Abstract
Acute lung injury can be worsened by inappropriate mechanical ventilation, and numerous experimental studies suggest that ventilator-induced lung injury is increased by excessive lung inflation at end inspiration or inadequate lung inflation at end expiration. Lung inflation depends not only on airway pressures from the ventilator but, also, pleural pressure within the chest wall. Although esophageal pressure (Pes) measurements are often used to estimate pleural pressures in healthy subjects and patients, they are widely mistrusted and rarely used in critical illness. To assess the credibility of Pes as an estimate of pleural pressure in critically ill patients, we compared Pes measurements in 48 patients with acute lung injury with simultaneously measured gastric and bladder pressures (Pga and Pblad). End-expiratory Pes, Pga, and Pblad were high and varied widely among patients, averaging 18.6 ± 4.7, 18.4 ± 5.6, and 19.3 ± 7.8 cmH2O, respectively (mean ± SD). End-expiratory Pes was correlated with Pga (P = 0.0004) and Pblad (P = 0.0104) and unrelated to chest wall compliance. Pes-Pga differences were consistent with expected gravitational pressure gradients and transdiaphragmatic pressures. Transpulmonary pressure (airway pressure − Pes) was −2.8 ± 4.9 cmH2O at end exhalation and 8.3 ± 6.2 cmH2O at end inflation, values consistent with effects of mediastinal weight, gravitational gradients in pleural pressure, and airway closure at end exhalation. Lung parenchymal stress measured directly as end-inspiratory transpulmonary pressure was much less than stress inferred from the plateau airway pressures and lung and chest wall compliances. We suggest that Pes can be used to estimate transpulmonary pressures that are consistent with known physiology and can provide meaningful information, otherwise unavailable, in critically ill patients.
Keywords: respiratory mechanics, esophageal balloon, mechanical ventilation, positive end-expiratory pressure, plateau pressure
acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are debilitating respiratory conditions that can be worsened by inappropriate airway pressures applied during mechanical ventilation (6). The ARDSnet Consortium demonstrated that mortality could be improved by reducing tidal volume (Vt) and by limiting the end-inspiratory plateau pressure (PPlat) to 30 cmH2O, thereby limiting the peak stress applied to the respiratory system and lung (1). Numerous experimental studies have shown that lung injury can also be reduced by maintaining sufficient positive end-expiratory pressure (PEEP) to prevent lung collapse at end expiration (8, 9, 11, 15, 22, 23, 26, 37–39, 49). Lung inflation depends on transpulmonary pressure (airway pressure − pleural pressure), which in turn depends on characteristics of the chest wall, as well as the lung. Unfortunately, pressures within the chest cavity are rarely measured in critical illness, and, as a result, ventilator pressures are rarely adjusted to account for the pressures outside the lung. In a recent clinical trial (45), we tested a strategy of measuring esophageal pressure (Pes) to estimate transpulmonary pressure and set the PEEP applied by the ventilator. This strategy was associated with significant improvements in lung function and a trend toward improved survival, suggesting that PEEP and end-expiratory transpulmonary pressure are important determinants of lung health in this population.
Pes has been used for decades to estimate an effective pleural pressure (Ppl) between the lung and chest wall in healthy subjects and patients who are upright (10, 20, 40, 43). Because pleural pressure varies from place to place because of the gravitational gradient and other factors (27), there is no single value of pleural pressure. However, there is always an “effective” value of pleural pressure that, if it were applied to the whole pleural surface, would result in the observed lung volume and flow. To distinguish local and effective pressures, in this report, Ppl will denote the theoretical, unmeasured, effective pleural pressure that probably approximates the local pleural pressure at midlung height in the supine posture. By extension, we define an effective transpulmonary pressure (Pl = airway pressure − Ppl) as a theoretical pressure difference between the airway opening and the pleural space (34). Pl is to be distinguished from the corresponding estimated transpulmonary pressure, usually measured with an esophageal balloon (Pl,es = airway pressure − Pes).
Despite its longstanding use in upright patients and healthy subjects, there is widespread skepticism that Pes measurements reflect Ppl in patients who are supine, especially those with critical illness (12, 21). In particular, Pes values measured in supine critically ill patients are often much greater than what many assume to be likely pleural pressures. In this report, we argue, to the contrary, that Pes can be used to estimate credible values of Ppl. To be sure, there are differences between Pes and the effective Ppl in the supine position caused by the weight of mediastinal contents and other factors. The possibility remains, however, that the inaccuracies in estimating Ppl are minor compared with the differences in Pes among patients. Imperfect measurements can still be meaningful and useful, as was demonstrated in our recent clinical study (45).
To explore the potential utility of Pes measurements in critical illness, we describe measurements in patients with ALI/ARDS and use Pes measurements to infer the pressures acting on the lung and chest wall. Our aim is to determine whether the values of Pes measured in our patients are consistent with expectations based on fundamental principles of lung and chest wall mechanics. We postulate that Pes is an indicator, albeit imperfect, of an effective Ppl in critically ill semirecumbent patients and discuss the implications of these measurements for management of mechanical ventilation in patients with ALI/ARDS.
