Abstract
Connective tissue growth factor (CTGF) participates in diverse fibrotic processes including glomerulosclerosis. The adenylyl cyclase agonist forskolin inhibits CTGF expression in mesangial cells by unclear mechanisms. We recently reported that the histone H3K79 methyltransferase disruptor of telomeric silencing-1 (Dot1) suppresses CTGF gene expression in collecting duct cells (J Clin Invest 117: 773–783, 2007) and HEK 293 cells (J Biol Chem In press). In the present study, we characterized the involvement of Dot1 in mediating the inhibitory effect of forskolin on CTGF transcription in mouse mesangial cells. Overexpression of Dot1 or treatment with forskolin dramatically suppressed basal CTGF mRNA levels and CTGF promoter-luciferase activity, while hypermethylating H3K79 in chromatin associated with the CTGF promoter. siRNA knockdown of Dot1 abrogated the inhibitory effect of forskolin on CTGF mRNA expression. Analysis of the Dot1 promoter sequence identified a CREB response element (CRE) at −384/−380. Overexpression of CREB enhanced forskolin-stimulated Dot1 promoter activity. A constitutively active CREB mutant (CREB-VP16) strongly induced Dot1 promoter-luciferase activity, whereas overexpression of CREBdLZ-VP16, which lacks the CREB DNA-binding domain, abolished this activation. Mutation of the −384/−380 CRE resulted in 70% lower levels of Dot1 promoter activity. ChIP assays confirmed CREB binding to the Dot1 promoter in chromatin. We conclude that forskolin stimulates CREB-mediated trans-activation of the Dot1 gene, which leads to hypermethylation of histone H3K79 at the CTGF promoter, and inhibition of CTGF transcription. These data are the first to describe regulation of the Dot1 gene, and disclose a complex network of genetic and epigenetic controls on CTGF transcription.
Keywords: chromatin, transcription factor, gene expression
connective tissue growth factor (CTGF), also known as CCN2, is a member of the CCN family of early response genes, which includes five members: Cyr61 (cysteine-61-rich protein), Nov (nephroblastoma overexpressed gene), WISP-1 (Wnt-1-induced secreted protein 1), WISP-2, and WISP-3 (29). CTGF is involved in diverse cellular functions, including cell cycle regulation, migration, adhesion, angiogenesis, and the formation of the extracellular matrix. CTGF is not expressed in the normal kidney, but its expression is induced in glomerulonephritis, glomerulosclerosis, and diabetic nephropathy, and its expression levels are correlated with both the progression and severity of renal fibrosis (4, 23, 27). CTGF has also been implicated in epithelial-mesenchymal transition in the kidney and in the renal inflammatory stimulus to diverse mediators (5). CTGF expression is induced by a number of factors involved in renal damage, such as transforming growth factor-β1 (13), angiotensin II (31, 39), aldosterone (31), and high concentrations of glucose (19). Conversely, agents that elevate intracellular cAMP have been reported to inhibit CTGF expression and cell proliferation in TGF-β1-stimulated renal fibroblasts and mesangial cells (3, 10, 14, 18).
For transcriptional activation or repression of a given gene in response to stimuli to occur, regulatory elements in the gene must recruit specific chromatin-modifying factors to their immediate vicinity (40). The degree to which chromatin structure is relaxed or condensed ultimately dictates the accessibility of nascent transcription factors and RNA polymerases to the specific enhancer, repressor, and promoter regions. Core histones contain lysine-rich domains that are substrates for covalent posttranslational modifications, including methylation, which alter chromatin in a location-specific manner (20, 32), and generate a “histone code” (30) that extends the information content of the DNA code. Among these modifications, the methylation of lysine residues in histones H3 and H4 is critical to the regulation of chromatin structure and gene expression (28). Dot1 has been shown to regulate gene expression by methylation of K79, a conserved, surface-exposed residue that lies within the globular domain of histone H3. The level of histone H3K79 methylation varies among some specific loci (22), suggesting that this modification may be targeted to specific genomic regions. In contrast to other known histone methyltransferases, Dot1 introduces multiple methyl groups on H3K79 via a distributive (also termed “nonprocessive”) kinetic mechanism (11), so that monomethylated (H3K79me1), dimethylated (H3K79me2), and trimethylated (H3K79me3) forms of H3K79 can be observed in chromatin (17, 33). The relative abundance of the methylation states depends on Dot1 activity, and global H3K79 methylation appears to be functionally more important than a specific H3K79 methylation state. How the expression of Dot1 itself is regulated in response to agents that alter its activity has not been explored.
We previously cloned and characterized the murine Dot1 gene (9). Our analysis of the 5′-flanking region of the murine Dot1 gene revealed a putative cAMP response element (CRE) half-site at −384TGACG−380. CREs have been identified in numerous gene promoters and generally consist of minor variations in the 8-bp palindrome 5′-TGANNTCA-3′ (21). The minimum sequence required for a functional CRE is the downstream half-site, 5′-NGTCA-3′, which is consistent with our observations of the −384/−380 site in the Dot1 promoter (9). The trans-activation of genes through a CRE occurs by binding of CRE-binding protein (CREB) phosphorylated at S133 to the CRE with recruitment of the coactivator CREB-binding protein (CBP) (7). CREB is a nuclear transcription factor belonging to the larger basic leucine zipper (bZIP) family of transcription factors. The canonical pathway that leads to CREB phosphorylation is the cAMP protein kinase A pathway, but several other protein kinase pathways have been shown to be involved in CREB activation, including phosphatidylinositol 3-kinase, phosphoinositide-dependent protein kinase 1, calmodulin-dependent protein kinase, and various members of the mitogen-activated protein kinase (6, 8, 15).
