Abstract
Transepithelial fluid secretion across the renal (Malpighian) tubule epithelium of the mosquito (Aedes aegypti) is energized by the vacuolar-type (V-type) H+-ATPase and not the Na+-K+-ATPase. Located at the apical membrane of principal cells, the V-type H+-ATPase translocates protons from the cytoplasm to the tubule lumen. Secreted protons are likely to derive from metabolic H2CO3, which raises questions about the handling of HCO3− by principal cells. Accordingly, we tested the hypothesis that a Cl/HCO3 anion exchanger (AE) related to the solute-linked carrier 4 (SLC4) superfamily mediates the extrusion of HCO3− across the basal membrane of principal cells. We began by cloning from Aedes Malpighian tubules a full-length cDNA encoding an SLC4-like AE, termed AeAE. When expressed heterologously in Xenopus oocytes, AeAE is both N- and O-glycosylated and mediates Na+-independent intracellular pH changes that are sensitive to extracellular Cl− concentration and to DIDS. In Aedes Malpighian tubules, AeAE is expressed as two distinct forms: one is O-glycosylated, and the other is N-glycosylated. Significantly, AeAE immunoreactivity localizes to the basal regions of stellate cells but not principal cells. Concentrations of DIDS that inhibit AeAE activity in Xenopus oocytes have no effects on the unstimulated rates of fluid secretion mediated by Malpighian tubules as measured by the Ramsay assay. However, in Malpighian tubules stimulated with kinin or calcitonin-like diuretic peptides, DIDS reduces the diuretic rates of fluid secretion to basal levels. In conclusion, Aedes Malpighian tubules express AeAE in the basal region of stellate cells, where this transporter may participate in producing diuretic rates of transepithelial fluid secretion.
Keywords: Malpighian tubules, chloride/bicarbonate exchange, immunohistochemistry, Xenopus oocytes, electrophysiology, solute-linked carrier 4
with their enriched expression and activity of an apical vacuolar (V-type) H+-ATPase (2, 52, 86), the principal cells of renal (Malpighian) tubules in the mosquito (Aedes aegypti) are similar to acid-secreting cells of the mammalian renal collecting tubule (85), mammalian male reproductive tract (10), turtle and amphibian urinary bladders (9), amphibian skin (17), and mammalian bone (76). In these acid-secreting cells, the apical V-type H+-ATPase is often opposed by a basolateral Cl/HCO3 anion exchanger of the solute-linked carrier 4 (SLC4) superfamily of bicarbonate transporters. The anion exchanger mediates the basolateral extrusion of intracellular HCO3−, thereby removing the intracellular HCO3− that was generated during the ionization of carbonic acid (H2CO3).
Disulfonic stilbene derivates, such as SITS and DIDS, are potent inhibitors of almost all SLC4 transporters (61). These negatively charged compounds are thought to interact with positively charged lysine residues on the external surface of SLC4 proteins (47, 83), perhaps blocking a conduit for anion translocation. Pharmacological studies using stilbene derivatives suggest the presence of Cl/HCO3 exchangers in Malpighian tubules of some insects. For example, the addition of SITS to the peritubular bath of isolated Aedes Malpighian tubules lowers the unstimulated rates of fluid secretion (25), and both peritubular SITS and DIDS inhibit the serotonin-stimulated rates of fluid secretion in isolated Malpighian tubules of Rhodnius prolixus (23, 29). In isolated Malpighian tubules of the cricket (Teleogryllus oceanicus), SITS inhibits unstimulated rates of fluid secretion in the distal and main segments (89); DIDS also inhibits secretion in the distal segment, but it paradoxically stimulates secretion in the main segment (89). Finally, X-ray microanalysis on larval Drosophila Malpighian tubules indicate that DIDS decreases the intracellular Cl− concentration ([Cl−]) in the basal cytoplasm of principal cells, which is consistent with the presence of a Cl/HCO3 exchanger (88).
To date, only one SLC4-like transporter has been cloned and characterized from any insect, i.e., the Na+-driven anion exchanger (NDAE) of Drosophila (62, 65). When expressed in Xenopus oocytes, NDAE mediates DIDS-sensitive cotransport of Na+ and HCO3− in exchange for Cl− and H+ (62), making it analogous in function to the Na+-driven Cl/HCO3 exchangers (NDCBE) that have been cloned and characterized from mammals and cephalopods (20, 49, 83). In principal cells of Drosophila Malpighian tubules, NDAE immunoreactivity colocalizes with the α-subunit of the Na+-K+-ATPase (65), but the role of NDAE in Malpighian tubule function remains enigmatic. Preliminary studies from our laboratory have identified mRNA transcripts encoding NDAE in Aedes Malpighian tubules (90), but we have been unable to measure detectable immunochemical expression of NDAE protein in the tubules (unpublished observations). Thus we have focused our present efforts on the cloning and characterization of the only other SLC4-like transporter found in insects, the putative Cl/HCO3 anion exchanger (AE).
The goal of the present study was to test the hypothesis that Aedes Malpighian tubules express a SLC4-like AE in the basal membrane of principal cells. In this study, we have cloned a cDNA from Aedes Malpighian tubules that encodes a putative AE (designated AeAE), which represents the first SLC4-like AE to be cloned from the Malpighian tubules of any insect. When expressed heterologously in Xenopus oocytes, AeAE mediates Na+-independent changes to intracellular pH that are sensitive to extracellular [Cl−] and DIDS. To our surprise, we did not detect AeAE immunoreactivity in principal cells of Aedes Malpighian tubules, but rather in the basal regions of the small stellate cells that intercalate between principal cells. Using the Ramsay assay of fluid secretion, we have shown that peritubular DIDS has no effects on the unstimulated rates of transepithelial fluid in isolated Aedes Malpighian tubules, but instead reverses the stimulatory effects of diuretic peptides (i.e., aedeskinin III and calcitonin-like peptide) on fluid secretion. These observations uncover a putative novel role of stellate cells during diuresis, namely, the coupling of AeAE transport activity at the basal membrane of stellate cells to the metabolic regulation of intracellular pH (and thereby activity of the V-type H+-ATPase) in principal cells.
MATERIAL AND METHODS
Mosquitoes and Tissue Isolations
A colony of mosquitoes (A. aegypti) was maintained as described previously (48). In this study, only adult females were used 3–7 days after their eclosion. The mosquitoes were anesthetized on ice, decapitated, and submerged in a Ringer solution containing the following (in mM): 150 NaCl, 3.4 KCl, 1.7 CaCl2, 1.8 NaHCO3, 1.0 MgSO4, 5 glucose, and 25 HEPES. The pH of the Ringer was titrated to 7.1 using 1 N NaOH. The entire digestive tract (including the Malpighian tubules) was removed by tugging on the final abdominal segment as described previously (48). The five Malpighian tubules were freed from the intestinal tract with fine forceps and subsequently used in molecular, immunochemical, and physiological studies as described below.
Synthesis of Malpighian Tubule cDNA
Approximately 150 Malpighian tubules were isolated from at least 30 mosquitoes and placed in a 1.5-ml low-adhesion microcentrifuge tube (USA Scientific, Ocala, FL) containing 1 ml of ice-cold Trizol reagent (Invitrogen, Carlsbad, CA). Total RNA was extracted from the tubules as described previously (87) and used as a template to generate two independent pools of single-stranded cDNA. For the first pool of cDNA (3′-cDNA), 1 μg of total Malpighian tubule RNA was reverse transcribed using Superscript III reverse transcriptase (Invitrogen) and a GeneRacer oligo(dT) primer (Invitrogen). Before the second pool of cDNA (5′-cDNA) was generated, the GeneRacer kit (Invitrogen) was used to enrich full-length RNA transcripts from 1 μg of total Malpighian tubule RNA and “cap” their 5′ ends with the generic GeneRacer RNA oligo. The full-length, capped RNA was then reverse transcribed using SuperScript III reverse transcriptase (Invitrogen) and random hexamer primers (Invitrogen). Both resulting cDNA pools (∼20 μl each) were aliquoted (2 μl) and stored at −80°C. Before use, an aliquot was thawed on ice and diluted 10-fold with nuclease-free H2O (Integrated DNA Technologies, Coralville, IA).
cDNA Cloning of the Aedes SLC4-Like Anion Exchanger
We used the recently published Aedes genome (44) to design PCR primers that were expected to 1) recognize the predicted open reading frame (ORF) of a SLC4-like anion exchanger (GenBank accession no. XM_001652813) and 2) detect any potential splice variation in the 5′ and 3′ ends of the anion exchanger transcripts analogous to that which occurs in mammalian SLC4 members (1, 8). The forward primer 1F (Table 1) was designed to bind to the 3′ end of the transcript (purple arrow in Supplemental Fig. 1) within a region that encodes the latter half of the predicted transmembrane domain. (Supplemental data for this article is available at the American Journal of Physiology-Regulatory, Integrative and Comparative Physiology website.) The reverse primer 1R (Table 1) was designed to bind to a region in the 5′ end of the transcript (orange arrow in Supplemental Fig. 1) within a region that encodes the end of the cytosolic NH2-terminal domain, downstream of the well-conserved ETARWIKFEE motif.
Table 1.
Primers used for cloning of AeAE cDNA
| Primer | Sequence, 5′–3′ |
|---|---|
| 1F | ATTCTAACCTTGGGAACATTCTCGCTGGCT |
| 1R | TCCCATCTCCTTCAGCTGCCGCCAAAA |
| 2F | TCTTCTACTACCGTTCGCCGCAAGCTTCCT |
| 2R | CAATCGTGACGAATAACGTGTTCCGGCTTT |
See text for discussion on use of specific primers.
The 3′-rapid amplification of cDNA ends (RACE) was performed on 0.5 μl of Malpighian tubule 3′-cDNA using forward primer 1F, a generic reverse primer (GeneRacer 3′-Primer; Invitrogen), and Platinum PCR Supermix HF (Invitrogen) following the touchdown thermocycling protocol of the GeneRacer kit (Invitrogen). The 5′-RACE was conducted on 0.5 μl of Malpighian tubule 5′-cDNA using a generic forward primer (GeneRacer 5′-Primer; Invitrogen), reverse primer 1R, and Platinum PCR Supermix HF (Invitrogen) following the same touchdown thermocycling protocol. To assess the PCRs, we separated 5 μl of the respective products by electrophoresis on 1% agarose gels containing ethidium bromide, which were then visualized under UV light.
The PCR products were TA-cloned into a pCR 4 or pCR 2.1 TOPO vector (Invitrogen) and transformed into TOP10 Escherichia coli (Invitrogen) following the manufacturer's protocols. At least five resulting colonies were selected for overnight culture in 5 ml of LB broth (containing 100 μg/ml ampicillin), and their respective plasmid DNA was isolated using a QIAprep Spin Miniprep kit (Qiagen, Valencia, CA). The plasmid DNA was submitted to the Cornell DNA Sequencing Center (Ithaca, NY) for sequencing in both the 5′ and 3′ directions.
After the sequences of the 5′ and 3′ ends of the Aedes AE cDNA were obtained, we designed additional forward and reverse primers (2F and 2R in Table 1) in the predicted 5′- and 3′-untranslated regions (UTRs), respectively, to amplify the entire ORF in a single PCR. This full-length PCR was performed on 0.5 μl of Malpighian tubule 3′-cDNA with primers 2F and 2R and Platinum PCR Supermix HF (Invitrogen) using the following cycling parameters: one cycle at 94°C for 2 min; 35 cycles at 94°C for 30 s, 65°C for 30 s, and 68°C for 4 min; and one cycle at 68°C for 10 min. The full-length PCR products were TA-cloned and sequenced as described above. A consensus sequence was generated from the sequencing of plasmid DNA isolated from five colonies and then submitted to GenBank (accession no. EU700988). From this point, we refer to this cDNA, and to the protein it encodes, as AeAE.
The ORF of AeAE was subcloned into a pGH19 Xenopus expression vector (81) and then sequenced in both the 5′ and 3′ directions. To generate a cDNA encoding enhanced green fluorescent protein (eGFP) fused to the COOH-terminal end of AeAE (i.e., AeAE-eGFP-pGH19 cDNA), we subcloned the ORF of eGFP onto the 3′ end of the AeAE ORF as described previously (56).
Antibodies
We hired 21st Century Biochemicals (Marlboro, MA) to raise and affinity purify polyclonal rabbit antibodies against two synthetic peptide fragments of the AeAE protein predicted from the cloned cDNA. The peptides correspond to amino acid residues of the putative cytosolic NH2- and COOH-terminal domains of AeAE, i.e., Thr44-Pro63 (AENt peptide) and Ile1204-Leu1220 (AECt peptide). The antisera were affinity purified with the respective synthetic peptides, resulting in the production of two affinity-purified polyclonal antibodies: anti-AENt and anti-AECt. To detect eGFP immunoreactivity in Xenopus oocytes, we purchased a monoclonal mouse antibody (JL-8) from Clontech (Mountain View, CA).
Heterologous Expression of AeAE in Xenopus Oocytes
The AeAE-pGH19 and AeAE-eGFP-pGH19 cDNAs were used as templates to synthesize capped RNA (cRNA) with a T7 mMessage mMachine kit (Ambion, Austin, TX). The resulting cRNAs were purified in nuclease-free H2O using an RNeasy MinElute Cleanup kit (Qiagen) and stored at −80°C.
Stage V and VI oocytes were isolated from Xenopus laevis as described by Romero et al. (60). The oocytes were injected with 28 nl of AeAE cRNA (1.0 ng/nl), AeAE-eGFP cRNA (1.0 ng/nl), or nuclease-free H2O. The injected oocytes were cultured at 16°C in the wells of a Falcon six-well tissue culture plate (Becton Dickson, Franklin Lakes, NJ) containing OR3 medium (60) for 5–10 days before experiments commenced.
The heterologous expression of the AeAE and AeAE-eGFP proteins was verified via Western blotting of oocyte membrane-protein fractions and/or in vivo fluorescence of intact oocytes, exactly as described previously (56). In brief, membrane-protein fractions were first isolated from cleared oocyte lysates by ultracentrifugation at 100,000 g for 60 min (4°C) using an OptimaMax ultracentrifuge (Beckman, Fullerton, CA) and then prepared for Western blotting (see Ref. 56 for details). The following antibodies were used in the Western blots: anti-AENt (0.1 μg/ml in blocking buffer), anti-AECt (0.2 μg/ml in blocking buffer), and JL-8 (1 μg/ml in blocking buffer). The composition of the blocking buffer is described below in Western Blotting of Malpighian Tubules. For in vivo fluorescence, the oocytes were examined with an OV100 (Olympus) fluorescence imaging system equipped (150-W xenon lamp; 460- to 490-nm excitation filter; 510- to 550-nm emission filter) in the Cornell Microscopy and Imaging Facility (Ithaca, NY).
Oocyte Electrophysiology
Table 2 lists the compositions of the solutions used in electrophysiological studies of Xenopus oocytes. When required, DIDS was dissolved in solution IV to a final concentration of 200 μM. Solutions I and II were held in 250-ml glass Erlenmeyer flasks, whereas solutions III, IV, and V were held in 250-ml glass aeration flasks under constant bubbling with 95% O2-5% CO2. All solutions were delivered by gravity to an RC-3Z oocyte chamber (Warner Instruments, Hamden, CT) at a flow rate of 4 ml/min. Before an experiment was conducted, oocytes were transferred to separate petri dishes containing solution I to examine their in vivo fluorescence. For voltage-clamping experiments (see e.g., Fig. 11), an oocyte was transferred to the RC-3Z oocyte chamber under superfusion with solution I. For the intracellular pH (pHi) experiments (see e.g., Fig. 12), an oocyte was acclimated for at least 2 h in ∼1.4 ml of solution III in a 1.5-ml low-adhesion microcentrifuge tube (USA Scientific) and then placed in the oocyte chamber under superfusion with solution III. Solutions passing through the oocyte chamber were changed with a Rheodyne Teflon 8-way rotary valve (model 5012; Rohnert Park, CA).
Table 2.