METHODS
This was a parallel physiological study of 48 patients enrolled in the EPVent Trial (45) at Beth Israel Deaconess Medical Center. In 51 of the 62 patients, initial measurements were made simultaneously with two recording systems, one for making clinical recordings used in the clinical trial to set ventilator parameters in the intervention group and another for recording physiological data for subsequent analysis. Eleven patients in the clinical trial were not studied with the physiological system because of scheduling problems. Here we report an analysis of all 48 physiological records in which technically adequate pressure, volume, and flow measurements were recorded during baseline (pretrial intervention) ventilation. (Three of the physiological recordings were not analyzed: 2 lacked data recorded during end-expiratory occlusion, and 1 lacked data recorded before trial intervention.) The study was approved by the Institutional Review Board of Beth Israel Deaconess Medical Center, and prior informed written consent was obtained from the patients or their healthcare proxy.
Physiological Measurements
Airflow was measured with a pneumotachograph (Fleisch no. 1, available from PhippsBird.com) and integrated to obtain tidal volume (Vt). Airway pressure was measured at the endotracheal tube. Gastric and esophageal pressures (Pga and Pes) were measured with an esophageal balloon-catheter (Ackrad Laboratories, available from CooperSurgical.com). The balloon (9.5 cm long, 2 cm perimeter) was filled with 0.5–1.0 ml of air. Frequency response of the system was adequate (without significant delays) up to 15 Hz. Pressure, volume, and flow measurements were displayed and recorded using a custom-written program and analyzed with Windaq software (Dataq.com).
Protocol
Patients were studied in the supine position, with the head of the bed elevated 30° (to prevent ventilator-associated pneumonia), on controlled mechanical ventilation with preintervention ventilator settings. They were sufficiently sedated to permit measurements of passive elastic recoil pressures of the respiratory system during mechanical ventilation. Bladder pressure (Pblad) was measured in 36 subjects via urinary catheter using standard techniques, with 50 ml of saline in the bladder and with the transducer referenced to the level of the pubic symphysis (32). In 35 subjects in whom it was possible, the esophageal balloon-catheter was first passed through the lower esophageal sphincter into the stomach for measurement of Pga at end expiration during mechanical ventilation. In all subjects, the balloon's tip was repositioned to 40 cm from the incisors for recording Pes using techniques validated previously (46). Balloon position in the lower esophagus, which could not be confirmed by the occlusion technique in patients who were not making respiratory efforts, was confirmed by the presence of cardiac pulsation in the pressure trace, by appropriate pressure deflections during mechanical ventilation and a gentle push on the abdomen, and, in one case, by chest X-ray. In subjects in whom the pressure was initially recorded in the stomach, a change in pressure and respiratory and cardiac waveforms during withdrawal confirmed movement of the balloon into the esophagus. Airway pressure, Pes, and Pl,es were measured during an end-inspiratory occlusion (EIO) to obtain Pplat, PesEIO, and Pl,esEIO, respectively. The corresponding pressures during an end-expiratory occlusion (EEO) yielded total PEEP (PEEPT), PesEEO, and Pl,esEEO. Lung elastance (El) was calculated as (Pl,esEIO − Pl,esEEO)/Vt, and chest wall elastance (Ecw) was calculated as (PesEIO − PesEEO)/Vt.
Statistical Analysis
Data are presented as means ± SD. Linear regression with the coefficient of determination and ANOVA (JMP, SAS Institute) were used as appropriate. Significance was assumed for P < 0.05.
RESULTS AND DISCUSSION
The subjects were seriously ill (45), and most were ventilated with a low Vt and high PEEP, consistent with current recommendations (1). Table 1 shows subject characteristics, blood gas values, and preintervention ventilator settings.
Table 1.
Subject characteristics and initial ventilator settings
| Characteristic/Setting | Value |
|---|---|
| Male gender, % | 60 |
| Age, yr | 52 ± 20 |
| Caucasian, % | 88 |
| Predicted body wt, kg | 66 ± 10 |
| APACHE II on admission, points | 26.6 ± 5.6 |
| Primary physiological injury, % | |
| Pulmonary | 22 |
| Abdominal pathology | 35 |
| Trauma/blood loss | 29 |
| Sepsis | 8 |
| Other | 6 |
| Arterial blood gas analysis at baseline | |
| pH | 7.33 ± 0.09 |
| Arterial Pco2, Torr | 41 ± 8 |
| Arterial Po2, Torr | 98 ± 38 |
| Arterial Po2-to-inspiratory O2 fraction ratio | 146 ± 57 |
| Ventilator settings at baseline | |
| Inspiratory O2 fraction | 0.70 ± 0.18 |
| Vt, ml | 443 ± 102 |
| Vt/predicted body wt, ml/kg | 6.8 ± 1.6 |
| Respiratory rate, min−1 | 24.9 ± 6.2 |
| PEEP, cmH2O | 12.6 ± 4.4 |
| Pplat, cmH2O | 30.5 ± 6.2 |
Values are means ± SD. APACHE II, Acute Physiology and Chronic Health Evaluation II; Vt, tidal volume; PEEP, positive end-expiratory pressure; Pplat, plateau pressure.
