Abstract
The risk for dementia, a major contributor to incapacitation and institutionalization, rises rapidly as we age, doubling every 5 y after age 65. Tens of millions of new Alzheimer's disease (AD) and other dementia cases are projected as elderly populations increase around the world, creating a projected dementia epidemic for which most nations are not prepared. Thus, there is an urgent need for prevention approaches that are safe, effective, and affordable. This review addresses the potential of one promising candidate, the (n-3) fatty acid docosahexaenoic acid (DHA), which appears to slow pathogenesis of AD and possibly vascular dementia. DHA is pleiotropic, acting at multiple steps to reduce the production of the β-amyloid peptide, widely believed to initiate AD. DHA moderates some of the kinases that hyperphosphorylate the τ-protein, a component of the neurofibrillary tangle. DHA may help suppress insulin/neurotrophic factor signaling deficits, neuroinflammation, and oxidative damage that contribute to synaptic loss and neuronal dysfunction in dementia. Finally, DHA increases brain levels of neuroprotective brain-derived neurotrophic factor and reduces the (n-6) fatty acid arachidonate and its prostaglandin metabolites that have been implicated in promoting AD. Clinical trials suggest that DHA or fish oil alone can slow early stages of progression, but these effects may be apolipoprotein E genotype specific, and larger trials with very early stages are required to prove efficacy. We advocate early intervention in a prodromal period with nutrigenomically defined subjects with an appropriately designed nutritional supplement, including DHA and antioxidants.
Introduction
There are no cures in sight for chronic diseases of aging, only increasingly expensive chronic treatments. A major shift from costly disease management toward prevention is now mandated because the U.S. and other developed and developing nations with aging populations face projections of unsustainable health care costs to pay for the health care of aging populations. Many of the most costly and debilitating conditions are neurodegenerative. The most prevalent forms of these diseases have polygenic influences interacting with aging and environmental risk factors: notably, stroke and vascular dementia, Parkinson's disease, and Alzheimer's disease (AD).4 These conditions develop slowly, with a prolonged prodromal pathological buildup of pathological lesions generally driven by combined risk factors. Intervention to prevent AD, the focus of this review, should ideally begin well before disease onset, during an insidious decade-long presymptomatic phase.
The most successful prevention approach is to block the factors initiating lesion pathogenesis, which can be accomplished in animal models, but that approach would be extremely difficult to test in the clinic because it would require very early intervention decades before clinical outcomes. Therefore, the best interventions will be those that are cheap, safe, pleiotropic, and with multiple potential benefits; for example, they may apply to common features of the chronic diseases of aging that we seek to prevent. While they may be less specific than novel drug and antibody approaches that are under intensive study for treatment, prevention interventions with diet and/or exercise may be more effective and are more practical with respect to costs and safety concerns.
AD pathogenesis
From the genetics of early onset AD, we have learned that it can be initiated by aggregates of the 42–amino acid β-amyloid (Aβ42) peptide derived from its amyloid precursor protein (APP). Aβ42 peptide is normally produced and cleared, but when this is out of balance because of genetics or aging, small increases in Aβ42 result in elevated small neurotoxic and synaptotoxic oligomer assemblies leading to massive accumulations of larger fibrillar amyloid plaque and vascular deposits. In the amyloid cascade hypothesis, pathological Aβ assemblies can cause excitotoxicity, oxidative damage, mitochondrial dysfunction, inflammation, and microglial activation, as well as activation of kinases that hyperphosphorylate the microtubule protein τ, leading to τ aggregates (1). The resultant τ oligomers further aggregate to form fibrils or paired helical filaments, which accumulate as β-sheet stabilized intraneuronal neurofibrillary tangles, a lesion correlated with AD progression and neuron loss. Animal model studies demonstrate that Aβ and τ pathology can both cause synaptic and neuronal dysfunction and loss. The relative contributions of these different pathological species to the prolonged and complex pathogenesis leading to regional neurodegeneration and a range of emerging symptoms remain controversial. Part of the controversy may arise from variation between regions and individuals with different risk factors and versions of the AD syndrome, but it is also likely that contributions of these lesions to the cascade are simply complex and regionally stage dependent. Whatever their individual contributions are at different stages, inflammation, oxidative damage, and protein aggregate accumulation are common features that occur early and accompany many of the neurodegenerative diseases of aging. Inflammation, oxidative damage, and lipid profiles have epidemiological evidence as factors modifying dementia risk, suggesting that they represent useful targets to explore for prevention.
