Abstract
Evidence suggests that loss of podocytes into urine contributes to development of glomerular diseases; shed podocytes are frequently viable and proliferate in culture conditions. To determine the phenotypic characteristics of viable urinary cells derived from human subjects, we established long-term urinary cell culture from two patients with focal segmental glomerulosclerosis and two healthy volunteers, via transformation with the thermosensitive SV40 large T antigen (U19tsA58) together with human telomerase (hTERT). Characterization of arbitrarily selected two clonal cell lines from each human subject was carried out. mRNA expression for the podocyte markers synaptopodin, nestin, and CD2AP were detected in all eight clones. Podocin mRNA was absent from all eight clones. The expression of nephrin, Wilms' tumor 1 (WT1), and podocalyxin mRNA varied among the clones, which may be due to transformation and/or cloning. These results suggest that podocyte cell lines can be established consistently from human urine. The generation of podocyte cell lines from urine of patients and healthy volunteers is novel and will help to advance studies of podocyte cell biology. Further improvements in the approaches to cell transformation and/or cell culture techniques are needed to allow cultured podocytes to fully reproduce in vivo characteristics.
Keywords: focal segmental glomerulosclerosis, SV40 large T antigen, human telomerase
there is strong evidence that podocyte damage and loss contribute to the initiation of glomerulosclerosis and progression of renal disease (21, 28). Mature podocytes are highly differentiated cells; it remains controversial as to whether they undergo replenishment under normal or pathological conditions (1, 21, 32). Podocyte depletion leads to nephron degeneration and renal tubular fibrosis that are closely correlated with decline in renal function (17, 18), and a reduction of the podocyte number is related to severity of glomerular injury (19, 20, 23, 27, 41).
Measurement of podocyte number or mRNA of podocyte proteins in urine sediment has been suggested as a useful tool for monitoring glomerular disease activity. Hara et al. (8–10), Nakamura et al. (25), and Habara et al. (7) measured the number of urinary podocytes using an antibody recognizing podocalyxin and reported that the excretion of podocalyxin-positive cells was increased in active glomerular diseases. Wang et al. (39, 40) and Szeto et al. (36) reported that mRNA expression of nephrin, podocin, and synaptopodin in urine sediment was correlated with proteinuria as well as reduced renal function in patients suffering from glomerular diseases. More recently, Sato et al. (34) investigated mRNA in urine sediment of a rat glomerular disease model and lupus nephritis patients and reported that upregulation of podocin and nephrin mRNA in urine sediment mirrored disease activity.
Urine-derived podocytes undergo proliferation under cell culture conditions. Vogelmann et al. (38) cultured urinary cells from humans, and Petermann et al. (29) and Yu et al. (44) studied proliferating urinary cells from rats. They detected podocyte markers in cultured urinary cells. Further study of urinary podocytes offers an open window to an understanding of glomerular disease. Several laboratories have established conditionally immortalized podocyte cell lines derived from isolated mouse or human glomeruli using the thermosensitive SV40 T antigen tsA58 (13, 24, 33, 43). Under growth-restricted conditions, these cells display the differentiated phenotype which mimics physiological status and have been widely used to study the biology of podocytes.
We have analyzed the phenotype of human urinary cells in primary culture and following transformation, using urine obtained from two focal segmental glomerulosclerosis (FSGS) patients and two healthy volunteers. After transformation of urinary cells by a thermosensitive SV40 T antigen (U19tsA58) and with human telomerase (hTERT), we carried out antibiotic selection and limiting dilution cloning, which yielded several clones for each subject. Notably, all of the clones and primary urinary cells analyzed in the current study were positive for several podocyte markers but negative for tubular cell markers, indicating that podocyte-derived cells are the major cell type in human urine that survives and proliferates in long-term cell culture. In further experiments, we found that immortalization with the SV40 T antigen (U19del89-97) that was not capable of benzimidazole 1 homolog (Bub-1) binding, together with hTERT, produced podocyte cell lines with a more differentiated podocyte phenotype.
METHODS
Human subjects.
Patients with FSGS and healthy volunteers gave informed consent to provide urine for research studies. The research protocol, Pathogenesis of Glomerulosclerosis (94-DK-0129), was approved by the National Institute of Diabetes and Digestive and Kidney Diseases Institutional Review Board (National Institutes of Health, Bethesda, MD). Estimated glomerular filtration rate (eGFR) was calculated using the four-variable Modification of Diet in Renal Disease equation. Renal diagnosis was made by renal biopsy carried out for clinical indications. We studied two patients: a male, age 48, with human immunodeficiency virus-associated collapsing glomerulopathy (eGFR 34 ml·min−1·1.73 m−2, proteinuria 1.99 g/day) and a male, age 46, with idiopathic FSGS (eGFR 55 ml·min−1·1.73 m−2, proteinuria 2.58 g/day). Healthy volunteers, two males (ages 36 and 29), were shown to lack proteinuria by urine dipstick. In confirmatory studies, we also studied urinary cells from another patient with idiopathic FSGS [male, age 38 (eGFR 103 ml·min−1·1.73 m−2, proteinuria 0.6 g/day)] and two additional healthy volunteers (a male, age 36, and a female, age 29).
Primary urinary cell culture.
Urinary cell culture was performed as previously described with minor modifications (38). Midstream urine was collected in sterile containers and centrifuged at 1,000 rpm for 5 min. Pelleted cellular material was washed with PBS twice and suspended in RPMI medium supplemented with 10% heat-inactivated FBS, insulin-transferrin-selenium G supplement (Invitrogen, Carlsbad, CA), and penicillin and streptomycin (Invitrogen). Cells were seeded on 75 cm2 type I collagen coated tissue culture flasks. After ∼3 wk, urine-derived cells were subcultured once by trypsin digestion and prepared for retrovirus infection, quantitative (q)RT-PCR, or immunofluorescence.
Additional podocyte cultures.
Mouse podocyte cell line AI cells were cultured as we described previously (13). Two additional mouse podocyte cell lines, provided from Peter Mundel (24) and Masanori Kitamura (43), were cultured in RPMI, including 10% heat-inactivated FBS with IFN-γ in 33°C under growth-permissive (GP) conditions or without IFN-γ in 37°C [growth-restrictive (GR) conditions]. A human podocyte cell line (AB8/13; provided by Moin Saleem) (33) was cultured in RPMI supplemented with 10% heat-inactivated FBS and insulin-transferrin-selenium G supplement (Invitrogen) under GP or GR conditions.
Retroviral infection.
Urinary cells were infected with retrovirus carrying the SV40 T antigen mutants U19tsA58 or U19del89-97 in combination with retrovirus encoding hTERT at 37°C (3, 12, 26). Beginning 72 h after infection with U19tsA58 and hTERT, cells were cultured at 33°C with 0.2 mg/ml G418 (selection for T antigen); after 7 days, the selected antibiotic was changed to 0.05 mg/ml hygromycin (selection for hTERT) for a further 7 days. Cells infected with U19del89-97 were cultured at 37°C under an identical selection regimen. Surviving cells were propagated, subcultured several times, and subjected to RT-PCR, qRT-PCR, magnetic bead separation with podocalyxin antibody, or limiting dilution cloning. Limiting dilution yielded several clones of transformed cells, from which two clones were randomly chosen and prepared for RT-PCR, Western blotting, or immunofluorescent staining.
