Abstract
OBJECTIVE
Pulmonary diseases associated with fibrosis, including scleroderma lung disease, are characterized by accumulation of T cells in the lungs. These cells are thought to facilitate lung fibrosis, but the exact mechanisms of their profibrotic action are not clear. Several αV-containing integrins, including αVβ3 and αVβ5 have been shown to directly activate TGF-β and promote collagen accumulation. Whether pulmonary T cells express profibrotic integrins and regulate collagen accumulation is unknown.
METHODS
Expression of integrins was assessed by immunohistochemistry in the lung tissue and by flow cytometry in bronchoalveolar lavage (BAL) from patients or from a CCL18 overexpression animal model of pulmonary T cell infiltration. Experiments in cell culture tested whether integrin-expressing T cells are profibrotic in co-cultures with pulmonary fibroblasts and through what possible mechanism.
RESULTS
Lymphocytes and integrin-positive cells were present in the lungs, and pulmonary T cells expressed integrins αVβ3 and αVβ5, in patients and in the animal model. Systemic administration of neutralizing anti-integrin αV antibody or genetic deficiency of integrin β3 in the CCL18 overexpression model significantly attenuated CCL18-driven pulmonary lymphocytic infiltration and collagen accumulation. Jurkat T cells overexpressing integrin αVβ3 or integrin αVβ5 in co-cultures with primary pulmonary fibroblasts stimulated collagen accumulation and Smad2 nuclear translocation. Neutralizing anti-TGF-β antibody attenuated the profibrotic effect of integrin-expressing T cells.
CONCLUSIONS
Pulmonary infiltrating T lymphocytes may express integrins αVβ3 and αVβ5 that are necessary for lymphocytic infiltration and T cell-associated TGF-β activation and collagen accumulation.
INTRODUCTION
Pulmonary fibrosis, or excessive accumulation of connective tissue in the lungs, is a severe and even deadly complication that occurs in a variety of diseases, such as the idiopathic interstitial pneumonias, the systemic connective tissue diseases, sarcoidosis, graft versus host disease, occupational or environmental lung diseases, and some rare genetic diseases (1). The exact causes of pulmonary fibrosis remain poorly understood, but the mechanisms of this devastating condition appear numerous and diverse, including inflammation-related and -unrelated processes.
An important commonality among various fibrotic diseases of the lungs is the frequent association with the excessive pulmonary accumulation of T lymphocytes. The T cells constitute a relatively minor population in a normal lung; this population expands numerically and undergoes phenotypic changes in association with lung inflammation and fibrosis (2). It remains unclear whether the infiltrating T lymphocytes promote fibrosis, accumulate in a futile attempt to counter it, or are innocent bystanders of ongoing response to pulmonary injury (2). Extensive data from animal models and limited observations in humans suggest that depending on specific phenotypic features of the infiltrating pulmonary T cells, their contribution may indeed be either pro- or antifibrotic (2). Pulmonary infiltration of T lymphocytes mediated by overexpression of a selective chemotactic factor CCL18 causes a moderate T lymphocyte-dependent accumulation of collagen (3), whereas in combination with bleomycin injury, the same CCL18-mediated T lymphocytic infiltration has a partially protective antifibrotic effect (4).
It is likely that the infiltrating lymphocytes mediate their profibrotic effect on pulmonary fibroblasts through cytokines, particularly the most potent profibrotic cytokine TGF-β, as well as Th2/Tc2 cytokines, chemokines, CD40 ligation, Fas-FasL and perforin-granzyme pathways (2,5–7). However, T lymphocytes of “proinflammatory” (TNF-α-expressing) or Th1 phenotype may also be protective and act antifibrotically (2). We and others have previously shown that T lymphocytes accumulate in the lungs of patients with scleroderma lung disease, and that these T cells appear to be activated and express a profibrotic pattern of cytokines, chemokines, and cell surface molecules (6,7). Pulmonary lymphocytic infiltration and collagen accumulation in patients with scleroderma lung disease may be driven by CCL18 that is a selective chemoattractant of T cells but not other cell types (3,4,8–11). Of important notice, the infiltrating pulmonary T lymphocytes in patients with scleroderma lung disease express various integrin chains, including integrin αV, when compared to scleroderma patients with no pulmonary involvement or healthy controls (7).
Recently, an novel integrin-dependent mechanism of fibrosis has been discovered, that depends on TGF-β activation by integrin αVβ6; the epithelium-restricted β6 −/− mice showed only a minor fibrotic response of lung to bleomycin administration compared with wild-type mice (12). Integrins are heterodimers, with eight β subunits and eighteen α subunits that associate into 24 known integrins. They mediate cell adhesion and play important role in a variety of cellular and extracellular processes, including survival, proliferation and migration (13). It appears that not only integrin αVβ6, but other αV-containing integrins, including αVβ1, αVβ3, αVβ5, αVβ8 may also activate latent TGF-β and act profibrotically (14–16). The activation of TGF-β may occur through binding of the RGD motif of the latency-associated peptide (LAP) (13), whereas integrin αVβ8 may also activate latent TGF-β by membrane-type 1-matrix metalloproteinase (MMP)-dependent degradation of LAP (16). Expression of integrins αVβ3 (17) or αVβ5 (18,19) on scleroderma but not normal fibroblasts has been recently implicated as a contributing factor maintaining the profibrotic autocrine TGF-beta signaling loop in scleroderma.