Our group and many others have tested potential prevention strategies using transgenic mouse models that express familial AD mutations and induce mild cognitive deficits that correlate with Aβ oligomerization and, unlike in AD, precede the plaque pathology (2,3). The first interventions examined using these models, amyloid vaccine (4) and the nonsteroidal antiinflammatory drugs (NSAIDs) (5), were effective against amyloid plaque pathogenesis, but both have had safety and efficacy issues. This has led to safer passive immunization approaches already in clinical trials, but it is unlikely to be widely used for prevention because of cost and safety issues. We have looked for safer pleiotropic alternatives to NSAID prevention approaches and explored both the polyphenolic NSAID/antioxidant curcumin and the (n-3) fatty acid docosahexaenoic acid (DHA) that we and others observed has neuroprotective, antioxidant, antiinflammatory, and Aβ42-reducing activities in vitro and in animal models.
DHA
Two major essential PUFA series, the (n-6) PUFA, with double bonds beginning at carbon number 6, and (n-3) PUFA, with double bonds beginning at carbon number 3, are regulated by dietary intake. Linoleic acid [18:2(n-6)] is obtained predominantly from plants and serves as a substrate of elongases and desaturases to produce the common animal tissue fat, arachidonic acid [AA; 20:4(n-6)]. AA is the (n-6) PUFA series substrate for the cyclooxygenases (COX) and lipoxygenases, which produce proinflammatory lipid mediators. Of the (n-3) PUFA series, a small percentage of α-linolenic acid [18:2(n-3)], common in plant sources like flaxseed, soy, nuts (e.g., walnuts), and other oily seeds, is elongated and desaturated to produce antiinflammatory long-chain (n-3) PUFA, including eicosopentaenoic acid [EPA; 20:5(n-3)] and DHA [22:6(n-3)]. The bulk of preformed intake of EPA + DHA is from fish, notably oily fish. Unlike EPA, DHA is in high concentration in brain and neuronal phospholipids where it may be as high as 35% of phosphatidylethanolamine (6). AA and DHA compete for esterification into the labile phospholipid SN-2 position, so that releasable SN-2 AA in brain membranes can be reduced by lowering the dietary (n-6):(n-3) PUFA ratio. Because of ubiquitous high intake of (n-6) fatty acids in Western diets, this is most readily accomplished by increasing the intake of preformed DHA from fish or supplements. The antiinflammatory effects of increasing (n-3) fatty acids intake are achieved by competitively reducing phospholipid incorporation of available AA and the (n-6):(n-3) fatty acid ratio in brain. For example, DHA reduces proinflammatory AA prostaglandin products from COX enzymes that are the targets of NSAID. The neuroinflammation that accompanies AD and NSAID has been repeatedly associated with reduced AD risk in epidemiological studies (7), indicating that the COX enzymes may be viable targets for AD interventions (8). However, results to date from studies using specific COX-2 inhibitors have suggested little efficacy with established AD or in initial results from a prevention trial that was halted due to safety concerns (9), although a long-term protective benefit in the Alzheimer's Disease Anti-Inflammatory Prevention Trial naproxen arm was reported (10).
DHA reduces epidemiological risk for cognitive decline and dementia
Our recent literature review found 9 epidemiological studies associating increased fish consumption with reduced risk for dementia, including AD. Furthermore, 8 out of 10 studies found that higher blood (n-3) fatty acids were associated with reduced cognitive decline (11).
The epidemiology is not entirely consistent. One report found that high fish consumption but not dietary (n-3) fatty acid intake appeared protective in the Chicago Health and Aging Project (12). Another study showed no risk reduction with increased RBC DHA but reduced dementia (4.3–5.1%) in those with high RBC PUFA and high whole blood mercury, a good correlate of higher long-term fish intake (13). One possible explanation for the lack of significant risk protection reported in the latter study with only small trends (P = 0.19) with RBC DHA levels (2.8–4.1% total PUFA) is that these DHA concentrations are lower than those reported in other studies showing significant risk reduction [e.g., DHA = 6.34 ± 1.1% with no decline vs. 5.89 ± 1.0% with decline (P = 0.04) (14) or DHA = 5.4 ± 1.2% in the protected fish oil group vs. 4.6 ± 1.0% without fish oil (15)]. It is also possible that measuring phosphatidylcholine (PC) DHA rather than total DHA may be a stronger predictor of risk reduction because, in the Framingham study, high plasma PC DHA corresponded to 47% risk reduction (16), and, in another study, only RBC PC DHA but not phosphatidylethanolamine DHA was lower in AD patients (400%) (17). Despite some inconsistencies in the epidemiology, metaanalysis assessing the quality of available epidemiology and preclinical studies concluded that clinical trials were warranted (18), and clinical trials are beginning to resolve the issue in favor of (n-3) fatty acid protection with some restrictions.
