Abstract
Purpose
To use a rabbit model of induced autoimmune dacryoadenitis to evaluate the efficacy of topical ophthalmic cyclosporine A (CsA).
Methods
Autoimmune dacryoadenitis was induced by injecting autologous peripheral blood lymphocytes, which had been activated in a mixed cell reaction with acinar cells isolated from one inferior lacrimal gland (LG), back into the donor animal's remaining inferior LG. Schirmer's test, tear breakup time, and rose Bengal staining were assessed. Animals with established disease were treated topically with either CsA or Endura twice daily for 5 months.
Results
Without treatment tear production and tear stability were abnormal for 6 months, and clear signs of ocular surface defects were evident. Severe immune cell infiltration was observed in the LG. Long-term CsA treatment increased tear production only slightly, but the severity of LG histopathology decreased noticeably. CD4+ T-cell infiltration of the LG was decreased and infiltration by MHC class II-expressing cells was also decreased. For the Endura-treated group tear production did not improve, rose Bengal scores remained high, and histopathology showed infiltration comparable to the untreated group, but by the end of the study the tear breakup time did improve.
Conclusions
The rabbit model of autoimmune dacryoadenitis had signs of chronic dry eye disease 6 months after induction of disease. Tear production improved slightly with CsA treatment and CD4+ T-cell infiltration decreased significantly in the LG. This suggests that some Sjögren's patients may benefit from long-term CsA treatment.
Introduction
Dry eye disease, or keratoconjunctivitis sicca, is the most common cause of patient visits to eye care specialists. Dry eye is generally thought to be caused by a combination of immunologic, genetic, hormonal, and environmental factors.1–3 Approximately 4 million patients have a severe form of dry eye associated with Sjögren's syndrome. The relationship between inflammation and dry eye is an important consideration in understanding dry eye symptoms, and this has led to the use of anti-inflammatory drugs, such as short-term corticosteroids and long-term cyclosporine.4,5 Chronic inflammation, associated with Sjögren's syndrome, is characterized by the presence of lymphocytes, which consist primarily of CD4+, CD8+ T cells and B cells,6,7 infiltrating the lacrimal gland (LG), releasing soluble mediators, including the proinflammatory cytokines IL-1β, IL-2, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α). These mediators cause exocrine quiescence and eventual destruction of the parenchyma.8 This cellular pathophysiology and resulting changes in the tear film damage the cornea, conjunctiva, and goblet cells.
Dry eye diseases are most commonly treated with punctual plugs or artificial tears, which either reduce drainage of the ocular surface fluid film or supplement it with aqueous solution and lessen its osmolarity,9,10 but the palliative benefits are short-lived. Kaswan and colleagues11–13 demonstrated that severe dry eye disease in dogs is associated with extensive lymphocytic infiltration of the LGs and often with the presence of rheumatoid factor, suggesting that the inflammation was autoimmune in nature. Plugfelder and colleagues14 and Baudouin and colleagues15 showed that inflammatory cytokines and markers of immune cell activation are elevated in the ocular surface fluid and conjunctivae of patients with dry eye disease.
In 1989 Kaswan and colleagues16 reported that treatment of a series of canine cases with topical ophthalmic cyclosporine A (CsA) led to marked increases in Schirmer's scores and improvements in the status of the ocular surface. Plugfelder has reported proinflammatory cytokines to be major mediators of the disease14 and has described the relationship between inflammation and dry eye and the need for therapeutic strategies to treat ocular inflammation.
In 2002 CsA became the first FDA-approved drug for treating the inflammatory cellular response that exists in the conjunctivae and LG. Long-term treatment of patients with dry eye syndrome with topical ophthalmic CsA was found to (1) reduce markers of conjunctival inflammation; (2) increase the number of conjunctival goblet cells17,18; (3) improve Schirmer's scores; and (4) reduce corneal staining.19
Despite its clinical success, relatively little is known about topical CsA's influences on autoimmune pathophysiology in the LGs. The purpose of this study was to use a previously described model of induced autoimmune dacryoadenitis in rabbits to evaluate whether long-term topical CsA treatment influences LG histopathology and immune cell marker expression commensurately with its amelioration of the clinical signs of dry eye disease.
