Abstract
This review summarizes the state of the current literature relating to the associations of lung disease on obesity and adipokines (proteins produced by adipose tissue) in humans. Obesity is an independent risk factor for asthma. Recent studies suggest that obesity is also an independent risk factor for chronic airflow obstruction, as is seen with chronic obstructive pulmonary disease (COPD). The mechanistic basis for these associations in humans is not established, although a possible role for adipokines has been invoked. Leptin, a proinflammatory adipokine, and adiponectin, an anti-inflammatory adipokine, are causally associated with asthma in mice. Although human studies are currently inconclusive, high-serum leptin and low-serum adiponectin concentrations predict asthma, independent of obesity, in select population groups, such as premenopausal women in the United States. In contradistinction, low-serum leptin and high-serum adiponectin concentrations are associated with stable COPD, although these associations are likely confounded by fat mass. Interestingly, leptin may promote systemic and airway inflammation in stable COPD patients. On the other hand, COPD may upregulate systemic and lung adiponectin expression. The precise mechanism and significance of the associations between these adipokines and lung disease at the current stage is confusing and frankly paradoxical in places. This area of research needs additional study that may open up novel therapeutic strategies for these lung diseases.
Keywords: asthma, chronic obstructive pulmonary disease, adipokine, leptin, adiponectin
there is an increasing prevalence of obesity [body mass index (BMI) ≥ 30 kg/m2] worldwide, particularly in the United States. Data from the two National Health and Nutrition Examination surveys show that the prevalence of obesity has increased among adults aged 20–74 yr in the United States from 15.0% (in the 1976–1980 survey) to 32.9% (in the 2003–2004 survey) (57). It is now known that adipose tissue is not an inert organ simply for energy storage, but regulates systemic inflammation via a variety of secreted proteins (called adipokines). While the associations of obesity and adipokines with cardiovascular, endocrine, and rheumatological diseases are well described, the respiratory effects of obesity and adipokines are less well known. This review will focus on the effect of obesity and adipokines on asthma and chronic obstructive pulmonary disease (COPD) in humans.
OBESITY AND ASTHMA
The prevalence of obesity and asthma is increasing concomitantly (23). Murine experiments have demonstrated a causal relationship between obesity and asthma (75, 76). It is possible that obesity causes asthma in humans as well (78).
Epidemiological Studies
Obesity is associated with asthma in both adults and children, as shown by an increasingly large number of cross-sectional, case-control, longitudinal, and weight intervention studies (1, 7, 9, 15, 16, 18, 22–24, 31, 46, 56, 58, 61, 66, 67, 71, 77, 81, 82, 85, 87, 93, 96). Some reports demonstrate that the obesity-asthma association is stronger among women and peripubertal girls (7, 15, 16, 18, 22, 31, 61, 71, 81, 85, 96), although other studies suggest that obese men and women are at equal risk for asthma (9, 58). Most of the prospective studies have demonstrated risk or odds ratios (OR) between 1.1 and 3.0, comparing the highest to the lowest BMI category with a dose-response effect of increasing BMI, leading to increasing odds of incident asthma (9). These studies also demonstrate that obesity antecedes the development of asthma (23).
Type of Asthma Associated with Obesity
Studies have evaluated the association between obesity and various clinical, physiological, and inflammatory phenotypes of asthma. The clinical phenotypes of asthma commonly studied include self-reported wheeze or provider diagnosis of asthma, use of asthma medications, visits to provider clinics, emergency rooms, or hospitalizations, as well as absenteeism from school or work (7, 15, 24, 56, 67, 77, 82, 93). The physiological phenotypes of asthma studied include peak expiratory flow rate variability, bronchodilator responsiveness of forced expiratory volume in 1 s (FEV1), and presence or extent of airway hyperreactivity (1, 16, 17, 66, 67, 81, 85). The inflammatory phenotypes of asthma studied include sputum cell counts and differentials, exhaled nitric oxide, and exhaled breath condensate measures, such as F2-isoprostanes (a measure of oxidant stress) (34, 37, 46, 94). Overall, it appears that obesity is more strongly associated with clinically defined phenotypes of asthma, compared with physiological and inflammatory phenotypes of asthma. Conversely, weight reduction in the obese subject with asthma is associated with improvement in clinical control and severity of asthma, but with little change in other physiological and inflammatory indexes of asthma (1, 46, 87). This observation is discussed in greater detail in the review articles by Shore (73a) and Mancuso (45a) in this issue of the journal. It is currently unclear whether obesity-associated asthma is a distinct phenotype of asthma. This issue is further discussed in the review article by Lugogo et al. (45b) in this issue of the journal.
Type of Obesity Associated with Asthma
It is not known whether asthma is related to a distinct obesity phenotype. Most studies use excess body mass (i.e., high BMI) to define the obesity phenotype. It is now known that high BMI is, in fact, a collection of various phenotypes, with some, but not all, relating to excess fat. A study in which both BMI and percent body fat (using bioelectrical impedance) was measured found a significant association between body fat and asthma in women (P = 0.04), but not in men (P = 0.75). Sood et al. studied the association of asthma on fat mass and lean mass (i.e., primarily skeletal muscle and viscera), as defined by dual-energy X-ray absorptiometry (DEXA) (82, 83). While DEXA accurately identifies relatively large physiological fat depots as fat mass, it inaccurately classifies smaller pathological fat depots within the skeletal muscle and viscera as lean mass. These studies suggest that excess lean mass may predict asthma better than excess fat mass among women (82, 83). A possible explanation is that pathological fat depots may be stronger predictors than physiological fat depots for asthma in women. Furthermore, lean mass in the truncal area may be the strongest predictor for asthma in women, compared with other regional distributions of DEXA-assessed fat and lean mass (unpublished observations by Sood A, Qualls C, Li R, Schuyler M, Beckett WS, Smith LJ, Thyagarajan B, Lewis CE, Jacobs DR).
Possible Mechanisms
The mechanistic basis for the association between obesity and asthma is not known, although mechanical, immunological, genetic, hormonal, and environmental pathways have all been proposed (78). It is further unclear which of these various pathways may be the dominant mechanism. Immunological pathways have recently received the greatest attention, with animal experiments confirming an immunological mechanism to the obesity-asthma association in mice (75, 76) [the findings from mice experiments are discussed in greater detail in the review article by Shore (73a) in this issue of the journal]. The immunological pathway invokes a possible role for proinflammatory adipokines (such as leptin) and anti-inflammatory adipokines (such as adiponectin). Although adipose tissue produces over 50 adipokines, the focus of this review is restricted to the role of leptin and adiponectin in human lung disease.
