Abstract
Objectives
Chronic stress with relative hypercortisolism has been associated with metabolic disease risk. Stress-reduction interventions may therefore hold promise for reducing such chronic disease risk in obese youth. The purpose of this study was to conduct a 4-week pilot intervention to determine whether stress-reduction Interactive Guided ImagerySM (IGI) could serve as an acceptable and effective stress-reduction modality in overweight Latino adolescents.
Design
Subjects (6 male/6 female, ages 14–17, body-mass index >95th percentile) were randomly assigned to the experimental guided imagery group (IGI, n = 6), or the nonintervention control group (C, n = 6). IGI subjects received four weekly 45-minute stress-reduction IGI sessions. Salivary cortisol was assessed immediately before and after each session. Acceptability was assessed by compliance and qualitative interviews.
Results
Subjects attended all sessions and expressed acceptance of the IGI intervention. There were significant within-group reductions in salivary cortisol in the IGI group in three of the four sessions, and no reductions in cortisol in the control group. For all four sessions combined, there was a significant between-group effect for the change in salivary cortisol in IGI versus C (p = 0.007). Effect sizes of cortisol change in IGI group were moderate to very high in the four sessions.
Conclusions
We conclude that IGI may be feasible and effective in acutely reducing salivary cortisol levels in overweight Latino adolescents. Future studies will need to determine whether stress-reduction IGI can result in longer-term reductions in chronic stress and measures of HPA activity.
Introduction
The prevalence of obesity is increasing dramatically in children, particularly among Latino youth.1 This is associated with a high prevalence of obesity-related morbidities such as type 2 diabetes, pre-diabetes, and metabolic syndrome in this population.2,3 Specifically, we have previously shown that ~32% of overweight Latino children and adolescents with a family history of type 2 diabetes have pre-diabetes,4,5 while ~30% have metabolic syndrome (a cluster of cardiovascular disease risk factors related to insulin resistance).6
Chronic stress may increase obesity-related disease risk through hyperactivity of the hypothalamic–pituitary– adrenal axis (HPA).7,8 Evidence supporting this hypothesis includes the findings that increased stress-related cortisol secretion is associated with features of the metabolic syndrome in both men,9 and women,10,11 and men with metabolic syndrome have increased urinary cortisol metabolite secretion.12 We have recently reported that among overweight Latino teenagers, those with metabolic syndrome have higher concentrations of morning serum cortisol than those without metabolic syndrome.13
There is evidence that adolescents of today may have significantly elevated chronic life stress. Twenge has shown that today's adolescents suffer from increased anxiety relative to those of the past.14 The transition into high school may be a time of particularly higher risk for emotional distress and disordered eating.15 Inner-city Latino adolescents are frequently exposed to specific environmental factors that have been linked to chronic stress, including crime, disturbed family and social connections,14 racial discrimination,15 and lower socioeconomic status.16 The increasing environmental stress upon today's urban, minority adolescents may, in the setting of increased availability of inexpensive, high caloric food and diminished physical activity, promote both general obesity (through lifestyle behaviors such as emotional overeating) and visceral obesity (through HPA axis activation and increased cortisol secretion). This suggests overweight Latino teens as an ideal population to study the relationships between chronic stress and obesity-related metabolic complications.
Insofar as chronic stress with increased cortisol levels may be associated with metabolic disease risk, stress-reduction interventions may hold promise for reducing such chronic disease risk in obese youth. Stress reduction mind–body modalities such as meditation, guided imagery, and hypnotherapy have been shown to reduce stress and affect other health outcomes favorably.17–19 For example, Pawlow and Jones showed acute reductions of salivary cortisol in response to brief progressive muscle relaxation.20 However, most reports of the benefits of mind–body stress reduction interventions have centered on adult, primarily white, populations. The use of such interventions in an inner-city teenage minority population naive to mind–body modalities has not been previously investigated. Furthermore, the effectiveness of guided imagery in general, and Interactive Guided ImagerySM (IGI) in particular, on lowering salivary cortisol levels has not been previously assessed.