Methods
Animals and reagents
Female New Zealand white rabbits, each weighing between 3.4 and 4 kg, were obtained from Irish Farms (Norco, CA). The animals were maintained and used in compliance with institutional guidelines and in accord with the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Ophthalmic Research. The research was approved by the institutional review board. Clinical examinations were performed on all eyes prior to any experimental procedures to establish baseline data and to exclude any animals with ocular defects. Topical CsA (Restasis™, ophthalmic emulsion, 0.05%) and vehicle (Endura™) were provided by Allergan (Irvine, CA). Schirmer strip paper was purchased from Rose Stone Enterprises (Alta Loma, CA). FUL-GLO fluorescein strips and rose Bengal strips were purchased from Akorn Inc Laboratories (Buffalo Grove, IL). Antibodies specific for rabbit CD4, CD8, and CD18 and MHC-II were purchased from Antigenix America (Huntington Station, NY) and antibodies specific for RTLA (rabbit T-lymphocyte antigen) were obtained from Cedarlane Laboratories (Hornby, Ontario, Canada). Species-specific secondary antibodies were obtained from Chemicon International (Temecula, CA). ABC reagent and aminoethyl-carbazole reagent were obtained from Vector Laboratories Inc. (Burlingame, CA).
Autologous mixed cell reaction and induction of autoimmune dacryoadenitis
Descriptions of LG excision, purification of acinar cells (pLGEC), and procedures for mixed cell reactions between pLGEC and autologous peripheral blood lymphocytes (PBL) have been previously published.7,20,21 pLGEC and PBL were cultured separately for 2 days prior to mixed cell reactions, which were performed in 12-well plates, using equal numbers (1 × 106 cells/mL) of PBL and γ-irradiated pLGEC (2,500 rad), and maintained for 5 days. Parallel mixed cell reactions were performed in 96-well plates under the same conditions, but using 1 × 105 cells of each type, to monitor PBL stimulation for each rabbit. 3H-Thymidine was added after 4 days, and cells were harvested 24 h later using a Brandel model 290 PHD™ sample harvester (Brandel, Gaithersburg, MD). An LS 6000 IC beta scintillation counter (Beckman Instruments, Inc., Fullerton, CA) was used to measure 3H-thymidine incorporation. A minimum of six wells was counted to obtain representative data. Animals yielding mixed cell reactions with stimulation indices >2 were used in the study.
Activated lymphocytes from the parallel cultures in 12-well plates were collected and 1 × 106 cells/mL injected into the central regions of the inferior right eye (OD) LGs of the respective donor rabbits as described previously. This results in detectable dry eye signs in all injected animals in 2–4 weeks as described below. Autoimmune dacryoadenitis was induced in 22 adult New Zealand white rabbits. Nine were treated twice daily in both eyes for 5 months with Restasis™, seven with Endura™, and six were untreated. The treatments began 28 days after disease induction in a double-blinded study. Here after these untreated animals are referred to as ID, Restasis™-treated animals are referred to as ID/Rx animals, and the Endura™-treated animals as ID/E. All eyes were assessed at 4-week intervals for tear production (Schirmer's test), tear breakup time and rose Bengal staining as previously described.6 Schirmer's tests without anesthesia were performed on both eyes. Tear breakup time (BUT) was evaluated by instilling fluorescein under examination with a slit-lamp biomicroscope equipped with a blue filter. A standardized grading system was used to score rose Bengal staining of the cornea. Generally clinical evaluations performed every 2 to 4 weeks are adequate to monitor prominent changes in our chronic disease model. Since topical CsA is known to have a slow therapeutic response, we opted to have the clinical examinations performed only once a month by the same person for this long-term study because of scheduling problems. We are presenting clinical data related to the OD eyes and not to the left (OS) eyes because the left inferior LGs were excised at the beginning of the study.
