Abstract
The transmembrane protein ferroportin (Fpn) is essential for iron efflux from the liver, spleen, and duodenum. Fpn is regulated predominantly by the circulating iron regulatory hormone hepcidin, which binds to cell surface Fpn, initiating its degradation. Accordingly, when hepcidin concentrations decrease, Fpn levels increase. A previous study found that Fpn levels were not elevated in copper-deficient (CuD) mice that had anemia, a condition normally associated with dramatic reductions in hepcidin. Lack of change in Fpn levels may be because CuD mice do not display reduced concentrations of plasma iron (holotransferrin), a modulator of hepcidin expression. Here, we examined Fpn protein levels and hepcidin expression in CuD rats, which exhibit reduced plasma iron concentrations along with anemia. We also examined hepcidin expression in anemic CuD mice with normal plasma iron levels. We found that CuD rats had higher liver and spleen Fpn levels and markedly lower hepatic hepcidin mRNA expression than did copper-adequate (CuA) rats. In contrast, hepcidin levels did not differ between CuD and CuA mice. To examine potential mediators of the reduced hepcidin expression in CuD rats, we measured levels of hepatic transferrin receptor 2 (TfR2), a putative iron sensor that links holotransferrin to hepcidin production, and transcript abundance of bone morphogenic protein 6 (BMP6), a key endogenous positive regulator of hepcidin production. Diminished hepcidin expression in CuD rats was associated with lower levels of TfR2, but not BMP6. Our data suggest that holotransferrin and TfR2, rather than anemia or BMP6, are signals for hepcidin synthesis during copper deficiency.
Introduction
Anemia and hepatic iron accumulation are well-known consequences of nutritional copper deficiency in humans and laboratory animals (1–3). The perturbations in iron metabolism arise because metabolic pathways involving the 2 metals are linked at least in part through the copper-containing proteins ceruloplasmin (Cp)6 and hephaestin (3). These proteins appear to aid in the release of iron into the plasma by working in concert with ferroportin (Fpn), the cell surface iron export protein. Fpn is expressed predominantly in reticuloendothelial macrophages of the liver and spleen and duodenal enterocytes (4,5). After Fpn exports iron, the Fe2+ must be oxidized to Fe3+ before it can bind to its transport protein transferrin. It is widely thought that circulating Cp, a ferroxidase, serves this function. A reduction in the activity of Cp, which carries 70–95% of the copper in the plasma, is one of the earliest manifestations of copper deficiency (6). Hephaestin, a transmembrane homolog of Cp, colocalizes with Fpn on the basolateral membrane of enterocytes (7), where it aids in the absorption of dietary iron (8). Studies show that copper-deficient rodents have reduced levels of duodenal hephaestin and impaired iron absorption (9,10). Taken together, the diminished activities of Cp and hephaestin in copper deficiency would seem to decrease the amount of Fe3+ released into the plasma from the liver, spleen, and duodenum. The resultant low plasma iron level would limit the adequate iron supply to the bone marrow, leading to anemia.
Cellular Fpn levels are regulated by iron through transcriptional and post-transcriptional events. Iron loading, for example, increases the levels of Fpn mRNA and heterogeneous nuclear RNA, consistent with increased transcription (11). Post-transcriptional regulation of Fpn is likely conferred by an iron-response element (IRE) located in the 5′ untranslated region of Fpn mRNA. Translational control of iron-related proteins by IRE and iron-regulatory proteins (IRP) is well defined (12). Under low iron conditions, IRP bind to the 5′ IRE, blocking mRNA translation. Iron loading promotes the degradation of IRP or their disassociation from the IRE, thus allowing for translation. Translational control of Fpn by iron is supported by studies using luciferase reporter gene constructs (13) and cell culture models of iron loading (14). Fpn at the cell surface is subject to an additional level of control through the circulating iron-regulatory hormone hepcidin. When hepcidin binds to Fpn at the plasma membrane, the hepcidin-Fpn complex is rapidly internalized and degraded within lysosomes (15). Hepcidin rapidly decreases cellular Fpn levels, even under conditions of iron loading, indicating that hepcidin is a more dominant regulator of Fpn than is iron.
Hepcidin production responds to a variety of stimuli, being upregulated by iron loading and inflammatory cytokines and downregulated in response to anemia, increased erythropoietic drive, and hypoxia (16). The induction of hepcidin involves a number of proteins, including Hfe, hemojuvelin, transferrin receptor 2 (TfR2), and bone morphogenic protein 6 (BMP6) (16). Among these proteins, TfR2 is thought to play a unique role by serving as a body iron sensor, relaying information from plasma iron (holotransferrin) to hepcidin synthesis (17). Recently, BMP6 has emerged as another key endogenous regulator of hepcidin production. Mice that lack BMP6 display reduced hepcidin expression and iron overload (18,19). Similar to TfR2, levels of BMP6 have been shown to be responsive to iron status, suggesting that BMP6 may also play a role in monitoring body iron requirements (20).
Because they are anemic yet display hepatic iron loading, copper-deficient animals provide a unique experimental model to interrogate the mediators of hepcidin as well as Fpn expression. Chung et al. (21) studied copper-deficient mice and found that hepatic Fpn protein levels were not higher despite anemia. However, hepcidin expression was not measured. Recently, Pyatskowit and Prohaska (22) reported that copper-deficient mice, although they are anemic, do not have low plasma iron concentrations. In contrast, copper-deficient rats on the same dietary treatment exhibit anemia and low plasma iron levels. Because a growing number of studies suggest that plasma iron is the signal that relays body iron status to hepcidin synthesis (17), we investigated hepcidin and Fpn expression in copper-deficient rats and mice. We also examined the relative contributions of various potential mediators of hepcidin expression.
