Abstract
Existing evidence links greater dietary intake of fish and (n-3) PUFA to better early brain development and lowered risk of cognitive disorders in late life. The mechanisms for these associations remain unclear and may be related to specific (n-3) fatty acids and may concern cognitive function generally rather than only early brain development and age-related cognitive dysfunction. In this investigation, we tested potential associations between (n-3) fatty acids in serum phospholipids and major dimensions of cognitive functioning in mid-life adults. Participants were 280 community volunteers between 35 and 54 y of age, free of major neuropsychiatric disorders, and not taking fish oil supplements. Dietary biomarkers were α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenonic acid (DHA) in serum phospholipids measured using GC. Five major dimensions of cognitive functioning were assessed with a 75-min battery of neuropsychological tests. In covariate adjusted regression models, higher DHA (mol %) was related to better performance on tests of nonverbal reasoning and mental flexibility, working memory, and vocabulary (P ≤ 0.05). These associations were generally linear. Associations between DHA and nonverbal reasoning and working memory persisted with additional adjustment for participant education and vocabulary scores (P ≤ 0.05). Neither EPA nor ALA was notably related to any of the 5 tested dimensions of cognitive performance. Among the 3 key (n-3) PUFA, only DHA is associated with major aspects of cognitive performance in nonpatient adults <55 y old. These findings suggest that DHA is related to brain health throughout the lifespan and may have implications for clinical trials of neuropsychiatric disorders.
Introduction
Optimal cognitive functioning is a primary component of health-related quality of life, and macro- and micronutrients are increasingly understood to affect the brain and cognitive processes. Recent research specifically indicates that the (n-3) PUFA are important nutrients during early brain development as well as during the development of late life cognitive decline and dementia (1–6). From a nutritional and biochemical standpoint, the 18-carbon (n-3) fatty acid, α-linolenic acid (ALA),8 is essential in the human diet and serves as precursor to the 20–22 carbon, eicosapentaenoic acid (EPA) and docosahexaenonic acid (DHA). Because the conversion of ALA into EPA and DHA is limited in humans, direct dietary intake of EPA and DHA in fish and other seafood may be necessary to achieve optimal blood and tissue concentrations (7). DHA is concentrated in the CNS membrane phospholipids and makes up 8% of the brain's dry weight (8).
During early brain development, low or no consumption of (n-3) PUFA is associated in laboratory studies with abnormal neurogenesis, neurotransmitter metabolism, and learning (1,5). Clinical research has similarly shown that the (n-3) PUFA are important during early brain development, with low intake resulting in poor neurological development and low IQ (1,2), and such associations may extend through adolescence (9). In addition, fish oil supplementation appears to have therapeutic effects on the cognitive and behavioral manifestations of attention deficit/hyperactivity disorder and related conditions (10,11). Finally, in late life, low fish intake predicts greater age-related cognitive dysfunction and elevated risk for Alzheimer's dementia (12–14). As the predominant (n-3) PUFA in the brain, DHA intake, in particular, would be expected to underlie these associations (15).
In the elderly with and without cognitive impairment, the effects of fish oil supplementation are unclear, however (16–18). Fish oil capsules typically contain more EPA than DHA and only 1 randomized clinical trial has administered a DHA-predominant supplement (16). This study and 1 other found no effect of the intervention in dementia patients but noted cognitive performance benefits in elderly participants with normal or near-normal cognitive functioning (16,18). These findings suggest that (n-3) PUFA intake may be related not to dementia per se but to cognitive performance more generally. Therefore, the current investigation examined the associations between blood levels of the essential (n-3) PUFA–ALA, EPA, and DHA–and major dimensions of cognitive function in 280 healthy persons in middle adulthood not taking fish oil supplements.
Participants and Methods
Data for this study were collected in the University of Pittsburgh Adult Health and Behavior (AHAB) project, which is a registry of general behavioral and biological measurements in mid-life community volunteers (30–54 y of age). Participants were recruited via mass mail solicitation from communities of southwestern Pennsylvania (principally Allegheny County). Exclusion criteria for the AHAB project included a reported history of atherosclerotic cardiovascular disease, chronic kidney or liver disease, cancer treatment in the preceding year, major neurological disorders, schizophrenia or other psychotic illness [according to the Diagnostic and Statistical Manual of Mental Disorders, 4th ed criteria and determined by structured clinical interview (19)], and in women, current pregnancy or perimenopausal menstrual irregularities. Participants were required to speak English as their primary language for at least the past 5 y.