Clinical trials
Four small completed clinical trials with (n-3) fatty acids (typically from fish oil) suggested possible protection, but only in mild cognitive impairment patients, whereas 2 trials with (n-3) fatty acids plus antioxidants or other nutrients (α-lipoate, L. Shinto, Oregon Health and Science University) and B vitamins plus UMP, a putative enhancer of DHA incorporation (Souvenaid, P. Scheltens, The Netherlands) have suggested possible efficacy in established AD [reviewed in (11)]. A large 6-mo placebo-controlled trial with 900 mg/d algal DHA, the Memory Improvement with DHA Study trial (485 participants with mild memory complaints), recently reported that DHA improved performance in participants with age-related cognitive decline, manifested as logical memory scores >1 SD below the younger adult mean using a computerized cognitive test, Cantab Paired Associate Learning or PAL, a visual-spatial episodic memory test (19). Error rates in the PAL were designed and reported to discriminate between probable early AD and depression, which had similar scores on the more established cognitive battery used for AD, the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAScog) (20). DHA was reported to cut the error rate in half on the PAL (P < 0.03), and the response correlated with blood levels of DHA, consistent with an effect of DHA on early AD. There were no serious adverse events.
An 18-mo Alzheimer Disease Cooperative Studies (ADCS) trial with 2 g/d algal DHA versus placebo examined progression in 402 randomized mild- to moderate-AD participants [mean age of 76 ± 8.7 y, minimental state exam (MMSE) = 20.7 ± 3.6, 59% apolipoprotein E4 (ApoE4) positive and 41% ApoE4 negative] reported no effect on cognitive test scores (ADAScog and MMSE) in participants with ApoE4, but possible slowing of progression by the same measures in participants with ApoE3 (21). The ADCS trial result was significant for ADAScog scores only in the ApoE3 subgroup (a widely accepted measure of cognitive decline based on subject performance) of treated versus untreated non-ApoE4 carriers (P = 0.0285). The ApoE4 negative planned comparison subgroup analysis was an exploratory, secondary analysis with no adjustment for multiple comparisons and requires independent confirmation. Although MMSE, another standardized test measure of cognitive decline, showed that the DHA group was associated with a similar slowed progression in the non-ApoE4 group, it did not alter scores that included rater assessments of global severity (clinical dementia rating) and behavioral disturbances (neuropsychiatric inventory and activities of daily living). Whether the possible DHA benefit on cognitive performance in the ApoE3 group was a result of random variation or a real effect will require further study. Statistical nonsignificance seems less likely to reflect mere chance because the result echoes the epidemiology, where 3 of the more recent studies found that risk reduction as a result of fish intake was less or even absent in ApoE4 carriers (11). Additional large clinical trials are underway and should assess the issues of very early intervention and nutrigenomic interactions. For example, in addition to modulation of trial outcomes by genetic variance in the lipid transporter ApoE, one can anticipate a contribution to a variable response due to low activity variants of the desaturases that convert plant source (n-3) fatty acids to long-chain, bioactive EPA and DHA (22).
DHA: Multiple mechanisms for AD prevention
The (n-3) fatty acid DHA has the NSAID-like antiinflammatory effects of lowering AA, as discussed above. Our group and others have shown that DHA can reduce production of Aβ from APP and Aβ42 accumulation in AD model mice (23–26) and cultured human neurons (27). One group reported that (n-3) fatty acids did not modify amyloid levels in the cerebral cortex or behavior, but this negative result was from an experiment using bigenic mutant PS1× APP transgenic mice where, for whatever reason, the diet did not alter brain fatty acids levels (28). This lack of a diet effect on brain fatty acid composition is in contrast with the positive studies, including our own, where DHA supplementation increased DHA and markedly reduced the (n-6):(n-3) PUFA ratio in the frontal cortex (29). The Aβ42 reduction may be due to multiple effects of DHA incorporated into brain phospholipid, such as changes in membrane and lipid raft structure and fluidity (30), which influence APP processing (31); a repression of presenilin 1 and, therefore, gamma secretase (26); or the induction of antiamyloidogenic chaperones for APP [SorLA (32)] and Aβ itself [transthyretin (33,34)]. A lipoxgenase product of DHA, neuroprotection D1, may mediate 1 or more of the effects to downregulate APP processing to Aβ (27).
