Abstract
Recent studies suggest that the cost of muscle contraction may be reduced in old age, which could be an important mediator of age-related differences in muscle fatigue under some circumstances. We used phosphorus magnetic resonance spectroscopy and electrically elicited contractions to examine the energetic cost of ankle dorsiflexion in 9 young (Y; 26 ± 3.8 yr; mean ± SD) and 9 older healthy men (O; 72 ± 4.6). We hypothesized that the energy cost of twitch and tetanic contractions would be lower in O and that this difference would be greater during tetanic contractions at f50 (frequency at 50% of peak force from force-frequency relationship) than at 25 Hz. The energy costs of a twitch (O = 0.13 ± 0.04 mM ATP/twitch, Y = 0.18 ± 0.06; P = 0.045) and a 60-s tetanus at 25 Hz (O = 1.5 ± 0.4 mM ATP/s, Y = 2.0 ± 0.2; P = 0.01) were 27% and 26% lower in O, respectively, while the respective force·time integrals were not different. In contrast, energy cost during a 90-s tetanus at f50 (O = 10.9 ± 2.0 Hz, Y = 14.8 ± 2.1 Hz; P = 0.002) was 49% lower in O (1.0 ± 0.2 mM ATP/s) compared with Y (1.9 ± 0.2; P < 0.001). Y had greater force potentiation during the f50 protocol, which accounted for the greater age difference in energy cost at f50 compared with 25 Hz. These results provide novel evidence of an age-related difference in human contractile energy cost in vivo and suggest that intramuscular changes contribute to the lower cost of contraction in older muscle. This difference in energetics may provide an important mechanism for the enhanced fatigue resistance often observed in older individuals.
Keywords: bioenergetics, magnetic resonance, dorsiflexors, twitch, ATP, force-frequency
during contractions, muscles convert chemical energy in the form of ATP to mechanical output. The ATP cost of a contraction is determined by the ATPases that are involved in force production (actomyosin ATPase) and ion transport (Ca2+ ATPase, Na+/K+-ATPase). Sustained muscle activity requires a balance between ATP consumption and production through aerobic and anaerobic bioenergetic pathways. Therefore, the ATP cost of a contraction likely plays an important role in determining muscle endurance performance.
Many aspects of muscle performance decline with old age. However, a body of literature now exists that indicates muscular endurance, or fatigue resistance, is enhanced in older compared with young adults (4, 11, 25, 29, 32). At least a portion of this enhanced fatigue resistance may be due to age-related differences in muscle bioenergetics. For example, in a study in which older adults fatigued less than young during progressive, isometric ankle dorsiflexion, older adults exhibited less accumulation of inorganic phosphate (Pi) and blunted acidosis, both of which are associated with muscle fatigue (29). Subsequent studies have shown that, during maximal voluntary contractions, older muscle is more reliant on oxidative phosphorylation and less reliant on glycolysis for ATP production than younger muscle (30, 31). Further, lower energy cost of contraction has been reported in older adults (30, 31) and senescent rats (22), while energy cost was actually higher in late middle-aged rats (22). The apparent reduction in total ATP cost during maximal muscle contractions in old age may contribute to the decreased reliance on nonoxidative pathways for energy provision and, ultimately, enhanced fatigue resistance in older humans.
Several factors may contribute to a reduced ATP cost of contraction in older muscle. First, aging is generally associated with selective atrophy of type II fibers (27, 35). While the proportion of type I fibers is similar (35) or higher (27) in old compared with young muscle, selective atrophy of the type II fibers dictates that type I fibers occupy a larger fraction of the total contractile volume of older muscle. The economy of force production is 3- to 4-fold higher in type I fibers than type II fibers (7, 21, 47, 49), due to differences in both the myosin ATPase (21, 47) and the cost of calcium handling (48, 49). Therefore, the age-related shift in fiber-type composition could reduce contractile costs in older muscle, as has been reported in senescent rats (22). Second, the age-related shift in fiber-type composition contributes to the slowing of muscle contractile properties (5, 40, 53), causing a leftward shift in the force-frequency relationship (1, 40). This shift results in greater summation of force at lower stimulation frequencies in older muscle and could reduce the energy required for ion transport (Ca2+ uptake and Na+ pumping), as fewer action potentials would be needed to maintain a given relative force. Therefore, age-related changes within the muscle (e.g., fiber-type composition) could work synergistically with changes in activation pattern (e.g., stimulation frequency) to reduce the ATP cost of contraction in older muscle, although the contribution of these mechanisms to the age-related difference in the energy cost of contraction has not been investigated.
The purpose of this study was to examine the potential mechanisms of an age-related change in the energy cost of muscle contraction. We used supramaximal, electrically stimulated contractions to ensure recruitment of all motor units and to manipulate stimulation frequency. First, we hypothesized that the ATP cost of single, unfused twitch contractions would be 1) lower in older than younger muscle, and 2) associated with slowed contractile properties. Second, we hypothesized that stimulation at a frequency that induces a fused tetanic contraction (25 Hz) would also result in a lower metabolic cost in older muscle. Finally, we hypothesized that these age-related differences would be amplified during tetanic stimulation at a frequency that produced 50% of peak tetanic force, based on the force-frequency relationship (f50). Stimulation at the f50 allowed us to separate the influence of stimulation frequency on energy cost from that of relative force output.