ADIPOKINES AND ASTHMA
Leptin and Asthma
Serum concentrations of leptin, an energy-regulating hormone, increase with obesity (62, 64). Leptin and leptin receptors are expressed by the lung (8, 12). Leptin has overall proinflammatory systemic effects that may be associated with asthma: it may stimulate the production of tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 from adipose tissue (6, 44), negatively modulate the function of regulatory T cells that are associated with asthma (92), and promote Th1 proliferation with increased production of interferon-γ (45, 49). Recent in vitro studies indicate that leptin also stimulates release of vascular endothelial growth factor by airway smooth muscle cells (72), although it does not affect airway smooth muscle cell proliferation (55). Vascular endothelial growth factor may stimulate subepithelial neovascularization and vascular permeability, a key finding in asthma (72).
Murine studies.
A causal role for leptin in asthma is supported by murine studies; administration of exogenous leptin in leptin-deficient mice augments airway hyperreactivity following allergen challenge, as well as lung inflammation following ozone exposure (74, 75).
Human studies.
Human data is, however, currently inconclusive regarding the independent association between serum leptin concentrations and risk for asthma (see Table 1) (27, 54, 79, 80). A large cross-sectional, population-based study in the United States showed a positive association between the highest quartile of serum leptin concentration and the risk for asthma in women [OR of 3.2, 95% confidence interval (CI) 1.3–7.7], independent of triceps skinfold thickness. On the other hand, a large population-based study by Jartti et al. (31) used a sequential nested case control study design set within an established Finnish cohort and did not show an independent association between asthma and serum leptin concentrations. Other studies done in children had significantly smaller sample sizes: some showing a positive association (27, 53), and others showing no association (35).
Table 1.
A summary of studies evaluating the leptin-asthma association
| Authors (Years and Country) (Ref. No.) | Subject Characteristics | Study Design Characteristics | Obesity-Asthma Association | Leptin-Asthma Association, Adjusted For Obesity | Comment |
|---|---|---|---|---|---|
| Studies involving children | |||||
| Guler et al. (2004, Turkey) (27) | 6 ± 3 yr, 135 subjects, 102 with asthma | Cross sectional | No BMI-asthma association | All (OR: 2.0; 95% CI: 1.1–3.6) with current asthma; stronger for boys than girls | Association stronger for atopic asthma than for nonatopic asthma |
| Nagel et al. (2009, Germany) (53) | 10 ± 2.2 yr, 462 subjects, 30 with asthma | Cross sectional | No BMI-asthma association | All (OR: 4.1; 95% CI: 1.3–12.5) for ever asthma; stronger for girls than boys | Association stronger for nonatopic asthma than for atopic asthma |
| Kim et al. (2008, Korea) (35) | 10 ± 2 yr, 240 subjects, 186 with asthma | Cross sectional | No association | No association with current asthma (methacholine confirmed) | |
| Studies involving adults | |||||
| Sood et al. (2006, US) (79) | 44 ± 1 yr, 5,876 subjects, 290 with asthma | Cross sectional, population based | BMI-asthma association present | Women with highest leptin quartile (OR: 3.2; 95% CI: 1.3–7.7) for current asthma; stronger in premenopausal women (OR: 3.6) | Results were adjusted for atopy; adjusting for different obesity measures affected association differently |
| Studies involving both children and adults | |||||
| Jartti et al. (2009, Finland) (31) | 1) 3–18 yr, 3,583 subjects, 64 with asthma; 2) 9–24 yr, 2,764 subjects, 101 with asthma; 3) 24–39 yr, 2,620 subjects, 121 with asthma | Sequential case control, population based | BMI-asthma association only at 24–39 yr age | No association with ever asthma | |
BMI, body mass index; OR, odds ratio; CI, confidence interval.
Among the studies showing a positive independent association between serum leptin concentrations and asthma, the association appears to be stronger in specific population subgroups (27, 54, 79, 80). These subgroups include prepubertal boys, peripubertal girls, and premenopausal women (27, 54, 79, 80). Prepubertal boys have lower serum leptin concentrations than girls of the same age and weight (65). On the other hand, peripubertal girls and premenopausal women have higher serum leptin concentrations than boys and men of the same age and weight, respectively, presumably due to the effect of sex hormones on leptin expression (2, 62, 64).
Interestingly, even among the positive studies, the obesity-asthma association does not appear to be entirely explained by serum leptin concentration alone (79). Sood et al. reported that the relationship between BMI and asthma in women was attenuated but remained of borderline significance, after adjustment for serum leptin concentrations (79). This suggests that serum leptin may explain only a small portion of the obesity-asthma association in women, implying that other mechanistic pathways may be involved as well.
The strength of the associations between serum leptin concentrations and asthma in positive studies is usually modest. For serum leptin, the strength of the association varies from OR of ∼2.0 to 4.1 for asthma (27, 54, 79), although OR of ∼6–13 have been described in subgroup analyses in some studies (54, 79).
Adiponectin and Asthma
Adiponectin is a predominantly anti-inflammatory adipokine. Adiponectin inhibits proinflammatory cytokines, such as TNF-α, IL-6, and nuclear factor-κB (3, 48, 101), as well as induces anti-inflammatory cytokines, such as IL-10 and IL-1 receptor antagonist (38, 100, 101). Adiponectin and all of the known receptors for adiponectin (AdipoR1, AdipoR2, T-cadherin, and calreticulin) are expressed on multiple cell types in the lung (30, 52, 91, 102). Adiponectin is also transported from blood into the alveolar lining fluid via the T-cadherin molecule on the endothelium (30).
Obesity and adiponectin.
Although central (visceral) adipocytes are the most important source of adiponectin (86), serum adiponectin concentrations do not increase with obesity as do serum leptin concentrations. On the contrary, there is a tendency for reduced serum adiponectin concentration among obese subjects (5). It is believed that hypoxia-related necrosis of adipocytes in obesity exposes naked lipid droplets to the interstitium (19). Macrophages collect around necrosing adipocytes and become functionally activated, forming syncytia (19). Macrophages in the adipose tissue produce TNF-α and IL-6, which, in turn, may directly inhibit the local production of adiponectin in a paracrine fashion (13), explaining the reduced systemic adiponectin concentrations seen with obesity.
