Abstract
Androgens appear to enhance, whereas estrogens mitigate, cardiac hypertrophy. However, signaling pathways in cells for short (3 min) and longer term (48 h) treatment with 17β-estradiol (E2) or 5α-dihydrotestosterone (DHT) are understudied. We compared the effect of adrenergic stimulation by norepinephrine (NE; 1 μM) alone or in combination with DHT (10 nM) or E2 (10 nM) treatment in neonatal rat ventricular myocytes (NRVMs) by cell area, protein synthesis, sarcomeric structure, gene expression, phosphorylation of extracellular signal-regulated (ERK), and focal adhesion kinases (FAK), and phospho-FAK nuclear localization. NE alone elicited the expected hypertrophy and strong sarcomeric organization, and DHT alone gave a similar but more modest response, whereas E2 did not alter cell size. Effects of NE dominated when used with either E2 or DHT with all combinations. Both sex hormones alone rapidly activated FAK but not ERK. Long-term or brief exposure to E2 attenuated NE-induced FAK phosphorylation, whereas DHT had no effect. Neither hormone altered NE-elicited ERK activation. Longer term exposure to E2 alone reduced FAK phosphorylation and reduced nuclear phospho-FAK, whereas its elevation was seen in the presence of NE with both sex hormones. The mitigating effects of E2 on the NE-elicited increase in cell size and the hypertrophic effect of DHT in NRVMs are in accordance with results observed in whole animal models. This is the first report of rapid, nongenomic sex hormone signaling via FAK activation and altered FAK trafficking to the nucleus in heart cells.
Keywords: focal adhesion kinase, nuclear-cytoplasmic shuttle, rapid hormone response, sarcomere organization
sustained cardiac hypertrophy is associated with increased risk for arrhythmias, sudden death, and heart failure (38, 62). Results from epidemiological studies indicate that prevalence of hypertrophy is lower in women (30, 40, 62) and that given the same mechanical load on the heart, women are less likely to develop hypertrophy than men (16). Left ventricular wall thickness increases after menopause (1) with greater relative wall thickness found in postmenopausal women compared with age-, race-, and weight-matched premenopausal women (33). Furthermore, evidence suggests that hormone replacement therapy reduces left ventricular mass in postmenopausal women (44, 45). In addition, healthy men have larger hearts than age-matched women even when indexed to body size, and the ventricular size divergence develops following puberty (23, 53). These findings suggest that being female and/or having female sex hormones diminishes the cardiac hypertrophic response. Alternatively, male sex or androgenic hormones may contribute to and enhance hypertrophy; a possibility supported by the finding that testosterone elicits cardiac hypertrophy in sham-operated and postmyocardial infarction (post-MI) male rats (46), and studies demonstrating testosterone and 5α-dihydrotestosterone (DHT) elicit hypertrophy in isolated neonatal rat ventricular myocytes (NRVMs) (3, 41).
To our knowledge, a direct comparison of the influence of male and female sex hormones on extracellular signal-induced hypertrophy in cardiac myocytes has not been conducted, and there has been no direct comparison of the effect of sex hormones on hypertrophic growth and signaling pathways. In this study, we examined the direct effect of 17β-estradiol (E2) and DHT on NRVM hypertrophy and the modulatory effect of sex hormones on norepinephrine (NE)-elicited hypertrophy. Myocardial hypertrophy develops in response to multiple initiating stimuli, including mechanical stress (from hemodynamic overload) and extracellular signals (such as catecholamines and growth factors). Mechanical stress is communicated into the cell via integrins that link the extracellular matrix to a network of intracellular structural and signal-transducing proteins, including a cytoplasmic tyrosine kinase called focal adhesion kinase (FAK). FAK phosphorylation and activation is well established to be associated with the hypertrophic response. Extracellular signals, including endothelin-1, angiotensin II, and the well-studied α-adrenergic agonist phenylephrine, and others activate multiple mitogen-activated protein kinases (MAP kinases), including extracellular signal-regulated kinase (ERK). ERK has been established to have an important role in cardiac cell hypertrophy. Activation of hypertrophic pathways via different initiating signals is not independent but has complex downstream interactions (see reviews, Refs. 6, 11, 13, 52).
Given that estrogen is considered protective in the context of hypertrophy and androgens are thought to elicit and/or contribute to cardiac hypertrophy; the aims of this study were 1) to compare the direct effects of E2 and DHT on cardiac hypertrophy in isolated NRVMs, 2) to compare the effect of these two sex hormones on NE-induced hypertrophy, 3) to compare the effect of E2 and DHT on the activation of kinases previously shown to be involved in transduction of hypertrophic signals (ERK and FAK), and 4) to determine whether male and female sex hormone-elicited effects on ERK and FAK are the result of rapid steroid signaling.
METHODS
Cell culture.
Hearts of 1- to 2-day-old Sprague-Dawley male and female rat pups (Harlan, Indianapolis, IN) were uses to isolate NRVM by stepwise collagenase digestions as reported previously (9). The cells were preplated for 1 h to reduce nonmyocyte contamination before incubation in phenol-free Dulbecco's modified Eagle's medium/nutrient mixture F-12 HAM (Sigma, St. Louis, MO) without l-glutamine and with standard amino acid concentrations, palmitic acid (2.56 mg/l) and linoleic acid (0.84 mg/l), penicillin G (100 IU/ml), streptomycin (0.1 mg/ml), and amphotericin (0.25 μg/ml) with 5% charcoal-dextran-filtered steroid-free fetal bovine serum (FBS; Hyclone; Logan, UT). Cytosine-β-d-arabino-furanoside (ARA-C; 5 μg/ml) was added to prevent fibroblast proliferation. After 24 h in a 5% CO2, 37°C incubator, unattached cells were removed by aspiration and the remaining cells were washed three times with ITS buffer [serum-free, phenol-free DMEM with the addition of insulin (5 μg/ml), transferrin (1 μg/ml), and selenium (5 ng/ml) (ITS Premix; BD Biosciences, Bedford, MA)]. The cells were maintained in ITS buffer with ARA-C and antibiotics throughout the experimental protocols. NRVM were plated at 30–60% confluency on 100-mm Primaria culture dishes (BD Bioscience) unless otherwise stated. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996) and were approved by the Institutional Animal Care and Use Committee.
Experimental protocols.