METHODS
Subjects
Nine young (26 ± 3.8 years; means ± SD) and 9 older (72 ± 4.6 years) healthy men participated in this study. All participants gave written informed consent. All procedures were approved by the appropriate review boards at the University of Massachusetts, Amherst, and Yale University School of Medicine, and conformed to the standards set by the Declaration of Helsinki.
All participants were nonsmokers, free of diabetes, and known cardiovascular, peripheral vascular [ankle-brachial index > 1; (37)], neuromuscular, or pulmonary disease; they did not take medications known to affect muscle function or blood flow (including antihypertensives, statins and central nervous system stimulants, or depressants). All participants were relatively sedentary (<60 min of structured physical activity per week). Overall, physical activity level was quantified using uniaxial accelerometers (Actigraph GT1M; Pensacola, FL) worn by each subject for 7 days. Activity counts for each day were corroborated with a physical activity log maintained by each subject. Daily accelerometer counts for a minimum of four representative days (including at least 1 weekend day) were averaged for each participant. Days were considered representative if 1) there were no gaps >2 h in the activity counts during waking hours, and 2) the pattern of activity was generally consistent with the activity log maintained by the subject, as determined by the same investigator for all participants.
Force-Frequency Relationship
All participants were tested on two occasions, separated by at least 48 h. The first session took place in the Muscle Physiology Laboratory at the University of Massachusetts, Amherst. Subjects lay supine with their leg secured in a custom-built apparatus designed to measure ankle dorsiflexion force, the details of which have been previously described (44). Briefly, the ankle was fixed in 30° of plantarflexion. Electrodes (10-mm gold disk; Grass Technologies, West Warwick, RI) were taped to the skin for supramaximal peroneal nerve stimulation, as well as over the belly and tendon of the tibialis anterior to record the electromyogram (EMG) signal. Current intensity (model DS7A; Digitimer, Hertfordshire, UK) was set to 115% of that required to achieve the maximal amplitude of the compound muscle action potential in response to a single pulse (500-μs pulse duration), ensuring maximal activation of all motor units in the tibialis anterior muscle.
Data collection was initiated by assessing baseline twitch, tetanic (50 Hz, 500-ms train), and maximal voluntary isometric contraction (MVC) force. During voluntary contractions, subjects were provided visual feedback and verbal encouragement to ensure a maximal effort. Voluntary contractions were repeated until two contractions were within 10% of each other, and a minimum of three were performed by each subject, with 2-min rest between MVCs. The highest value was recorded as the baseline MVC.
Next, the force-frequency relationship was established for a subset of subjects (n = 7 young, 8 older). Supramaximal stimulation trains with frequencies of 1, 2, 5, 7, 10, 12, 14, 17, 20, 25, 30, 40, 50, and 75 Hz were delivered in random order, with ∼20 s of rest between each train. Tetanic trains lasted 1 s, while the 1-and 2-Hz trains lasted 4 and 2 s, respectively, to ensure that twitches did not summate at these frequencies. Finally, subjects were familiarized with the contraction protocols that were employed during the metabolic testing session.
The force signal was sampled at 500 Hz for MVCs and 2,500 Hz for all stimulated contractions during the first session. Force data were analyzed using a custom-designed analysis program (MATLAB, MathWorks, Natick, MA). To reflect whole-muscle contractile properties (12, 56), maximal rates of force development (RFD) and relaxation (RFR) and half-relaxation time were calculated for the 50-Hz tetanic contraction. RFD and RFR are expressed as a percentage of the peak force achieved during the contraction. This approach accounts for the fact that the RFD is higher when force production is higher and allows direct comparison among individuals with different torque-generating capacities (39). The force-frequency relationship was fit with a sigmoid function, and the f50 was calculated as the frequency that would produce 50% of maximal force.
Metabolic Testing
The second testing session took place in the Magnetic Resonance Research Center at the Yale University School of Medicine. Participants avoided strenuous physical activity for 24 h, and caffeine for 6 h, prior to testing. A probe assembly contaning a coplanar 7-cm 1H circular and 3 × 5 cm 31P elliptical surface coil was secured over the belly of the tibialis anterior muscle. Stimulating electrodes were taped over the peroneal nerve, and the foot was secured in a nonmagnetic exercise apparatus, as in the preliminary testing session. Because EMG was not available during the metabolic testing session, current intensity was set at 115% of that required to produce maximum twitch force. Participants lay supine in a 4.0 Tesla whole-body superconducting magnet (Bruker Biospin, Rheinstetten, Germany). Transverse gradient-echo scout images were obtained to ensure correct positioning of the limb in the isocenter of the magnet and to select a region of interest within the tibialis anterior muscle for localized shimming on the muscle water peak using the FASTMAP method (45). The shimming procedure was successful in producing narrow peaks, as indicated by the mean full width of the phosphocreatine (PCr) peak at half of its height (10.2 ± 6.1 Hz).