A subset of 308 AHAB participants enrolled in supplementary data collections that included serum fatty acid analysis. Additional exclusion criteria for this component of AHAB participation included diabetes, severe hypertension [blood pressure (BP) ≥ 180/110 mm Hg], severe obesity (BMI ≥ 40 kg/m2), renal insufficiency (serum creatinine >160 μmol/L), excessive alcohol consumption (>260 g alcohol/wk), and use of fish oil supplements or antihypertensive, diabetic, lipid-lowering, antiarrhythmic, glucocorticoid, weight-loss, and psychotropic medications. A total of 283 individuals met these criteria and also had a stored fasting serum sample available for fatty acid analysis. Three of the 283 serum samples produced undetectable chromatogram peaks, leaving 280 participants for inclusion in the current study.
Participants completed a battery of psychosocial, lifestyle, and demographic questionnaires, and a project nurse conducted a medical history and medication use interview and measured several standard risk factors, including BP and BMI (kg/m2). A morning blood sample was obtained after an overnight fast. Following centrifugation, serum samples were stored at −80°C until analysis. Whereas AHAB data collection occurred over multiple laboratory sessions, the above procedures as well as the neuropsychological testing were all completed on the same day. Informed consent was obtained in accordance with the guidelines of the University of Pittsburgh Institutional Review Board.
Determination of serum phospholipid fatty acid composition
Fasting serum samples were stored at −80°C until analysis. Serum phospholipid fatty acid composition was determined by capillary GC as described elsewhere (20). DHA, EPA, and ALA levels are expressed as percentages of the total fatty acid pool (weight or mol %). Intra- and interassay CV were 2.0–9.2% and 1.9–9.6%, respectively, for all major serum fatty acids and PUFA.
Cognitive testing
Research associates, trained and supervised by a licensed neuropsychologist (C.M.R.), administered a neuropsychological test battery comprised of selected subtests from the Wechsler Memory Scale, 3rd edition (WMS-3) (21) and the Wechsler Abbreviated Intelligence Scale (WASI) (22), and 4 more specialized measures of cognitive function. The test scores were grouped according to the dimension of cognitive functioning that each test primarily assesses.
Nonverbal reasoning and mental flexibility.
This dimension of cognitive functioning included the WASI Matrix reasoning test and the Trail making test. The Matrix reasoning test uses a multiple choice format that presents a series of matrices containing increasingly complex visual pattern arrays. Participants are required to select the element that best fits the matrix. This task assesses nonverbal abstract problem-solving and reasoning by analogy. The Trail making test measures mental flexibility and complex psychomotor speed. In Part A, participants draw a line connecting randomly distributed numbers 1 through 25 in ascending order. In Part B they perform a similar task but must alternate between numbers and letters (1-A-2-B, etc.) as quickly as possible. The primary measure of mental flexibility is Part B time minus Part A time.
Attention and concentration.
This dimension of cognitive functioning included the Stroop interference test and the Digit Vigilance test. The Stroop interference test measures mental flexibility, response inhibition, attention, and some aspects of executive function by requiring participants to name the color of the ink in which a color word is printed (e.g. the word “red” is printed in green ink; participants would say “green”). An interference score is derived from the timed measures. In the Digit Vigilance test, participants visually scan a page of numbers and cross out every designated target (the number 6). The time required provides a measure of processing speed and sustained attention.
General or episodic memory.
Four subtests from the WMS-3 (21) assessed visual and verbal episodic memory: logical memory, verbal paired associates, faces, and family pictures. Each test is untimed and scored based upon the number of correct responses.
Working memory.
Four subtests from WMS-3 (21) assessed working memory: letter number sequencing, spatial span, forward digit span, and backward digit span. Each test is untimed and scored based upon the number of correct responses.
Verbal knowledge and processing
This dimension of cognitive functioning was tested with the WASI Vocabulary test and the Verbal fluency test. In the WASI Vocabulary test (22), participants are asked to orally define 34 increasingly difficult words. This test measures expressive vocabulary and verbal knowledge or semantic memory. The Verbal fluency test requires participants spontaneously generate as many words as possible beginning with designated letters of the alphabet. The response measure is the number of words produced in 60 s for each of 3 letters (F, A, and S). The test measures verbal processing efficiency and is sensitive to damage to frontal and temporal brain areas.