Immunohistopathology
Rabbits were sacrificed 6 months postinjection and the remaining OD LGs were removed. One part was fixed in 10% formalin and embedded in paraffin, and paraffin-embedded sections were stained with hematoxylin and eosin (H&E) and examined in a double-blind fashion. Another part was embedded in OCT and cryosectioned at 7 μm for immunostaining. Sections were fixed in chilled acetone, air-dried, rehydrated in phosphate-buffered saline, and blocked in 5% BSA for 15 min.22 The sections were incubated at room temperature for 1 h with the primary antibody at the following dilutions: mouse anti-rabbit CD4 (1:200), mouse anti-rabbit CD8 (1:200), mouse-anti-rabbit CD18 (1:1,000), goat anti-rabbit RTLA (1:300), and mouse anti-rabbit MHC-II (1:100). Sections were rinsed and incubated for 60 min with appropriate secondary antibodies. After rinsing, the sections were quenched in 0.3% H2O2 in 40% methanol for 15 min and incubated in ABC reagent for 30 min, rinsed three times, and developed with AEC. The sections were again rinsed, counterstained with hematoxylin, and mounted for photography. The positive cells showed an intense brown color in the blue hematoxylin background. Entire sections were scanned and analyzed with the Analysis 3.0 (Olympus Soft Imaging System Lake Wood, CO) automated cellular imaging system. This combination of a proprietary, color-based imaging technology with automated microscope provides quantitative data, including percent positive with intensity scoring and area measurement.
Statistical analysis
Data collected from clinical analysis and Olympus Soft Imaging System were subjected to paired t-test or signed rank sum test using SAS version 9.1 (SAS Inst, Cary, NC) and analysis of variance (ANOVA). Multiple comparisons to the normal group were adjusted using Dunnett's correction.
Results
Tear production
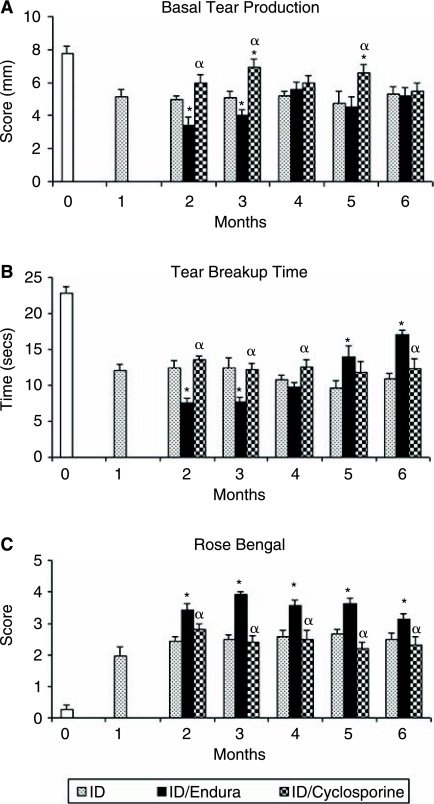
A significant decrease (P < 0.001) in basal tear production was established in OD LG by 1 month after induction of autoimmune dacryoadenitis (Fig. 1A). Tear production in OD LG of the ID group remained low and unchanged throughout the duration of the study. Tear production in OD LG of ID/Rx animals increased slightly by the end of month 2 and were statistically significant there after except in month 4. Tear production in the Endura-treated OD eyes was significantly less than the other study groups during months 2 and 3.
FIG. 1.
(A) Basal tear production. Schirmer's test was performed on the right (OD) and left (OS) eyes as previously described6 on 22 rabbits (Day 0) and then stimulated lymphocytes were injected into the contralateral iLG to induce disease. Schirmer's test was repeated every 4 weeks (ID n = 6), induced disease with cyclosporine A (CsA) treatment (ID/Rx n = 9), and induced disease with Endura treatment (ID/E n = 7). An asterisk indicates a significant difference between the ID and ID/Rx animals. An α indicates a significant difference between ID/E and ID/Rx. The values were calculated using independent sample t-tests. (B) Tear breakup time. Tear breakup time demonstrates tear instability. Slit-lamp examination was performed in both groups before surgery (Day 0) and every 4 weeks postinjection. The group designations and statistical analysis were the same as in A. (C) Rose Bengal staining score. Detection of ocular surface defects on OD was evaluated with rose Bengal stain as previously described6; and the intensity of staining of the medial and lateral bulbar conjunctiva and the cornea was scored. The designations for groups and statistical analysis are the same as in A.