Chemical compositions of oocyte recording solutions
| Solution |
|||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| NaCl | 96 | 0 | 63 | 0 | 0 |
| Na-gluconate | 0 | 96 | 0 | 63 | 0.096 |
| NMDG-Cl | 0 | 0 | 0 | 0 | 62.904 |
| NaHCO3 | 0 | 0 | 33 | 33 | 0 |
| NMDG-HCO3 | 0 | 0 | 0 | 0 | 33 |
| KCl | 2 | 0.07 | 2 | 0.07 | 0.07 |
| K-gluconate | 0 | 1.93 | 0 | 1.93 | 1.93 |
| MgCl2 | 1 | 0 | 1 | 0 | 0 |
| Mg-gluconate | 0 | 2 | 0 | 2 | 2 |
| CaCl2 | 1.8 | 0 | 1.8 | 0 | 0 |
| Ca-gluconate | 0 | 3.6 | 0 | 3.6 | 3.6 |
| HEPES | 5 | 5 | 5 | 5 | 5 |
Chemical composition of recording solutions I–V is given in mM. The pH of all solutions was adjusted to 7.5 with NaOH or N–methyl-d-glucamine (NMDG)-OH. The osmolality of all solutions was adjusted to 195 ± 5 mosmol/kgH2O by adding double-distilled H2O or mannitol. Solutions III, IV, and V were constantly bubbled with 95% O2-5% CO2.
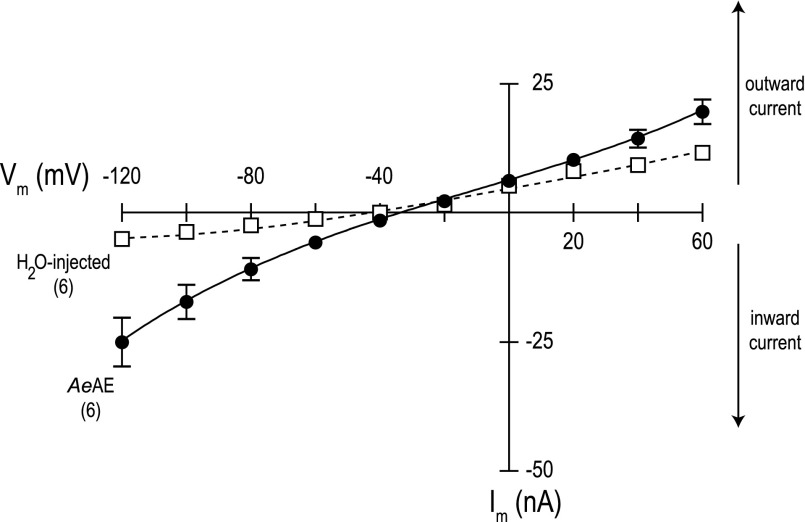
Fig. 11.
Current-voltage (I-V) plots of AeAE and H2O-injected oocytes. Negative Im values represent the net movement of positive charge into or negative charge out of the cell (inward current), whereas positive Im values represent outward current. Data were acquired in a normal Cl− solution with nominal CO2/HCO3− (solution I, Table 2). Values are means ± SE based on the number of oocytes shown in parentheses. Missing error bars indicate values too small to illustrate. The dashed line connecting open boxes represents the I-V relationship of the H2O-injected oocytes, whereas the solid line connecting filled circles represents the I-V relationship of the AeAE oocytes.
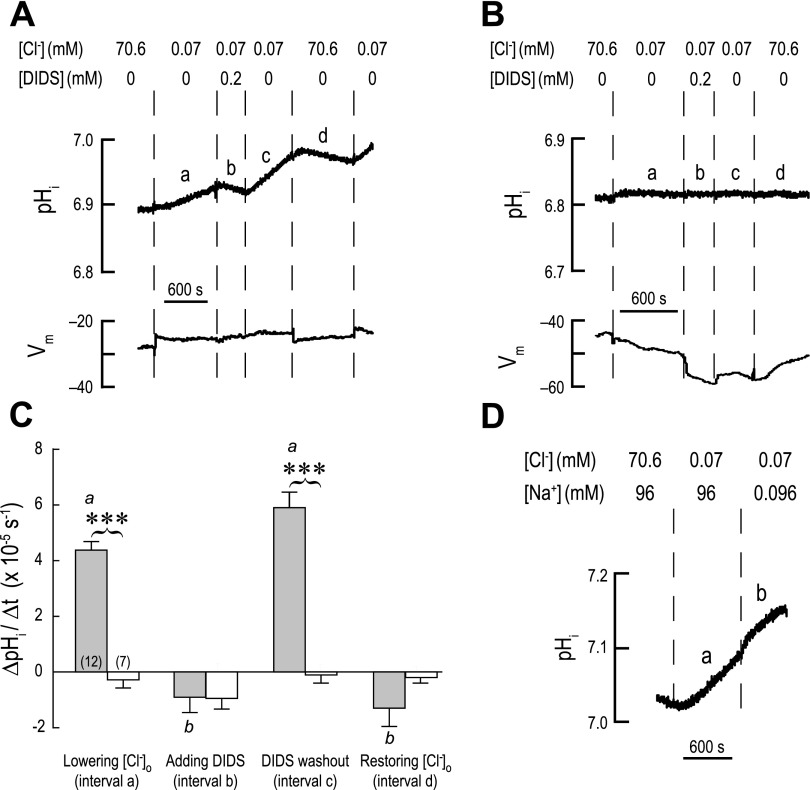
Fig. 12.
Cl− dependence, DIDS sensitivity, and Na+ independence of intracellular alkalinization in AeAE oocytes. A: representative recordings of pHi and membrane potential (Vm) in an AeAE oocyte exposed to a 5% CO2/33 mM HCO3− solution (solution III, Table 2) for 2 h prior. Extracellular concentrations (in mM) of Cl− and DIDS are indicated. When extracellular [Cl−] was lowered, it was replaced by gluconate. Solution changes are indicated by dashed vertical lines. Intervals a–d in the pHi trace indicate the intervals where rates of pHi change (ΔpHi/Δt) were measured. B: representative recordings of pHi and Vm in a H2O-injected oocyte, using a protocol similar to that described in A. C: summary of ΔpHi/Δt measurements. Shaded bars represent ΔpHi/Δt values of AeAE oocytes (number of oocytes shown in parentheses) during the intervals identified in A. The open bars represent H2O-injected oocytes at similar intervals. Values are means ± SE. Brackets connecting shaded and open bars represent comparisons in unpaired t-tests resulting in significant differences (***P < 0.001). a,bP < 0.001, categorization of the means of the AeAE oocytes as determined by a repeated-measures ANOVA and Newman-Keuls posttest. D: representative recording of pHi in an AeAE oocyte that examines the Na+ dependence of AeAE transport. Extracellular concentrations (in mM) of Cl− and Na+ are indicated. A total of 6 AeAE oocytes were evaluated using this protocol.
Oocytes injected with 28 nl of AeAE-eGFP cRNA (1.0 ng/nl) were used in electrophysiological experiments, because the fluorescence of eGFP allowed us to verify the heterologous expression of AeAE for each oocyte studied. Oocytes injected with 28 nl of nuclease-free H2O served as controls. pHi and membrane potential (Vm) were recorded with pH-sensitive and voltage-sensitive microelectrodes, respectively, exactly as described previously (56).
In the typical voltage-clamping experiment, the oocyte was clamped at the spontaneous Vm before a current-voltage (I-V) plot controlled by the Clampex module of the pCLAMP software (Molecular Devices) was acquired, as described previously (55). Thereafter, the extracellular [Cl−] was changed and Vm was allowed to stabilize for 90 s. The oocyte was then voltage clamped at this value before an additional I-V plot was acquired. In the typical pHi experiment, the initial pHi and Vm values were allowed to stabilize for ∼5 min before the solution change protocols shown in Fig. 12 were initiated.
Western Blotting of Malpighian Tubules
Membrane fractions.
Malpighian tubules were collected from at least 40 female mosquitoes and placed in a 1.5-ml microcentrifuge tube containing 1 ml of ice-cold Ringer solution. After the Ringer was aspirated, the tubules were snap frozen in liquid nitrogen and stored at −80°C until analysis. We prepared a membrane fraction of Malpighian tubules using a previously published protocol (5) that was modified in the two following ways. First, the tubules were thawed on ice in 100 μl of ice-cold Ringer solution supplemented with Halt protease inhibitor cocktail (Pierce Biotechnology, Rockford, IL) and EDTA (5 mM). Second, after the 100,000 g centrifugation, the supernatant containing cytosolic protein was discarded, whereas the pellet containing the membrane proteins was immediately resuspended in 50–100 μl of ice-cold Ringer solution (containing protease inhibitors). The resuspended membrane fraction was measured for total protein using a bicinchoninic acid protein assay (Pierce Biotechnology). An appropriate volume of a 5× Laemmli sample buffer (36) was added to the resuspended membrane fractions, which were then boiled at 100°C for 5 min.
Crude lysates.
Malpighian tubules were collected from at least 10 female mosquitoes and snap frozen as described above. The lysates were prepared by homogenizing the tubules in either 40 μl of a 1× Laemmli sample buffer or 30 μl of a high-urea buffer containing the following: 4 M urea, 0.1% Triton X-100, 0.1% SDS, 150 mM NaCl, and 10 mM Tris · HCl, pH 7.4 (43, 82). The high-urea lysates were then supplemented with an appropriate volume of a 5× Laemmli sample buffer. Both the Laemmli and high-urea lysates were boiled at 100°C for 5 min.
SDS-PAGE and immunoblotting.
Approximately 30 μg of membrane or crude lysate protein from Malpighian tubules were separated by molecular mass on a denaturing 8% polyacrylamide resolving gel (4% stacking gel) using an XCell SureLock mini cell electrophoresis unit (Invitrogen). After electrophoresis, the stacking gel was discarded and the separated proteins in the resolving gel were transferred to an Immunoblot polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA) using an XCell II blot module (Invitrogen) according to the manufacturer's protocol.
To detect AeAE immunoreactivity, the PVDF membrane was 1) washed three times (5 min each) with Tween-Tris-buffered saline (TTBS; 10 mM Tris · HCl, 150 mM NaCl, and 0.01% Tween 20, pH 7.4), 2) blocked for 1 h with 5% nonfat dry milk dissolved in TTBS (blocking buffer), and 3) incubated overnight at 4°C with the anti-AENt or anti-AECt antibody (1 μg/ml or 2 μg/ml, respectively, in blocking buffer). On the following day, the PVDF membrane was 1) washed three times (5 min each) with TTBS, 2) incubated for 1.5 h with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Pierce Biotechnology) diluted 1:25,000 in blocking buffer, and 3) washed three times (5 min each) with TTBS. To visualize binding of the antibodies, a luminescent substrate of HRP (SuperSignal West Pico, Pierce Biotechnology) was applied to the PVDF membrane, and the luminescent signal was detected with X-ray film (Pierce Biotechnology).
Glycosylation Studies
An enzymatic approach was used to determine the glycosylation state of heterologously expressed AeAE in Xenopus oocytes and endogenously expressed AeAE in Malpighian tubules. To remove N-linked glycans, we performed the following steps on 25–30 μg of oocyte membrane or crude tubule lysate protein: 1) denaturation in a glycoprotein denaturing buffer (0.5% SDS, 0.04 M dithiothreitol) for 10 min at 100°C; 2) neutralization in a G7 reaction buffer (0.05 M NaPO4, pH 7.5) supplemented with 1% Nonidet P-40; and 3) deglycosylation for 2–3 h at 37°C after the addition of 1,000 units of PNGase F (all from New England Biolabs, Ipswich, MA). To remove O-linked glycans, we followed the same procedure, but O-glycosidase (200,000 units; New England Biolabs) and neuraminidase (125 units; New England Biolabs) were used instead of PNGase F. In some experiments, all three enzymes were used simultaneously. The enzymatic reactions were terminated by adding 5× Laemmli sample buffer (36) and boiling the samples at 100°C for 5 min. Western blotting was then performed as described above.
Malpighian Tubule Immunohistochemistry
For immunolocalization studies of AeAE expression, 150 isolated Malpighian tubules (from 30 female mosquitoes) were fixed and processed for routine paraffin embedding exactly as described in a previous study (56). The 4-μm sections were adhered to ProbeOn Plus glass slides (Fisher Scientific, Hampton, NH), deparaffinized, rehydrated, peroxide treated, and blocked exactly as described previously (56).
After blocking, the sections were incubated overnight at 4°C in a humidified chamber with one of the following: 1) phosphate-buffered saline (PBS) supplemented with casein (Vector Laboratories, Burlingame, CA) as a negative control, 2) the anti-AENt antibody (10 μg/ml in PBS-casein), or 3) the anti-AECt antibody (10 μg/ml in PBS-casein). For antibody preadsorption experiments, the anti-AE antibodies (1 μg/ml) were incubated with their respective immunogenic peptides for 45 min at 37°C in a 7:1 molar ratio (peptide:antibody) before being placed on the tissue sections. The PBS consisted of the following (in mM): 145 NaCl, 3.2 NaH2PO4, and 7.2 Na2HPO4 (pH 7.3).
On the following day, the sections were rinsed three times with PBS and then washed in PBS for 5 min before being incubated for 20 min with a biotinylated goat anti-rabbit secondary antibody (Vector Laboratories) diluted 1:100 in PBS-casein. To detect binding of the antibodies, we treated the sections with streptavidin-HRP (Vector Laboratories) followed by a chromogenic substrate of HRP (Vector Laboratories), as described previously (56). The sections were counterstained with Harris's hematoxylin (Electron Microscopy Sciences, Hatfield, PA) and covered with a cover glass for viewing on an AX70 compound microscope (Olympus, Melville, NY).
Ramsay Fluid Secretion Assays
Rates of transepithelial fluid secretion were measured in isolated Malpighian tubules following the method of Ramsay (58). In brief, a Malpighian tubule from a female mosquito was transferred to a Ringer droplet (50 μl) covered with light mineral oil (Fisher Scientific). With the aid of a glass hook, the open proximal end of the tubule was pulled into the oil, leaving the distal blind end in the Ringer droplet. With the use of this approach, fluid secreted by the tubule exits the lumen into the oil as a droplet. The volume of secreted fluid in the droplet is calculated by measuring its diameter with an ocular micrometer.
In some experiments, unstimulated secretion rates were first measured over 30 min, and then DIDS was added to the Ringer droplet at a final concentration of 200 μM. The secretion rate was then measured over the next 30 min. In other experiments, unstimulated secretion rates were first measured over 30 min, and then aedeskinin III (AKIII) or calcitonin-like peptide from Anopheles gambiae (CLP; Anoga-DH31) was added to the Ringer droplet to yield a concentration of 10−6 M. After secretion rates were measured for 30 min in the presence of AKIII or CLP, DIDS was added to the Ringer droplet at a final concentration of 200 μM, and the ensuing secretion rate was measured for 30 min. The AKIII was synthesized and provided by the laboratory of Nachman (97). The CLP was a gift of Prof. David A. Schooley (University of Nevada).
Statistics
Statistical tests were performed using GraphPad Prism 4 (GraphPad Software, San Diego, CA). Comparisons between two groups were evaluated with unpaired or paired t-tests. To compare means among more than two groups, a repeated-measures or one-way analysis of variance (ANOVA) was used. If needed, multiple comparisons were performed with a Newman-Keuls post test.
RESULTS
Molecular Cloning of the AeAE Transcript From Aedes Malpighian Tubules
Using RT-PCR on Aedes Malpighian tubules, we cloned an AeAE cDNA. As illustrated in Fig. 1A, the 4,415-bp transcript consists of a 5′-UTR of 204 bp, an ORF of 3,732 bp, and a 3′-UTR of 479 bp (including a polyadenosine tail of 21 bp). Neither the 5′-RACE nor the 3′-RACE revealed evidence of alternative splicing of the AeAE transcripts expressed by Malpighian tubules.