Participants completed a series of contraction protocols while in the magnet. For each protocol, 31P-MRS data acquisition was initiated 2 min after a 3- or 4-s MVC, which “warmed up” the muscle, and continued for 76 s before (8 dummy scans plus 60-s rest), during, and for 10 min after each contraction. Pulse parameters were as follows: 100-μs hard pulse with a nominal 60° flip angle, 2-s repetition time, 2,048 data points, 8-kHz spectral width. All subjects performed 1) a 16-s MVC, used to determine oxidative capacity (Vmax) from the kinetics of PCr recovery following the contraction, and 2) 5 min of evoked twitch contractions at 2 Hz. A subset of participants (n = 7 young, 8 older) performed two additional stimulated contractions; 3) 60 s of continuous 25-Hz stimulation, and 4) 90 s of continuous stimulation at f50, determined from each individual's force-frequency relationship. During the metabolic testing session, the force signal from all contractions was recorded at 500 Hz. Force data were analyzed using the same analysis program described above. For the twitch protocol, peak force and force·time integrals (FTI) were determined as the average for all twitches in the protocol. For the tetanic protocols, fatigue was defined as end force/peak force.
Spectral Analysis and Metabolite Quantitation
Spectral analysis was performed using NUTS software (Acorn NMR, Livermore, CA). Free induction decays were averaged to improve signal-to-noise. Time resolution for the 16-s MVC was 60 s at rest, 4 s during the contraction, 8 s during the first 5 min of recovery, and 30 s during the last 5 min of recovery. During stimulated contractions, time resolution was 10 s for 2 Hz and f50 protocols, and 12 s for the 25-Hz protocol. Time resolution during recovery from stimulated contractions was 30 s.
Averaged spectra were apodized using an exponential function corresponding to 10-Hz line-broadening, zero-filled, and Fourier transformed. After manual phasing, the underlying broad peak due to the phosphorus in bone was removed with a polynomial fit to obtain a flat baseline. Peaks corresponding to phosphocreatine (PCr), inorganic phosphate (Pi), phosphomonoesters (PME), and γ-ATP were then fit with Lorentzian-shaped curves to quantify their respective areas. Two peaks were used to fit γ-ATP and, where peak-splitting was evident, Pi (30). Splitting of the Pi peak occurred in most subjects during the tetanic contraction protocols.
To calculate metabolite concentrations from 31P-MRS spectra, peak areas were corrected for partial saturation effects. In a subset of subjects (n = 8), 24 spectra were obtained under partially saturated (TR = 2 s) and fully relaxed (TR = 30 s) conditions. The spectra for each condition were averaged, and correction factors were derived for PCr (1.97), Pi (1.89), and γ-ATP (1.83). Metabolite concentrations were calculated by assuming that resting [ATP] = 8.2 mM (18) and [PCr] + [Pi] = 42.5 mM, which is based on the assumptions that [PCr] + [creatine] = 42.5 (18), and Pi and creatine exhibit a 1:1 stoichioimetry due to the creatine kinase reaction and the impermeability of the cell to Pi (28). Intramuscular pH was calculated from the chemical shift (σ) of Pi relative to PCr (50). Adenosine diphosphate (ADP) concentration was calculated from the equilibrium of the creatine kinase reaction and the stoichiometry of free Cr and Pi (33):
| (1) |
The equilibrium constant of the creatine kinase reaction was corrected for the effect of pH, assuming a free magnesium concentration of 1 mM (14).
Muscle Oxidative Capacity
The recovery of PCr following the 16-s MVC was fit with a monoexponential function. Oxidative capacity (Vmax, mM ATP/s) was calculated as the product of the rate constant from the monoexponential function (kPCr) and the resting concentration of PCr, as described previously (30, 38). Although the kinetics of PCr recovery can be limited by oxygen availability in exercise-trained individuals (19), this is not the case in sendentary subjects (20), such as those studied here. Furthermore, Wray and colleagues (57) recently reported no impairment in postexercise PCr recovery despite reduced end-exercise perfusion in older adults, demonstrating that PCr recovery is not affected by age-related reductions in blood flow. Together with the body of literature using this method to measure in vivo muscle oxidative capacity, these results indicate that, under the conditions of the current study, PCr recovery provides a valid means of assessing oxidative capacity in young and older sedentary individuals.
ATP Cost of Twitch Contractions
The ATP cost of a twitch contraction was estimated from the initial rate of decline of PCr during 5 min of 2-Hz stimulation (13). In one subject, technical difficulties necessitated shortening the protocol, and twitch cost was calculated from 2.5 min of contractions. At the onset of twitch contractions, PCr initially falls as it buffers ATP, then reaches a steady-state level as ATP synthesis by oxidative phosphorylation and glycolysis increase to match the rate of ATP consumption. The time course of decline in PCr during stimulated twitches was fit with an exponential function (13):
| (2) |
where [PCr]ss refers to the steady-state concentration of PCr, Δ[PCr] refers to the amplitude of the response, t is time, and λ is a rate constant describing the kinetics of the fall in [PCr]. The ATP cost of an individual twitch (mM/twitch) was calculated as follows:
| (3) |
where the numerator is the rate of change in PCr at time = 0, derived from the exponential fit in Eq. 2. The relatively slow onset of both oxidative phosphorylation (15) and anaerobic glycolysis (8) dictate that net PCr breakdown is the only source of ATP regeneration at this first time point. Because ATP levels in the cell are stable under these conditions, the rate of PCr hydrolysis must equal the rate of ATP hydrolysis (13).