Materials and Methods
Animal care and treatments
Pregnant Holtzman rats, male weanling rats, and pregnant Hsd:ICR (CD-1) outbred albino mice were purchased from Harlan Sprague Dawley. Rodents were offered a copper-deficient diet (Teklad Laboratories) based on the AIN-76A diet formulation but modified by omitting cupric carbonate from the mineral mix as described previously (23). This diet contained 0.36 mg Cu/kg and 53 mg Fe/kg by chemical analysis. Copper-adequate (CuA) rodents were given deionized water with copper sulfate (20 mg Cu/L) to drink. Copper-deficient (CuD) rodents drank copper-free deionized water.
Two nutritional paradigms of copper deprivation were used in these studies: a postweaning and perinatal (gestational/lactational) model. In the postweaning model, male rats at postnatal d (P) 19 started treatments for 30 d. At P49, rats were killed and blood, liver, and spleen samples were harvested. In the perinatal rat model, dams started the treatments on embryonic d (E) 7. Male offspring were killed at P25 and blood and tissue samples were harvested. In the perinatal mouse model, dams started the treatments on E17. At either P20 or P29, male pups were killed and blood and tissue samples were harvested. In both perinatal models, pups were weaned at P20 and maintained on the same treatments as their dams. All animals had free access to diet and drinking water and were maintained at 24°C with 55% relative humidity on a 12-h-light cycle (0700–1900 h light). All protocols were approved by the University of Minnesota Animal Care Committee.
Biochemical analyses
Hemoglobin was determined spectrophotometrically as metcyanhemoglobin (24). Plasma activity of Cp was measured by following oxidation of o-dianisidine at 37°C (25). Portions of liver and diet were wet digested with HNO3 (Trace Metal Grade, Fisher Scientific) and the residue was dissolved in 0.1 mol/L HNO3. Samples were analyzed for copper and iron by flame atomic absorption spectroscopy (model 1100 B, Perkin-Elmer). Plasma iron was measured by flame atomic absorption spectroscopy following treatment with trichloroacetic acid (26). Liver nonheme iron levels were determined colorimetrically after acid digestion of tissues (27).
Western blot analyses
Antibodies.
Affinity-purified rabbit anti-mouse/rat Fpn peptide antibody was generated and validated as described previously (28). The following antibodies were purchased commercially: rabbit anti-scavenger receptor B1 (SR-B1), SR-B1 (Novus Biologicals); rabbit anti-copper chaperone for superoxide dismutase (CCS), CCS, and rabbit anti-TfR2, TfR2 (Santa Cruz Biotechnology); and mouse monoclonal anti-α-tubulin, Clone B-5–1-2 (Sigma-Aldrich).
Preparation of membrane fractions and tissue homogenates.
Crude membrane fractions were used for Western blot analysis of the integral membrane proteins Fpn, SR-B1, and TfR2. To isolate membranes, liver or spleen samples were placed in ice-cold buffer (20 mmol/L HEPES, 1 mmol/L EDTA, 300 mmol/L mannitol, pH 7.4) containing protease inhibitors (Complete Mini Protease inhibitor cocktail, Roche) and were homogenized by dounce homogenization. The homogenate was centrifuged at 10,000 × g for 10 min at 4°C to pellet insoluble cell debris. The resulting supernatant was centrifuged at 100,000 × g for 30 min at 4°C. The membrane pellet was resuspended in HEPES EDTA mannitol buffer. Tissue homogenates were used for Western blot analysis of the cytosolic proteins CCS and tubulin. Tissues were homogenized in lysis buffer (10 mmol/L Tris, pH 7.2) containing protease inhibitors (Complete Mini Protease inhibitor cocktail, Roche). Homogenates were centrifuged at 13,000 × g for 10 min at 4°C to remove insoluble cell debris. Total protein concentration was determined colorimetrically by using the RC DC protein assay (Bio-Rad).
SDS-PAGE and immunoblotting.
Proteins were mixed with Laemmli buffer (1× final concentration) and electrophoretically separated on a 7.5% SDS-polyacrylamide gel. Before loading into the gel, all samples (except those analyzed for Fpn) were boiled for 5 min at 95°C. Separated proteins were transferred to an Optitran nitrocellulose membrane (Schleicher and Schuell). The resulting blot was preincubated for 1 h with blocking buffer [5% nonfat dry milk in Tris-buffered saline, pH 7.4, containing 0.01% Tween 20 (TBS-T)], followed by incubation overnight with primary antibody at 4°C. The blot was then washed in TBS-T and incubated for 40 min with secondary antibody, either horseradish peroxidase-linked donkey anti-rabbit IgG (GE Healthcare UK Limited) or horseradish peroxidase-linked goat anti-mouse IgG (Invitrogen). After washing in TBS-T and TBS, immunoreactivity was visualized using enhanced chemiluminescence (SuperSignal West Pico, Pierce) and X-ray film. To indicate lane loading, blot was stripped for 5 min in 0.5 mol/L glycine (pH 2.8), 0.5 mol/L NaCl, washed in TBS, blocked for 1 h in blocking buffer, then reprobed as above, but with either anti-SR-B1 or anti-α-tubulin as primary antibody. Immunoreactive band intensities on X-ray film were quantified by densitometry by using GeneTools software (SynGene).