RT-PCR and qRT-PCR.
Total RNA of the urinary cells (primary cells, transformed bulk cells, or clones) for each podocyte cell line was purified with an miRNeasy Mini Kit (Qiagen, Valencia, CA), and cDNA was synthesized using the Superscript III First-Strand Synthesis System for RT-PCR (Invitrogen). RT-PCR was performed with Platinum PCR SuperMix High-Fidelity (Invitrogen). Corresponding primers are shown in Table 2. The absence of genomic DNA contamination was confirmed by observation of no amplification following cDNA synthesis and PCR using primers for β-actin in the absence of reverse transcriptase (RT−).
Table 2.
Pairs of primers used in this study
| Gene Name (Alternative Name) | Accession No. | Sequence (5′->3′) | Exon Position | Product Size, bp |
|---|---|---|---|---|
| NPHS1 (Nephrin) | NM_004646.2 | F: CGCAGGAGGAGGTGTCTTATTC | E26 | 234 |
| R: CGGGTTCCAGAGTGTCCAAG | E28 | |||
| NPHS2 (Podocin) | NM_014625.2 | F: GGGAATCAAAGTGGAGAGAATAG | E6 | 223 |
| R: CAGAGACTGAAGGGTGTGGAG | E8 | |||
| WT1 | NM_000378.3, NM_024424.2, NM_024425.2, NM_024426.3 (transcript variant A, B, C, D) | F: ATAACCACACAACGCCCATC | E6 | 127 |
| R: TCAGATGCCGACCGTACAAG | E7 | |||
| PDCXL (Podocalyxin) | NM_001018111.2, NM_005397.3 (transcript variant 1, 2) | F: CTCCCAGAGGAAGGACCAG | E8 | 136 |
| R: CCCGTTGAGGCTGACCAC | E9 | |||
| CD2AP | NM_012120.2 | F: AAGGGTGGCTGGAAGGAGAAC | E2 | 122 |
| R: ATGCCTTTCCCGTTTGATGG | E3 | |||
| NES(Nestin) | NM_006617.1 | F: CTGGAGCAGGAGAAACAGG | E2 | 249 |
| R: TGGGAGCAAAGATCCAAGAC | E4 | |||
| SYNPO (Synaptopodin) | NM_001109974.1, NM_007286.4 (transcript variant 1, 2) | F: AGGTAGGCGTGGAGGAGG | E2 | 182 |
| R: GAGGTTCTGGTTGGGTTTGG | E2 | |||
| AQP1 | NM_198098.1 | F: TCTCTGTAGCCCTTGGACACC | E2 | 175 |
| R: GCCAGGATGAAGTCGTAGATGAG | E4 | |||
| AQP2 | NM_000486.5 | F: CGGCTGCTCTATGAATCCTG | E3 | 150 |
| R: CTCTTGGCTGGCGGAAAC | E4 | |||
| UMOD (Uromodulin) | NM_003361.2, NM_001008389.1 (transcript variant 1, 2) | F: CCCATGCCACTTACAGCAAC | E7 | 163 |
| R: CCGCCCACTCTGATGTTTAG | E8 | |||
| SLC12A3 (TSC) | NM_000339.2, NM_001126107.1, NM_001126108.1 (transcript variant 1, 2, 3) | F: GCTTCGGCTGGGTCAAGG | E2 | 180 |
| R: TGGTGGAGATGGCTGAGATG | E4 | |||
| SLC12A1 (NKCC2) | NM_000338.2 | F: TGGCTGTTGCTATGTATGTGG | E6 | 167 |
| R: CTTTGCCTCCCATTCCATTC | E7 | |||
| VWF (von Willebrand factor) | NM_000552.3 | F: TGCCTCCAAAGGGCTGTATC | E5 | 261 |
| R: CACCACTGTTCTCCACTGCTC | E6 | |||
| ACTB* (β-Actin) | NM_001101.3 | F: TCGTGCGTGACATTAAGGAG | E4 | 178 |
| R: AGGAAGGAAGGCTGGAAGAG | E4 | |||
| ACTB† (β-Actin) | NM_001101.3 | F: AGCACAGAGCCTCGCCTTTG | E1 | 81 |
| R: AGCGCGGCGATATCATCATC | E2 |
Shown are the primer pairs used for RT-PCR and quantitative (q)RT-PCR. F, forward; R, reverse; AQP1, aquaporin-1. The primer pairs for nephrin, WT1, and podocalyxin were used for both RT-PCR and qRT-PCR. The other primer pairs were used only for RT-PCR. The non-exon-spanning primer pair for ACTB (*) was used for RT-PCR including an RT(−) reaction to rule out genomic DNA contamination. The exon-spanning primer pair for ACTB (†) was used for qRT-PCR.
In separate experiments, cDNA synthesis was performed with a High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA), and qRT-PCR was carried out in the ABI Prism 7900HT Sequence Detection System (Applied Biosystems) with Power-SYBR green PCR Master Mix (Applied Biosystems). Relative amounts of indicated genes were calculated by the delta-delta Ct method and normalized to β-actin.
Western blotting.
Urinary cell clones were harvested by trypsin digestion and washed with PBS twice by centrifugation. Cell pellets were lysed in RIPA lysis buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 2.5% deoxycholic acid, 1% NP-40, 1 mM EDTA) with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN), and protein concentration was determined by BCA protein assay (Pierce, Rockford, IL). Total cell lysate (equal to 20 μg of protein) was separated on NuPAGE 7% Tris-acetate gels (Invitrogen) under reducing conditions. The following primary antibodies were used: mouse monoclonal ant-SV40 T antigen antibody, Ab-2 (EMD Biosiences, Gibbstown, NJ) at 1:1,000; mouse monoclonal anti-Wilms' tumor 1 (WT1) antibody, clone 6F-H2 (Millipore, Billerica, MA) at a dilution of 1:500; mouse monoclonal anti-podocalyxin antibody, clone 18.29 (Millipore) at 1:200; rabbit polyclonal anti-CD2AP antibody (provided from Dr. Andrey S. Shaw) at 1:2,000; and mouse monoclonal anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO). Signal was detected with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce).
Immunofluorescent staining and phalloidin staining.
Primary urinary cells forming colonies grown on type I collagen-coated tissue culture dishes or urinary cell clones cultured on type I collagen-coated coverslips in six-well dishes were fixed by ethanol-acetone (1:1) for 20 min at −20°C or 4% paraformaldehyde in PBS for 30 min at room temperature followed by permeabilization with 0.2% Triton X-100 in PBS for 10 min. After incubation with a blocking solution (2% FBS, 2% BSA, 0.2% gelatin in PBS) for 30 min, cells were probed with mouse monoclonal anti-synaptopodin antibody, clone G1D4 (Fitzgerald Industries International, Concord, MA) ready to use, rabbit polyclonal anti-nestin antibody (Millipore) at a dilution of 1:200, mouse monoclonal anti-WT1 antibody (Millipore) at 1: 100, mouse monoclonal anti-podocalyxin antibody (Millipore) at 1:50, or rabbit polyclonal anti-von Willebrand factor (Dako) at 1:400 as primary antibodies for 1 h at room temperature. Signals were visualized by incubating cells with Alexa Fluor 488-conjugated anti-mouse or anti-rabbit secondary antibody (Invitrogen). To visualize F-actin, cells were fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.2% Triton X-100 in PBS for 10 min, and incubated with Alexa Fluor 594-phalloidin (Invitrogen) at a dilution of 1:100 for 1 h.