ATP Cost of Tetanic Contractions
The ATP cost of tetanic contractions was calculated from the rates of ATP synthesis through the net breakdown of PCr by the creatine kinase reaction (ATPCK), oxidative phosphorylation (ATPOX), and glycolysis (ATPGLY) (28, 30, 55). ATPCK (mM/s) was determined from the rate of decrease in PCr during the contraction:
| (4) |
ATPOX was determined based on the assumption that the rate of oxidative phosphorylation is controlled by the phosphorylation potential with a Km of 0.11 (55):
| (5) |
ATPGLY was calculated from the changes in pH after correction for intracellular buffering (β), proton efflux (νeff), and changes in PCr and ATPOX (30, 55):
| (6) |
where dpH/dt and dPCr/dt refer to the changes in pH and PCr, respectively, with time, and θ is the proton stoichiometry coefficient for the creatine kinase reaction:
| (7) |
The correction factor m accounts for the production of protons during oxidative ATP synthesis:
| (8) |
The calculation of β (slykes) and νeff are discussed in detail elsewhere (28, 30, 55). Briefly, total β was calculated as the sum of the individual contributions from Pi, PME, bicarbonate, and inherent buffering, which was calculated from the initial change in pH and PCr during contraction. Proton efflux rates during contraction were estimated on the basis of the proportionality between pH and proton efflux calculated during recovery.
The total rate of ATP production (mM ATP/s) during tetanic contractions was calculated as the sum of the rates from each of the three pathways:
| (9) |
Total ATP cost (mM ATP) was calculated for each time interval during the contraction and summed.
Statistical Analysis
Our main hypotheses regarding the difference in the energy cost of twitch and tetanic contractions between young and older men were tested using two-tailed, unpaired t-tests, while linear regression was used to determine the relationship between the energy cost of a twitch and contractile (half-relaxation time) and metabolic (end-contraction pH) properties. The relative contributions to ATP production by each of the three pathways were compared across groups using two-tailed, unpaired t-tests, with Bonferroni correction for multiple comparisons.
Additional analyses were made to fully characterize the groups and included two-tailed, unpaired t-tests to compare subject characteristics, force, and contractile properties, f50, fatigue, and PCr-fitting parameters during the twitch contraction protocol. The Wilcoxon rank sums test was used to compare groups for physical activity counts, and PCr and pH at rest, all of which exhibited skewed distributions. For one-way comparisons, exact P values and, when data were normally distributed, 95% confidence intervals on the differences between groups are presented.
The force-frequency relationship was analyzed using a two-way ANOVA for repeated measures that included tests for main effects of age and frequency, as well as an age-by-frequency interaction. Similarly, the changes in metabolite levels during the tetanic contraction protocols were analyzed using a mixed-models ANOVA for repeated measures that included tests for main effects of age and time, as well as an age-by-time interaction. Post hoc pairwise comparisons for the force-frequency relationship and metabolic changes were made using an F-test, with Bonferroni correction for multiple comparisons. Unless otherwise stated, data are presented as means ± SD.
RESULTS
Descriptive data are provided in Table 1. Young and older men were similar in height and body mass. Overall physical activity level was higher in the young group [median = 233 counts·day−1·1,000−1, interquartile range (IQR) = 209, 265] than in the older group (median = 149, IQR = 132, 156; P = 0.021), although none of the subjects participated in regular physical exercise. There was no difference between the groups in the rate constant for PCr recovery (kPCr) following a 16-s MVC, or in muscle oxidative capacity (Vmax).
Table 1.
Subject characteristics and muscle oxidative capacity
| Young | Older | 95% CI | P | |
|---|---|---|---|---|
| Height, cm | 174 ± 6 | 177 ± 7 | −3, 9 | 0.319 |
| Body mass, kg | 80 ± 9 | 86 ± 8 | −2, 14 | 0.115 |
| kPCr, s−1 | 0.026 ± 0.005 | 0.026 ± 0.005 | −0.002, 0.006 | 0.342 |
| Vmax, M ATP·s−1 | 0.96 ± 0.14 | 1.08 ± 0.06 | −0.11, 0.34 | 0.301 |
Values are mean ± SD. 95% confidence intervals for the difference between groups are presented for each variable. kPCr, rate constant from monoexponential fit of phosphocreatine (PCr) recovery following 16 s maximal voluntary isometric contraction (MVC); Vmax, muscle oxidative capacity.