Murine studies.
Exogenous adiponectin administration prevents the development of allergen-induced airway hyperreactivity in mice (76). Adiponectin also inhibits vascular smooth muscle proliferation in mice, but does not affect airway smooth muscle proliferation (51, 72).
Human studies.
Like leptin, human data on the independent association between serum adiponectin concentrations and asthma are currently inconclusive (see Table 2) (31, 35, 54, 80). One study each of children and adults showed a protective association between serum adiponectin concentrations and risk for asthma, independent of BMI (54, 80). These studies further suggest that the protective association may be stronger in specific population subgroups, such as peripubertal girls and premenopausal women (54, 80). On the other hand, Jartti et al., in a large Finnish cohort, did not show an independent association between serum adiponectin and ever asthma (31). Jartti et al. also found no independent association between the ratio of serum leptin to adiponectin (ostensibly reflecting the inflammatory balance of adipokines) with patients who reported ever having asthma (31).
Table 2.
A summary of studies evaluating the adiponectin-asthma association
| Authors (Years and Country) (Ref. No.) | Subject Characteristics | Study Design Characteristics | Obesity-Asthma Association | Adiponectin-Asthma Association, Adjusted for Obesity | Comments |
|---|---|---|---|---|---|
| Studies involving children | |||||
| Nagel et al. (2009, Germany) (53) | 10 ± 2.2 yr, 462 subjects, 30 with asthma | Cross sectional | No association | β-coefficient of 0.6 between low levels of log adiponectin and ever asthma (P = 0.20) | Association significant only for nonatopic asthma (β coefficient of 1.4; P < 0.01) |
| Kim et al. (2008, Korea) (35) | 10 ± 2 yr, 240 subjects, 186 with asthma | Cross sectional | No association | No association with current asthma (methacholine confirmed) | |
| Studies involving adults | |||||
| Sood et al. (2008, US) (80) | 44 ± 1 yr, 5,876 subjects, 290 with asthma | Cross sectional, population based | BMI-asthma association present | Premenopausal women (OR: 0.5; 95% CI: 0.3–0.8 in the highest tertile with current asthma) | |
| Studies involving children and adults | |||||
| Jartti et al. (2009, Finland) (31) | 1) 3–18 yr, 3,583 subjects, 64 with asthma; 2) 9–24 yr, 2,764 subjects, 101 with asthma; 3) 24–39 yr, 2,620 subjects, 121 with asthma | Sequential case control, population based | BMI-asthma association only at 24–39 yr age | No association with ever asthma | |
The strength of the associations described between serum adiponectin and asthma in positive studies is usually modest. For adiponectin, OR of ∼0.5 for asthma has been described in the highest tertile of serum adiponectin concentration in premenopausal women, compared with the lowest tertile (80). Furthermore, like leptin, the obesity-asthma association does not appear to be explained by serum adiponectin alone (80), implying multiplicity of mechanistic pathways for the obesity-asthma association.
General Comments on Human Studies of Adipokines and Asthma
The phenotype of asthma studied above is usually clinical, based on self-report, parental report, or physician report of ever or current asthma (27, 54, 79, 80). Interestingly, the only study that used a physiological phenotype of asthma (based on methacholine confirmed airway hyperreactivity) did not show significant associations between serum adipokines and asthma (35). The association of adipokines with inflammatory phenotypes of asthma has not been studied. Furthermore, studies do not show a consistent relationship of adipokines with either atopic or nonatopic variants of asthma (27, 54, 79). Data on the association of adipokines with intermediate asthma-related phenotypes is limited. One study showed a positive association between serum leptin and serum IgE concentrations (27), and another showed a negative association between serum adiponectin and atopic dermatitis and eczema in children (54).
Among the studies showing significant associations between adipokines and asthma, the strength of the association is stronger among peripubertal girls and premenopausal women (27, 54, 79, 80). Sex-related factors may, therefore, interact with the adipokine-asthma association, unlike the murine studies in this field. Although presumed to be female sex hormones, the specific sex-related factor that may be important for these associations is not known. Interestingly, there is a marked sexual dimorphism of the distribution of adiponectin isoforms in humans. Women have greater proportion of high-molecular-weight (HMW) isoform; and lower proportion of the low-molecular-weight (LMW) isoform than men (70). Recent studies suggest that the HMW isoform of adiponectin is the most biologically active form of adiponectin in regulating insulin resistance. It is possible that HMW isoform of adiponectin may also be more active than other adiponectin isoforms for asthma.
The role of systemic adipokines in early childhood has not been adequately studied. One longitudinal study of 740 German children reported that cord blood leptin levels did not predict wheeze during the first 2 yr of life (63). In contrast, adiponectin concentrations in the cord blood of term infants were associated with an increased risk of asthma or obstructive bronchitis during the first 2 yr of life in children born from atopic mothers (63). It is, therefore, possible that serum adipokines may have different inflammatory effects in the perinatal period, compared with later in life.
Suggestions for Future Research in Human Adipokine-Asthma Associations
Heterogeneity of obesity and asthma phenotypes.
All human adipokine-asthma studies have used excess body mass to define the obesity phenotype. As discussed previously, a large value for BMI is, in fact, a collection of various phenotypes, with some but not all relating to adiposity. The strength of the leptin-asthma association was noted to change when adjusted for different obesity measures: the association strengthened when adjusted for triceps skinfold thickness and weakened when adjusted for BMI (79). Future studies in this area, therefore, need to take into account the heterogeneity of obesity phenotypes.
Similarly, the phenotype of asthma studied is usually clinical, based on self-report, parental report, or physician report, and varies from ever to current asthma (27, 54, 79, 80). Studies evaluating additional clinical phenotypes, including indexes of asthma control and severity, are needed. Additionally, physiological and inflammatory phenotypes of asthma need to be studied. Furthermore, the potential interaction between atopy and adipokines on asthma needs further evaluation.
Direction of association.