Measurement of mRNA levels
Relative transcript abundances of hepcidin, Fpn, and BMP6 were quantified by using quantitative RT-PCR analysis. Briefly, total RNA was isolated from liver by using RNABee (Tel-Test). Isolated RNA was treated with DNaseI (Turbo DNA-free kit, Ambion) to remove any contaminating genomic DNA. First-strand cDNA was synthesized from the isolated RNA by using the High-Capacity cDNA Archive kit (Applied Biosystems). Quantitative RT-PCR was performed using iQ SYBRGreen Supermix (Bio-Rad) and an Applied Biosystems 7300 real-time PCR system. Quantitation of mRNA was determined by comparison to standard curves generated by 4 10-fold serial dilutions of standard cDNA. Levels of mRNA were normalized to that of 18S rRNA, cyclophilin B, or glyceraldehyde 3-phosphate dehydrogenase. The following primer sequences were used: rat hepcidin: 5′-GGCAGAAAGCAAGACTGATGAC-3′ (forward) and 5′-ACAGGAATAAATAATGGGGCG-3′ (reverse); rat Fpn: 5′-CCGTGAACTTGAATGTGAACAAG-3′ (forward) and 5′-CGGAAGGGTTCTGCGATCT-3′ (reverse); rat 18S: 5′-CGAGGAATTCCCAGTAAGTGC-3′ (forward) and 5′-CCATCCAATCGGTAGTAGCG-3′ (reverse); rat BMP6: 5′-CGCCGCAATCCTCCTCTT-3′ (forward) and 5′-CTTTTGCATCTCCCGCTTCT-3′ (reverse); rat cyclophilin B: 5′-CGGACAGCCGGGACAA-3′ and 5′-TTCGATCTTGCCACACTCTACAA-3′ (reverse); mouse hepcidin: 5′- GCCTGAGCAGCACCACCTAT-3′ (forward) and 5′-TTCTTCCCCGTGCAAAGG-3′ (reverse); mouse glyceraldehyde 3-phosphate dehydrogenase: 5′-TTCCTACCCCCAATGTATCCG-3′ (forward) and 5′-ACCACCCTGTTGCTGTAGCCA-3′ (reverse).
Statistics
Data represent means ± SEM. Unless otherwise noted, group means were compared by Student's unpaired t test using PRISM (version 4.02 for Windows, GraphPad) software. Variance equality was evaluated by using the F-test. Data sets with unequal variances were ln transformed to normalize variance prior to statistical analysis.
Results
Effect of copper deficiency on body weight, Cp activity, and tissue copper and iron concentrations.
Phenotypic and biochemical characteristics of the CuD and CuA rats and mice indicated changes due to dietary treatments (Table 1). In rats, a month of dietary copper restriction in the postweaning (P49) model lowered body weight by 30% (P < 0.05) compared with CuA rats. It is possible that the lower body weights of CuD rodents reflect lower food intakes; however, food intakes were not measured. Body weights of CuD rats and mice in the perinatal model (P25 and P20, respectively) were similar to CuA animals. All CuD rodents had markedly diminished diamine oxidase activity of the plasma cuproprotein Cp (P < 0.001) compared with CuA rodents. Liver copper concentrations of CuD rodents were <10% of CuA rodents (P < 0.001). Copper deficiency was associated with a doubling of liver iron concentrations in all models (P < 0.05).
TABLE 1.
Body weight, Cp activity, and liver copper and iron concentrations in CuA and CuD rats and mice1
| Postweaning rat | Perinatal rat | Perinatal mouse | ||
|---|---|---|---|---|
| Body weight, g | CuA | 260 ± 10.6 | 80.1 ± 7.0 | 11.1 ± 1.5 |
| CuD | 184 ± 3.4* | 61.9 ± 3.2 | 10.5 ± 1.0 | |
| Cp activity,units/L | CuA | 185 ± 16.1 | 76.9 ± 8.9 | 41.0 ± 3.6 |
| CuD | 0* | 0* | 0.5 ± 0.2* | |
| Liver Cu, nmol/g | CuA | 62.2 ± 3.1 | 102.3 ± 6.3 | 415.4 ± 45.6 |
| CuD | 4.7 ± 0.6* | 6.3 ± 1.6* | 29.9 ± 3.1* | |
| Liver Fe, μmol/g | CuA | 1.2 ± 0.1 | 0.6 ± 0.04 | 0.8 ± 0.1 |
| CuD | 3.1 ± 0.4* | 1.0 ± 0.1* | 1.9 ± 0.2* |
Values are means ± SEM, n = 4–6. *Different from respective CuA group, P < 0.05.
Comparison of hemoglobin and plasma iron between CuD rats and mice.
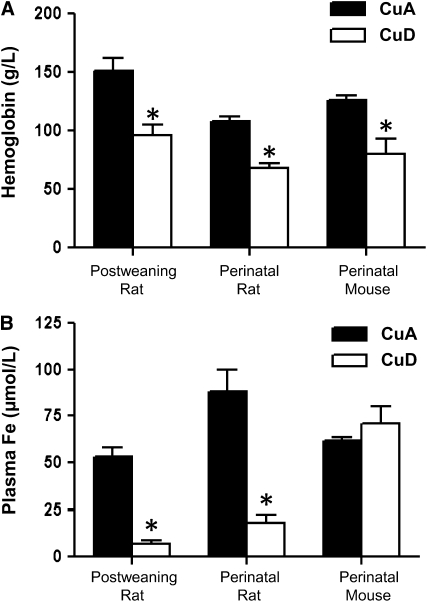
CuD rats and mice were anemic, with ∼40% lower hemoglobin levels (P < 0.05) than CuA rodents (Fig. 1A). In CuD rats, there was a marked reduction (P < 0.001) in plasma iron concentrations (Fig. 1B). In contrast, anemic CuD mice had plasma iron concentrations that did not differ from those of CuA mice.
FIGURE 1 .
Hemoglobin (A) and plasma iron (B) concentrations in CuA and CuD rats and mice on P49 in the postweaning rat model, P25 in the perinatal rat model, and P20 in the perinatal mouse model. Values represent means ± SEM, n = 4–6. *Different from respective CuA group, P < 0.05.