Tear breakup time
Tear BUT values for all OD eyes were decreased significantly by month 1, and values remained at ∼50% of normal for the duration of the study (Fig. 1B), irrespective of whether animals were or were not treated. As for tear BUT values for CsA-treated animals, there appeared to be only a slight beneficial trend for OD eyes compared to untreated ID eyes. Tear BUT was most improved in the Endura-treated eyes in months 5 and 6.
Rose Bengal staining
Corneal staining increased significantly in OD eyes by the end of month 1 (Fig. 1C). Ocular surface staining of ID and ID/Rx eyes peaked by month 2 and remained relatively constant at this level until the end of the study. Relative to both the ID and ID/Rx groups, significantly more staining was observed in Endura-treated eyes beginning in month 2 and continuing to the end of the study.
Histopathology
Inferior LG (iLG) from normal animals displayed occasional small foci of immune cell infiltrates (Fig. 2A), whereas the untreated iLG of ID animals were heavily infiltrated even 6 months after the induction of disease. Frequently immune cell infiltrates were concentrated around ducts and venules (Fig. 2B). Some lobules of ID iLG showed degenerating acini, while other lobules in the same gland did not contain infiltrates. Plasma cells were also numerous in the ID group. The infiltrates in the Endura-treated group were similar in number of lymphocytes and foci to those seen in the untreated ID group (Fig. 2C). iLG of CsA-treated animals had much less pathology compared to the animals not treated with CsA (Fig. 2D). Some small foci of lymphocytes were observed around ducts and venules, but there was no evidence of any dramatic infiltration.
FIG. 2.
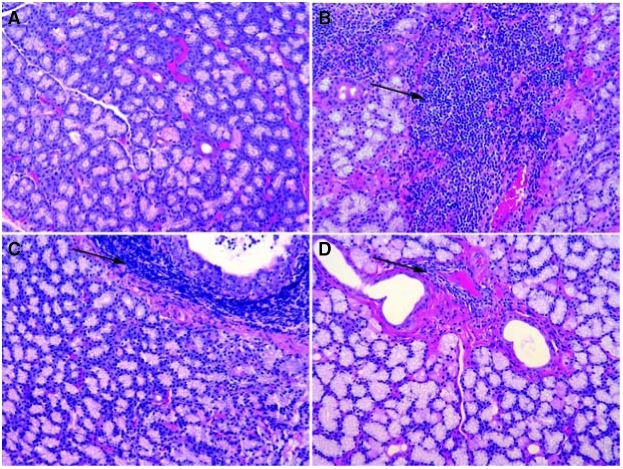
Histopathology with H&E stain iLG. (A) Normal iLG showed occasional small foci of infiltration. (B) After 6 months lymphocytic infiltration in the iLG was substantial even in interacinar connective tissue. (C) Sections from ID/E iLG at 6 months postinjection showed inflammation very similar to ID group after Endura treatment. (D) iLG from CsA-treated group showed small to medium foci around ducts and venules and these are not frequent as seen in ID and ID/E groups.
Since only OS iLG were removed for isolation of pLGEC, and activated lymphocytes were injected only into OD iLG, it was possible to evaluate histopathology in noninjected superior LG (sLG) of ID and treated animals. Normal sLG resembled normal iLG (Fig. 3A). sLG from ID animals consistently showed moderate infiltration (Fig. 3B) and infiltration of sLG of the Endura-treated group was similar to that of ID group (Fig. 3C). Only one of nine OD sLGs of treated animals had swirling acini and associated lymphocytes as seen in iLG of untreated animals (Fig. 3D).
FIG. 3.
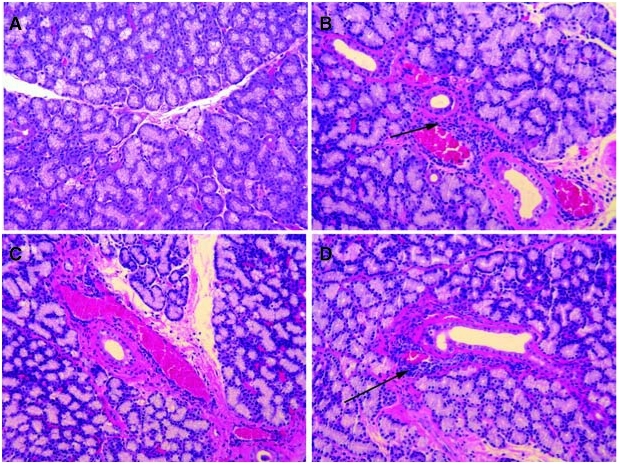
Histopathology with H&E stain sLG. (A) Superior LG (sLG) sections from normal sLG was similar to iLG. (B) sLG from the ID group showed infiltrating cells around ducts and venules. (C) The ID/E group showed infiltration but not as common as seen in ID group. (D) Sections of sLG from ID/Rx rabbits rarely displayed immune cells compared to ID animals.