To examine the effect of sex hormones on NE-elicited hypertrophy and to answer the question whether long-term or short-term hormone exposure is necessary for the modulation of the NE-induced hypertrophic response, we develop three protocols. Protocol 1 was designed to answer our first two aims listed above, i.e., to determine the effect of sex hormones on NRVM hypertrophy and to determine the effect of sex hormones on NE-induced NRVM hypertrophy. In protocol 1, NRVMs were exposed to NE (1 μM) in the presence or absence of E2 (10 nM) or DHT (10 nM) for 48 h. Forty-eight hours of exposure to NE have been shown by others to reliably produce NRVM hypertrophy (54). Protocol 2 was designed to answer the third aim, i.e., to determine whether sex hormones alter NE-elicited activation of kinases shown to be involved in hypertrophic signaling. NE-elicited kinase activation is a rapid response, whereas genomic steroid signaling (alteration in gene expression) may require hours to days. Therefore, after exposure to E2 or DHT (or no treatment) for 48 h, NRVM were stimulated with NE for 2 min. Protocol 3 was designed to answer the fourth aim, i.e., to determine whether short-term sex hormone exposure (rapid steroid signaling) altered NE-elicited kinase activation. Cells were incubated for 48 h without treatment, followed by exposure to E2 or DHT for 1 min and then to NE (or no treatment) for another 2 min. Because early evidence suggests that the NE-elicited hypertrophic response is mediated through α1-adrenergic receptors (54), phenylephrine (PE) is frequently used experimentally as a hypertrophic signal. However, others have found that both α- and β-adrenergic receptors are involved in mediating the hypertrophic response (5). We chose to examine the effect of sex hormones on NE-elicited hypertrophy in NRVMs because it is a clinically relevant, endogenous mixed α- and β-adrenergic hypertrophic stimulus, and there is no known endogenous α-adrenergic ligand. However, we added PE (10 μM) as a positive control in all experiments. Thus all protocols included the following groups: no treatment (negative control) and exposure to NE alone, E2 alone, E2 and NE, DHT alone, DHT and NE, or PE alone.
Drug treatment.
Drugs were dissolved in phosphate-buffered saline (PBS). E2 required dimethyl sulfoxide (DMSO) at a final concentration of <0.001% to facilitate solution. E2, DHT, and NE were obtained from Sigma (St. Louis, MO) and were added to 100-mm culture dishes in 100-μl aliquots.
Cell surface area analysis.
Myocyte surface area was determined in cells exposed to sex hormones and/or NE for 48 h (protocol 1). The concentration of DMSO was shown to have no effect on cell surface area. Cells were visualized by light microscopy (Nikon Microphot-FXA) with a ×40 objective. Four randomly chosen fields of cells were selected from each 100-mm culture dish (2 dishes per treatment per experiment) and digitally photographed using a Spot RT charge-coupled device camera (Diagnostic Instruments). Cells within each field were outlined, and two-dimensional area was obtained using ImageJ software (National Institutes of Health, Bethesda, Maryland). Approximately 50 cells were measured for each treatment in each experiment, and the final mean surface area for each treatment was determined from three separate experiments.
Tritiated-leucine incorporation.
NRVM were plated on 30-mm Primaria cell culture dishes. Sex hormones, NE, and 0.5 μCi of [3H]leucine {l-[3,4,5-3H(N)]; PerkinElmer Life Sciences, Boston, MA} were added together to the cells in 2 ml of ITS buffer. Forty-eight hours later (protocol 1), the cells were washed three times with PBS and then incubated with shaking at room temperature in PBS (0.5 ml) with sodium dodecylsulfate (SDS; 10%) and trichloroacetic acid (TCA; 5%). Cells were scraped off the dishes, and a 0.25-ml sample was taken for liquid scintillation counting in Econo-Safe liquid scintillation fluid (Research Products International, Mt. Prospect, IL). Counting error was <2%.
Determination of mRNA.
NRVM were treated with E2, DHT, and NE (48 h, protocol 1). RNA was isolated using Trizol (Invitrogen) according to the manufacturer's instructions, and concentration was determined by NanoDrop spectrophotometry (Thermo Fischer). Complementary DNA was synthesized from 1 μg of RNA using the SuperScript III First-Strand synthesis system (Invitrogen). Real-time PCR was run in triplicate on 10 ng of cDNA per well with ribosomal RNA (18S) as an endogenous control and detected by TaqMan chemistry (fluorogenic 5′ nuclease chemistry) using the AB7300 real-time PCR system (Applied Biosystems). TaqMan probe accession numbers (Applied Biosystems) for each RNA of interest are as follows: brain natriuretic peptide (BNP), Rn00580641_m1; atrial natriuretic peptide (ANP), Rn00561661_m1; skeletal actin, Rn00570060_g1; and β-myosin heavy chain (β-MHC), Rn000568328_m1. The comparative threshold cycle (CT) method was used for relative quantification.
Western blotting.
After described NE and hormone treatments, cells were rinsed with cold PBS and then scraped in ice-cold lysis buffer with sodium vanadate (1 mM). Protein concentrations were assessed using the Bradford assay (Pierce, Rockford, IL), and then equal amounts of protein were separated by 4–20% SDS-PAGE and transferred to nitrocellulose membranes (Hybond; Amersham, Arlington Heights, IL). Proteins were probed with appropriate antibodies, including FAK mouse antibody (BD Transduction, Lexington, KY), antibody to recognize phosphorylated FAK at tyrosine 397 (FAK pY397 rabbit; Biosource, Camarillo, CA), ERK1/2 rabbit antibody (Promega, Madison, WI), and ERK1/2 pTEpY rabbit antibody (Promega). Corresponding horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies were visualized by enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL). The bands corresponding to specified proteins were quantified by laser densitometry. Samples were normalized to GAPDH antibody to insure equal loading.
Immunochemistry and image analysis.
NRVM were washed in Ca2+- and Mg2+-free PBS. Fixation was carried out by submersion in 4% paraformaldehyde-PBS (Fisher, Fairlawn, NJ) for 10 min at room temperature, followed by a wash in 70% ethanol for 5 min. Cells were stored in 70% ethanol at −20°C until use. Immunostaining was performed as previously described using anti-FAK antibody (BD Transduction, CA), Alexa-488 phalloidin (Molecular Probes, Carlsbad, CA), phospho-anti-FAK pY397 antibody (Biosource), and appropriate fluorescence-labeled secondary antibodies. Membranes were then mounted on glass slides with 4′,6′-diamidino-2-phenylindole nuclear stain (DAPI; Vector Laboratories, Burlingame, CA) as an antifade agent. Fluorescently labeled cells were then viewed using a Zeiss model LSM 510 laser scanning confocal microscope.
Data analysis.
All values are means ± SE, with n = 3–4 separate experiments. Data were analyzed using one-way ANOVA followed by a Bonferroni multiple comparison post hoc test using GraphPad Prism statistical software (San Diego, CA). Differences among means were considered significant at P < 0.05.
RESULTS
Sex hormones differentially alter cell size and protein synthesis and modulate NE-induced changes in hypertrophic indicators (protocol 1).
