Abstract
Few studies have quantified lower limb muscle activity over 24 h using electromyographic signals (EMG). None have described the changes in EMG duration and intensity when data are analyzed with different thresholds. Continuous bilateral EMG recordings were made from vastus lateralis (VL) in 10 subjects (20–48 yr) for 24 h. Before and after this recording, voluntary quadriceps forces and VL EMG at 25%, 50%, 75%, and 100% of the maximal voluntary contraction (MVC), percentage voluntary activation (twitch interpolation), and compound action potentials (M-waves) were recorded. Offline, the 24-h EMG integrals (IEMG, 10-ms time constant) were normalized to the MVC IEMG. Total EMG duration and mean IEMG ranged from 1–3 h and 3.2–12.1% MVC, respectively, when the data were analyzed using the baseline (+3 SD) as threshold. When analysis was done with progressively higher thresholds, from baseline up to 4% MVC, the total EMG duration declined curvilinearly. In some cases the decline in duration was 50–60% for a 1% MVC threshold increment. The mean 24-h IEMG increased by 1.5–2% MVC for each 1% MVC threshold increment. Hence, a small change in the analysis threshold may result in large changes in 24-h EMG duration but moderate changes in mean IEMG. Our findings suggest that VL was active for a short amount of time and at low intensities over 24 h.
Keywords: electromyogram, long-term electromyogram recording, voluntary activation, electromyogram threshold, maximal voluntary contraction
the extent to which skeletal muscles are used during everyday living is not well understood. The most direct approach to characterizing daily muscle activity is to record long-term electromyographic (EMG) activity (1, 10, 11, 13, 18, 23, 26). In humans, long-term EMG recordings of upper limb muscles have been made during various occupations to identify risk factors for muscular complaints (24, 26, 34). Howe and Rafferty (15) compared quadriceps EMG activity between patients with knee osteoarthritis and healthy controls, while Jakobi et al. (16) characterized the differences in EMG activity between healthy older adults and one person who had sustained a stroke. Investigators have also compared daily EMG among upper and lower limb muscles in healthy adults and found that the amount of activity can vary widely between muscles and individuals (up to 6-fold) (7, 18, 23, 30).
Although differences in daily EMG activity between individuals or muscles are often attributed to biological and behavioral factors (13, 18, 23), few investigators have addressed comprehensively the role that methodological factors may play. Differences in the threshold used to separate EMG from the baseline, and the maximal EMG used to normalize the data, would be expected to alter the derived EMG duration and intensity. For example, in a study of nocturnal trapezius muscle activity, Mork and Westgaard (25) reported that setting the threshold visually was a more sensitive criterion than the same absolute threshold for all individuals because it captured more EMG activity. Monster and colleagues (23) used a threshold equal to 8% of the 90th percentile of the amplitude distribution in the analysis of daytime EMG recordings of agonist-antagonist muscle pairs. Depending on the activities performed during the recording period, the 90th percentile may reflect widely different contraction intensities across individuals. In most of the recent studies of long-term EMG in the lower limb, a relative threshold (2% of the MVC) was used to analyze the data (16, 18, 30), although a value of 10% has also been employed (15). Some low-level activity may have been unaccounted for in these previous investigations since laboratory experiments have shown that activity below 2% MVC occurs during different phases of gait (5, 6, 8) and postural tasks (27).
Activation levels during an MVC are also often <100% (2, 9, 21). Thus both the MVC EMG and derived thresholds (%MVC) are likely to be underestimated. Twitch interpolation (29) has not been used to assess muscle activation during MVCs recorded during studies of long-term EMG. Hence, the relationships between MVC EMG, the threshold for separating EMG from baseline noise, and the duration and intensity of muscle activity derived from 24-h EMG data are not well understood.
The purpose of this study was to quantify vastus lateralis (VL) muscle activity over the course of a typical day in healthy adults by recording EMG continuously for 24 h. The two primary aims were 1) to quantify the total duration of EMG and mean EMG intensity (integrated EMG, IEMG) over 24 h, as well as the individual IEMG-duration profiles; and 2) to determine the percentage change in 24-h EMG duration and mean IEMG that results from analyzing the data with different thresholds.
MATERIALS AND METHODS
Subjects.
The subjects were five men and five women, 33 ± 3 yr old (range 21–48 yr old). They were students or employees of the University of Miami. A few participants regularly engaged in recreational exercise, but none of the subjects were highly trained. Informed written consent was obtained from each subject before the experiment, which involved a 24-h EMG recording and laboratory measurements before and after that recording. The Institutional Review Board of the University of Miami approved this study, and the procedures conformed to the standards set by the Declaration of Helsinki.
Twenty-four-hour EMG recording.