Inflammation was not observed in the salivary gland, parotid gland, liver, kidney, and spleen in any of the animals with induced disease. This finding suggests that the auto-adoptively transferred immunopathology spreads from the injected iLG to sLG and conjunctivae, but not to other tissues commonly involved in patients with Sjögren's syndrome.
Immunohistochemistry
Representative images of cryosections of iLG from ID and ID/Rx animals immunostained for CD4, CD8, MHC-II, RTLA, and CD18 are presented in Figure 4. Quantitative data from the immunohistochemical analyses are presented in Table 1. Treatment reduced CD4+ T cells from 4.4% to 0.75% (P = 0.004). Treatment also decreased MHC-II-expressing cells (P < 0.01), but the relative change, that is, from 8.5% to 6.6%, was less dramatic than for CD4+ cells. The values for CD8+ and CD18+ cells did not differ between the two groups. RTLA+ cells were more abundant in the ID-treated group (11.4%) than in the ID group (6.2%).
FIG. 4.
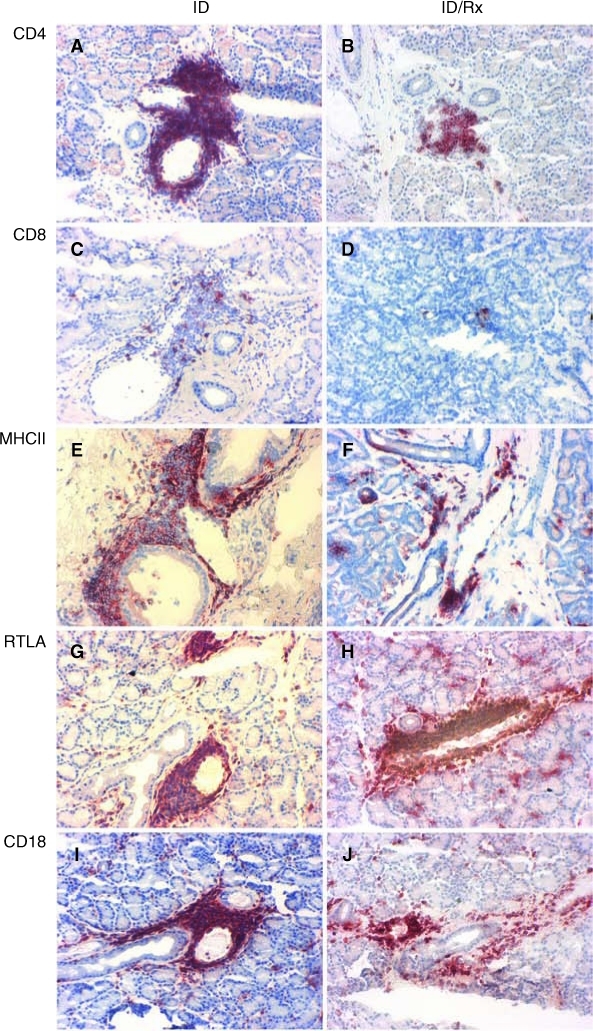
Immunohistochemical staining of cell markers. At 6 months more CD4+ T cells were detected in dense foci around iLG ducts and venules of untreated animals (A) than CsA-treated animals (B). CD8+ T cells were occasionally seen around ducts or venules of iLGs from untreated (C) compared to treated animals (D), but these cells were never abundant in either group. Areas with intense staining for MHC-II protein were more evident around venules and ducts, infiltrating into the interstitium around LG acini of untreated animals (E) and less in rabbits treated with cyclosporine (F). Fewer RTLA+ cells were seen in iLG of untreated animals (G) than in treated animals (H). Distribution of CD18 cells resembled that of RTLA cells in untreated (I) and treated (J) animals. There was no significant difference between the two groups in the total percentage of the CD18+ T cells.