Esophageal Pressure
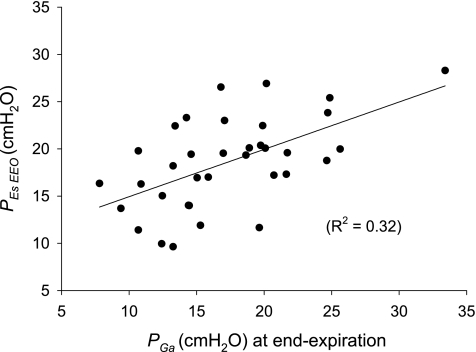
Pes values were quite high and variable among patients. Whereas pleural pressure inferred from Pes in upright subjects is usually subatmospheric at end exhalation, PesEEO in our patients averaged 18.6 ± 4.7 cmH2O, and PesEIO averaged 22.3 ± 5.0 cmH2O. These PesEEO values are higher than those of 10 healthy supine subjects at relaxed functional residual capacity (3.3 ± 3.2 cmH2O) (47). They are also higher than the average 8.0 ± 3.1 cmH2O in 10 supine subjects of normal weight without lung disease studied under anesthesia and paralysis during ventilation without PEEP (2a). Several arguments suggest that these high values in patients are consistent with known pathophysiology. First, in the supine posture, Pes is known to overestimate Ppl at midlung height by ∼5 cmH2O, presumably, because of the weight of mediastinal contents (17, 25, 33, 36, 47). Therefore, the effective pleural pressure is estimated to be ∼5 cmH2O lower than Pes. Second, abdominal pressure inferred from bladder pressure is variable and greater than normal in many critically ill patients (31, 32), suggesting that Ppl and Pes might also be greater than normal. If the high Pes values we measured reflect real elevations in intrathoracic and pleural pressures, we would expect them to be associated with commensurately high values of Pga and Pblad, which are not subject to the effects of mediastinal weight and inhomogeneous lung mechanics, which are thought to affect Pes in patients. This was, in fact, the case. The average value of end-expiratory Pga was 18.4 ± 5.6 cmH2O, which is greater than the average 5.6 ± 3.8 cmH2O measured in six nonobese patients without lung disease who were anesthetized and ventilated without PEEP before elective surgery (2a). Pblad averaged 19.3 ± 7.8 cmH2O, which is greater than the normal range (∼12–15 cmH2O) in subjects in the supine position, with the head of the bed elevated 30° (13). Furthermore, Pga and Pblad were positively correlated with PesEEO (R2 = 0.32, P = 0.0004 and R2 = 0.18, P = 0.0104, respectively; Fig. 1), so those patients with higher PesEEO tended to have higher Pga and Pblad. The similar magnitude and correlation of PesEEO with Pga and Pblad support the hypothesis that all these measurements reflect a common, variable elevation of pressure within the coelomic cavity in many critically ill patients.
Fig. 1.
Esophageal pressure at end-expiratory occlusion (PesEEO) vs. end-expiratory gastric pressure (Pga) in all subjects. Pressures are of similar magnitudes and are correlated. Solid line, line of regression.
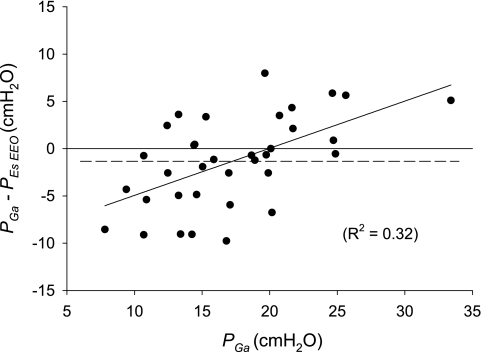
The difference between PesEEO and Pga in individuals (Pga − Pes) ranged from −9.8 to 8.0 cmH2O and averaged −1.3 ± 4.8 cmH2O (Fig. 2). The Pga-PesEEO difference is changed in opposite directions by gravitational pressure gradients (13) and transdiaphragmatic pressure. Because the lower esophagus is dorsal to the gastric air bubble, we would expect Pes to be higher than Pga in supine subjects because of the gravitational pressure gradient in the intervening tissues (including that due to the weight of mediastinal contents noted above). On the other hand, passive tension in the diaphragm causes a transdiaphragmatic pressure. We would expect that, in patients with high abdominal pressure, the diaphragm would be stretched, increasing its passive tension and increasing the Pga-PesEEO difference. This expectation is borne out by the positive correlation of the Pga-PesEEO difference with Pga, higher Pga values being associated with higher Pga-PesEEO difference (R2 = 0.32, P = 0.0004; Fig. 2), although mathematical coupling of independent and dependent variables could also explain this result. Other causes of interindividual differences between Pga and Pes include local shear-induced pressure gradients that accompany the deformation of shape-stable viscera (29), variations in gastric and esophageal muscle tone, and gravitational pressure differences associated with differences in body position (5). We suggest that Pga and Pes differ by amounts consistent with variations due to gravitational pressure gradients, deformation of solid tissues, mediastinal weight, and passive diaphragmatic tension; the latter dominates when Pga is high.
Fig. 2.
Difference between Pga and Pes (Pga − PesEEO) vs. Pga, all at end expiration. Dashed line, mean difference; solid horizontal line, line of regression. Pressure difference tended to be greater in atients with higher Pga, suggesting that greater tension in the passive diaphragm had caused greater transdiaphragmatic pressure.
Transpulmonary Pressures at End-Expiratory Occlusion
Because PesEEO values were high, the end-expiratory Pl,esEEO values tended to be low, averaging −2.8 ± 4.9 cmH2O, and Pl,esEEO was less than zero in most patients (Fig. 3). Pl,esEEO was much less than the simultaneously measured airway pressure (i.e., PEEPT), which averaged 15.7 ± 4.2 cmH2O.
Fig. 3.
Transpulmonary pressure at end-expiratory occlusion (Pl,esEEO) vs. simultaneously measured airway pressure [i.e., total positive end-expiratory pressure (PEEPT)]. Pl,esEEO is usually substantially less than PEEPT. Solid line, line of regression.