The skin over the distal portion of the VL was shaved, abraded, and cleansed with alcohol. Self-adhering electrodes (Superior Silver, no. 626SS, Uni-Patch, Wabasha, MN) were trimmed (1 cm × 3 cm) and positioned over the distal third of the muscle bilaterally in a bipolar configuration (4-cm interelectrode distance). Biceps femoris, tibialis anterior, and medial gastrocnemius EMG were also collected bilaterally, but these data are not reported here. A ground electrode (1 cm × 3 cm) was positioned on each of the four muscles bilaterally, 4 cm proximal to the other two electrodes. The electrodes were covered with Hypafix surgical tape. The VL signals were amplified on site (×400; Motion Lab Systems, Baton Rouge, LA), filtered (30 Hz–1 kHz), and sampled continuously at 1 kHz for 24 h to a 1-GB flashcard in a microcontroller-based, digital data-logger. This device was custom made, battery powered, and portable (32). During the 24-h recording, the device was usually carried in a hip pack worn about the subject's waist. However, during sleep and some periods of stationary work the pack was placed beside the subject. The participants wore compression stockings for the 24-h period to secure all electrodes and cables. They reported that this recording set-up did not encumber them or restrict their daily activities. M-waves recorded before and after the 24-h recordings were similar (Fig. 1C), which suggests an absence of fatigue.
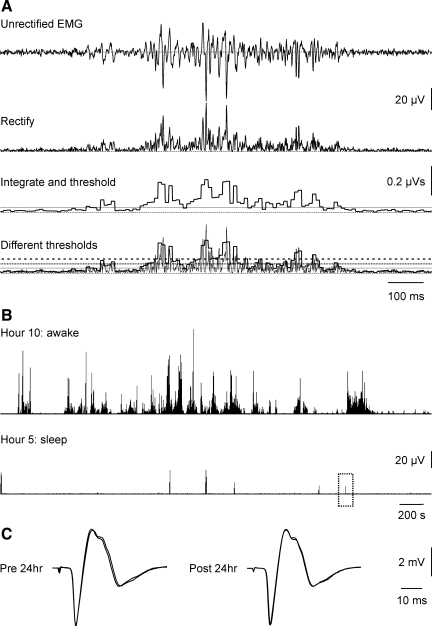
Fig. 1.
Data processing. A: records were rectified, integrated over 10-ms intervals, and defined as EMG if the integral crossed the baseline threshold [solid line; thresholds for 2% and 3% maximal voluntary contraction (MVC) are in dotted and dashed lines]. B: rectified EMG data from the right vastus lateralis (VL) of 1 subject during awake (hour 10) and sleep time (hour 5). One contraction during sleep (dotted box) has been expanded in A (top trace), then processed (bottom traces). C: examples of 2 maximal M-waves (overlaid) recorded from the same subject before and after the 24-h recording.
Pre-post 24-h measurements.
Subjects sat on a custom-designed padded plinth with the trunk stabilized with a hip strap. The angle between the trunk and thigh was ∼100° and the knee was flexed to 100°, placing the quadriceps under stretch (0° = full extension). A padded strap was placed around the shin just proximal to the ankle joint. A cable connected this strap to a force transducer (no. 363-B10-200-25P1, Revere Transducers, Tustin, CA) that was secured to the frame of the plinth. The strain gauge was calibrated with known weights and gave a linear response (R2 = 0.99).
To stimulate the femoral nerve, pulses (50-μs duration, model DS7H, Digitimer stimulator, Hertfordshire, UK) were applied over the inguinal crease via a bipolar bar electrode (1-cm discs, 3-cm spacing; Teca, Pleasantville, NY) mounted on a plastic holder, and manually held in place. The amplitude of submaximal VL M-waves (recorded with the same electrodes in place, as described above) was monitored to determine the optimal site for stimulation. Single pulses were applied every 5 s at progressively greater amplitude (10-mA increments) until a maximal M-wave was attained. That is, no further increase in M-wave amplitude occurred despite five increments in current amplitude. Another five M-waves were recorded using a level of current that was 20% above that used to generate the maximal M-wave.
Three MVCs of each quadriceps muscle were recorded before and after the 24-h recording. Each contraction lasted 5 s and was followed by a 1.5-min rest. Subjects were strongly encouraged to give their best effort during each contraction and feedback of the force trace was provided to them on an oscilloscope. After completion of the MVCs, a total of three 5-s contractions was performed with each VL at target forces of 25%, 50%, and 75% of the MVC force. Two single supramaximal pulses (1 s apart) were applied over the femoral nerve during each contraction, and immediately after, to determine the percentage activation of quadriceps (29) and to record the M-waves. The order in which the right and left legs was tested was balanced across subjects for the pre-24-h measurements. This order was reversed for the post-24-h testing.
Data collection.
The evoked and voluntary EMG signals were amplified, filtered between 30 Hz and 1 kHz (Astro-Medical, model P511, West Warwick, RI), and sampled at 3 kHz. Force signals were amplified, filtered (DC-100 Hz; model 2310, Vishay Measurements Group, Malvern, PA), and sampled at 375 Hz. All signals were displayed on a monitor and stored on-line using a SC/Zoom acquisition and analysis system (Physiology section, Umeå University, Sweden). Sine waves (1 mV) were recorded to the laboratory equipment and to the portable data logger for signal calibration between the two systems.