Cell selection by magnetic beads.
Immortalized bulk urinary cells were subjected to magnetic bead separation using a miniMACS separation unit (Miltenyi Biotec, Auburn, CA) with a monoclonal mouse anti-podocalyxin antibody (Millipore) according to the manufacturer's instructions. Positive and negative selection were carried out twice in series, to maximize cell purity. Separated cells were expanded, passaged twice, and prepared for qRT-PCR.
Proliferation assay.
Urinary cells (10 × 104) were plated on six-well culture plates coated with type I collagen. At various time points, the number of viable cells was counted in a hemocytometer under the microscope by trypan blue dye exclusion.
Statistics.
Statistical analyses were carried out using Prism 5 software (Graphpad Software, San Diego, CA), and significance was evaluated by one-way ANOVA, using Bonferroni testing for intergroup comparisons. A P value of <0.05 was taken as significant.
RESULTS
Urinary podocytes from healthy volunteers and FSGS patients are viable and proliferate in culture.
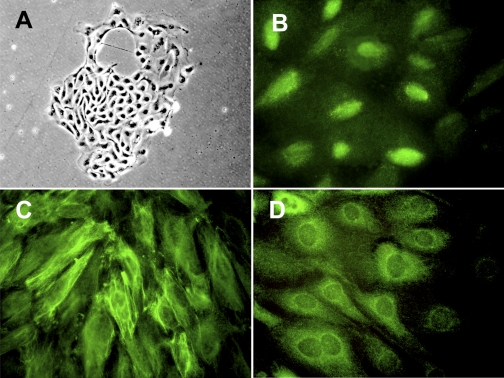
We cultured urine sediment cells to confirm that podocytes are present in urine and can proliferate in vitro. As shown in Fig. 1A, small cell colonies with typical epithelial cell morphology appeared at 1–2 wk after the start of culture. These cells typically stopped proliferating after 4–5 wk. At 3–4 wk of culture, we characterized these cells and carried out subculture and transformation. These proliferating cells were obtained consistently from two healthy volunteers and two FSGS patients. Immunofluorescent staining of cell colonies of a healthy volunteer revealed that podocyte markers WT1 (Fig. 1B), nestin (Fig. 1C), and synaptopodin (Fig. 1D) were expressed in urine-derived cells. These results indicate that podocyte-derived cells constitute a sizeable fraction of the cells that survive in long-term human urinary cell cultures.
Fig. 1.
Proliferating primary urinary cells display podocyte markers. Urinary cells from a healthy volunteer were cultured in growth medium on a type I collagen-coated dish. Colony formation observed at 2 wk after start of culture demonstrated a cobblestone appearance under phase-contrast microscopy (A). Immunofluorescent staining identified Wilms' tumor 1 (WT1) in nuclei (B), nestin in cytoplasm with a filamentous pattern (C), and synaptopodin in cytoplasm with a partially filamentous and partially homogenous pattern (D) in the cells. Original magnification: ×200 (A); ×400 (B–D).
Conditionally transformed urinary cells from FSGS patients express podocyte markers.
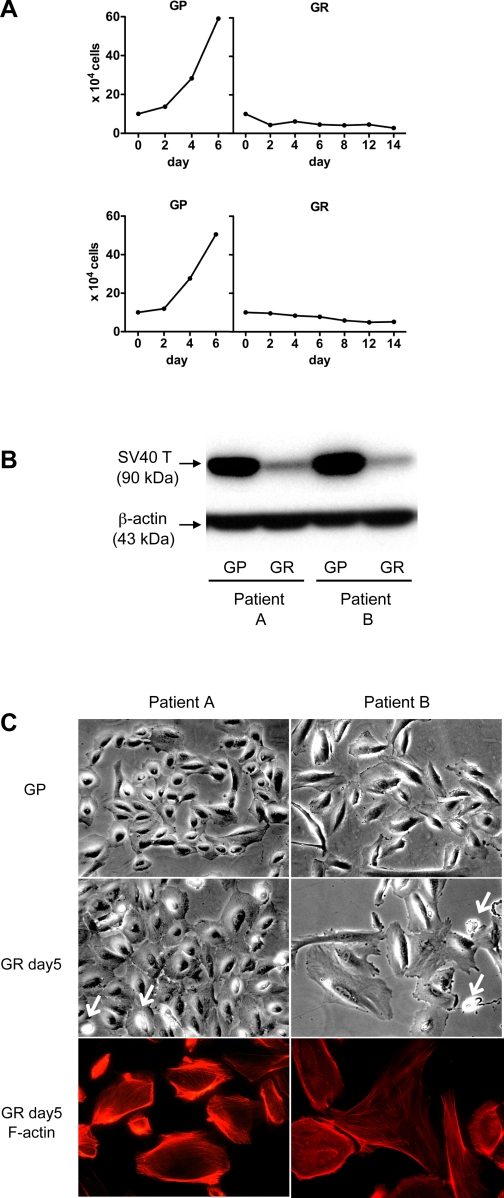
We infected cultured urinary cells from two FSGS patients with retrovirus vectors carrying a thermosensitive SV40 T antigen (U19tsA58) and hTERT. After antibiotic selection and limiting dilution cloning, we obtained several conditionally transformed urinary cell clones. The growth curve of a representative urinary cell clone obtained from each of two FSGS patients is presented in Fig. 2A, and Western blotting for SV40 T antigen is shown in Fig. 2B. At 33°C (growth-permissive condition; GP), cell numbers increased over time (Fig. 2) and SV40 T antigen was expressed at high levels (Fig. 2B). As expected, at 37°C (GR condition), cell numbers gradually fell and immunodetectable-SV40 T antigen was present at much lower levels.
Fig. 2.
Establishment of temperature-sensitive urinary cell lines from 2 focal segmental glomerulosclerosis (FSGS) patients. A: primary urinary cells from each of two FSGS patients were transformed by retrovirus vector encoding thermo-sensitive SV40-T antigen (U19tsA58) and human telomerase (hTERT). Selection with G418 and hygromycin, and subsequent limiting dilution cloning, yielded several cellular clones per each patient. The growth curve of a representative urinary cell clone from each patient indicates that the cell number continuously increased under growth-permissive conditions (GP), whereas cell number slowly decreased under growth-restricted conditions (GR). B: high expression under GP condition and attenuation under GR condition of SV40 T antigen in cells from each patient was determined by Western blotting. β-Actin served as a loading control. C: representative urinary cell clone from each FSGS patient under GP or GR conditions at day 5 is presented under phase-contrast microscopy, and Alexa 594-phalloidin staining for cells from each patient under GR conditions is shown. Clones from both patients, when grown under GP conditions, were small, with a cobblestone appearance similar to primary urinary cells. Under GR condition, cells from both patients became larger and pyknotic nuclei were frequently observed (arrows). Cells from patient A retained a cuboidal shape, whereas some cells from patient B show irregular polygonal shapes. Cells from each subject showed prominent cortical actin with few stress fibers, although cortical actin was less abundant in polygonal cells from patient B. Original magnification: ×400.