Table 1.
Immunohistochemical Analysis of Inflammatory Cells in Lacrimal Glands With Induced Disease With and Without Cyclosporine Treatment
| Group | CD4 (P < 0.004) | CD8 (P > 0.32) | MHC-II (P < 0.012) | RTLA (P < 0.005) | CD18 (P < 0.11) |
|---|---|---|---|---|---|
| Untreated (ID) mean positive percentage ± SD | 4.41 ± 0.9 | 0.67 ± 0.2 | 8.5 ± 0.4 | 6.2 ± 0.9 | 8.7 ± 0.6 |
| CsA-treated (ID/Rx) mean positive percentage ± SD | 0.75 ± 0.2 | 0.39 ± 0.1 | 6.6 ± 0.5 | 11.4 ± 1.7 | 7.5 ± 0.4 |
Compared to ID group, the CsA-treated group (ID/Rx) had a significant decrease of CD4+ (P < 0.008), MHC-II (P < 0.012), and RTLA (P < 0.005), and no significant change in CD18+ (P < 0.11).
Discussion
Our findings with CsA 0.05% ophthalmic emulsion, the only drug currently approved by the U.S. Food and Drug Administration for treating severe dry eye, are similar to findings of improved Schirmer's scores in dry eye patients, and consistent with the clinical experience that patients frequently require long-term treatment before they experience significant improvements.23 In contrast, the ocular surface pathology in our rabbit model is more robust than the LG disease, and apparently, more robust than the ocular surface pathology in most patients with dry eye disease. In this study we successfully established chronic dry eye disease that persisted 6 months. Previously we had monitored the disease pathogenesis for only 2 months.7 The duration of treatment is an important consideration. For example, Lemp reported in a human study with CsA a significant increase in Schirmer's test score after 6 months.23 However, in another study primary Sjögrens syndrome patients failed to respond to CsA treatment with regard to tear production in a short follow-up period of 3 months.24 For our therapeutic evaluation of CsA, we opted to start treatment 4 weeks after inducing disease based upon our previous studies.7,25 At this time animals were divided into matched study groups based upon rose Bengal vital staining scores and tear dynamics status, which signified that dry eye disease had been established.
The observation that several weeks of treatment are necessary before lacrimal function improves, and the finding in this study that ocular surface defects detected by rose Bengal staining did not improve despite the apparent partial restoration of lacrimal function suggest that CsA is not simply immunosuppressive, but that it prevents CD4+ effector T-cell activation from being maintained. Topical CsA was known to inhibit the activity of nuclear factors of activated T cells and also to block activation of antigen-specific T cells.26 In dry eye associated with graft-versus-host disease (GVHD), the Schirmer's scores did not change with 1 month of CsA treatment, but the vital staining score and the tear BUT improved significantly.27 Another GVHD patient study showed improvement in Schirmer's scores in 3 months.28 There was variation in the response to CsA treatment in different patient groups due to the difference in the cause of the disease, duration of treatment, and the severity of inflammation.
The normal CD4 to CD8 T-cell ratio in LGs has been reported to be 1:2 for humans29 and rabbits,7 but changes to 4:1 for Sjögren's patients and in the LGs of a murine model.30 In the current study, the ratio was 6:1 for untreated rabbits with induced autoimmune dacryoadenitis; however, following long-term treatment with CsA the ratio reverted back to 1:2. Signs of ocular surface disease did not improve despite the decrease of lacrimal histopathology. One could speculate that an extended treatment period might eventually improve the clinical outcome to a detectable level.