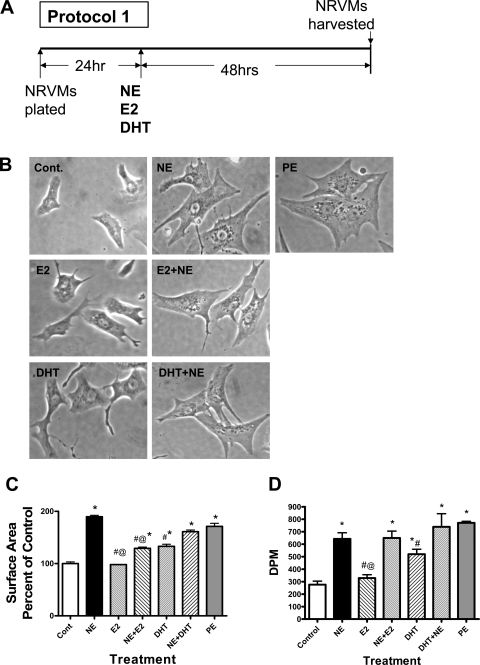
NE (1 μM, 48 h) and PE (10 μM, 48 h) treatment of NRVMs was associated with a similar degree of cellular hypertrophy (Fig. 1A), which was confirmed by calculating cell surface area using ImageJ software (Fig. 1B). E2 alone did not alter cell surface area, whereas DHT caused a small but significant increase (136 ± 4% of control). E2 significantly attenuated the NE-induced increase in cell size, whereas DHT had no significant effect. Treatment with either E2 or DHT alone consistently resulted in the appearance of long, thin filopodia extending from the cells (Fig. 1A).
Fig. 1.
Effect of sex hormones on norepinephrine (NE)-induced cardiac hypertrophy. Cells were treated with sex hormones alone, NE alone, or NE with sex hormones for 48 h (protocol 1). E2, 17β-estradiol; DHT, 5α-dihydrotestosterone; NVRMs, neonatal rat ventricular myocytes. A: schematic illustration of protocol 1. B: light microscopy showing cells at ×40 magnification. PE, phenylephrine. C: 2-dimensional cell surface area as a percentage of control. Bars represents means ± SE of determinations from 3 separate experiments. D: NRVMs were treated with E2, DHT, and NE in the presence of 0.5 μCi of [3H]leucine for 48 h. Cells were harvested, and 3H incorporation was determined by liquid scintillation counting. Bars represent mean (±SE) disintegrations per minute (DPM) (n = 3). Except for E2, all treatments are significantly different from control *P < 0.05, significantly different from control (no treatment). #P < 0.05, significantly different from NE. @P < 0.05, significant difference between E2 and DHT treatment in either the presence or absence of NE.
Incorporation of [3H]leucine into NRVMs was used to represent a measure of cellular protein synthesis (Fig. 1C). Forty-eight hours of NE exposure induced a greater than twofold increase in [3H]leucine incorporation. Tritiated leucine incorporation in the presence of E2 alone was similar to control, whereas all of the other treatments (E2 + NE, DHT alone, and DHT + NE) significantly increased incorporation. DHT stimulated [3H]leucine incorporation to a greater degree than E2. However, neither E2 nor DHT reduced the NE-elicited increase in [3H]leucine incorporation into cellular proteins.
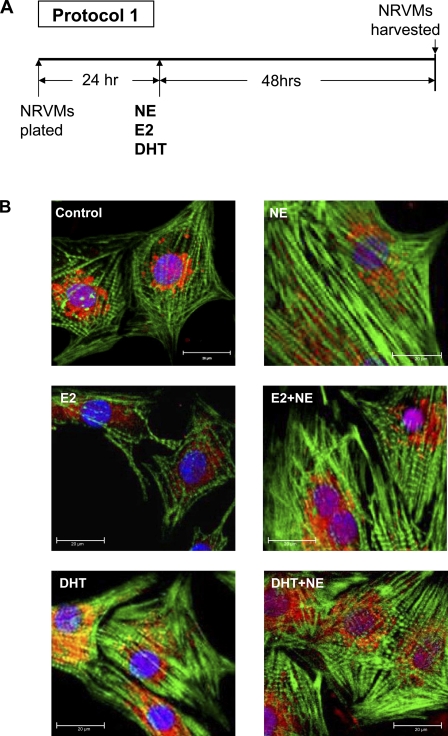
Sex hormones and/or NE treatment induces sarcomeric reorganization (protocol 1).
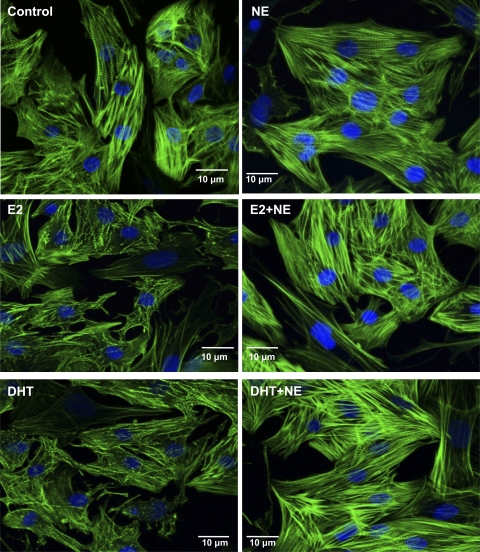
Confocal images of NRVMs counterstained with FITC-conjugated phalloidin, which stained actin filaments, revealed an NE-elicited increase in actin stress fibers (Fig. 2). In the absence of NE, both sex hormones appeared to reduce sarcomeric organization compared with control. However, the striations reappeared when NE stimulation was combined with either hormone treatment.
Fig. 2.
Effect of sex hormones and/or NE treatment on NRVM sarcomeric organization. Cells were treated with sex hormones alone, NE alone, or NE with sex hormones for 48 h (protocol 1). Confocal micrographs of myocytes after incubation with E2, DHT, and/or NE for 48 h. Actin is visualized with FITC-conjugated phalloidin stain (green), and the nucleus is visualized by 4′,6′-diamidino-2-phenylindole staining (blue).
Sex hormone effects and sex hormone-induced modulation of NE-elicited alterations in expression of hypertrophic gene markers (protocol 1).