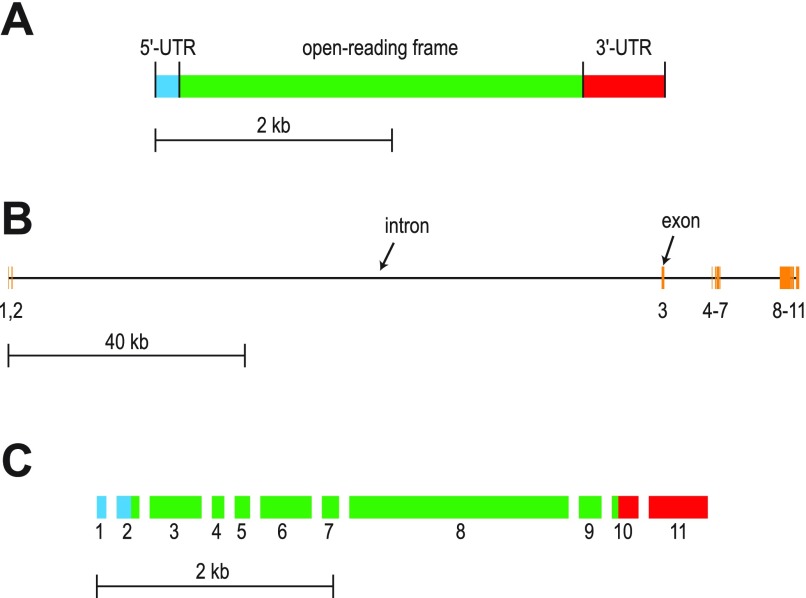
Fig. 1.
Maps of the cDNA cloned from Aedes Malpighian tubules that encodes a putative AE (designated AeAE cDNA) and the AeAE gene. A: map of the AeAE cDNA showing the relative lengths of the 5′-untranslated region (UTR; blue), open reading frame (green), and 3′-UTR (red). B: map of the AeAE gene showing the distribution of the exons (orange vertical bars). Exon numbers are indicated. Horizontal black bars represent introns. Exact genomic positions and/or lengths of the exons and introns are listed in Table 3. C: fractured map of the AeAE cDNA showing how each exon contributes to the features and length of the cDNA shown in A.
To elucidate the organization of the exons and introns that comprise the AeAE gene, we mapped the cloned AeAE cDNA onto the Aedes genomic data (44). As shown in Fig. 1B, the AeAE gene consists of 11 exons separated by 10 introns that together span over 136.5 kb in region “supercontig 1.28” of the Aedes genome. Table 3 lists the exact genomic positions and lengths of these exons and introns. Figure 1C illustrates how each of the 11 exons in the AeAE gene contributes to the structural features of the transcript noted in Fig. 1A.
Table 3.
Positions and lengths of the exons and introns comprising the AeAE gene
| Exon | Start Position | End Position | Length of Exon, bp | Length of Following Intron, bp |
|---|---|---|---|---|
| 1 | 303,213 | 303,295 | 83 | 443 |
| 2 | 303,739 | 303,919 | 181 | 112,229 |
| 3 | 416,149 | 416,596 | 448 | 8,180 |
| 4 | 424,777 | 424,883 | 107 | 468 |
| 5 | 425,352 | 425,484 | 133 | 63 |
| 6 | 425,548 | 425,990 | 443 | 62 |
| 7 | 426,053 | 426,199 | 147 | 10,273 |
| 8 | 436,473 | 438,367 | 1895 | 62 |
| 9 | 438,430 | 438,624 | 195 | 70 |
| 10 | 438,695 | 438,923 | 229 | 340 |
| 11 | 439,264 | 439,796 | 533 |
All positions are relative to region “supercontig 1.28” of the Aedes genome.
A comparison of the cloned AeAE cDNA sequence with the corresponding data from the Aedes genome revealed a total of 15 nucleotide differences (Supplemental Fig. 1). Twelve of the differences are of no consequence to the predicted amino acid sequence (asterisks in Supplemental Fig. 1). However, three of the differences are consecutive (red bar in Supplemental Fig. 1) and result in two nonsynonymous changes to the predicted amino acid sequence (i.e., Val1157Pro in our cDNA vs. Glu1157Ser in the genome). We have found our cDNA sequence in the contested region to be highly reliable, because it is identical among 1) the five full-length AeAE cDNAs used to generate the consensus sequence and 2) the five partial cDNAs that were sequenced from the 3′-RACE. Moreover, the two nonsynonymous amino acids encoded by our AeAE cDNA are completely conserved with the comparable residues in the predicted AEs and NDAEs from the genomes of other mosquitoes (i.e., Culex pipiens and A. gambiae; data not shown).
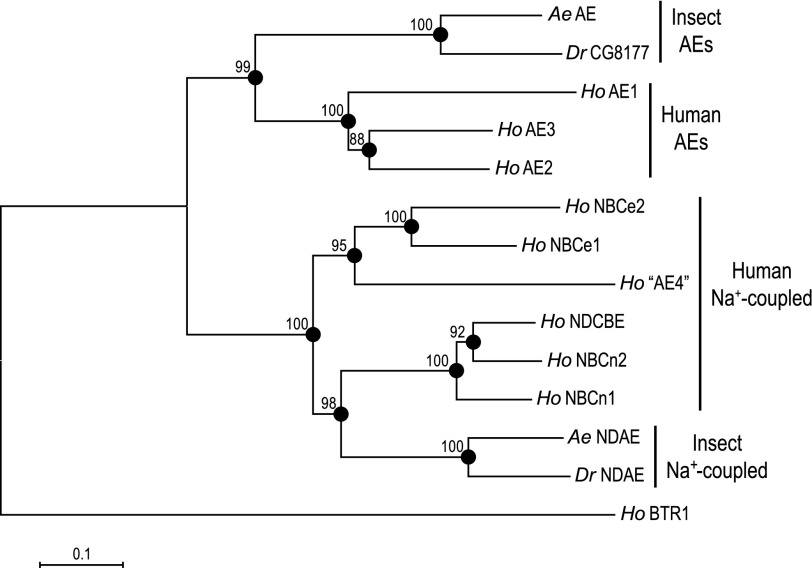
The ORF of the AeAE cDNA encodes a predicted protein of 1,243 amino acids (139.1 kDa) that on average shares ∼39% amino acid identity with the SLC4 Na+-independent AEs and ∼33% identity with the SLC4 Na+-coupled HCO3− transporters, both of humans. The neighbor-joining phylogenetic tree (Fig. 2) shows how the predicted amino acid sequence of AeAE relates to those of other representative SLC4 proteins from insects and humans. The tree indicates that the AeAE protein 1) shares a common node with the predicted AE of Drosophila (CG8177) and 2) occurs within a larger branch of the tree that includes the human AEs (Fig. 2). Among the human AEs, AeAE is more closely related to AE2 and AE3 than to AE1 (Fig. 2). The Na+-coupled SLC4 members of insects and humans occur in their own branch of the tree that is distinct from the AEs (Fig. 2).
Fig. 2.
Phylogenetic relationship of AeAE to other SLC4 proteins. A neighbor-joining phylogenetic tree of selected insect (Aedes, Ae; Drosophila, Dr) and human (Ho) SLC4 proteins. The tree was generated with MEGA 4 software (77) using Poisson-corrected distance estimates. The nodes of branches are indicated by filled circles for which bootstrap scores (from 1,000 replicates) are provided. The total branch length between 2 proteins represents the proportion of amino acids that differ between them. The scale bar represents a branch length that corresponds to a proportional difference of 0.1 (i.e., a 10% difference in amino acids). Human BTR1 (HoBTR1, SLC4A11) is the outgroup. Accession numbers are as follows: AeAE, EU700988; AeNDAE, ACH96582; DrCG8177, NP_996034; DrNDAE, NP_723263.2; HoAE1, AAH99628; HoAE2, EAW54048; HoAE3, AAI46657; Ho“AE4”, NP_113655; HoBTR1, NP_114423; HoNBCe1, NP_001128214; HoNBCe2, NP_067019; HoNBCn1, ACH61961; HoNBCn2, BAB18301; HoNDCBE, ABJ09587.
Predicted AeAE Protein Possesses Hallmark Features of SLC4 Proteins
In a broad review of the SLC4 transporter family, Romero et al. (61) identified several commonalities shared among the AEs and Na+-coupled HCO3− transporters, including their predicted topology in the plasma membrane, the presence of N-glycosylation, and their inhibition by stilbene derivatives such as DIDS. Below, we evaluate these features and others in the primary sequence of AeAE.
Transmembrane segments and topology prediction.
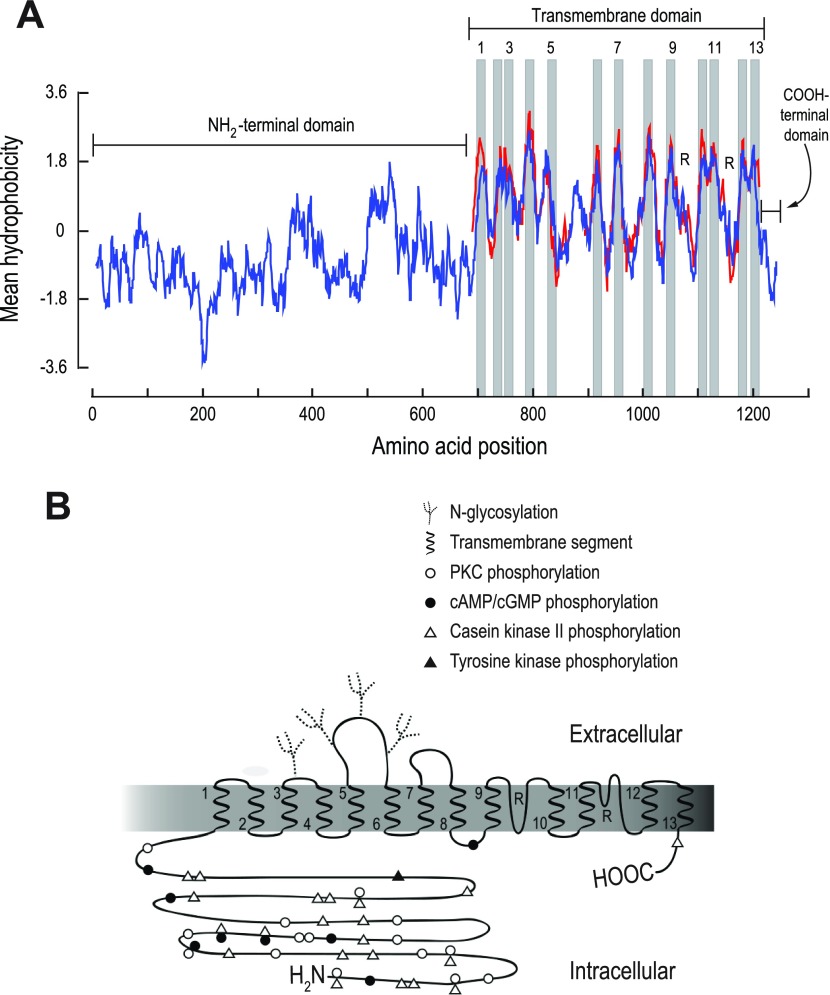
A Kyte-Doolittle hydropathy plot of the predicted AeAE protein (blue trace in Fig. 3A) demonstrates that AeAE has the typical structural features of SLC4-like proteins (61). That is, AeAE contains 1) a long, hydrophilic NH2-terminal domain of ∼700 residues, 2) a transmembrane (TM) domain of ∼500 residues, and 3) a short, hydrophilic COOH-terminal domain of ∼ 40 residues (Fig. 3A).
Fig. 3.
Predicted membrane topology of the AeAE protein. A: Kyte-Doolittle hydropathy plots (window size = 15) of AeAE (blue trace) and the transmembrane (TM) domain of human AE1 (red trace) as generated and aligned using BioEdit Sequence Alignment Editor software, version 7 (24). Breaks in the red trace represent gaps introduced by the sequence alignment. Shaded regions are predicted TM segments, for which every other is numbered. R, predicted reentrant loops. B: hypothesized topology map of AeAE based on hydropathy plot in A and the experimentally derived topology of human AE1 (13, 93, 94). The TM segments are numbered at their emerging ends. Putative posttranslational modifications and regulatory sites are also indicated (see text and Fig. 4 for details).
The red trace in Fig. 3A shows a hydrophobicity plot of the TM domain of human AE1, for which a detailed topology map exists (13, 93, 94), aligned with that of AeAE. The similar hydropathy profiles shared between the TM domains of AeAE and human AE1 indicate that these proteins likely share a similar membrane topology. On the basis of the topology model for human AE1 (13, 93, 94), we assigned 13 putative TM segments (shaded bars in Fig. 3A) and 2 reentrant loops (R in Fig. 3A) to the AeAE protein. The resulting topological model of AeAE is illustrated in Fig. 3B. The amino acid residues that correspond to the TM segments are identified by the numbered horizontal bars in Fig. 4.
Fig. 4.
Annotated amino acid sequence of AeAE. The amino acids of AeAE (accession no. EU700988) were aligned with those of human AE2 (HoAE2; accession no. AAC50964.1) and human AE3 (HoAE3; accession no. NP_963868.2) using the ClustalW algorithm (38). The residue shading was performed using BioEdit Sequence Alignment Editor software, version 7 (24), with a threshold of 100%, where black shading indicates identical residues and gray shading indicates similar residues. Numbered horizontal bars identify the regions of predicted TM segments that are depicted in Fig. 3. The red boxes at the ends of TM segments 3, 5, and 12 outline regions that contain lysine residues (in red) associated with sensitivity to stilbene derivates such as DIDS. The 4 blue boxes (3 in the NH2-terminal domain and 1 between TM segments 9 and 10) outline regions associated with the sensitivity of murine AE2 to intracellular (pHi) and/or extracellular pH (pHo). Specific residues (within and outside the blue boxes) that are involved with the regulation of AE2 by pH are colored as follows: green, involved with regulation by pHo; yellow, involved with regulation by pHi; orange, involved with regulation by pHo and pHi. The corresponding residues of AeAE and HoAE3 are colored identically if they are conserved (i.e., identity, charge, or hydrophobicity). Symbols are as defined in Fig. 3B. Data on pH sensitivity are from Refs. 35 and 69–75.
Within the TM domain, a feature of particular interest is the relatively large exofacial loop that connects TM segments 5 and 6 (i.e., the 5–6 loop). As shown in Fig. 4, the amino acid residues that comprise this loop in AeAE (Y838–P907) are deficient in cysteine, as also is the case for human AE2 and AE3. In contrast, the corresponding 5–6 loops of all Na+-coupled HCO3− transporters that have been cloned from vertebrates, insects, and squid possess four highly conserved cysteine residues (55, 61).
Predicted N-glycosylation sites.
Using an in silico ProScan analysis (16), we identified four putative sites for N-glycosylation of the AeAE protein. As shown in Figs. 3B and 4, the exofacial loop connecting TM segments 3 and 4 (i.e., the 3–4 loop) possesses a single predicted N-glycosylation site, whereas the 5–6 loop contains three such sites.
Predicted DIDS-binding sites.
Negatively charged stilbene derivates, such as DIDS and SITS, are thought to bind to and inhibit SLC4 proteins by interacting with positively charged lysine (K) residues at the ends of TM segments 3, 5, and 12 (47, 83). That is, the collective lysine residues of these three TM segments are hypothesized to form a single binding pocket for a stilbene molecule (47, 83). As indicated by the red boxes in Fig. 4, AeAE has no such lysines at the end of TM segment 3 but has one at the end of TM segment 5 (K833) and another at the end of TM segment 12 (K1186). The location of K833 in AeAE is analogous to that of K558 in mouse erythroid AE1 and K559 in human NBCe1-A, which both play important roles in the reversible binding of DIDS to their respective transporters (32, 41).
Other predicted regulatory sites.
Previous studies have demonstrated that phosphorylation events can influence the activity (18), stoichiometry (21), and trafficking (80) of SLC4 proteins. As shown in Figs. 3B and 4, we have identified numerous putative phosphorylation sites in AeAE using an in silico ProScan analysis (16). The large NH2-terminal domain contains almost all of the predicted sites, whereas one putative site was found in each of the TM and COOH-terminal domains (Figs. 3B and 4).
Mammalian AE2 displays a profound sensitivity to both pHi and extracellular pH (pHo) (28, 31, 39, 68). The laboratory of Alper (1) has identified several regions within AE2 that contribute to its pH sensitivity. These regions are indicated by the blue boxes in Fig. 4. In addition, Alper's group has identified individual amino acid residues within and outside of the above regions that are involved with the pH sensitivity of AE2 (1). These residues are colored as indicated in the Fig. 4 legend. Note that the amino acid sequence of AeAE shares a high degree of homology and/or identity with these regions and residues (Fig. 4).