Effect of postweaning copper deficiency on Fpn levels in rats.
The liver and spleen are the 2 major suppliers of iron into the plasma. The iron is derived mainly from the recycling of erythrocyte hemoglobin iron by reticuloendothelial macrophages of the liver (Kupffer cells) and splenic red pulp (29). These macrophages phagocytose senescent erythrocytes and catabolize heme to liberate iron, which is released via Fpn into the plasma. To assess the capacity for iron release into the plasma, we measured Fpn in liver and spleen.
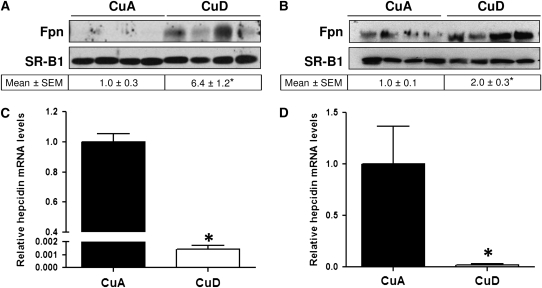
Western blot analysis of liver membrane fractions from rats in the postweaning model revealed a marked upregulation of Fpn levels in CuD rats relative to CuA controls (Fig. 2A). Equivalent protein loading among lanes was confirmed by determining the levels of SR-B1, an integral membrane protein. Densitometric analysis of Fpn-immunoreactive bands indicated a 5-fold higher (P < 0.01) hepatic Fpn level in CuD rats (Fig. 2A). Relative Fpn mRNA levels were also higher (P < 0.05) in CuD rats (1.4 ± 0.13) than in CuA rats (1.0 ± 0.07; data not shown).
FIGURE 2 .
Hepatic Fpn protein (A,B) and hepcidin mRNA (C,D) levels in postweaning (A,C) and perinatal (B,D) CuA and CuD rats. To indicate protein loading in A and B, each blot was stripped and reprobed for SR-B1, an integral membrane protein. Values below Western blots indicate relative intensities of Fpn-immunoreactive bands. Relative hepcidin mRNA levels in postweaning (C) and perinatal (D) copper deficiency. Values represent means ± SEM, n = 4. *Different from respective CuA group, P < 0.05.
To confirm and extend the observations of the effects of copper deficiency on Fpn levels in postweaning rats, a second experiment was performed in rats by restricting copper to the pregnant dams starting at E7. The effect of copper deficiency on iron biology is greatly affected by the age at which the copper is limited. For example, brain iron is affected only in pups but not following postweaning copper deficiency (30). Male offspring were killed at P25 and liver and spleen Fpn levels were measured. Similar to the postweaning model of Cu deficiency, perinatal Cu deficiency resulted in higher Fpn levels (P < 0.05) in the liver (Fig. 2B). However, hepatic Fpn mRNA expression did not differ between CuD and CuA rats (data not shown).
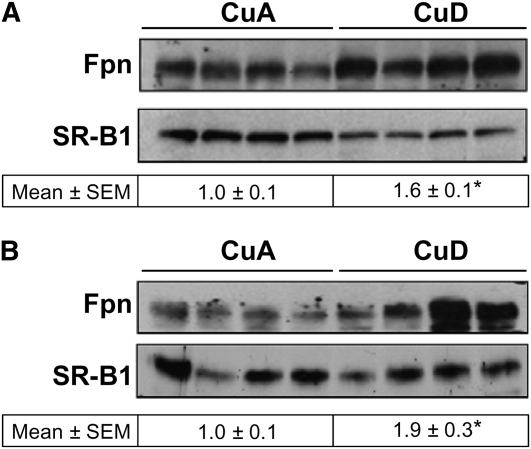
Because Fpn levels are controlled chiefly by hepcidin, we measured hepcidin expression in the liver, the main source of the circulating peptide. We found that hepatic hepcidin mRNA levels in postweaning CuD rats were 0.1% those of the CuA controls (P < 0.0001) and in perinatal CuD rats, 2% of the controls (P < 0.05) (Fig. 2D). The markedly lower levels of hepatic hepcidin are consistent with the much higher levels of hepatic Fpn (Fig. 2A,B). Likewise, Fpn levels in spleen were 60–90% higher (P < 0.05) in CuD rats compared with CuA rats (Fig. 3A,B).
FIGURE 3 .
Splenic Fpn protein levels in postweaning CuA and CuD rats. Western blot analysis of splenic Fpn in postweaning (A) and perinatal (B) rats. To indicate protein loading, the blot was stripped and reprobed for SR-B1. Values below Western blots (A,B) indicate relative intensities of Fpn-immunoreactive bands. Values represent mean ± SEM, n = 4. *Different from respective CuA group, P < 0.05.
To confirm copper-dependent changes in protein levels, we measured CCS, a sensitive indicator of copper status. Levels of CCS in liver, heart, kidney, and brain are higher following copper deficiency (31). Similar to previous reports, we observed markedly higher (P < 0.001) levels of CCS in the liver and spleen of CuD rats relative to CuA controls (Supplemental Fig. 1).
Effect of perinatal copper deficiency on Fpn levels in mice.
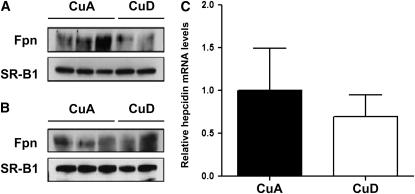
A previous study found that Fpn mRNA and protein levels were not upregulated in the liver of mice following perinatal copper deficiency (21). Because this result contrasts sharply with the clear upregulation of Fpn we observed in the liver of CuD rats, we analyzed several samples from CuA and CuD mice obtained from a study similar to the experimental model by Chung et al. (21). We found that liver Fpn levels did not differ between CuD and CuA mice, consistent with the results by Chung et al. (21) (Fig. 4A). Splenic Fpn levels also did not differ between CuD and CuA mice (Fig. 4B). Importantly, hepatic and splenic Fpn levels clearly were not higher in Cu-deficient mice, as they were in rats. Analysis of CuD (n = 6) and CuA (n = 5) mice further revealed no differences in hepcidin mRNA abundance (Fig. 4C).