We considered a possibility that pulmonary infiltrating T lymphocytes may express integrins in association with fibrosis (7), and that such expression may be a driving force contributing to the prolonged T lymphocytic infiltration (3) and/or connective tissue accumulation in the lungs. We previously reported that the expression of integrin αVβ6 heterodimer on the surface of pulmonary T cells is minimally increased in patients with scleroderma (7). Therefore, this study focused on the potential pathophysiological role of T cells that express other profibrotic integrins, αVβ3 and αVβ5. Based on observations in patients with diffuse parenchymal lung disease, in an animal model of pulmonary fibrosis, and in in vitro experiments, we report here that expression of integrins αVβ3 and/or αVβ5 on pulmonary T lymphocytes may regulate the extent of lymphocytic infiltration and the degree of pulmonary fibrosis, whereas T lymphocytes that do not express integrins may be not involved in the fibrotic regulation process.
PATIENTS, MATERIALS, AND METHODS
Patients and Patient Samples
Patients with systemic sclerosis (SSc) or idiopathic pulmonary fibrosis (IPF) were recruited from the University of Maryland Medical Center, the Baltimore VA Medical Center, and the Johns Hopkins School of Medicine. The study has been approved by the Institutional Review Boards at the University of Maryland and at the Johns Hopkins University.
Explant lungs were obtained from a SSc patient and two IPF patient during transplantation procedure. The explant lungs were immediately delivered to the Department of Pathology, sectioned, and 1 × 1 ×1 cm cubes excised for histological and immunohistochemical analyses from each lobe. Also, two explant lung from separate normal donors that were initially planned for but ultimately not used for transplantation have been sectioned to obtain tissue samples in a similar fashion. Additional five sections of lung tissue from SSc patients were kindly provided by Dr. Elena Tourkina (Medical University of South Carolina).
The BAL procedures with differential cell counts in 25 SSc patients and five healthy volunteers were done as previously described (6). The BAL samples were immediately delivered on ice to the research laboratory and processed. Where indicated, CD3+ T cells were purified from BAL samples using positive selection with non-activating magnetic beads as previously described (7). Flow cytometry assays confirmed ≥ 93% purity of these cells; the remaining cells were mostly alveolar macrophages.
Murine In Vivo CCL18 Overexpression Model
Wild-type C57BL/6 mice or integrin β3-deficient mice (Jackson Laboratory, Bar Harbor, ME) mice were infected intratracheally with replication-deficient adenoviral constructs that either did or did not (as a control) encode CCL18 as previously described (3,4). The mice were thus challenged with pulmonary overexpression of CCL18 for 14 days to attract T cells to the lungs (3,4). Expression of CCL18 was confirmed by ELISA of BAL fluids and lung homogenates. Where indicated, mice were treated daily from day 3 to day 13 with intraperitoneal injections of neutralizing antibody against integrin αV (BD Pharmingen, San Diego, CA) at 30 μg per injection. Control CCL18 overexpressing mice were similarly treated with isotype-matching control immunoglobulin. Procedures for obtaining mouse lung tissue samples and BAL were as previously described (3,4).
Primary Pulmonary Fibroblast Cultures and Jurkat T cells
Normal human primary pulmonary fibroblast cultures derived from adult donors were purchased from Cambrex (Walkersville, MD). Fibroblast cultures were maintained in T75 culture flasks in humidified atmosphere of 5% CO2 at 37° C in high serum tissue culture medium, which was DMEM supplemented with 2 mM glutamine, 2 mM sodium pyruvate, 50 mg/liter gentamicin, and 10% bovine calf serum (all from Invitrogen, Carlsbad, CA). Cell cultures used in experiments were preincubated for 24 h in similar conditions, using low-serum (0.5% dialyzed bovine calf serum with no TGF-β detectable by ELISA) medium supplemented with 0.28 mM ascorbic acid and 0.2 mM β-aminopropionitrile (Sigma, St. Louis, MO) in addition to the mentioned reagents. The cell culture medium for all experiments was the same low-serum medium. In all experiments, fibroblast cell lines were tested in passages three to seven. For the experiments, fibroblasts were plated at 3.5 × 105 cells/well in 6-well plates (Costar), incubated overnight in 3 ml/well of high serum medium, and then for 24 h in low serum medium.
Jurkat T cell line (ATCC, Manassas, VA) was cultured as recommended by the supplier and co-transfected with human ITGAV- and ITGB3-encoding plasmids, or human ITGAV- and ITGB5-encoding plasmids (Origene, Rockville, MD), or transfected with control non-coding plasmid utilizing Amaxa electroporation. In the co-culture experiments, 2 × 106 transfected T cells/well were co-cultured with primary fibroblasts in low serum medium for 48 hrs.