Together, the results of the above bioinformatic analyses lead us to hypothesize that AeAE is 1) a Na+-independent Cl/HCO3 exchanger, 2) inhibited by DIDS, 3) N-glycosylated, 4) regulated by pHi and/or pHo, and 5) regulated by various kinases. The first three hypotheses are explored in following sections; the latter two are not addressed directly in the present study because they are beyond its scope.
Heterologous Expression of AeAE in Xenopus Oocytes
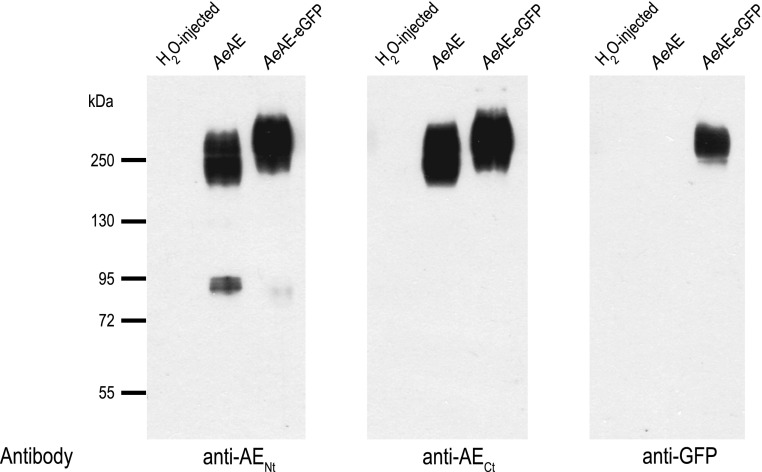
As described in materials and methods, affinity-purified anti-AENt and anti-AECt polyclonal antibodies were produced against peptides corresponding to portions of the cytosolic NH2-terminal and cytosolic COOH-terminal domains of AeAE, respectively. To test these antibodies and confirm heterologous expression in Xenopus oocytes, we performed Western blotting on total membrane fractions isolated from Xenopus oocytes injected with H2O, AeAE cRNA, or AeAE-eGFP cRNA. As shown in Fig. 5, the anti-AENt and anti-AECt antibodies did not detect proteins in the H2O-injected oocytes but exhibited very similar patterns of immunoreactivity in the AeAE and AeAE-eGFP oocytes. That is, the antibodies labeled intense, broad bands of protein from ∼170 to >250 kDa in the AeAE oocytes and from ∼200 to >250 kDa in the AeAE-eGFP oocytes. Importantly, a monoclonal anti-GFP antibody recognized a band of protein in the AeAE-eGFP oocytes similar to that detected by the anti-AENt and anti-AECt antibodies (Fig. 5).
Fig. 5.
Heterologous expression of AeAE in Xenopus oocytes. Western blots of total membrane fractions isolated from Xenopus oocytes 6 days after injection with H2O, AeAE cRNA (28 ng), or AeAE-enhanced green fluorescent protein (eGFP) cRNA (28 ng). The antibodies used against the NH2 (anti-AENt)- and COOH-terminal (anti-AECt) AeAE peptides and GFP are indicated. Migrations of the molecular mass markers (in kDa) are indicated at left.
The anti-AENt antibody also detected weaker bands of protein between 72 and 95 kDa in the AeAE and AeAE-eGFP oocytes (Fig. 5). These bands likely represent truncated versions of the AeAE and AeAE-eGFP proteins (via proteolysis and/or mistranslation), because neither the anti-AECt nor the anti-GFP antibodies detected comparable bands.
Glycosylation of heterologously expressed AeAE.
As shown in Fig. 5, the immunoreactivities of AeAE and AeAE-eGFP begin at 170 and 200 kDa, respectively, when expressed in Xenopus oocytes. The larger size of AeAE-eGFP is expected given that the molecular mass of eGFP is ∼30 kDa. However, in both cases, the sizes of the immunoreactivities are at least 30 kDa greater than that expected from the protein encoded by the AeAE and AeAE-eGFP cRNAs (i.e., 139 kDa and 169 kDa respectively). Thus we aimed to determine whether the larger than expected molecular mass is due to glycosylation of the AeAE protein.
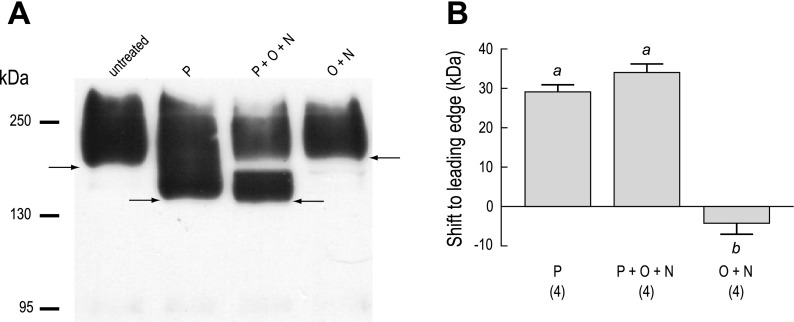
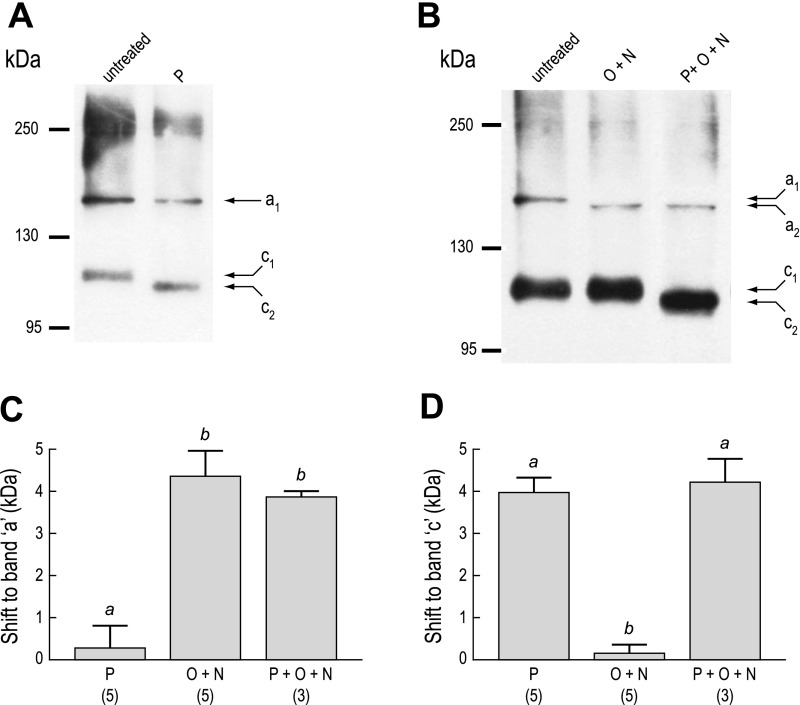
Figure 6 shows the effects of glycosidases on the anti-AENt immunoreactivity in membrane fractions of AeAE oocytes. Treating the membrane fractions with PNGase F, which selectively removes N-linked glycans from proteins, shifted the leading edge of the AeAE immunoreactivity by ∼30 kDa (P in Fig. 6A), relative to untreated membrane fractions. Incubating the membrane fractions with PNGase F and a mixture of O-glycosidase and neuraminidase (the latter 2 enzymes remove common types of O-linked glycans from proteins) produced a shift comparable to that observed with PNGase F alone but also resulted in a distinct disappearance of immunoreactivity just below 170 kDa (P + O + N in Fig. 6A). This disappearance was observed consistently in three additional experiments. Treating the membrane fractions with only the O-glycosidase and neuraminidase (O + N in Fig. 6A) did not affect the migration or the appearance of the immunoreactivity, compared with that of the untreated membrane fractions. Figure 6B summarizes the shifts (in kDa) observed to the leading edge of the AeAE immunoreactivity after each enzymatic treatment.
Fig. 6.
Glycosylation of heterologously expressed AeAE in Xenopus oocytes. A: Western blot of total membrane fractions isolated from AeAE oocytes (6 days postinjection). The membrane fractions were either untreated or exposed to one of the following enzymatic treatments: 1) PNGase F (P); 2) a mixture of PNGase F, O-glycosidase, and neuraminidase (P + O + N); or 3) a mixture of O-glycosidase and neuraminidase (O + N). The anti-AENt antibody was used to detect AeAE immunoreactivity. Arrows indicate the leading edge of the immunoreactivity. Migrations of the molecular mass markers (in kDa) are indicated at left. B: summary of the effects of enzymatic deglycosylation on the migration of the AeAE immunoreactivity. Shaded bars represent the shift (in kDa) to the leading edge. Values are means ± SE based on the number of measurements shown in parentheses. a,bP < 0.001, categorization of the means as determined by a repeated-measures ANOVA and Newman-Keuls posttest.
Expression of AeAE Immunoreactivity in Aedes Malpighian Tubules
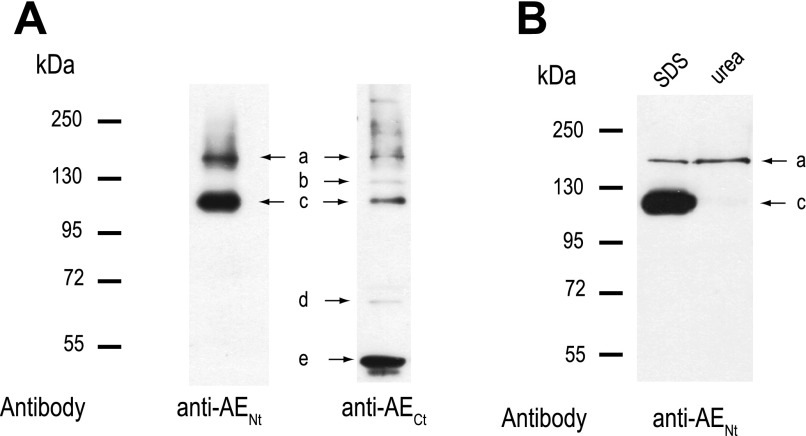
Figure 7A shows representative Western blots using the anti-AENt and anti-AECt antibodies on membrane fractions isolated from Aedes Malpighian tubules. The anti-AENt antibody detected two bands of protein (Fig. 7A). Band a runs at ∼150 kDa, which is close to the predicted size of the AeAE monomer (∼139 kDa), whereas band c runs at ∼111 kDa. The immunoreactivity of both bands was quenched when the anti-AENt antibody was preadsorbed with its immunogenic peptide (Supplemental Fig. 2A). The anti-AECt antibody detected the same proteins that correspond to bands a and c (Fig. 7A) and also bands of protein at ∼125, ∼67, and <55 kDa (bands b, d, and e in Fig. 7A, respectively). Preadsorption of the anti-AECt antibody with its immunogenic peptide reduces the immunoreactivity of bands a, b, c, and e, but not band d (Supplemental Fig. 2B).
Fig. 7.
Expression of AeAE immunoreactivity in female Aedes Malpighian tubules. A: representative Western blots of total membrane protein (30 μg/lane) isolated from Malpighian tubules of adult Aedes females. Labeled arrows indicate the protein bands (a–e) displaying AeAE immunoreactivity. The antibodies used are indicated. Migrations of the molecular mass markers (in kDa) are indicated at left. B: representative Western blot of anti-AENt immunoreactivity in crude Malpighian tubule lysates (30 μg protein/lane) denatured under standard Laemmli (SDS) or high-urea conditions.
The simplest explanation for the additional bands of protein detected by the anti-AECt antibody, with the exception of band d, is that they are proteolytic fragments of bands a and/or c missing the NH2-terminal epitope recognized by the anti-AENt antibody. In support of this explanation, the anti-AENt antibody detected several bands of weak immunoreactivity between 95 and 55 kDa that were not detected by the anti-AECt antibody when the X-ray film was overexposed to the chemiluminescent signal emitted from the PVDF (data not shown).
The above experiments indicate that the anti-AENt antibody primarily detected two intact forms of the AeAE protein (i.e., bands a and c), which both possess the NH2-terminal and COOH-terminal epitopes recognized by the anti-AENt and anti-AECt antibodies, respectively. Furthermore, in preliminary Western blotting experiments, we observed that the anti-AENt antibody 1) was more sensitive at detecting AeAE immunoreactivity in Malpighian tubules and 2) exhibited less background staining relative to the anti-AECt antibody (data not shown). For these reasons, the anti-AENt antibody was used in the following Western blotting experiments that aimed to decipher potential biochemical differences between bands a and c.
Effects of denaturation on AeAE immunoreactivity.
To determine whether the 111-kDa band c represents a fraction of the 150-kDa AeAE protein (band a) that ran anomalously on SDS-PAGE, because of a conformation that is resistant to the standard Laemmli denaturation at 100°C (36), we enhanced the denaturation of Malpighian tubule proteins by adding a high-urea buffer (43, 82). Compared with the standard Laemmli denaturation (SDS), the tubule protein denatured in the high-urea buffer resulted in a single, discrete band of AeAE immunoreactivity that corresponds to the 150-kDa band a (Fig. 7B).
To examine the effects of a weaker denaturation on the migration of the AeAE protein on SDS-PAGE, we incubated the tubule proteins in the standard Laemmli buffer at 37°C instead of 100°C. As shown in Supplemental Fig. 3, denaturing at 37°C resulted in two broad, diffuse bands of AeAE immunoreactivity that correspond to bands a and c.
Glycosylation of AeAE.
We next examined the effects of glycosidases on the anti-AENt immunoreactivity in Malpighian tubules to determine whether differences in glycosylation are associated with the 150-kDa “Laemmli-sensitive” (band a) and 111-kDa “Laemmli-resistant” (band c) forms of AeAE. As shown in Fig. 8A, removing N-linked glycans with PNGase F did not affect the migration of the 150-kDa band a, but the 111-kDa band c migrated further by ∼4 kDa (c2 in Fig. 8A), relative to untreated lysates. In contrast, Fig. 8B demonstrates that removing O-linked glycans with a mixture of O-glycosidase and neuraminidase enhanced the migration of band a by ∼4 kDa (a2 in Fig. 8B), relative to untreated lysates, but did not affect the migration of band c. Simultaneous treatment of tubule lysates with PNGase F and a mixture of O-glycosidase and neuraminidase (Fig. 8B) did not cause additional effects. Figure 8, C and D, summarizes the migration shifts observed to bands a and c, respectively, after the enzymatic treatments.
Fig. 8.
Glycosylation of AeAE in female Aedes Malpighian tubules. Western blots were performed using crude lysates from Malpighian tubules of adult Aedes females. A: lysates were either untreated or exposed to PNGase F (P). B: lysates were either untreated or exposed to one of the following enzymatic treatments: 1) O + N or 2) P + O + N. The anti-AENt antibody was used to detect AeAE immunoreactivity. Labeled arrows indicate the migrations of bands a and c; migrations of the molecular mass markers (in kDa) are indicated at left. In both A and B, diffuse immunoreactivity of ∼250 kDa appears, which may represent aggregates of the AeAE protein. C: summary of the effects of enzymatic deglycosylation on the migration of band a. Shaded bars represent the shift (in kDa) to band a. Values are means ± SE based on the number of measurements shown in parentheses. a,bP < 0.01, categorization of the means as determined by a 1-way ANOVA and Newman-Keuls posttest. D: same experiment described in C, but for band c.