Under GP conditions, cells from both patients exhibited cobblestone morphology, typical of epithelial cells; Fig. 2C shows the morphology of representative cell clones. Under GR conditions, the cell clones from patient A maintained a cuboidal morphology, whereas cell clones from patient B developed a more irregular shape than from patient A. Some cells retained a cuboidal shape whereas others exhibited polygonal or multipolar shapes. Under GR conditions, compared with GP conditions, cells from both patients were enlarged and certain cells showed pyknotic nuclei suggestive of apoptosis (Fig. 1C, arrows).
F-actin staining for cells under GR conditions was carried out (Fig. 2C). Cells from each patient manifested thick cortical actin bundles with moderate stress fiber formation, whereas some cells, especially cells with polygonal or multipolar shapes from patient B, manifested diminished cortical actin.
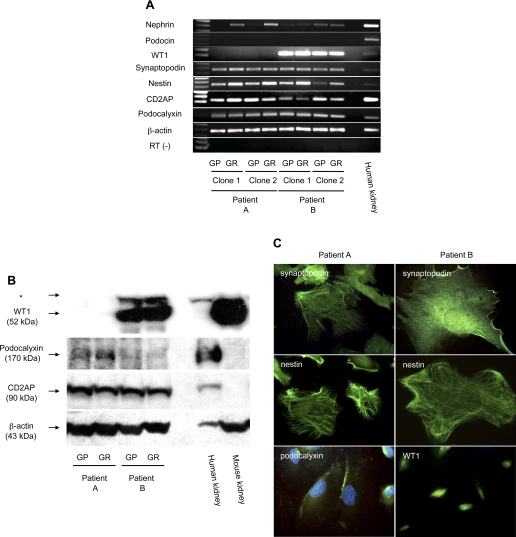
We next analyzed expression of podocyte markers in transformed urinary cell clones by RT-PCR (Fig. 3A and Table 1). Nephrin mRNA was expressed in all four cell lines, although the expression level was low. Podocin mRNA was not detected in any cell line. WT1 mRNA was absent from cell lines from patient A, whereas both cell lines from patient B expressed WT1 mRNA strongly. Podocalyxin and CD2AP mRNA were positive in all clones tested. Surprisingly, podocytes were not consistently more differentiated under GR conditions. While nephrin mRNA expression rose in both clones from patient A under GR conditions, nephrin mRNA was unaffected in both clones from patient B under GR conditions. Nestin mRNA expression rose to some extent in all cells under GR conditions, although the technique is not quantitative. The other markers were unaffected by the shift to GR conditions. By Western blotting, WT1 protein was absent in cell lines from patient A, whereas it was strongly expressed in both cell lines from patient B (Fig. 3B). Podocalyxin protein was expressed in cell lines from both patients, at lower levels in cell lines from patient B. CD2AP was also detected in cell lines from both patients. Thus mRNA and protein expression were congruent. Immunofluorescent staining revealed that synaptopodin was distributed in a partially filamentous and partially homogenous pattern, and nestin was stained as fine filaments in cytoplasm (Fig. 3C). Podocalyxin was expressed on the cell surface in both patients (Fig. 3C; only cells from patient A are shown), and WT1 was detected in the nucleus of a cell line from patient B (Fig. 3C).
Fig. 3.
Characteristic podocyte molecules were identified in urinary cell clones from FSGS patients. Two urinary cell clones from each FSGS patient under GP or GR conditions were analyzed for indicated podocyte molecules by RT-PCR (A). Nephrin mRNA was weakly detected in each clone from both patients. WT1 mRNA was absent in urinary cells from patient A in contrast to high expression in patient B. Synaptopodin, nestin, CD2AP, and podocalyxin mRNA was detected in each clone examined. A human kidney was used as a positive control for each RT-PCR reaction. β-Actin served as an internal control. Reaction for β-actin without RT (RT−) was performed to rule out the possibility of genomic DNA contamination. One representative clone from each FSGS patient was selected, and Western blotting for indicated podocyte-associated markers was performed (B). WT1 was negative in cells from patient A, whereas strongly positive in cells from patient B. The nonspecifc bands are marked with an asterisk (*). Podocalyxin was detected in both cells, although expression in cells from patient B is weak. CD2AP was expressed in each. A human kidney and mouse kidney served as a positive control, and β-actin served as a loading control. Lack of WT1 in the human kidney is probably due to low sensitivity of the assay. Anti-podocalyxin antibody and anti-CD2AP antibody used in this study do not react in the mouse. Immunofluorescent staining for synaptopodin, nestin, podocalyxin, or WT1 in one representative clone from each of patient A or patient B under GR condition is displayed (C). Synaptopodin was detected with a pattern that is partially homogenous and partially filamentous. Nestin was expressed with a filamentous pattern. Podocalyxin was identified on the cell surface, and WT1 was located in nuclei. Original magnification for synaptopodin, nestin, and podocalyxin: ×600; WT1, ×400. Nuclei were counterstained with 4′-6-diamidino-2-phenylindole in podocalyxin staining.
Table 1.
Podocyte marker expression
| CD2AP |
Nestin |
Synaptopodin |
Nephrin |
Podocalyxin |
WT1 |
Podocin |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Line | Subject | mRNA | Protein | mRNA | Protein | mRNA | Protein | mRNA | Protein | mRNA | Protein | mRNA | Protein | mRNA | Protein |
| FSGS patient A | 1 | + | + | + | + | + | + | + | ND | + | + | − | − | − | ND |
| 2 | + | ND | + | ND | + | ND | + | ND | + | ND | − | − | − | ND | |
| FSGS patient B | 1 | + | + | + | + | + | + | + | ND | + | + | + | + | − | ND |
| 2 | + | ND | + | ND | + | ND | + | ND | + | ND | + | + | − | ND | |
| Healthy volunteer A | 1 | + | + | + | + | + | + | − | ND | − | ND | + | − | − | ND |
| 2 | + | ND | + | ND | + | ND | + | ND | − | ND | + | ND | − | ND | |
| Healthy volunteer B | 1 | + | + | + | + | + | + | + | ND | + | ND | + | — | − | ND |
| 2 | + | ND | + | ND | + | ND | + | ND | + | ND | + | ND | − | ND | |
Two cell lines were obtained from subjects with focal segmental glomerulosclerosis (FSGS) and healthy volunteers. WT1, Wilms' tumor 1. In cells cultured under growth-restricted conditions, mRNA expression was determined by PCR, and protein expression was determined by Western blotting and/or immunostaining. Results are presented as present (+), absent (−), or not determined (ND).