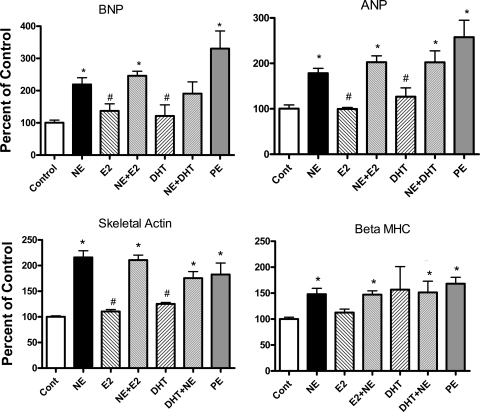
The effect of sex hormones and/or NE and PE on the expression of hypertrophic marker genes was examined (Fig. 3). RNA was isolated from NRVMs continuously exposed to sex hormones alone, to NE or PE alone, or to E2+NE or DHT+NE for 48 h. PE served as a positive control for the expected increases in BNP, ANP, skeletal actin, and β-MHC. Both PE and NE treatment significantly increased the abundance of BNP, ANP, skeletal actin, and β-MHC. Neither E2 nor DHT treatment alone significantly altered mRNA level of marker genes. Neither sex hormone altered the NE-elicited increase in expression of the aforementioned genes.
Fig. 3.
Effect of sex hormones on NE-elicited alterations in expression of marker hypertrophic genes. NRVMs were treated with sex hormones alone, NE alone, or NE with sex hormones for 48 h (protocol 1) and then harvested to determine levels of brain natriuretic peptide (BNP), atrial natriuretic peptide (ANP), skeletal actin, and β-myosin heavy chain (β-MHC) mRNA by real-time RT-PCR. Bars represent mean (±SE) mRNA as a percentage of control value from 4 separate experiments. *P < 0.05, significantly different from control. #P < 0.05, significantly different from NE alone.
Long-term (48 h) sex hormone exposure in the presence or absence of long-term (48 h) NE exposure alters ERK and FAK phosphorylation (protocol 1).
Previous reports have shown that FAK and various MAP kinases become activated in cultured cardiac myocytes in response to hypertrophic agonists and mechanical stimulation. Therefore, we examined the effects of E2 and DHT alone on phosphorylation of ERK (p44 and p42) and FAK (pY397) and the influence of sex hormones on NE-elicited activation of these kinases. Long-term (48 h) exposure of NRVMs to NE or PE alone, sex hormones alone, or sex hormone and NE all resulted in a decrease in total ERK phosphorylation compared with control. The reduction in phosphorylation ranged from 24 to 36% with no significant differences among the treatment groups. Forty-eight hours of exposure to NE, PE, sex hormones alone, or sex hormones in the presence of NE also resulted in a nonsignificant reduction in FAK phosphorylation at the tyrosine 397 residue (15–25%). Again, there were no significant differences among the treatment groups (data not shown).
Long-term (48 h) sex hormone exposure directly alters FAK activation and modulates rapid (2 min) NE-elicited FAK phosphorylation (protocol 2).
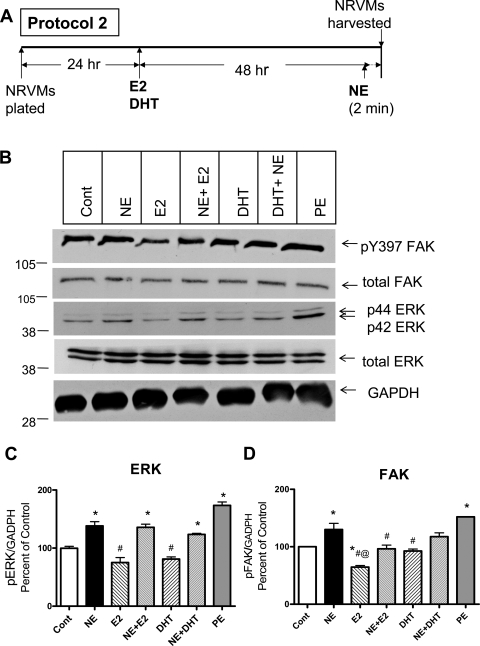
To examine effect of sex hormones on rapid NE-elicited activation of ERK and FAK, we exposed NRVM to sex hormones for 48 h and then to NE for 2 min (Fig. 4). Two-minute exposure of NRVMs to NE and PE significantly stimulated ERK and FAK phosphorylation. As in the previous experiment, 48 h of exposure to E2 and DHT alone reduced ERK phosphorylation (75 ± 8 and 81 ± 4% of control, respectively), but the effect was not significant. Exposure to E2 alone decreased FAK phosphorylation to 65 ± 3% of control, whereas DHT alone did not significantly alter FAK phosphorylation (93 ± 4% of control). E2 had no effect on NE-induced activation of ERK but significantly reduced NE-elicited FAK activation. In contrast, DHT had no effect on the NE-elicited activation of either kinase.
Fig. 4.
Effect of long-term (48 h) sex hormone exposure on rapid (2 min) NE-elicited alterations in ERK and focal adhesion kinase (FAK) phosphorylation. NRVMs were incubated with either E2 or DHT for 48 h and then stimulated with NE (or diluent, control) for 2 min (protocol 2). A: schematic illustration of protocol 2. B: typical Western blots. C and D: mean (±SE) phosphorylated total ERK (pERK; C) and phosphorylated FAK (pFAK; D) normalized to GADPH and expressed as a percentage of control from 3 separate experiments. *P < 0.05, significantly different from control. #P < 0.05, significantly different from NE alone. @P < 0.05, significant difference between sex hormone treatments.
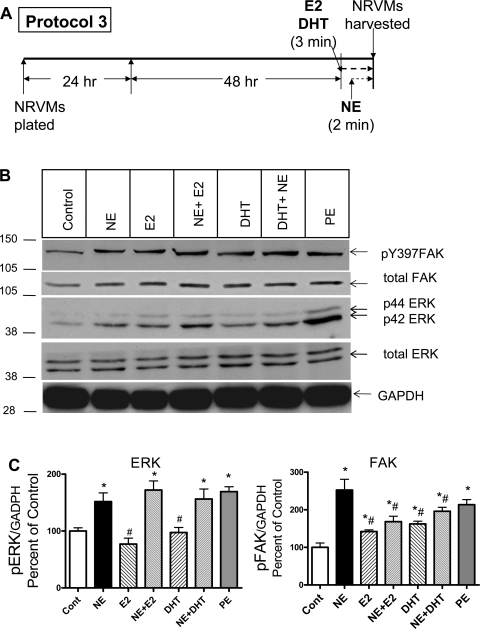
Short-term (3 min) sex hormone exposure alters FAK phosphorylation and modulates rapid (2 min) NE-elicited FAK activation (protocol 3).
To determine whether the effect of sex hormones alone and the effect of sex hormones on NE-elicited activation of ERK and FAK could be the result of a rapid signal transduction mechanism rather than a classic genomic signal transduction mechanism (alteration in gene expression), we exposed myocytes to sex hormone for just 1 min and then to NE for an additional 2 min (Fig. 5). Short-term exposure to sex hormones had no direct influence on ERK phosphorylation, but both E2 and DHT significantly increased FAK phosphorylation compared with control. With respect to the effect of short-term sex hormone exposure on NE-elicited kinase phosphorylation, neither hormone altered the NE-elicited increase in ERK phosphorylation, but E2 significantly reduced NE-induced FAK phosphorylation (31 ± 6% of control).