Localization of AeAE Immunoreactivity in Aedes Malpighian Tubules
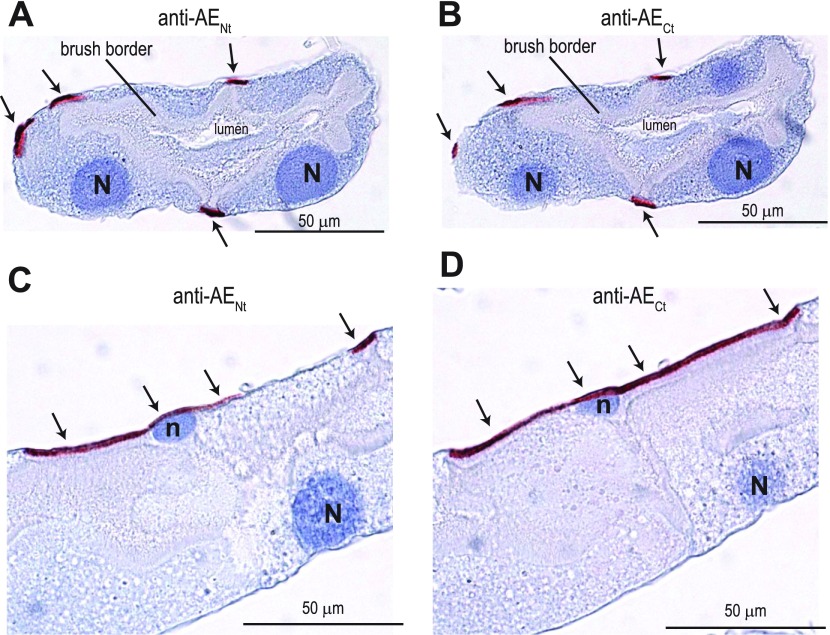
The tubule sections shown in Fig. 9, A and B, demonstrate that both the anti-AENt and anti-AECt antibodies labeled the same regions of the tubule. That is, the immunoreactivity occurred in small strips along the basal aspect of the tubule epithelium (arrows in Fig. 9, A and B). The labeling was clearly not associated with the basal surface of the large principal cells; instead it occurred between principal cells. This pattern is consistent with the localization of AeAE to the stellate cells that intercalate between principal cells in the distal portions of the tubule epithelium (e.g., Ref. 52).
Fig. 9.
Localization of AeAE immunoreactivity in consecutive sections of female Aedes Malpighian tubules. Representative immunoperoxidase labeling of AeAE is shown in isolated Malpighian tubules from adult Aedes females. The antibodies used are indicated. The sections in A and B are from the same Malpighian tubule, taken 4 μm apart, and provide transverse sections through the armlike projections of stellate cells; the sections in C and D are as described in A and B but provide longitudinal sections through the armlike projections of a stellate cell. The arrows indicate red staining associated with the immunolabeling. The blue counterstain (hematoxylin) labels nuclei and provides contrast. N, the nucleus of a principal cell; n, the nucleus of a stellate cell.
The localization of AeAE to stellate cells is verified by the images shown in Fig. 9, C and D, where the section plane occurs through the cell body of a stellate cell. The immunolabeling was subnuclear and occurred along the entire basal surface of the stellate cell (arrows in Fig. 9, C and D). In addition, Fig. 9, C and D, reveals the distinct armlike projections of the stellate cell that radiate laterally from its cell body (see Ref. 52). The narrow striplike appearance of the immunoreactivity shown in Fig. 9, A and B, likely represents cross sections through such armlike projections.
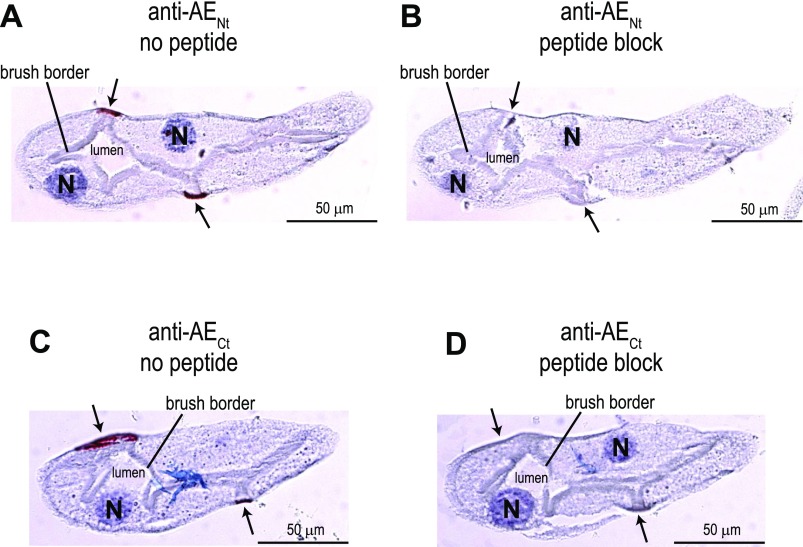
To confirm the specificity of the immunostaining in Aedes Malpighian tubules, we conducted peptide-blocking experiments. Typical experiments are shown in Fig. 10. When either the anti-AENt or anti-AECt antibody was placed on a tubule section in the absence of the respective immunogenic peptide, the labeling of stellate cells was observed (arrows in Fig. 10, A and C). However, when the antibodies were preincubated with their immunogenic peptides, the immunoreactivity in stellate cells was quenched (arrows in Fig. 10, B and D).
Fig. 10.
Effect of antibody preadsorption on AeAE immunolabeling in consecutive sections of Aedes Malpighian tubules. Representative immunoperoxidase labeling of AeAE is shown in isolated Malpighian tubules from adult Aedes females. The antibodies used and the presence of immunogenic peptides are indicated. The sections in A and B are from the same Malpighian tubule, taken 4 μm apart; the sections in C and D are likewise. In A and C, the arrows indicate red staining associated with the immunolabeling. In B and D, the arrows indicate the cells that are stained in A and C, respectively. The blue counterstain (hematoxylin) labels nuclei and provides contrast.
Functional Characterization of AeAE Expressed Heterologously in Xenopus Oocytes
As was shown in Fig. 5, Xenopus oocytes express the AeAE and AeAE-eGFP proteins after injection with their respective cRNAs. Furthermore, the AeAE-eGFP oocytes display a robust fluorescent signal over their entire surface that is not detectable in AeAE or H2O-injected oocytes (data not shown). Since the fluorescence permits noninvasive screening of the oocytes for AeAE expression, we used only AeAE-eGFP oocytes in the following studies for functionally characterizing AeAE.
Effects of extracellular CO2/HCO3− on the steady-state properties of AeAE-expressing and H2O-injected oocytes.
As reported in Table 4, the steady-state pHi of AeAE-expressing (“AeAE” hereafter) and H2O-injected oocytes were similar when acclimated to a solution containing nominal CO2/HCO3−. In contrast, the resting Vm of AeAE oocytes was significantly depolarized by ∼13 mV compared with that of H2O-injected oocytes (Table 4). When acclimated to a solution containing 5% CO2/33 mM HCO3−, AeAE oocytes had both a 1) greater resting pHi by ∼0.1 units and 2) depolarized Vm by ∼14 mV compared with H2O-injected oocytes (Table 4).
Table 4.
Steady-state properties of AeAE-expressing and H2O-injected oocytes acclimated to nominal CO2/HCO3−- or 5% CO2/33 mM HCO3−-containing solutions
| Oocytes | Bath Solution Composition, %CO2/mM HCO3− | pHi | Vm, mV |
|---|---|---|---|
| AeAE | Nominal/nominal | 7.17 ± 0.03 (13) | −34.4 ± 2.7* (13) |
| H2O injected | Nominal/nominal | 7.15 ± 0.02 (10) | −47.7 ± 3.6 (10) |
| AeAE | 5%/33 mM | 6.95 ± 0.02† (12) | −26.9 ± 1.5† (12) |
| H2O injected | 5%/33 mM | 6.84 ± 0.02 (7) | −40.9 ± 3.0 (7) |
Values are means ± SE (n = no. in parentheses) represnting steady-state properties of AeAE-expressing and H2O-injected oocytes acclimated to nominal CO2/HCO3− (solution I, Table 2) or 5% CO2/33 mM HCO3− (solution III, Table 2)-containing solutions.
P < 0.01;
P < 0.001, significant difference from H2O-injected oocytes.
I-V relationships of AeAE and H2O-injected oocytes.
Previous studies in Xenopus oocytes have shown that the heterologous expression of some SLC4 anion exchangers depolarizes the resting Vm due to the presence of an anion conductance intrinsic to the exchanger (19, 51). To determine whether the depolarized resting Vm of AeAE oocytes is due to the presence of such an anion conductance, we examined the I-V relationships of AeAE and H2O-injected oocytes in solutions containing normal (103.6 mM) or low concentrations (0.07 mM) of Cl− (both with nominal CO2/HCO3−).
As shown in Fig. 11, the I-V relationship of the AeAE oocytes in a normal Cl− solution was curvilinear and displayed slightly greater voltage dependence (i.e., conductance) compared with that of H2O-injected oocytes. In particular, the membrane conductance of the AeAE oocytes (compared with that of H2O-injected oocytes) was most distinct at the hyperpolarizing holding potentials (−120 to −80 mV) and less apparent at the depolarizing holding potentials (+20 to +60 mV). These voltage dependences were reflected in the chord conductances of the oocytes calculated at holding potentials (VH) of −120 and +60 mV (Table 5). That is, when VH = −120 mV, the chord conductance of the AeAE oocytes in normal Cl− solution was approximately fourfold greater than that of the H2O-injected oocytes in normal Cl− solution (Table 5). In contrast, when VH = +60 mV, the chord conductance of the AeAE oocytes in normal Cl− solution was only approximately twofold greater than that of the H2O-injected oocytes in normal Cl− solution (Table 5). The reversal potential (Erev) of AeAE oocytes in normal Cl− solution was shifted positive by ∼10 mV compared with that of H2O-injected oocytes in normal Cl− solution (Table 5).
Table 5.
I-V parameters in AeAE and H2O-injected oocytes acclimated to normal Cl− or low-Cl− solutions with nominal CO2/HCO3−
|
gi, nS |
||||
|---|---|---|---|---|
| Oocytes | Solution | VH = −120 mV | VH = +60 mV | Erev, mV |
| AeAE | normal Cl− | 285.4 ± 48.9† | 210.7 ± 28.9* | −32.9 ± 2.3* |
| H2O injected | normal Cl− | 68.1 ± 3.4 | 111.6 ± 4.6 | −43.5 ± 3.1 |
| AeAE | low Cl− | 231.9 ± 38.2† | 124.2 ± 16.3‡ | −32.3 ± 1.9 |
| H2O injected | low Cl− | 58.9 ± 4.5 | 89.8 ± 4.5§ | −38.12 ± 4.0 |
Values are means ± SE (n = 5 oocytes in each group) representing current-voltage (I-V) parameters in AeAE and H2O-injected oocytes acclimated to normal Cl− (solution I, Table 2) or low-Cl− (solution II, Table 2) solutions with nominal CO2/HCO3−. gi, chord conductance at the indicated holding potential (VH) according to Ref. 26.
P < 0.05;
P < 0.01, significant difference from H2O-injected.
P < 0.05;
P < 0.01, significant difference from value in normal Cl− solution.
After the bath was switched to the low-Cl− solution, the chord conductances (when VH = −120 mV) of the AeAE oocytes and H2O-injected oocytes did not change significantly compared with those in normal Cl− (Table 5). In contrast, when VH = +60 mV, the chord conductances of the AeAE and H2O-injected oocytes 1) decreased significantly by ∼40 and ∼30%, respectively, in the low-Cl− solution relative to those in normal Cl− and 2) were not significantly different from one another (Table 5). The Erev values of neither the AeAE oocytes nor the H2O-injected oocytes changed significantly in the low Cl− solution (Table 5).
Sensitivity of AeAE-mediated HCO3− transport to Cl− and DIDS.
When acclimated to the 5% CO2/33 mM HCO3− solution, AeAE oocytes had a resting pHi elevated by ∼0.1 units relative to that of the H2O-injected oocytes (Table 4), consistent with enhanced HCO3− uptake in the AeAE oocytes. To explore the mechanism of this HCO3− uptake in light of the expected anion exchange mediated by AEs of the SLC4 family, we examined the effects of extracellular [Cl−] and DIDS on the pHi and Vm of AeAE oocytes.
A typical experiment is shown in Fig. 12A. The AeAE oocyte was initially superfused with a 5% CO2/33 mM HCO3− solution containing 70.6 mM Cl− (solution III, Table 2) after exposure to this solution for 2 h prior. During interval a (Fig. 12A), the [Cl−] in the bath solution was lowered 1,000-fold by replacement with gluconate (solution IV, Table 2) to create a gradient favoring the exit of Cl− from the cell. As expected for the presence of a Cl/HCO3 exchanger, pHi began to slowly alkalinize (interval a in Fig. 12A), consistent with AeAE-mediated uptake of HCO3− in exchange for Cl−. Regarding Vm, the lowering of [Cl−] caused a small depolarization of ∼5 mV (Fig. 12A), which is consistent with the small effects of lowering extracellular [Cl−] on the I-V parameters of the AeAE oocytes described above (Table 5).
Subsequently, the addition of the stilbene derivate DIDS (200 μM) to the bath abruptly halted the Cl−-dependent alkalinization of pHi and then slowly reversed the pHi trajectory, reflecting intracellular acidification (b in Fig. 12A). Thus DIDS appears to block the uptake of HCO3− mediated by AeAE. Washing out the DIDS (interval c in Fig. 12A) resulted in an immediate reprisal of the alkalinization of pHi, albeit at a slightly greater rate than before (compare intervals a and c in Fig. 12A). Regarding Vm, the addition and washout of DIDS had no detectable effects.
Restoring the normal extracellular [Cl−] after washing out DIDS blunted the alkalinization of pHi initially, and then pHi began to slowly acidify (interval d in Fig. 12A). The solution change also repolarized Vm to the initial voltage (Fig. 12A). The subsequent lowering of [Cl−] allowed the alkalinization of pHi to resume, verifying that the AeAE transporter was still active (Fig. 12A).
Repeating the above protocol on a H2O-injected oocyte had nominal effects on the pHi trajectory (Fig. 12B) compared with those observed in the AeAE oocyte. Regarding Vm, the lowering or restoring of normal extracellular [Cl−] resulted in gradual hyperpolarizations and repolarizations of ∼5 mV, respectively (intervals a and d in Fig. 12B). The addition of DIDS to the bath resulted in a relatively abrupt hyperpolarization of ∼5 mV, which was partially reversible on DIDS washout (intervals b and c in Fig. 12B). These small changes to Vm are similar to those observed in previous studies that have measured the Vm of H2O-injected oocytes after changing extracellular [Cl−] or applying DIDS (34, 50, 55, 83, 84).
Figure 12C summarizes the measurements of rates of pHi change (ΔpHi/Δt) in AeAE (shaded bars) and H2O-injected (open bars) oocytes. The ΔpHi/Δt measurements of AeAE oocytes were significantly greater than those of the H2O-injected oocytes during low extracellular [Cl−] in the absence of DIDS (intervals a and c in Fig. 12C). When DIDS was added or the normal extracellular [Cl−] was restored, the ΔpHi/Δt values for AeAE and H2O-injected oocytes were similar (intervals b and d in Fig. 12C). Together, these data are consistent with AeAE mediating DIDS-sensitive Cl/HCO3 exchange.
Sensitivity of AeAE-mediated alkalinization to Na+.
To examine whether the Cl−-dependent alkalinization of pHi mediated by AeAE is Na+ dependent, we measured pHi in a subset of AeAE oocytes where first the extracellular [Cl−] was lowered to initiate the AeAE-mediated intracellular alkalinization (interval a in Fig. 12D) and then the extracellular [Na+] was lowered 1,000-fold (interval b in Fig. 12D) by replacement with N-methyl-d-glucamine (solution V; Table 2). Of the six AeAE oocytes in this series of experiments, the alkalinization rate of 10.86 ± 1.83 × 10−5 s−1 in the presence of normal extracellular [Na+] was statistically indistinguishable from that of 11.55 ± 2.85 × 10−5 s−1 in the lowered extracellular [Na+] (paired t-test; P = 0.67). These data indicate that the Cl/HCO3 exchange activity of AeAE is not dependent on Na+.