FIGURE 4 .
Fpn protein and hepcidin mRNA levels in CuA and CuD mice. Western blot analysis of hepatic Fpn (A) and splenic Fpn (B) in perinatal mice. To indicate protein loading, the blot was stripped and reprobed for SR-B1. (C) Hepatic hepcidin mRNA abundance. Values represent mean ± SEM, n = 4.
Potential mediators of hepcidin expression.
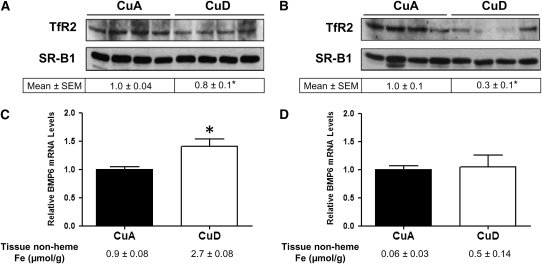
Hepatic hepcidin mRNA levels in CuD rats were markedly lower than in CuA rats in both the perinatal model and the postweaning model of Cu deficiency. We took advantage of these 2 independent, yet similar studies to examine potential mediators of hepcidin expression. We focused our attention on TfR2 and BMP6, because they are known to be responsive to plasma iron (holotransferrin) concentrations (32) and body iron levels (20), both of which differed between CuA and CuD rats. In postweaning deficiency, TfR2 levels were 25% lower (P < 0.05) in CuD rats compared with CuA rats (Fig. 5A). In the perinatal deficiency model, TfR2 levels were 75% lower (P < 0.001) in CuD rats compared with CuA rats (Fig. 5B). To demonstrate the depression in TfR2 protein levels that occurs when plasma iron levels are low, liver membrane extracts from iron-adequate (FeA) and iron-deficient (FeD) rats were analyzed (Supplemental Fig. 2A). TfR2 abundance was markedly lower in FeD compared with FeA rats. In postweaning copper deficiency, BMP6 mRNA levels were 40% higher (P < 0.05) in CuD rats compared with CuA rats (Fig. 5C). In the perinatal deficiency model, BMP6 mRNA levels did not differ between CuD and CuA rats (Fig. 5D). To demonstrate iron-dependent modulation of BMP6, we measured levels of hepatic BMP6 transcripts in FeA, FeD, and iron-overloaded rats (Supplemental Fig. 2B). Compared with FeA controls, the relative expression of hepatic BMP6 was higher in iron-overloaded rats and lower in FeD rats.
FIGURE 5 .
Hepatic TfR2 protein and BMP6 mRNA levels in CuA and CuD rats. Western blot analysis of liver TfR2 in postweaning (A) and perinatal (B) copper deficiency. To indicate protein loading, the blot was stripped and reprobed for SR-B1. Values below Western blots indicate relative intensities of TfR2-immunoreactive bands. Relative BMP6 mRNA levels in postweaning (C) and perinatal (D) copper deficiency. Values represent mean ± SEM, n = 4. *Different from respective CuA group, P < 0.05.
Discussion
The anemia and perturbations in iron metabolism resulting from Cu deficiency have been studied for over 80 y (3). The last decade has witnessed a resurgence of interest in the metabolic links between iron and copper metabolism, mainly due to the identification of the key proteins that participate in iron trafficking and systemic iron homeostasis. Data from the present study reveal that anemic CuD rats display higher Fpn protein levels in liver and spleen. The higher Fpn levels were observed in 2 independent studies utilizing different paradigms of nutritional copper limitation (postweaning or perinatal copper deficiency). In contrast, this study and a previous one (21) documented that anemic CuD mice do not exhibit elevated tissue Fpn levels. Here, we further demonstrate that hepcidin expression was not lower in anemic CuD mice as it was in rats. These data suggest that the differential Fpn response between the 2 species arises mainly from differences in hepcidin regulation. In rats, Fpn levels were most likely elevated in CuD, because levels of hepcidin—a negative regulator of Fpn—were markedly diminished. The 40% higher Fpn mRNA levels in postweaning CuD rats may have also contributed to higher Fpn protein abundance but probably does not fully account for the 5-fold higher protein levels. This does not seem to be the case, however, in the perinatal model, because Fpn mRNA levels did not differ between CuD and CuA animals. We speculate that Fpn mRNA levels were elevated in the postweaning CuD rats, because they had accumulated more hepatic iron than did the younger, perinatal CuD rats (3.12 ± 0.4 vs 1.01 ± 0.1 μmol Fe/g liver).
Given the key role of hepcidin in regulating whole-body iron metabolism, many studies have sought to identify the physiologic and molecular drivers of hepcidin expression. Initial studies by Nicolas et al. (33) demonstrated that anemia induced by phlebotomy or phenylhydrazine-induced hemolysis results in a dramatic decrease in liver hepcidin mRNA levels. Moreover, the suppressive effect of anemia on hepcidin expression was observed in phenylhydrazine-treated mice that were loaded with iron (which independently increases hepcidin expression), indicating that the anemia is a more dominant regulator of hepcidin. Our observation that anemic CuD rats have markedly lower hepatic hepcidin mRNA levels despite elevated liver iron concentrations further supports the dominant effect of anemia over iron on hepcidin expression. However, the CuD mice in our study were also anemic but did not display reduced hepcidin expression, suggesting that anemia is not the primary regulator of hepcidin. Studies of anemic mice in which erythropoiesis was inhibited demonstrate that erythropoietic activity is a more important driver of hepcidin suppression than is anemia (34). Accordingly, future studies will need to determine whether erythropoietic activity is compromised in CuD mice.