Studies examining the association between adipokines and human asthma are usually of cross-sectional nature and, therefore, unable to determine direction of association. Mouse experiments support a bidirectional association: allergen inhalation affects serum adipokines, and exogenous adipokine administration affects airway changes of asthma. A small interventional study suggests that serum adipokines in sensitized humans with asthma are unaffected by acute bronchoprovocation from inhalation of a specific allergen (i.e., with skin sensitization) (84). This suggests that the acute effect of serum adipokines on asthma in humans may be unidirectional i.e., serum adipokine levels may affect asthma instead of themselves being acutely affected by asthma. The chronic effect of asthma on serum adipokine concentrations, however, needs further study.
Need for adequately powered studies.
It is imperative that studies of systemic adipokine-asthma association be adequately powered. The power of a study may be affected by overall sample size, sample size in relevant population subgroups, effect size of the association, prevalence of asthma, and severity of obesity. The strength of the adipokine-asthma associations is usually modest, being stronger in specific population subgroups. Furthermore, several adipokine-asthma studies done outside the United States do not show an obesity-asthma association (27, 31, 35, 54). It is possible that these studies are underpowered because of lower prevalence and severity of obesity in those countries, relative to the United States (27, 31, 35, 54).
Role of airway adipokines.
The role of airway adipokines in asthma remains to be established. It is possible that airway leptin concentrations may not reflect serum leptin concentrations. A recent study showed a negative correlation between plasma and sputum concentrations of leptin in patients with COPD, suggesting that airway leptin may be synthesized in the lung itself and not leaked from the plasma pool through the microvasculature into the lung (10, 97). Furthermore, the bronchial epithelium of subjects with asthma showed a decreased expression of leptin and its receptor, compared with healthy controls (12). Similarly, airway adiponectin concentrations may not reflect serum adiponectin concentrations. Preliminary murine studies suggest that, while serum adiponectin concentrations are higher in female mice than male mice, airway adiponectin concentrations are similar between male and female mice. Furthermore, serum adiponectin in mice is different from the airway adiponectin in terms of isomeric distribution. While adiponectin is present as the LMW, medium-molecular weight, and HMW isoforms in the serum, bronchoalveolar lavage fluid contains the HMW isoform in disproportionately large amounts (104). It is not known if similar differences between serum and airway adiponectin concentrations exist among humans.
OBESITY AND COPD
COPD is a progressive respiratory disorder characterized by the presence of airflow limitation due to chronic bronchitis or emphysema (4). While chronic bronchitis is defined in clinical terms and emphysema is defined in anatomical terms, these two phenotypes are not always easily distinguishable (47). Increased body fat is typically, although not always, seen with chronic bronchitis, whereas emphysema is typically associated with loss of fat and/or lean mass (26, 39, 73).
Risk for COPD
The effect of obesity on risk for COPD has not been well studied. Limited epidemiological data suggest that overweight or obese status may protect against the risk for developing emphysema-dominant COPD phenotype in men (29, 32, 42, 98). However, recent studies suggest that this may not be entirely true. A recent large-population-based French study (n = 121,965) showed that abdominal obesity was independently associated with spirometric airflow obstruction in both sexes (overall OR = 1.1; 95% CI 1.04–1.2), although it was unclear whether those with airflow obstruction had asthma, chronic bronchitis, or emphysema diagnoses (43). Similarly, a large Chinese population-based study (n = 7,358) carefully excluded those with asthma and showed that abdominal obesity was independently associated with spirometric airflow obstruction (adjusted OR = 1.4; 95% CI 1.1–1.8) (41). The use of different definitions of COPD may be one reason for the apparent differences in results between various studies (47).
COPD Mortality
Limited epidemiological data suggest that overweight or obese status may protect against mortality from COPD (32, 42, 98). The large Copenhagen City Heart study suggested such a protective effect in mortality in severe COPD, not in mild or moderate disease (98). The Korean Cancer Prevention Study similarly showed that the risk of death from respiratory causes and specifically from COPD decreased progressively with increasing BMI (32). These studies were limited by the relatively small number of deaths from respiratory causes in obese individuals with COPD, about two-thirds of deaths in individuals with COPD result from nonrespiratory causes anyway (50). Furthermore, these study populations did not have the type and severity of obesity currently prevalent in the United States. It should also be pointed out that there is no plausible reason why adiposity should protect against mortality in COPD (42). This phenomenon, well-characterized in other chronic diseases, such as congestive heart failure, is referred to as the “obesity paradox.” A recent study indicates that lean mass index is a better predictor of mortality than BMI in moderate to severe COPD, whereas fat mass index is not a significant prognostic indicator (69). It is, therefore, possible that excess lean mass (and not excess fat mass) contributes to the survival advantage in the COPD subject with greater BMI, although this still remains to be proven.
Physiological Derangements of COPD
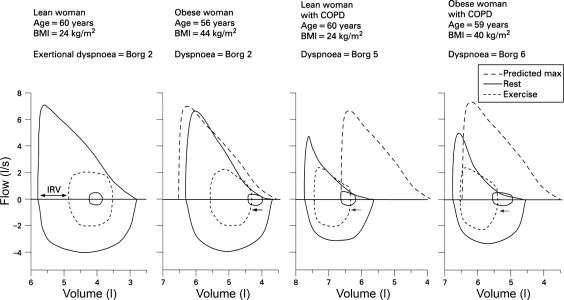
The physiological derangements in obesity and in COPD are well understood when each is considered in isolation, but few studies have explored the pathophysiological derangements that occur when both conditions coexist in the same individual, as is increasingly the case. Clinical experience indicates that obese patients with COPD have worse symptoms and activity limitation than normal-weight patients with COPD (60, 89). Figure 1 shows the maximal and tidal flow-volume loops for a series of age-matched women: a lean woman with normal lung function, an obese woman with normal lung function, a lean woman with COPD, and an obese woman with COPD (25). While the end-expiratory lung volume in the lean woman with normal lung function was decreased during exercise, all of the remaining women experienced dynamic hyperinflation during exercise. Dynamic hyperinflation occurs when patients commence inhalation before full exhalation has been achieved, leading to air trapping or increased end-expiratory lung volume. Dynamic hyperinflation explains most of the variation in dyspnea intensity in diseases with airflow limitation (59). Compared with women with either obesity or COPD alone in Fig. 1, dynamic hyperinflation during exercise was greater in the woman with both conditions. Dynamic hyperinflation occurring in a setting of increased ventilatory requirement results in an earlier mechanical limitation of ventilation with greater dyspnea and exercise intolerance in the patient with both obesity and COPD than in the patient with either condition alone (25).
