Abstract
Our lab has shown that left circumflex coronary artery (LCX) perivascular adipose tissue (PAT) blunts endothelin-1 (ET-1)-induced maximal contractions in normal pigs on low- and high-fat diets. Other studies report that PAT exerts anticontractile effects on agonist-induced arterial contraction via release of a relaxing factor that acts on the underlying vasculature. The purpose of this study was to test the hypotheses that PAT blunts LCX contraction in familial hypercholesterolemic pigs and that exercise training (Ex) augments this anticontractile effect. Male familial hypercholesterolemic pigs were divided into Ex (n = 13) and sedentary (Sed) (n = 15) groups. LCX reactivity to angiotensin II (ANG II), bradykinin (BK), ET-1, and sodium nitroprusside (SNP) was evaluated in vitro with intact or removed PAT in Sed and Ex familial hypercholesterolemic pigs. LCX relaxation induced by BK and SNP was not altered by Ex or PAT removal. LCX contractions stimulated by ANG II and ET-1 were not significantly altered by Ex or PAT removal across doses; however, Ex did act to significantly reduce ET-1 maximal contractions in familial hypercholesterolemic pig LCX compared with Sed familial hypercholesterolemic pig LCX, independent of PAT (P < 0.05). We conclude that LCX PAT in Sed and Ex familial hypercholesterolemic pigs exerts no substantial anticontractile influence over LCX vasomotor responses to endogenous constrictors such as ANG II and ET-1. Our results suggest that exercise training significantly reduces familial hypercholesterolemic pig LCX maximal contractile responses to the endogenous constrictor ET-1, independent of PAT.
Keywords: adipocyte-derived relaxing factor, anticontractile effect, perivascular adipose tissue dysfunction
adipose tissue is no longer viewed as a simple reservoir for energy storage and thermoregulation but rather as a complex, indispensable, active metabolic and endocrine organ (17, 34, 44). Recent evidence indicates that there are differences in the endocrine function of adipose tissues that are specific to their anatomic location (i.e., subcutaneous or visceral) (7, 9). Adipose tissue surrounding the vasculature or perivascular adipose tissue (PAT) is thought to provide structural support to underlying arteries. It is surprising that the role of PAT has been largely overlooked in the field of atherosclerosis regarding its influence on vascular function, given that some vessels most susceptible to atherosclerosis are covered in PAT (i.e., coronaries). Several studies reveal a paracrine release from normal PAT of a heat-labile (11) adipocyte-derived relaxing factor (ADRF) that induces relaxation of vascular smooth muscle (6, 21, 24, 40). This action is thought to be mediated through the opening of K+ channels in vascular smooth muscle cells (6, 40). Several of these studies demonstrate in rodents a blunted contractile response to constrictors such as angiotensin II (21), phenylephrine (21), and endothelin-1 (40) in vessels with PAT compared with vessels without PAT independent of actions mediated by leptin (21), an adipokine known to cause vasorelaxation (20, 26, 35).
The role of PAT in contributing to human coronary artery graft patency is also the subject of current debate (3, 18). Conventionally, vascular grafts are harvested and surgically grafted with surrounding adipose tissue intact. Studies suggest that removal of surrounding adipose tissue results in functionally different vessels (2) and may increase the risk of perioperative vasospasm (36). In vitro studies in human internal thoracic arteries have revealed a nitric oxide (NO)- and prostacyclin-independent PAT anticontractile effect specific to the PAT depot (10, 24).
Previously, our lab has shown that left circumflex coronary artery PAT blunts endothelin-1 responses in normal pigs on low- and high-fat diets (32) and that physical activity/exercise training exerts a beneficial effect on vasomotor function in hypercholesterolemic pig coronary arteries (37). Hypercholesterolemia is a well-established risk factor for atherosclerosis, and numerous animal models of hypercholesterolemia exist that involve diets high in cholesterol or saturated fat. However, a particularly unique line of familial hypercholesterolemic pigs were developed by Rapacz and coworkers (29, 31) that exhibit spontaneous hypercholesterolemia when fed a cholesterol-free, low-fat diet. Currently, scant data exist concerning the idea that a primary prevention therapy such as exercise training imposes beneficial effects on PAT endocrine function in porcine models of chronic disease such as familial hypercholesterolemia. The present study was aimed to test the hypotheses that PAT of familial hypercholesterolemic pigs has a blunted anticontractile effect on left circumflex coronary artery responses compared with our previously reported results in normal pigs on low- or high-fat diets (32), and that exercise training would augment or improve the anticontractile effect exerted by PAT in familial hypercholesterolemic pig left circumflex coronary arteries.
METHODS
Experimental animals and design.
