diabetes-induced albuminuria, glomerular hyperfiltration, and mesangial pathology. Moreover, the majority of modified mouse lines generated by gene targeting are produced using embryonic stem cells derived from substrains of 129, and inbred diabetic animals could be generated with a simple cross. Furthermore, despite substantial elevation in their serum glucose levels, the 129/SvEv-Ins2+/C96Y animals remained vigorous and healthy throughout the study period. On the other hand, the DBA/2 mice, which had the highest levels of albuminuria, did not gain weight normally, appeared frail, and a significant percentage did not survive to the end of the study. Several of the experimental animals also developed renal pathological lesions resembling pyelonephritis, which could confound interpretation of their kidney phenotype.
Our studies provide clear-cut evidence for strain-specific genetic modifiers affecting kidney phenotypes associated with the Ins2+/C96Y mutation. These include differences in susceptibility to albuminuria and mesangial pathology, as well as variable renal and glomerular hypertrophic responses. To assess the heritability of these traits and focusing on albuminuria, we carried out an intercross between the strain with relative resistance to albuminuria, C57BL/6, and one of the more susceptible strains, DBA/2. The level of albumin excretion in the F1 Akita mice generated by this intercross was very similar to the parental DBA/2 line, suggesting a dominant pattern of inheritance for albumin susceptibility alleles. However, the enhanced mesangial pathology in the F1 mice most closely resembled the C57BL/6 parental line, indicating that genetic control of diabetic mesangial expansion may be distinct from that of albuminuria. Based on the observed segregation of these traits in the F1 animals, mapping of susceptibility loci could be possible through F1 intercrosses or backcrosses with the individual parental lines. Finally, because the F1 mice are easily generated by a single cross between commercially available C57BL/6-Ins2+/C96Y and DBA/2-Ins2+/+ mice, these animals may be useful as a mouse model of type I diabetes developing relatively high levels of albuminuria along with mesangial expansion.
In summary, we have generated novel lines of inbred Ins2+/C96Y mice and have demonstrated strong genetic modifiers influencing albuminuria and renal injury. These strains could be used to map these naturally occurring modifiers of renal disease in diabetes. We suggest that the 129/SvEv-Ins2+/C96Y line may be particularly useful for further model development, especially in combination with other genetic manipulations carried out in embryonic stem cells derived from 129 substrains that may accelerate or exaggerate the development of glomerulosclerosis and renal failure in diabetes.
GRANTS
This work was performed as part of the Animal Models of Diabetic Complications Consortium (AMDCC) supported by U01 DK076136-02.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Arnlov J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D'Agostino RB, Vasan RS. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 112: 969–975, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Breyer MD, Bottinger E, Brosius FC, 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005 [DOI] [PubMed] [Google Scholar]
- 2b.Brosius FC, III, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T. Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drummond K, Mauer M. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 51: 1580–1587, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290: F214–F222, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Gurley SB, Coffman TM. The renin-angiotensin system and diabetic nephropathy. Semin Nephrol 27: 144–152, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Hackbarth H, Hackbarth D. Genetic analysis of renal function in mice. 1. Glomerular filtration rate and its correlation with body and kidney weight. Lab Anim 15: 267–272, 1981 [DOI] [PubMed] [Google Scholar]
- 8.Hem A, Smith AJ, Solberg P. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret and mink. Lab Anim 32: 364–368, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Ishola DA, Jr, van der Giezen DM, Hahnel B, Goldschmeding R, Kriz W, Koomans HA, Joles JA. In mice, proteinuria and renal inflammatory responses to albumin overload are strain-dependent. Nephrol Dial Transplant 21: 591–597, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA 92: 3521–3525, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25: 1111–1115, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Krolewski AS, Warram JH, Christlieb AR, Busick EJ, Kahn CR. The changing natural history of nephropathy in type I diabetes. Am J Med 78: 785–794, 1985 [DOI] [PubMed] [Google Scholar]
- 13.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest 74: 1143–1155, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mogensen CE. Early glomerular hyperfiltration in insulin-dependent diabetics and late nephropathy. Scand J Clin Lab Invest 46: 201–206, 1986 [DOI] [PubMed] [Google Scholar]
- 15.Mogensen CE, Vestbo E, Poulsen PL, Christiansen C, Damsgaard EM, Eiskjaer H, Froland A, Hansen KW, Nielsen S, Pedersen MM. Microalbuminuria and potential confounders. A review and some observations on variability of urinary albumin excretion. Diabetes Care 18: 572–581, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Mueller PW, Rogus JJ, Cleary PA, Zhao Y, Smiles AM, Steffes MW, Bucksa J, Gibson TB, Cordovado SK, Krolewski AS, Nierras CR, Warram JH. Genetics of Kidneys in Diabetes (GoKinD) study: a genetics collection available for identifying genetic susceptibility factors for diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol 17: 1782–1790, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettitt DJ, Saad MF, Bennett PH, Nelson RG, Knowler WC. Familial predisposition to renal disease in two generations of Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 33: 438–443, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Rudberg S, Persson B, Dahlquist G. Increased glomerular filtration rate as a predictor of diabetic nephropathy—an 8-year prospective study. Kidney Int 41: 822–828, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Salzler HR, Griffiths R, Ruiz P, Chi L, Frey C, Marchuk DA, Rockman HA, Le TH. Hypertension and albuminuria in chronic kidney disease mapped to a mouse chromosome 11 locus. Kidney Int 72: 1226–1232, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 42: 1050–1065, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Satko SG, Langefeld CD, Daeihagh P, Bowden DW, Rich SS, Freedman BI. Nephropathy in siblings of African Americans with overt type 2 diabetic nephropathy. Am J Kidney Dis 40: 489–494, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Sheehan S, Tsaih SW, King BL, Stanton C, Churchill GA, Paigen B, DiPetrillo K. Genetic analysis of albuminuria in a cross between C57BL/6J and DBA/2J mice. Am J Physiol Renal Physiol 293: F1649–F1656, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Trevisan R, Viberti G. Genetic factors in the development of diabetic nephropathy. J Lab Clin Med 126: 342–349, 1995 [PubMed] [Google Scholar]
- 26.US Renal Data System USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007 [Google Scholar]
- 27.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 103: 27–37, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang TJ, Evans JC, Meigs JB, Rifai N, Fox CS, D'Agostino RB, Levy D, Vasan RS. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation 111: 1370–1376, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Weibel ER. Stereological Methods: Practical Methods for Biological Morphometry London, UK: Academic, 1979 [Google Scholar]
- 30.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 46: 887–894, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Zager PG, Kriz W, Freedman BI. The Family Investigation of Nephropathy, and Diabetes Research Group. Genetic Determinants of Diabetic Nephropathy: The Family Investigation of Nephropathy and Diabetes (FIND). J Am Soc Nephrol 14: S202–S204, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest 77: 1925–1930, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]