In addition to reducing Aβ production, DHA is neuroprotective. Aging APP transgenic mice fed a diet high in (n-6) fatty acids, but deficient in (n-3) fatty acids, showed increased caspase activation, dramatic loss of the AD-sensitive dendritic spine marker drebrin, and other excitatory synaptic markers, whereas DHA supplements protected caspase, activation, drebrin, PSD-95, and CaMKII-α loss and cognitive deficits (29,35). Proposed mechanisms for DHA's neuroprotective effects include increasing survival signaling through the PI3-K/Akt pathway by increasing membrane docking through pleckstrin homology domains (36), increasing a neuroprotective DHA enzymatic metabolite, neuroprotectin D1 (27), and increasing brain-derived neurotrophic factor (37) and reducing oxidative damage (38) as well as multiple additional neuroprotective and cardiovascular protective activities (11).
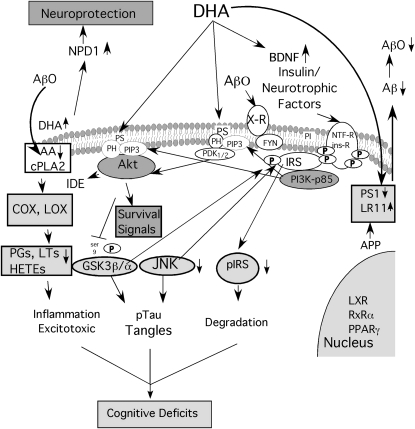
Of particular interest, AD patients and AD animal models have synaptic and dendritic loss that may begin with a postsynaptic attack by Aβ oligomers (39), whereas DHA shows strong synaptic protection from preformed Aβ oligomers in vitro (40), consistent with protection of the dendritic spine marker drebrin in APP Tg mice (35). Perhaps surprisingly, the Aβ oligomer synaptic attack may be related to a seemingly disconnected target for nutritional treatment, type II diabetes. Classic insulin-resistant diabetes is a risk factor for developing AD, and insulin signaling defects in AD brain are a focus for treatment in AD (41). Insulin/neurotrophic factor signaling protects from Aβ oligomer toxicity, but the signal transduction pathways involved appear defective in AD and AD models (42,43) and may be blunted by Aβ aggregates in vitro (44). Thus, there is increasing evidence for an AD defect in insulin-like signaling that may limit glucose utilization, synaptic plasticity, and survival signaling. In fact, clinical trials with glitazone insulin sensitizing drugs (PPARγ agonists) have already had some clinical trial success in ApoE3 but not ApoE4 carriers (41,45). This is reminiscent of the apparent ApoE dependence of the ADCS clinical trial with DHA. DHA and its derivatives can be PPAR agonists, but whether this is physiological remains unclear (46). There are other pathways for DHA effects on insulin-like signaling. One relevant mechanism is hyperphosphorylation of insulin receptor substrate (IRS), an adaptor protein coupling insulin and other neurotrophic factor signaling to PI3-K/Akt survival signaling upstream of c-jun N-terminal kinase (JNK) and glycogen synthase kinase 3 beta (GSK3beta). Aβ oligomer–activated τ kinases JNK and GSK3β also cause hyperphosphorylation of IRS, resulting in resistance to insulin/neurotrophic factor signaling, but the (n-3) fatty acid DHA protects from the accumulation of both phospho-IRS and phospho-τ (similar to that seen in neurons in AD brain) in both oligomer-treated neurons and AD model triple transgenic mice (40). Because DHA increases neuroprotective brain-derived neurotrophic factor and also reduces Aβ and Aβ-induced insulin/trophic factor resistance, it should have a potent pleiotropic protective effect against Aβ's synaptotoxic and neurotoxic activities. Figure 1 summarizes some of these anti-Alzheimer pathways.
FIGURE 1 .