Castrated male adult (11–14 mo of age) Rapacz familial hypercholesterolemic pigs (29, 31) used in this study were purchased from the University of Wisconsin Swine Research and Teaching Center. Pigs were randomly assigned into exercise trained (Ex, n = 13) or cage confined/sedentary (Sed, n = 15) groups and had ad libitum access to water. Pigs were pair fed the University of Wisconsin gestation diet (15, 29), a corn- and soybean-based cholesterol-free, 3% fat diet. All experimental protocols were approved by the Animal Care and Use Committee at the University of Missouri. Pigs were housed in rooms maintained at 20–23°C with a 12:12-h light-dark cycle. Experimental treatments lasted for 16–20 wk, during which Sed pigs were restricted to their enclosures (2 × 4-m pens) and did not exercise and Ex pigs underwent an exercise training regime on treadmills as previously described (32, 42, 43). Briefly, exercise training on treadmill involved a 5-min warm-up, and then pigs ran at 5 mph for 15 min and at 3 mph for 20–30 min. Intensity and duration of exercise bouts increased steadily so that by week 10 of training the pigs ran 85 min/day, 5 days/wk. The 85-min training bouts consisted of a 5-min warm-up, a 15-min sprint run at 6–8 mph, a 60-min endurance run at 4–6 mph, and a 5-min cooldown. Efficacy of the training program was determined from measurements of endurance time (from the treadmill exercise before and after test) and measurements of pig heart weight-to-body weight ratios.
In vitro assessment of vessel reactivity.
At the end of the 16- to 20-wk training program, pigs were anesthetized with intramuscular ketamine-xylazine, and intravenous pentothal for deep anesthesia, and the heart was removed to achieve euthanasia. Hearts were placed in iced Krebs bicarbonate buffer on removal for dissection of coronary artery samples. Epicardial and perivascular coronary adipose tissue was carefully dissected from the heart, and wet weights were measured on a balance. Samples of the left circumflex branch of the left coronary artery were then taken for examination of vasomotor function. Arterial segments from 3 to 4 mm in length of the left circumflex coronary artery from pigs in each group were dissected. PAT was either removed or left intact on the arterial segments. To avoid any confounding differences between proximal and distal portions of the artery, the surrounding adipose tissue was dissected off of every other arterial ring. Vessel segments were then mounted on wires connected to force transducers that measure grams of tension, and the wires with vessels on them were then lowered into a 20-ml bath containing Krebs bicarbonate buffer maintained at 37°C with a gas mixture of 95% O2 and 5% CO2. Vessels were allowed 1 h to equilibrate to Krebs bicarbonate buffer. Before dose-response curves, coronary rings were stretched to a length that produced maximal force stimulated by 50 mM KCl. After rings were stretched to their optimal length and had acquired a stable baseline tension, changes in tension to 80 mM KCl were assessed in all rings twice. Both angiotensin II (10−10–10−6 M) and endothelin-1 (10−10–10−8 M) were used to assess vasoconstriction in a dose-response manner. In all vessels, angiotensin II dose responses were examined first, followed by endothelin-1. Once stable tension was reached subsequent to application of the highest dose of endothelin-1 for the endothelin-1 dose-response curve, a vasorelaxation curve for bradykinin (10−11–10−6 M) was produced in a dose-response manner without washout between the end of the endothelin-1 curve and the beginning of the bradykinin curve. Bradykinin was used to assess endothelium-dependent relaxation. After the bradykinin curve, a series of washes were performed totaling 50 min. Endothelin-1 (10−8 M) was then added to reconstrict all vessels, and sodium nitroprusside (10−10–10−4 M) was then used to assess endothelium-independent relaxation. After the sodium nitroprusside dose-response curve, the bathing solution was changed to a calcium-free buffer and then measured after 30 min of minimal tension. Change in force was measured from the force transducer in response to cumulatively increasing doses of agonist, with washouts occurring between each dose-response protocol, except for between bradykinin and endothelin-1 protocols.
Solutions and drugs.
The Krebs bicarbonate buffer solution contained (in mM) 131.5 NaCl, 5.0 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 11.2 glucose, 20.8 NaHCO3, 0.003 propranolol, and 0.025 EDTA. The solution was aerated with 95% O2 and 5% CO2 (pH 7.4) and maintained at 37°C. Angiotensin II was purchased from Bachem Americas, and all other drugs and chemicals were purchased from Sigma Chemical.
Statistical analysis.
Differences between groups regarding serum lipid and glucose data, heart weight, body weight, heart weight-to-body weight ratio, cardiac adipose tissue content, treadmill performance data, ring characteristics, and maximal endothelin-1 responses were determined via an unpaired t-test where P < 0.05 was considered significant. All unpaired t-test data were analyzed in GraphPad Prism v5.0a.
Nonparametric statistical methods were used to perform a series of tests on dose-response data because examination of residual plots from a three-factor analysis of variance model indicated that the assumption of normality of the error terms was suspect. Specifically, group differences at each dose-treatment combination (where treatment is vessel with or without PAT) were looked at with the Wilcoxon rank sum test. Treatment differences at each dose-group combination (where groups are Sed and Ex pigs) were also examined with the Wilcoxon signed-rank test on the differences. A false discovery rate adjustment was used for multiple tests in view of the large number of tests considered. Results with the false discovery rate at 0.05 or lower were considered significant.
RESULTS
Experimental animals.