Histological and Immunohistochemical Analyses of human and mouse lung tissues were performed as previously described (3,4). Sections (5µm) of the explant lung were paraffin-embedded, and stained with H&E or with Masson-Trichrome to reveal collagen. Antibodies for human integrins αV and β3 were from Santa Cruz Biotechnology (Santa Cruz, CA), and for human αVβ5 heterodimers were from Chemicon (Temecula, CA) (quality anti-human antibodies for immunohistochemical analyses of integrin αVβ3 heterodimer expression were not commercially available). Antibodies for mouse integrins αV, β3, and β5 were from Santa Cruz Biotechnology (anti-mouse antibodies for immunohistochemical analyses of integrin αVβ3 or αVβ5 heterodimers were not commercially available).
Flow Cytometric Analyses
For flow cytometric analyses, BAL cells from humans or mice were obtained as described above and stained with fluorescence-labeled antibodies or corresponding isotype control antibodies (BD PharMingen, San Diego, CA). Cellular fluorescence was measured with a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Expression of human integrin αVβ3 (ITGAVB3), αVβ5 (ITGAVB5), or αVβ6 (ITGAVB6) heterodimers (US Bioilogical, MA) were measured on BAL CD3+CD4+ or CD3+CD8+ cells from individual patients (with no sample pooling). BAL samples from patients and controls were also characterized flowcytometrically for expression of CD45RA, CD45RO, CD69, CD25, and HLA DR on CD3+CD4+ or CD3+CD8+ cells. Many of the commercially available antibodies tested in our laboratory for mouse ITGAV-containing integrin heterodimers did not allow for high quality specific resolution of expressing cells in flow cytometry assays (not shown). Therefore, expression of monomer integrins αV (ITGAV), β3 (ITGB3), or β5 (ITGB5) was measured on pulmonary CD3+ lymphocytes from BAL of individual (no sample pooling) control or CCL18 overexpressing mice, using antibodies from Santa Cruz Biotechnology (Santa Cruz, CA).
Collagen Assays
Collagen levels were measured in cell culture supernates using Western blotting or 14C-proline incorporation assays, as previously described (3,4,9–11). Equal numbers of fibroblasts in all cultures in each experiment were confirmed with CellTiter 96 Aqueous assays (Promega, Madison, WI).
Nuclear Translocation of Smad2
In some experiments, fibroblasts were transfected with a Smad2-GFP construct (a kind gift of Dr. Andrew Chantry, University of East Anglia, UK) using electroporation technique (Amaxa, Gaithersburg, MD). The intracellular localization of the construct was assessed by confocal or fluorescent microscopy. Nuclear and cytoplasmic fractions of fibroblasts were prepared as previously described (10), and total Smad2/3 protein was evaluated by Western blotting using an antibody from Cell Signaling Technology (Danvers, MA).
Statistical Analyses
Data were processed used Statistica software (StatSoft, Tulsa, OK). Groups were compared using two-tailed Student's t-test, Mann-Whitney U-test, or multivariate ANOVA analyses as indicated in the text. Categorical measures were compared using chi-square statistics. Pearson correlation analyses were used to examine pair-wise associations between variables. Differences with P ≤ 0.05 were considered significant in all analyses.
RESULTS
Expression of Integrins on Pulmonary T lymphocytes
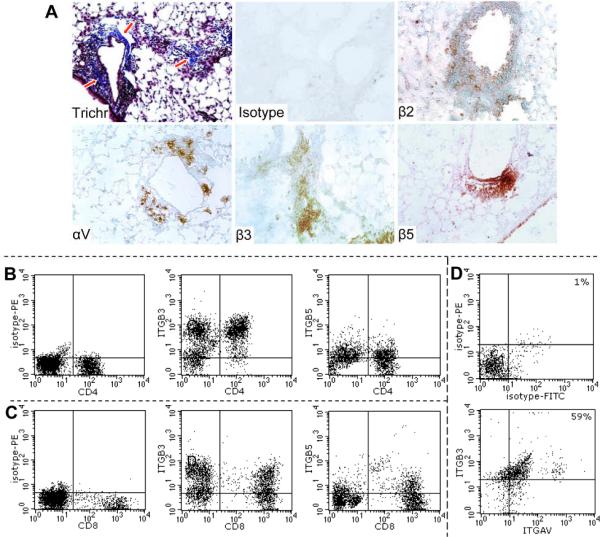
To begin addressing the issue of possible contribution of integrin-expressing T cells in the lungs of patients with interstitial lung disease, initial immunohistochemical experiments were performed. Histological examination of the healthy lungs reveals rare if any T lymphocytes present in the interstitium or alveolar space, whereas numerous cells positive for CD3, CD4, and CD8 were present in the lungs of SSc (Figure 1A) and IPF (Supplemental Figure 1) patients. Also, cells positive for integrins αV or β3, as well as cells positive for integrin αVβ5 heterodimers, were present in the lungs of six SSc and two IPF patients (Figure 1, anti-human antibodies suitable for immunohistochemistry against αVβ3 heterodimers are not commercially available). No staining was observed with the matching isotype control antibodies; rare T cells (maximum 1–2 per microscopic field) were observed in the healthy lungs. These observations suggest that T cells and integrin-expressing cells are present in the lungs of patients with interstitial lung diseases. Confocal microscopy of the SSc lung sections revealed co-expression of CD3 and integrin β3 on numerous cells (Figure 1B).