Therefore, the purpose of this study was to conduct a 4-week pilot intervention to determine whether stress-reduction IGI could serve as an acceptable and effective stress-reduction modality in overweight, Latino adolescents. Our primary hypotheses were that stress-reduction IGI would (1) be acceptable to this population, and (2) result in acute decreases in salivary cortisol levels.
Materials and Methods
Participants were boys and girls aged 14–16 years, who were in the 9th grade or higher. All were of Latino heritage per parental self-report of all four biological grandparents being Latino, similar to entry criteria of other studies by our research group relating to chronic metabolic disease risk in overweight Latino adolescents.4,21,22 Other entry criteria included overweight status, defined as body mass index (BMI) percentile greater than the 95th percentile for age and sex using CDC 2000 criteria.23 Participants were excluded if they had participated in any weight loss program within the previous 6 months, had a serious chronic illness, had a medical condition or were taking medication that would affect body composition or insulin sensitivity/secretion, had participated regularly in any mind–body stress reduction or related practices in the past, had a clinically diagnosed psychiatric or eating disorder, or were cognitively or language/hearing impaired.
Weight and height were measured in triplicate using a beam medical scale and wall-mounted stadiometer, to the nearest 0.1 kg and 0.1 cm, respectively. BMI percentile was calculated using software EpiInfo, Version 3.3.2 (Centers for Disease Control and Prevention, Atlanta, GA). Tanner pubertal stage was assigned based on breast stage for girls and pubic hair stage for boys by a licensed pediatric care provider.24,25 This study was approved by the Institutional Review Board of the University of Southern California (USC). Written parental consent and youth assent were obtained prior to initiation of any study procedures.
Interactive Guided Imagery intervention
Subjects were randomly assigned using a random number table to either IGI (Experimental Group) or nonintervention (Control Group). Participants in the experimental group received a weekly 45-minute IGI session for 4 consecutive weeks, conducted after school on weekdays, generally beginning between 4 and 5:30 PM. Imagery sessions were conducted individually for each participant by a single certified Interactive Guided ImagerySM practitioner (MW) (Academy for Guided Imagery, Malibu, CA).
The IGI method utilizes the subject's personalized images to promote health through several standardized, yet adaptable, techniques including, for the purposes of this study, relaxation/stress reduction. The IGI facilitator's goal is to enable the subject to engage his/her own images that are symbolic of his/her specific health or life issues, in order to develop health-directed insights, health-promoting behavior changes, or direct physiologic changes.26 For this study, the four 45-minute IGI sessions utilized standard stress-reduction techniques of IGI, with each session building successively on the previous sessions. Session 1 included focused relaxation breathing and an explanation of the basic mind–body principles underlying guided imagery. In Session 2, head-to-toe progressive muscle relaxation was added, characterized by focused attention and subsequent relaxation of each successive muscle group, synchronized to the focused relaxation breathing. In Session 3, relaxed-place imagery was added, which involves a facilitated exploration of an image of a safe, comfortable place specific to the participant. For this exercise, following an induction of relaxed breathing and muscle relaxation, the subject invites an image of a place that represents just relaxation and freedom from stress. The facilitator then guides the subject through an exploration of this image through dialogue with the subject who is continually engaged with the image. Exploration of the image includes sensory recruitment (visual, auditory, olfactory, tactile, and kinesthetic), particularly focusing on linking elements of relaxation in the image to the physiologically relaxed state simultaneously being experienced by the subject. In the final weekly session, conditioned relaxation was added, a technique in which participants access their relaxed-placed image more quickly by linking the appearance of the image with a single deep “signal” breath, rather than with an extended breathing–muscle relaxation induction.27 Participants were instructed to practice at home the stress-reduction techniques they had learned in each IGI session for 10 minutes, twice a day, in between weekly sessions. Practice logs were given to participants to record the times of their practice as well as a qualitative description of their imagery practice experience.