Statistical analysis
All statistical analyses were performed using SPSS v15. Digit completion time, DHA, and EPA were log-transformed to better approximate a normal distribution. Two-step multiple regression analysis was used to examine the relationships between blood levels of fatty acids and aspects of cognitive functioning. For this, standard covariates of age, gender, and race were entered in step 1, and each fatty acid variable of interest was entered in step 2. Dependent measures were the performance scores on each of the neuropsychological tests. Composite measures (based on the mean of standardized scores) were generated for general memory and working memory according to the WMS-3 guidelines. Other test scores were not combined, because each test has unique features and scores were not highly correlated with one another (Pearson r2 < 0.20 for all).
Potential interactions between fatty acid variables and both gender and age were tested in these linear regression models. Independent variables were mean-centered and the interaction variables were calculated as the product of 2 variables of interest and then added to the model for analysis. Individual differences in health literacy may affect diet choices, and fish oil consumption can lower BP, which could, in turn, affect cognitive function (23). Therefore, we explored the potential influence of subject education attainment and resting BP on our primary hypotheses in supplementary regression models.
Finally, potential nonlinear associations between the fatty acid levels and cognitive functioning were examined by regressing each cognitive variable on a nonlinear function of the fatty acid variables. These functions were generated by transforming the raw fatty acid variables into logarithmic, quadratic, cubic, and square root predictor variables. Significance was defined as P ≤ 0.05.
Results
A review of their characteristics (Table 1) indicates that participants were mid-life adults, predominantly white, and generally healthy, although tending to be overweight. The large majority had attended at least some college. The primary analyses (Table 2) revealed that, among the 3 examined (n-3) PUFA, DHA was most related to cognitive function. Higher DHA (mol %) was associated with proportionally better performance on tests of both nonverbal reasoning and mental flexibility. DHA was similarly associated with the composite working memory score and with scores on 3 of the 4 individual working memory tests. Attention, concentration, and general memory did not vary notably as a function of DHA, whereas among the tests of verbal knowledge and processing, vocabulary was also proportional to DHA.
TABLE 1.
Participants' characteristics
| Characteristics | |
|---|---|
| Participants, n | 280 |
| Age, y | 44.6 ± 6.71 |
| Sex, % male | 48 |
| Race, % white | 89 |
| Education, % | |
| High school equivalent or less | 11 |
| Technical training or some college | 30 |
| Bachelor or graduate degree | 59 |
| Current smoker, % | 15 |
| Alcohol, g/wk | 36 ± 51 |
| BMI, kg/m2 | 26.2 ± 4.3 |
| Systolic BP, mm Hg | 114 ± 12 |
| Diastolic BP, mm Hg | 77 ± 9 |
| Serum fatty acids, % total phospholipid fatty acids | |
| ALA | 0.16 (0.00–0.51) |
| DHA | 1.52 (0.50–4.08) |
| EPA | 0.49 (0.07–3.70) |
Values are means ± SD, mean (range), or percentages.
TABLE 2.