Figure 1.
Histochemical and immunohistochemical analyses of paraffin sections of a healthy lung (Ctrl) and of an explant lung from a patient with scleroderma lung disease (SSc). A. The sections were stained with hematoxylin and eosin (H&E), trichrome (Trichr), or immunohistochemically (brown color) for CD3, CD4, CD8, integrins αV, β3, and αVβ5 heterodimer as indicated. Notice profound inflammatory infiltration in association with deposition of extracellular matrix, presence of T lymphocytes, and of the cells expressing integrins. Magnification ×200 in all panels. Randomly selected panels are shown. B. Confocal microscopy of SSc lung sections stained for CD3 (FITC conjugated), integrin β3 (TRITC conjugated), and merged images at indicated magnifications.
The subsequent experiments were performed to evaluate whether integrins are indeed expressed on the surface of pulmonary lymphocytes as suggested by our previous analyses of mRNA expression in these cells (7). Flow cytometry assays of BAL cells from 25 scleroderma patients and five healthy non-smoking control volunteers were performed. Characteristic of these patients and controls are shown in Supplemental Table 1.
BAL cells were stained for integrin αVβ3, αVβ5, or αVβ6 heterodimers, as well as for markers of T cells (CD3, CD4, CD8). There was no expression of these integrins on T cells from healthy controls (Figure 2A,B). Integrins αVβ3 and αVβ5, but not αVβ6, were expressed on as many as 50% of T cells from the lungs of scleroderma patients (Figure 2A,B). In 14 of 25 scleroderma patients, more than 10% of BAL T cells were positive for integrins αVβ3 or αVβ5 (Supplemental Figure 2). Similar flow cytometry assays revealed that integrins αVβ3 and αVβ5 were not expressed on blood T cells from healthy controls or scleroderma patients. Regression analyses of the percent positive BAL CD4+αVβ3+, CD4+αVβ5+, CD8+αVβ3+, and CD8+αVβ5+ cells showed strong pair-wise positive correlation (P < 0.01 for each regression; r2 values indicated in Supplemental Figure 2), but no correlation with CD4+ or CD8+ cells positive for HLA-DR, CD25, CD45RA, CD45RO, or CD69 (P > 0.05; r2 not exceeding 0.08 in any case). There was no correlation between percent integrin-positive BAL T cells and percent macrophages, lymphocytes, neutrophils, or eosinophils in BAL (P > 0.05; r2 not exceeding 0.15 in any case). There was no correlation with percent predicted forced vital capacity (P > 0.05; r2 not exceeding 0.01 in any case) or percent predicted diffusing capacity for carbon monoxide (P > 0.05; r2 not exceeding 0.04 in any case). ANOVA analyses showed that patients' groups did not differ (P > 0.05) in percent integrin-positive BAL T cells depending on their gender, race, age, or combinations of these characteristics.
Figure 2.
Expression of integrins in pulmonary T lymphocytes. A,B, Flow cytometry for integrin αVβ3 and αVβ5 heterodimers on BAL T cells (gated on CD3+ cells) from an SSc patient (red) and a healthy volunteer (blue). Staining with isotype control antibody is shown in black. C,D, RT-Q-PCR of RNA from BAL T cells of two SSc patients (red) and two healthy volunteers (blue), using specific primers for 18S rRNA (reference RNA), or for integrin β3 mRNA (C) or integrin β5 mRNA (D). Negative controls with the primers for corresponding integrins are shown in black. Close overlap of 18S rRNA amplification curves indicates equal total RNA concentration in all samples. Amplification of integrin mRNAs occurred earlier in the samples from SSc patients than in the samples from healthy volunteers, suggesting higher steady-state levels of mRNA.
To validate selected results of flow cytometry experiments described above, reverse-transcriptase real-time polymerase chain reaction assays for integrin β3 mRNA were performed, using mRNA purified from BAL T cells that were enriched by positive selection with non-activating magnetic beads as previously described (7). The levels of integrin β3 mRNA were significantly higher in BAL T cells of SSc patients that in healthy volunteers (Figure 2C).
Thus, pulmonary T lymphocytes express integrins αVβ3 and αVβ5 in patients with systemic sclerosis but not in healthy individuals.
BAL T cells Express Integrins in the Pulmonary CCL18 Overexpression Animal Model
We then considered that pulmonary levels of a T cell-selective chemokine CCL18, as well as pulmonary levels of T cells, are significantly elevated in the lungs of patients with SSc and other pulmonary fibrotic diseases (reviewed in 2). We have recently introduced a new animal model based on adenoviral delivery of CCL18 gene to the lungs in vivo (3,4). The overexpression of CCL18 in vivo manifests in pulmonary T lymphocytic infiltration and T cell-dependent activation of TGF-β and accumulation of collagen (2–4). This model is more suitable for the current study than a more traditional bleomycin model of pulmonary fibrosis, because the bleomycin model is known to be T cell-independent (2,20,21). Similarly to humans with interstitial lung disease (see Figure 1,2), the infiltrating cells in the CCL18 overexpression model expressed integrins αV, β3, and β5 (Figure 3A). Stainings were performed for individual integrin chains because quality antibodies against mouse integrins αVβ3 or αVβ5 were not commercially available. To confirm that such expression occurs specifically on T cells, flow cytometry assays of BAL cells were performed (Figure 3B–D). Again, commercially available antibodies for mouse αV-containing integrin heterodimers did not allow for high quality specific resolution of expressing cells in flow cytometry assays, therefore staining was performed for individual integrin chains.