Salivary Cortisol
At the beginning and end of each 45-minute session, experimental subjects provided a salivary cortisol sample. Research staff phoned control group subjects to ensure collection of salivary cortisol samples at home at the same time of day as those collected from the IGI group. Control samples were successfully obtained on 92% of occasions. Salivary cortisol level reflects circulating free (unbound) plasma cortisol and is thus a good indicator of the physiologically active form of the hormone.28 Saliva was obtained using the Salivette® system (Sarstedt, Newton, NC). A dry cotton swab was placed in the mouth for 2 minutes, yielding approximately 1 mL of passively absorbed saliva. Wet swabs were kept at room temperature, typically for 1–2 hours, until transferred to the laboratory the same evening of collection, then stored overnight at 4°C. Control subjects immediately placed collected samples in their home refrigerator until retrieved that same evening by study staff, and then processed as per the experimental group samples. The morning following collection, Salivettes were centrifuged at 2500 revolutions per minute for 10 minutes, and saliva supernatant was then frozen at −70°C until assayed. Cortisol in saliva is very stable in vitro,29 and prior validation studies in our laboratory had shown salivary cortisol levels are stable through at least two freeze-thaw cycles (correlation of fresh versus twice-frozen/thawed samples = 0.97, p < 0.001). Samples were assayed for cortisol using an automated enzyme immunoassay (Tosoh AIA 600 II analyzer, Tosoh Bioscience, Inc., South San Francisco, CA) in the General Clinical Research Center Core Laboratory. The assay sensitivity was 0.02 μg/dL, interassay coefficient of variation (CV) was 7.8%, and intra-assay CV was 3.4%.
Other measures
Baseline outcome measures were obtained during an outpatient visit in the USC General Clinical Research Center (GCRC). Perceived Stress was assessed using a 17-item version of the Perceived Stress Scale.30 Stressful life-events over the past year were measured using a 65-item checklist developed and validated in a mostly Latino population of middle school students.31 Fasting serum cortisol was measured using an automated enzyme-linked immunoassay (Tosoh AIA 600 II analyzer). Acceptability of the guided imagery intervention to this study population was assessed by compliance with imagery session visits, home practice logbook records, and qualitative questioning of individual subjects at the beginning and completion of each session.
Data analysis
Comparison of age, Tanner stage, and BMI percentile in controls and IGI groups were performed to assure that randomization to experimental group was effective. Any notable differences (p < 0.10, due to small sample sizes) were controlled for in further group comparative analyses. Within-group acute changes in salivary cortisol were compared pre- and postsession using paired t tests for each of the individual sessions. Between-group comparisons of acute change in salivary cortisol were made using multiple linear regression analysis, controlling for any sample differences in demographics and pre-session salivary cortisol level. A p value <0.05 was taken to indicate statistical significance. Because making statistical inferences with small sample sizes is challenging, emphasis was also placed on quantifying clinical relevance with the use of effect sizes. Effect sizes were calculated as the ratio of the difference in the group means to the pooled standard deviation; they provide an indication of the strength of a difference between groups. The following standard guidelines suggested by Cohen (1988)32 were used to determine the strength of effects: <0.4 = “small,” 0.4–0.7 = “moderate,” 0.7–1.0 = “high” (or “large”), and >1.0 = “very high” (or “very large”). All statistical tests were performed using SPSS (Version 11.0; SPSS Inc., Chicago, IL).33
Results
Comparison of group characteristics
According to Table 1, there were no significant differences in age, gender, BMI, or BMI percentile between the two randomized groups. The experimental group had slightly less advanced pubertal stage (p < 0.1). There were no significant differences between groups in baseline levels of stress as assessed by either perceived stress or life events scales. Morning serum cortisol did not differ between the groups.
Table 1.
Baseline Subject Characteristics
| IGI (n = 6) | Control (n = 6) | |
|---|---|---|
| Age (yrs) | 16.1 ± 0.6 | 15.8 ± 1.1 |
| Gender (F/M) | 3/3 | 3/3 |
| Tanner stage | 4.0 ± 0.9 | 4.8 ± 0.4a |
| BMI (kg/m2) | 36.2 ± 6.3 | 33.2 ± 6.4 |
| BMI percentile | 98.5 ± 1.1 | 97.1 ± 2.2 |
| Perceived stress | 14.7 ± 7.2 | 21.0 ± 5.5 |
| Stressful life events | 17.2 ± 10.1 | 16.2 ± 6.9 |
| Fasting serum cortisol (μg/dL) | 7.3 ± 2.4 | 6.8 ± 2.1 |
Between-group comparisons by independent t test: p = 0.07. IGI, Interactive guided ImagerySM; BMI, body–mass index.