Analysis of pre-post measurements.
The largest M-wave areas (from the first 2 phases of the potentials, defined by isoelectric crossings) recorded before and after the 24-h period were compared in each leg to assess the electrode integrity over long recordings. For MVC and submaximal target force contractions, a 2-s portion of the EMG just before the first interpolated twitch was rectified and integrated (IEMG) every 10 ms. The MVC (target force) IEMG was defined as the largest mean of 20 consecutive integrals over this 2-s period irrespective of whether it was recorded during the pre- or post-24-h period. The maximal quadriceps force was taken to be the highest level achieved over this 2-s period. Interpolated forces were calculated as the difference between the evoked peak force and force onset. The percentage activation = [1 − (Ts/Tr)] × 100%, where Ts was the interpolated force evoked by the first stimulus during the MVC or target force, and Tr was the mean of the peak forces of the two postcontraction twitches. Individual plots of normalized force-IEMG and percentage activation-IEMG were generated.
Processing and analysis of 24-h EMG.
The surface EMG signals were processed using a custom program written in MatLab (version 7, MathWorks Natick, MA) and DADiSP (DSP Development, Newton, MA). The 24-h data were first time-aligned to reflect the 24-h clock. Midnight was time 0, with midnight to 1 AM as hour 1. The EMG data were filtered (30-Hz high pass, 60-Hz notch) to remove movement artifacts and noise from the line voltage. The EMG signals were then examined at time resolutions ranging from tens of seconds to several minutes to identify and remove large artifacts. The number of artifacts was minimal in all subjects, amounting to <0.5% of the total EMG duration recorded over 24 h. All signals were then rectified and integrated every 10 ms (Fig. 1A). This time was the average duration of human thenar motor unit potentials, and potentials from motor units were the minimal signal we expected to record (33). The total duration of EMG activity over 24 h was calculated as the product of the number of integrals above threshold and integral duration. Each integral above threshold was divided by the MVC IEMG to provide a measure of relative contraction intensity (%MVC IEMG).
EMG thresholds for 24-h recordings.
The thresholds used to separate the EMG from the baseline were based on absolute (baseline noise) and relative (%MVC) criteria. To determine the magnitude of the baseline noise, a 30-s segment of quiet baseline (i.e., no EMG activity) was rectified and integrated (10-ms time constant) from hours 5, 10, 15, 20, and 24. The mean (+3 SD) integral of these five segments represented the baseline threshold for the channel and satisfactorily separated the baseline noise from the EMG (12). The appropriateness of each baseline threshold was verified by scrolling through computer displays of overlaid rectified EMG, the corresponding integrals, and a line representing the threshold (Fig. 1A). To quantify the changes in the derived EMG duration and IEMG (%MVC) resulting from using different thresholds, the 24-h data were reanalyzed using thresholds corresponding to 2%, 2.5%, 3%, 3.5%, and 4% MVC in all subjects.
Statistics.
Paired t-tests were used to evaluate the effects of different thresholds (baseline, 2%–4% MVC) on 24-h EMG duration and IEMG. Right-left and pre-post comparisons of M-wave area, MVC IEMG, MVC force, and percentage activation were determined using paired t-tests and intraclass correlation coefficients (ICC). Since the data recorded from the right VL were not significantly different from the respective values recorded from the left VL, mean data for both legs are presented. Pearson product-moment correlation was applied to examine relationships between measurements. Significance was taken as P < 0.05. Mean values (±1 SE) are reported.
RESULTS
Twenty-four-hour EMG.
Complete recordings for 24 h were obtained from 9 of 10 subjects. In one participant the final 4 awake hours were not recorded due to a battery failure. EMG duration and mean IEMG for these 4 h were assumed to equal the average awake values recorded in this subject. Based on a brief exit interview and review of an activity log completed during the 24 h, subjects spent most of the day attending lectures or working at a computer station. Some completed work-related tasks standing at a work bench. Subjects walked for brief periods (e.g., to campus buildings or the bus) but did not participate in any sport or fitness training. Over 24 h, subjects were awake for an average of 14.8 ± 0.7 h (range: 11.8–17.2 h) and the sleep period lasted 9.1 ± 0.7 h (6.8–12.2 h). Typical examples of EMG recorded during an awake hour and the relative paucity of EMG present during a sleep hour are shown in Fig. 1B.
Twenty-four-hour EMG duration.