AD pathways targeted by DHA. AD is initiated by increased levels of Aβ-42 derived from APP, which traffics to a membrane compartment where secretase enzymes, including a complex dependent on presenilin 1 (PS1) generate Aβ. The chaperone LR11 traffics APP away from the secretases. DHA reduces Aβ production, reportedly by increasing LR11 and reducing PS1, leading to less Aβ and, thus, fewer toxic Aβ oligomers (AβO). DHA also increases levels of neuroprotectin D1 (NPD1) and neuroprotective brain-derived neurotrophic factor (BDNF). It improves signaling of BDNF, insulin, and other neurotrophic factors (NTF) through their receptors [NTF-R and insulin receptor (ins-R)], which autophosphorylate (P) at Tyr residues to couple to protective signaling via adaptor proteins, such as IRS. For example, IRS binds the p85 subunit of phosphatidylinositol 3-kinase (PI3K) to generate PIP3 lipid, which promotes binding and activation of downstream PDK and Akt kinases through their pleckstrin homology (PH) domains. DHA facilitates this activation by increasing phosphatidylserine (PS), which accelerates PH domain membrane binding. Activated Akt increases the Aβ protease, insulin degrading enzyme (IDE), inhibits the τ kinase GSK3β, and acts on multiple survival signals to promote neuron growth and survival. AβO acts on unidentified receptors (N-methyl-d-aspartate, integrin, and Prp), which signal through fyn to rac to cause toxicity via another τ kinase, JNK. JNK serves a priming kinase for GSK3β, also a τ kinase. Together, they phosphorylate and inactivate IRS, which leads not only to its rapid degradation and insulin/neurotrophic factor resistance, but also to accumulation of τ oligomers and tangles. In summary, DHA protects against these toxic pathways, reducing pJNK, ptau, and pIRS. DHA also reduces brain AA levels to limit AβO-induced increases AA via cytosolic phospholipase A2 (cPLA2) activation. Less AA means less downstream prostaglandin (PG), leukotriene (LT), and hydroxyeicosatetraenoic acid (HETE) products of COX and lipoxygenase (LOX). These AA products are implicated in inflammation and excitotoxicity, which, together with τ pathology and trophic signaling deficits, contribute to synaptic and cognitive deficits.
Thus, DHA protects against AD by reducing the initiating Aβ42 toxin as well as suppressing synaptotoxicity via τ kinase activation and the impact of these kinases on at least 2 important substrates, τ and IRS. Although in principal DHA should also reduce brain AA metabolites and inflammation, to date there is little or no evidence that the (n-3) fatty acid reduces biomarkers for neuroinflammation in cerebrospinal fluid of AD patients (47).
Pleiotropic activities needed for AD prevention
While DHA or fish oil have many potentially beneficial and potent effects, both clinical trial and animal model data suggest that the benefits are real but limited and may be improved by combining DHA with other nutrients. Because of its 6 double bonds, DHA is highly susceptible to lipid peroxidation, and lipid peroxidation products of DHA are elevated in brains of AD patients (48,49). Because DHA alone does not appear sufficient to control neuroinflammation in our models (G. M. Cole, G. P. Lim, and S. A. Frautschy, unpublished results) and it is readily oxidized, we advocate combining it with an antiinflammatory/antioxidant agent, such as curcumin (35). A small clinical trial has already produced positive results with fish oil plus lipoate (50). The Souvenaid trial with fish oil/DHA, UMP, B vitamins, and antioxidants represents another apparently successful approach (51)
Recognizing the need for an antioxidant and the limited efficacy of DHA as an NSAID, our group has focused on curcumin (diferulomethane), which was identified as the yellow pigment in the curry spice turmeric, a polyphenolic antioxidant consisting of 2 methoxyphenol groups linked by a β-diketone bridge. In turmeric, curcumin acts as good food preservative inhibitor of lipid peroxidation. It should protect DHA from lipid peroxidation, known to be elevated in AD and by Aβ aggregates. As a turmeric extract, curcumin has potent antiinflammatory activity contributing to a long history of use in traditional Asian and Ayruvedic medicine. It is the Indian version of vitamin E and aspirin, rolled into 1 molecule. In AD models, curcumin reduced pro-inflammatory cytokines, oxidative damage, and amyloid β protein and cognitive deficits (52). The limited bioavailability of curcumin in supplement preparations has stymied clinical trials (53), but this delivery problem has been solved with new lipidated formulations (54) that are already in clinical trials for cancer and AD. The combination of curcumin plus (n-3) fatty acids appears synergistic in reducing defects in a triple transgenic AD model (55) and in other AD models (our unpublished results). Many other combinations are possible, and we anticipate that cocktails combining DHA with other nutrients will prove useful for AD prevention.