Acceptance of intervention
All subjects in the experimental group completed the 4-week intervention, with 100% compliance in attendance of all weekly imagery sessions (i.e., there were no missed sessions). In general, the imagery sessions were subjectively well received by all 6 subjects in the experimental group, as indicated by qualitative postintervention evaluations. Specific characteristic statements when asked their experience of the IGI stress-reduction sessions included: “cool”; “It was really relaxing”; “It felt good—took the tension away”; and, “I was able to concentrate on my math test better.” Despite their enthusiasm for the IGI sessions, compliance with recommended twice-daily home imagery practice was poor. Review of home practice log entries showed that most participants reported practicing only a few times a week. Most practiced only once a day at most, typically for 5–10 minutes per practice session.
Within-group comparisons
Table 2 reports the salivary cortisol levels for the two groups before and after each weekly session, as well as the change in salivary cortisol across each individual session. Within the IGI group, there were significant declines in salivary cortisol across sessions 2, 3, and 4. The magnitude of the change remained fairly consistent across the 4 weeks, with no significant differences in the magnitude of decline between any two individual sessions and no general trend in degree of change in salivary cortisol across the 4 weeks (p = 0.33). There were no significant changes in salivary cortisol within the Control group across any of the sessions.
Table 2.
Change in Salivary Cortisol Following Stress-Reduction Guided Imagery
| |
Guided Imagery group |
Control group |
||||
|---|---|---|---|---|---|---|
| |
Salivary cortisol (μg/dL) |
Salivary cortisol (μg/dL) |
||||
| Session number | Pre-session | Post-session | Change | Pre-session | Post-session | Change |
| 1 | 0.83 ± 0.17 | 0.64 ± 0.19 | −0.19 ± 0.30 | 0.56 ± 0.29 | 0.52 ± 0.25 | −0.04 ± 0.25 |
| 2 | 0.66 ± 0.26 | 0.52 ± 0.12 | −0.14 ± 0.15** | 0.68 ± 0.25 | 0.55 ± 0.20 | −0.12 ± 0.17 |
| 3 | 0.66 ± 0.16 | 0.48 ± 0.10 | −0.18 ± 0.09* | 0.50 ± 0.16 | 0.40 ± 0.14 | −0.10 ± 0.14 |
| 4 | 0.61 ± 0.19 | 0.44 ± 0.16 | −0.18 ± 0.08*a | 0.52 ± 0.31 | 0.50 ± 0.28 | −0.01 ± 0.28a |
Between-group comparisons: ap < 0.10.
Data reported are unadjusted means ± standard deviation for salivary cortisol.
Within-group comparisons: *p < 0.01; **p < 0.10.
Between-group comparisons
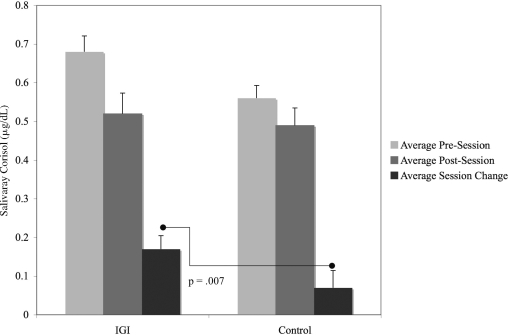
When comparing the degree of decline in acute salivary cortisol across individual sessions between the experimental and control group, there was a trend toward a treatment group effect seen in Session 4 (p = 0.099). When the changes in salivary cortisol for all four sessions were combined, there was a significant between-group effect for the change in salivary cortisol in IGI versus Controls, controlling for Tanner stage and repeated measures within individuals (Fig. 1, p = 0.007).
FIG. 1.