Standardized β from linear regression models of serum phospholipid fatty acids and cognitive function test scores in middle-aged adults
| DHA |
EPA |
ALA |
||||
|---|---|---|---|---|---|---|
| β1 | P | β | P | β | P | |
| Nonverbal reasoning and mental flexibility | ||||||
| Matrix reasoning | 0.202 | <0.001 | 0.072 | 0.22 | −0.085 | 0.15 |
| Trail making2 | 0.116 | 0.043 | 0.042 | 0.47 | −0.094 | 0.10 |
| Attention and concentration | ||||||
| Stroop interference | 0.039 | 0.52 | 0.016 | 0.80 | 0.001 | 0.99 |
| Digit vigilance2 | 0.079 | 0.17 | 0.089 | 0.13 | 0.087 | 0.13 |
| General memory | ||||||
| Composite | 0.080 | 0.17 | 0.032 | 0.58 | 0.090 | 0.12 |
| Logical memory | 0.122 | 0.035 | 0.035 | 0.56 | 0.082 | 0.16 |
| Verbal paired associates | 0.101 | 0.091 | 0.035 | 0.57 | −0.084 | 0.12 |
| Faces | 0.041 | 0.49 | 0.025 | 0.68 | 0.074 | 0.21 |
| Family pictures | −0.011 | 0.85 | −0.0028 | 0.64 | 0.094 | 0.11 |
| Working memory | ||||||
| Composite | 0.163 | 0.005 | 0.113 | 0.054 | −0.020 | 0.73 |
| Letter-number sequencing | 0.137 | 0.019 | 0.041 | 0.48 | −0.059 | 0.32 |
| Spatial span | 0.144 | 0.010 | 0.124 | 0.038 | −0.006 | 0.92 |
| Forward digit span | 0.092 | 0.12 | 0.034 | 0.58 | 0.008 | 0.90 |
| Backward digit span | 0.177 | 0.002 | 0.146 | 0.014 | −0.006 | 0.93 |
| Verbal knowledge and processing | ||||||
| Vocabulary | 0.169 | 0.002 | 0.056 | 0.32 | −0.020 | 0.72 |
| Verbal fluency | 0.073 | 0.22 | 0.003 | 0.97 | 0.019 | 0.75 |
All associations were adjusted for age, gender, and race.
The signs of the coefficients for trail making and digit vigilance were reversed to be consistent with other tests such that higher scores always indicate better performance.
Analyses of the second major, long-chain (n-3) fatty acid, EPA, revealed very limited relation to cognitive function (Table 2). EPA was a significant predictor of a subset of working memory test scores but was unrelated to other aspects of cognitive functioning. Finally, the precursor of these long-chain fatty acids, ALA, was not found to have any association with the administered performance measures. No gender or age interactions were noted in any of the foregoing analyses.
Role of BP.
BP may confound or mediate these revealed associations, because high BP is linked to cognitive dysfunction and dietary intake of (n-3) fatty acids can lower BP. In this sample, diastolic, but not systolic, BP correlated inversely with matrix reasoning, digit vigilance, general memory, and verbal fluency (r = −0.14 to −0.20; P < 0.05). Therefore, the primary results were reexamined including diastolic BP in regression models and all findings related to DHA remained significant.
Role of health literacy.
Better-educated individuals may, by virtue of greater health literacy, choose to eat fish more frequently than other persons. The positive association between DHA and vocabulary score in the current analyses supports this thesis. Accordingly, we repeated the regression analyses with the additional covariates of educational attainment (8 levels) and vocabulary test score. Phospholipid DHA remained a significant predictor of matrix reasoning performance and the working memory composite score, whereas trail making was no longer related to DHA.
Dose-response relationship.
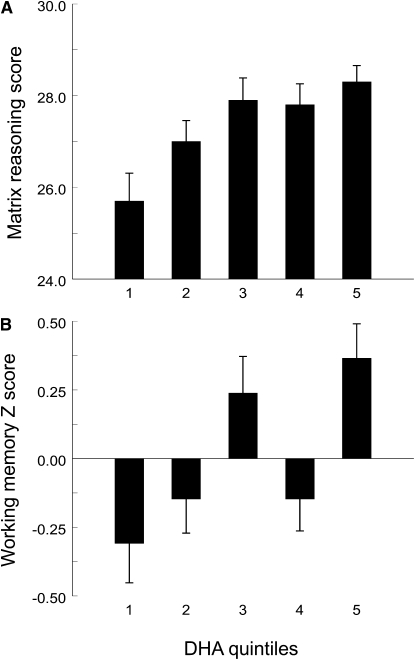
To illustrate the dose-response relationship, we examined performance scores as a function of DHA quintile (Fig. 1). Matrix reasoning and working memory composite results were chosen for this purpose due to their robustness in the above analyses and are representative of the pattern also with trail making and vocabulary. Although the relationships were not entirely stepwise, the patterns were generally linear. For completeness, nonlinear models were compared with the linear model for each dependent measure. Explained variance in the nonlinear models was either less than or within 1% greater than that of the linear models, leading us to conclude that linear models are adequate for this data set.
FIGURE 1 .