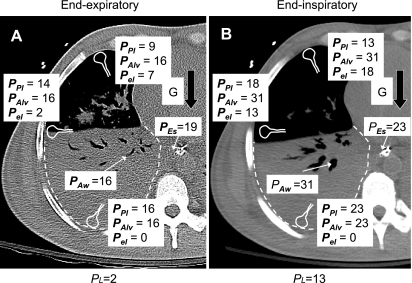
How could Pl,esEEO reasonably be negative in these patients? Some mechanisms that could affect the relations between Pl, Pl,esEEO, and PEEPT are depicted in Fig. 4A. First, because Pes is increased in the supine posture by the weight of mediastinal contents (see above), Pes overestimates Ppl at midlung height, and Pl,esEEO underestimates Pl by ∼5 cmH2O. Second, the gravitational gradient in pleural pressure, which would be more important with edematous lungs, could permit the upper (nondependent) lung regions to have a locally positive transpulmonary pressure.
Fig. 4.
Possible mechanisms determining pressures in the thorax at end-expiratory (A) and end-inspiratory (B) occlusion. End-inspiratory and end-inspiratory CT scans at a level 7 cm below the carina in a patient with ARDS show extensive dependent consolidation of the lung, with large airways patent. Pressures shown in central airway (Paw) and esophagus (Pes) are the average measured values in all subjects. Possible local pressures are also shown for alveolar regions in upper, middle, and lower lung, including pleural pressure (Ppl), alveolar pressure (Palv), and elastic recoil pressure (Pel = Palv − Ppl). Ppl in middle lung determines effective transpulmonary pressure (Pl), which is shown below scans. Ppl at mid lung height is assumed to be 5 cmH2O lower than Pes, because gravity (G), acting on mediastinal contents, compresses the esophagus. Ppl values in upper and lower lung differ from Ppl at mid lung because of the gravitational pressure gradient, which is assumed to be 0.6 cmH2O/cm height throughout this edematous lung. In middle and upper lung, airways are open, Palv = Paw, and local elastic recoil pressure is equal to local transpulmonary pressure. In lower lung, small airways are closed and/or alveoli contain viscous fluid, preventing Palv from equilibrating with Paw, so Palv = Ppl, and local elastic recoil pressure is nil.
Pl is defined as the pressure difference from airway to pleural space, so a negative Pl implies that pleural pressure is greater than airway pressure. Note that a negative Pl does not imply that the elastic recoil pressure of the lung (alveolar pressure − pleural pressure) is negative; recoil pressure is likely to be positive, even at low lung volume (24). Instead, a negative Pl implies that airway closure and/or alveolar flooding have prevented alveolar pressure from equilibrating with airway pressure, and alveolar pressure becomes equal to the local pleural pressure. Airway closure is a normal occurrence in older people at low lung volumes (2, 3), and static negative transpulmonary pressures at very low volumes have been demonstrated in healthy breath-hold divers who have employed glossopharyngeal exsufflation to empty their lungs below residual volume (28). Airway closure probably occurs in all lungs (of terrestrial mammals) that are held at substantially negative transpulmonary pressure (e.g., by application of a highly negative pressure at the trachea). Thus statically negative values of Plare likely when pressures in the thorax and abdomen are pathologically elevated.
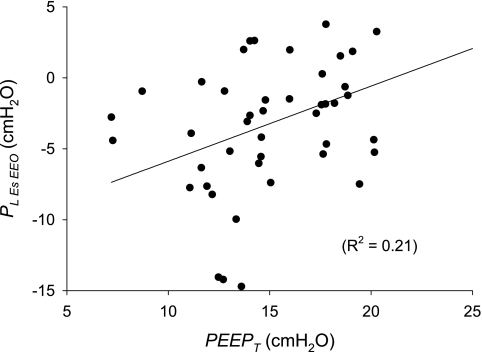
Pl,esEEO, the difference between PEEPT and PesEEO, is a measure of the pressure distending the lung at end expiration and is controlled clinically by adjustment of PEEP. In our patients, Pl,esEEO was negatively correlated with PesEEO (R2 = 0.39, P < 0.0001) and positively correlated with PEEPT (R2 = 0.21, P = 0.0011; Fig. 3), suggesting that the most important determinant of the interindividual differences in Pl,esEEO (and, presumably, also end-expiratory lung volume) was PesEEO, which depends on the chest wall, not the PEEP applied by the ventilator.
Transpulmonary Pressure at End-Inspiratory Occlusion (Plateau)
As with Pl,esEEO, Pl,esEIO values were low, averaging 8.3 ± 6.2 cmH2O and ranging from −5.8 to 26.6 cmH2O. These values are much lower than the simultaneously measured airway pressures (Pplat), which averaged 30.5 ± 6.2 cmH2O. How could Pl,esEIO be so low (sometimes less than zero) at end inflation in a critically ill subject? Possible mechanisms are depicted in Fig. 4B. The weight of mediastinal contents lowers Pl,esEIO relative to Pl, so Pl,esEIO could be approximately −5 cmH2O without implying a negative effective Pl. However, even after we accounted for effects of mediastinal weight, many of our estimated static transpulmonary pressures at midlung height (Pl) are still negative. How could end-inspiratory Pl be negative? The gravitational gradient in pleural pressure could permit the upper lung regions to have a locally positive transpulmonary pressure, while the “effective” Pl at midlung height remained slightly negative. Furthermore, transpulmonary pressure would be higher during inspiratory flow than during the end-inspiratory occlusion, permitting ventilation, even if Pl,esEIO was negative. These arguments suggest that the range of Pl,esEIO values in our subjects (after correction for mediastinal weight) could reflect similar values of Pl.