Average pre- and postsession salivary cortisol for control and Interactive Guided ImagerySM (IGI) groups (four sessions combined). N = 24 observations for IGI group; N = 22 observations for the Control Group. Post–pre difference was −0.17 ± 0.17 for IGI and −0.07 ± 0.21 for controls. Group comparison of difference was p = 0.007, using repeated-measures analysis of covariance adjusting for Tanner stage.
Effect sizes
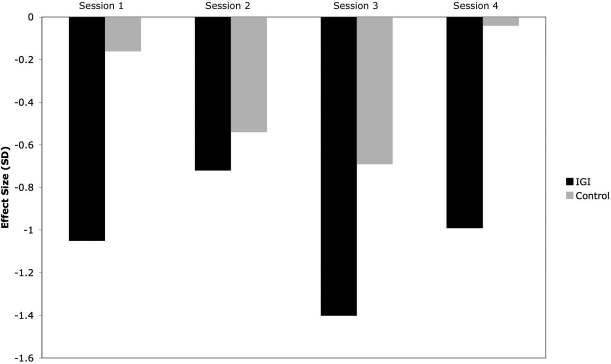
Measures of effect size of change within each session were high to very high in the experimental group, ranging from −0.72 to −1.4 (Fig. 2). In all but one session (Session 2), there was a mean decrease of 1 standard deviation or more in salivary cortisol from pre- to post-IGI session. Effect sizes were small to moderate within the control group (ranging from −0.04 to −0.69). There was a moderate effect (−0.54) between groups in the pre-post change when all sessions were combined (adjusted for repeated measures).
FIG. 2.
Effect size of salivary cortisol change across individual sessions. Bars represent effect sizes [(Mpost – Mpre)/SD-pooled] for change in salivary cortisol at each session. IGI, Interactive Guided Imagery,SM; SD, standard deviation.
Discussion
We sought in this pilot study to demonstrate the acceptability and effectiveness of acutely lowering salivary cortisol levels of stress-reduction IGI in an overweight Latino adolescent subject population. Our results demonstrate that the guided imagery was generally acceptable as well as enjoyed by this group of youth, and that it resulted in moderate to large acute decreases in salivary cortisol. High to very high effect sizes were seen in each of the four imagery sessions, and were reasonably consistent across sessions. To our knowledge, these results are the first to suggest that IGI can acutely lower salivary cortisol, and that IGI may be an acceptable and effective stress-reduction intervention in this adolescent population.
Prior work in adults suggests that chronic stress resulting in increased activity of the HPA axis may play an important role in obesity-related disease risk. The HPA axis is a prime mediator of the physiologic stress response, and chronic stress, via neuroendocrine mechanisms producing subtle hypercortisolism, may result in a “pseudo-Cushingoid” obesity phenotype characterized by visceral adiposity, insulin resistance, and metabolic syndrome.8,34,35 Supporting this hypothesis, it has been shown that cynomolgus monkeys subjected to chronic social stress over 2 years demonstrated hypercortisolism.36 Among obese identical twins, high visceral fat was associated with increased urine cortisol and increased psychosocial stress.37 Obese women with abdominal obesity show higher urinary free cortisol excretion, along with other markers of hypercortisolism, compared to both controls and women with peripheral, nonabdominal obesity.38,39 Finally, adults with abdominal obesity and/or metabolic syndrome have been shown to have increased stress-related salivary cortisol secretion,9,10 increased excretion of urinary cortisol metabolites,12 and other measures of HPA hyperactivity.40
There are far fewer pediatric data relating cortisol to obesity or obesity-related complications. Reinehr has shown that obese, insulin-resistant youth have higher morning cortisol levels, and cortisol decreases with weight-reduction and improved insulin sensitivity.41 We have recently shown that overweight Latino youth with metabolic syndrome have higher morning serum cortisol levels than those without metabolic syndrome.13 Our findings of a significant acute decrease in salivary cortisol with a biologically large effect size suggest that IGI may represent a promising mind–body therapy for reducing exposure to the relatively higher cortisol levels that may contribute to obesity-related disease risk in chronically stressed individuals. It remains an area for future investigation to determine whether the acute reductions in cortisol occasioned by IGI in this study would, over time, also be reflected in changes in other measures of cortisol that have been linked to obesity-related diseases, such as morning cortisol,13 stress-related cortisol secretion,9 or urinary cortisol levels.12
The fact that IGI was successful in acutely reducing salivary cortisol is consistent with similar effects by other related mind–body interventions. Progressive muscle relaxation, music therapy, and mindful meditation have all been shown to produce significant reductions in measures of cortisol and/or stress.18–20 Thus, the effect of IGI may not be specific to this form of mind–body therapy, but may reflect the similarity of relaxation methodologies utilized by all of these mind–body therapies. Nonetheless, our findings of acceptability of the IGI intervention in this population are encouraging that IGI could be utilized effectively as a stress-reduction treatment in these youth.