In the present report, we mechanistically tested the involvement of Dot1 in CTGF transcription in response to forskolin in mouse mesangial cells (MMCs). We hypothesized that Dot1 modifies chromatin associated with the CTGF promoter to basally suppress CTGF transcription and that forskolin accentuates this suppression. We further hypothesized that forskolin stimulates binding of CREB to a CRE in the proximal promoter of Dot1, resulting in trans-activation of Dot1.
MATERIALS AND METHODS
Cell culture, reagents, and plasmids.
MMCs (ATCC CRL-1927) were maintained in complete medium: Ham's F12 plus DMEM supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 5% FBS. The MMC lines that stably express the proximal 3.8 kb of the murine CTGF promoter fused to the firefly luciferase coding region were maintained in complete medium supplemented with 400 μg/ml Zeocin (Invitrogen). Vehicle or forskolin (1 μM; Sigma) was added to the cells as indicated in the text and figure legends. Oligonucleotides were custom synthesized by SigmaGenosys (The Woodlands, TX). Lipofectamine 2000 reagent was from Invitrogen (Carlsbad, CA). The Dual-Luciferase Reporter Assay System and the luciferase vectors pGL3-Basic and pRL-SV40 were from Promega (Madison, WI). The BCA protein estimation kit was from Pierce Chemical (Rockford, IL). Plasmids CMV500, CREB, the dominant-negative CREB mutant A-CREB (1), the constitutively active CREB mutant CREB-VP16 (25, 38), and pCREBdLZ-VP16, which is identical to CREB-VP16 except that the DNA-binding and dimerization domains of CREB are deleted (25), were a gift from Dr. D. D. Ginty (Department of Neuroscience, Howard Hughes Medical Institute, The Johns Hopkins University School of Medicine, Baltimore, MD). pEGFPC3-mDot1, which contains the murine Dot1 cDNA in pEGFP-C3, has been previously characterized by our laboratory (42). The murine CTGF promoter/enhancer (pGL3-3.8CTGF, nucleotides −3874 to −1) and the proximal 5′-flanking region of the murine Dot1 gene (pGL3-0.5Dot1, nucleotides −505 to +319) were PCR amplified from mouse genomic DNA and then cloned into the pGL3-basic vector at XhoI and HindIII sites. Site-directed mutation of the −384/−380 CREB-binding element (5′-CAGAAAAAGAAAATTGACGGGAAGGGAAGAGAAAG-3′ replaced with 5′-CAGAAAAAGAAAATgacacGGAAGGGAAGAGAAAG-3′ mutations in lower case letters; Fig. 1) in pGL3-0.5Dot1 was accomplished by PCR amplification, using pGL3-0.5Dot1 DNA as a template and the QuikChange Multi site-directed mutagenesis kit (Strategene, La Jolla, CA) according to the manufacturer's manual, to yield pGL3-0.5Dot1ΔCRE−384/−380. pcDNA3.1-3.8CTGF-luc was constructed by amplifying the 3.8-kb murine CTGF 5′-flanking region together with the firefly luciferase coding region from pGL3-3.8CTGF and cloning the final amplicon into the XhoI and EcoRV sites of pcDNA3.1-Zeo(−) (Clontech). The authenticity of the DNA insert was confirmed by sequence analysis. All plasmid inserts were sequenced to verify their authenticity, and to confirm the presence of the desired mutations and the absence of spurious mutations.
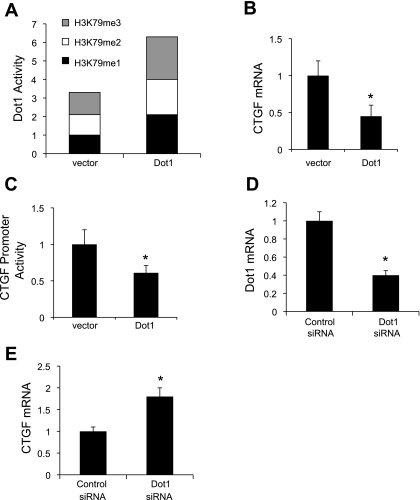
Fig. 1.
Dot1 represses endogenous connective tissue growth factor (CTGF) mRNA expression and basal CTGF promoter activity in mouse mesangial cells (MMCs). A: overexpressed Dot1 increases H3K79 methylation states in acid extracts of histone proteins. MMCs were transiently transfected with pEGFPC3 (vector) or pEGFPC3-mDot1 (Dot1). Acid extracts of histone proteins were then prepared and assayed for H3K79 methylation states with a fluorometric assay as described in materials and methods. The H3K79me1 value for vector-transected cells was set at 1, and the other values were normalized to it; n = 3. B: overexpressed Dot1 downregulates basal CTGF mRNA expression and promoter activity in mesangial cells. MMCs were transiently transfected with pEGFPC3 (vector) or pEGFPC3-mDot1 (Dot1) as in A, and total RNA was prepared for analysis of CTGF mRNA levels by quantitative RT-PCR. *P < 0.05 vs. vector, n = 4. C: luciferase assay demonstrating that Dot1 overexpression suppresses expression of a stably incorporated CTGF promoter-luciferase construct in MMCs. A mesangial cell line harboring stably transfected pGL3Zeocin-3.8CTGF was transiently transfected with pEGFPC3 (vector) or pEGFPC3-mDot1 (Dot1). Twenty-four hours later, cell lysates were prepared, and the firefly luciferase activity of each sample was normalized to its protein content to generate the “CTGF promoter activity.” The relative luciferase activity of the vector-transfected cells was designated as 1 and utilized to determine the relative level and the significance of the other samples. *P < 0.05 vs. vector-transfected controls, n = 4. D: mRNA analysis showing siRNA knockdown of Dot1 expression in MMCs. MMCs were treated with scrambled control siRNA or Dot1-specific siRNA and then subjected to qRT-PCR analysis. *P < 0.05 vs. scrambled siRNA-transfected controls, n = 4. E: mRNA analysis showing that siRNA knockdown of Dot1 upregulates expression of endogenous CTGF mRNA expression in MMCs. MMCs were treated with scrambled control siRNA or Dot1-specific siRNA and then subjected to qRT-PCR analysis of endogenous CTGF mRNA expression. *P < 0.05 vs. scrambled siRNA, n = 3.