Baseline Characteristics and the Force-Frequency Relationship
Data for baseline MVC and contractile properties from tetanic contractions are presented in Table 2. Baseline MVC tended to be lower in the older group (17%), and maximal tetanic force was 25% lower. Consistent with previous studies (29), peak tetanic force was lower than MVC force in both groups, and the ratio of peak tetanic to MVC force was not different in the two groups [0.76 ± 0.13 vs. 0.70 ± 0.15 for young (n = 7) and older (n = 8), respectively; P = 0.43]. The maximum RFD during 50-Hz tetanus was not different in the two groups, but the maximal RFR was slower, and the half-relaxation time was longer in the older group.
Table 2.
Baseline muscle force, tetanic properties, and f50
| Young | Older | 95% CI | P | |
|---|---|---|---|---|
| Baseline MVC, N | 380 ± 66 | 317 ± 75 | −137, −10 | 0.087 |
| Tetanic force, N | 281 ± 62 | 211 ± 39 | −127, −12 | 0.020 |
| Max RFD, % peak force·ms−1 | 0.54 ± 0.07 | 0.50 ± 0.11 | −0.14, 0.06 | 0.392 |
| Max RFR, % peak force·ms−1 | 1.31 ± 0.19 | 0.93 ± 0.20 | 0.17, 0.60 | 0.002 |
| Half-relax time, ms | 106 ± 5 | 149 ± 15 | 30, 55 | <0.001 |
| f50, Hz | 14.8 ± 2.1 | 10.9 ± 2.0 | −6.2, −1.7 | 0.002 |
Values are mean ± SD. 95% confidence intervals for the difference between groups are presented for each variable. MVC, maximal voluntary isometric contraction (n = 9 young and 9 older); Tetanic force from 75 Hz stimulation (n = 7 young and 8 older); contractile properties from the 50 Hz tetanus (n = 8 young and 8 older), including max RFD, maximum rate of force development; max RFR, maximum rate of force relaxation; half-relax time; f50, frequency calculated to produce 50% of maximal tetanic force (n = 7 young and 8 older).
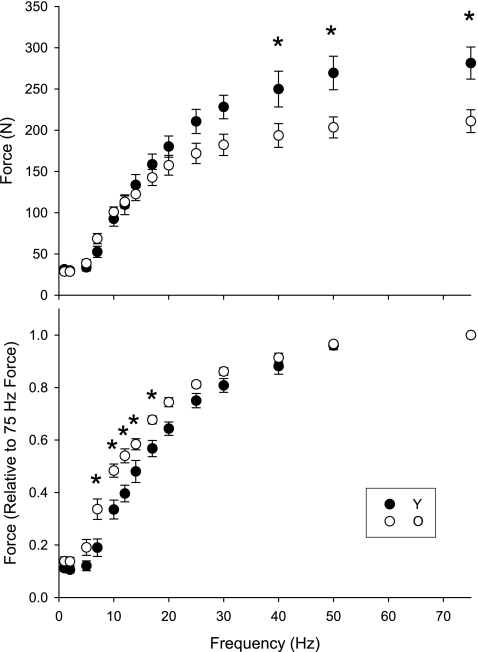
The force-frequency relationships for both groups are shown in Fig. 1. The analysis indicated significant age-by-frequency interactions for both the absolute (top, P < 0.001) and normalized (bottom, P < 0.001) curves. Post hoc analysis revealed that absolute force was lower in the older group during tetanic stimulation at ≥40 Hz. Normalized force was higher in older muscle during tetanic stimulation from 7 to 20 Hz. This shift in the force-frequency relationship is reflected in the f50, which was 26% lower in the older group (Table 2).
Fig. 1.
Dorsiflexor muscle force-frequency relationship for seven young (solid symbols) and eight older men (open symbols). Force is expressed in absolute units (top) and relative to peak force obtained during 75 Hz stimulation (bottom). Note the relative leftward shift in the relationship for the older men, which resulted in a significantly lower f50 (Table 1). Data are expressed as mean ± SE; *P ≤ 0.001 between groups.