Our first objective of this pilot study was to demonstrate the acceptability of the use of IGI in a group of inner-city youth at high risk for obesity-related complications. We could find nothing in the literature to guide us in this direction. In our extensive clinical experience, this population is generally not aware of, and does not utilize, mind–body stress-reduction methodologies. However, our anecdotal use of these techniques with adolescents in our clinics suggested to us that these youth would be responsive to an IGI intervention. In addition, we have carried out preliminary focus-group qualitative studies, which strongly suggested that these youth would respond well to stress-reduction IGI (data not shown). On the whole, our results indicate that the 4-week intervention was quite well accepted and even enjoyed by the imagery study group. The 100% compliance for the guided imagery sessions, in which no participant missed any of the sessions, and very positive qualitative participant evaluations, support this conclusion. Besides enjoyment of the novel intervention, attendance compliance was aided by our use of friendly, bilingual research staff, and by providing transportation to the sessions when needed. Despite their enthusiasm for the IGI sessions, compliance with the home imagery practice portion of the intervention was poor. Thus, in the future, focusing more efforts on increasing compliance with stress-reduction imagery practice at home should be a major goal of any intervention hoping to effect physiologically significant long-term reductions in cortisol levels.
The strengths of this novel study relate primarily to its demonstrated effects in providing a physiologically effective and well accepted stress-reduction intervention in a chronically understudied group that is at very high risk of obesity-related complications, and for whom there is a paucity of information regarding effective interventions. The results thus suggest that guided imagery may hold promise as an effective mode of therapeutic intervention in this population. The treatment was relaxing, enjoyable, and non-invasive, and the physiological response was immediate and reproducible week to week. Our current groups of subjects were equivalent in levels of baseline stress, so that these variables were unlikely to confound our results regarding salivary cortisol changes in response to guided imagery. Statistically and biologically (effect size) significant reductions in salivary cortisol were demonstrated in the imagery group despite the small numbers of subjects, indicating it is a potentially powerful modality that can produce physiologically significant results.
The main limitation of this study is the small sample size. However, this project was designed as a pilot study with the aim of determining feasibility and effect sizes. Both of these goals were realized, in addition to the significant within-group effect on salivary cortisol in the imagery group. Although there were suggestions of between-group differences in salivary cortisol, the small sample size undoubtedly prevented statistically significant between-group differences to be demonstrated, other than the trend seen in Session 4 (IGI versus Control difference in salivary cortisol change <0.1), and when all session cortisol values were combined (Fig. 1, p = 0.001). Another weakness was that salivary cortisol was obtained from the control group in their home environment, not entirely identical to that of the imagery study group. It is likely that the drop in salivary cortisol in control subjects across session time, more modest than in the imagery group, was related to the normal diurnal decline of cortisol in the late afternoon, plus a possible decrease in psychological stress as the participants came home from school and relaxed in their home environment. The different environments between groups may also partly explain the appearance of lower presession cortisol values in the control group compared to the imagery group, in that it is conceivable the imagery group experienced higher presession cortisols due to transient anticipatory stress as they entered a new, unfamiliar experience: the imagery sessions. We also cannot exclude a “floor effect” in the control group, whereby starting from a relatively lower baseline cortisol, significant further reductions were unlikely. However, these factors seem unlikely to have accounted for our results, since much lower salivary cortisol levels at 10 PM (averaging 0.22 ± 0.05 μg/dL; data from ongoing studies in this population) argue against the “floor effect,” the presession cortisols were not statistically different between the groups (reaching a trend only in Session 3, p < 0.1), and because our analyses of cortisol change were adjusted for baseline (i.e., presession) cortisol. It is also unlikely that the Tanner stage differences between the groups accounted for these differences, because for the group as a whole (n = 12) there was no relationship between Tanner stage and salivary cortisol measured at 6 PM (bivariate correlation r = −0.08, p = 0.8). Another way that the control group may not be directly comparable to the intervention group is that they did not receive the same individualized attention that the IGI group did. It could be argued that some of the effectiveness of IGI in this study could be due to the nonspecific effect of participants receiving special attention by friendly and caring research staff. To correct for these limitations, it will be important for future studies to include an adequate sample size to determine between-group effects of IGI, as well as ensure more equal treatment of control and study groups in terms of environmental exposure and study team contact variables.