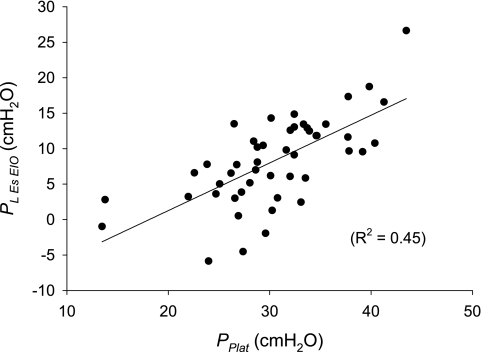
Pl,esEIO values were not only positively correlated with Pplat (R2 = 0.45, P < 0.0001; Fig. 5) but, also, negatively correlated with PesEIO (R2 = 0.18, P = 0.0026). Thus, although end-inspiratory transpulmonary pressure is largely determined by airway pressure, it is also importantly influenced by the pressure across the chest wall and cannot be adequately predicted from Pplat alone.
Fig. 5.
Estimated transpulmonary pressure at end-inspiratory occlusion (Pl,esEIO) vs. simultaneously measured airway pressure (Pplat). Pl,esEIO is substantially less than Pplat. Solid line, line of regression.
Chest Wall Elastance
Ecw was, on average, slightly greater than normal, 8.7 ± 4.7 cmH2O/l, but it ranged widely from 1.3 to 21.8 cmH2O/l. Perhaps surprisingly, Ecw was not correlated with PesEEO, implying that the slope of the pressure-volume curve of the chest wall was not correlated with the position of the curve relative to the pressure axis. Therefore, high levels of Pes at end-expiration are not, in general, associated with high Ecw. Conversely, high Ecw values are not, in general, associated with particularly high levels of PesEEO. Ecw was also not correlated with Pplat or Pl,esEIO.
Implications of the Findings for Ventilator Management in ALI/ARDS
The findings presented above constitute a dilemma. If we maintain that the high Pes values measured in our subjects are artifactual (12, 21), we run the risk of ignoring important information that could be clinically useful (4). In particular, the well-documented elevations in abdominal pressure in some patients with critical illness suggest that there might be similar elevations in pleural pressure that would affect the lungs. Alternatively, if we accept the measured values of Pes and Pl,es as meaningful, if somewhat flawed, estimates of Ppl and Pl (after a correction of ∼5 cmH2O), we can use these estimates to assess respiratory mechanics in ARDS/ALI. Below, we consider implications of our findings for management of mechanical ventilation.
Setting PEEP to set end-expiratory lung volume.
Maintaining inflation of the lungs at end expiration is critical for oxygenation and minimization of atelectrauma (44). Usually, lung inflation at end expiration is adjusted by setting PEEP with the ventilator. However, we found that Pl,esEEO, which depends largely on the pressure across the chest wall, was variable and often substantially lower than the simultaneously measured airway pressure (PEEPT). It follows that measurement of the pressure across the chest wall (PesEEO) and calculation of Pl,esEEO can help in the choice of an appropriate PEEP in such patients. This strategy was used in our earlier clinical trial (45).
The finding that pleural pressures vary widely among patients implies that the risks and benefits of applied PEEP are likely to vary from patient to patient. A given high level of PEEP in an individual with a high pleural pressure could improve oxygenation and protect the lung from repetitive alveolar collapse, whereas the same level of PEEP in an individual with low pleural pressure could expose the lung to injurious stress at end inspiration. This may account for the difficulty of determining an optimal level of applied PEEP in clinical trials (18). Effects of PEEP on the lungs cannot be fully appreciated without consideration of the effects of the chest wall on transpulmonary pressure.
Ecw and Pes.
The chest wall in ARDS has been described as sometimes having low compliance (19). High intrathoracic pressures and low chest wall compliance can be caused by abdominal distension (42). However, high pressures and low compliance need not occur together. Note that elastance, the inverse of compliance, is the change in pressure for a given change in volume and does not predict the pressure at any volume. Our finding that Ecw was not universally elevated is consistent with previous findings in 70 patients with acute respiratory failure (46) and challenges a common perception that high Pes values are due to low chest wall compliance. The state of inflation of the lung depends more on the value of Pes than on the increase in Pes during tidal inflation (elastance). Therefore, the lack of correlation between PesEEO and Ecw underscores the importance of measuring the resting values of Pes, not merely the tidal excursions in Pes used to calculate Ecw.
Lung stress in ALI/ARDS and the effects of the chest wall.
The end-inspiratory airway pressure, Pplat, is often used as an index of lung stress during mechanical ventilation and as a means to assess lung injury in ALI/ARDS (1). Checkley et al. (6) recently analyzed preenrollment ventilation parameters in 2,451 patients with ALI/ARDS entered into several of the ARDSnet Trials. They showed that higher preenrollment Pplat values strongly predicted mortality and reasoned that the reciprocal relationship between Pplat and respiratory system compliance suggests that high Pplat and low compliance provide similar information, both indicating lung injury (16). Thus, for a given Vt, less compliant lungs would result in a greater increase in airway pressure and higher Pplat.