Serum lipid analysis was performed on an Olympus AU400 chemistry analyzer by personnel at the Veterinary Medical Diagnostic Laboratory of the University of Missouri and were found to be elevated compared with previously reported values for this model of hyperlipidemia (29). Unexpectedly, Sed familial hypercholesterolemic pigs had significantly lower total cholesterol than Ex familial hypercholesterolemic pigs (308.9 ± 16.28 vs. 402.5 ± 24.48 mg/dl, respectively), whereas triglyceride levels did not differ between Sed and Ex familial hypercholesterolemic pigs (60.75 ± 7.69 vs. 64.23 ± 7.37 mg/dl, respectively) (Table 1). No differences were found between Sed (113.8 ± 7.2 mg/dl) and Ex (115.8 ± 9.9 mg/dl) serum glucose levels (Table 1).
Table 1.
Pig characteristics
| Sed | Ex | |
|---|---|---|
| Serum lipids and glucose, mg/dl | ||
| Total cholesterol | 308.9 ± 16.28 | 402.5 ± 24.48* |
| Triglycerides | 60.75 ± 7.69 | 64.23 ± 7.37 |
| Glucose | 113.8 ± 7.2 | 115.8 ± 9.9 |
| Epicardial adipose tissue content, g | ||
| Total coronary | 4.10 ± 0.36 | 4.99 ± 0.57 |
| Total noncoronary | 0.75 ± 0.16 | 1.55 ± 0.30* |
| Total epicardial | 4.85 ± 0.40 | 6.54 ± 0.82 |
| Other characteristics | ||
| Heart weight, g | 207.9 ± 10.13 | 250.7 ± 15.43* |
| Body weight, kg | 62.97 ± 3.22 | 61.87 ± 3.35 |
| Heart weight/body weight, g/kg | 3.33 ± 0.09 | 4.04 ± 0.09* |
| Prestress test, min | 22.17 ± 1.34 | 23.96 ± 0.91† |
| Poststress test, min | 19.72 ± 0.77 | 29.37 ± 0.69* |
Data are means ± SE; n = 13–16 for blood profile and other characteristics, n = 5–8 for epicardial adipose tissue content. Sed, sedentary familial hypercholesterolemic (FH) pigs; Ex, exercise-trained FH pigs. *P < 0.05 for Sed vs. Ex comparison at the measured characteristic; *P < 0.05 for Ex prestress test vs. Ex poststress test comparison.
Group differences between epicardial adipose tissue content were only evident when measuring total epicardial, noncoronary adipose tissue, where Ex familial hypercholesterolemic pigs had 1.55 ± 0.30 g compared with Sed familial hypercholesterolemic pigs at 0.75 ± 0.16 g (Table 1). Total epicardial fat tended to be lower in Sed familial hypercholesterolemic pigs versus Ex familial hypercholesterolemic pigs (4.85 ± 0.40 vs. 6.54 ± 0.82 g, respectively) but did not achieve significance (P = 0.0626; Table 1). No significant differences were found between groups in any of the measured vessel characteristics; therefore neither exercise nor PAT removal significantly altered the structural characteristics of the left circumflex coronary arteries in the familial hypercholesterolemic pigs.
Although body weights did not differ between Sed and Ex familial hypercholesterolemic pigs (Sed 62.97 ± 3.22 kg and Ex 61.87 ± 3.35 kg), the efficacy of the exercise training program was evident in several other characteristics of the Ex familial hypercholesterolemic pigs as shown in Table 1. Heart weights (g) and heart weight-to-body weight ratios (g/kg) were significantly lower in Sed versus Ex familial hypercholesterolemic pigs (Sed 207.9 ± 10.13 g, Ex 250.7 ± 15.43 g and Sed 3.33 ± 0.09 g/kg, Ex 4.04 ± 0.09 g/kg, respectively). Also consistent with the efficacy of the training program is the finding that the duration of running in the exercise stress test was significantly increased in the Ex familial hypercholesterolemic pigs between the pre- and post-treadmill training program from 23.96 ± 0.91 to 29.37 ± 0.69 min, whereas there was no increase in the Sed familial hypercholesterolemic pigs (22.17 ± 1.34 and 19.72 ± 0.77 min).
Effects of perivascular adipose tissue and exercise on coronary in vitro vasorelaxation.