Conclusions
In conclusion, IGI shows promise as a feasible and effective modality in acutely reducing salivary cortisol levels in overweight Latino adolescents. Future studies will need to extend these results to determine whether stress-reduction IGI can result in more long-term reductions in chronic stress and measures of HPA activity. The long-term effectiveness of IGI as an effective intervention for obesity-related disease risk in this population remains to be demonstrated.
Acknowledgments
This study was supported by grant 5 M01 RR00043-46 from National Center for Research Resources/National Institutes for Health (NCRR/NIH). NCRR had no further role in study design, execution of study, analyses, or manuscript preparation. We would like to thank the GCRC nursing staff for their help. Finally, we would like to acknowledge and thank the study subjects and their families for their participation.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Hedley AA. Ogden CL. Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Weiss R. Dziura J. Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. NEJM. 2004;350:2362. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbloom AL. Joe JR. Young RS. Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 4.Weigensberg MJ. Ball GD. Shaibi GQ, et al. Decreased beta-cell function in overweight Latino children with impaired fasting glucose. Diabetes Care. 2005;28:2519–2524. doi: 10.2337/diacare.28.10.2519. [DOI] [PubMed] [Google Scholar]
- 5.Goran MI. Bergman RN. Avila Q, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. 2004;89:207. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 6.Cruz ML. Weigensberg MJ. Huang TT, et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. 2004;89:108. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 7.Bjorntorp P. Rosmond R. Hypothalamic origin of the metabolic syndrome X. Ann NY Acad Sci. 1999;892:297–307. doi: 10.1111/j.1749-6632.1999.tb07803.x. [DOI] [PubMed] [Google Scholar]
- 8.Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: Neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24(suppl 2):S50. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- 9.Rosmond R. Dallman MF. Bjorntorp P. Stress-related cortisol secretion in men: Relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83:1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- 10.Epel ES. McEwen B. Seeman T, et al. Stress and body shape: Stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62:623. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Pasquali R. Vicennati V. Activity of the hypothalamic-pituitary-adrenal axis in different obesity phenotypes. Int J Obes Relat Metab Disord. 2000;24(suppl 2):S47. [PubMed] [Google Scholar]
- 12.Brunner EJ. Hemingway H. Walker BR, et al. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: Nested case-control study [see comment] Circulation. 2002;106:2659–2665. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- 13.Weigensberg M. Relationship between metabolic syndrome and morning serum cortisol in overweight Latino youth. J Clin Endocrinol Metab. 2008;93:1372–1378. doi: 10.1210/jc.2007-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Twenge JM. The age of anxiety? Birth cohort change in anxiety and neuroticism, 1952–1993. J Pers Soc Psychol. 2000;79:1007. doi: 10.1037//0022-3514.79.6.1007. [DOI] [PubMed] [Google Scholar]
- 15.Stein KF. Hedger KM. Body weight and shape self-cognitions, emotional distress, and disordered eating in middle adolescent girls. Arch Psychiatr Nurs. 1997;11:264. doi: 10.1016/s0883-9417(97)80017-9. [DOI] [PubMed] [Google Scholar]
- 16.Williams DR. Race, socioeconomic status, and health: The added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896:173. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- 17.Gruzelier JH. A review of the impact of hypnosis, relaxation, guided imagery and individual differences on aspects of immunity and health. Stress. 2002;5:147. doi: 10.1080/10253890290027877. [DOI] [PubMed] [Google Scholar]