Western diets, cardiovascular risk, and vascular dementia
While the most frequent cause of late onset dementia is AD, the second most frequent cause is vascular dementia. In fact, the risk factors for AD and cardiovascular disease (CVD) are generally shared (56), and CVD risk factors accelerate AD (57). One consequence is that varying with the population, perhaps one-third of all dementia cases is mixed dementia (58). Thus, any reasonable approach to reducing AD and dementia risk is to address the CVD risk factors. One of the most easily addressed risk factors for CVD is low intake of marine (n-3) fatty acids, which is typical of Western diets (59–61).
Conclusions
In conclusion, although there are many new AD treatment approaches under development, these are likely to be costly and have significant side effect issues. Prevention of dementia requires much greater safety and very few or no adverse side effects. Fish oil/DHA appears to be efficacious against AD with multiple pleiotropic activities in preclinical models and with some initial success in clinical trials with early (pre-AD) intervention. Larger trials in minimally cognitively impaired patients are warranted. The major advantages with DHA and related combined nutritional approaches with DHA and natural antioxidant (lipoate), polyphenolic (curcumin), or similar interventions are that individually these approaches have safety and side effect track records, broad spectrum utility in preclinical models, and low cost. There is a real opportunity to use them for prevention.
Acknowledgments
G.M.C. and S.A.F. played equal roles in designing and implementing animal experiments, securing funding, and writing and editing this manuscript. G.M.C. wrote the first draft, which was edited and rewritten by S.A.F. Both authors read and approved the final manuscript.
Published as a supplement to The Journal of Nutrition. Presented as part of the symposium “DHA and Neurodegenerative Disease: Models of Investigation” given at the Experimental Biology 2009 meeting, April 19, 2009 in New Orleans, LA. This symposium was sponsored the American Society for Nutrition and supported by an unrestricted educational grant from Martek Bioscience. The symposium was chaired by Jay Whelan and Robert K. McNamara. Guest Editor for this symposium publication was Cathy Levenson. Guest Editor disclosure: no conflicts to disclose.
This work was supported by National Center for Complementary and Alternative Medicine NIH R01AT3008, NIH National Institute on Aging RO1 AG13471, VA Merit and the Mary S. Easton Alzheimer's Center, and Easton Drug Discovery Consortium.
Author disclosures: G.M. Cole and S.A. Frautschy, no conflicts of interest.
Abbreviations used: AA, arachidonic acid; Aβ-42, amyloid β-protein 42; AD, Alzheimer's disease; ADAScog, Alzheimer's Disease Assessment Scale-cognitive subscale; ADCS, Alzheimer Disease Cooperative Studies; Apo, apolipoprotein; APP, amyloid precursor protein; CDR, clinical dementia rating; COX, cyclooxygenase; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosopentaenoic acid; GSK3, glycogen synthase kinase 3; IRS, insulin receptor substrate; JNK, c-jun N-terminal kinase; MMSE, minimental state examination; NSAID, nonsteroidal antiinflammatory drugs; PAL, Paired Associate Learning; PC, phosphatidylcholine.