Dose-response relationship between serum phospholipid DHA (mole %) and cognitive performance scores. Panels A and B present matrix reasoning and working memory results, respectively. Values are means ± SE adjusted for age, sex and race, by DHA quintile (n = 56 for each quintile). DHA means for the quintiles are 0.80, 1.11, 1.39, 1.73, 2.57 mole %, respectively. ANOVA revealed a significant effect of DHA quintile on matrix reasoning (P = 0.002) and working memory (P = 0.001).
Discussion
The (n-3) PUFA are emerging as important nutrients for optimal brain development and for possible protection against brain senescence. Though not yet directly tested, it is plausible that insufficient dietary intake is related to relatively poor cognitive abilities or performance throughout the lifespan and that such effects are attributable specifically to DHA. The current investigation examined in healthy, mid-life adults potential associations between cognitive performance and serum phospholipid levels of the 3 key (n-3) PUFA. DHA was related to cognitive functioning, with higher blood levels associated with better scores on tests of nonverbal reasoning, mental flexibility, working memory, and vocabulary. In contrast, EPA was marginally associated (P = 0.054) only with working memory and ALA was unrelated to any measure of cognitive performance. The positive findings for DHA were noted in analyses controlling for age, gender, and race and were not attributable to confounding by BP and, for the most part, withstood additional adjustment for education and vocabulary. Hence, the findings of the present analyses suggest that the (n-3) fatty acids, and specifically DHA, play a role in major aspects of cognitive functioning during middle adulthood.
Observational and experimental studies conducted in human infants have linked greater pre- and postnatal supply of long-chain fatty acids to better neurodevelopmental outcomes (2,24). At the other end of the human lifespan, age-related cognitive decline is inversely related to self-reported fish intake and blood concentrations of (n-3) fatty acids (13,14,25). Preliminary clinical trial evidence suggests that fish oil supplementation improves general cognitive function in elderly patients with at most mild baseline impairment but not in those with dementia (16,18).
The role of the (n-3) fatty acids in cognitive function after early brain development but before senescence remains relatively unexplored. Supplement trials to date in children have been negative, although the dose (26) and duration (27) may have been inadequate. Two epidemiologic studies of middle-aged European and American adults examined cognitive performance in relation to self-reported fatty fish intake (28) and fatty acid analyses (29). Both found the (n-3) fatty acids were related to cognitive performance and specifically suggest that these nutrients are related to psychomotor speed and mental efficiency, but not general memory. The single, small trial of nonelderly adults found, in a limited neuropsychological battery, that supplementation with EPA and DHA improved simple reaction time and performance on a sustained attention test that taps working memory (30). The current study did not find associations between (n-3) fatty acids and attention per se but otherwise corroborates this prior research. Working memory, not carefully assessed previously, was strongly related to phospholipid DHA levels, and this finding is consistent with an association between (n-3) fatty acids and general mental efficiency.
There is ample evidence of diverse roles played by (n-3) fatty acids in the brain but as yet scant research linking their specific biochemical effects to select aspects of human behavior. DHA is the predominant (n-3) fatty acid in the brain, and evidence from multiple species indicates that the brain concentration varies in relation to dietary consumption (31–33). Its concentration is greatest in gray matter and is proportional to regional metabolic activity (31). Most EPA and DHA are found in phospholipids within cell membranes, mitochondria, and synaptosomes; here they exert physiochemical effects on membrane-bound receptors and other proteins. Both EPA and DHA also serve as precursors to eicosanoids, docosanoids, resolvins, and related compounds while also serving as ligands to a variety of nuclear receptors (34). DHA, in particular, increases cell viability via neuroprotective and antiapoptotic mechanisms (35,36) while also promoting dendritic arborization and synaptogenesis (37).
Some neurotransmitter specificity is revealed in rodent experiments where dietary (n-3) deprivation reduces serotonin content and turnover in the frontal cortex and receptor density in the hippocampus (38) and reduces dopamine-associated regulatory proteins, such as tyrosine hydroxylase (39). Other studies in animals have found that (n-3) fatty acid deprivation reversibly impairs learning and working memory along with hippocampal long-term potentiation (40,41). In healthy adults, cingulate cortex, hippocampus, and amygdala gray matter volumes were found to be greatest in persons reporting the highest intake of EPA and DHA, while among depressed patients, phospholipid DHA levels correlated positively with metabolic rate in the temporoparietal cortex and negatively with metabolism in the prefrontal cortex and anterior cingulate gyrus (42). In this study, serum phospholipid DHA was related to mental reasoning and flexibility and working memory, and these cognitive functions involve the neural structures noted above and generally comprise the limbic system and frontal/prefrontal cortex (43).