With Pes measurements, it is theoretically possible to measure end-inspiratory lung stress directly. When airways are open and the central airways communicate with alveoli, Pl,esEIO is an estimate of the average end-inspiratory stress of the lung parenchyma (i.e., the elastic recoil pressure of the lung) (34).
In a recent report, Chiumello et al. (7) analyzed the stress applied to the lungs of patients with ALI/ARDS and patients with normal lungs by estimating transpulmonary pressure with an esophageal balloon. However, they equated the end-inspiratory stress applied to the lung with the tidal increase in transpulmonary pressure from that at relaxation volume, discounting the initial (baseline) pressure by subtraction. This approach is common in studies of critically ill patients (14, 19, 30, 35, 41, 42, 48). However, we reasoned that the prestress before inflation might be quantitatively important. For example, the end-inspiratory parenchymal stress after a tidal inflation from a low initial stress (e.g., after inflation from a low lung volume) would be less than that after inflation from a high initial stress (after inflation from a high lung volume), even though the changes in stress might be identical. Similarly, if the lungs were being compressed by a high Ppl at end expiration, the transpulmonary pressure could increase substantially to initiate inflation without causing high end-inspiratory pressures or stress.
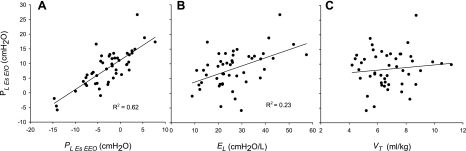
With this in mind, we evaluated the possible contribution of prestress to end-inspiratory lung stress in our patients. Parenchymal stress at end inflation can be assessed by measuring the transpulmonary pressure, Pl,esEIO = Pl,esEEO + El × Vt, and in any individual the parenchymal stress would be increased by raising Pl,esEEO, El, or Vt. We sought to determine how interindividual differences in postinflation Pl,esEIO depend on differences in each of these variables among our subjects. Pl,esEIO values were correlated with Pl,esEEO (R2 = 0.62, P < 0.0001; Fig. 6A), correlated with El (R2 = 0.23, P = 0.0006; Fig. 6B), and not correlated with Vt (Fig. 6C). Therefore, in our cohort, lung parenchymal stress at end inflation was most importantly determined by Pl,esEEO, the prestress before inflation. Pl,esEEO was not evaluated by Chiumello et al. (7) because of concerns about artifacts in PesEEO, but this practice ignores the influence of pressure inside the chest wall, which could be important.
Fig. 6.
Static end-inspiratory transpulmonary pressure (Pl,esEIO = Pl,esEEO + El × Vt, where El is lung elastance and Vt is tidal volume), which is the parenchymal stress after inflation, plotted against the 3 parameters that together determine its value. Pl,esEIO is moderately correlated with Pl,esEEO (A), correlated with El (b), and not correlated with Vt (C).
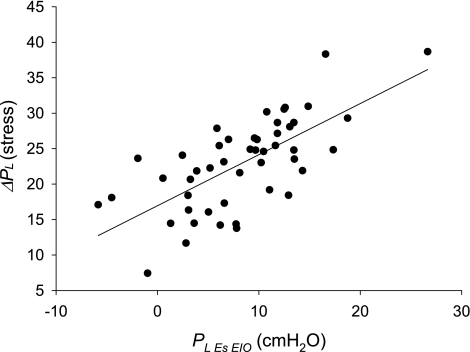
To assess the contribution of prestress to the estimation of end-inspiratory parenchymal stress, we compared directly measured values of end-inspiratory transpulmonary pressure in our subjects with values calculated using the equation of Chiumello et al. (7): ΔPl(stress) = ΔPaw × El/(El + Ecw), where ΔPaw is the tidal increase in airway pressure (i.e., Pplat − PEEPT). The end-inspiratory lung stress calculated by this equation, which does not account for prestress, averages 22.9 ± 6.5 cmH2O and ranges from 7.4 to 38.7 cmH2O, which is much higher than the directly measured Pl,esEIO, which averaged 8.3 ± 6.2 cmH2O and ranged from −5.8 to 26.6 cmH2O (Fig. 7). Even if we assume that Pes is 5 cmH2O higher than pleural pressure measured at midlung height, as in our earlier clinical trial (45), the two estimates of lung stress differ by 9.6 ± 5.0 cmH2O. Theoretically, the directly measured end-inspiratory transpulmonary pressure (Pl,esEIO) is a measure of end-inspiratory lung parenchymal stress, whereas ΔPl(stress) of Chiumello et al. is a measure of the increase in stress from relaxation volume, which depends only on El and inflation volume. Thus, ΔPl(stress) provides an incomplete measure of the effects of the chest wall on lung stress.
Fig. 7.
Lung stress calculated using Eq. 2 of Chiumello et al. (7), ΔPl(stress), plotted against directly measured transpulmonary pressure (Pl,esEIO). ΔPl(stress) does not account for prestress before inflation and is much greater than Pl,esEIO.
GRANTS
This research was supported in part by National Heart, Lung, and Blood Institute Grant HL-52586.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. Frederic G. Hoppin, Jr., for contributions to the revision.