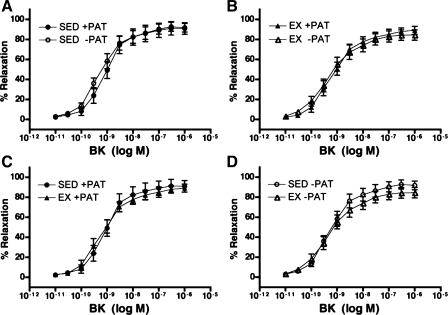
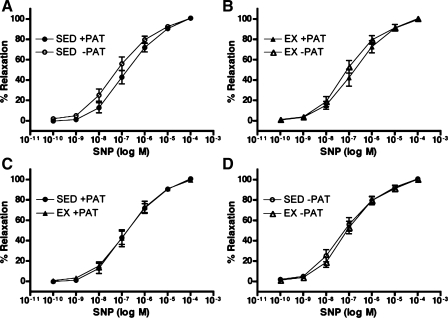
To evaluate the effects of endogenous PAT-derived factors and exercise on coronary vasorelaxation responses, isometric tension studies were performed in isolated porcine left circumflex coronary arteries with and without PAT in both Sed and Ex groups. Consistent with our recent data (32), PAT did not effect vasorelaxation responses of familial hypercholesterolemic porcine left circumflex coronary artery to bradykinin as shown in Fig. 1, A and B. Contrary to our previous findings (37), exercise training did not exert any significant effects on bradykinin-induced relaxation in Ex familial hypercholesterolemic pigs with or without PAT compared with Sed familial hypercholesterolemic pigs with or without PAT (Fig. 1, C and D). Sodium nitroprusside was used to assess endothelium-independent relaxation in familial hypercholesterolemic pig left circumflex coronary arteries, and neither PAT nor exercise training influenced left circumflex coronary artery endothelium-independent relaxation (Fig. 2, A and B, and Fig. 2, C and D, respectively), consistent with our previous findings (32, 37).
Fig. 1.
Bradykinin (BK)-induced % relaxation following preconstriction with endothelin-1 (ET-1) in left circumflex coronary artery (LCX) from sedentary (Sed) and exercise-trained (Ex) familial hypercholesterolemic (FH) pigs with (+PAT) and without (−PAT) perivascular adipose tissue. A and B: effects of PAT removal on BK-induced % relaxation in Sed (A) and Ex (B) groups. C and D: effects of exercise training on BK-induced % relaxation in Sed and Ex groups with (C) and without (D) PAT. Data presented are means ± SE; n = 13–15 for all graphs. Statistical analysis did not reveal any significant treatment (A and B) or group (C and D) differences in artery responses across BK doses.
Fig. 2.
Sodium nitroprusside (SNP)-induced % relaxation after preconstriction with ET-1 in LCX from Sed and Ex FH pigs with and without PAT. A and B: effects of PAT removal on SNP-induced % relaxation in Sed (A) and Ex (B) groups. C and D: effects of exercise training on SNP-induced % relaxation in Sed and Ex groups with (C) and without (D) PAT. Data presented are means ± SE; n = 13–15 for all graphs. Statistical analysis did not reveal any significant treatment (A and B) or group (C and D) differences in artery responses across SNP doses.
Effects of perivascular adipose tissue and exercise on coronary in vitro vasocontraction.
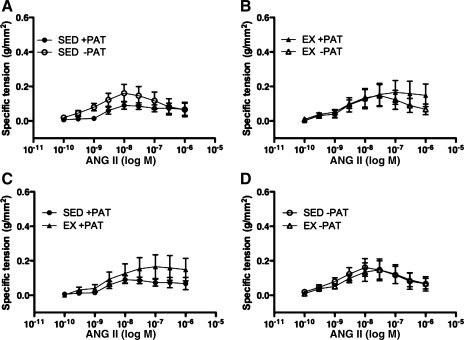
To evaluate the influence of endogenous PAT-derived factors and exercise on coronary vasocontractile responses, isometric tension studies were performed in isolated porcine left circumflex coronary arteries with and without PAT in both Sed and Ex groups. In opposition to our hypothesis, familial hypercholesterolemic pig left circumflex coronary artery vasocontractile responses to angiotensin II were not significantly different after PAT removal in either Sed or Ex groups (Fig. 3, A and B). Exercise training also did not appear to exert any influence on left circumflex coronary artery vasocontractile responses to angiotensin II independent of PAT removal (Fig. 3, C and D).
Fig. 3.
Angiotensin II (ANG II)-induced contraction in LCX from Sed and Ex FH pigs with and without PAT. A and B: effects of PAT removal on ANG II-induced contraction in Sed (A) and Ex (B) groups. C and D: effects of exercise training on ANG II-induced contraction in Sed and Ex groups with (C) and without (D) PAT. Data presented are mean ± SE grams of tension per square millimeter of artery wall; n = 13–15 for all graphs. Statistical analysis did not reveal any significant treatment (A and B) or group (C and D) differences in artery responses across ANG II doses.
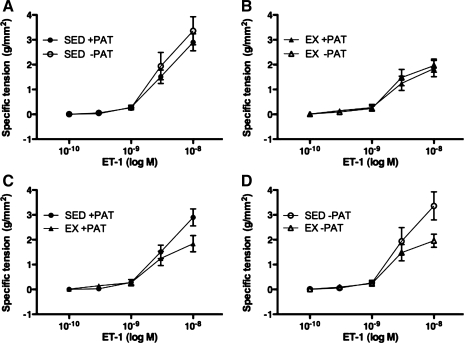
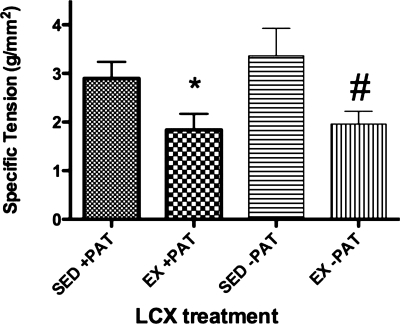
Contrary to our earlier findings (32) and to our hypothesis for the present study, PAT removal had no effect on familial hypercholesterolemic pig left circumflex coronary artery vasocontractile responses to endothelin-1 across doses (Fig. 4, A and B). Exercise training also had no effect on familial hypercholesterolemic pig left circumflex coronary artery vasocontractile responses to endothelin-1 across doses (Fig. 4, C and D); however, left circumflex coronary artery endothelin-1 maximal responses were significantly lower in the Ex familial hypercholesterolemic pigs compared with the Sed familial hypercholesterolemic pigs (Fig. 5).