Transient and stable transfections.
MMCs or stably transfected MMCs were seeded in 24-well plates and grown to 90–95% confluency in complete medium without antibiotics and cotransfected the following day using the LipoFectamine 2000 reagent according to the manufacturer's protocol and a total of 1 μg/well of plasmid DNAs. The amount of transfected DNA was kept constant by addition of appropriate amounts of the parental empty vector. Transfection efficiencies were normalized by cotransfection with 20 ng/well of the Renilla luciferase expression plasmid pRL-SV40 to correct for transfection efficiency. Six hours after cotransfection, complete medium was changed with charcoal-stripped medium. Sixteen hours later, charcoal-stripped medium was added with vehicle or forskolin. Cell lysates were then prepared at various intervals as indicated in the text. The firefly and Renilla luciferase activities in 100-μl lysate samples were measured as previously described in our laboratory (41). The ratio of firefly to Renilla luciferase activities was reported and taken as an index of promoter activity. Each determination was performed in triplicate, and the mean value was recorded as a single independent observation. Three to four independent observations were conducted for each experimental protocol. For stable transfections, MMCs were seeded in six-well plates and transfected with pcDNA3.1-3.8CTGFCTGF-luc using LipofectAMINE 2000 reagent according to the manufacturer's protocol. Twenty-four hours after transfection, the cells were split, and 400 μg/ml Zeocin (Invitrogen) was added for selection. Surviving clonal cell lines were expanded and tested for presence of the transfected gene by PCR and for firefly luciferase activity, which was measured and normalized to protein concentration. Multiple positive cell lines were expanded for subsequent experiments.
Acid extraction of histone proteins and measurement of Dot1 activity.
Confluent cells on 150-mm-diameter dishes were harvested in lysis buffer (PBS containing 0.5% Triton X-100, 2 mM PMSF, and 0.02% NaN3) for 10 min on ice. After centrifugation at 3,000 rpm for 5 min at 4°C, the supernatants were resuspended in extraction buffer (0.5 N HCl +10% glycerol) on ice for 30 min. After centrifugation, 8 vol of acetone were added and the suspension was incubated at −20°C overnight. The histone extract protein was dissolved in water after centrifugation and quantified and stored at −20°C for further analysis. Mono-, di-, and trimethyl histone H3K79 activities in the histone extracts were measured using the fluorometric EpiQuik Global Pan-methyl Histone H3K79 Quantification Kit (Epigentek Group, Brooklyn, NY) using 485-nm excitation and 528-nm emission according to the manufacturer's protocol. Standard curves of fluorescence vs. H3K79 protein concentrations were generated from positive control samples provided with the kit, and these data were used to calculate the Dot1 activity in the experimental samples over the linear range of the assay.
Dot1 silencing by RNA interference.
A negative control siRNA (catalog no. 12935-300) and mouse Dot1 siRNA [a mixture of 3 Stealth/siRNA duplex oligoribonucleotides: 1) 5′-GGAAGTGGATGAAATGGTATGGAAA-3′, 5′-TTTCCATACCATTTCATCCACTTCC-3′; 2) 5′-TGTCTGGCCTGGCTTTGCCTGATTA-3′, 5′-TAATCAGGCAAAGCCAGGCCAGACA-3′; and 3) 5′-GGAACGGCCTCTAGGACTAACTAAT-3′, 5′-ATTAGTTAGTCCTAGAGGCCGTTCC-3′] were purchased from Invitrogen. Cells were grown in 24-well plates and transfected with 20 pmol of negative control or Dot1 siRNAs using LipofectAMINE reagent. Six hours after transfection, medium was replaced with complete medium containing 5% charcoal-stripped FBS. After another 18 h, cells were treated as detailed in the text and figure legends, and total cell lysates were harvested for measurement of luciferase activity. Transfection optimization and the efficiency of siRNA knockdown of Dot1 were determined by qRT-PCR. Data are means ± SE from four independent experiments, with each experiment carried out in triplicate.
qRT-PCR and ChIP-qPCR.
Quantitative real-time RT-PCR assays were performed using the SYBR GreenER qPCR SuperMix Universal (Invitrogen), primers to amplify +542 to +793 of the CTGF cDNA (sense: 5′-ACCGACTGGAAGACACATTTGG-3′; antisense: 5′-CAGGCTTGGCGATTTTAGGTG-3′), nucleotides +593 to +810 of the Dot1 cDNA (sense: 5′-ACCGACTGGAAGACACATTTGG-3′; antisense: 5′-CAGGCTTGGCGATTTTAGGTG-3′), or nucleotides +238 to +757 of the GAPDH cDNA (sense: 5′-CCACTAACATCAAATGGGGTGAGG-3′; antisense: 5′-TACTTGGCAGGTTTCTCCAGGC-3′), and nucleotides +130 to +360 of the β-actin cDNA (sense: 5′-ATGGTGGGAATGGGTCAGAAGGAC-3′; antisense: 5′-GGTCATCTTTTCACGGTTGGC-3′), and cDNA synthesized using AccuScript High Fidelity RT-PCR system (Stratagene) from total RNA isolated with TRIzol reagent from MMCs as in our previous work (37). The specificity of the primers was analyzed by agarose gel and melting curve analysis. Standard curves were plotted with the threshold cycles vs. log template quantities. For quantification, CTGF or Dot1 expression was normalized to the expressed level of β-actin or GAPDH, as indicated in the text in figure legends, from the same sample. ChIP-qPCR assays were performed according to the manufacturer's instructions (Upstate) and as previously described (45), using 10 μg of antibodies directed against CREB (Santa Cruz Biotechnology, Santa Cruz, CA), or H3K79me1, H3K79me2, H3K79me3 (each from Abcam, Cambridge, MA), or 5 μl of rabbit nonimmune serum; eluted DNA fragments were purified to serve as templates for PCR with primers constructed to amplify the region with −3491 to −3070 of CTGF (forward 5′-GAGCTGAGTACATCATCTCAC-3′; reverse 5′-GGACATTCAAGACATTCACAG-3′) or −582 to −162 of Dot1 (forward 5′-GGTCAGACTGGTCAACAGAATG-3′; reverse 5′-CTTACGCACGCAGCGGCGCACG-3′). qPCRs were run in triplicate and the values were transferred into copy numbers of DNA using a standard curve of genomic DNA with known copy numbers. The resulting transcription values were then normalized for primer pair amplification efficiency using the qPCR values obtained with input DNA. Data are means ± SE from four independent experiments, with each experiment carried out in triplicate.