Figure 3.
Lung histology and T cell flow cytometry in the CCL18 overexpression model. A, Trichrome staining of lung sections (×200) showed infiltration (nuclei stain in purple) and collagen fibers (blue, indicated by red arrows) in response to CCL18 overexpression. Immunohistocologically (brown staining), isotype control antibody did not stain tissue sections, whereas infiltrates that consist predominantly of T cell stained for integrins β2 (used as positive control), αV, β3, and β5. Minimal to no changes were observed in control mice infected with a similar non-coding (NULL) adenovirus virus. B,C, Isotype control antibodies for integrins β3, β5, or αV do not stain pulmonary T cells, whereas CD4+ and CD8+ T cells stain for integrins β3 (ITGB3) and β5 (ITGB5) as indicated. D, Integrins β3 and αV are co-expressed on pulmonary T cells. The gating was on CD3+ (CyChrome-labeled antibody) cells; percent of double-positive cells indicated in the upper right quadrants. Most of the T cells in the CCL18-overexpressing mice are double-positive, suggesting that both integrin αV and integrin β3 are co-expressed on the same T cells, consistent with the notion that these chains may form heterodimers on T cells.
Thus, pulmonary T lymphocytes express integrins not only in patients with interstitial lung disease but also in the animal model of CCL18-mediated pulmonary T lymphocytic infiltration and fibrosis.
Expression of Integrins is Required for Lymphocytic Infiltration and Collagen Accumulation
To determine whether integrin expression may be a determining factor in T lymphocytic infiltration and collagen accumulation in the lungs, two types of experiments have been conducted. First, CCL18 overexpressing mice were treated systemically with integrin αV-blocking antibody. Such treatment significantly attenuated lymphocytic infiltration and collagen accumulation compared to CCL18 overexpressing mice treated with isotype control antibody (Figure 4). Second, adenovirus-mediated CCL18 overexpression was performed in integrin β3 deficient mice. In contrast to wild-type mice, the ITGB3 −/− had minimal, if any, accumulation of lymphocytes or collagen in their lungs (Figure 4). Thus, the expression of integrins is necessary for T cell infiltration into the lungs and associated collagen accumulation in CCL18 overexpressing mice.
Figure 4.
Effects of treatment with neutralizing anti-integrin αV (ITGAV) antibody or of integrin β3 (ITGB3) deficiency on the lungs of CCL18 overexpressing mice. A, Trichrome stain of histological sections reveals collagen in blue. Wild type or ITGB3-knockout mice challenged with AdV-NULL virus were indistinguishable from healthy control mice (Ctrl). Overexpression of CCL18 in WT mice (WT) caused lymphocytic infiltration and collagen accumulation (arrows). ITGB3 KO mice overexpressing CCL18 (KO) showed minimal, if any, cellular infiltration or collagen accumulation. WT mice overexpressing CCL18 and treated with the anti-ITGAV neutralizing antibodies (Ab) had significantly attenuated degree of infiltration and collagen accumulation (arrowhead). B, Percent lymphocytes in the BAL of WT or ITGB3 KO mice instilled with the NULL non-coding adenovirus or the CCL18-encoding virus, and treated with anti-ITGAV antibody or corresponding isotype Ig control as indicated. The majority of other BAL cells were macrophages, with occasional neutrophils (not exceeding 1.5%) or bronchial epithelial cells (not exceeding 3%). C, Total lung collagen (measured as hydroxyproline content) in mice treated as described above. All experiments shown in this figure were repeated on two independent occasions, with consistent result, 5–6 animals per group each time.
Effects of Integrin αVβ3- or αVβ3-Overexspressing Jurkat Cells on Primary Fibroblast Cultures
To directly establish whether T cells expressing integrin αVβ3 or integrin αVβ5 may act profibrotically, human integrin chains αV together with β3 or integrin chains αV together with β5 were overexpressed in a T cell line (Jurkat). Flow cytometry assays for αVβ3 or αVβ5 heterodimers confirmed the overexpression (Figure 5A,B). Such an approach was used because the absolute amounts of primary pulmonary T cells derived from the patients' BAL samples are usually limited (6–8). Obtaining necessary amounts of integrins-expressing primary T cell for cell culture assays would be technically challenging. Pulmonary fibroblasts were cultured alone (Fib), with integrin-encoding constructs-transfected Jurkat cells (Fib+Itg), or with Jurkat cells transfected with the equivalent amounts of the blank non-coding plasmids (Fib+Blank) (Figure 5C,D). Cell viability assays revealed that such transfections did not cause a cytotoxic effect on Jurkat cells. Western blotting assays of fibroblast culture supernates for collagen type I revealed that integrin-expressing but not mock-transfected T lymphocytes co-cultured with fibroblasts stimulated an increase in collagen levels (Figure 5C,D).