Footnotes
- Vt
- Tidal volume
- PEEP
- Positive end-expiratory (airway) pressure
- PEEPT
- PEEP measured during end-expiratory occlusion
- Pplat
- Plateau (airway) pressure during end-inspiratory occlusion
- Ppl
- True pleural pressure at midlung height
- Pl
- True transpulmonary pressure at midlung height (using Ppl)
- Pl,es
- Pl measured with an esophageal balloon
- Pl,esEEO
- Pl,es measured during end-expiratory occlusion
- Pl,esEIO
- Pl,es measured during end-inspiratory occlusion (plateau)
- Pes
- Esophageal pressure
- PesEEO
- Pes measured during end-expiratory occlusion
- PesEIO
- Pes measured during end-inspiratory occlusion
- Pga
- Gastric pressure
- Pblad
- Bladder pressure
- El
- Lung elastance
- Ecw
- Chest wall elastance
REFERENCES
- 1. Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Anthonisen NR, Danson J, Robertson PC, Ross WR. Airway closure as a function of age. Respir Physiol 8: 58–65, 1969 [DOI] [PubMed] [Google Scholar]
- 2a. Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol 108: 212–218, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bode FR, Dosman J, Martin RR, Ghezzo H, Macklem PT. Age and sex differences in lung elasticity and in closing capacity in nonsmokers. J Appl Physiol 41: 129–135, 1976 [DOI] [PubMed] [Google Scholar]
- 4. Brander L, Ranieri VM, Slutsky AS. Esophageal and transpulmonary pressure help optimize mechanical ventilation in patients with acute lung injury. Crit Care Med 34: 1556–1558, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Cheatham ML, De Waele JJ, De Laet I, De Keulenaer B, Widder S, Kirkpatrick AW, Cresswell AB, Malbrain M, Bodnar Z, Mejia-Mantilla JH, Reis R, Parr M, Schulze R, Puig S. The impact of body position on intra-abdominal pressure measurement: a multicenter analysis. Crit Care Med 37: 2187–2190, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Checkley W, Brower R, Korpak A, Thompson BT. Effects of a clinical trial on mechanical ventilation practices in patients with acute lung injury. Am J Respir Crit Care Med 177: 1215–1222, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, Tallarini F, Cozzi P, Cressoni M, Colombo A, Marini JJ, Gattinoni L. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med 178: 346–355, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med 160: 109–116, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Chu EK, Whitehead T, Slutsky AS. Effects of cyclic opening and closing at low- and high-volume ventilation on bronchoalveolar lavage cytokines. Crit Care Med 32: 168–174, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Colebatch HJ, Greaves IA, Ng CK. Exponential analysis of elastic recoil and aging in healthy males and females. J Appl Physiol 47: 683–691, 1979 [DOI] [PubMed] [Google Scholar]
- 11. D'Angelo E, Pecchiari M, Baraggia P, Saetta M, Balestro E, Milic-Emili J. Low-volume ventilation causes peripheral airway injury and increased airway resistance in normal rabbits. J Appl Physiol 92: 949–956, 2002 [DOI] [PubMed] [Google Scholar]
- 12. de Chazal I, Hubmayr RD. Novel aspects of pulmonary mechanics in intensive care. Br J Anaesth 91: 81–91, 2003 [DOI] [PubMed] [Google Scholar]
- 13. De Keulenaer BL, De Waele JJ, Powell B, Malbrain ML. What is normal intra-abdominal pressure and how is it affected by positioning, body mass and positive end-expiratory pressure? Intensive Care Med 35: 969–976, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Downie JM, Nam AJ, Simon BA. Pressure-volume curve does not predict steady-state lung volume in canine lavage lung injury. Am J Respir Crit Care Med 169: 957–962, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Duggan M, McCaul CL, McNamara PJ, Engelberts D, Ackerley C, Kavanagh BP. Atelectasis causes vascular leak and lethal right ventricular failure in uninjured rat lungs. Am J Respir Crit Care Med 167: 1633–1640, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Ferguson ND, Frutos-Vivar F, Esteban A, Anzueto A, Alia I, Brower RG, Stewart TE, Apezteguia C, Gonzalez M, Soto L, Abroug F, Brochard L. Airway pressures, tidal volumes, and mortality in patients with acute respiratory distress syndrome. Crit Care Med 33: 21–30, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Ferris BG, Jr, Mead J, Frank NR. Effect of body position on esophageal pressure and measurement of pulmonary compliance. J Appl Physiol 14: 521–524, 1959 [Google Scholar]
- 18. Frutos-Vivar F, Ferguson ND, Esteban A. Mechanical ventilation: quo vadis? Intensive Care Med 35: 775–778, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Gattinoni L, Pelosi P, Suter PM, Pedoto A, Vercesi P, Lissoni A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med 158: 3–11, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Gibson GJ, Pride NB, O'Cain C, Quagliato R. Sex and age differences in pulmonary mechanics in normal nonsmoking subjects. J Appl Physiol 41: 20–25, 1976 [DOI] [PubMed] [Google Scholar]
- 21. Hager DN, Brower RG. Customizing lung-protective mechanical ventilation strategies. Crit Care Med 34: 1554–1555, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Halter JM, Steinberg JM, Schiller HJ, DaSilva M, Gatto LA, Landas S, Nieman GF. Positive end-expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/derecruitment. Am J Respir Crit Care Med 167: 1620–1626, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Herrera MT, Toledo C, Valladares F, Muros M, Diaz-Flores L, Flores C, Villar J. Positive end-expiratory pressure modulates local and systemic inflammatory responses in a sepsis-induced lung injury model. Intensive Care Med 29: 1345–1353, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Hoppin FG, Jr, Stothert JC, Jr, Greaves IA, Lai YL, Hildebrandt J. Lung recoil: elastic and rheological properties. In: Handbook of Physiology. The Respiratory System. Mechanics of Breathing. Bethesda, MD: Am. Physiol. Soc., 1986, sect. 3, vol. III, pt. 1, chapt. 13, p. 195–215 [Google Scholar]