The number of cells positive for RTLA was greater than the combined numbers of CD4+ and CD8+ cells in both untreated and treated LGs. The number of RTLA+ cells also increased after 5 months of treatment. This disparity suggests that the rabbit LG may contain large numbers of double-negative T cells, which in other species typically express the alternative, γ, δ, form of the T-cell antigen receptor. Rabbit-specific reagents that could be used to test this conjecture are not yet available. However, it may be appropriate to note that γ, δ T cells are thought to perform immunoregulatory rather than effector functions in the intestinal epithelium. One hypothesis that can account for the present findings is that CsA treatment leads to the generation of regulatory cells that are able to prevent the ongoing activation of CD4+ effector T cells in the signaling milieu of the LG, but whose actions are abrogated by the inflammatory signaling milieu of the ocular surface. Immunohistochemical analysis of inflammatory cells in LGs of animals treated with Endura was not performed, so we cannot comment on this aspect.
In summary, experimentally induced chronic autoimmune dacryoadenitis treated long term with CsA resulted in a slight improvement of clinical features and reduced histopathology in the LG of this animal model. This suggests that some Sjögren's patients may benefit from long-term CsA treatment supplemented with an adjunctive therapy targeting ocular surface disease.
Acknowledgments
The authors wish to thank Laurie LaBree Dustin for statistical analysis and Susan Clarke for editorial assistance. This work was supported by a restricted and an unrestricted grant from Allergan, NIH grants EY12689, EY005801, EY10550, EY03040, and an unrestricted grant from Research to Prevent Blindness, Inc.
Author Disclosure Statement
All authors (except M.D.T. and S.C.Y.) have no competing interests and therefore have nothing to declare. M.D.T. and S.C.Y. are paid consultants for Allergan. M.D.T. is a recipient of a restricted grant from Allergan, and A.K.M. is the recipient of an unrestricted research grant from Allergan. The protocol and the resulting manuscript submitted for publication were prepared without any input from Allergan.
References
- 1.Fox R.I. Kang H.I. Pathogenesis of Sjogren's syndrome. Rheum. Dis. Clin. North Am. 1992;18:517–538. [PubMed] [Google Scholar]
- 2.Lemp M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- 3.Sullivan D.A. Edwards J.A. Androgen stimulation of lacrimal gland function in mouse models of Sjogren's syndrome. J. Steroid Biochem. Mol. Biol. 1997;60:237–245. doi: 10.1016/s0960-0760(96)00190-2. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson D. Tauber J. Reis B.L. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology. 2000;107:967–974. doi: 10.1016/s0161-6420(00)00035-x. [DOI] [PubMed] [Google Scholar]
- 5.Sall K. Stevenson O.D. Mundorf T.K., et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–639. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 6.Barabino S. Dana M.R. Dry eye syndromes. Chem. Immunol. Allergy. 2007;92:176–184. doi: 10.1159/000099268. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Z. Stevenson D. Schechter J.E., et al. Lacrimal histopathology and ocular surface disease in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:25–32. doi: 10.1097/00003226-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Niederkorn J.Y. Stern M.E. Pflugfelder S.C., et al. Desiccating stress induces T cell-mediated Sjogren's Syndrome-like lacrimal keratoconjunctivitis. J. Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 9.Pflugfelder S.C. Tseng S.C. Sanabria O., et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38–56. doi: 10.1097/00003226-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Pflugfelder S.C. Solomon A. Dursun D., et al. Dry eye and delayed tear clearance: “a call to arms.”. Adv. Exp. Med. Biol. 2002;506:739–743. doi: 10.1007/978-1-4615-0717-8_104. [DOI] [PubMed] [Google Scholar]
- 11.Kaswan R.L. Martin C.L. Chapman W.L., Jr. Keratoconjunctivitis sicca: histopathologic study of nictitating membrane and lacrimal glands from 28 dogs. Am. J. Vet. Res. 1984;45:112–118. [PubMed] [Google Scholar]