The total duration of EMG activity over 24 h (total EMG integration time above baseline threshold) ranged from 1.0 to 3.0 h (mean ± SE: 2.0 ± 0.2 h; n = 10), corresponding to 4.2–12.5% of the day (mean, 8.6 ± 0.9%). The majority of this activity (>99%) occurred during the awake period. Total EMG duration corresponded to 13.3 ± 1.1% of the awake period. EMG activity during the sleep period ranged from 0.2 to 12.5 min or 0.01 to 0.86% of the day (mean, 4.5 ± 1.2 min). The 24-h EMG duration was not significantly different between the five men (1.7 ± 0.25 h) and five women (2.3 ± 0.28 h) (P = 0.13), consistent with results from VL using burst analysis (18).
The baseline noise (i.e., no EMG) was very similar across subjects, averaging 0.055 ± 0.001 μV·s (range, 0.048–0.060) or 5.5 ± 0.1 μV (4.8–6.0). The baseline (+3 SD) threshold was 0.068 ± 0.002 μV·s (range, 0.060–0.080) corresponding to 1.7 ± 0.2% MVC IEMG (range, 0.9–2.6, n = 10). Across subjects, the 24-h duration was not significantly related to the baseline threshold (μV·s or %MVC), the MVC IEMG (μV·s), or voluntary muscle activation. These findings suggest that the likely source of the variability in 24-h duration is real subject differences in daily physical activity rather than variation in the MVC.
Mean 24-h IEMG.
To assess the intensity of EMG activity over 24 h, the IEMG above baseline threshold was divided by the MVC IEMG. The mean IEMG for 24 h ranged from 3.2 to 12.1% MVC (mean, 6.7 ± 1.0%, n = 10). For the awake period, the mean IEMG ranged from 3.2 to 12.2% (mean, 6.9 ± 1.0%). These values were similar to the corresponding data for the sleep period (3.7–10.5%; mean, 6.4 ± 0.8%, P > 0.05). Similar to the study by Kern and colleagues (18), the 24-h mean IEMG was not significantly different between the men (5.6 ± 1.1% MVC) and women (7.8 ± 1.6% MVC) (P = 0.30).
Twenty-four-hour IEMG-duration profile.
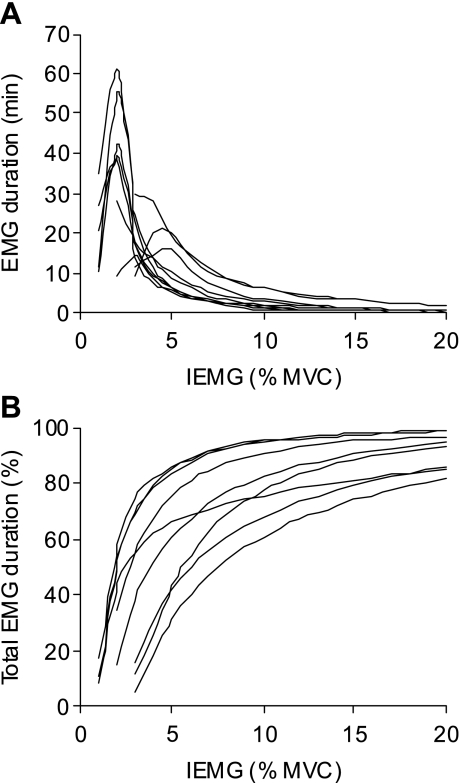
Individual subject plots of 24-h EMG duration (expressed in minutes and as a percentage of the total EMG duration) vs. IEMG (%MVC) are displayed in Fig. 2. The majority of activity was of low intensity. On average, 66 ± 6% (range: 39–86) of the total EMG duration occurred at 5% MVC IEMG or less (Fig. 2B). The corresponding values at and below 10%, 20%, and 50% MVC IEMG were 84 ± 4% (64–95), 93 ± 2% (85–99), and 99 ± 0.4% (97–100) of the total EMG duration, respectively. On average, 6.2 ± 1.8% (1.0–14) of the total EMG duration, corresponding to a mean duration of 8.9 ± 3.2 min (1.1–23), occurred above 20% MVC IEMG. Only 0.1 ± 0.06% (0.005–0.5) of the total EMG duration, corresponding to a maximum of 1 min, was above 80% MVC IEMG.
Fig. 2.
Duration-intensity relationships. Individual subject plots (n = 9) of 24-h EMG duration in minutes (A), and as a cumulative percentage of the total EMG duration (B), at a given EMG integral (IEMG) (bin width: 1% MVC). In A, the EMG duration for the weakest bin of each subject reflects a threshold that was close to the cutoff for that bin. Data for 1 subject were excluded because the last 4 h of data were not recorded.
Effects of different thresholds on 24-h EMG duration and intensity.