The findings in this report suggest that DHA may be the (n-3) fatty acid most closely related to cognitive function and this finding is consonant with some prior work on cognitive aging and dementia (44,45). Conversely, the null findings for ALA suggest that intake of this precursor to EPA and DHA may not have important effects on cognitive functioning. Nonetheless, ALA did not vary widely in this sample and the potential effects of ALA on cognitive functioning have not been tested experimentally in humans. Although EPA was generally unrelated to cognition in the current analyses, dietary intake of EPA has been associated with mood and impulsivity in community samples (46,47) as well as in psychiatric and forensic populations in both observational studies and clinical trials (48). Therefore, the literature as a whole suggests that DHA and EPA may have different behavioral effects. Though still quite speculative, this may reflect the primary role of EPA as a precursor to antiinflammatory eicosanoids, in contrast to DHA's greater role as a neurotrophic/neuroprotective factor and modulator of membrane function and oxidative stress (1,5,34,49).
The findings from these analyses derive from cross-sectional data and, therefore, cannot establish that any association between the (n-3) fatty acids on cognitive performance is causal. While this and prior observational studies of (n-3) fatty acids and cognitive function have generally found associations that withstand adjustment for obvious confounding factors, additional intervention-based experiments and randomized clinical trials in both healthy and clinical samples are warranted. Taken together, research to date suggests that specifically, DHA may favorably affect cognitive performance and may do so throughout the life course.
Acknowledgments
M.F.M, C.M.R., and S.B.M. designed research and project conception; J.K.Y. provided essential materials and reagents; M.F.M, C.M.R., and L.S. analyzed data and had primary responsibility for final content. S.M.C., M.F.M., and J.K.Y. conducted research; M.F.M, C.M.R., L.S. J.K.Y., S.M.C., and S.B.M. wrote the paper. All authors read and approved the final manuscript.
Supported by US NIH grants PO1 40962 and T32 HL007560.
Author disclosures: S. M. Conklin, J. K. Yao, and M. F. Muldoon received a $9000 research grant from Isodis Natura to support serum fatty acid determinations used for a separate study. C. M. Ryan, L. Sheu, and S. B. Manuck, no conflicts of interest.
Abbreviations used: AHAB, Adult Health and Behavior; ALA, α-linolenic acid; BP, blood pressure; DHA, docosahexaenonic acid; EPA, eicosapentaenoic acid; WASI, Wechsler Abbreviated Intelligence Scale; WMS-3, Wechsler Memory Scale, 3rd ed.
References
- 1.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008;1237:35–43. [DOI] [PubMed] [Google Scholar]
- 2.Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–85. [DOI] [PubMed] [Google Scholar]
- 3.Farquharson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW. Infant cerebral-cortex phospholipid fatty-acid composition and diet. Lancet. 1992;340:810–3. [DOI] [PubMed] [Google Scholar]
- 4.Oken E, Osterdal ML, Gillman MW, Knudsen VK, Halldorsson TI, Strom M, Bellinger DC, Hadders-Algra M, Michaelsen KF, et al. Associations of maternal fish intake during pregnancy and breastfeeding duration with attainment of developmental milestones in early childhood: a study from the Danish National Birth Cohort. Am J Clin Nutr. 2008;88:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boudrault C, Bazinet RP, Ma DW. Experimental models and mechanisms underlying the protective effects of n-3 polyunsaturated fatty acids in Alzheimer's disease. J Nutr Biochem. 2009;20:1–10. [DOI] [PubMed] [Google Scholar]
- 6.Das UN. Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer's disease–but how and why? Prostaglandins Leukot Essent Fatty Acids. 2008;78:11–9. [DOI] [PubMed] [Google Scholar]
- 7.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:S1467–76. [DOI] [PubMed] [Google Scholar]