- 18.Schneider N. Schedlowski M. Schurmeyer TH. Becker H. Stress reduction through music in patients undergoing cerebral angiography. Neuroradiology. 2001;43:472. doi: 10.1007/s002340000522. [DOI] [PubMed] [Google Scholar]
- 19.Speca M. Carlson LE. Goodey E. Angen M. A randomized, waitlist controlled clinical trial: The effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosom Med. 2000;62:613. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Pawlow LA. Jones GE. The impact of abbreviated progressive muscle relaxation on salivary cortisol. Biol Psychol. 2002;60:1. doi: 10.1016/s0301-0511(02)00010-8. [DOI] [PubMed] [Google Scholar]
- 21.Cruz ML. Weigensberg MJ. Huang TT, et al. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 22.Goran MI. Bergman RN. Avila Q, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab. 2004;89:207–212. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 23.Charts CfDCaPCg. Centers for Disease Control and Prevention: CDC Growth Charts. www.cdc.gov/nchs/about/major/nhanes/growthcharts www.cdc.gov/nchs/about/major/nhanes/growthcharts
- 24.Marshall WA. Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall WA. Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossman ML. Guided Imagery for Self-Healing. Tiburon, CA: HJ Kramer, New World Library; 2000. [Google Scholar]
- 27.Bresler D. Free Yourself from Pain Series, DB101. New York: Alpha Books; 2003. Conditioned Relaxation. [Google Scholar]
- 28.Laudat MH. Cerdas S. Fournier C, et al. Salivary cortisol measurement: A practical approach to assess pituitary-adrenal function. J Clin Endocrinol Metab. 1988;66:343–348. doi: 10.1210/jcem-66-2-343. [DOI] [PubMed] [Google Scholar]
- 29.Clow A. Thorn L. Evans P. Hucklebridge F. The awakening cortisol response: Methodological issues and significance. Stress. 2004;7:29. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- 30.Cohen S. Kamarck T. Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385. [PubMed] [Google Scholar]
- 31.Booker CL. Gallagher P. Unger JB. Ritt-Olson A. Johnson CA. Stressful life events, smoking behavior, and intentions to smoke among a multiethnic sample of sixth grader. Ethn Health. 2004;9:369–397. doi: 10.1080/1355785042000285384. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 33.SPSS Mac OS X Version. 11.0 ed. Chicago: SPSS Inc.; 2005. [Google Scholar]
- 34.Bjorntorp P. Rosmond R. Neuroendocrine abnormalities in visceral obesity. Int J Obes Rel Metab Disord. 2000;24(suppl 2):S80. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- 35.Charmandari E. Kino T. Souvatzoglou E. Chrousos GP. Pediatric stress: Hormonal mediators and human development. Horm Res. 2003;59:161. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- 36.Shively CA. Laber-Laird K. Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 37.Marniemi J. Kronholm E. Aunola S, et al. Visceral fat and psychosocial stress in identical twins discordant for obesity. J Intern Med. 2002;251:35. doi: 10.1046/j.1365-2796.2002.00921.x. [DOI] [PubMed] [Google Scholar]
- 38.Marin P. Darin N. Amemiya T, et al. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41:882. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- 39.Pasquali R. Cantobelli S. Casimirri F, et al. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J Clin Endocrinol Metab. 1993;77:341–346. doi: 10.1210/jcem.77.2.8393881. [DOI] [PubMed] [Google Scholar]
- 40.Pasquali R. Vicennati V. Cacciari M. Pagotto U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann N Y Acad Sci. 2006;1083:111–128. doi: 10.1196/annals.1367.009. [DOI] [PubMed] [Google Scholar]
- 41.Reinehr T. Andler W. Cortisol and its relation to insulin resistance before and after weight loss in obese children. Horm Res. 2004;62:107–112. doi: 10.1159/000079841. [DOI] [PubMed] [Google Scholar]