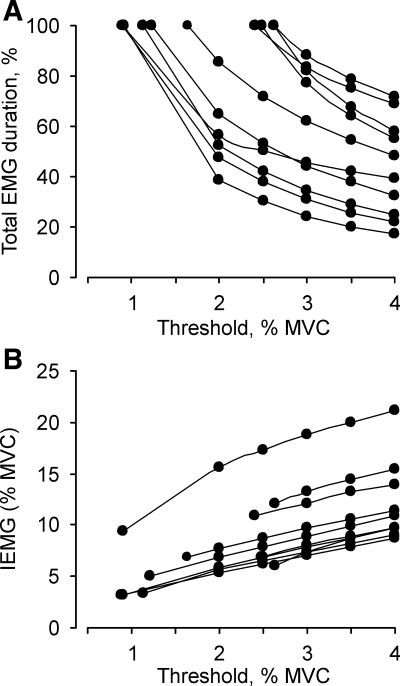
The effects of applying different EMG thresholds on 24-h EMG duration and mean IEMG (%MVC) are shown in Fig. 3 for all subjects. With application of progressively higher thresholds, from baseline up to 4% MVC, the derived 24-h EMG duration declined in a curvilinear manner (Fig. 3A). The magnitude of the reduction in EMG duration for a 1% MVC threshold increment varied across subjects. For example, in two subjects the EMG duration was shortened by 95 min (60% less) and 50 min (50% less) when data were processed using a 2% MVC threshold compared with a baseline (∼1% MVC) threshold (Fig. 3A, lowest two traces). In others, EMG duration declined by 20% for a 1% increase in threshold above the baseline threshold (∼2.5% MVC, Fig. 3A, two upper most traces). Although the threshold-related drop in relative EMG duration was less in these two subjects, this still reflected a 20- to 30-min reduction in activity. On average, the EMG duration was 13.5% less (range, 6–25%) when the 24-h data were processed with a 4% MVC threshold compared with a 3% MVC threshold (n = 10, P < 0.05). The corresponding data for a 3% vs. a 2% threshold was 17.5% (range, 11–24%, n = 6). The mean IEMG increased by 1.5–2% MVC for each 1% MVC increment in threshold (Fig. 3B). For example, the mean IEMG increased from 10 ± 1.1% MVC to 12 ± 1.2% MVC when data were processed using thresholds of 3% and 4% MVC (n = 10, P < 0.05), and from 7.8 ± 1.6% MVC to 9.9 ± 1.8% MVC for thresholds of 2% and 3%, respectively (n = 6).
Fig. 3.
Analysis with different EMG thresholds. Twenty-four-hour EMG duration (A) and IEMG (B) for all 10 subjects when analysis was done with baseline (the lowest threshold), and 2%, 2.5%, 3%, 3.5% MVC, and 4% MVC thresholds. Each line represents combined data from both legs for a given subject.
Voluntary muscle activation.
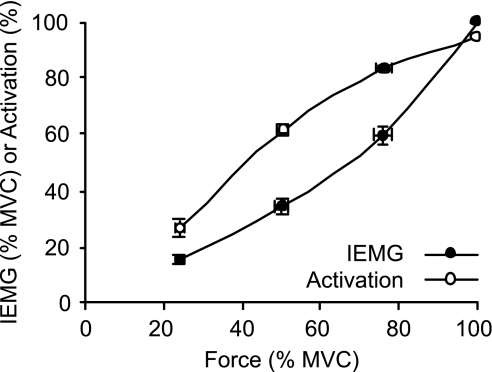
Mean voluntary activation was 95.2 ± 0.6% (range 91.3–97.9%, n = 10). During MVCs, there was a threefold range in the recorded maximal IEMG across subjects (2.3–8.4 μV·s, mean 4.6 ± 0.7 μV·s). The intersubject differences can be partially attributed to peripheral factors since the MVC IEMG was positively correlated with the maximal M-wave area (R2 = 0.74, P = 0.001). Group mean normalized force-activation and force-IEMG relationships are plotted together in Fig. 4.
Fig. 4.
Group mean (±SE, n = 10) normalized force-EMG and force-percentage activation relationships.
Maximal M-waves.
Maximal M-waves evoked from the VL of a subject before and after the 24-h recording are shown in Fig. 1C. The pre- and post-24-h M-wave integrals were similar. For the group, there were no significant differences between the pre- and post-24-h M-wave integrals in either leg. The pre-post ICC for M-wave area was above 0.97 for both legs. The repeatability of the M-waves before and after the 24-h recording provides strong evidence of electrode integrity during the 24-h recording.
DISCUSSION
This study demonstrates that the VL was active for a short amount of time and at low intensities during a typical 24-h period. The mean 24-h EMG duration and IEMG were only 2 h and 6.7% MVC, respectively. Less than 1% (or 4.5 min) of the 24-h EMG duration occurred during the sleep period. Furthermore, the mean IEMG during the awake and sleep periods were similar (6.9% MVC and 6.4% MVC, respectively). In all subjects, over 80% of the total EMG duration was below 20% MVC. No more than 4 min of EMG was above 50% MVC and 1 min above 80% MVC. Given that the mean EMG amplitude during gait and static postures is <15% MVC (5, 8, 27), our EMG profiles are a reasonable description of daily muscle activity for people with relatively sedentary life styles.
Effect of different EMG thresholds on 24-h EMG duration and intensity.