Fig. 4.
ET-1-induced contraction in LCX from Sed and Ex FH pigs with and without PAT. A and B: effects of PAT removal on ET-1-induced contraction in Sed (A) and Ex (B) groups. C and D: effects of exercise training on ET-1-induced contraction in Sed and Ex groups with (C) and without (D) PAT. Data presented are mean ± SE grams of tension per square millimeter of artery wall; n = 13–15 for all graphs. Statistical analysis did not reveal any significant treatment (A and B) or group (C and D) differences in artery responses across ET-1 doses.
Fig. 5.
Maximal ET-1 responses of FH pig LCX in Sed and Ex pigs with or without PAT. Values presented are mean ± SE maximal responses to ET-1 in grams of tension per square millimeter of artery wall; n = 13–15. *P < 0.05 for Sed +PAT vs. Ex +PAT; #P < 0.05 for Sed −PAT vs. Ex −PAT.
Maximal left circumflex coronary artery contractile responses to 80 mM KCl were not significantly different as illustrated in Table 2, again indicating that neither exercise nor PAT removal significantly altered the structural characteristics of the left circumflex coronary arteries in the familial hypercholesterolemic pig.
Table 2.
Vessel characteristics
| Sed +PAT | Sed −PAT | Ex +PAT | EX −PAT | |
|---|---|---|---|---|
| Outer diameter, mm | 2.67 ± 0.15 | 2.65 ± 0.15 | 2.71 ± 0.16 | 2.70 ± 0.16 |
| Inner diameter, mm | 1.28 ± 0.05 | 1.25 ± 0.04 | 1.22 ± 0.06 | 1.20 ± 0.07 |
| Wall thickness, mm | 0.69 ± 0.07 | 0.70 ± 0.07 | 0.75 ± 0.07 | 0.75 ± 0.07 |
| Axial length, mm | 4.28 ± 0.19 | 4.08 ± 0.19 | 4.45 ± 0.19 | 3.90 ± 0.14 |
| 80 mM KCl specific tension, g/mm2 | 4.07 ± 0.49 | 4.25 ± 0.43 | 3.37 ± 0.40 | 3.84 ± 0.74 |
Left circumflex coronary artery data presented are means ± SE; n = 13–15 for all treatments. PAT, perivascular adipose tissue. Statistical analysis did not reveal any significant treatment differences in vessel characteristics.
DISCUSSION
The purpose of the present investigation was to reveal whether PAT of familial hypercholesterolemic pigs demonstrates a blunted anticontractile effect on left circumflex coronary artery responses compared with our previously reported results from normal pigs and pigs on a high-fat diet (32). We further proposed that given reported beneficial effects of exercise training on hypercholesterolemic pig coronary artery vasomotor function (37), exercise training would augment the anticontractile effect exerted by PAT on familial hypercholesterolemic pig left circumflex coronary artery vasomotor function. The results indicate that PAT had no effect on left circumflex coronary artery endothelium-dependent (Fig. 1, A and B) and endothelium-independent (Fig. 2, A and B) relaxation responses in the familial hypercholesterolemic pig left circumflex coronary artery, either Sed or Ex. Additionally, PAT also had no significant effect on left circumflex coronary artery contractile responses to vasoconstrictors (Figs. 3, A and B, and 4, A and B) in either Sed or Ex familial hypercholesterolemic pigs. Also, no effects of exercise training on familial hypercholesterolemic pig left circumflex coronary artery endothelium-dependent (Fig. 1, C and D) and endothelium-independent (Fig. 2, C and D) relaxation were observed, independent of PAT. In addition, exercise training did not appear to influence familial hypercholesterolemic pig left circumflex coronary artery contractile responses to the endogenous constrictors angiotensin II and endothelin-1 across doses, independent of PAT (Figs. 3, C and D, and 4, C and D, respectively). However, exercise training did significantly reduce familial hypercholesterolemic pig left circumflex coronary artery maximal contractile responses to endothelin-1 compared with Sed familial hypercholesterolemic pig left circumflex coronary artery maximal contractile responses to endothelin-1 (Fig. 5). This effect of exercise on endothelin-1 contraction was independent of PAT. When taken together, these results suggest that left circumflex coronary artery PAT in either Sed or Ex familial hypercholesterolemic pigs exerts no substantial anticontractile influence over left circumflex coronary artery vasomotor responses to endogenous constrictors such as angiotensin II and endothelin-1. Our results also indicate that exercise training significantly reduces familial hypercholesterolemic pig left circumflex coronary artery maximal contractile responses to the endogenous constrictor endothelin-1, independent of PAT.