Fig. 1.
Maximal and tidal flow-volume loops for a series of age-matched women: a lean woman with normal lung function, an obese woman with normal lung function, a lean woman with chronic obstructive pulmonary disease (COPD) (forced expiratory volume in 1 s: 46% predicted), and an obese woman with COPD (forced expiratory volume in 1 s: 45% predicted). Inner tidal loops are shown at rest (solid inner loops) and at ventilations of ∼32 l/min (dashed loops), the latter corresponding to the symptom-limited peak of exercise for both women with COPD. While the end-expiratory lung volume was decreased during exercise in the healthy lean woman, the other women experienced dynamic pulmonary hyperinflation (leftward arrows). The dynamic hyperinflation in women with both obesity and COPD was greater than that for the women with either condition alone. Additional flow and volume constraints on tidal volume expansion in the women with COPD limited the peak ventilation that could be attained during exercise. In women with COPD, a critically reduced inspiratory reserve volume limited any further increase in tidal volume during exercise and resulted in severe exertional dyspnea. Ratings of dyspnea intensity using the modified 10-point Borg scale are shown at comparable ventilations during exercise. [Reproduced with permission from Franssen et al. (25).]
Interestingly, exercise intolerance in patients with COPD may be associated with the relative distribution of fat and lean mass. In early COPD, gain in fat mass may have a relatively greater negative impact on physical performance than loss of lean mass (21). On the other hand, in more advanced disease, loss of lean mass may have a relatively greater negative impact on physical performance than alterations in fat mass (69).
ADIPOKINES AND COPD
Leptin and COPD
There are very few human studies that have examined the association between systemic or airway leptin and COPD, and most have not adjusted for the confounding effect of fat mass. Most studies demonstrate overall lower serum leptin concentrations in stable COPD patients compared with healthy controls, likely reflecting the relatively lower fat mass in advanced COPD (33, 90, 99). Despite these observations, several studies suggest that both circulating and airway leptin are associated with greater systemic and airway inflammation and lower lung function in stable COPD.
Systemic inflammation.
Circulating leptin may promote systemic inflammation in stable COPD. Schols et al. (68) reported a positive association between plasma concentrations of leptin and soluble TNF receptor-55, a marker of systemic inflammation in stable patients with emphysema, after adjustment for fat mass, although another study could not corroborate these findings (73).
Lung function.
It is possible that serum leptin concentrations adversely affect human lung function, independent of obesity. Kim et al. (35) showed weak negative correlations in primarily asthmatic children between serum leptin concentrations and FEV1 and forced midexpiratory flow rate values (r = −0.2, P ≤ 0.01 for both analyses) without adjustment for BMI. A recent study has found an association between leptin receptor polymorphisms and rate of lung function decline in a population of smokers with COPD, after adjustment for smoking and change in BMI (28).
Acute exacerbations.
Acute exacerbations of COPD may be associated with a transient increase in serum concentrations of leptin (14, 20, 40). This disturbance might be induced by the systemic inflammatory response, as well as by the systemic corticosteroid treatment associated with acute COPD exacerbation (20). The increased circulating leptin concentrations during acute exacerbations, in turn, are thought to induce anorexia and subsequent weight loss in depleted patients with COPD (20, 68). In contradistinction, serum leptin concentrations are not thought to induce anorexia and weight loss in stable COPD (103).
Airway leptin.
The role of airway leptin in COPD is also not well studied. Broekhuizen at al. (10) showed a positive unadjusted association between sputum concentrations of leptin and other inflammatory markers (sputum C-reactive protein and total TNF-α) in stable patients with COPD. Bruno et al. (11) demonstrated greater expression of leptin within the bronchial submucosa in COPD than in healthy controls, along with greater expression of activated T lymphocytes, particularly of the CD8+ type, suggesting a role of leptin in regulating airway inflammation in COPD. This study further noted an inverse correlation between level of leptin expression in bronchial submucosa and FEV1 and FEV1/forced vital capacity (FVC) values in patients with COPD, without adjustment for BMI (11).
Adiponectin and COPD
Genetically induced adiponectin deficiency in normal-weight mice maintained on a normal diet results in an increased expression of TNF-α and matrix metalloproteinases in alveolar macrophages and alveolar simplification and/or enlargement due to abnormal postnatal development of the lung, resembling an emphysema-like phenotype (88). This suggests that systemic adiponectin may have an independent protective effect on the lung through inhibition of alveolar macrophage-related inflammation in mice. It is not known if the same is true in humans.
Risk for COPD.
There is limited data in humans evaluating the predictive effect of serum adiponectin concentrations on risk for COPD, independent of obesity. Contrary to the direction of association in the above-described mouse study, two small cross-sectional human studies have demonstrated that serum adiponectin concentrations in male COPD patients were higher than that in controls (36, 95). These studies were limited by their inability to evaluate the confounding effect of fat mass on this association and by their exclusion of female patients with COPD. Another study showed that expression of adiponectin in airway epithelial cells in patients with emphysema was greater than in healthy (disproportionately female) controls (52). Furthermore, acute exacerbation of COPD was associated with greater serum adiponectin concentrations than stable disease in a small study of 36 male COPD patients (36). It is, therefore, possible that development of COPD, particularly acute exacerbation of COPD, upregulates systemic and lung adiponectin expression, at least in men. The direction of the adiponectin-COPD association, however, needs to be better established in future prospective studies.
Lung function.
Association studies between serum adiponectin concentrations and lung function have yielded conflicting results. In a large unpublished population-based study (Thyagarajan B, Jacobs DR, Smith LJ, Kalhan R, Gross MD, Sood A, unpublished observations) of 2,056 young participants of both sexes, serum adiponectin concentrations were positively associated with FVC and FEV1 without any significant change in FEV1-to-FVC ratio, independent of obesity. An additional small study of primarily asthmatic children showed a weak positive correlation between serum adiponectin concentration and percent predicted forced midexpiratory flow rate (r = 0.2; P = 0.006), without adjustment for obesity (35). On the other hand, two small previous cross-sectional studies of 31 and 15 patients with stable and established COPD, without adjustment for obesity, showed a lack of correlation between spirometric lung function and serum adiponectin concentrations (36, 95). One of the above studies also demonstrated a positive unadjusted association between serum adiponectin concentrations and residual volume (95).