Figure 5.
Overexpression of integrins αVβ3 or αVβ5 on Jurkat cells makes them profibrotic. A,B, Flow cytometry with the antibodies against indicated heterodimers after transfection with plasmids encoding the indicated integrin chains (black line) or after transfection with non-coding plasmid (grey filled histogram). C,D, Western blotting for collagen type I of fibroblast cultures (Fib), or co-cultures with Jurkat cells transfected with the non-coding plasmid (Fib+blank) or transfected to express the indicated integrins (in duplicate in for each heterodimer). The bars in the densitograms match the individual Western blotting bands shown in the gels below. E, Metabolic incorporation of 14C-proline as a surrogate measure of new collagen synthesis, CPM ± SD of triplicate cultures. Fibroblasts were cultured in the medium without (Med) or with anti-TGF-β monoclonal antibody 1D11 as indicated. Alternatively, fibroblasts were co-cultured with Jurkat T cells transfected to overexpress integrin αVβ5, without (αVβ5) or with anti-TGF-β antibody (αVβ5+1D11). All experiments in this figure were confirmed on at least three independent occasions for each integrins αVβ3 or αVβ5, with consistent results.
The Effect of Integrin-Expressing T cells on Collagen Production in Cell Cultures is Mediated by TGF-β
The upregulation of collagen in the co-cultures of integrin-transfected Jurkat cells and primary fibroblasts (Figure 5C,D) was confirmed in 14C-proline metabolic incorporation assays reflective of new collagen production rates by fibroblasts (Figure 5E). Jurkat cells were transfected to express integrin αVβ5 and stimulated collagen upregulation in co-cultures with primary fibroblasts (Figure 5E). Importantly, anti-TGF-β blocking antibody (clone 1D11) at the recommended dose of 1 μg/ml had minimal effect on collagen production in fibroblast cultures alone, but significantly attenuated the upregulated collagen levels in co-cultures with Jurkat cells transfected to overexpress integrin αVβ5 (Figure 5E). This observation suggested that the effect of integrin-expressing T cells on collagen production in fibroblasts is TGF-β-dependent. However, ELISA assays revealed no increase in total TGF-β and no detectable freely diffusible active TGF-β in these co-cultures (not shown). The latter observation is consistent with the notion that integrin-mediated activation of TGF-β is a surface-associated event (13) and that activated TGF-β may be cell surface-bound or become internalized by fibroblasts. Therefore, the experiments aimed to determine whether intracellular signaling characteristic of TGF-β may be activated in fibroblasts in co-cultures with integrin-expressing Jurkat T cells. Primary adult lung fibroblasts were transfected with functional Smad2-GFP construct (22) and co-cultured for 48 hrs with Jurkat cells overexpressing integrins αVβ3 or αVβ5 (Figure 6A,B; confocal microscopy was used to definitively confirm nuclear translocation of Smad2, whereas fluorescent microscopy provided comparable quality of resolution and was used for quantitative purposes). In parallel, non-transfected primary pulmonary fibroblasts were similarly co-cultured with Jurkat cells overexpressing these integrins, and the relative levels of Smad2/3 in the nucleus and cytoplasm were assessed by Western blotting (Figure 6C). Increased nuclear accumulation of Smad2 suggests that TGF-β is indeed involved in such regulation (Figure 6). Additionally, expression of α-smooth muscle actin was increased in fibroblasts co-cultured with integrin-overexpressing Jurkat cells, indicative of fibroblast activation (Supplemental Figure 3).
Figure 6.

Nuclear translocation of Smad2 in primary adult lung fibroblasts co-cultured with Jurkat cells overexpessing integrins αVβ3 or αVβ5, or transfected with blank non-coding plasmid. A, Confocal microscopy of primary fibroblasts transfected with Smad2-GFP construct and co-cultured with Jurkat cells. Fibroblasts co-cultured with the blank plasmid-transfected Jurkat cells (upper row) remained quiescent, not distinguishable from fibroblasts cultured alone, and showed Smad2 either lacking in the nuclei (white arrows in the upper row) or evenly distributed between the nucleus and cytoplasm. In contrast, fibroblasts co-cultured with Jurkat cells that overexpressed integrins αVβ3 or αVβ5 showed numerous nuclei with predominant accumulation of Smad2 (yellow arrows in the lower row). B, Numerically, fibroblasts co-cultured with integrin-overexpressing Jurkat cells had significantly increased numbers of Smad2-positive nuclei (indicated with asterisks in the bar-graph on the right side, mean ± SD, p ≤ 0.05, repeated on two independent occasions, 100 randomly selected cells counted in duplicate for each condition). C, Western blotting for total Smad2/3, β-actin, or histone H1 (indicated on the left) in the nuclear (N) and cytoplasmic (C) fractions (indicated at the bottom) of fibroblasts stimulated with rhTGF-β (positive control) or co-cultured with primary Jurkat cells transfected with the constructs indicated on top. Repeated on two separate occasions, with consistent results.