Data analysis.
Quantitative data are expressed as means ± SE and were analyzed for statistical significance by one-way ANOVA. P values <0.05 were taken as significant.
RESULTS
Dot1 represses endogenous CTGF mRNA expression and basal CTGF promoter activity in MMC.
Our previous studies demonstrated that overexpressed Dot1 inhibited CTGF mRNA expression in mIMCD3 cells (44, 45) and HEK 293T cells (24). To formally test whether Dot1 regulates CTGF gene transcription in MMCs, we manipulated the Dot1 expression level in MMCs through overexpression and siRNA knockdown, and then measured the effects on endogenous CTGF mRNA expression, as measured by qRT-PCR, and, in separate experiments, on the activity of a CTGF promoter-reporter construct stably incorporated in MMCs. These cell lines provided a system with which to correlate changes in endogenous CTGF mRNA levels, CTGF promoter-luciferase activity, and chromatin modifications in vivo.
To verify overexpression of Dot1 in the transfections, we assessed Dot1 activity toward histone H3K79 methylation in acid extracts of histone proteins from the transfected cells using a quantitative H3K79 methyltransferase activity assay. As seen in Fig. 1A, histones of vector-transfected controls exhibited relatively comparable levels of each of the three H3K79 methylation states, whereas cells transfected with Dot1 showed a near doubling of Dot1 activity for each of the three H3K79 methylation states, verifying appropriate overexpression. Dot1 overexpression resulted in ∼50% lower levels of endogenous CTGF mRNA expression (Fig. 1B) and ∼40% lower luciferase activity generated by pcDNA3.1-3.8CTGF-luc in the cell lines (Fig. 1C) compared with controls.
In reciprocal experiments, Dot1 knockdown was accomplished by RNA interference. qRT-PCR determinations indicated that the knockdown efficiency of Dot1a mRNA was ∼60% (Fig. 1D), and this was accompanied by a near doubling of endogenous CTGF mRNA as measured by qRT-PCR (and normalized to the levels of β-actin mRNA obtained by qRT-PCR in the same samples) compared with cells transfected with negative control siRNA (Fig. 1E). The levels of β-actin mRNA, used as a specificity control, were invariant across the samples.
Forskolin inhibits CTGF mRNA expression and CTGF promoter activity while inducing H3K79 methylation states at the CTGF promoter in vivo.
Forskolin has been shown to inhibit CTGF gene expression in several cell types (26), but its effects on mesangial cells have not been reported. We tested whether forskolin inhibited CTGF mRNA expression and CTGF promoter activity in MMCs. As seen in Fig. 2A, forskolin inhibited endogenous CTGF mRNA levels by ∼90% beginning at 3 h (the earliest time point measured) and continuing through 24 h (the latest time point measured). The inhibitory effects of forskolin on CTGF promoter activity were more delayed: CTGF promoter activity was ∼45% less than its time control at 6 h, and ∼75% lower at 24 h of treatment (Fig. 2B). In contrast, forskolin induced global H3K79 methylation at the CTGF promoter as measured by ChIP-qPCR (Fig. 2C). In control experiments, only negligible amounts of chromatin containing the −3491 to −3070 of CTGF were immunoprecipitated by the control IgG (data not shown). Thus, there appears to be an early (within 6 h) nongenomic effect of forskolin to inhibit CTGF mRNA expression, and a later effect to inhibit CTGF transcription.
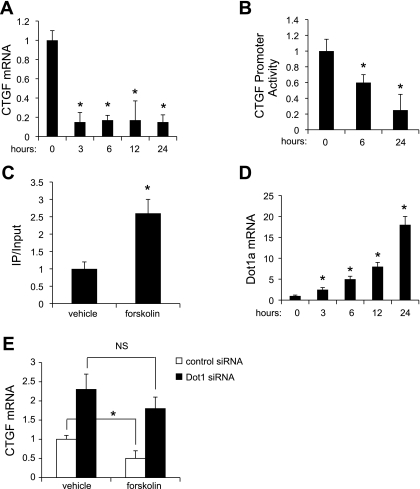
Fig. 2.