It is interesting that PAT did not exert any effect on familial hypercholesterolemic pig left circumflex coronary artery contractile responses (Figs. 3, A and B, and 4, A and B), contrary to our previous study (32), in which PAT blunted endothelin-1 responses in normal pigs on low- and high-fat diets. A major difference between this present study and the previous one is the extent of atherosclerosis developed between the different porcine models used. The Rapacz familial hypercholesterolemic pigs used in this study are known to develop extensive atherosclerotic plaques and lesions (29, 31), whereas the previously used Yucatan miniature swine develop only very early-stage vascular disease when fed a high-fat diet (38). While it is possible that atherosclerosis present in the familial hypercholesterolemic pig coronary arteries masked the anticontractile effects of PAT on left circumflex coronary artery contractile responses, it is also possible that the coronary PAT is altered in familial hypercholesterolemic pigs so that it no longer releases relaxing factors. While the previous report did not present KCl-induced contraction data, it did report no effects of PAT on acetylcholine-induced contractile force, suggesting that there were no differences in smooth muscle responses contributing to the differences in endothelin-1 responses with and without PAT (32). However, our findings are consistent with other studies in which diminished anticontractile effects of PAT were previously reported in noncoronary artery disease models. Greenstein et al. (12) demonstrated in obese humans with metabolic syndrome that gluteal artery PAT had diminished anticontractile effects that were linked to local inflammation and hypoxia. Models of rodent hypertension have also been shown to possess blunted PAT anticontractile effects in the aorta and mesenteric arteries associated with elevations in blood pressure (19, 45).
Chatterjee et al. (1) and others (30, 34) have suggested that PAT can develop “PAT dysfunction” under inflammatory conditions such as high-fat diet and hypercholesterolemia where paracrine release of atheroprotective factors such as ADRF, adiponectin, and leptin decreases while release of atheroprone factors such as interleukin-6, TNF-α, and monocyte chemoattractant protein-1 increases. Given the more advanced state of atherosclerosis in the familial hypercholesterolemic pig than in our previous experiments, one could speculate that left circumflex coronary artery PAT has undergone this type of phenotypic shift, as suggested by Chatterjee et al. (1) and demonstrated by current investigations (8, 12), which would diminish the left circumflex coronary artery PAT anticontractile effect on left circumflex coronary artery function in the familial hypercholesterolemic pig. This speculation, however, warrants further investigation as to the mechanistic basis for a lack of PAT anticontractile effect on familial hypercholesterolemic pig left circumflex coronary artery function. It is worth noting that differences in characteristics between vessels cannot account for this lack of PAT effect on left circumflex coronary artery contractile responses because no differences were found between characteristics of vessels with PAT and without PAT in Sed or Ex familial hypercholesterolemic pig groups (Table 2).
Consistent with our previous finding (32) was the lack of PAT effect on familial hypercholesterolemic pig left circumflex coronary artery responses to endothelium-dependent relaxation. These results are contrary to recent studies conducted by Payne et al. (27, 28) in which PAT was found to depress endothelium-dependent relaxation in coronary arteries. It is not clear to us why our studies reveal no effect of PAT on endothelium-dependent relaxation whereas their results show clear evidence of an effect of PAT on endothelium-dependent relaxation. The most obvious difference between these studies and ours is that they used normal dogs and our experiments used pigs with variable levels of atherosclerosis. It seems that the effects of PAT may be different in dogs than in pigs and/or the presence of abnormal lipid levels in the pigs could alter the effects of PAT versus normal dogs. The presence of advanced atherosclerosis in the familial hypercholesterolemic pigs, not present in the canine models used by Payne et al. (27, 28), might alter the PAT given that atherosclerosis is a well-known major risk factor for endothelial dysfunction independent of PAT (4, 14, 41). Consequently, removal of PAT may not exert as great an influence on vasomotor function in a model of advanced atherosclerosis compared with a nondiseased animal because of the depressed endothelial function related to the atherosclerosis.
Exercise training had no effect on familial hypercholesterolemic pig left circumflex coronary artery endothelium-dependent relaxation responses in the present study, independent of PAT (Fig. 1, C and D). This is contrary to our previous study (37) in which exercise training improved endothelial function in Ex versus Sed hypercholesterolemic pig coronaries. Surprisingly, total epicardial, noncoronary adipose tissue content and total plasma cholesterol and triglycerides (Table 1) were significantly higher in the Ex familial hypercholesterolemic pig group than in the Sed familial hypercholesterolemic pig group. Previously we observed no differences between the amount of PAT in Sed and Ex groups and observed that Ex did not alter serum lipids in the other porcine model (32). Several investigators have reported that epicardial adipose tissue volume is strongly associated with the presence and extent of coronary atherosclerosis and systemic inflammation (5, 13, 23, 33), and this could account for the lack of beneficial effects of Ex on left circumflex coronary artery relaxation in the present study. However, the exact mechanism by which Ex would act to modify epicardial adipose tissue is difficult to conclude. The elevated lipid levels in the Ex familial hypercholesterolemic pigs compared with Sed familial hypercholesterolemic pigs could account for the lack of positive effects of exercise training on left circumflex coronary artery relaxation, because hyperlipidemia is a well-established risk factor for endothelial dysfunction (4, 14, 41). Furthermore, lipid levels in Ex familial hypercholesterolemic pigs from this present study were also much higher than those in the previously studied Ex high-fat-diet pigs (37) and much higher than the normal- and high-fat-diet Yucatan pig lipid levels (32).