In summary, conditionally transformed urinary cell lines from two FSGS patients expressed various characteristic podocyte markers at the RNA and protein levels.
Conditionally transformed urinary cell lines from healthy volunteers exhibit a similar phenotype to those from FSGS patients.
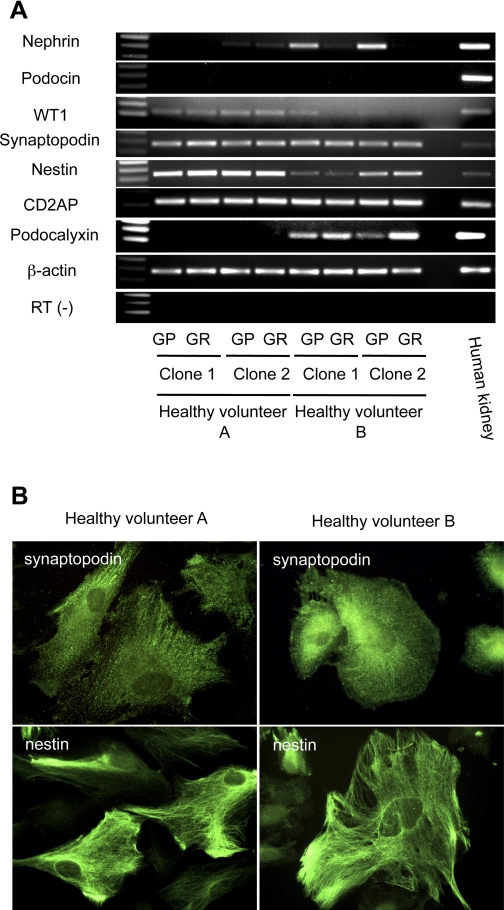
We next established two urinary cell lines from each of two healthy volunteers. As expected, cell lines grew under GP conditions and not GR conditions, and Western blotting showed much more expression of SV40 T antigen under GP conditions than GR conditions (data not shown). Podocyte marker expression was examined by RT-PCR (Fig. 4A and Table 1). Nephrin mRNA expression was absent in one clone and very low in another clone from healthy volunteer A, and low in two clones obtained from healthy volunteer B. Unexpectedly, the expression level of nephrin mRNA under GR conditions was lower than under GP conditions in healthy volunteer B, suggesting these cells under GR conditions failed to differentiate. Podocin mRNA was absent in all four clones, as with FSGS patient clones. CD2AP mRNA and, at a lower level, WT1 mRNA, were detected in all four cell lines, while podocalyxin mRNA was detected only in cell lines from healthy volunteer B. By Western blotting, CD2AP protein was expressed in one representative clone from each healthy volunteer (data not shown). However, in contrast to the results of RT-PCR, WT1 protein was not detected in clones from either patient, indicating that the expression level is below the sensitivity of the Western blot, or, alternatively, that mRNA was not efficiently translated (data not shown). Immunostaining revealed that synaptopodin was expressed in clones from each healthy volunteer, and nestin was expressed in each (Fig. 4B). In summary, conditionally immortalized urinary cell lines from healthy volunteers expressed podocyte markers (Table 1). mRNA and protein of synaptopodin and nestin were detected, but podocin mRNA was absent in cell clones from all four individuals. The expression of other podocyte markers was, however, heterogeneous among four human subjects, and there were no consistent differences between cells from FSGS patients and healthy volunteers.
Fig. 4.
Podocyte markers are also expressed in urinary cell clones from healthy volunteers. Four urinary cell clones from 2 healthy volunteers grown under GP or GR conditions were analyzed with RT-PCR for podocyte markers (A). Nephrin mRNA was weakly expressed in 1 of 2 clones of healthy volunteer A and 2 clones from healthy volunteer B. Low expression of WT1 mRNA was exhibited in both healthy volunteers. Synaptopodin, nestin, and CD2AP mRNA was expressed in each healthy volunteer. Podocalyxin mRNA was absent from cells from healthy volunteer A but present in healthy volunteer B. A human kidney served as a positive control for each RT-PCR. RT-PCR for β-actin was used as an internal control. Reaction for β-actin without RT (RT−) was carried out to confirm the absence of genomic DNA contamination. Immunofluorescent staining for synaptopodin or nestin in 1 representative clone from each of healthy volunteer A or healthy volunteer B is shown (B). Synaptopodin was stained in each cell clone with a pattern that is partially homogenous and partially filamentous. Nestin was exhibited in both human subjects with a filamentous pattern. Original magnification: ×600.
Conditionally transformed urinary cells do not express tubular cell markers and an endothelial cell marker.
To further characterize the podocyte-like cell lines, we next examined markers for each tubular segment and for endothelial cells in all eight cell clones (Supplementary Fig. 1A, patients A and B; 1B, healthy volunteers A and B; all supplementary material for this article is available on the journal web site) by RT-PCR (15, 16). Surprisingly, aquaporin-1 (AQP1) mRNA, a water channel expressed in proximal tubules, was amplified in all the clones. Uromodulin (UMOD), also known as Tamm-Horsfall glycoprotein, and the Na-K-2Cl cotransporter (NKCC2) are localized in the thick ascending limb of Henle; mRNAs were not detected in any cell clones. The thiazide-sensitive NaCl cotransporter (TSC; SLC12A3) is identified in the distal convoluted tubule; mRNA was not amplified in any clones. AQP2 is localized in the collecting duct; mRNA was not amplified in any clone. von Willebrand factor (VWF), a marker for endothelial cells, mRNA was not expressed in any clones. We also performed immunofluorescent staining for VWF in transformed bulk urinary cells from each human subject and confirmed the absence of this protein (data not shown).
We analyzed expression of these tubular cell markers except AQP1 in urinary primary cells and transformed bulk cells (i.e., before cloning) as well as clones from the FSGS patients to evaluate the possibility that transformation or single cell cloning diminished the tubular cell marker expression (Supplementary Fig. 1C, patient A, and 1D, patient B). Primary cell cultures, transformed bulk cells, and two clones from both patients did not express the tubular cell markers or VWF, suggesting that tubular epithelial cells or endothelial cells were absent in urine from these individuals or did not survive and proliferate in culture.
In vivo, AQP1 expression has been reported in proximal tubules and mesangial cells but not in podocytes (6, 22). We next examined AQP1 mRNA expression in three mouse conditionally immortalized podocyte cell lines (Supplementary Fig. 2A) and one human cell line (Supplementary Fig. 2B) generated from isolated glomeruli, all of which have been previously published by other investigators and which were generously provided to us (13, 24, 33, 43). AQP1 mRNA was detected in all four podocyte cell lines, suggesting that podocyte-like cells in vitro express AQP1 mRNA.
Transformation by SV40 T antigen U19tsA58 and hTERT alters gene expression of urinary cells.