Effects of DIDS on Transepithelial Fluid Secretion in Aedes Malpighian Tubules
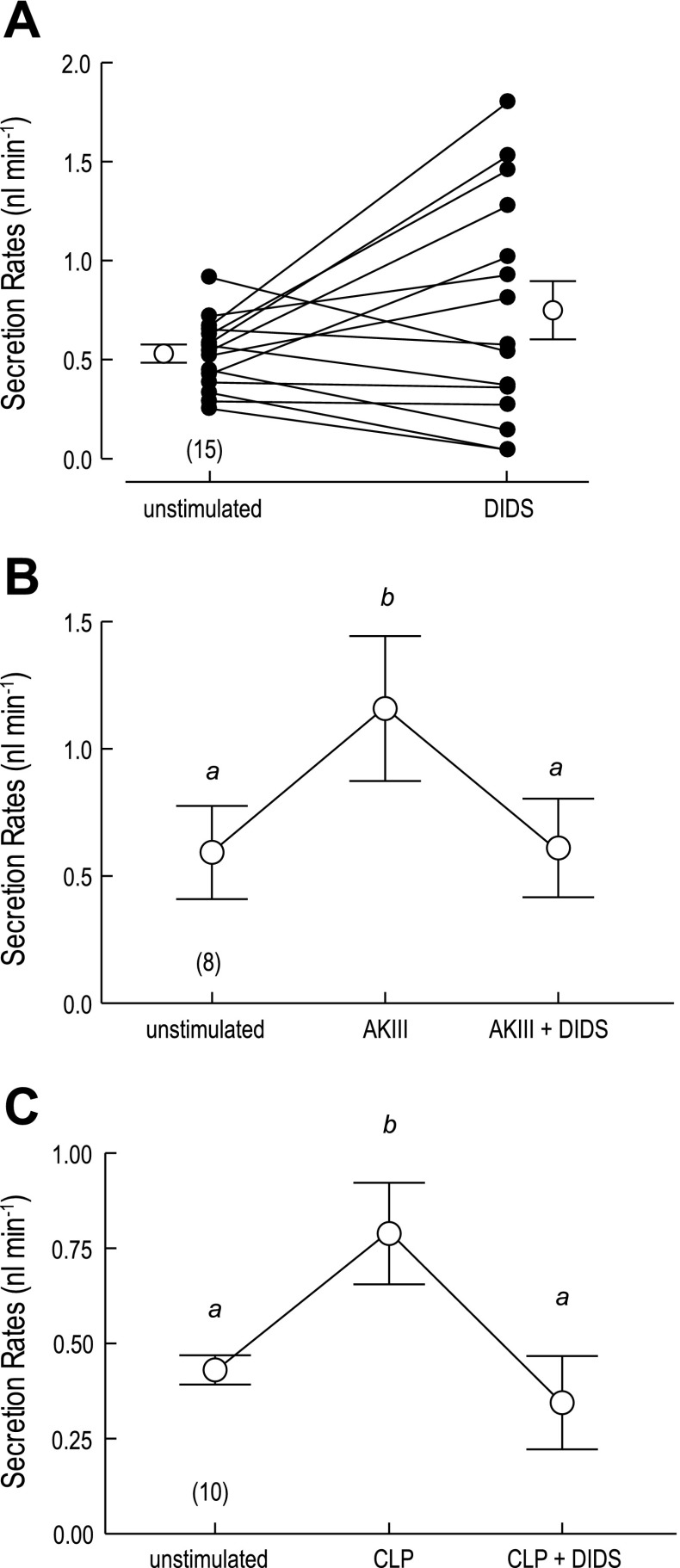
To determine whether AeAE plays a role in transepithelial fluid secretion mediated by Aedes Malpighian tubules, we used the method of Ramsay to examine the effects of DIDS on the rates of fluid secretion. Isolated Malpighian tubules spontaneously secrete fluid at a mean rate of 0.53 ± 0.05 nl/min (open circle above “unstimulated” in Fig. 13A). Adding DIDS to the Ringer bath (200 μM) slightly increased the mean secretion rate to 0.75 ± 0.15 nl/min (open circle above “DIDS” in Fig. 13A), but this increase was not significant (P = 0.12, paired t-test). A closer look at the individual data points for each tubule (filled circles in Fig. 13A) shows that DIDS had variable effects on the unstimulated tubules; i.e., in some cases the unstimulated flow rates were enhanced by adding DIDS, whereas in others the unstimulated flow rates remained the same or decreased. These variable effects of DIDS on unstimulated tubules are similar to those reported previously by us for SITS, another stilbene derivative (25).
Fig. 13.
Effects of DIDS on fluid secretion rates of isolated Aedes Malpighian tubules. A: effect of 200 μM DIDS in unstimulated Malpighian tubules. Lines connect control (unstimulated) and experimental (DIDS) data (filled circles) of each tubule for paired comparisons. Transepithelial fluid secretion rates were measured in the absence and presence of DIDS for 30 min each. Open circles indicate mean (±SE) secretion rates. The number of paired tubule measurements is shown in parenthesis. B: effect of 200 μM DIDS on the diuresis stimulated by aedeskinin III (AKIII; 10−6 M). Each Malpighian tubule was first studied for 30 min under unstimulated conditions, then in the presence of AKIII, and finally in the presence of AKIII and DIDS. C: effect of 200 μM DIDS on the diuresis stimulated by Anopheles calcitonin-like peptide (CLP; 10−6 M) following the experimental protocol in B. a,bP < 0.01, categorization of the means as determined by a repeated-measures ANOVA and Newman-Keuls posttest.
We next examined the effects of DIDS on tubules stimulated with the diuretic peptide AKIII; the second messenger of such kinin diuretic peptides is Ca2+ (92). As shown in Fig. 13B, adding AKIII to the peritubular Ringer bath (10−6 M) significantly increased the mean fluid secretion rate from 0.59 ± 0.18 nl/min in the unstimulated period to 1.16 ± 0.28 nl/min in the presence of AKIII. The subsequent addition of DIDS to the Ringer bath (200 μM) significantly reduced the mean fluid secretion rate to 0.61 ± 0.19 nl/min (Fig. 13B), which is not significantly different from the unstimulated rates of secretion.
A similar effect of DIDS on reversing diuretic rates of fluid secretion was observed when the diuresis was induced with the CLP of A. gambiae (10−6 M); the second messenger of CLP is cAMP (15). As shown in Fig. 13C, adding CLP to the peritubular Ringer bath significantly increased the mean fluid secretion rate from 0.41 ± 0.03 to 0.81 ± 0.15 nl/min. The subsequent addition of DIDS to the Ringer bath significantly reduced the mean fluid secretion rate to 0.38 ± 0.13 nl/min (Fig. 13C), which, again, is not significantly different from the unstimulated rates of secretion.
DISCUSSION
Cloning of the AeAE cDNA and Elucidation of the AeAE Gene
We have cloned a full-length cDNA from Aedes Malpighian tubules that encodes a SLC4-like Na+-independent AE, which to our knowledge is the first such AE to be cloned from the Malpighian tubules of any insect. Remarkably, the gene behind the ∼4.4-kb AeAE transcript is over 136.5 kb in length and includes an intron of over 112 kb (Fig. 1 and Table 3). To put the size of this intron in perspective, the entire orthologous gene in Drosophila (CG8177) spans just over 22.7 kb (//www.ncbi.nlm.nih.gov/projects/mapview), and the entire gene of human AE2 spans only ∼17 kb (42). The large intron in Aedes likely explains why the initial computer-based prediction of the AeAE gene (GenBank accession no. XM_001652813) failed to associate exons 1 and 2 with this gene.
A length of the AeAE gene that is approximately sixfold greater than that of the Drosophila gene CG8177 is consistent with the genomic findings of Nene et al. (44), who reported that on average, the protein-coding genes of Aedes are four to six times longer than those of Drosophila. The increased gene lengths in Aedes are primarily attributed to the accumulation of transposable elements within introns (44). The large intron separating exons 2 and 3 of AeAE is a case in point.
AeAE Protein is Glycosylated
Heterologous expression in Xenopus oocytes.
As demonstrated by the Western blots in Fig. 5, Xenopus oocytes readily translate the AeAE and AeAE-eGFP cRNAs into their respective proteins. Notably, both proteins are detected as broad bands of immunoreactivity that begin at molecular masses ∼30 kDa greater than expected. This finding, combined with the observation that the AeAE primary sequence contains a total of four putative N-glycosylation sties (Figs. 3B and 4), led us to investigate whether Xenopus oocytes glycosylate the AeAE protein. The glycosylation studies in Fig. 6 demonstrate that the AeAE protein is both N-glycosylated and O-glycosylated by Xenopus oocytes. Interestingly, the data suggest that the removal of N-linked glycans from AeAE with PNGase F is necessary before the O-linked glycans can be accessed by the O-glycosidase and neuraminidase.
Expression in Aedes Malpighian tubules.
In contrast to the AeAE expressed heterologously in Xenopus oocytes, the AeAE protein expressed in Malpighian tubules migrates on SDS-PAGE as two distinct immunoreactive forms: the 150-kDa band a and the 111-kDa band c (Fig. 7A). Both of these forms possess the epitopes recognized by the anti-AENt and anti-AECt antibodies (Fig. 7A), which indicates that the 111-kDa band is not a proteolytic fragment of the 150-kDa band.
The denaturation experiments in Malpighian tubules (Fig. 7B and Supplemental Fig. 3) suggest that the 111-kDa band represents a pool of the 150-kDa AeAE protein with a robust secondary or tertiary structure not fully denatured under the standard Laemmli conditions (i.e., SDS, a reducing agent, 100°C), resulting in its anomalous migration on SDS-PAGE (e.g., see Ref. 59). When the denaturation of Malpighian tubule proteins is enhanced by adding a high-urea buffer, the 111-kDa Laemmli-resistant form of AeAE appears to fully denature, because the AeAE immunoreactivity occurs as a single, discrete 150-kDa band (Fig. 7B). In contrast, when the denaturation of tubule proteins is weakened by lowering the temperature from 100°C to 37°C, both the 111-kDa Laemmli-resistant and the 150-kDa Laemmli-sensitive forms of AeAE appear to run anomalously on SDS-PAGE as indicated by their broad, diffuse immunoreactivities near 111 and 150 kDa, respectively (Supplemental Fig. 3).
The glycosidase experiments in Malpighian tubules (Fig. 8) indicate that the sensitivity of AeAE to the standard Laemmli denaturation (at 100°C) is associated with its glycosylation state; the 150-kDa Laemmli-sensitive form of AeAE is O-glycosylated but not N-glycosylated, whereas the 111-kDa Laemmli-resistant form is N-glycosylated (Fig. 8). We cannot rule out the possibility that the latter form is also O-glycosylated, because it is not completely denatured under the conditions of the glycosidase treatment; i.e., potential O-linked glycans may be sterically “hidden” from the O-glycosidase and/or neuraminidase. However, the above results indicate that the presence of N-glycosylation is unique to the 111-kDa Laemmli-resistant form of AeAE. It remains to be determined whether this Laemmli-resistant, N-glycosylated form of AeAE represents 1) a pool of the AeAE protein that is processed independently of the Laemmli-sensitive, O-glycosylated form or 2) a downstream maturational state of the Laemmli-sensitive, O-glycosylated form.
Sites of glycosylation.
Our bioinformatic analyses indicate that one predicted N-glycosylation site occurs in the short 3–4 loop of AeAE, whereas three such sites occur in the longer 5–6 loop (Figs. 3B and 4). In view of its short length, the 3–4 loop is unlikely to serve as a substrate for N-glycosylation (37). Instead, at least one of the predicted sites along the 5–6 loop is likely to be N-glycosylated, especially since this loop is N-glycosylated in other SLC4 proteins such as vertebrate NBCe1, mammalian NBCn2 (SLC4A10), mammalian AE2, and trout AE1 (12, 14, 27, 95). In human AE1, the 7–8 loop is N-glycosylated (30, 78), but predicted N-glycosylation sites do not occur in this loop of AeAE (Figs. 3B and 4).
We are unaware of reliable algorithms to predict the location of O-linked glycosylation sites on insect proteins. However, the 5–6 loop may provide a suitable substrate given the presence of several serine and threonine residues on the 5–6 loop of AeAE (Fig. 4). Moreover, the 5–6 loops of amphibian NBCe1 and mammalian AE2 are thought to be sites of O-glycosylation (14, 95, 96).
In general, the functional significance of glycosylation to SLC4 proteins is not well understood and appears to be protein specific. For mammalian AE1 and NBCe1, glycosylation is not necessary for its functional expression (11, 14), but the N-glycosylation of AE1 has been suggested to facilitate its correct folding in the plasma membrane (22). In contrast, the functional expression of mammalian NBCn2 (SLC4A10) requires its full glycosylation (12).
AeAE Immunoreactivity is Enriched in Stellate Cells of Aedes Malpighian Tubules
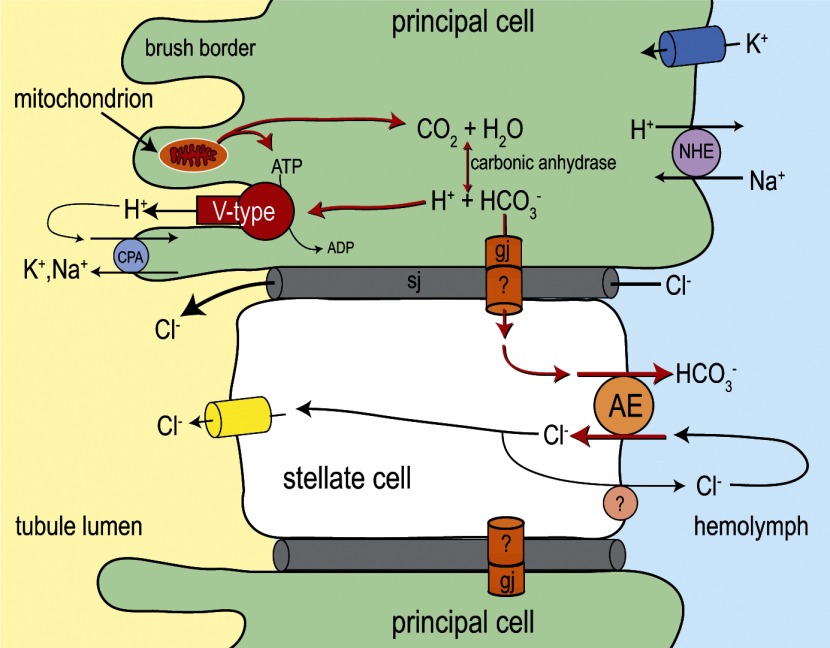
We have located the expression of AeAE immunoreactivity in Malpighian tubules to the stellate cells and not the principal cells. The presence of detectable AeAE immunoreactivity in only stellate cells is validated by 1) the identical staining patterns produced by the anti-AENt and anti-AECt antibodies (Fig. 9) and 2) the blocking of stellate cell immunolabeling by preincubating the anti-AE antibodies with their respective immunogenic peptides (Fig. 10). As shown in Fig. 9, the localization of AeAE in stellate cells is clearly basal, likely in the extensive basal membrane infoldings and/or vesicles near the basal membrane (4, 45). To our knowledge, AeAE is the first example of an exchange-mediated transporter to be expressed exclusively in stellate cells of mosquito Malpighian tubules.
Functional Characterization of AeAE Protein in Xenopus Oocytes
Lack of an anion conductance.
The Xenopus oocytes expressing AeAE-eGFP (hereafter referred to as AeAE oocytes) are characterized by a Vm that is significantly depolarized relative to that of H2O-injected oocytes, which led us to examine whether AeAE possesses an intrinsic anion channel. Such a channel has been observed in Xenopus oocytes expressing trout AE1 (19) or human AE1Δ(6:7) (51); the latter protein is an engineered construct of human AE1 missing TM segments 6 and 7 (51). Although AeAE oocytes exhibit significantly greater chord conductances and positively shifted Erev values compared with H2O-injected oocytes (Fig. 11 and Table 5), we are reluctant to consider these differences as evidence of an intrinsic channel within AeAE for the following reasons.
First, the absolute magnitudes of the whole cell currents are rather small, <25 nA in our study (Fig. 11) compared with several microamperes in oocytes expressing trout AE1 or human AE1Δ(6:7) (19, 51). Second, the chord conductances of the AeAE oocytes are small, only two- to fourfold greater than those of H2O-injected oocytes (Fig. 11 and Table 5). In contrast, the slope conductances of oocytes expressing trout AE1 or human AE1Δ(6:7) are 25- to 100-fold greater than those of H2O-injected oocytes (19, 51). Third, reducing the extracellular [Cl−] has no effect on the Erev of AeAE oocytes and a minimal effect on chord conductances (Table 5). In contrast, removing extracellular Cl− from oocytes expressing human AE1Δ(6:7) shifts their Erev by ∼23 mV to more cell-positive values and blunts their slope conductance at depolarizing holding potentials (51).