At the molecular level, TfR2 is an important regulator of hepcidin expression. Mutations in TfR2 have been linked to low hepcidin levels and iron overload in humans (35). Transgenic mice with a targeted orthologous mutation in TfR2 also do not produce sufficient hepcidin and develop iron overload similar to the human disease (36). The generation of liver-specific TfR2-knockout mice further revealed that the liver is the primary site of TfR2 activity and that hepatic TfR2 is required for hepcidin expression (37). TfR2 may regulate hepcidin expression through its interaction with holotransferrin. Studies of cultured hepatoma cells demonstrate that holotransferrin increases TfR2 levels in a dose-responsive manner (32,38). The effect requires the iron-bound ligand, because neither iron-depleted transferrin (apotransferrin) nor nontransferrin-bound iron affects TfR2 levels. In vivo, hepatic TfR2 levels have been shown to be high in iron-loaded rats and low in iron-deficient animals (32). A direct link between holotransferrin and hepcidin was demonstrated in primary mouse hepatocytes by showing that hepcidin mRNA levels increased in parallel with increasing iron saturation of transferrin (39). Collectively, these observations support a model in which TfR2 levels reflect plasma transferrin saturations. In this way, TfR2 “senses” body iron status and modulates hepcidin expression accordingly.
In our 2 rat studies, the CuD animals had lower hepcidin mRNA and plasma iron levels than did CuA controls. These changes were associated with decreased levels of TfR2, in agreement with the model that TfR2 levels reflect transferrin saturation. It thus seems probable that the reduction in TfR2 levels contributes to the downregulation of hepcidin production in these animals. The iron phenotype of CuD rats is somewhat analogous to that of hypotransferrinemic (trfhpx/hpx) mice. Trfhpx/hpx mice display anemia, hepatic iron loading, and low transferrin saturation. Trfhpx/hpx mice, like CuD rats, also have decreased hepcidin expression (40) and reduced hepatic TfR2 levels (32). In addition to TfR2-associated downregulation of hepcidin synthesis, erythroid-derived signaling molecules that are released during states of enhanced erythropoiesis (41,42) may independently contribute to the hepcidin suppression in anemic Trfhpx/hpx mice and CuD rats.
BMP6 is another key endogenous positive regulator of hepcidin production. BMP6 administration has been shown to increase hepcidin mRNA levels and reduce serum iron concentrations in mice (18). Moreover, dietary iron dose responsively increases hepatic BMP6 mRNA levels in parallel to hepcidin mRNA levels (20). Less clear, however, is whether BMP6 levels increase in response to hepatic iron accumulation or to elevated serum iron concentrations. Data from our postweaning copper deficiency study suggest that it is the former, for CuD rats had higher liver iron levels but lower plasma iron concentrations. It is important to note that despite the elevated levels of BMP6 in postweaning CuD rats (or no change in BMP6 levels in perinatal CuD rats), hepcidin levels were markedly diminished, indicating that other factors (including TfR2) predominantly mediate hepcidin expression in this animal model.
Although CuD rats had higher levels of the iron-export protein, Fpn, their livers did not become depleted of iron. In fact, hepatic iron concentrations were elevated in these animals. At the same time, the CuD rats also had lower plasma iron concentrations. The elevation in liver iron, coupled with diminished plasma iron, suggests that copper deficiency impairs the normal iron release from the liver into the plasma. Using cell culture models, De Domenico et al. (43) recently reported that iron export and cell-surface expression of Fpn required the presence of glycosylphosphatidyl-inositol-linked Cp, a membrane-bound form of Cp. In the absence of glycosylphosphatidyl-inositol-Cp, Fpn was rapidly internalized and degraded. Such a mechanism seems unlikely in our CuD rats (which displayed no plasma Cp activity), because Fpn was not degraded; instead, its levels were elevated in both liver and spleen. Regardless of the mechanism of impaired release, the low plasma iron concentrations in CuD rats limit iron supply to the bone marrow, contributing to the anemia. The anemia of the CuD mice is more complicated; it cannot be attributed to a simple limitation in iron supply to the bone marrow, because plasma iron levels were normal. These animals also had essentially no plasma diamine oxidase Cp activity, suggesting that Cp is not required to maintain normal plasma iron concentrations in CuD mice. Indeed, Cp-null mice have been shown to have normal plasma iron levels without anemia (44), indicating that sufficient iron release and erythropoiesis can occur in the absence of Cp. Iron mobilization is unaffected in CuD mice, most likely because serum ferroxidase activity is not lower during copper deficiency as it is in rats (45). Furthermore, mice appear to have ferroxidase activity that is not associated with Cp, as demonstrated in early studies of copper deficiency and recently confirmed in Cp-null mice (45,46). One study in mice did, however, report low serum iron concentrations following 6 wk of copper deprivation (10). It was suggested in that study and others with rats that the anemia of copper deficiency resulted from decreased intestinal hephaestin activity, leading to inefficient iron absorption and systemic iron deficiency (9,10). Although we did not measure intestinal hephaestin or iron absorption in our study, the normal plasma iron levels and elevated hepatic iron concentrations in our CuD mice suggest that iron absorption was not significantly impaired. Furthermore, even if hephaestin function is marginally impaired in CuD mice, it does not affect whole body iron accumulation (22). It therefore seems that the anemia we observed in CuD mice arose not from iron limitation, but from a defect in uptake/utilization by the bone marrow. Consistent with this possibility are studies showing that iron administration did not correct the anemia of older CuD mice (47). Further research is encouraged to identify the cause of copper-deficiency anemia in mice.