- 25. Knowles JH, Hong SK, Rahn H. Possible errors using esophageal balloon in determination of pressure-volume characteristics of the lung and thoracic cage. J Appl Physiol 14: 525–530, 1959 [Google Scholar]
- 26. Ko SC, Zhang H, Haitsma JJ, Cheng KC, Li CF, Slutsky AS. Effects of PEEP levels following repeated recruitment maneuvers on ventilator-induced lung injury. Acta Anaesthesiol Scand 52: 514–521, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Lai-Fook SJ. Pleural mechanics and fluid exchange. Physiol Rev 84: 385–410, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Loring SH, O'Donnell CR, Butler JP, Lindholm P, Jacobson F, Ferrigno M. Transpulmonary pressures and lung mechanics with glossopharyngeal insufflation and exsufflation beyond normal lung volumes in competitive breath-hold divers. J Appl Physiol 102: 841–846, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Loring SH, Yoshino K, Kimball WR, Barnas GM. Gravitational and shear-associated pressure gradients in the abdomen. J Appl Physiol 77: 1375–1382, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Maggiore SM, Jonson B, Richard JC, Jaber S, Lemaire F, Brochard L. Alveolar derecruitment at decremental positive end-expiratory pressure levels in acute lung injury: comparison with the lower inflection point, oxygenation, and compliance. Am J Respir Crit Care Med 164: 795–801, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Malbrain ML. Abdominal pressure in the critically ill: measurement and clinical relevance. Intensive Care Med 25: 1453–1458, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, Japiassu A, Kurtop E, De Keulenaer BL, Daelemans R, Del Turco M, Cosimini P, Ranieri M, Jacquet L, Laterre PF, Gattinoni L. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med 30: 822–829, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Mead J, Gaensler EA. Esophageal and pleural pressures in man, upright and supine. J Appl Physiol 14: 81–83, 1959 [DOI] [PubMed] [Google Scholar]
- 34. Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 28: 596–608, 1970 [DOI] [PubMed] [Google Scholar]
- 35. Mergoni M, Martelli A, Volpi A, Primavera S, Zuccoli P, Rossi A. Impact of positive end-expiratory pressure on chest wall and lung pressure-volume curve in acute respiratory failure. Am J Respir Crit Care Med 156: 846–854, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Milic-Emili J, Mead J, Turner JM. Topography of esophageal pressure as a function of posture in man. J Appl Physiol 19: 212–216, 1964 [DOI] [PubMed] [Google Scholar]
- 37. Monkman SL, Andersen CC, Nahmias C, Ghaffer H, Bourgeois JM, Roberts RS, Schmidt B, Kirpalani HM. Positive end-expiratory pressure above lower inflection point minimizes influx of activated neutrophils into lung. Crit Care Med 32: 2471–2475, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149: 1327–1334, 1994 [DOI] [PubMed] [Google Scholar]
- 39. Nakazawa K, Yokoyama K, Yamakawa N, Makita K. Effect of positive end-expiratory pressure on inflammatory response in oleic acid-induced lung injury and whole-lung lavage-induced lung injury. J Anesth 21: 47–54, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Pare PD, Brooks LA, Bates J, Lawson LM, Nelems JM, Wright JL, Hogg JC. Exponential analysis of the lung pressure-volume curve as a predictor of pulmonary emphysema. Am Rev Respir Dis 126: 54–61, 1982 [DOI] [PubMed] [Google Scholar]
- 41. Pelosi P, Cereda M, Foti G, Giacomini M, Pesenti A. Alterations of lung and chest wall mechanics in patients with acute lung injury: effects of positive end-expiratory pressure. Am J Respir Crit Care Med 152: 531–537, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Ranieri VM, Brienza N, Santostasi S, Puntillo F, Mascia L, Vitale N, Giuliani R, Memeo V, Bruno F, Fiore T, Brienza A, Slutsky AS. Impairment of lung and chest wall mechanics in patients with acute respiratory distress syndrome: role of abdominal distension. Am J Respir Crit Care Med 156: 1082–1091, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Salazar E, Knowles JH. An analysis of pressure-volume characteristics of the lungs. J Appl Physiol 19: 97–104, 1964 [DOI] [PubMed] [Google Scholar]
- 44. Slutsky AS. Lung injury caused by mechanical ventilation. Chest 116: 9S–15S, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Talmor D, Sarge T, Malhotra A, O'Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 359: 2095–2104, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Talmor D, Sarge T, O'Donnell CR, Ritz R, Malhotra A, Lisbon A, Loring SH. Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med 34: 1389–1394, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Washko GR, O'Donnell CR, Loring SH. Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol 100: 753–758, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Wright PE, Bernard GR. The role of airflow resistance in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 139: 1169–1174, 1989 [DOI] [PubMed] [Google Scholar]
- 49. Wyszogrodski I, Kyei-Aboagye K, Taeusch HW, Jr, Avery ME. Surfactant inactivation by hyperventilation: conservation by end-expiratory pressure. J Appl Physiol 38: 461–466, 1975. [DOI] [PubMed] [Google Scholar]