- 8.Muskiet FA, van Goor SA, Kuipers RS, Velzing-Aarts FV, Smit EN, Bouwstra H, Dijck-Brouwer DA, Boersma ER, Hadders-Algra M. Long-chain polyunsaturated fatty acids in maternal and infant nutrition. Prostaglandins Leukot Essent Fatty Acids. 2006;75:135–44. [DOI] [PubMed] [Google Scholar]
- 9.Aberg MA, Aberg N, Brisman J, Sundberg R, Winkvist A, Toren K. Fish intake of Swedish male adolescents is a predictor of cognitive performance. Acta Paediatr. 2009;98:555–60. [DOI] [PubMed] [Google Scholar]
- 10.Vaisman N, Kaysar N, Zaruk-Adasha Y, Pelled D, Brichon G, Zwingelstein G, Bodennec J. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: effect of dietary n-3 fatty acids containing phospholipids. Am J Clin Nutr. 2008;87:1170–80. [DOI] [PubMed] [Google Scholar]
- 11.Sinn N, Bryan J, Wilson C. Cognitive effects of polyunsaturated fatty acids in children with attention deficit hyperactivity disorder symptoms: a randomised controlled trial. Prostaglandins Leukot Essent Fatty Acids. 2008;78:311–26. [DOI] [PubMed] [Google Scholar]
- 12.Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, Alperovitch A. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69:1921–30. [DOI] [PubMed] [Google Scholar]
- 13.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849–53. [DOI] [PubMed] [Google Scholar]
- 14.Nurk E, Drevon CA, Refsum H, Solvoll K, Vollset SE, Nygard O, Nygaard HA, Engedal K, Tell GS, et al. Cognitive performance among the elderly and dietary fish intake: the Hordaland Health Study. Am J Clin Nutr. 2007;86:1470–8. [DOI] [PubMed] [Google Scholar]
- 15.Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545–50. [DOI] [PubMed] [Google Scholar]
- 16.Freund-Levi Y, Basun H, Cederholm T, Faxen-Irving G, Garlind A, Grut M, Vedin I, Palmblad J, Wahlund LO, et al. Omega-3 supplementation in mild to moderate Alzheimer's disease: effects on neuropsychiatric symptoms. Int J Geriatr Psychiatry. 2008;23:161–9. [DOI] [PubMed] [Google Scholar]
- 17.van de Rest O, Geleijnse JM, Kok FJ, van Staveren WA, Dullemeijer C, Olderikkert MG, Beekman AT, de Groot CP. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71:430–8. [DOI] [PubMed] [Google Scholar]
- 18.Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, Stewart R, Huang SY. The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1538–44. [DOI] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders, research version, non-patient edition. New York: New York State Psychiatric Institute, Biometrics Research Department; 1996.
- 20.Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res. 2000;42:7–17. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D. Wechsler Memory Scale. 3rd ed. San Antonio (TX): Psychological Corporation; 1997.
- 22.Wechsler. WASI manual. San Antonio (TX): Psychological Corporation; 1999.
- 23.Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–9. [DOI] [PubMed] [Google Scholar]
- 24.Eilander A, Hundscheid DC, Osendarp SJ, Transler C, Zock PL. Effects of n-3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: a review of human studies. Prostaglandins Leukot Essent Fatty Acids. 2007;76:189–203. [DOI] [PubMed] [Google Scholar]
- 25.van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007;85:1142–7. [DOI] [PubMed] [Google Scholar]
- 26.Osendarp SJ, Baghurst KI, Bryan J, Calvaresi E, Hughes D, Hussaini M, Karyadi SJ, van Klinken BJ, van der Knaap HC, et al. Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 parallel, randomized, placebo-controlled studies in Australia and Indonesia. Am J Clin Nutr. 2007;86:1082–93. [DOI] [PubMed] [Google Scholar]
- 27.Ryan AS, Nelson EB. Assessing the effect of docosahexaenoic acid on cognitive functions in healthy, preschool children: a randomized, placebo-controlled, double-blind study. Clin Pediatr (Phila). 2008;47:355–62. [DOI] [PubMed] [Google Scholar]
- 28.Kalmijn S, van Boxtel MPJ, Ocke M, Verschuren WMM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62:275–80. [DOI] [PubMed] [Google Scholar]
- 29.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2007;85:1103–11. [DOI] [PubMed] [Google Scholar]
- 30.Fontani G, Corradeschi F, Felici A, Alfatti F, Migliorini S, Lodi L. Cognitive and physiological effects of omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur J Clin Invest. 2005;35:691–9. [DOI] [PubMed] [Google Scholar]