The above observations suggest that the depolarization of Vm and increased membrane conductance in AeAE oocytes do not stem from an intrinsic anion conductance. The changes more likely reflect a slightly increased “background” conductance or “leak” current, such as that observed in Xenopus oocytes heterologously expressing other SLC4 proteins from mammals and insects (62, 64, 84).
Cl−/HCO3− exchange.
The bioinformatic analyses of the AeAE amino acid sequence (and other known AEs) led us to hypothesize that AeAE is a Cl/HCO3 exchanger that is sensitive to DIDS and independent of Na+ gradients. Our experimental observations of pHi in AeAE oocytes (Table 4 and Fig. 12) support this hypothesis. First, the mean resting pHi of AeAE oocytes acclimated to a 5% CO2/33 mM HCO3− solution is significantly higher than that of comparably treated H2O-injected oocytes (Table 4), which reflects an enhanced uptake of extracellular HCO3− in the AeAE oocytes. Similar findings occur in the resting pHi of Xenopus oocytes heterologously expressing human AE1 (51) and zebrafish AE2 (66) when bathed in solutions containing CO2/HCO3−.
Second, as expected from a Cl/HCO3 exchanger, reducing the extracellular [Cl−] in the presence of 5% CO2/33 mM HCO3− alkalinizes the pHi of AeAE oocytes (Fig. 12). The Cl−-induced intracellular alkalinization is a classic feature of SLC4 Cl/HCO3 exchangers (33, 40, 51, 63, 66, 68). Moreover, the Cl−-induced alkalinization is 1) blocked by DIDS, 2) reversed by raising extracellular [Cl−], and 3) impervious to extracellular [Na+] (Fig. 12). Thus AeAE functions as a Na+-independent, DIDS-sensitive Cl/HCO3 exchanger. Changes in extracellular [Cl−] and the addition and removal of DIDS have minimal effects on the Vm of AeAE oocytes, indicating that AeAE mediates electroneutral anion exchange.
DIDS Blocks Fluid Secretion in Stimulated Malpighian Tubules
In isolated Aedes Malpighian tubules, adding 200 μM DIDS to the peritubular Ringer solution, a concentration that blocks the activity of AeAE expressed in Xenopus oocytes, does not have significant effects on the mean fluid secretion rates of unstimulated tubules (Fig. 13A). However, once tubules are stimulated to secrete fluid at diuretic rates by adding either AKIII or CLP, DIDS significantly inhibits fluid secretion rates to the levels of unstimulated tubules (Fig. 13, B and C). These effects of DIDS on Aedes Malpighian tubules are analogous to those of bumetanide, which does not affect the secretion rates of unstimulated tubules but blocks the enhanced secretion rates induced by dibutyryl-cAMP (25). As described below, the similar effects of DIDS on Aedes Malpighian tubules stimulated with either AKIII or CLP suggest that the transport activity of AeAE in stellate cells is associated with regulating the enhanced metabolism of the tubule epithelium under diuretic conditions.
Hypothetical Role of AeAE in Malpighian Tubule Physiology
A striking result of the present study is the exclusive localization of AeAE immunoreactivity to stellate cells of Aedes Malpighian tubules (Figs. 9 and 10). This finding raises questions about the physiological role of AeAE in the basal membrane of stellate cells. On first reflection, stellate cells do not appear to contribute much to transepithelial secretion, because principal cells are five times more numerous and considerably larger than stellate cells in Aedes Malpighian tubules (7). However, our previous discovery of Cl− channels in apical membranes of stellate cells (45) now takes on significance when viewed together with the basal Cl/HCO3 exchanger discovered in this study.
As illustrated in Fig. 14, the two transport systems provide a potential transcellular pathway for the transepithelial secretion of Cl−. That is, Cl− enters the stellate cell from the hemolymph via electroneutral exchange with HCO3− and then exits the cell into the tubule lumen through apical membrane Cl− channels, supporting the proposed model of transcellular Cl− secretion by stellate cells in Drosophila Malpighian tubules (46). Whether this model applies to transepithelial Cl− secretion by Aedes Malpighian tubules is questionable. For one reason, DIDS, the known blocker of AeAE transport, has on average no effect on unstimulated rates of transepithelial fluid secretion (Fig. 13A). For another reason, electrophysiological correlates of diuretic fluid secretion, such as those induced by kinin diuretic peptides, are observed in very short segments of isolated perfused Malpighian tubules that contain only two or three principal cells and no stellate cells (91). Thus the activity of AeAE in stellate cells does not appear to be an important route for transepithelial Cl− secretion in Aedes Malpighian tubules.
Fig. 14.
Hypothesized metabolic model of stellate cell function in Aedes Malpighian tubules. The illustration shows a stellate cell intercalated between 2 principal cells. As emphasized by the red arrows, we propose that AeAE contributes primarily to the handling of intracellular HCO3− that is generated by the mitochondrial metabolism of principal cells during diuretic conditions. AeAE also may contribute to a transcellular pathway for Cl− secretion, but this is considered a minor role (see text for details). A recycling mechanism for Cl− across the basal membrane of stellate cells cannot be ruled out. Principal and stellate cells are shown to be connected via 1) paracellular septate junctions (sj), which provide the primary pathway for transepithelial Cl− secretion (3), and 2) intercellular gap junctions (gj), which provide a putative pathway for the transport of HCO3− to stellate cells. Previous studies in mosquito Malpighian tubules have shown evidence of an apical V-type H+-ATPase in the brush border of principal cells (52, 86), a carbonic anhydrase in principal cells (67), gap junctions in principal cells (87), basal K+ channels in principal cells (6), a basal Na/H exchanger (NHE) in principal cells (53, 57), and apical Cl− channels in stellate cells (45). The molecular identity of the apical cation/H+ antiporter (CPA) has yet to be identified (56). See Ref. 7 for a detailed review of the transepithelial transport mechanisms in mosquito Malpighian tubules.
Ruling out stellate cells as a transepithelial pathway for Cl− secretion begs an explanation for why DIDS only inhibits diuretic rates of transepithelial fluid secretion (Fig. 13, B and C). Significantly, DIDS does not inhibit transepithelial fluid secretion completely; it inhibits only the diuretic increase of fluid secretion, regardless of the diuretic agent (i.e., AKIII or CLP). Since kinin diuretic peptides target an increase in transepithelial Cl− secretion via the second messenger Ca2+ (92), and CLP targets the increase in transepithelial Na+ secretion via the second messenger cAMP (15), the common reversal of the diuretic secretion rates by DIDS may reflect inhibition of a stimulatory mechanism common to both types of diuresis, such as the increased rates of cellular metabolism during diuretic fluid secretion.
In particular, elevated rates of metabolism associated with diuresis reflect increased rates of ATP synthesis by mitochondria for fueling the apical V-type H+-ATPase (79). As shown in Fig. 14, increased rates of mitochondrial respiration generate CO2, and consequently, carbonic acid, which hydrolyzes into H+ and HCO3− via carbonic anhydrase. Petzel et al. (54) suggested that the metabolic generation of CO2 may explain the acidification of principal cells they observed in Aedes Malpighian tubules stimulated by dibutyryl-cAMP. Since H+ is a substrate of the apical V-type H+-ATPase, the acidification of principal cells is expected to stimulate this proton pump and thereby the extrusion of cations into the tubule lumen (54).
Petzel et al. (54) reasoned further that the activation of a mechanism that removes metabolic HCO3− from principal cells may support the cellular acidification, thereby promoting the stimulation of the V-type H+-ATPase and diuretic rates of fluid secretion. In the present study, we have provided evidence for such a HCO3− extrusion mechanism in the stellate cells of Malpighian tubules, i.e., the Cl/HCO3 exchanger AeAE. The absence of this transporter in principal cells suggests that stellate cells may be metabolically coupled to principal cells to support the elevated metabolism during diuresis. As illustrated in Fig. 14, HCO3− produced in principal cells may be transported to stellate cells through gap junctions for its absorption via AeAE. It remains to be determined whether the gap junctions that we have detected between principal cells (87) also connect principal cells with stellate cells.
Perspectives and Significance
In conclusion, the present study demonstrates that stellate cells of Aedes Malpighian tubules express a SLC4-like, DIDS-sensitive Cl/HCO3 exchanger, AeAE. In addition, our findings point to a novel role of stellate cells and AeAE in the metabolic regulation of intracellular pH (and thereby V-type H+-ATPase activity) in the Malpighian tubule epithelium during diuretic fluid secretion. Thus AeAE may play a critical role in the renal processing of blood meals taken by female mosquitoes, which makes it a potentially intriguing molecule to target for genetic and/or chemical pest management strategies. Future studies examining the possibility of functional cross talk between stellate cells and principal cells in mosquito Malpighian tubules are bound to provide exciting new insights into the mechanisms of transepithelial fluid transport in this epithelium as well as the regulation of V-type H+-ATPase activity in other transporting epithelia.
GRANTS
Financial support from the following sources made this work possible: National Institute of Diabetes and Digestive and Kidney Diseases Grant K01 DK080194-01 (to P. M. Piermarini) and National Science Foundation Grant IBN 0078058 (to K. W. Beyenbach). The contributions of L. F. Grogan were in part supported by the Cornell University Summer Veterinary Leadership Program. The contributions of L. Wang were conducted as part of an undergraduate honor's thesis.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank the laboratory of Prof. William A. Horne (Clinical Sciences) at the Cornell University College of Veterinary Medicine for generously supplying the Xenopus oocytes used in this study. We are also indebted to the laboratory of Dean Michael I. Kotlikoff (Cornell University College of Veterinary Medicine) for providing equipment used in Western blotting. Last, we thank Patricia Fisher (Cornell University), Mary Lou Norman (Cornell University), and Kimberly A. Schiller (Cornell Veterinary Leadership Program) for excellent technical assistance; Michael G. Janech (Medical University of South Carolina), Andrea Quaroni (Cornell University), and Laura Sirot (Cornell University) for invaluable consultations regarding the interpretation of Western blots; and Mark D. Parker (Case Western Reserve) for helpful insights regarding the electrophysiological characterization of anion exchangers in Xenopus oocytes.
REFERENCES
- 1. Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol 212: 1672–1683, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beyenbach KW. Energizing epithelial transport with the vacuolar H+-ATPase. News Physiol Sci 16: 145–151, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Beyenbach KW. Regulation of tight junction permeability with switch-like speed. Curr Opin Nephrol Hypertens 12: 543–550, 2003. [DOI] [PubMed] [Google Scholar]
- 4. Beyenbach KW. Transport mechanisms of diuresis in Malpighian tubules of insects. J Exp Biol 206: 3845–3856, 2003. [DOI] [PubMed] [Google Scholar]
- 5. Beyenbach KW, Baumgart S, Lau K, Piermarini PM, Zhang S. Signaling to the apical membrane and to the paracellular pathway: changes in the cytosolic proteome of Aedes Malpighian tubules. J Exp Biol 212: 329–340, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beyenbach KW, Masia R. Membrane conductances of principal cells in Malpighian tubules of Aedes aegypti. J Insect Physiol 48: 375–386, 2002. [DOI] [PubMed] [Google Scholar]
- 7. Beyenbach KW, Piermarini PM. Osmotic and ionic regulation in insects. In: Osmotic and Ionic Regulation: Cells and Animals, edited by Evans DH. Boca Raton, FL: CRC, 2009, p. 231–293. [Google Scholar]
- 8. Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol 212: 1697–1706, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown D, Breton S. Mitochondria-rich, proton-secreting epithelial cells. J Exp Biol 199: 2345–2358, 1996. [DOI] [PubMed] [Google Scholar]
- 10. Brown D, Smith PJ, Breton S. Role of V-ATPase-rich cells in acidification of the male reproductive tract. J Exp Biol 200: 257–262, 1997. [DOI] [PubMed] [Google Scholar]
- 11. Casey JR, Pirraglia CA, Reithmeier RA. Enzymatic deglycosylation of human band 3, the anion transport protein of the erythrocyte membrane. Effect on protein structure and transport properties. J Biol Chem 267: 11940–11948, 1992. [PubMed] [Google Scholar]
- 12. Chen LM, Kelly ML, Rojas JD, Parker MD, Gill HS, Davis BA, Boron WF. Use of a new polyclonal antibody to study the distribution and glycosylation of the sodium-coupled bicarbonate transporter NCBE in rodent brain. Neuroscience 151: 374–385, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheung JC, Li J, Reithmeier RA. Topology of transmembrane segments 1–4 in the human chloride/bicarbonate anion exchanger 1 (AE1) by scanning N-glycosylation mutagenesis. Biochem J 390: 137–144, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi I, Hu L, Rojas JD, Schmitt BM, Boron WF. Role of glycosylation in the renal electrogenic Na+-HCO3− cotransporter (NBCe1). Am J Physiol Renal Physiol 284: F1199–F1206, 2003. [DOI] [PubMed] [Google Scholar]
- 15. Coast GM, Garside CS, Webster SG, Schegg KM, Schooley DA. Mosquito natriuretic peptide identified as a calcitonin-like diuretic hormone in Anopheles gambiae (Giles). J Exp Biol 208: 3281–3291, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Combet C, Blanchet C, Geourjon C, Deleage G. NPS@: network protein sequence analysis. Trends Biochem Sci 25: 147–150, 2000. [DOI] [PubMed] [Google Scholar]
- 17. Ehrenfeld J, Klein U. The key role of the H+ V-ATPase in acid-base balance and Na+ transport processes in frog skin. J Exp Biol 200: 247–256, 1997. [DOI] [PubMed] [Google Scholar]
- 18. Espiritu DJ, Bernardo AA, Robey RB, Arruda JA. A central role for Pyk2-Src interaction in coupling diverse stimuli to increased epithelial NBC activity. Am J Physiol Renal Physiol 283: F663–F670, 2002. [DOI] [PubMed] [Google Scholar]
- 19. Fievet B, Gabillat N, Borgese F, Motais R. Expression of band 3 anion exchanger induces chloride current and taurine transport: structure-function analysis. EMBO J 14: 5158–5169, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. J Biol Chem 276: 8358–8363, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Gross E, Hawkins K, Pushkin A, Sassani P, Dukkipati R, Abuladze N, Hopfer U, Kurtz I. Phosphorylation of Ser982 in the sodium bicarbonate cotransporter kNBC1 shifts the HCO3−: Na+ stoichiometry from 3:1 to 2:1 in murine proximal tubule cells. J Physiol 537: 659–665, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groves JD, Tanner MJ. Role of N-glycosylation in the expression of human band 3-mediated anion transport. Mol Membr Biol 11: 31–38, 1994. [DOI] [PubMed] [Google Scholar]
- 23. Gutierrez AM, Hernandez CS, Whittembury G. A model for fluid secretion in Rhodnius upper Malpighian tubules (UMT). J Membr Biol 202: 105–114, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98, 1999. [Google Scholar]