Although it was anticipated that the 24-h EMG duration and IEMG would be sensitive to changes in the threshold, no one has addressed this issue systematically. Our findings demonstrate a curvilinear decline in total EMG duration as the threshold used to analyze the data was progressively increased. In some cases, total EMG duration was 50–60% less when data were analyzed with a higher threshold (i.e., 2% MVC) compared with the lower baseline threshold (i.e., 1% MVC) (Fig. 3A). Hence in these individuals, a large amount of low-intensity activity would be excluded if a 2% MVC threshold was applied to characterize the data. This finding is consistent with an earlier report where visually derived thresholds in each subject captured more EMG activity than the same threshold for all subjects (25). Since previous investigators have used thresholds equal to 2% MVC and greater, a significant amount of lower amplitude activity may not have been accounted for in some of their subjects (15, 16, 18, 24, 30). The mean 24-h IEMG (%MVC) increased linearly with the application of higher thresholds. Thus, for each 1% MVC increment in threshold, the mean 24-h IEMG increased by about 1.5–2% MVC. Our findings reveal that a slight change in threshold of 1–2% MVC leads to a relatively large percentage change in 24-h duration and a moderate change in 24-h IEMG.
Careful consideration should be given to the threshold used since the derived 24-h duration and intensity may impact the physiological and clinical conclusions. For example, chronic electrical stimulation studies reveal that as little as 7.2 min, or 0.5% of 24 h, is sufficient to alter muscle properties (19). This amount of activity is less than the differences in EMG duration associated with a 1% MVC change in threshold in the present study. Ultimately, the type of analysis and thresholds employed will be determined by various factors including the study purpose, recording quality, whether the MVC can be measured, and practical matters such as software capabilities. For example, if the purpose of the study is to characterize daily low-level activity associated with postural work, then it may be prudent to use the lowest possible baseline threshold in each subject rather than a threshold derived from the MVC (25).
A number of human studies have characterized long-term EMG activity as a series of bursts and/or gaps (18, 24, 26). We cannot conclude definitively whether the 24-h EMG duration and intensity and threshold-related effects described here would alter dramatically if the recordings were analyzed using burst analysis. However, daytime EMG amplitude (%MVC) derived using the present approach and burst analysis were compared in the trapezius and three back muscles by Mork and Westgaard (24). At a given threshold (i.e., 2% MVC), they found that the mean burst amplitude was 1–3% MVC higher than the mean amplitude of all of the activity. If the total EMG duration derived from the two approaches is equal, then the pattern of threshold-related changes in EMG is likely to be similar.
MVC EMG.
The recorded MVC EMG is a critical measurement because changes in the threshold (%MVC) influence both 24-h EMG duration and IEMG. Various approaches have been employed to determine the MVC EMG in studies of long-term EMG. In the case of the quadriceps, one study did record force (18), but none have employed twitch interpolation (15, 16). In the present study, the percentage activation of the quadriceps averaged 95%. Although the recorded MVC IEMG was probably less than maximal and is likely indicative of suboptimal motor unit recruitment and/or discharge rates (2, 9, 21, 28, 29, 31), the 24-h EMG duration was independent of the absolute or relative baseline threshold, the MVC, and voluntary activation. Thus the range of EMG durations measured in this study probably reflects genuine differences in muscle activity across subjects.
EMG normalization.
The surface EMG reflects neural drive to the muscle (e.g., motor unit recruitment, rate coding, synchronization) and peripheral processes, including electrode location and configuration, and signal cancellation (3). Indeed, in our subjects, the absolute MVC IEMG and M-wave area were significantly related (R2 = 0.74), meaning that those with larger M-wave areas tended to have greater MVC IEMG. Usually, the EMG signal is normalized to minimize variation induced by the electrode site and to allow data from different subjects (muscles) to be more accurately compared (4). Options for reference EMG values include a specific percentile, peak, or mean value associated with locomotor activity (23), or a value obtained during an isometric or dynamic MVC or submaximal contraction (4, 18, 35). Some may question the validity of normalizing daily EMG activity to an isometric contraction as done here rather than a dynamic contraction. However, it can be argued that isometric contractions occur frequently during the day for postural maintenance and during dynamic tasks such as climbing stairs. Furthermore, some have concluded that normalizing data to a dynamic (isokinetic) MVC rather than an isometric MVC does not reduce intra- or interindividual variability in gait EMG, or provide a more representative measure of muscle activation (4).
In the present study, the 24-h EMG was normalized to the mean of the highest 20 consecutive integrals (i.e., a 200-ms period) recorded during the MVC. This approach to normalization is similar to previous studies of daily quadriceps EMG activity, where the peak MVC EMG recorded over 100 ms was defined as the maximal value (15, 16, 18). However, a higher MVC EMG can be employed by using shorter segments and/or defining the maximal value in a different way. For example, when the MVC IEMG was defined as the top 10% of integrals over 2 s of the MVC (i.e., twenty 10-ms integrals that were not necessarily consecutive), the MVC IEMG was 1.5 times (or 50%) greater compared with the value based on 20 consecutive integrals. Assuming that the top 10% of integrals is less attenuated from signal cancellation than consecutive integrals over a longer time (17), the former may be closer to the true MVC EMG during maximal efforts.