- 31.Brenna JT, Diau GY. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot Essent Fatty Acids. 2007;77:247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rapoport SI, Purdon D, Shetty HU, Grange E, Smith Q, Jones C, Chang MC. In vivo imaging of fatty acid incorporation into brain to examine signal transduction and neuroplasticity involving phospholipids. Ann N Y Acad Sci. 1997;820:56–73. [DOI] [PubMed] [Google Scholar]
- 33.Hirashima F, Parow AM, Stoll AL, Demopulos CM, Damico KE, Rohan ML, Eskesen JG, Zuo CS, Cohen BM, et al. Omega-3 fatty acid treatment and T-2 whole brain relaxation times in bipolar disorder. Am J Psychiatry. 2004;161:1922–4. [DOI] [PubMed] [Google Scholar]
- 34.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–55. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee PK, Chawla A, Loayza MS, Bazan NG. Docosanoids are multifunctional regulators of neural cell integrity and fate: significance in aging and disease. Prostaglandins Leukot Essent Fatty Acids. 2007;77:233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukiw WJ, Bazan NG. Docosahexaenoic acid and the aging brain. J Nutr. 2008;138:2510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cansev M, Wurtman RJ, Sakamoto T, Ulus IH. Oral administration of circulating precursors for membrane phosphatides can promote the synthesis of new brain synapses. Alzheimers Dement. 2008;4:S153–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levant B, Ozias MK, Davis PF, Winter M, Russell KL, Carlson SE, Reed GA, McCarson KE. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: Interactions with reproductive status in female rats. Psychoneuroendocrinology. 2008;33:1279–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuperstein F, Eilam R, Yavin E. Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency. J Neurochem. 2008;106:662–71. [DOI] [PubMed] [Google Scholar]
- 40.Tanabe Y, Hashimoto M, Sugioka K, Maruyama M, Fujii Y, Hagiwara R, Hara T, Hossain SM, Shido O. Improvement of spatial cognition with dietary docosahexaenoic acid is associated with an increase in Fos expression in rat CA1 hippocampus. Clin Exp Pharmacol Physiol. 2004;31:700–3. [DOI] [PubMed] [Google Scholar]
- 41.Chung WL, Chen JJ, Su HM. Fish oil supplementation of control and (n-3) fatty acid-deficient male rats enhances reference and working memory performance and increases brain regional docosahexaenoic acid levels. J Nutr. 2008;138:1165–71. [DOI] [PubMed] [Google Scholar]
- 42.Sublette ME, et al. Plasma polyunsaturated fatty acids and regional cerebral glucose metabolism in major depression. Prostaglandins, Leukot Essent Fatty Acids. 2009;80:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jennings JR, van der Veen FM, Meltzer CC. Verbal and spatial working memory in older individuals: a positron emission tomography study. Brain Res. 2006;1092:177–89. [DOI] [PubMed] [Google Scholar]
- 44.Whalley LJ, Deary IJ, Starr JM, Wahle KW, Rance KA, Bourne VJ, Fox HC. n-3 Fatty acid erythrocyte membrane content, APOE varepsilon4, and cognitive variation: an observational follow-up study in late adulthood. Am J Clin Nutr. 2008;87:449–54. [DOI] [PubMed] [Google Scholar]
- 45.Morris MC. Docosahexaenoic acid and Alzheimer disease. Arch Neurol. 2006;63:1527–8. [DOI] [PubMed] [Google Scholar]
- 46.Conklin SM, Harris JI, Manuck SB, Yao JK, Hibbeln JR, Muldoon MF. Serum omega-3 fatty acids are associated with variation in mood, personality and behavior in hypercholesterolemic community volunteers. Psychiatry Res. 2007;152:1–10. [DOI] [PubMed] [Google Scholar]
- 47.Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF. High omega-6 and low omega-3 fatty acids are associated with depressive symptoms and neuroticism. Psychosom Med. 2007;69:932–4. [DOI] [PubMed] [Google Scholar]
- 48.Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163:969–78. [DOI] [PubMed] [Google Scholar]
- 49.Chapkin RS, McMurray DN, Davidson LA, Patil BS, Fan YY, Lupton JR. Bioactive dietary long-chain fatty acids: emerging mechanisms of action. Br J Nutr. 2008;100:1152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]