Forskolin inhibits CTGF mRNA expression and promoter activity as it increases H3K79me3 methylation associated with chromatin at the CTGF 5′-flanking region and Dot1 mRNA expression. A: mesangial cells were treated with vehicle or forskolin (1 μM) for the indicated number of hours, and total RNA was harvested for analysis of CTGF and β-actin mRNAs from the same sample levels by quantitative RT-PCR. The CTGF mRNA value was normalized to that of β-actin, which was invariant under the 2 conditions. *P < 0.05 vs. vehicle-treated cells, n = 4. B: luciferase assay demonstrating that forskolin inhibits the activity of the stably incorporated CTGF promoter-luciferase construct in a time-dependent manner. *P < 0.05 vs. vehicle-treated cells, n = 3. C: ChIP-qPCR analysis of H3K79me3 occupancy at the CTGF 5′-flanking region. Mesangial cells were treated with vehicle or forskolin (1 μM) for 24 h. ChIP assays were then performed with antibody directed against H3K79me3 or nonimmune IgG followed by qPCR with a primer set designed to amplify −3491 to −3070 of the CTGF gene. The value for the final amplicon/input DNA with anti-H3K79me3 from the vehicle-treated cells was set as 1, and the value from the forskolin-treated cells was normalized to it. Values are means ± SE of 3 separate experiments, each performed in triplicate. D: Dot1 mRNA levels were measured in mesangial cells treated with forskolin (1 μM) for the indicated number of hours by quantitative RT-PCR. The levels were normalized to that of β-actin, which was invariant under the 2 conditions. *P < 0.05 vs. vehicle-treated cells, n = 4. E: Dot1 knockdown abrogates the ability of forskolin to inhibit CTGF mRNA expression. Mesangial cells were transfected with scrambled control siRNA or Dot1-specific siRNA. The transfected cells were treated with vehicle or forskolin (1 μM) for 24 h, RNA was then harvested, and quantitative RT-PCR was used to determine CTGF mRNA levels. *P < 0.05 vs. vehicle-treated cells, n = 5. NS, not significant.
Given the forskolin induction of global H3K79 methylation at the CTGF promoter (Fig. 2C), we tested whether forskolin induces Dot1 gene expression. Indeed, forskolin treatment resulted in a time-dependent increase in Dot1 mRNA levels (Fig. 2D), which paralleled the progressive decrements in CTGF promoter activity (Fig. 2B). To determine whether Dot1 is a principal mediator of the late effect of forskolin inhibition of CTGF transcription, we tested the effects of forskolin on endogenous CTGF mRNA expression as measured by qRT-PCR in cells transfected with control or Dot1-specific siRNAs (Fig. 2E). We hypothesized that Dot1 knockdown would limit the ability of forskolin to inhibit CTGF mRNA expression. As seen in Fig. 2E, transfection of Dot1 siRNA promoted an ∼2.3-fold increase in CTGF mRNA compared with control siRNA, and forskolin did not significantly inhibit this induction, whereas it did inhibit CTGF induction of the cells transfected with control siRNA.
Forskolin enhances CREB-mediated trans-activation of the Dot1 promoter.
Forskolin is known to act through the cAMP-CREB pathway to activate gene transcription. We analyzed the Dot1 proximal 5′-flanking region with TESS (Transcription Element Search System at //www.cbil.upenn.edu/tess/) and noted several consensus binding sites for transcription factors, including a potential CREB response element at −384/−380 (Fig. 3A). ChIP assays coupled with qPCR were then performed on MMCs treated with vehicle or forskolin for 24 h to examine whether CREB binds to this region of the Dot1 promoter region in MMCs in vivo, and whether forskolin enhances binding. Chromatin containing this region was consistently immunoprecipitated by anti-CREB antibody, and this was significantly enhanced by forskolin treatment of the cells (Fig. 3B). In contrast, only negligible amounts of chromatin containing the Dot1 proximal promoter region were immunoprecipitated by the control IgG (data not shown). These results indicate that forskolin increases CREB occupancy of the Dot1 proximal promoter region in chromatin of MMCs.
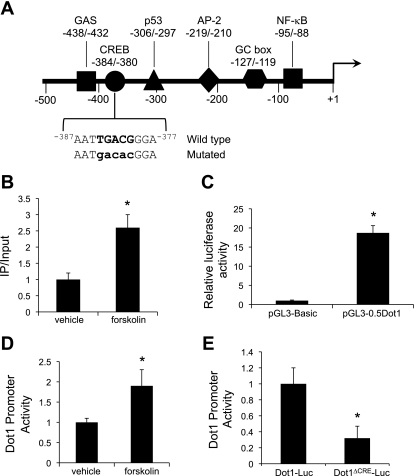
Fig. 3.
A: map of the proximal 5′-flanking region of the murine Dot1 gene. Consensus sites for the binding of selected transcription factors are indicated. Sequence of the −384/−380 region and the mutated sequence (mutations in lower case letters) used for trans-activation assays are also indicated. B: ChIP-qPCR analysis of CRE-binding protein (CREB) occupancy at the Dot1 proximal promoter. MMCs were treated with vehicle or forskolin (1 μM) for 24 h. ChIP assays were then performed with antibody directed against CREB or nonimmune IgG followed by qPCR with a primer set designed to amplify −582 to −162 of the Dot1 gene. The value for the final amplicon/input DNA with anti-CREB from the vehicle-treated cells was set as 1, and the value from the forskolin-treated cells was normalized to it. Values are means ± SE of 3 separate experiments, each performed in triplicate. C: luciferase assay showing functional promoter activity of the transiently transfected pGL3-0.5Dot1 construct. pGL3-Basic or pGL3-0.5Dot1 was cotransfected with pRL-SV40 into MMCs, and firefly and Renilla luciferase activities were measured in triplicate for each sample. Firefly luciferase activity was normalized to that of Renilla luciferase activity to generate “Dot1 promoter activity.” *P < 0.05 vs. pGL3-Basic-transfected cells, n = 3. D: luciferase activity showing forskolin (1 μM for 24 h) induction of pGL3-0.5Dot1. MMCs were cotransfected with pGL3-0.5Dot1 and pRL-SV40 in MMCs, and the cells were treated with vehicle or forskolin (1 μM) for 24 h. Firefly luciferase activity was normalized to that of Renilla luciferase activity to generate “Dot1 promoter activity,” measured in triplicate for each sample. *P < 0.05 vs. vehicle-treated cells, n = 3. E: functional analysis of the −384/−380 CRE in the proximal promoter of the murine Dot1 gene. Core nucleotides of the CRE in the pGL3-0.5Dot1 were mutated by point mutagenesis to create pGL3-0.5Dot1Δ−384/−380 (Dot1Δ−384/−380-Luc) as described in materials and methods. The construct or pGL3-0.5Dot1 was cotransfected with pRL-SV40 into MMCs, and firefly and Renilla luciferase activities were measured in triplicate for each sample. Firefly luciferase activity was normalized to that of Renilla luciferase activity to generate Dot1 promoter activity. Error bars indicate ±SE. *P ≤ 0.05 vs. pGL3-0.5Dot1 (Dot1-Luc), n = 3.