Although exercise training did not have any apparent effect on familial hypercholesterolemic pig left circumflex coronary artery contractile responses to angiotensin II or endothelin-1 across doses (Figs. 3, C and D, and 4, C and D), exercise training did act to significantly reduce familial hypercholesterolemic pig left circumflex coronary artery maximal responses to endothelin-1 (Fig. 5). These results are consistent with previous findings that the effects of exercise training decrease vascular reactivity to endothelin-1 in pig coronaries (16, 25) and in the human vasculature (22, 39). Further studies will be needed to determine a mechanistic basis for the observed decrease in Ex familial hypercholesterolemic pig left circumflex coronary artery maximal responses to endothelin-1; however, it is of note that differences in characteristics between vessels cannot account for this effect of exercise training on left circumflex coronary artery contractile responses because no differences were found between characteristics of vessels from Sed or Ex familial hypercholesterolemic pig groups, with or without PAT (Table 2).
At present, no investigation has examined whether exercise training imposes beneficial effects on PAT endocrine function in porcine models of chronic disease such as familial hypercholesterolemia. We showed that in familial hypercholesterolemic pig left circumflex coronary artery the surrounding PAT had no observable effect on left circumflex coronary artery vasomotor function. We also found that exercise training exerted no influence on familial hypercholesterolemic pig left circumflex coronary artery PAT control of left circumflex coronary artery vasomotor function. However, exercise training did significantly reduce familial hypercholesterolemic pig left circumflex coronary artery maximal contractile responses to endothelin-1 compared with Sed familial hypercholesterolemic pig left circumflex coronary artery maximal contractile responses to endothelin-1, independent of PAT. Together these results indicate that left circumflex coronary artery PAT in either Sed or Ex familial hypercholesterolemic pigs exerts no substantial anticontractile influence over left circumflex coronary artery vasomotor responses to endogenous constrictors such as angiotensin II and endothelin-1. For future studies it will be important to characterize the left circumflex coronary artery PAT of the familial hypercholesterolemic pig to evaluate the amount of inflammation present in this tissue and determine the reason that this PAT does not release an adipose-derived relaxing substance.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant PO1-HL-52490.
DISCLOSURES
The authors are not aware of financial conflict(s) with the subject matter or materials discussed in this article with any of the authors, or any of the authors' academic institutions or employers.
ACKNOWLEDGMENTS
We thank Pam Thorne and Dave Harah for their technical assistance. We also acknowledge Dr. Richard Madsen for assisting with the statistical analysis of the data presented in this article.
REFERENCES
- 1. Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res 104: 541–549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deja MA, Golba KS, Malinowski M, Wos S, Kolowca M, Biernat J, Kajor M, Spyt TJ. Skeletonization of internal thoracic artery affects its innervation and reactivity. Eur J Cardiothorac Surg 28: 551–557, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Del Campo C. Pedicled or skeletonized? A review of the internal thoracic artery graft. Tex Heart Inst J 30: 170–175, 2003 [PMC free article] [PubMed] [Google Scholar]
- 4. Dilaveris P, Giannopoulos G, Riga M, Synetos A, Stefanadis C. Beneficial effects of statins on endothelial dysfunction and vascular stiffness. Curr Vasc Pharmacol 5: 227–237, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Djaberi R, Schuijf JD, van Werkhoven JM, Nucifora G, Jukema JW, Bax JJ. Relation of epicardial adipose tissue to coronary atherosclerosis. Am J Cardiol 102: 1602–1607, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol 286: H1107–H1113, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Dusserre E, Moulin P, Vidal H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim Biophys Acta 1500: 88–96, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Eiras S, Teijeira-Fernandez E, Shamagian LG, Fernandez AL, Vazquez-Boquete A, Gonzalez-Juanatey JR. Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue. Cytokine 43: 174–180, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 145: 2273–2282, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Gao YJ, Zeng ZH, Teoh K, Sharma AM, Abouzahr L, Cybulsky I, Lamy A, Semelhago L, Lee RM. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg 130: 1130–1136, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci 25: 647–653, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119: 1661–1670, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, Tittus J, Parhofer K, Becker C, Reiser M, Knez A, Leber AW. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol 29: 781–786, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 1: 183–198, 2005 [PMC free article] [PubMed] [Google Scholar]