References
- 1.Cummings JL, Cole G. Alzheimer disease. JAMA. 2002;287:2335–8. [DOI] [PubMed] [Google Scholar]
- 2.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation and amyloid plaques in transgenic mice. Science. 1996;274:99–102. [DOI] [PubMed] [Google Scholar]
- 3.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CC, Yang A, Gallagher M, Ashe KH. A specific amyloid-β assembly in the brain impairs memory. Nature. 2006;440:352–7. [DOI] [PubMed] [Google Scholar]
- 4.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–7. [DOI] [PubMed] [Google Scholar]
- 5.Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J Neurosci. 2000;20:5709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salem N, Jr., Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–59. [DOI] [PubMed] [Google Scholar]
- 7.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NE, Eikelenboom P, Emmerling M, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–47. [DOI] [PubMed] [Google Scholar]
- 9.Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, Mullan M. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laino C. In follow-up analysis of clinical trial, NSAIDs seem to preserve cognitive function in patients with healthy brains. Neurology Today. 2009;9:21–2. [Google Scholar]
- 11.Cole GM, Ma QL, Frautschy SA. Omega-3 fatty acids and dementia. Prostaglandins Leukot Essent Fatty Acids. 2009;81:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849–53. [DOI] [PubMed] [Google Scholar]
- 13.Kroger E, Verreault R, Carmichael PH, Lindsay J, Julien P, Dewailly E, Ayotte P, Laurin D. Omega-3 fatty acids and risk of dementia: the Canadian Study of Health and Aging. Am J Clin Nutr. 2009;90:184–92. [DOI] [PubMed] [Google Scholar]
- 14.Heude B, Ducimetiere P, Berr C. Cognitive decline and fatty acid composition of erythrocyte membranes–the EVA Study. Am J Clin Nutr. 2003;77:803–8. [DOI] [PubMed] [Google Scholar]
- 15.Whalley LJ, Fox HC, Wahle KW, Starr JM, Deary IJ. Cognitive aging, childhood intelligence, and the use of food supplements: possible involvement of n-3 fatty acids. Am J Clin Nutr. 2004;80:1650–7. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545–50. [DOI] [PubMed] [Google Scholar]
- 17.Selley ML. A metabolic link between S-adenosylhomocysteine and polyunsaturated fatty acid metabolism in Alzheimer's disease. Neurobiol Aging. 2007;28:1834–9. [DOI] [PubMed] [Google Scholar]
- 18.Maclean CH, Issa AM, Newberry SJ, Mojica WA, Morton SC, Garland RH, Hilton LG, Traina SB, Shekelle P. Effects of omega-3 fatty acids on cognitive function with aging, dementia, and neurological diseases. Evid Rep Technol Assess (Summ). 2005;114:1–3. [PMC free article] [PubMed] [Google Scholar]
- 19.Yurko-Mauro K. Cognitive and Cardiovascular Benefits of Docosahexaenoic Acid in Aging and Cognitive Decline. Curr Alzheimer Res. Epub Jan 21. [DOI] [PubMed]
- 20.Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ. Early detection and differential diagnosis of Alzheimer's disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12:265–80. [DOI] [PubMed] [Google Scholar]
- 21.Quinn JF. A clinical trial of docosahexaenoic acid (DHA) for the treatment of Alzheimer's disease. Alzheimers Dementia. 2009;5(4) supplement: P84. [Google Scholar]
- 22.Rzehak P, Heinrich J, Klopp N, Schaeffer L, Hoff S, Wolfram G, Illig T, Linseisen J. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 (FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br J Nutr. 2009;101:20–6. [DOI] [PubMed] [Google Scholar]
- 23.Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Jr., Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oksman M, Iivonen H, Hogyes E, Amtul Z, Penke B, Leenders I, Broersen L, Lutjohann D, Hartmann T, Tanila H. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol Dis. 2006;23:563–72. [DOI] [PubMed] [Google Scholar]
- 25.Hooijmans CR, Rutters F, Dederen PJ, Gambarota G, Veltien A, van Groen T, Broersen LM, Lutjohann D, Heerschap A, et al. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD). Neurobiol Dis. 2007;28:16–29. [DOI] [PubMed] [Google Scholar]
- 26.Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L, LaFerla FM. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci. 2007;27:4385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arendash GW, Jensen MT, Salem N, Jr., Hussein N, Cracchiolo J, Dickson A, Leighty R, Potter H. A diet high in omega-3 fatty acids does not improve or protect cognitive performance in Alzheimer's transgenic mice. Neuroscience. 2007;149:286–302. [DOI] [PubMed] [Google Scholar]
- 29.Calon F, Lim GP, Morihara T, Yang F, Ubeda O, Salem NJ, Frautschy SA, Dietary GMC. n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer's disease. Eur J Neurosci. 2005;22:617–26. [DOI] [PubMed] [Google Scholar]
- 30.Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev. 2005;45:559–79. [DOI] [PubMed] [Google Scholar]