- 12.Kaswan R.L. Martin C.L. Dawe D.L. Rheumatoid factor determination in 50 dogs with keratoconjunctivitis sicca. J. Am. Vet. Med. Assoc. 1983;183:1073–1075. [PubMed] [Google Scholar]
- 13.Kaswan R.L. Martin C.L. Dawe D.L. Keratoconjunctivitis sicca: immunological evaluation of 62 canine cases. Am. J. Vet. Res. 1985;46:376–383. [PubMed] [Google Scholar]
- 14.Pflugfelder S.C. Anti-inflammatory therapy of dry eye. Ocul. Surf. 2003;1:31–36. doi: 10.1016/s1542-0124(12)70005-8. [DOI] [PubMed] [Google Scholar]
- 15.Baudouin C. Liang H. Amplifying factors in ocular surface diseases: apoptosis. Ocul. Surf. 2005;3:S194–S197. doi: 10.1016/s1542-0124(12)70254-9. [DOI] [PubMed] [Google Scholar]
- 16.Kaswan R.L. Salisbury M.A. Ward D.A. Spontaneous canine keratoconjunctivitis sicca. A useful model for human keratoconjunctivitis sicca: treatment with cyclosporine eye drops. Arch. Ophthalmol. 1989;107:1210–1216. doi: 10.1001/archopht.1989.01070020276038. [DOI] [PubMed] [Google Scholar]
- 17.Kunert K.S. Tisdale A.S. Gipson I.K. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch. Ophthalmol. 2002;120:330–337. doi: 10.1001/archopht.120.3.330. [DOI] [PubMed] [Google Scholar]
- 18.Pflugfelder S.C. De Paiva C.S. Villarreal A.L., et al. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27:64–69. doi: 10.1097/ICO.0b013e318158f6dc. [DOI] [PubMed] [Google Scholar]
- 19.Pflugfelder S.C. Antiinflammatory therapy for dry eye. Am. J. Ophthalmol. 2004;137:337–342. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Guo Z. Song D. Azzarolo A.M., et al. Autologous lacrimal-lymphoid mixed-cell reactions induce dacryoadenitis in rabbits. Exp. Eye Res. 2000;71:23–31. doi: 10.1006/exer.2000.0855. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Z. Stevenson D. Ritter T., et al. Expression of IL-10 and TNF-inhibitor genes in lacrimal gland epithelial cells suppresses their ability to activate lymphocytes. Cornea. 2002;21:210–214. doi: 10.1097/00003226-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Trousdale M.D. Zhu Z. Stevenson D., et al. Expression of TNF inhibitor gene in the lacrimal gland promotes recovery of tear production and tear stability and reduced immunopathology in rabbits with induced autoimmune dacryoadenitis. J. Autoimmune Dis. 2005;2:6. doi: 10.1186/1740-2557-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemp M.A. Management of dry eye disease. Am. J. Manag. Care. 2008;14:S88–S101. [PubMed] [Google Scholar]
- 24.Hyon J.Y. Lee Y.J. Yun P.Y. Management of ocular surface inflammation in Sjogren's syndrome. Cornea. 2007;26:S13–S15. doi: 10.1097/ICO.0b013e31812f6782. [DOI] [PubMed] [Google Scholar]
- 25.Thomas P.B. Zhu Z. Selvam S., et al. Autoimmune dacryoadenitis and keratoconjunctivitis induced in rabbits by subcutaneous injection of autologous lymphocytes activated ex vivo against lacrimal antigens. J. Autoimmun. 2008;31:116–122. doi: 10.1016/j.jaut.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duncan N. Craddock C. Optimizing the use of cyclosporin in allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38:169–174. doi: 10.1038/sj.bmt.1705404. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y. Ogawa Y. Dogru M., et al. Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2008;41:293–302. doi: 10.1038/sj.bmt.1705900. [DOI] [PubMed] [Google Scholar]
- 28.Lelli G.J., Jr. Musch D.C. Gupta A., et al. Ophthalmic cyclosporine use in ocular GVHD. Cornea. 2006;25:635–638. doi: 10.1097/01.ico.0000208818.47861.1d. [DOI] [PubMed] [Google Scholar]
- 29.Pepose J.S. Akata R.F. Pflugfelder S.C., et al. Mononuclear cell phenotypes and immunoglobulin gene rearrangements in lacrimal gland biopsies from patients with Sjogren's syndrome. Ophthalmology. 1990;97:1599–1605. doi: 10.1016/s0161-6420(90)32372-2. [DOI] [PubMed] [Google Scholar]
- 30.Oak J.S. Deane J.A. Kharas M.G., et al. Sjogren's syndrome-like disease in mice with T cells lacking class 1A phosphoinositide-3-kinase. Proc. Natl. Acad. Sci. USA. 2006;103:16882–16887. doi: 10.1073/pnas.0607984103. [DOI] [PMC free article] [PubMed] [Google Scholar]