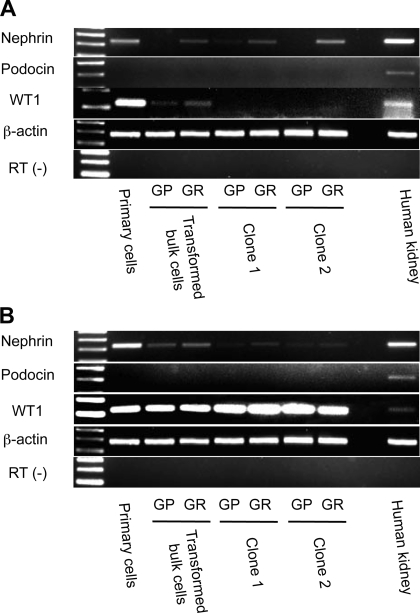
As described above, there was heterogeneity of podocyte marker expression across all eight cell lines studied. We next evaluated the role of the viral oncogene transformation and cell cloning in producing this heterogeneity. SV40 T antigen transformation is associated with genomic instability (30, 31), and furthermore the procedure of cloning by limiting dilution may select for cells that do not represent faithfully their parent populations. To evaluate this possibility, we compared podocyte marker mRNA expression by RT-PCR from cells obtained from FSGS patients at three stages of selection: primary urinary cell cultures, transformed bulk cell cultures, and podocyte cell clones. Nephrin mRNA and WT1 mRNA were expressed in primary urinary cells of patient A (Fig. 5A) and patient B (Fig. 5B), but expression was reduced in immortalized bulk cells. WT1 mRNA disappeared after single-cell cloning in patient A clones but was preserved in patient B clones. Podocin mRNA was consistently negative among cells at each stage.
Fig. 5.
U19tsA58 alters gene expression of urinary cells. Primary urinary cells, transformed bulk urinary cells, and 2 representative urinary cell clones from patient A (A) or patient B (B) were examined by RT-PCR for indicated podocyte components. In cells from both patients, nephrin mRNA was strongly expressed in primary cells, but expression was reduced in transformed bulk cells and clones. WT1 mRNA disappeared after single cell cloning in urinary cells from patient A but persisted in patient B in both clones. Podocin mRNA was negative at each step, from primary cells to clones in cells from both patients. Human kidney tissue was used as a positive control, and β-actin served as a loading internal control. Contamination of genomic DNA was excluded by no observation of amplification in reaction for β-actin without RT (RT−).
These results indicate that SV40 T antigen transformation and single-cell cloning did indeed affect podocyte marker expression, with reduced expression of nephrin and WT1 mRNA. By contrast, podocin mRNA was consistently absent in all the urinary cells analyzed, including primary cells. We performed RT-PCR for podocin using urinary primary cell cultures from two additional healthy volunteers and one FSGS patient and confirmed the absence of podocin mRNA (data not shown), whereas the expression of other podocyte molecules was generally similar to the two primary cultures described herein.
Podocalyxin expression is not related to WT1 or nephrin mRNA expression by urinary cells.
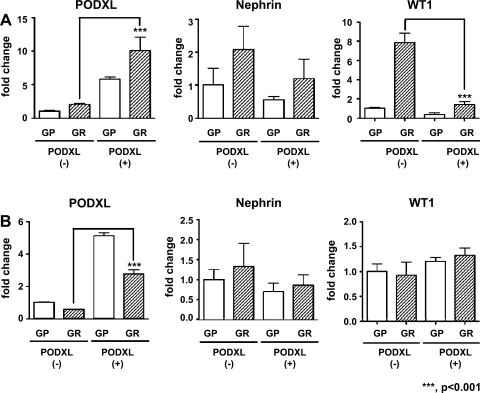
We next examined cell heterogeneity in transformed bulk cultures (there were insufficient cells in the primary cultures for this technique). We performed magnetic bead separation with podocalyxin antibody, as this technique requires a cell surface protein. As shown in Fig. 6, podocalyxin mRNA was considerably increased in the podocalyxin-positive fraction compared with the podocalyxin-negative fraction in urinary cells from both patients (Fig. 6A, patient A; 6B, patient B), confirming that the cell fractionation was successful. On the other hand, the expression level of nephrin mRNA was not significantly different between podocalyxin-positive cells and podocalyxin-negative cells from both FSGS patients. Furthermore, unexpectedly, WT1 mRNA expression in podocalyxin-positive cells was significantly lower than in podocalyxin-negative cells from patient A, whereas WT1 mRNA was similar in both cell fractions from patient B. Thus isolation of podocalyxin-positive cells failed to increase the expression of nephrin or WT1 mRNA. These results suggest that transformed urinary cells in bulk culture, while heterogeneous with regard to podocalyxin expression, cannot be clearly separated into podocyte-like and non-podocyte-like cells.
Fig. 6.
Expression of podocalyxin is not related to nephrin or WT1 expression in urinary cells. Immortalized bulk urinary cells from FSGS patient A (A) or FSGS patient B (B) were divided into a podocalyxin-positive fraction (PODXL +) and negative fraction (PODXL −) by magnetic bead separation with anti-podocalyxin antibody. Each fraction was propagated and subcultured twice. RNA samples were collected under GP and GR conditions and analyzed with quantitative (q) RT-PCR for podocalyxin, nephrin, or WT1. Podocalyxin mRNA was significantly higher in the PODXL-positive fraction than in the PODXL-negative fraction in cells from both human subjects, indicating that the separation was successfully performed. The expression of nephrin mRNA was not significantly different between PODXL-positive and PODXL-negative fractions in cells from both patients. WT1 mRNA in the PODXL-positive fraction was downregulated compared with the PODXL-negative fraction in healthy volunteer A, whereas no significant difference was observed between the PODXL-positive and PODXL-negative fractions in cells from healthy volunteer B. Data were normalized to expression of β-actin mRNA, and means ± SD, relative to PODXL-negative cells cultured under GR conditions, are presented.
Transformation by SV40 large T antigen that does not bind Bub1 preserves podocyte markers of urinary cells.
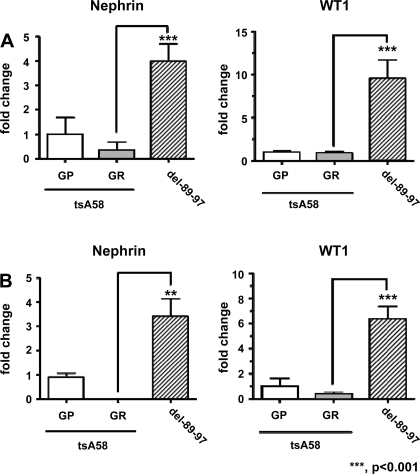
We found that podocyte-like cells lost certain markers after transformation by thermo-sensitive SV40 T antigen (U19tsA58) and hTERT. SV40 T antigen disrupts genome integrity in part by binding the cellular protein budding inhibited by Bub1 (11). We next transformed urinary cells from healthy volunteers, using SV40 T antigen mutant U19dl89–97 (which does not bind Bub1) plus hTERT, and compared these cells with those obtained by transformation with U19tsA58 plus hTERT. As shown in Fig. 7, nephrin and WT1 mRNA expression was considerably higher in cells transformed by U19del89-97 compared with those transformed by U19tsA58 with hTERT under GR conditions (Fig. 7A, healthy volunteer A; 7B, healthy volunteer B). The results were consistent among cells derived from two healthy volunteers. These results indicate that transformation by an SV40 T antigen mutant that does not bind Bub1, in combination with hTERT, results in cells that retain podocyte marker expression at higher levels compared with the SV40 T antigen that retains Bub1 binding.