Fig. 5.
Effect of short-term sex hormone exposure on NE-elicited ERK and FAK phosphorylation. One minute after exposure to sex hormones, NRVMs were stimulated with NE or diluent for 2 min (protocol 3). A: schematic illustration of protocol 3. B: typical Western blots. C and D: mean (±SE) phosphorylated total ERK (C) or FAK (D) normalized to GADPH and expressed as a percentage of control from 3 separate experiments. *P < 0.05, significantly different from control. #P < 0.05, significantly different from NE alone.
Effect of 48-h sex hormone exposure and/or 48-h NE treatment on phosphorylated FAK localization (protocol 1).
Association of the focal adhesion complex, the integrin receptor, and the actin cytoskeleton suggests an important role for FAK in the regulation of sarcomere length during cardiac myocyte remodeling. We examined the subcellular localization of phosphorylated FAK by confocal microscopy in NRVMs treated with NE in the presence or absence of sex hormones. Images of immunostained NRVMs (Fig. 6) show that under control conditions, pY397 FAK (stained red) localized around the cell nucleus (blue) with a limited amount of phosphorylated FAK within the nucleus. After 48 h of NE treatment phosphorylated FAK was still localized around the nucleus but was also distributed to a limited degree along the sarcomeres closer to the nucleus. Much less phosphorylated FAK was found in E2-treated cells, and essentially none was located in the cell nucleus. However, in the presence of NE and E2, phosphorylated FAK appeared to be highly localized within the nucleus as demonstrated by the violet (red + blue) color. In DHT-treated cells, phosphorylated FAK was localized primarily around the nucleus, whereas in cells treated with DHT and NE, phosphorylated FAK was localized both within and around the nucleus.
Fig. 6.
Effect of sex hormone and NE treatment on FAK phosphorylation and localization. Distribution of phosphorylated FAK was examined in NRVMs treated with sex hormones alone, NE alone, or NE with sex hormones for 48 h (protocol 1). A: schematic illustration of protocol 1. B: distribution of phosphorylated FAK after 48 h of treatment with NE alone and sex hormone in the presence or absence of NE. Images are confocal micrographs of immunostained NRVMs. Cell nuclei are stained blue, phosphorylated FAK (Y397) is immunostained red, and actin is stained green.
DISCUSSION
Cardiac hypertrophy is mediated by mechanical stressors such as volume or pressure overload, by extracellular signals, and by interaction between these two stimuli (53). Many animal models have been created to investigate the effect of sex and sex hormones on the development of cardiac hypertrophy, and although there are exceptions (15, 26, 64), the results overwhelmingly suggest that being female and/or having estrogenic hormones reduces the response to hypertrophic stimuli, slows progression to failure, and improves survival. Animal models of hypertrophy elicited by gene overexpression [e.g., phospholamban (22), β2-adrenergic receptor (29), or tumor necrosis factor receptor (34)] or by hemodynamic pressure or volume overload have shown male rodents progress more rapidly to hypertrophy compared with females (12, 25, 31, 57), and ovariectomy mitigates the female advantage (12). Attenuation of pressure overload hypertrophy was found to depend on E2 (63) and to be mediated by estrogen receptor-β in female mice (55). In contrast to the effects of estrogen, evidence indicates that androgenic hormones elicit hypertrophy and accelerate progression to myocardial dilation and failure (41, 47). Testosterone increases cardiac hypertrophy and dilation and worsens function in female rats and mice post-MI, and estrogen attenuates or abolishes these effects (18, 28, 60).
Despite many investigations of sex difference in the development of cardiac hypertrophy and the potential role of sex hormones, to our knowledge this is the first study to compare the direct effects of E2 and DHT on NRVM hypertrophy and to directly compare the effect of male and female sex hormones on NE-induced hypertrophy in a cardiac cell culture model. Exposure of NRVM to NE for 48 h produced the expected hypertrophy, strong sarcomeric organization, and enhanced expression of hypertrophic marker genes. Estrogen exposure did not directly alter cell size or any other indicator of hypertrophy. DHT elicited a hypertrophic response as indicated by an increase in cell size and [3H]leucine incorporation, but the response was more modest than that observed with NE. DHT did not influence the NE-elicited hypertrophy. In contrast, E2 significantly reduced the NE-induced increase in cell size. Our findings are in accordance with the results of others who have investigated the hypertrophic effects of E2 or DHT/testosterone separately in cultured cardiac cells and in whole animal models. They also are congruent with studies demonstrating antihypertrophic effects of E2 in humans. The results confirm dimorphic sex hormone effects exerted at the cellular level by direct comparison in the same isolated cardiomyocyte model and suggest the sexually dimorphic pattern of cardiac hypertrophy is at least partially mediated by the direct cellular effects of sex hormones.
We used several different experimental measures commonly associated with cardiac hypertrophy in this investigation. The primary definition of cardiac hypertrophy is “augmentation of ventricular mass as a result of increased myocyte size” (17). Thus an increase in cell size would be considered the most robust measure. However, other markers generally considered indicative of hypertrophy include increased protein synthesis, increased sarcomeric organization, and the reexpression of fetal genes (27). Assuming these are valid indicators, the results of our investigation raise several questions, such as why does DHT increase cell size and [3H]leucine incorporation but appear to reduce sarcomeric organization and fail to increase the expression of fetal genes? And, why does E2 mitigate the NE-induced increase in cell size but not reduce NE-elicited increase in fetal gene expression and [3H]leucine incorporation? Our results also contrast with others demonstrating that E2 increases ANP expression (4, 49) and DHT (but not testosterone) stimulates cellular ANP secretion (41) in NRVMs. Although our results do not agree with those of some others, their validity is supported by the predicted PE- and NE-induced increase in expression of ANP, skeletal actin, BNP, and β-MHC. Potential explanations for these conflicting results can only be speculative. One possibility is that DHT alone produces a “physiological” hypertrophy but that NE and PE (which elicited all of the markers of hypertrophy) produce a “pathological” hypertrophy. Evidence for dissociation of expression of gene markers in response to pathological stimuli from hypertrophy induced by physiologic stimuli is found in exercise studies whereby increased expression of classic hypertrophic gene markers were not found in hearts with an exercise-induced increase in mass (10, 24, 43). Dissociation between the cell size response and other markers of hypertrophy and differences between our results and those of others also could be related to culture conditions, doses of NE or sex hormones (37, 71), and/or the presence or absence of concurrent β-adrenergic stimulation (7, 27, 69). In our investigation, we used Primaria culture dishes (abrogating the requirement for fibronectin or laminin for cell attachment), charcoal-dextran-filtered FBS for cell plating, and serum-free defined medium for cell incubation. However, defining the mechanism underlying the dissociation of indicators of hypertrophy would require further study.