Twitch Contractions
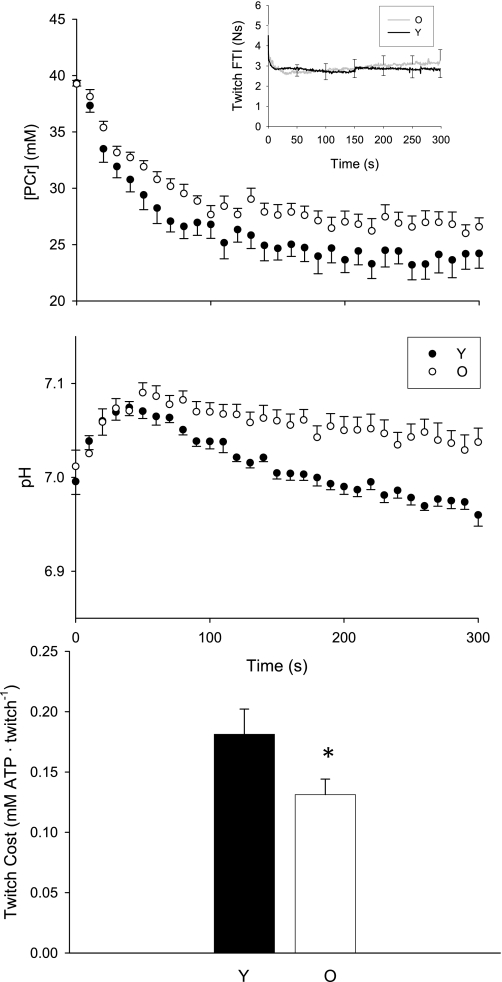
At rest, muscle [PCr] was similar in young (median = 39.6 mM; IQR = 39.2, 39.9) and older (39.4 mM; IQR = 39.0, 39.9) groups (P = 0.895), as was pH [young: 7.00 (6.97, 7.03); old: 7.00 (6.98, 7.02); P = 0.757]. During the 2-Hz protocol, average twitch force and FTI were similar in young and older muscle (Table 3; Fig. 2, inset). During 5 min of stimulation at 2 Hz, the fall in PCr (Fig. 3) was closely approximated by the monoexponential fit (r2 = 0.89 ± 0.05 and 0.87 ± 0.08 for Y and O, respectively; P = 0.506), and the rate constant for the fall in PCr (λ) was not different in the two groups (Table 3). However, the change in PCr from rest to the end of stimulation (Δ[PCr]) tended to be larger, and the steady-state value ([PCr]SS) tended to be lower, in the young group compared with the older group (Table 3, Fig. 2, middle). At the end of the twitch contractions, intracellular pH was lower in young muscle than in older muscle (Table 3, Fig. 2).
Table 3.
Twitch properties and metabolic variables from 2-Hz protocol
| Young | Older | 95% CI | P | |
|---|---|---|---|---|
| Force | ||||
| Average twitch force, N | 23 ± 9 | 22 ± 7 | −8, 8 | 0.940 |
| Average twitch FTI, Ns | 2.5 ± 1.2 | 2.9 ± 1.1 | −0.8, 1.6 | 0.504 |
| Metabolism | ||||
| pH end-exercise | 6.96 ± 0.03 | 7.04 ± 0.04 | 0.03, 0.12 | 0.001 |
| PCr onset λ, s−1 | 0.023 ± 0.005 | 0.020 ± 0.007 | −0.009, 0.004 | 0.430 |
| [PCr]SS, mM | 23.9 ± 2.9 | 26.5 ± 2.7 | −0.19, 5.4 | 0.065 |
| Δ[PCr], mM | 15.6 ± 2.6 | 13.1 ± 2.5 | −5.0, −0.14 | 0.062 |
| Twitch cost, mM·Twitch−1 | 0.180 ± 0.06 | 0.131 ± 0.04 | −0.097, −0.001 | 0.045 |
Values are mean ± SD, and 95% confidence intervals for the difference between groups are presented. Twitch force and force·time integrals (FTI) are averages from entire 2-Hz protocol; PCr onset λ (rate constant for fall in PCr), [PCr]SS (steady-state PCr concentration achieved during twitch contractions), and Δ[PCr] (amplitude of the fall in PCr during twitch contractions) from monoexponential fit of the PCr during 2 Hz protocol.
Fig. 2.
Twitch protocol. PCr (top), pH (middle), and ATP cost of a single twitch (bottom) in young (solid symbols, black line) and older (open symbols, gray line) men. Top, inset: FTI for all 600 twitches. Data are presented as means ± SE. *P = 0.05.
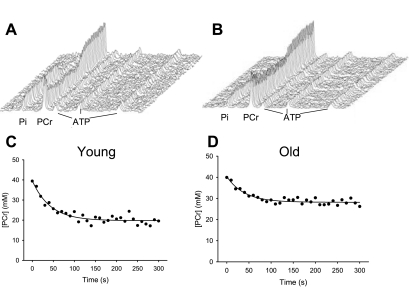
Fig. 3.
Representative stack plots (top) and changes in PCr (bottom) during a 2-Hz twitch protocol for 1 young (A, C) and 1 older (B, D) participant. Exponential fits are shown. Twitch cost = 0.24 and 0.15 mM·twitch-1 for the young and older man, respectively.
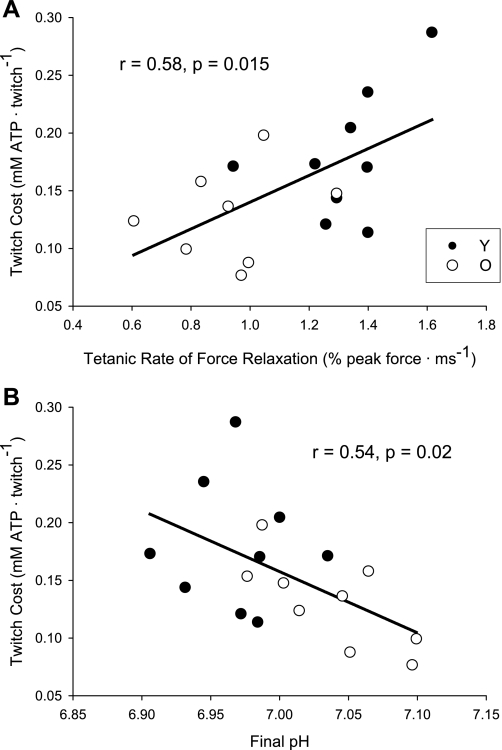
The metabolic cost of a twitch contraction was ∼27% lower in older muscle than in young muscle (Table 3; Fig. 2, bottom) and was positively correlated with the maximum RFR for all subjects combined (Fig. 4A). Additionally, the cost of a twitch was inversely correlated with pH at the end of the protocol (Fig. 4B).