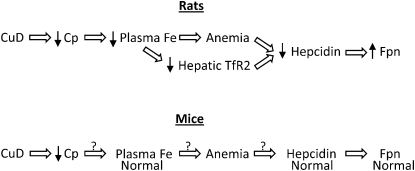
Figure 6 summarizes the effects of CuD on hepcidin regulation in rats and mice. In rats, CuD causes a reduction in plasma iron concentrations, which limits iron mobilization to the bone marrow, resulting in anemia. During anemia, hepcidin expression decreases, but the molecular mechanisms remain poorly defined. A reduction in plasma iron concentrations (holotransferrin) is associated with lower levels of hepatic TfR2, a known positive regulator of hepcidin expression. Thus, lower levels of TfR2 likely contribute to the downregulation of hepcidin expression in CuD rats. In contrast to rats, CuD mice do not display reduced plasma iron concentrations, although they have anemia. Despite the anemia, however, hepcidin levels are normal. The nature of the differences in hepcidin regulation between rats and mice remains to be determined.
FIGURE 6 .
Effect of CuD on hepcidin regulation in rats and mice. In rats, CuD decreases Cp ferroxidase activity, which likely reduces plasma iron concentrations. Low plasma iron levels limit iron supply to the bone marrow, resulting in anemia, a suppressor of hepcidin expression. Diminished plasma iron concentrations are associated with lower levels of hepatic TfR2, a putative body iron sensor that modulates hepcidin expression according to plasma iron concentrations. Although CuD decreases Cp activity in mice, plasma iron levels are normal, possibly due to additional plasma ferroxidases. The anemia in CuD does not appear to result from limited iron supply to the bone marrow but rather to a defect in bone marrow iron utilization. Yet despite the anemia, CuD mice have normal levels of hepcidin, possibly related to normal plasma iron levels. The normal levels of hepcidin in anemic, CuD mice likely accounts for the normal Fpn levels in these animals. The question marks identify gaps in our understanding of these relationships.
Supplementary Material
Acknowledgments
M.D.K. and J.R.P. designed research; S.J., M.B., H.N., and J.R.P. conducted research; S.J., M.B., M.D.K., and J.R.P. analyzed data; M.D.K., S.J., and J.R.P. wrote the paper; and M.D.K. had primary responsibility for final content. All authors read and approved the final manuscript.
Supported in part by the NIH grant DK080706 (M.D.K.) and HD039708 and HD055423 (J.R.P.).
Author disclosures: S. Jenkitkasemwong, M. Broderius, H. Nam, J. R. Prohaska, and M. D. Knutson, no conflicts of interest.
Supplemental Figures 1 and 2 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: BMP6, bone morphogenic protein 6; CCS, copper chaperone for superoxide dismutase; Cp, ceruloplasmin; CuA, copper-adequate; CuD, copper-deficient; E, embryonic day; FeA, iron-adequate; FeD, iron-deficient; Fpn, ferroportin; IRE, iron-response element; IRP, iron-regulatory protein; P, postnatal day; SR-B1, scavenger receptor B1; TBS-T, Tris-buffered saline with Tween 20; TfR2, transferrin receptor 2; trfhpx/hpx, hypotransferrinemic.
References
- 1.Danks DM. Copper deficiency in humans. Annu Rev Nutr. 1988;8:235–57. [DOI] [PubMed] [Google Scholar]
- 2.Videt-Gibou D, Belliard S, Bardou-Jacquet E, Troadec MB, Le Lan C, Jouanolle AM, Loreal O, Rivalan J, Brissot P. Iron excess treatable by copper supplementation in acquired aceruloplasminemia: a new form of secondary human iron overload? Blood. 2009;114:2360–1. [DOI] [PubMed] [Google Scholar]
- 3.Fox PL. The copper-iron chronicles: the story of an intimate relationship. Biometals. 2003;16:9–40. [DOI] [PubMed] [Google Scholar]
- 4.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–12. [DOI] [PubMed] [Google Scholar]
- 5.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. [DOI] [PubMed] [Google Scholar]
- 6.Lee GR, Nacht S, Lukens JN, Cartwright GE. Iron metabolism in copper-deficient swine. J Clin Invest. 1968;47:2058–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han O, Kim EY. Colocalization of ferroportin-1 with hephaestin on the basolateral membrane of human intestinal absorptive cells. J Cell Biochem. 2007;101:1000–10. [DOI] [PubMed] [Google Scholar]
- 8.Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195–9. [DOI] [PubMed] [Google Scholar]
- 9.Reeves PG, Demars LC, Johnson WT, Lukaski HC. Dietary copper deficiency reduces iron absorption and duodenal enterocyte hephaestin protein in male and female rats. J Nutr. 2005;135:92–8. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Huang G, Su T, Gao H, Attieh ZK, McKie AT, Anderson GJ, Vulpe CD. Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J Nutr. 2006;136:1236–41. [DOI] [PubMed] [Google Scholar]
- 11.Aydemir F, Jenkitkasemwong S, Gulec S, Knutson MD. Iron loading increases ferroportin heterogeneous nuclear RNA and mRNA levels in murine J774 macrophages. J Nutr. 2009;139:434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenstein RS. Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annu Rev Nutr. 2000;20:627–62. [DOI] [PubMed] [Google Scholar]
- 13.Lymboussaki A, Pignatti E, Montosi G, Garuti C, Haile DJ, Pietrangelo A. The role of the iron responsive element in the control of ferroportin1/IREG1/MTP1 gene expression. J Hepatol. 2003;39:710–5. [DOI] [PubMed] [Google Scholar]