Fig. 7.
U19del89-97 preserves podocyte markers in urinary cells. Primary urinary cells from healthy volunteer A (A) or healthy volunteer B (B) were transformed by SV40 T antigens U19tsA58 or U19del89-97 (lacking Bub1 binding), and cultured under GP and GR conditions. Nephrin and WT1 mRNA expression was examined by qRT-PCR. Expression of nephrin and WT1 mRNA in cells transformed by U19del89-97 was significantly higher than U19tsA58. Data were normalized to expression of β-actin mRNA. Means ± SD, relative to cells transformed by U19tsA58 cultured under GR conditions, are displayed.
DISCUSSION
In the present study, we have established conditionally transformed urinary podocyte cell lines from FSGS patients and healthy volunteers by transducing the cells with thermosensitive SV40 T antigen and hTERT. Previously, four groups have established transformed podocyte cell lines from isolated human glomeruli (4, 5, 33, 37). These cell lines were originated from kidneys surgically removed for cancer, obstructive nephropathy, and Denys-Drash syndrome, whereas in the present study podocyte cell lines were derived from urine obtained from patients with glomerular disease and also healthy volunteers. Vogelmann et al. (38) established primary cell cultures from human urine and showed expression of podocyte markers; Petermann et al. (29) and Yu et al. (44) did similar studies of rat urine. In the present work, we demonstrate the ability to reproducibly culture podocyte-like urinary cells from FSGS patients (who would be expected to shed increased numbers of podocytes in urine) and from healthy volunteers (from whom the shedding of podocytes is perhaps more surprising), and to generate transformed podocyte cell lines. This is the first report to establish and characterize urinary podocyte cell lines. The ability to routinely establish podocyte cell lines, using urine obtained from particular patients and individuals with distinct genotypes, will provide a useful tool for studies in podocyte biology, including biomarker analysis and medication sensitivity.
Synaptopodin, nestin, and CD2AP were expressed by all cell lines. However, podocin was consistently absent in every urinary cell, and expression of other markers were diverse among the urinary cell clones. Urinary podocytes may undergo irreversible dedifferentiation under the artificial conditions of culture. Petermann et al. (29) reported that cultured rat urinary cells expressed WT1 and podocin mRNA, although nephrin and synaptopodin mRNA were absent. In contrast, we detected synaptopodin mRNA in all of urinary cell clones, nephrin and WT1 in some clones, but did not observe podocin mRNA in primary cells, transformed bulk cells, or cell clones. These discrepancies could be caused by difference in species or methodologies. Vogelmann et al. (38) reported cultured primary human urinary cells expressed synaptopodin, WT1, podocalyxin, and podocin. Our results differ in that none of the cells we cultured expressed podocin.
The undesirable effect of transformation by U19tsA58 together with hTERT may contribute to the loss of certain differentiation markers. U19tsA58 has been used for conditionally immortalization of human primary cells in combination with hTERT, as well as rodent primary cells as a single transforming gene (12, 26). Most reported podocyte cell lines from mice and humans were transformed by tsA58 (13, 24, 33, 43). The effects of this SV40 T antigen on podocyte marker expression were inconsistent among human subjects. SV40 transformation is known to alter cell gene expression, and the regulation is cell specific (2). When we transformed urinary cells with SV40 T antigen (U19del89-97) that does not bind Bub1, we observed higher expression of podocyte markers.
Serial passage may also contribute to the loss of podocyte markers. Katsuya et al. (14), in studies of rat primary podocyte culture derived from isolated glomeruli, found that nephrin, podocin, and podocalyxin mRNAs were dramatically decreased after subculture. They also found that synaptopodin and CD2AP mRNAs were retained after subculture, which are consistent with our results.
There were certain differences among the cell lines that we studied. Podocytes from patient A lacked WT1 protein and RNA expression in both clones in contrast to podocytes from patient B, but primary podocytes from patient A expressed WT1 mRNA. Both urinary cell clones from the healthy volunteer A lacked podocalyxin RNA expression, and one of the clones from volunteer A did not express nephrin mRNA, in contrast to volunteer B. However, there is no reason to believe that there is any marked podocyte phenotype difference in vivo between these healthy volunteers. We believe that expression differences for nephrin, WT1, and podocalyxin among podocyte clones most commonly emerge as a result of transformation and/or cloning, rather than reflecting a persistence of an in vivo phenotype.
All of the urinary cell clones were positive for AQP1 mRNA, raising the possibility that these cells could be proximal tubular cells. Although we cannot completely exclude this possibility, our findings that several podocyte cell lines previously reported also expressed AQP1 mRNA support our interpretation that podocytes express AQP1 mRNA in culture.
Mesangial cells and glomerular endothelial cells, the other two cell types in glomeruli are separated from the urinary space by the glomerular basement membrane (GBM). As expected, urinary cells did not express the endothelial cell marker VWF. Mesangial cells are reported to form multilayers in culture (42) and manifest typical F-actin stress fiber formation (35). In this study, primary and transformed urinary cells manifested typical monolayers (Figs. 1A and 2C), and most transformed urinary cells showed cortical F-actin (Fig. 2D) rather than stress fibers. These findings suggest that mesangial cells are not a source of urinary cells.
In an attempt to obtain more homogeneous podocyte cultures, we used podocalyxin expression to sort bulk-transformed urinary cells but found no consistent differences in nephrin or WT1 mRNA expression in podocyalxyin-positive and podocalyxin-negative cell populations. These findings suggest that the podocalyxin expression level varies among cells that express other podocyte markers and suggest the limitations of relying on podocalyxin expression to identify podocytes, at least in cell culture.
In summary, we have demonstrated that podocyte cell lines can be routinely established from urine of both FSGS patients and healthy volunteers. mRNA and protein of synaptopodin, nestin, and CD2AP were consistently expressed in all urinary podocyte cell lines, and podocin mRNA was absent. Although the alteration of gene expression by transformation remains a problem, we believe that this problem may be overcome with further work and that urinary podocyte cell lines will prove to be increasingly useful tools for studying pathogenesis and mechanisms of podocyte injury.
GRANTS
This study was supported by the Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases (project ZO1 DK043308).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
Podocyte cell lines were generously provided by Moin Saleem, University of Bristol (Bristol, UK); Peter Mundel (University of Miami, Miami, FL); and Masanori Kitamura, University of Yamanashi (Yamanashi, Japan). Anti-CD2AP antibody was generously provided by Andrey Shaw (Washington University, St. Louis, MO). We are grateful to Hideko Takahashi and Huiyan Lu for technical assistance and to Hua Zhou for a critical review of the manuscript.