To evaluate the functional role of CREB binding to the −384/−380 element, we performed a series of Dot1 promoter-luciferase activity assays. First, we verified that pGL3-0.5Dot1 contains functional promoter elements (Fig. 3C). MMCs were transiently transfected with pGL3-0.5Dot1 or pGL3-Basic as a control along with the Renilla luciferase expression plasmid pRL-SV40. After 24 h, the firefly and Renilla luciferase activities were measured and their ratios were determined and used as an index of the Dot1 promoter activity. The value for the vector control was set at 1, and the other values were normalized to this. As shown in Fig. 3C, robust luciferase activity was evident in MMCs transfected with pGL3-0.5Dot1 compared with controls, indicating that the pGL3-0.5Dot1 construct contained functional promoter elements. We next tested the effects of vehicle or forskolin on the activity of pGL3-0.5Dot1. Forskolin treatment for 24 h resulted in an approximate doubling of Dot1 promoter activity compared with controls (Fig. 3D). Finally, we introduced site-specific mutations (depicted in Fig. 3A) into pGL3-0.5Dot1 to generate pGL3-0.5Dot1ΔCRE−384/−380. The wild-type and mutant constructs were transiently transfected along with the Renilla luciferase expression plasmid pRL-SV40 into MMCs. As seen in Fig. 3C, site-directed mutation of the −384/−380 CREB site of the Dot1 promoter resulted in 70% lower levels of basal promoter activity compared with the wild-type pGL3-0.5Dot1.
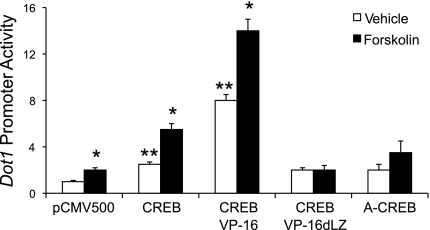
In trans-activation assays, we tested pGL3-0.5Dot1 activity after overexpression of wild-type CREB or CREB mutants: CREB-VP16 (a constitutively active CREB mutant), CREBdLZ-VP16 (a mutant of CREB-VP16 that lacks the CREB DNA-binding domain), and A-CREB (a dominant-negative CREB mutant). These plasmids or the empty parent vector were cotransfected with pGL3-0.5Dot1 and pRL-SV40. The total amounts of DNA transfected were held constant. Twenty-four hours later, the cells were treated with vehicle or forskolin for 24 h before lysates were harvested for luciferase assays. The value generated by the cotransfection of the control plasmid was set to 1, and the luciferase values obtained in the other transfections were normalized to this value. As seen in Fig. 4, overexpression of CREB enhanced basal and, to a greater extent, forskolin-stimulated activity of pGL3-0.5Dot1. As expected, CREB-VP16 strongly induced pGL3-0.5Dot1 activity in the vehicle-treated cells to the level seen for CREB overexpression in forskolin-treated cells. In contrast, the basal and forskolin-stimulated induction of pGL3-0.5Dot1 was negligible in cells transfected with CREBdlZ-VP16 or A-CREB (Fig. 4).
Fig. 4.
CREB trans-activates the proximal promoter of the murine Dot1 gene in MMCs. pGL3-0.5Dot1 reporter construct and pRL-SV40 were cotransfected with the expression vector for CREB, a constitutively active CREB mutant (CREB-VP16), CREBdLZ-VP16, the CREB dominant-negative mutant A-CREB, or an insertless mammalian expression vector containing the cytomegalovirus promoter CMV500. Twenty-four hours after transfection, cell lysates were prepared and firefly and Renilla luciferase activities in lysates of the cells were assayed. Firefly luciferase activity was normalized to Renilla luciferase activity. The bar graph plots the luciferase activities in the various transfection conditions. Error bars indicate ±SE. *P ≤ 0.05 vs. vehicle-treated pCMV500-transfected cells; **P ≤ 0.05 vs. forskolin-treated pCMV500-transfected cells, n = 3.
DISCUSSION
In this report, we provide the first evidence for chromatin-based regulation of CTGF transcription, and for mediators that regulate Dot1 transcription. We observed that forskolin, a well-studied agent that inhibits CTGF gene expression, does so in large part through reciprocal effects on Dot1-mediated H3K79 methylation at the CTGF promoter in MMCs: forskolin enhances CREB-mediated trans-activation of the Dot1 promoter, which translates into hypermethylation of H3K79, and inhibition of CTGF transcription. The degree to which Dot1 suppresses basal CTGF expression is substantial, since siRNA knockdown of Dot1 doubled CTGF mRNA levels (Fig. 1B). Moreover, Dot1 appears to be the principal mediator of the late (>6 h) effect of forskolin to inhibit CTGF transcription, since siRNA knockdown of Dot1 prevented forskolin inhibition of CTGF (Fig. 2E). Presumably, the same mechanism may be operative in other cell types, since our early work showed that forskolin induced H3K79 methylation of bulk histones harvested from mIMCD3 collecting duct cells (9).