- 31.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma QL, Teter B, Ubeda OJ, Morihara T, Dhoot D, Nyby MD, Tuck ML, Frautschy SA, Cole GM. Omega-3 fatty acid docosahexaenoic acid increases SorLA/LR11, a sorting protein with reduced expression in sporadic Alzheimer's disease (AD): relevance to AD prevention. J Neurosci. 2007;27:14299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarzman AL, Gregori L, Vitek MP, Lyubski S, Strittmatter WJ, Enghilde JJ, Bhasin R, Silverman J, Weisgraber KH, et al. Transthyretin sequesters amyloid b protein and prevents amyloid formation. Proc Natl Acad Sci USA. 1994;91:8368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puskas LG, Kitajka K, Nyakas C, Barcelo-Coblijn G, Farkas T. Short-term administration of omega 3 fatty acids from fish oil results in increased transthyretin transcription in old rat hippocampus. Proc Natl Acad Sci USA. 2003;100:1580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr., et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA. 2005;102:10858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr., Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. [DOI] [PubMed] [Google Scholar]
- 38.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21:1457–67. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L, Ma QL, Calon F, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, et al. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–42. [DOI] [PubMed] [Google Scholar]
- 40.Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, Chen PP, Hudspeth B, Chen C, et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci. 2009;29:9078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole GM, Frautschy SA. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer's Disease. Exp Gerontol. 2007;42:10–21. [DOI] [PubMed] [Google Scholar]
- 43.Moloney AM, Griffin RJ, Timmons S, O'Connor R, Ravid R, O'Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2008;31:224–43. [DOI] [PubMed] [Google Scholar]
- 44.Tong L, Balazs R, Thornton PL, Cotman CW. Beta-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. J Neurosci. 2004;24:6799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 2006;6:246–54. [DOI] [PubMed] [Google Scholar]
- 46.Gani OA. Are fish oil omega-3 long-chain fatty acids and their derivatives peroxisome proliferator-activated receptor agonists? Cardiovasc Diabetol. 2008;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freund-Levi Y, Hjorth E, Lindberg C, Cederholm T, Faxen-Irving G, Vedin I, Palmblad J, Wahlund LO, Schultzberg M, et al. Effects of omega-3 fatty acids on inflammatory markers in cerebrospinal fluid and plasma in Alzheimer's disease: the OmegAD study. Dement Geriatr Cogn Disord. 2009;27:481–90. [DOI] [PubMed] [Google Scholar]
- 48.Nourooz-Zadeh J, Liu EHC, Yhlen B, Anggard EE, Halliwell B. F4-Isoprostanes as specific marker of docosahexaenoic acid peroxidation in Alzheimer's disease. J Neurochem. 1999;72:734–40. [DOI] [PubMed] [Google Scholar]
- 49.Montine KS, Quinn JF, Zhang J, Fessel JP, Roberts LJ 2nd, Morrow JD, Montine TJ. Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem Phys Lipids. 2004;128:117–24. [DOI] [PubMed] [Google Scholar]
- 50.Shinto L, Quinn J, Montine T, Baldauf-Wagner SB, Bourdette D, Oken DB, Kaye J. Omega-3 fatty acids and lipoic acid in Alzheimer's disease. Neurology. 2008;70:A393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheltens P, Kamphuis PJ, Verhey FR, Olde Rikkert MG, Wurtman RJ, Wilkinson D, Twisk JW, Kurz A. Efficacy of a medical food in mild Alzheimer's disease: A randomized, controlled trial. Alzheimers Dement. 2010. Jan;6 (1):1–10.e1. [DOI] [PubMed] [Google Scholar]
- 52.Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lao CD, Ruffin MT 4th, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J Pharmacol Exp Ther. 2008;326:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Q, Dong Y, Huang L, Yang S, Chen W. Study of cerebrovascular reserve capacity by magnetic resonance perfusion weighted imaging and photoacoustic imaging. Magn Reson Imaging. 2009;27:155–62. [DOI] [PubMed] [Google Scholar]
- 56.Rosendorff C, Beeri MS, Silverman JM. Cardiovascular risk factors for Alzheimer's disease. Am J Geriatr Cardiol. 2007;16:143–9. [DOI] [PubMed] [Google Scholar]
- 57.Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72:368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cleland LG, Caughey GE, James MJ, Proudman SM. Reduction of cardiovascular risk factors with longterm fish oil treatment in early rheumatoid arthritis. J Rheumatol. 2006;33:1973–9. [PubMed] [Google Scholar]
- 60.Lee JH, O'Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega-3 fatty acids for cardioprotection. Mayo Clin Proc. 2008;83:324–32. [DOI] [PubMed] [Google Scholar]
- 61.Griffin BA. How relevant is the ratio of dietary n-6 to n-3 polyunsaturated fatty acids to cardiovascular disease risk? Evidence from the OPTILIP study. Curr Opin Lipidol. 2008;19:57–62. [DOI] [PubMed] [Google Scholar]