DISCUSSION
We report in this work that pulmonary T lymphocytes express integrins αVβ3 and αVβ5 in humans with interstitial lung disease, particularly in scleroderma (see Figures 1,2), and in the animal model of CCL18-mediated infiltration and T cell-dependent collagen accumulation (see Figures 3,4). Although the expression of integrins αVβ3 and αVβ5 on subpopulations of T cells has been discovered more than a decade ago (23–26), its role in the disease processes, particularly in regulating inflammation and the balance of connective tissue, remains unclear. The results of our study suggest that expression of these integrins on pulmonary lymphocytes may explain their previously described persistent infiltration in the lungs (3). We report that antibody-mediated neutralization or genetic deficiency of these integrins abrogates lymphocytic infiltration and related collagen accumulation in the lungs (see Figure 4). In vitro experiments revealed that T cells overexpressing these integrins are directly profibrotic on cultured primary human pulmonary fibroblasts, likely through a TGF-β-dependent mechanism (see Figures 5,6). Consistent with such notion, pulmonary infiltration of T cells in the CCL18 overexpression model is associated with local TGF-β activation and collagen accumulation (3). A possibility was considered that the mechanism of TGF-β activation and collagen accumulation may be due to the regulatory nature (Treg) of the infiltrating T cells. However, immunohistochemical analyses and flow cytometry assays in the CCL18 overexpression model revealed that FoxP3+ cells were present in the infiltrates and BAL, but they were rare (7 ± 1% of BAL T cells by flow cytometry) and not different percent-wise between CCL18 overexpression alone or in combination with bleomycin models (not shown). Thus, the regulation of fibrosis by T cells likely occurs in no apparent association with FoxP3 expression.
These results suggest that although FoxP3-negative (not a classical Treg phenotype) and not actively producing TGF-β (not a Th3 phenotype), pulmonary T cells that express integrins may act by activating latent TGF-β. Based on this observation, such cells perhaps may be broadly classified as regulatory T cells, with an implied antiinflammatory effect. Overall, the effects of T lymphocytic infiltration in the lungs are likely diverse and depend on the specific nature of local inflammatory milieu as well as on the specific T cell phenotype. Data in humans and in animal models suggest that pulmonary T cells may facilitate accumulation of collagen, attenuate pulmonary fibrosis, or be non-participating bystanders in the fibrotic process (2). Our data suggest that in patients with diffuse parenchymal lung disease, particularly in scleroderma patients, T lymphocytes may accumulate in the lungs in a futile attempt to attenuate autoimmune process and inflammation. They express integrins αVβ3 and αVβ5 to ensure adhesion, persistent presence in the lungs, and TGF-β activation. The latter factor, a potent immunosuppressive and antiinflammatory regulator, is also the most potent profibrotic cytokine. As a result, integrin-expressing T cells likely exert a profibrotic effect on the lung. One implication of such logic is that systemic or local pulmonary depletion of T cells may have a therapeutic antifibrotic effect, yet a side effect of such hypothetical therapy may be undesired attenuation of T cells' antiinflammatory action.
In conclusion, pulmonary infiltrating T lymphocytes may express integrins αVβ3 and αVβ5. Such expression likely explains pulmonary lymphocytic infiltration, persistence of T cells in the lungs, and T cell-associated TGF-β activation and collagen accumulation.
Supplementary Material
Acknowledgments
Supported by NIH R01HL054163, R03AR054946 and Arthritis Foundation, Maryland Chapter
REFERENCES
- 1.Schwarz MI, King TE., Jr. Interstitial lung diseases. 4th ed. B.C. Decker; Hamilton, ON, Canada: 2003. [Google Scholar]
- 2.Luzina IG, Todd NW, Iacono AT, Atamas SP. Roles of T lymphocytes in pulmonary fibrosis. J Leukoc Biol. 2008;83(2):237–44. doi: 10.1189/jlb.0707504. [DOI] [PubMed] [Google Scholar]
- 3.Luzina IG, Papadimitriou JC, Anderson R, Pochetuhen K, Atamas SP. Induction of prolonged infiltration of T lymphocytes and transient T lymphocyte-dependent collagen deposition in mouse lungs following adenoviral gene transfer of CCL18. Arthritis Rheum. 2006;54(8):2643–55. doi: 10.1002/art.21950. [DOI] [PubMed] [Google Scholar]
- 4.Pochetuhen K, Luzina IG, Lockatell V, Choi J, Todd NW, Atamas SP. Complex regulation of pulmonary inflammation and fibrosis by CCL18. Am J Pathol. 2007;171(2):428–37. doi: 10.2353/ajpath.2007.061167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atamas SP, Luzina IG, Dai H, Wilt SG, White B. Synergy between CD40 ligation and IL-4 on fibroblast proliferation involves IL-4 receptor signaling. J Immunol. 2002;168(3):1139–45. doi: 10.4049/jimmunol.168.3.1139. [DOI] [PubMed] [Google Scholar]