REFERENCES
- 1.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantalupo PG, Saenz-Robles MT, Rathi AV, Beerman RW, Patterson WH, Whitehead RH, Pipas JM. Cell-type specific regulation of gene expression by simian virus 40 T antigens. Virology 386: 183–191, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotsiki M, Lock RL, Cheng Y, Williams GL, Zhao J, Perera D, Freire R, Entwistle A, Golemis EA, Roberts TM, Jat PS, Gjoerup OV. Simian virus 40 large T antigen targets the spindle assembly checkpoint protein Bub1. Proc Natl Acad Sci USA 101: 947–952, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delarue F, Virone A, Hagege J, Lacave R, Peraldi MN, Adida C, Rondeau E, Feunteun J, Sraer JD. Stable cell line of T-SV40 immortalized human glomerular visceral epithelial cells. Kidney Int 40: 906–912, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol 158: 1723–1731, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgas K, Rumballe B, Wilkinson L, Chiu HS, Lesieur E, Gilbert T, Little MH. Use of dual section mRNA in situ hybridisation/immunohistochemistry to clarify gene expression patterns during the early stages of nephron development in the embryo and in the mature nephron of the adult mouse kidney. Histochem Cell Biol 130: 927–942, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Habara P, Mareckova H, Sopkova Z, Malickova K, Zivorova D, Zima T, Tesar V. A novel method for the estimation of podocyte injury: podocalyxin-positive elements in urine. Folia Biol (Praha) 54: 162–167, 2008 [PubMed] [Google Scholar]
- 8.Hara M, Yamamoto T, Yanagihara T, Takada T, Itoh M, Adachi Y, Yoshizumi A, Kawasaki K, Kihara I. Urinary excretion of podocalyxin indicates glomerular epithelial cell injuries in glomerulonephritis. Nephron 69: 397–403, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Hara M, Yanagihara T, Kihara I. Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron 89: 342–347, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Hara M, Yanagihara T, Kihara I, Higashi K, Fujimoto K, Kajita T. Apical cell membranes are shed into urine from injured podocytes: a novel phenomenon of podocyte injury. J Am Soc Nephrol 16: 408–416, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Hein J, Boichuk S, Wu J, Cheng Y, Freire R, Jat PS, Roberts TM, Gjoerup OV. Simian virus 40 large T antigen disrupts genome integrity and activates a DNA damage response via Bub1 binding. J Virol 83: 117–127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jat PS, Sharp PA. Cell lines established by a temperature-sensitive simian virus 40 large-T-antigen gene are growth restricted at the nonpermissive temperature. Mol Cell Biol 9: 1672–1681, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kajiyama H, Titus S, Austin CP, Chiotos K, Matsumoto T, Sakairi T, Kopp JB. Tetracycline-inducible gene expression in conditionally immortalized mouse podocytes. Am J Nephrol 29: 153–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsuya K, Yaoita E, Yoshida Y, Yamamoto Y, Yamamoto T. An improved method for primary culture of rat podocytes. Kidney Int 69: 2101–2106, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, Uchida S, Okamura HO, Marumo F, Sasaki S. Human CLC-KB gene promoter drives the EGFP expression in the specific distal nephron segments and inner ear. J Am Soc Nephrol 13: 1992–1998, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Kriz W, Hartmann I, Hosser H, Hahnel B, Kranzlin B, Provoost A, Gretz N. Tracer studies in the rat demonstrate misdirected filtration and peritubular filtrate spreading in nephrons with segmental glomerulosclerosis. J Am Soc Nephrol 12: 496–506, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Kriz W, Hosser H, Hahnel B, Gretz N, Provoost AP. From segmental glomerulosclerosis to total nephron degeneration and interstitial fibrosis: a histopathological study in rat models and human glomerulopathies. Nephrol Dial Transplant 13: 2781–2798, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD. Podocytopenia and disease severity in IgA nephropathy. Kidney Int 61: 1475–1485, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, Remuzzi G, Remuzzi A. Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol 174: 797–807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maunsbach AB, Marples D, Chin E, Ning G, Bondy C, Agre P, Nielsen S. Aquaporin-1 water channel expression in human kidney. J Am Soc Nephrol 8: 1–14, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant 15: 1379–1383, 2000 [DOI] [PubMed] [Google Scholar]
- 26.O'Hare MJ, Bond J, Clarke C, Takeuchi Y, Atherton AJ, Berry C, Moody J, Silver AR, Davies DC, Alsop AE, Neville AM, Jat PS. Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells. Proc Natl Acad Sci USA 98: 646–651, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Petermann AT, Krofft R, Blonski M, Hiromura K, Vaughn M, Pichler R, Griffin S, Wada T, Pippin J, Durvasula R, Shankland SJ. Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int 64: 1222–1231, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, Milchgrub S, Smith AL, Souza RF, Gilbey L, Zhang X, Gandia K, Vaughan MB, Wright WE, Gazdar AF, Shay JW, Minna JD. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res 64: 9027–9034, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Reddel RR, Salghetti SE, Willey JC, Ohnuki Y, Ke Y, Gerwin BI, Lechner JF, Harris CC. Development of tumorigenicity in simian virus 40-immortalized human bronchial epithelial cell lines. Cancer Res 53: 985–991, 1993 [PubMed] [Google Scholar]
- 32.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, Chang JW, Wiggins JE, Lienczewski C, Kretzler M, Wiggins RC. Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol 20: 1041–1052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J 1: 272–281, 1987 [DOI] [PubMed] [Google Scholar]
- 36.Szeto CC, Lai KB, Chow KM, Szeto CY, Yip TW, Woo KS, Li PK, Lai FM. Messenger RNA expression of glomerular podocyte markers in the urinary sediment of acquired proteinuric diseases. Clin Chim Acta 361: 182–190, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Viney RL, Morrison AA, van den Heuvel LP, Ni L, Mathieson PW, Saleem MA, Ladomery MR. A proteomic investigation of glomerular podocytes from a Denys-Drash syndrome patient with a mutation in the Wilms tumour suppressor gene WT1. Proteomics 7: 804–815, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol 285: F40–F48, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang G, Lai FM, Lai KB, Chow KM, Kwan BC, Li PK, Szeto CC. Urinary messenger RNA expression of podocyte-associated molecules in patients with diabetic nephropathy treated by angiotensin-converting enzyme inhibitor and angiotensin receptor blocker. Eur J Endocrinol 158: 317–322, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Wang G, Lai FM, Tam LS, Li KM, Lai KB, Chow KM, Li KT, Szeto CC. Messenger RNA expression of podocyte-associated molecules in urinary sediment of patients with lupus nephritis. J Rheumatol 34: 2358–2364, 2007 [PubMed] [Google Scholar]
- 41.White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, De Cosmo S, Viberti G. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes 51: 3083–3089, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Wilson HM, Stewart KN. Glomerular epithelial and mesangial cell culture and characterization. Methods Mol Med 107: 269–282, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Yamauchi K, Takano Y, Kasai A, Hayakawa K, Hiramatsu N, Enomoto N, Yao J, Kitamura M. Screening and identification of substances that regulate nephrin gene expression using engineered reporter podocytes. Kidney Int 70: 892–900, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol 16: 1733–1741, 2005 [DOI] [PubMed] [Google Scholar]