General Comments on Human Studies of Adipokines and COPD
Human studies examining the association between adipokines and COPD suffer from similar limitations as those examining the association with asthma. These limitations include the following: small number of studies, cross-sectional nature of studies that prevents determination of the direction of association; small sample size that raises concerns for power; lack of sex-specific analysis; lack of adjustment for confounding effects of fat and fat-free mass; lack of differentiation between the emphysema-dominant and chronic bronchitis-dominant phenotypes of COPD; and inadequate assessment of airway adipokine status.
SUMMARY
Obesity is an independent risk factor for asthma. Furthermore, although emphysema is classically associated with low fat mass, recent studies suggest that obesity is an independent risk factor for chronic airflow obstruction as well. COPD subjects with greater BMI may have greater dyspnea and activity limitation but paradoxically lower mortality than COPD subjects with lower BMI. The mechanistic basis for these associations in humans is not established, although a possible role for adipokines has been invoked. The adipokines leptin and adiponectin are causally associated with asthma in mice. Although human studies are currently inconclusive, high-serum leptin and low-serum adiponectin concentrations predict asthma, independent of obesity, in select population groups, such as premenopausal women in the United States. In contradistinction, low-serum leptin and high-serum adiponectin concentrations are associated with stable COPD, although these associations are likely confounded by fat mass. Interestingly, leptin may promote systemic and airway inflammation in stable COPD patients. On the other hand, COPD may upregulate systemic and lung adiponectin expression, at least in men. The precise mechanism and significance of the associations between adipokines and lung disease at the current stage is confusing and frankly paradoxical in places. This area of research needs additional study that may open up novel therapeutic strategies for these lung diseases.
GRANTS
This work was supported in part by National Institutes of Health (NIH) National Center for Research Resources Grant M01-RR-00997 and NIH Grant 1 K23 HL 094531-01 A1/A.
DISCLOSURES
I am not aware of financial conflict(s) with the subject matter or materials discussed in this manuscript with any of the authors, or any of the authors' academic institutions or employers.
REFERENCES
- 1.Aaron SD, Fergusson D, Dent R, Chen Y, Vandemheen KL, Dales RE. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest 125: 2046–2052, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Ahmed ML, Ong KK, Morrell DJ, Cox L, Drayer N, Perry L, Preece MA, Dunger DB. Longitudinal study of leptin concentrations during puberty: sex differences and relationship to changes in body composition. J Clin Endocrinol Metab 84: 899–905, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-κB activation and IL-6 production and increases PPARγ2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol 288: R1220–R1225, 2005. [DOI] [PubMed] [Google Scholar]
- 4.[Anon]. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med 152: S77–S121, 1995 [PubMed] [Google Scholar]
- 5.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17: 4–12, 2006 [PubMed] [Google Scholar]
- 7.Beckett WS, Jacobs DR, Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med 164: 2045–2050, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Bergen HT, Cherlet TC, Manuel P, Scott JE. Identification of leptin receptors in lung and isolated fetal type II cells. Am J Respir Cell Mol Biol 27: 71–77, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 175: 661–666, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broekhuizen R, Vernooy JH, Schols AM, Dentener MA, Wouters EF. Leptin as local inflammatory marker in COPD. Respir Med 99: 70–74, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Bruno A, Chanez P, Chiappara G, Siena L, Giammanco S, Gjomarkaj M, Bonsignore G, Bousquet J, Vignola AM. Does leptin play a cytokine-like role within the airways of COPD patients? Eur Respir J 26: 398–405, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Bruno A, Pace E, Chanez P, Gras D, Vachier I, Chiappara G, La Guardia M, Gerbino S, Profita M, Gjomarkaj M. Leptin and leptin receptor expression in asthma. J Allergy Clin Immunol 124: 230–237, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab 285: E527–E533, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Calikoglu M, Sahin G, Unlu A, Ozturk C, Tamer L, Ercan B, Kanik A, Atik U. Leptin and TNF-alpha levels in patients with chronic obstructive pulmonary disease and their relationship to nutritional parameters. Respiration 71: 45–50, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med 159: 2582–2588, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med 163: 1344–1349, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Celedon JC, Palmer LJ, Litonjua AA, Weiss ST, Wang B, Fang Z, Xu X. Body mass index and asthma in adults in families of subjects with asthma in Anqing, China. Am J Respir Crit Care Med 164: 1835–1840, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol 155: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46: 2347–2355, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Creutzberg EC, Wouters EF, Vanderhoven-Augustin IM, Dentener MA, Schols AM. Disturbances in leptin metabolism are related to energy imbalance during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 162: 1239–1245, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Eisner MD, Blanc PD, Sidney S, Yelin EH, Lathon PV, Katz PP, Tolstykh I, Ackerson L, Iribarren C. Body composition and functional limitation in COPD. Respir Res 8: 7, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figueroa-Munoz JI, Chinn S, Rona RJ. Association between obesity and asthma in 4–11 year old children in the UK. Thorax 56: 133–137, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol 115: 897–909, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Mannino DM, Redd SC, Mokdad AH, Mott JA. Body mass index and asthma incidence among USA adults. Eur Respir J 24: 740–744, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Franssen FM, O'Donnell DE, Goossens GH, Blaak EE, Schols AM. Obesity and the lung: 5. Obesity and COPD. Thorax 63: 1110–1117, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest 122: 1256–1263, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol 114: 254–259, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Hansel NN, Gao L, Rafaels NM, Mathias RA, Neptune ER, Tankersley C, Grant AV, Connett J, Beaty TH, Wise RA, Barnes KC. Leptin receptor polymorphisms and lung function decline in COPD. Eur Respir J 34: 103–110, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harik-Khan RI, Fleg JL, Wise RA. Body mass index and the risk of COPD. Chest 121: 370–376, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 