- 15. Hasler-Rapacz J, Prescott MF, Von Linden-Reed J, Rapacz JM, Jr, Hu Z, Rapacz J. Elevated concentrations of plasma lipids and apolipoproteins B, C-III, and E are associated with the progression of coronary artery disease in familial hypercholesterolemic swine. Arterioscler Thromb Vasc Biol 15: 583–592, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Jones AW, Rubin LJ, Magliola L. Endothelin-1 sensitivity of porcine coronary arteries is reduced by exercise training and is gender dependent. J Appl Physiol 87: 1172–1177, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89: 2548–2556, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Khuri SF. To skeletonize the internal thoracic artery or not? Is that the question? Circulation 114: 754–756, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Lee RM, Ding L, Lu C, Su LY, Gao YJ. Alteration of perivascular adipose tissue function in angiotensin II-induced hypertension. Can J Physiol Pharmacol 87: 944–953, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d'Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes 49: 293–297, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J 16: 1057–1063, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Maeda S, Sugawara J, Yoshizawa M, Otsuki T, Shimojo N, Jesmin S, Ajisaka R, Miyauchi T, Tanaka H. Involvement of endothelin-1 in habitual exercise-induced increase in arterial compliance. Acta Physiol (Oxf) 196: 223–229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O'Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J 30: 850–856, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malinowski M, Deja MA, Golba KS, Roleder T, Biernat J, Wos S. Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin-independent anticontractile factor. Eur J Cardiothorac Surg 33: 225–231, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Merkus D, Houweling B, Mirza A, Boomsma F, van den Meiracker AH, Duncker DJ. Contribution of endothelin and its receptors to the regulation of vascular tone during exercise is different in the systemic, coronary and pulmonary circulation. Cardiovasc Res 59: 745–754, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Mohammed MM, Myers DS, Sofola OA, Hainsworth R, Drinkhill MJ. Vasodilator effects of leptin on canine isolated mesenteric arteries and veins. Clin Exp Pharmacol Physiol 34: 771–774, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via PKC-beta-dependent phosphorylation of nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H460–H465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Payne GA, Borbouse L, Bratz IN, Roell WC, Bohlen HG, Dick GM, Tune JD. Endogenous adipose-derived factors diminish coronary endothelial function via inhibition of nitric oxide synthase. Microcirculation 15: 417–426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prescott MF, McBride CH, Hasler-Rapacz J, Von Linden J, Rapacz J. Development of complex atherosclerotic lesions in pigs with inherited hyper-LDL cholesterolemia bearing mutant alleles for apolipoprotein B. Am J Pathol 139: 139–147, 1991 [PMC free article] [PubMed] [Google Scholar]
- 30. Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev 8: 253–261, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Rapacz J, Hasler-Rapacz J, Taylor KM, Checovich WJ, Attie AD. Lipoprotein mutations in pigs are associated with elevated plasma cholesterol and atherosclerosis. Science 234: 1573–1577, 1986 [DOI] [PubMed] [Google Scholar]
- 32. Reifenberger MS, Turk JR, Newcomer SC, Booth FW, Laughlin MH. Perivascular fat alters reactivity of coronary artery: effects of diet and exercise. Med Sci Sports Exerc 39: 2125–2134, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 117: 605–613, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J 153: 907–917, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Sahin AS, Bariskaner H. The mechanisms of vasorelaxant effect of leptin on isolated rabbit aorta. Fundam Clin Pharmacol 21: 595–600, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Sarabu MR, McClung JA, Fass A, Reed GE. Early postoperative spasm in left internal mammary artery bypass grafts. Ann Thorac Surg 44: 199–200, 1987 [DOI] [PubMed] [Google Scholar]
- 37. Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JW, Price E, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol 96: 1114–1126, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Turk JR, Henderson KK, Vanvickle GD, Watkins J, Laughlin MH. Arterial endothelial function in a porcine model of early stage atherosclerotic vascular disease. Int J Exp Pathol 86: 335–345, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50: 403–409, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Verlohren S, Dubrovska G, Tsang SY, Essin K, Luft FC, Huang Y, Gollasch M. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension 44: 271–276, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Wilkinson I, Cockcroft JR. Cholesterol, lipids and arterial stiffness. Adv Cardiol 44: 261–277, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Woodman CR, Ingram D, Bonagura J, Laughlin MH. Exercise training improves femoral artery blood flow responses to endothelium-dependent dilators in hypercholesterolemic pigs. Am J Physiol Heart Circ Physiol 290: H2362–H2368, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woodman CR, Turk JR, Williams DP, Laughlin MH. Exercise training preserves endothelium-dependent relaxation in brachial arteries from hyperlipidemic pigs. J Appl Physiol 94: 2017–2026, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci 54: 1847–1856, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Zeng ZH, Zhang ZH, Luo BH, He WK, Liang LY, He CC, Su CJ. The functional changes of the perivascular adipose tissue in spontaneously hypertensive rats and the effects of atorvastatin therapy. Clin Exp Hypertens 31: 355–363, 2009. [DOI] [PubMed] [Google Scholar]