- 14.Knutson MD, Vafa MR, Haile DJ, Wessling-Resnick M. Iron loading and erythrophagocytosis increase ferroportin 1 (FPN1) expression in J774 macrophages. Blood. 2003;102:4191–7. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. [DOI] [PubMed] [Google Scholar]
- 16.Lee PL, Beutler E. Regulation of hepcidin and iron-overload disease. Annu Rev Pathol. 2009;4:489–515. [DOI] [PubMed] [Google Scholar]
- 17.Fleming RE. Iron sensing as a partnership: HFE and transferrin receptor 2. Cell Metab. 2009;9:211–2. [DOI] [PubMed] [Google Scholar]
- 18.Andriopoulos B Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478–81. [DOI] [PubMed] [Google Scholar]
- 20.Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112:1503–9. [DOI] [PubMed] [Google Scholar]
- 21.Chung J, Prohaska JR, Wessling-Resnick M. Ferroportin-1 is not upregulated in copper-deficient mice. J Nutr. 2004;134:517–21. [DOI] [PubMed] [Google Scholar]
- 22.Pyatskowit JW, Prohaska JR. Copper deficient rats and mice both develop anemia but only rats have lower plasma and brain iron levels. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prohaska JR, Brokate B. The timing of perinatal copper deficiency in mice influences offspring survival. J Nutr. 2002;132:3142–5. [DOI] [PubMed] [Google Scholar]
- 24.Prohaska JR. Changes in tissue growth, concentrations of copper, iron, cytochrome oxidase and superoxide dismutase subsequent to dietary or genetic copper deficiency in mice. J Nutr. 1983;113:2048–58. [DOI] [PubMed] [Google Scholar]
- 25.Prohaska JR. Changes in Cu,Zn-superoxide dismutase, cytochrome c oxidase, glutathione peroxidase and glutathione transferase activities in copper-deficient mice and rats. J Nutr. 1991;121:355–63. [DOI] [PubMed] [Google Scholar]
- 26.Olson AD, Hamlin WB. A new method for serum iron and total iron-binding capacity by atomic absorption spectrophotometry. Clin Chem. 1969;15:438–44. [PubMed] [Google Scholar]
- 27.Torrance JD, Bothwell TH. A simple technique for measuring storage iron concentrations in formalinised liver samples. S Afr J Med Sci. 1968;33:9–11. [PubMed] [Google Scholar]
- 28.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA. 2005;102:1324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutson M, Wessling-Resnick M. Iron metabolism in the reticuloendothelial system. Crit Rev Biochem Mol Biol. 2003;38:61–88. [DOI] [PubMed] [Google Scholar]
- 30.Prohaska JR, Gybina AA. Rat brain iron concentration is lower following perinatal copper deficiency. J Neurochem. 2005;93:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prohaska JR, Broderius M, Brokate B. Metallochaperone for Cu,Zn-superoxide dismutase (CCS) protein but not mRNA is higher in organs from copper-deficient mice and rats. Arch Biochem Biophys. 2003;417:227–34. [DOI] [PubMed] [Google Scholar]
- 32.Robb A, Wessling-Resnick M. Regulation of transferrin receptor 2 protein levels by transferrin. Blood. 2004;104:4294–9. [DOI] [PubMed] [Google Scholar]
- 33.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camaschella C, Roetto A, Cali A, De Gobbi M, Garozzo G, Carella M, Majorano N, Totaro A, Gasparini P. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25:14–5. [DOI] [PubMed] [Google Scholar]
- 36.Fleming RE, Ahmann JR, Migas MC, Waheed A, Koeffler HP, Kawabata H, Britton RS, Bacon BR, Sly WS. Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis. Proc Natl Acad Sci USA. 2002;99:10653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallace DF, Summerville L, Subramaniam VN. Targeted disruption of the hepatic transferrin receptor 2 gene in mice leads to iron overload. Gastroenterology. 2007;132:301–10. [DOI] [PubMed] [Google Scholar]
- 38.Johnson MB, Enns CA. Diferric transferrin regulates transferrin receptor 2 protein stability. Blood. 2004;104:4287–93. [DOI] [PubMed] [Google Scholar]
- 39.Lin L, Valore EV, Nemeth E, Goodnough JB, Gabayan V, Ganz T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007;110:2182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy CN, Weinstein DA, Andrews NC. 2002 E. Mead Johnson Award for Research in Pediatrics Lecture: the molecular biology of the anemia of chronic disease: a hypothesis. Pediatr Res. 2003;53:507–12. [DOI] [PubMed] [Google Scholar]
- 41.Tanno T, Porayette P, Sripichai O, Noh SJ, Byrnes C, Bhupatiraju A, Lee YT, Goodnough JB, Harandi O, et al. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–101. [DOI] [PubMed] [Google Scholar]
- 43.De Domenico I, Ward DM, di Patti MC, Jeong SY, David S, Musci G, Kaplan J. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 2007;26:2823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA. 1999;96:10812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prohaska JR. Comparison between dietary and genetic copper deficiency in mice: copper-dependent anemia. Nutr Res. 1981;1:159–67. [Google Scholar]
- 46.Gray LW, Kidane TZ, Nguyen A, Akagi S, Petrasek K, Chu YL, Cabrera A, Kantardjieff K, Mason AZ, et al. Copper proteins and ferroxidases in human plasma and that of wild-type and ceruloplasmin knockout mice. Biochem J. 2009;419:237–45. [DOI] [PubMed] [Google Scholar]
- 47.Prohaska J, Bailey W, Cox D. Failure of iron injection to reverse copper-dependent anemia in mice. In: Mills CF, Bremner I, Chesters JK, editors. Trace elements in man and animals-TEMA 5. Farnham Royal, United Kingdom: Commonwealth Agricultural Bureaux; 1985. p. 27–32.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.