101: 10308–10313, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jartti T, Saarikoski L, Jartti L, Lisinen I, Jula A, Huupponen R, Viikari J, Raitakari OT. Obesity, adipokines and asthma. Allergy 64: 770–777, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, Samet JM. Body-mass index and mortality in Korean men and women. N Engl J Med 355: 779–787, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Karakas S, Karadag F, Karul AB, Gurgey O, Gurel S, Guney E, Cildag O. Circulating leptin and body composition in chronic obstructive pulmonary disease. Int J Clin Pract 59: 1167–1170, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Kazaks A, Uriu-Adams JY, Stern JS, Albertson TE. No significant relationship between exhaled nitric oxide and body mass index in people with asthma. J Allergy Clin Immunol 116: 929–930; author reply 930, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Kim KW, Shin YH, Lee KE, Kim ES, Sohn MH, Kim KE. Relationship between adipokines and manifestations of childhood asthma. Pediatr Allergy Immunol 19: 535–540, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Kirdar S, Serter M, Ceylan E, Sener AG, Kavak T, Karadag F. Adiponectin as a biomarker of systemic inflammatory response in smoker patients with stable and exacerbation phases of chronic obstructive pulmonary disease. Scand J Clin Lab Invest 69: 219–224, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Komakula S, Khatri S, Mermis J, Savill S, Haque S, Rojas M, Brown L, Teague GW, Holguin F. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir Res 8: 32, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 109: 2046–2049, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Kurosaki H, Ishii T, Motohashi N, Motegi T, Yamada K, Kudoh S, Jones RC, Kida K. Extent of emphysema on HRCT affects loss of fat-free mass and fat mass in COPD. Intern Med 48: 41–48, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Kythreotis P, Kokkini A, Avgeropoulou S, Hadjioannou A, Anastasakou E, Rasidakis A, Bakakos P. Plasma leptin and insulin-like growth factor I levels during acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med 9: 11, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam KB, Jordan RE, Jiang CQ, Thomas GN, Miller MR, Zhang WS, Lam TH, Cheng KK, Adab P. Airflow obstruction and the metabolic syndrome: the Guangzhou Biobank Cohort Study. Eur Respir J. In press. [DOI] [PubMed] [Google Scholar]
- 42.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 160: 1856–1861, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Leone N, Courbon D, Thomas F, Bean K, Jego B, Leynaert B, Guize L, Zureik M. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med 179: 509–516, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. FASEB J 12: 57–65, 1998 [PubMed] [Google Scholar]
- 45.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394: 897–901, 1998 [DOI] [PubMed] [Google Scholar]
- 45a.Mancuso P. Obesity and lung inflammation. J Appl Physiol (October 29, 2009). doi:10.1152/japplphysiol.00781.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45b.Lugogo NL, Kraft M, Dixon AE. Does obesity produce a distinct asthma phenotype? J Appl Physiol (October 29, 2009). doi:10.1152/japplphysiol.00845.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maniscalco M, Zedda A, Faraone S, Cerbone MR, Cristiano S, Giardiello C, Sofia M. Weight loss and asthma control in severely obese asthmatic females. Respir Med 102: 102–108, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Mannino DM. Chronic obstructive pulmonary disease: definition and epidemiology. Respir Care 48: 1185–1193, 2003 [PubMed] [Google Scholar]
- 48.Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology 40: 177–184, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Matarese G, La Cava A, Sanna V, Lord GM, Lechler RI, Fontana S, Zappacosta S. Balancing susceptibility to infection and autoimmunity: a role for leptin? Trends Immunol 23: 182–187, 2002 [DOI] [PubMed] [Google Scholar]
- 50.McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax 62: 411–415, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin-deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol 41: 397–406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller M, Cho JY, Pham A, Ramsdell J, Broide DH. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J Immunol 182: 684–691, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol 20: 81–88 2009. [DOI] [PubMed] [Google Scholar]
- 54.Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol 20: 81–88, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Nair P, Radford K, Fanat A, Janssen LJ, Peters-Golden M, Cox PG. The effects of leptin on airway smooth muscle responses. Am J Respir Cell Mol Biol 39: 475–481, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Nathell L, Jensen I, Larsson K. High prevalence of obesity in asthmatic patients on sick leave. Respir Med 96: 642–650, 2002. [DOI] [PubMed] [Google Scholar]
- 57.National Center for Health Statistics. Chartbook on Trends in the Health of Americans, Health, United States, 2006. Hyattsville, MD: Public Health Service, 2006 [PubMed] [Google Scholar]
- 58.Nystad W, Meyer HE, Nafstad P, Tverdal A, Engeland A. Body mass index in relation to adult asthma among 135,000 Norwegian men and women. Am J Epidemiol 160: 969–976, 2004 [DOI] [PubMed] [Google Scholar]
- 59.O'Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis 148: 1351–1357, 1993 [DOI] [PubMed] [Google Scholar]
- 60.Ramachandran K, McCusker C, Connors M, Zuwallack R, Lahiri B. The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chron Respir Dis 5: 205–209, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Romieu I, Avenel V, Leynaert B, Kauffmann F, Clavel-Chapelon F. Body mass index, change in body silhouette, and risk of asthma in the E3N cohort study. Am J Epidemiol 158: 165–174, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab 81: 3424–3427, 1996 [DOI] [PubMed] [Google Scholar]
- 63.Rothenbacher D, Weyermann M, Fantuzzi G, Brenner H. Adipokines in cord blood and risk of wheezing disorders within the first two years of life. Clin Exp Allergy 37: 1143–1149, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Ruhl CE, Everhart JE. Leptin concentrations in the United States: relations with demographic and anthropometric measures. Am J Clin Nutr 74: 295–301, 2001 [DOI] [PubMed] [Google Scholar]
- 65.Savino F, Fissore MF, Grassino EC, Nanni GE, Oggero R, Silvestro L. Ghrelin, leptin and IGF-I levels in breast-fed and formula-fed infants in the first years of life. Acta Paediatr 94: 531–537, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Schachter LM, Peat JK, Salome CM. Asthma and atopy in overweight children. Thorax 58: 1031–1035, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax 56: 4–8, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schols AM, Creutzberg EC, Buurman WA, Campfield LA, Saris WH, Wouters EF. Plasma leptin is related to proinflammatory status and dietary intake in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 160: 1220–1226, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 147: 1151–1156, 1993 [DOI] [PubMed] [Google Scholar]