Fig. 4.
Associations between twitch cost and contractile and metabolic properties. Associations between twitch cost and tetanic rate of force relaxation (n = 17; RFR not available for one O) (A), and end-contraction pH in Y (solid symbols) and O (open symbols) (B).
Tetanic Contractions
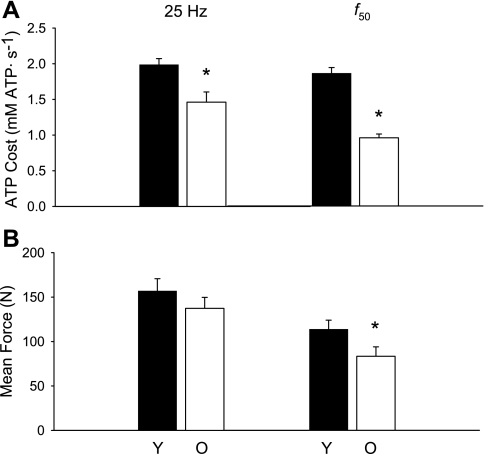
The force traces for the two tetanic protocols are shown in Fig. 5, top expressed relative to force from the 25-Hz tetanus for the purpose of comparison. Peak force during continuous 25-Hz stimulation was not different between young and older groups (P = 0.303). Force fell during the contraction (Fig. 5, top left), and younger muscle tended to fatigue to a greater degree (end force/peak force; 0.56 ± 0.15) than older muscle (0.70 ± 0.11; P = 0.058). Although mean force, averaged over the entire 60-s contraction, was 12% lower in the older group, this was not significantly different between groups (P = 0.290; Fig. 6B).
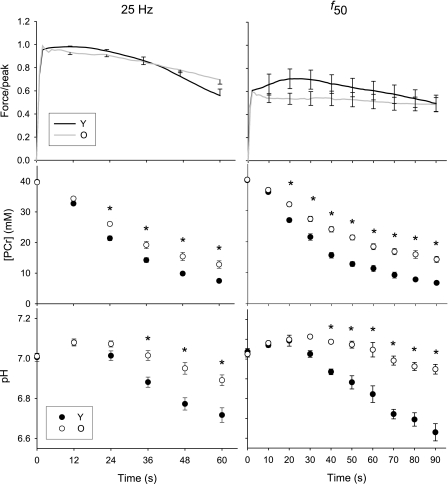
Fig. 5.
Force, PCr, and pH during tetanic protocols. Force, relative to 25 Hz peak (top), PCr (middle), and pH (bottom) for the 25 Hz (left) and f50 (right) protocols in Y (solid symbols, black line) and O (open symbols, gray line), respectively. Note the different duration for the two contractions. f50 = 14.8 ± 2.1 and 10.9 ± 2.0 Hz for Y and O, respectively. Data are presented as means ± SE. *P = 0.001.
Fig. 6.
Force and ATP cost during tetanic protocols. ATP cost (A) and mean force (B), averaged over the duration of the contraction, for the 25 Hz (left) and f50 (right) protocols in Y (solid bars) and O (open bars). Data are presented as means ± SE. *P < 0.05.
Prior to the 25-Hz contraction, resting phosphorus metabolites and pH were similar in young and older men (Fig. 5, left middle). During the contraction, PCr fell in both groups (P < 0.001), and to a greater degree in the young men (P < 0.001 for both main effect of time and age-by-time interaction). Post hoc analysis revealed that PCr was lower in young men at time ≥24 s. After an initial alkalosis, pH fell in both groups (P < 0.001; Fig. 5, bottom left). Young men exhibited greater acidosis than older men (P = 0.002 for effect of time, P < 0.0001 for age-by-time interaction), and post hoc analysis revealed that pH was lower in young men at time ≥36 s. [ATP] was not different between the groups (P = 0.591) and did not change during this contraction (P = 0.745; data not shown).
The purpose of stimulating at the f50 was to adjust for the leftward shift in the force-frequency curve in older muscle and thereby compare young and older muscle at similar relative forces, as elicited by different pulse frequencies. The force pattern during this 90-s contraction differed in the two groups (Fig. 5, top right). The young muscle potentiated, while the older muscle did not. After the initial potentiation, force fell, and fatigue was not different in the two groups (0.68 ± 0.21 vs. 0.76 ± 0.16 for young and old, respectively; P = 0.447). Peak force was 24% lower (P = 0.048), and average force for the entire 90-s contraction tended to be lower (27%, P = 0.062), in the older group than the younger group (Fig. 6B).