Dot1 is an evolutionarily conserved histone methyltransferase. In yeast, Dot1-mediated H3K79 methylation is associated with telomere silencing, meiotic checkpoint control, and DNA damage response (36). The biological function of H3K79 methylation in mammals, however, remains poorly understood. Dot1 knockout mice do not survive, and embryos exhibit multiple developmental abnormalities, including growth impairment, angiogenesis defects in the yolk sac, and cardiac dilation. Renal defects were not reported (16). Transcriptome analysis of Dot1-deficient embryonic stem cells revealed 535 genes whose expression profile is altered compared with wild-type cells (2). Of these, the number of genes with known functions in the cell cycle, cellular proliferation, and differentiation is disproportionately high. Thus, involvement of Dot1 in cell cycle progression in mesangial cells is of potential pathogenetic interest.
Terada et al. (31) implicated NF-κB in the activation of CTGF transcription, although they did not directly test whether NF-κB directly activated the CTGF promoter. Since NF-κB can either activate or inhibit transcription at target genes, our finding that the Dot1 proximal promoter contains a putative κB element (Fig. 3A) suggests the possibility that the NF-κB effect on CTGF transcription may have been indirect, and mediated through NF-κB binding to, and inhibiting, Dot1 promoter activity and hence H3K79 methylation at the CTGF promoter. Our finding that CREB trans-activates the Dot1 promoter via the −384/−380 CRE (Fig. 3E) and that forskolin stimulates CREB occupation at this promoter region in chromatin in vivo (Fig. 3B) explains, at least partially, the late effects of forskolin to inhibit, via Dot1, CTGF promoter activity (Fig. 2B). Since, in contrast to other known methylated lysines of histones, no demethylase specific for methylated H3K79 has yet been identified, despite exhaustive investigations, regulation of Dot1 gene expression itself is likely a key mechanism for controlling H3K79 methylation states in chromatin at target gene promoters. Characterizing the mechanisms underlying the early and pronounced nongenomic effects of forskolin to inhibit CTGF mRNA expression will require further inquiry, but this presumably reflects destabilizing the mRNA. For example, forskolin has been shown to rapidly (within 6 h) inhibit the vascular smooth muscle type I angiotensin II receptor by destabilizing its mRNA (34). If such an effect occurs in mesangial cell CTGF expression, its coordination with the transcriptional inhibition we observed would provide both rapid and sustained silencing of CTGF gene expression. Further studies will be needed to address this hypothesis.
While much attention has focused on histone-modifying enzymes and their dynamic regulation of chromatin and gene transcription, very little is known about how these important enzymes are themselves regulated. Our characterization of the Dot1 proximal promoter and its regulation by forskolin and CREB represents one of the first, if only, demonstrations of the transcriptional regulation of the epigenetic modifier (in this case, Dot1) itself. Our results raise the interesting paradox that chromatin must navigate to alter CTGF transcription after forskolin treatment: first, chromatin must adopt an “open” configuration so that Dot1 can be actively transcribed, and then it is faced with the newly transcribed and translated Dot1 acting to “close” it to silence the CTGF gene. Moreover, our findings provide a novel example of reciprocal genomic responses of histone modifier (transcriptional activation of Dot1) and its target gene (transcriptional repression of CTGF) linked via epigenetic changes (histone H3K79 methylation).
Understanding the regulation of Dot1 and its biological effects on mesangial cells likely will reveal new mechanisms and targets in renal diseases characterized by mesangial cell proliferation. We postulate that pathological conditions that lead to inactivation of the Dot1 pathway and consequent derepression of CTGF transcription could accelerate mesangial cell pathology in fibrotic glomerular diseases associated with CTGF induction.
GRANTS
This work was supported by the National Institutes of Health Grant R01-DK-075065.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Ahn S, Olive M, Aggarwal S, Krylov D, Ginty DD, Vinson C. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol 18: 967–977, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry ER, Krueger W, Jakuba CM, Veilleux E, Ambrosi DJ, Nelson CE, Rasmussen TP. ES cell cycle progression and differentiation require the action of the histone methyltransferase Dot1L. Stem Cells 27: 1538–1547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black SA, Jr, Palamakumbura AH, Stan M, Trackman PC. Tissue-specific mechanisms for CCN2/CTGF persistence in fibrotic gingiva: interactions between cAMP and MAPK signaling pathways, and prostaglandin E2-EP3 receptor mediated activation of the c-JUN N-terminal kinase. J Biol Chem 282: 15416–15429, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blom IE, van Dijk AJ, Wieten L, Duran K, Ito Y, Kleij L, deNichilo M, Rabelink TJ, Weening JJ, Aten J, Goldschmeding R. In vitro evidence for differential involvement of CTGF, TGFbeta, and PDGF-BB in mesangial response to injury. Nephrol Dial Transplant 16: 1139–1148, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Burns WC, Kantharidis P, Thomas MC. The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs 185: 222–231, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci 28: 436–445, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 365: 855–859, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Conkright MD, Montminy M. CREB: the unindicted cancer co-conspirator. Trends Cell Biol 15: 457–459, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Craig JC, Schumacher MA, Mansoor SE, Farrens DL, Brennan RG, Goodman RH. Consensus and variant cAMP-regulated enhancers have distinct CREB-binding properties. J Biol Chem 276: 11719–11728, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: downregulation by cAMP. FASEB J 13: 1774–1786, 1999 [PubMed] [Google Scholar]