Comparison to workday EMG.
Daytime recordings have been made from lower limb muscles including the VL (15, 18, 23, 30). Various thresholds were used to analyze the data, including 8% of the 90th percentile of the amplitude distribution derived from the recording (23) or 10% MVC (15). A few investigators analyzed the EMG activity as a series of bursts, in which a burst was defined as EMG greater than 2% MVC threshold and lasting longer than 0.1 s (16, 18, 30).
The present data derived from the awake period (mean, 14.8 h, n = 10) can be compared with previous VL recordings, albeit cautiously because of the different types of analysis and thresholds employed (15, 18, 23). Using the baseline threshold (mean = 1.7% MVC), we found that the VL was active for 13.3% of the awake period, on average. Others reported similar or slightly lower values for EMG duration during ∼8- to 12-h recordings, including 12% (15), 10% (18), and 9% of the recording period (23), equivalent to 45–90 min of activity. For EMG intensity, the mean IEMG during the awake period was 6.9% MVC in our subjects. This value is similar to the EMG intensities of Howe and Rafferty (15). However, Kern and coworkers (18) obtained a mean burst amplitude of 17% MVC in their subjects. Although we have no direct evidence, their participants may have been more mobile (i.e., walking and climbing stairs) over the course of the day compared with the majority of our subjects (Fig. 3B). Others report the EMG of the VL is less than 10% MVC during standing (27), modulates between 10% and 30% MVC during walking (5, 8), and averages ∼30% MVC during stair climbing (14). We may also have captured relatively more low-level activity because the awake period included postwork activities up to the time the sleep period commenced (18).
Our findings demonstrate that the VL is relatively inactive over 24 h. Most contractions were of low intensity. Interestingly, similar findings were apparent for the VL in caged monkeys (i.e., active for 6.2% of 24 h) (13). In the present study, there was also a threefold range in 24-h EMG duration and intensity over 24 h. This variation likely reflects differences in the amount and types of habitual tasks performed as well as how (i.e., motor strategy) the tasks are performed (26). Differences in motor unit (fiber type) composition could also contribute to the intersubject variation in 24-h EMG, but this issue remains equivocal (18, 23). Furthermore, the 24-h EMG duration was most sensitive to changes in the threshold used for analysis, with smaller effects on 24-h EMG intensity. In future studies of long-term EMG, standardized procedures should be employed to record the MVC EMG, including the percentage activation where possible. Data from different studies can be compared if the baseline thresholds are normalized to the MVC.
The relative amount of low and high IEMG (%MVC) activity observed in the present study's participants suggests that there was a preponderance of weak force contractions and a dearth of strong contractions over the course of the day. This IEMG-duration profile likely means that most of the daily activities were accomplished by activating low-threshold motor units with limited recruitment of high-threshold units. The long-term consequences of what can be described as a relatively sedentary muscle activity profile are uncertain. However, it is conceivable that limited recruitment of the highest threshold units over a period of many years could contribute to age-related weakness and muscle atrophy (20, 22, 28). Thus the determination of 24-h activity profiles using EMG, as described here, has many potential applications in research pertaining to the understanding of muscle plasticity in health, disease, and injury.