Our study is the first to compare the direct effects of E2 and DHT on ERK and FAK phosphorylation, as well as to examine the comparative effects of male and female sex hormones on NE-induced ERK and FAK activation in NRVMs. Our results suggest that long-term exposure to sex hormones tends to decrease the relative activation of both FAK and ERK. In contrast, short-term exposure (3 min) to E2 (and DHT) directly increases FAK phosphorylation, indicating the sex hormones are signaling though a rapid signal transduction pathway, rather than, or in addition to, a classic steroid genomic pathway. Such nongenomic effects have been previously documented in cardiac myocytes (14, 65). The rapid direct effects involve FAK, but not ERK, activation. This is the first report of rapid signaling by sex hormones through FAK phosphorylation in heart cells. However, rapid sex hormone signaling involving FAK activation has been previously demonstrated to play a role in cancer cell metastasis (35, 50).
Results of our study demonstrate that E2 (but not DHT) reduces NE-elicited hypertrophy as measured by NRVM size. E2 (but not DHT) also mitigates NE-induced FAK phosphorylation regardless of whether the NRVMs were exposed to the hormone briefly (1 min) or long-term (48 h) before NE stimulation. Neither sex hormone altered NE-induced ERK phosphorylation. Whether there is a relationship between the E2-mediated reduction in cell size and the ability of E2 to attenuate NE-elicited FAK phosphorylation remains to be seen.
Despite increasing knowledge regarding mechanisms of cardiac adaptation to hypertrophic stimuli such as increased workload and extracellular signals, there are significant sex differences that remain poorly understood. Many signaling kinases have been implicated in hypertrophy with subsequent stimulation of downstream effectors that regulate gene expression and cell growth. Mechanosensors such as FAK and phosphatidylinositol 3-kinase, as well as its downstream target, Akt, are likely candidates for these modifiers. For example, the serine/threonine protein kinase Akt when expressed as a transgene in constitutively active form in the heart has been shown to induce hypertrophy that is greater in females than in males when normalized to body weight (42). We compared the effect of sex hormones on the activation of two kinases (FAK and ERK) previously shown to be involved in the transduction of hypertrophic signals. FAK is closely associated with transduction of mechanical stimuli via the cytoskeleton (11), whereas ERK is associated with receptor-mediated signaling from extracellular ligands (51, 68). Cross talk between the two signaling kinases has been clearly demonstrated (55, 61).
Nuclear-cytoplasmic shuttling may be a mechanism by which the rapid translocation of FAK from the cytoplasm to the nucleus and perinuclear region occurs. FAK (and ERK) phosphorylation initiates the Ras/MAPK pathway that dissociates a cytoplasmic complex enabling FAK entry into the nucleus for hypertrophic gene expression (67, 70). Many other focal adhesion-associated proteins, including zyxin, paxillin, and muscle LIM protein (8, 67), shuttle to the nucleus, where they regulate chromatin structure, transcription, mRNA processing, and export. Interestingly, FAK accumulates in the myocyte nucleus of failing hearts of spontaneously hypertensive rats (67). In our study, long-term exposure to E2 decreased the amount of cellular phosphorylated FAK as measured by immunostaining, yet E2-exposed, NE-treated cells (as well as DHT-exposed, NE-treated cells) had a substantial amount of phosphorylated FAK in the nucleus. At this juncture, we cannot associate any of these findings with the pro- or antihypertrophic effect of male or female sex hormones. Further studies are necessary to determine whether alterations in FAK activation and/or translocation are important to the sex hormones' pro- or antihypertrophic protective effects.
We chose to examine the effect of sex hormones on NE-elicited hypertrophy because 1) it has been suggested that NE is a primary neurohormone linking hemodynamic demand with cardiac hypertrophy (48, 58); 2) evidence suggests that both α- and β-adrenergic receptors are involved in the hypertrophic response (5); 3) NE is an endogenous α- and β-adrenergic agonist; and 4) results of our previous investigations (19, 20, 66) and those of others (32, 36, 59) have revealed that both α- and β-adrenergic receptor expression is sexually dimorphic and modified by male and female sex hormones. These differences in receptor expression could theoretically alter both signal transduction and hypertrophic cellular response, a possibility requiring further study.
In summary, the results of this study demonstrate 1) E2 mitigates NE-elicited NRVM hypertrophy, but DHT does not; 2) DHT induces NRVM hypertrophy; 3) brief exposure to both E2 and DHT activate FAK, suggesting the sex hormones activate a rapid (nongenomic) signaling pathway associated with FAK phosphorylation; 4) neither sex hormone stimulates ERK phosphorylation, and they do not modulate NE-elicited ERK phosphorylation; 5) E2, but not DHT, attenuates NE-induced FAK phosphorylation; and 6) cellular localization of phosphorylated FAK is altered by sex hormones.
A strength of this study is that the hypertrophic response and signaling effects of E2 and DHT were compared under identical conditions, utilizing an isolated myocyte model that eliminates indirect hemodynamic and other non-cardiac cell effects of the steroid hormones. The model also eliminates potential interactive effects of sex hormones with other neuroendocrine axes, such as the hypothalamic pituitary growth hormone axis (2), that could influence the hypertrophic response. This study is limited by the lack of confirmation of estrogenic and androgenic effects with inactive steroid analogs or steroid receptor antagonists to inhibit observed responses. It also is limited by the use of NRVM from male and female rat pups as sex-dependent differences in gene expression (2), and sexually dimorphic responses to sex hormones have been demonstrated (21). Determining whether FAK signaling is mechanistically involved in E2-mediated antihypertrophic effects requires further study and eventual hypothesis testing in the whole animal where nonmyocyte cardiac cells, endocrine systems, and hemodynamics influence the outcome. Until then, the clinical debate continues with regard to whether hormone replacement therapy has a positive or negative impact on cardiac disease in women and men (39, 56). Our findings are important because they provide further insight on the comparative direct effects of sex hormones on cardiac muscle cell hypertrophy, which is a risk factor for heart disease.
GRANTS
These studies were supported in part by National Heart, Lung, and Blood Institute Grant HL62426 and National Institute of Nursing Research Grant P30 NR009014 from the Center for Reducing Risks in Vulnerable Populations.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health and a grant from the Dr. Ralph and Marian Falk Medical Research Trust (to Loyola University Chicago, Stritch School of Medicine).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Allen M. Samarel for his intellectual contributions and Dr. Lianzhi Gu for technical expertise.