- 11.Frederiks F, Tzouros M, Oudgenoeg G, van Welsem T, Fornerod M, Krijgsveld J, van Leeuwen F. Nonprocessive methylation by Dot1 leads to functional redundancy of histone H3K79 methylation states. Nat Struct Mol Biol 15: 550–557, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Gauer S, Segitz V, Goppelt-Struebe M. Aldosterone induces CTGF in mesangial cells by activation of the glucocorticoid receptor. Nephrol Dial Transplant 22: 3154–3159, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Gore-Hyer E, Shegogue D, Markiewicz M, Lo S, Hazen-Martin D, Greene EL, Grotendorst G, Trojanowska M. TGF-β and CTGF have overlapping and distinct fibrogenic effects on human renal cells. Am J Physiol Renal Physiol 283: F707–F716, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Heusinger-Ribeiro J, Eberlein M, Wahab NA, Goppelt-Struebe M. Expression of connective tissue growth factor in human renal fibroblasts: regulatory roles of RhoA and cAMP. J Am Soc Nephrol 12: 1853–1861, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal 16: 1211–1227, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Jones B, Su H, Bhat A, Lei H, Bajko J, Hevi S, Baltus GA, Kadam S, Zhai H, Valdez R, Gonzalo S, Zhang Y, Li E, Chen T. The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure. PLoS Genet 4: e100–0190, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacoste N, Utley RT, Hunter JM, Poirier GG, Cote J. Disruptor of telomeric silencing-1 is a chromatin-specific histone H3 methyltransferase. J Biol Chem 277: 30421–30424, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Lin SL, Chen RH, Chen YM, Chiang WC, Lai CF, Wu KD, Tsai TJ. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. J Am Soc Nephrol 16: 2702–2713, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Makino H, Mukoyama M, Sugawara A, Mori K, Suganami T, Yahata K, Fujinaga Y, Yokoi H, Tanaka I, Nakao K. Roles of connective tissue growth factor and prostanoids in early streptozotocin-induced diabetic rat kidney: the effect of aspirin treatment. Clin Exp Nephrol 7: 33–40, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Merika M, Thanos D. Enhanceosomes. Curr Opin Genet Dev 11: 205–208, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci USA 83: 6682–6686, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng HH, Ciccone DN, Morshead KB, Oettinger MA, Struhl K. Lysine-79 of histone H3 is hypomethylated at silenced loci in yeast and mammalian cells: a potential mechanism for position effect variegation. Proc Natl Acad Sci USA 100: 1820–1825, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen TQ, Tarnow L, Jorsal A, Oliver N, Roestenberg P, Ito Y, Parving HH, Rossing P, van Nieuwenhoven FA, Goldschmeding R. Plasma connective tissue growth factor is an independent predictor of end-stage renal disease and mortality in type 1 diabetic nephropathy. Diabetes Care 31: 1177–1182, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Reisenauer MR, Anderson M, Huang L, Zhang Z, Zhou Q, Kone BC, Morris AP, Lesage GD, Dryer SE, Zhang W. AF17 competes with AF9 for binding to Dot1a to upregulate transcription of epithelial Na+ channel α. J Biol Chem 284: 35659–35669, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 286: 2358–2361, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Ricupero DA, Rishikof DC, Kuang PP, Poliks CF, Goldstein RH. Regulation of connective tissue growth factor expression by prostaglandin E2. Am J Physiol Lung Cell Mol Physiol 277: L1165–L1171, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Riser BL, Denichilo M, Cortes P, Baker C, Grondin JM, Yee J, Narins RG. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol 11: 25–38, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 76: 75–100, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev 19: 133–144, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Strahl BD, Allis CD. The language of covalent histone modifications. Nature 403: 41–45, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Terada Y, Kuwana H, Kobayashi T, Okado T, Suzuki N, Yoshimoto T, Hirata Y, Sasaki S. Aldosterone-stimulated SGK1 activity mediates profibrotic signaling in the mesangium. J Am Soc Nephrol 19: 298–309, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umlauf D, Goto Y, Feil R. Site-specific analysis of histone methylation and acetylation. Methods Mol Biol 287: 99–120, 2004 [DOI] [PubMed] [Google Scholar]
- 33.van Leeuwen F, Gafken PR, Gottschling DE. Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell 109: 745–756, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Nickenig G, Murphy TJ. The vascular smooth muscle type I angiotensin II receptor mRNA is destabilized by cyclic AMP-elevating agents. Mol Pharmacol 52: 781–787, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int 70: 1914–1919, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Wysocki R, Javaheri A, Allard S, Sha F, Cote J, Kron SJ. Role of Dot1-dependent histone H3 methylation in G1 and S phase DNA damage checkpoint functions of Rad9. Mol Cell Biol 25: 8430–8443, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu X, Zhang W, Kone BC. CREB trans-activates the murine H+-K+-ATPase alpha2 subunit gene. Am J Physiol Cell Physiol 287: C903–C911, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Yan G, Chen X, Bancroft C. A constitutively active form of CREB can activate expression of the rat prolactin promoter in nonpituitary cells. Mol Cell Endocrinol 101: R25–R30, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3. Hypertension 54: 877–884, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Zhang D, Yu ZY, Cruz P, Kong Q, Li S, Kone BC. Epigenetics and the control of epithelial sodium channel expression in collecting duct. Kidney Int 75: 260–267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang D, Li S, Cruz P, Kone BC. Sirtuin 1 functionally and physically interacts with disruptor of telomeric silencing-1 to regulate alpha-ENaC transcription in collecting duct. J Biol Chem 284: 20917–20926, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Hayashizaki Y, Kone BC. Structure and regulation of the mDot1 gene, a mouse histone H3 methyltransferase. Biochem J 377: 641–651, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Xia X, Jalal DI, Kuncewicz T, Xu W, Lesage GD, Kone BC. Aldosterone-sensitive repression of ENaCα transcription by a histone H3 lysine-79 methyltransferase. Am J Physiol Cell Physiol 290: C936–C946, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Xia X, Reisenauer MR, Hemenway CS, Kone BC. Dot1a-AF9 complex mediates histone H3 Lys-79 hypermethylation and repression of ENaCalpha in an aldosterone-sensitive manner. J Biol Chem 281: 18059–18068, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, Xia X, Reisenauer MR, Rieg T, Lang F, Kuhl D, Vallon V, Kone BC. Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel alpha. J Clin Invest 117: 773–783, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]