GRANTS
This research was funded by National Institutes of Health Grant NS-30226, State of Florida Specification Appropriation No. 595, and The Miami Project to Cure Paralysis.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Alaimo MA, Smith JL, Roy RR, Edgerton VR. EMG activity of slow and fast ankle extensors following spinal cord transection. J Appl Physiol 56: 1608–1613, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Behm D, Power K, Drinkwater E. Comparison of interpolation and central activation ratios as measures of muscle inactivation. Muscle Nerve 24: 925–934, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bigland-Ritchie B. EMG/force relations and fatigue of human voluntary contractions. Exerc Sport Sci Rev 9: 75–117, 1981 [PubMed] [Google Scholar]
- 4.Burden AM, Trew M, Baltzopoulos V. Normalisation of gait EMGs: a re-examination. J Electromyogr Kinesiol 13: 519–532, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ciccotti MG, Kerlan RK, Perry J, Pink M. An electromyographic analysis of the knee during functional activities. I. The normal profile. Am J Sports Med 22: 645–650, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Dubo HI, Peat M, Winter DA, Quanbury AO, Hobson DA, Steinke T, Reimer G. Electromyographic temporal analysis of gait: normal human locomotion. Arch Phys Med Rehabil 57: 415–420, 1976 [PubMed] [Google Scholar]
- 7.Edgerton VR, McCall GE, Hodgson JA, Gotto J, Goulet C, Fleischmann K, Roy RR. Sensorimotor adaptations to microgravity in humans. J Exp Biol 204: 3217–3224, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Ericson MO, Nisell R, Ekholm J. Quantified electromyography of lower-limb muscles during level walking. Scand J Rehabil Med 18: 159–163, 1986 [PubMed] [Google Scholar]
- 9.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Hensbergen E, Kernell D. Circadian and individual variations in duration of spontaneous activity among ankle muscles of the cat. Muscle Nerve 21: 345–351, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Hensbergen E, Kernell D. Daily durations of spontaneous activity in cat's ankle muscles. Exp Brain Res 115: 325–332, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol 101: 511–519, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Hodgson JA, Wichayanuparp S, Recktenwald MR, Roy RR, McCall G, Day MK, Washburn D, Fanton JW, Kozlovskaya I, Edgerton VR. Circadian force and EMG activity in hindlimb muscles of rhesus monkeys. J Neurophysiol 86: 1430–1444, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Hortobagyi T, Mizelle C, Beam S, DeVita P. Old adults perform activities of daily living near their maximal capabilities. J Gerontol A Biol Sci Med Sci 58: M453–M460, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Howe TE, Rafferty D. Quadriceps activity and physical activity profiles over long durations in patients with osteoarthritis of the knee and controls. J Electromyogr Kinesiol 19: e78–e83, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Jakobi JM, Edwards DL, Connelly DM. Utility of portable electromyography for quantifying muscle activity during daily use. Gerontology 54: 324–331, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Keenan KG, Farina D, Maluf KS, Merletti R, Enoka RM. Influence of amplitude cancellation on the simulated surface electromyogram. J Appl Physiol 98: 120–131, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Kern DS, Semmler JG, Enoka RM. Long-term activity in upper- and lower-limb muscles of humans. J Appl Physiol 91: 2224–2232, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Kernell D, Donselaar Y, Eerbeek O. Effects of physiological amounts of high- and low-rate chronic stimulation on fast-twitch muscle of the cat hindlimb. II. Endurance-related properties. J Neurophysiol 58: 614–627, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Klein CS, Rice CL, Marsh GD. Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol 91: 1341–1349, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Kooistra RD, de Ruiter CJ, de Haan A. Conventionally assessed voluntary activation does not represent relative voluntary torque production. Eur J Appl Physiol 100: 309–320, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Monster AW, Chan H, O'Connor D. Activity patterns of human skeletal muscles: relation to muscle fiber type composition. Science 200: 314–317, 1978 [DOI] [PubMed] [Google Scholar]
- 24.Mork PJ, Westgaard RH. Long-term electromyographic activity in upper trapezius and low back muscles of women with moderate physical activity. J Appl Physiol 99: 570–578, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Mork PJ, Westgaard RH. The association between nocturnal trapezius muscle activity and shoulder and neck pain. Eur J Appl Physiol 92: 18–25, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Nordander C, Hansson GA, Rylander L, Asterland P, Byström JU, Ohlsson K, Balogh I, Skerfving S. Muscular rest and gap frequency as EMG measures of physical exposure: the impact of work tasks and individual related factors. Ergonomics 43: 1904–1919, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Okada M. An electromyographic estimation of the relative muscular load of different human postures. J Hum Ergol 1: 75, 1972 [PubMed] [Google Scholar]
- 28.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve 22: 1094–1103, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Rutherford OM, Jones DA, Newham DJ. Clinical and experimental application of the percutaneous twitch superimposition technique for the study of human muscle activation. J Neurol Neurosurg Psychiatry 49: 1288–1291, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shirasawa H, Kanehisa H, Kouzaki M, Masani K, Fukunaga T. Differences among lower leg muscles in long-term activity during ambulatory condition without any moderate to high intensity exercise. J Electromyogr Kinesiol 19: e50–e56, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Taylor JL, de Haan A, Gerrits KH, de Ruiter CJ. Point: Counterpoint “The interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol 107: 354–355, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Tepavac D, Thomas CK, Winslow J, Klein CS, Gan X, Dikshit A. Long-term surface EMG recording system. Proceedings of BMES Annual Meeting, Nashville: Biomedical Engineering Society, 2003, p. 24 [Google Scholar]
- 33.Thomas CK, Johansson RS, Bigland-Ritchie B. EMG changes in human thenar motor units with force potentiation and fatigue. J Neurophysiol 95: 1518–1526, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Thorn S, Søgaard K, Kallenberg LA, Sandsjo L, Sjøgaard G, Hermens HJ, Kadefors R, Forsman M. Trapezius muscle rest time during standardised computer work—a comparison of female computer users with and without self-reported neck/shoulder complaints. J Electromyogr Kinesiol 17: 420–427, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Yang JF, Winter DA. Electromyography reliability in maximal and submaximal isometric contractions. Arch Phys Med Rehabil 64: 417–420, 1983. [PubMed] [Google Scholar]