- 25. Hegarty JL, Zhang B, Pannabecker TL, Petzel DH, Baustian MD, Beyenbach KW. Dibutyryl cAMP activates bumetanide-sensitive electrolyte transport in Malpighian tubules. Am J Physiol Cell Physiol 261: C521–C529, 1991. [DOI] [PubMed] [Google Scholar]
- 26. Helman SI, Thompson SM. Interpretation and use of electrical equivalent circuits in studies of epithelial tissues. Am J Physiol Renal Fluid Electrolyte Physiol 243: F519–F531, 1982. [DOI] [PubMed] [Google Scholar]
- 27. Hubner S, Michel F, Rudloff V, Appelhans H. Amino acid sequence of band-3 protein from rainbow trout erythrocytes derived from cDNA. Biochem J 285: 17–23, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Humphreys BD, Jiang L, Chernova MN, Alper SL. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. Am J Physiol Cell Physiol 267: C1295–C1307, 1994. [DOI] [PubMed] [Google Scholar]
- 29. Ianowski JP, O'Donnell MJ. Electrochemical gradients for Na+, K+, Cl− and H+ across the apical membrane in Malpighian (renal) tubule cells of Rhodnius prolixus. J Exp Biol 209: 1964–1975, 2006. [DOI] [PubMed] [Google Scholar]
- 30. Jay DG. Glycosylation site of band 3, the human erythrocyte anion-exchange protein. Biochemistry 25: 554–556, 1986. [DOI] [PubMed] [Google Scholar]
- 31. Jiang L, Stuart-Tilley A, Parkash J, Alper SL. pHi and serum regulate AE2-mediated Cl−/HCO3− exchange in CHOP cells of defined transient transfection status. Am J Physiol Cell Physiol 267: C845–C856, 1994. [DOI] [PubMed] [Google Scholar]
- 32. Kietz D, Bartel D, Lepke S, Passow H. pH-dependence of inhibition by H2DIDS of mouse erythroid band 3-mediated Cl− transport in Xenopus oocytes. The effect of oligonucleotide-directed replacement of Lys-558 by an Asn residue. Biochim Biophys Acta 1064: 81–88, 1991. [DOI] [PubMed] [Google Scholar]
- 33. Kopito RR, Lee BS, Simmons DM, Lindsey AE, Morgans CW, Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell 59: 927–937, 1989. [DOI] [PubMed] [Google Scholar]
- 34. Kurita Y, Nakada T, Kato A, Doi H, Mistry AC, Chang MH, Romero MF, Hirose S. Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am J Physiol Regul Integr Comp Physiol 294: R1402–R1412, 2008. [DOI] [PubMed] [Google Scholar]
- 35. Kurschat CE, Shmukler BE, Jiang L, Wilhelm S, Kim EH, Chernova MN, Kinne RK, Stewart AK, Alper SL. Alkaline-shifted pHo sensitivity of AE2c1-mediated anion exchange reveals novel regulatory determinants in the AE2 N-terminal cytoplasmic domain. J Biol Chem 281: 1885–1896, 2006. [DOI] [PubMed] [Google Scholar]
- 36. Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 37. Landolt-Marticorena C, Reithmeier RA. Asparagine-linked oligosaccharides are localized to single extracytosolic segments in multi-span membrane glycoproteins. Biochem J 302: 253–260, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2. Bioinformatics 23: 2947–2948, 2007. [DOI] [PubMed] [Google Scholar]
- 39. Lee BS, Gunn RB, Kopito RR. Functional differences among nonerythroid anion exchangers expressed in a transfected human cell line. J Biol Chem 266: 11448–11454, 1991. [PubMed] [Google Scholar]
- 40. Lindsey AE, Schneider K, Simmons DM, Baron R, Lee BS, Kopito RR. Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc Natl Acad Sci USA 87: 5278–5282, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lu J, Boron WF. Reversible and irreversible interactions of DIDS with the human electrogenic Na/HCO3 cotransporter NBCe1-A: role of lysines in the KKMIK motif of TM5. Am J Physiol Cell Physiol 292: C1787–C1798, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Medina JF, Acin A, Prieto J. Molecular cloning and characterization of the human AE2 anion exchanger (SLC4A2) gene. Genomics 39: 74–85, 1997. [DOI] [PubMed] [Google Scholar]
- 43. Nazeer K, Janech MG, Lin JJ, Ryan KJ, Arthur JM, Budisavljevic MN. Changes in protein profiles during course of experimental glomerulonephritis. Am J Physiol Renal Physiol 296: F186–F193, 2009. [DOI] [PubMed] [Google Scholar]
- 44. Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, LI S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O'Leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316: 1718–1723, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Connor KR, Beyenbach KW. Chloride channels in apical membrane patches of stellate cells of Malpighian tubules of Aedes aegypti. J Exp Biol 204: 367–378, 2001. [DOI] [PubMed] [Google Scholar]
- 46. O'Donnell MJ, Rheault MR, Davies SA, Rosay P, Harvey BJ, Maddrell SH, Kaiser K, Dow JA. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol Regul Integr Comp Physiol 274: R1039–R1049, 1998. [DOI] [PubMed] [Google Scholar]
- 47. Okubo K, Kang D, Hamasaki N, Jennings ML. Red blood cell band 3. Lysine 539 and lysine 851 react with the same H2DIDS (4,4′-diisothiocyanodihydrostilbene-2,2′-disulfonic acid) molecule. J Biol Chem 269: 1918–1926, 1994. [PubMed] [Google Scholar]
- 48. Pannabecker TL, Hayes TK, Beyenbach KW. Regulation of epithelial shunt conductance by the peptide leucokinin. J Membr Biol 132: 63–76, 1993. [DOI] [PubMed] [Google Scholar]
- 49. Parker MD, Bouyer P, Daly CM, Boron WF. Cloning and characterization of novel human SLC4A8 gene products encoding Na+-driven Cl−/HCO3− exchanger variants NDCBE-A, -C, and -D. Physiol Genomics 34: 265–276, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parker MD, Tanner MJ. The disruption of the third extracellular loop of the red cell anion exchanger AE1 does not affect electroneutral Cl−/HCO3− exchange activity. Blood Cells Mol Dis 32: 379–383, 2004. [DOI] [PubMed] [Google Scholar]
- 51. Parker MD, Young MT, Daly CM, Meech RW, Boron WF, Tanner MJ. A conductive pathway generated from fragments of the human red cell anion exchanger AE1. J Physiol 581: 33–50, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Patrick ML, Aimanova K, Sanders HR, Gill SS. P-type Na+/K+-ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. J Exp Biol 209: 4638–4651, 2006. [DOI] [PubMed] [Google Scholar]
- 53. Petzel DH. Na+/H+ exchange in mosquito Malpighian tubules. Am J Physiol Regul Integr Comp Physiol 279: R1996–R2003, 2000. [DOI] [PubMed] [Google Scholar]
- 54. Petzel DH, Pirotte PT, Van Kerkhove E. Intracellular and luminal pH measurements of Malpighian tubules of the mosquito Aedes aegypti: the effects of cAMP. J Insect Physiol 45: 973–982, 1999. [DOI] [PubMed] [Google Scholar]
- 55. Piermarini PM, Choi I, Boron WF. Cloning and characterization of an electrogenic Na/HCO3− cotransporter from the squid giant fiber lobe. Am J Physiol Cell Physiol 292: C2032–C2045, 2007. [DOI] [PubMed] [Google Scholar]
- 56. Piermarini PM, Weihrauch D, Meyer H, Huss M, Beyenbach KW. NHE8 is an intracellular cation/H+ exchanger in renal tubules of the yellow fever mosquito Aedes aegypti. Am J Physiol Renal Physiol 296: F730–F750, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pullikuth AK, Aimanova K, Kang'ethe W, Sanders HR, Gill SS. Molecular characterization of sodium/proton exchanger 3 (NHE3) from the yellow fever vector, Aedes aegypti. J Exp Biol 209: 3529–3544, 2006. [DOI] [PubMed] [Google Scholar]
- 58. Ramsay JA. Active transport of potassium by the Malpighian tubules of insects. J Exp Biol 93: 358–369, 1953. [Google Scholar]
- 59. Rath A, Glibowicka M, Nadeau VG, Chen G, Deber CM. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc Natl Acad Sci USA 106: 1760–1765, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol 274: F425–F432, 1998. [DOI] [PubMed] [Google Scholar]
- 61. Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflügers Arch 447: 495–509, 2004. [DOI] [PubMed] [Google Scholar]
- 62. Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM. Cloning and characterization of a Na+-driven anion exchanger (NDAE1). A new bicarbonate transporter. J Biol Chem 275: 24552–24559, 2000. [DOI] [PubMed] [Google Scholar]
- 63. Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Immunolocalization of the electrogenic Na+-HCO3− cotransporter in mammalian and amphibian kidney. Am J Physiol Renal Physiol 276: F27–F38, 1999. [DOI] [PubMed] [Google Scholar]
- 64. Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney electrogenic Na+-HCO3− cotransporter, rkNBC, expressed in oocytes. Am J Physiol Renal Physiol 277: F611–F623, 1999. [DOI] [PubMed] [Google Scholar]
- 65. Sciortino CM, Shrode LD, Fletcher BR, Harte PJ, Romero MF. Localization of endogenous and recombinant Na+-driven anion exchanger protein NDAE1 from Drosophila melanogaster. Am J Physiol Cell Physiol 281: C449–C463, 2001. [DOI] [PubMed] [Google Scholar]
- 66. Shmukler BE, Kurschat CE, Ackermann GE, Jiang L, Zhou Y, Barut B, Stuart-Tilley AK, Zhao J, Zon LI, Drummond IA, Vandorpe DH, Paw BH, Alper SL. Zebrafish slc4a2/ae2 anion exchanger: cDNA cloning, mapping, functional characterization, and localization. Am J Physiol Renal Physiol 289: F835–F849, 2005. [DOI] [PubMed] [Google Scholar]
- 67. Smith KE, Vanekeris LA, Linser PJ. Cloning and characterization of AgCA9, a novel alpha-carbonic anhydrase from Anopheles gambiae Giles sensu stricto (Diptera: Culicidae) larvae. J Exp Biol 210: 3919–3930, 2007. [DOI] [PubMed] [Google Scholar]
- 68. Sterling D, Casey JR. Transport activity of AE3 chloride/bicarbonate anion-exchange proteins and their regulation by intracellular pH. Biochem J 344: 221–229, 1999. [PMC free article] [PubMed] [Google Scholar]
- 69. Stewart AK, Chernova MN, Kunes YZ, Alper SL. Regulation of AE2 anion exchanger by intracellular pH: critical regions of the NH2-terminal cytoplasmic domain. Am J Physiol Cell Physiol 281: C1344–C1354, 2001. [DOI] [PubMed] [Google Scholar]
- 70. Stewart AK, Chernova MN, Shmukler BE, Wilhelm S, Alper SL. Regulation of AE2-mediated Cl− transport by intracellular or by extracellular pH requires highly conserved amino acid residues of the AE2 NH2-terminal cytoplasmic domain. J Gen Physiol 120: 707–722, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stewart AK, Kerr N, Chernova MN, Alper SL, Vaughan-Jones RD. Acute pH-dependent regulation of AE2-mediated anion exchange involves discrete local surfaces of the NH2-terminal cytoplasmic domain. J Biol Chem 279: 52664–52676, 2004. [DOI] [PubMed] [Google Scholar]
- 72. Stewart AK, Kurschat CE, Alper SL. Role of nonconserved charged residues of the AE2 transmembrane domain in regulation of anion exchange by pH. Pflügers Arch 454: 373–384, 2007. [DOI] [PubMed] [Google Scholar]
- 73. Stewart AK, Kurschat CE, Burns D, Banger N, Vaughan-Jones RD, Alper SL. Transmembrane domain histidines contribute to regulation of AE2-mediated anion exchange by pH. Am J Physiol Cell Physiol 292: C909–C918, 2007. [DOI] [PubMed] [Google Scholar]
- 74. Stewart AK, Kurschat CE, Vaughan-Jones RD, Alper SL. Putative re-entrant loop 1 of AE2 transmembrane domain has a major role in acute regulation of anion exchange by pH. J Biol Chem 284: 6126–6139, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stewart AK, Kurschat CE, Vaughan-Jones RD, Shmukler BE, Alper SL. Acute regulation of mouse AE2 anion exchanger requires isoform-specific amino acid residues from most of the transmembrane domain. J Physiol 584: 59–73, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Supanchart C, Kornak U. Ion channels and transporters in osteoclasts. Arch Biochem Biophys 473: 161–165, 2008. [DOI] [PubMed] [Google Scholar]
- 77. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599, 2007. [DOI] [PubMed] [Google Scholar]
- 78. Tanner MJ, Martin PG, High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J 256: 703–712, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Terhzaz S, Southall TD, Lilley KS, Kean L, Allan AK, Davies SA, Dow JA. Differential gel electrophoresis and transgenic mitochondrial calcium reporters demonstrate spatiotemporal filtering in calcium control of mitochondria. J Biol Chem 281: 18849–18858, 2006. [DOI] [PubMed] [Google Scholar]
- 80. Toye AM. Defective kidney anion-exchanger 1 (AE1, band 3) trafficking in dominant distal renal tubular acidosis (dRTA). Biochem Soc Symp 47–63, 2005. [DOI] [PubMed] [Google Scholar]
- 81. Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269: 92–95, 1995. [DOI] [PubMed] [Google Scholar]
- 82. Velez JC, Bland AM, Arthur JM, Raymond JR, Janech MG. Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol Renal Physiol 293: F398–F407, 2007. [DOI] [PubMed] [Google Scholar]
- 83. Virkki LV, Choi I, Davis BA, Boron WF. Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am J Physiol Cell Physiol 285: C771–C780, 2003. [DOI] [PubMed] [Google Scholar]
- 84. Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+-HCO3− cotransporter (NBCe2). Am J Physiol Cell Physiol 282: C1278–C1289, 2002. [DOI] [PubMed] [Google Scholar]
- 85. Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar-ATPase. Physiol Rev 84: 1263–1314, 2004. [DOI] [PubMed] [Google Scholar]
- 86. Weng XH, Huss M, Wieczorek H, Beyenbach KW. The V-type H+-ATPase in Malpighian tubules of Aedes aegypti: localization and activity. J Exp Biol 206: 2211–2219, 2003. [DOI] [PubMed] [Google Scholar]
- 87. Weng XH, Piermarini PM, Yamahiro A, Yu MJ, Aneshansley DJ, Beyenbach KW. Gap junctions in Malpighian tubules of Aedes aegypti. J Exp Biol 211: 409–422, 2008. [DOI] [PubMed] [Google Scholar]
- 88. Wessing A, Zierold K, Bertram G. Carbonic anhydrase supports electrolyte transport in Drosophila Malpighian tubules. Evidence by X-ray microanalysis of cryosections. J Insect Physiol 43: 17–28, 1997. [DOI] [PubMed] [Google Scholar]
- 89. Xu W, Marshall AT. Effects of inhibitors and specific ion-free salines on segmental fluid secretion by the Malpighian tubules of the black field cricket Teleogryllus oceanicus. J Insect Physiol 45: 835–842, 1999. [DOI] [PubMed] [Google Scholar]
- 90. Yamahiro A, Piermarini PM, Beyenbach KW. Identification of Na-driven anion exchanger (NDAE) splice variants from Malpighian (renal) tubules of the adult yellow-fever mosquito (Abstract). FASEB J 22: 757. 722, 2008. [Google Scholar]
- 91. Yu MJ, Beyenbach KW. Effects of leucokinin-VIII on Aedes Malpighian tubule segments lacking stellate cells. J Exp Biol 207: 519–526, 2004. [DOI] [PubMed] [Google Scholar]
- 92. Yu MJ, Beyenbach KW. Leucokinin activates Ca2+-dependent signal pathway in principal cells of Aedes aegypti Malpighian tubules. Am J Physiol Renal Physiol 283: F499–F508, 2002. [DOI] [PubMed] [Google Scholar]
- 93. Zhu Q, Casey JR. The substrate anion selectivity filter in the human erythrocyte Cl−/HCO3− exchange protein, AE1. J Biol Chem 279: 23565–23573, 2004. [DOI] [PubMed] [Google Scholar]
- 94. Zhu Q, Lee DW, Casey JR. Novel topology in C-terminal region of the human plasma membrane anion exchanger, AE1. J Biol Chem 278: 3112–3120, 2003. [DOI] [PubMed] [Google Scholar]
- 95. Zolotarev AS, Chernova MN, Yannoukakos D, Alper SL. Proteolytic cleavage sites of native AE2 anion exchanger in gastric mucosal membranes. Biochemistry 35: 10367–10376, 1996. [DOI] [PubMed] [Google Scholar]
- 96. Zolotarev AS, Townsend RR, Stuart-Tilley A, Alper SL. HCO3−-dependent conformational change in gastric parietal cell AE2, a glycoprotein naturally lacking sialic acid. Am J Physiol Gastrointest Liver Physiol 271: G311–G321, 1996. [DOI] [PubMed] [Google Scholar]
- 97. Zubrzak P, Williams H, Coast GM, Isaac RE, Reyes-Rangel G, Juaristi E, Zabrocki J, Nachman RJ. β-amino acid analogs of an insect neuropeptide feature potent bioactivity and resistance to peptidase hydrolysis. Biopolymers 88: 76–82, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.