- 6.Atamas SP, Yurovsky VV, Wise R, Wigley FM, Goter Robinson CJ, Henry P, Alms WJ, White B. Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum. 1999;42(6):1168–78. doi: 10.1002/1529-0131(199906)42:6<1168::AID-ANR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Luzina IG, Atamas SP, Wise R, Wigley FM, Choi J, Xiao HQ, White B. Occurrence of an activated, profibrotic pattern of gene expression in lung CD8+ T cells from scleroderma patients. Arthritis Rheum. 2003;48(8):2262–74. doi: 10.1002/art.11080. [DOI] [PubMed] [Google Scholar]
- 8.Luzina IG, Atamas SP, Wise R, Wigley FM, Xiao HQ, White B. Gene expression in bronchoalveolar lavage cells from scleroderma patients. Am J Respir Cell Mol Biol. 2002;26(5):549–57. doi: 10.1165/ajrcmb.26.5.4683. [DOI] [PubMed] [Google Scholar]
- 9.Atamas SP, Luzina IG, Choi J, Tsymbalyuk N, Carbonetti NH, Singh IS, Trojanowska M, Jimenez SA, White B. Pulmonary and activation-regulated chemokine stimulates collagen production in lung fibroblasts. Am J Respir Cell Mol Biol. 2003;29(6):743–9. doi: 10.1165/rcmb.2003-0078OC. [DOI] [PubMed] [Google Scholar]
- 10.Luzina IG, Tsymbalyuk N, Choi J, Hasday JD, Atamas SP. CCL18-stimulated upregulation of collagen production in lung fibroblasts requires Sp1 signaling and basal Smad3 activity. J Cell Physiol. 2006;206(1):221–8. doi: 10.1002/jcp.20452. [DOI] [PubMed] [Google Scholar]
- 11.Luzina IG, Highsmith K, Pochetuhen K, Nacu N, Rao JN, Atamas SP. PKCalpha mediates CCL18-stimulated collagen production in pulmonary fibroblasts. Am J Respir Cell Mol Biol. 2006;35(3):298–305. doi: 10.1165/rcmb.2006-0033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 13.Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev. 2005;24(3):395–402. doi: 10.1007/s10555-005-5131-6. [DOI] [PubMed] [Google Scholar]
- 14.Munger JS, Harpel JG, Giancotti FG, Rifkin DB. Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphavbeta1. Mol Biol Cell. 1998;9(9):2627–38. doi: 10.1091/mbc.9.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludbrook SB, Barry ST, Delves CJ, Horgan CM. The integrin alphavbeta3 is a receptor for the latency-associated peptides of transforming growth factors beta1 and beta3. Biochem J. 2003;369(Pt 2):311–8. doi: 10.1042/BJ20020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002 Apr 29;157(3):493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol. 2005;175(11):7708–18. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- 18.Asano Y, Ihn H, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin in the establishment of autocrine TGF-beta signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol. 2006;126(8):1761–9. doi: 10.1038/sj.jid.5700331. [DOI] [PubMed] [Google Scholar]
- 19.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin-mediated activation of latent transforming growth factor beta1 in autocrine transforming growth factor beta signaling in systemic sclerosis fibroblasts. Arthritis Rheum. 2005;52(9):2897–905. doi: 10.1002/art.21246. [DOI] [PubMed] [Google Scholar]
- 20.Helene M, Lake-Bullock V, Zhu J, Hao H, Cohen DA, Kaplan AM. T cell independence of bleomycin-induced pulmonary fibrosis. J Leukoc Biol. 1999;65:187–95. doi: 10.1002/jlb.65.2.187. [DOI] [PubMed] [Google Scholar]
- 21.Janick-Buckner D, Ranges GE, Hacker MP. Effect of cytotoxic monoclonal antibody depletion of T-lymphocyte subpopulations on bleomycin-induced lung damage in C57BL/6J mice. Toxicol Appl Pharmacol. 1989;100:474–84. doi: 10.1016/0041-008x(89)90295-0. [DOI] [PubMed] [Google Scholar]
- 22.Wicks SJ, Lui S, Abdel-Wahab N, Mason RM, Chantry A. Inactivation of smad-transforming growth factor beta signaling by Ca(2+)-calmodulin-dependent protein kinase II. Mol Cell Biol. 2000;20:8103–11. doi: 10.1128/mcb.20.21.8103-8111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber DJ, Pereira P, Huang SY, Pelletier C, Tonegawa S. Expression of alpha v and beta 3 in-tegrin chains on murine lymphocytes. Proc Natl Acad Sci USA. 1996;93:14698–703. doi: 10.1073/pnas.93.25.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halvorson MJ, Coligan JE, Sturmhofel K. The vitronectin receptor (alpha V beta 3) as an example for the role of integrins in T lymphocyte stimulation. Immunol Res. 1996;15:16–29. doi: 10.1007/BF02918281. [DOI] [PubMed] [Google Scholar]
- 25.Brando C, Shevach EM. Engagement of the vitronectin receptor (alpha V beta 3) on murine T cells stimulates tyrosine phosphorylation of a 115-kDa protein. J Immunol. 1995;154:2005–11. [PubMed] [Google Scholar]
- 26.Huang S, Endo RI, Nemerow GR. Upregulation of integrins alpha v beta 3 and alpha v beta 5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–63. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