- 70.Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology 149: 2270–2282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax 54: 396–402, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shin JH, Kim JH, Lee WY, Shim JY. The expression of adiponectin receptors and the effects of adiponectin and leptin on airway smooth muscle cells. Yonsei Med J 49: 804–810, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shin KC, Chung JH, Lee KH. Effects of TNF-alpha and leptin on weight loss in patients with stable chronic obstructive pulmonary disease. Korean J Intern Med 22: 249–255, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73a.Shore SA. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol (October 29, 2009). doi:10.1152/japplphysiol.00749.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shore SA, Rivera-Sanchez YM, Schwartzman IM, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol 95: 938–945, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol 115: 103–109, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 118: 389–395, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med 162: 1477–1481, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Sood A. Does obesity weigh heavily on the health of the human airway? J Allergy Clin Immunol 115: 921–924, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Sood A, Camargo CA, Jr, Ford ES. Association between leptin and asthma in adults. Thorax 61: 300–305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sood A, Cui X, Qualls C, Beckett WS, Gross MD, Steffes MW, Smith LJ, Jacobs DR., Jr Association between asthma and serum adiponectin concentration in women. Thorax 63: 877–882, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sood A, Dawson BK, Eid W, Eagleton LE, Henkle JQ, Hopkins-Price P. Obesity is associated with bronchial hyper-responsiveness in women. J Asthma 42: 847–852, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Sood A, Qualls C, Arynchyn A, Beckett WS, Gross MD, Steffes MW, Smith LJ, Holvoet P, Thyagarajan B, Jacobs DR., Jr Obesity-asthma association: is it explained by systemic oxidant stress? Chest 136: 1055–1062, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sood A, Qualls C, Arynchyn A, Beckett WS, Smith LJ, Thyagarajan B, Sidney S, Jacobs DR. Asthma is associated with both fat and lean mass in women (Abstract). Am J Respir Crit Care Med 179: A5510, 2009. [Google Scholar]
- 84.Sood A, Qualls C, Seagrave J, Stidley C, Berwick M, Archibeque T, Schuyler M. Effect of specific allergen inhalation on serum adiponectin in human asthma. Chest 135: 287–294, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sood A, Verhulst S, Varma A, Eagleton L, Henkle J, Hopkins-Price P. Association of excess weight and degree of airway responsiveness in asthmatics and non-asthmatics. J Asthma 43: 1–6, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Steffes MW, Gross MD, Schreiner PJ, Yu X, Hilner JE, Gingerich R, Jacobs DR., Jr Serum adiponectin in young adults–interactions with central adiposity, circulating levels of glucose, and insulin resistance: the CARDIA study. Ann Epidemiol 14: 492–498, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Stenius-Aarniala B, Poussa T, Kvarnstrom J, Gronlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 320: 827–832, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Summer R, Little FF, Ouchi N, Takemura Y, Aprahamian T, Dwyer D, Fitzsimmons K, Suki B, Parameswaran H, Fine A, Walsh K. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol 294: L1035–L1042, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swinburn CR, Cooper BG, Mould H, Corris PA, Gibson GJ. Adverse effect of additional weight on exercise against gravity in patients with chronic obstructive airways disease. Thorax 44: 716–720, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takabatake N, Nakamura H, Abe S, Hino T, Saito H, Yuki H, Kato S, Tomoike H. Circulating leptin in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159: 1215–1219, 1999 [DOI] [PubMed] [Google Scholar]
- 91.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest 117: 375–386, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taleb S, Herbin O, Ait-Oufella H, Verreth W, Gourdy P, Barateau V, Merval R, Esposito B, Clement K, Holvoet P, Tedgui A, Mallat Z. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol 27: 2691–2698, 2007 [DOI] [PubMed] [Google Scholar]
- 93.Thomson CC, Clark S, Camargo CA., Jr Body mass index and asthma severity among adults presenting to the emergency department. Chest 124: 795–802, 2003 [DOI] [PubMed] [Google Scholar]
- 94.Todd DC, Armstrong S, D'Silva L, Allen CJ, Hargreave FE, Parameswaran K. Effect of obesity on airway inflammation: a cross-sectional analysis of body mass index and sputum cell counts. Clin Exp Allergy 37: 1049–1054, 2007 [DOI] [PubMed] [Google Scholar]
- 95.Tomoda K, Yoshikawa M, Itoh T, Tamaki S, Fukuoka A, Komeda K, Kimura H. Elevated circulating plasma adiponectin in underweight patients with COPD. Chest 132: 135–140, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Varraso R, Siroux V, Maccario J, Pin I, Kauffmann F. Asthma severity is associated with body mass index and early menarche in women. Am J Respir Crit Care Med 171: 334–339, 2005 [DOI] [PubMed] [Google Scholar]
- 97.Vernooy JH, Drummen NE, van Suylen RJ, Cloots RH, Moller GM, Bracke KR, Zuyderduyn S, Dentener MA, Brusselle GG, Hiemstra PS, Wouters EF. Enhanced pulmonary leptin expression in patients with severe COPD and asymptomatic smokers. Thorax 64: 26–32, 2009 [DOI] [PubMed] [Google Scholar]
- 98.Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sorensen TI, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med 173: 79–83, 2006 [DOI] [PubMed] [Google Scholar]
- 99.Wang QY, Zhang H, Yan X, Kang J, Yu RJ. [Serum resistin and leptin in patients with chronic obstructive pulmonary disease and their relationship to nutritional state]. Zhonghua Jie He He Hu Xi Za Zhi 28: 445–447, 2005 [PubMed] [Google Scholar]
- 100.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun 323: 630–635, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun 316: 924–929, 2004 [DOI] [PubMed] [Google Scholar]
- 102.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13: 332–339, 2007 [DOI] [PubMed] [Google Scholar]
- 103.Yang YM, Sun TY, Liu XM. The role of serum leptin and tumor necrosis factor-alpha in malnutrition of male chronic obstructive pulmonary disease patients. Chin Med J (Engl) 119: 628–633, 2006. [PubMed] [Google Scholar]
- 104.Zhu M, Hug C, Kashara DI, Johnston RA, Williams AS, Verbout NG, Si H, Jastrab J, Srivastava A, Williams ES, Ranscht B, Shore SA. Impact of adiponectin deficiency on pulmonary responses to acute ozone exposure in mice. Am J Respir Cell Mol Biol 2009November13 [DOI] [PMC free article] [PubMed] [Google Scholar]