REFERENCES
- 1.Agabiti-Rosei E, Muiesan ML. Left ventricular hypertrophy and heart failure in women. J Hypertens Suppl 20: S34–S38, 2002 [PubMed] [Google Scholar]
- 2.Ahluwalia A, Clodfelter KH, Waxman DJ. Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic acid microarray analysis. Mol Endocrinol 18: 747–760, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Altamirano F, Oyarce C, Silva P, Toyos M, Wilson C, Lavandero S, Uhlen P, Estrada M. Testosterone induces cardiomyocyte hypertrophy through mammalian target of rapamycin complex 1 pathway. J Endocrinol 202: 299–307, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Babiker FA, De Windt LJ, van Eickels M, Thijssen V, Bronsaer RJ, Grohe C, van Bilsen M, Doevendans PA. 17beta-estradiol antagonizes cardiomyocyte hypertrophy by autocrine/paracrine stimulation of a guanylyl cyclase A receptor-cyclic guanosine monophosphate-dependent protein kinase pathway. Circulation 109: 269–276, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Barki-Harrington L, Perrino C, Rockman HA. Network integration of the adrenergic system in cardiac hypertrophy. Cardiovasc Res 63: 391–402, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Barry SP, Davidson SM, Townsend PA. Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol 40: 2023–2039, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Bishopric NH, Kedes L. Adrenergic regulation of the skeletal alpha-actin gene promoter during myocardial cell hypertrophy. Proc Natl Acad Sci USA 88: 2132–2136, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boateng SY, Belin RJ, Geenen DL, Margulies KB, Martin JL, Hoshijima M, de Tombe PP, Russell B. Cardiac dysfunction and heart failure are associated with abnormalities in the subcellular distribution and amounts of oligomeric muscle LIM protein. Am J Physiol Heart Circ Physiol 292: H259–H269, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Boateng SY, Lateef SS, Mosley W, Hartman TJ, Hanley L, Russell B. RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am J Physiol Cell Physiol 288: C30–C38, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Boluyt MO, Cirrincione GM, Loyd AM, Korzick DH, Parker JL, Laughlin MH. Effects of gradual coronary artery occlusion and exercise training on gene expression in swine heart. Mol Cell Biochem 294: 87–96, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovasc Res 70: 422–433, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Brower GL, Gardner JD, Janicki JS. Gender mediated cardiac protection from adverse ventricular remodeling is abolished by ovariectomy. Mol Cell Biochem 251: 89–95, 2003 [PubMed] [Google Scholar]
- 13.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res 91: 776–781, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Buitrago C, Massheimer V, de Boland AR. Acute modulation of Ca2+ influx on rat heart by 17beta-estradiol. Cell Signal 12: 47–52, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Cabral AM, Antonio A, Moyses MR, Vasquez EC. Left ventricular hypertrophy differences between male and female renovascular hypertensive rats. Braz J Med Biol Res 21: 633–635, 1988 [PubMed] [Google Scholar]
- 16.Carroll JD, Carroll EP, Feldman T, Ward DM, Lang RM, McGaughey D, Karp RB. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation 86: 1099–1107, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Catalucci D, Latronico MV, Ellingsen O, Condorelli G. Physiological myocardial hypertrophy: how and why? Front Biosci 13: 312–324, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Cavasin MA, Sankey SS, Yu AL, Menon S, Yang XP. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol 284: H1560–H1569, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Chu SH, Goldspink P, Kowalski J, Beck J, Schwertz DW. Effect of estrogen on calcium-handling proteins, beta-adrenergic receptors, and function in rat heart. Life Sci 79: 1257–1267, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Chu SH, Sutherland K, Beck J, Kowalski J, Goldspink P, Schwertz D. Sex differences in expression of calcium-handling proteins and beta-adrenergic receptors in rat heart ventricle. Life Sci 76: 2735–2749, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol 20: 1333–1351, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Dash R, Schmidt AG, Pathak A, Gerst MJ, Biniakiewicz D, Kadambi VJ, Hoit BD, Abraham WT, Kranias EG. Differential regulation of p38 mitogen-activated protein kinase mediates gender-dependent catecholamine-induced hypertrophy. Cardiovasc Res 57: 704–714, 2003 [DOI] [PubMed] [Google Scholar]
- 23.de Simone G, Devereux RB, Daniels SR, Meyer RA. Gender differences in left ventricular growth. Hypertension 26: 979–983, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Diffee GM, Seversen EA, Stein TD, Johnson JA. Microarray expression analysis of effects of exercise training: increase in atrial MLC-1 in rat ventricles. Am J Physiol Heart Circ Physiol 284: H830–H837, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol 32: 1118–1125, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Drolet MC, Lachance D, Plante E, Roussel E, Couet J, Arsenault M. Gender-related differences in left ventricular remodeling in chronic severe aortic valve regurgitation in rats. J Heart Valve Dis 15: 345–351, 2006 [PubMed] [Google Scholar]
- 27.Du XJ. Divergence of hypertrophic growth and fetal gene profile: the influence of beta-blockers. Br J Pharmacol 152: 169–171, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frantz S, Hu K, Widder J, Weckler B, Scheuermann H, Bauersachs J, Ertl G, Callies F, Allolio B. Detrimental effects of testosterone on post-myocardial infarction remodelling in female rats. J Physiol Pharmacol 58: 717–727, 2007 [PubMed] [Google Scholar]
- 29.Gao XM, Agrotis A, Autelitano DJ, Percy E, Woodcock EA, Jennings GL, Dart AM, Du XJ. Sex hormones and cardiomyopathic phenotype induced by cardiac beta 2-adrenergic receptor overexpression. Endocrinology 144: 4097–4105, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Gardin JM, Siscovick D, Anton-Culver H, Lynch JC, Smith VE, Klopfenstein HS, Bommer WJ, Fried L, O'Leary D, Manolio TA. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation 91: 1739–1748, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Gardner JD, Brower GL, Janicki JS. Gender differences in cardiac remodeling secondary to chronic volume overload. J Card Fail 8: 101–107, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Golden KL, Marsh JD, Jiang Y. Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Horm Metab Res 36: 197–202, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Hinderliter AL, Sherwood A, Blumenthal JA, Light KC, Girdler SS, McFetridge J, Johnson K, Waugh R. Changes in hemodynamics and left ventricular structure after menopause. Am J Cardiol 89: 830–833, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Kadokami T, McTiernan CF, Kubota T, Frye CS, Feldman AM. Sex-related survival differences in murine cardiomyopathy are associated with differences in TNF-receptor expression. J Clin Invest 106: 589–597, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallergi G, Agelaki S, Markomanolaki H, Georgoulias V, Stournaras C. Activation of FAK/PI3K/Rac1 signaling controls actin reorganization and inhibits cell motility in human cancer cells. Cell Physiol Biochem 20: 977–986, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Kam KW, Qi JS, Chen M, Wong TM. Estrogen reduces cardiac injury and expression of beta1-adrenoceptor upon ischemic insult in the rat heart. J Pharmacol Exp Ther 309: 8–15, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Kilic A, Javadov S, Karmazyn M. Estrogen exerts concentration-dependent pro-and anti-hypertrophic effects on adult cultured ventricular myocytes. Role of NHE-1 in estrogen-induced hypertrophy. J Mol Cell Cardiol 46: 360–369, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Malkin CJ, Jones TH, Channer KS. Testosterone in chronic heart failure. Front Horm Res 37: 183–196, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Marcus R, Krause L, Weder AB, Dominguez-Meja A, Schork NJ, Julius S. Sex-specific determinants of increased left ventricular mass in the Tecumseh Blood Pressure Study. Circulation 90: 928–936, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK, Green GE, Schiebinger RJ. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation 98: 256–261, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem 277: 22896–22901, 2002 [DOI] [PubMed] [Google Scholar]
- 43.McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol 34: 255–262, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Miya Y, Sumino H, Ichikawa S, Nakamura T, Kanda T, Kumakura H, Takayama Y, Mizunuma H, Sakamaki T, Kurabayashi M. Effects of hormone replacement therapy on left ventricular hypertrophy and growth-promoting factors in hypertensive postmenopausal women. Hypertens Res 25: 153–159, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Modena MG, Muia N, Jr, Aveta P, Molinari R, Rossi R. Effects of transdermal 17beta-estradiol on left ventricular anatomy and performance in hypertensive women. Hypertension 34: 1041–1046, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Nahrendorf M, Frantz S, Hu K, von zur Muhlen C, Tomaszewski M, Scheuermann H, Kaiser R, Jazbutyte V, Beer S, Bauer W, Neubauer S, Ertl G, Allolio B, Callies F. Effect of testosterone on post-myocardial infarction remodeling and function. Cardiovasc Res 57: 370–378, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Olsson MC, Palmer BM, Leinwand LA, Moore RL. Gender and aging in a transgenic mouse model of hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 280: H1136–H1144, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Ostman-Smith I. Cardiac sympathetic nerves as the final common pathway in the induction of adaptive cardiac hypertrophy. Clin Sci (Lond) 61: 265–272, 1981 [DOI] [PubMed] [Google Scholar]
- 49.Pedram A, Razandi M, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem 280: 26339–26348, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Planas-Silva MD, Bruggeman RD, Grenko RT, Stanley Smith J. Role of c-Src and focal adhesion kinase in progression and metastasis of estrogen receptor-positive breast cancer. Biochem Biophys Res Commun 341: 73–81, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Ravingerova T, Barancik M, Strniskova M. Mitogen-activated protein kinases: a new therapeutic target in cardiac pathology. Mol Cell Biochem 247: 127–138, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res 47: 23–37, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Shub C, Klein AL, Zachariah PK, Bailey KR, Tajik AJ. Determination of left ventricular mass by echocardiography in a normal population: effect of age and sex in addition to body size. Mayo Clin Proc 69: 205–211, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Simpson P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circ Res 56: 884–894, 1985 [DOI] [PubMed] [Google Scholar]
- 55.Skavdahl M, Steenbergen C, Clark J, Myers P, Demianenko T, Mao L, Rockman HA, Korach KS, Murphy E. Estrogen receptor-beta mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol 288: H469–H476, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Stevenson JC. Hormone replacement therapy and cardiovascular disease revisited. Menopause Int 15: 55–57, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Tamura T, Said S, Gerdes AM. Gender-related differences in myocyte remodeling in progression to heart failure. Hypertension 33: 676–680, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Tarazi RC, Sen S, Saragoca M, Khairallah P. The multifactorial role of catecholamines in hypertensive cardiac hypertrophy. Eur Heart J 3, Suppl A: 103–110, 1982 [DOI] [PubMed] [Google Scholar]
- 59.Thawornkaiwong A, Preawnim S, Wattanapermpool J. Upregulation of beta 1-adrenergic receptors in ovariectomized rat hearts. Life Sci 72: 1813–1824, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Tivesten A, Bollano E, Nystrom HC, Alexanderson C, Bergstrom G, Holmang A. Cardiac concentric remodelling induced by non-aromatizable (dihydro-)testosterone is antagonized by oestradiol in ovariectomized rats. J Endocrinol 189: 485–491, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Torsoni AS, Constancio SS, Nadruz W, Jr, Hanks SK, Franchini KG. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ Res 93: 140–147, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J 141: 334–341, 2001 [DOI] [PubMed] [Google Scholar]
- 63.van Eickels M, Grohe C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 17beta-estradiol attenuates the development of pressure-overload hypertrophy. Circulation 104: 1419–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 64.van Eickels M, Patten RD, Aronovitz MJ, Alsheikh-Ali A, Gostyla K, Celestin F, Grohe C, Mendelsohn ME, Karas RH. 17-beta-estradiol increases cardiac remodeling and mortality in mice with myocardial infarction. J Am Coll Cardiol 41: 2084–2092, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Vicencio JM, Ibarra C, Estrada M, Chiong M, Soto D, Parra V, Diaz-Araya G, Jaimovich E, Lavandero S. Testosterone induces an intracellular calcium increase by a nongenomic mechanism in cultured rat cardiac myocytes. Endocrinology 147: 1386–1395, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Vizgirda VM, Wahler GM, Sondgeroth KL, Ziolo MT, Schwertz DW. Mechanisms of sex differences in rat cardiac myocyte response to beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol 282: H256–H263, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Wang JG, Miyazu M, Xiang P, Li SN, Sokabe M, Naruse K. Stretch-induced cell proliferation is mediated by FAK-MAPK pathway. Life Sci 76: 2817–2825, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Wang Y. Mitogen-activated protein kinases in heart development and diseases. Circulation 116: 1413–1423, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamazaki T, Komuro I, Yazaki Y. Signalling pathways for cardiac hypertrophy. Cell Signal 10: 693–698, 1998 [DOI] [PubMed] [Google Scholar]
- 70.Yi XP, Zhou J, Huber L, Qu J, Wang X, Gerdes AM, Li F. Nuclear compartmentalization of FAK and FRNK in cardiac myocytes. Am J Physiol Heart Circ Physiol 290: H2509–H2515, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Zhan E, Keimig T, Xu J, Peterson E, Ding J, Wang F, Yang XP. Dose-dependent cardiac effect of oestrogen replacement in mice post-myocardial infarction. Exp Physiol 93: 982–993, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]