During the contraction at f50, PCr fell (Fig. 5, middle right) to a greater degree in the younger men, as indicated by the main effect of age and the age-by-time interaction (P < 0.001 for both). Post hoc analysis revealed that PCr was lower in the young group at time ≥20 s. Intracellular pH (Fig. 5, bottom right) fell during the contraction (P < 0.001) to a greater extent in young muscle (P < 0.001 for main effect of age and age-by-time interaction), and post hoc analysis revealed that pH was lower at time ≥40 s in the younger muscle. [ATP] was similar in the two groups (P = 0.997) and did not change during the contraction (P = 0.527; data not shown).
Energy Cost and ATP Production During Tetanic Contractions
The ATP cost of the 25 Hz contraction, averaged over the entire 60 s, was 26% lower in the older group than in the younger group (Fig. 6A, left; P = 0.001). Because the young muscle fatigued more during this 60-s contraction, we also compared the ATP cost of contraction during the first 24 s of stimulation, prior to the onset of fatigue. At 24 s, relative force was similar in the two groups (0.94 ± 0.04 vs. 0.90 ± 0.02 for young and old, respectively, P = 0.20). As for the full 60-s contraction, the ATP cost for the first 24 s of the contraction was again lower in older men (1.5 ± 0.2 mM ATP/s vs. 1.0 ± 0.2 for young and old, respectively, P < 0.001). The ATP cost of the f50 contraction, averaged over the entire 90 s, was 49% lower in older men (Fig. 6A, right; P < 0.001).
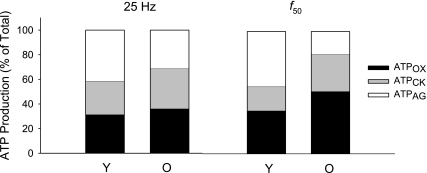
During the 25-Hz protocol, there were no age-related differences in the relative contribution of the creatine kinase reaction, glycolysis, and oxidative phosphorylation to total ATP production (P > 0.20 for all pathways; Fig. 7 left). In contrast, during the f50 protocol, older muscle generated relatively more ATP by oxidative phosphorylation (P = 0.002; Fig. 7, right) and the creatine kinase reaction (P < 0.001), and relatively less by glycolysis (P = 0.002).
Fig. 7.
Relative contribution of ATP-producing pathways. The relative contribution of oxidative phosphorylation (ATPOX; black), the creatine kinase reaction (ATPCK; gray), and glycolysis (ATPGLY; open) for the 25 Hz (left) and f50 (right) protocols. There were no age-related differences in the relative contribution of each of the pathways to total ATP production during the 25-Hz contraction, while older muscle generated relatively more ATP by oxidative phosphorylation and the creatine kinase reaction, and relatively less by glycolysis, compared with young during the f50 contraction (P ≤ 0.002).
DISCUSSION
In support of our first hypothesis, the results of this study indicate that the ATP cost of an evoked twitch contraction was ∼27% lower in older compared with young muscle, despite similar twitch force and FTI. Thus, a portion of the energetic differences observed previously between young and old muscle during voluntary contractions (29–31) may be due to a lower energy cost of contraction in old muscle. Further, twitch cost was positively correlated with the maximum rate of force relaxation following a tetanic contraction, suggesting that a portion of the age-related differences in the energy cost of contraction may be related to changes in fiber-type composition.
The second part of this study investigated the impact of a shift in the force-frequency relationship on the energy cost of contractions in young and older muscle. When young and older muscle was stimulated at the same absolute frequency (25 Hz), we found that the ATP cost of contraction was 26% lower in the older muscle. When we stimulated young and older muscle at the same point on their respective force-frequency curves (f50; 14.8 Hz and 10.9 Hz for young and old, respectively), we found that the difference in metabolic cost across groups was nearly doubled (49% lower in older muscle). The greater age-related difference in ATP cost at f50, compared with 25 Hz, can be explained by the difference in force output; mean force was 12% lower in the older compared with the young muscle during 25-Hz stimulation, but 24% lower in the older muscle at f50 (Fig. 6). This analysis suggests that the leftward shift in the force-frequency relationship and corresponding lower frequency of stimulation in the older group had a negligible additional effect on the age-related difference in the energy cost of contraction.
Twitch Contractions
We used twitch contractions to examine contractile cost because they provide a measure of cost that is independent of activation pattern (3, 13). During 5 min of 2-Hz stimulation, PCr and pH decreased to a greater extent in younger compared with older muscle, despite similar twitch force and FTI. We calculated the ATP cost of twitch contractions from the fall in PCr using an approach that has been validated in animal studies (13). Our estimates for the energetic cost of a twitch contraction (0.13 and 0.18 mM·twitch−1 for old and young, respectively) are similar to those previously reported for human muscle [0.15 mM·twitch−1 (3)], and consistent with values obtained from cat muscle measured by 31P-MRS [0.10 and 0.22 mM·twitch−1 for soleus and biceps, respectively, (17)] and rat hindlimb calculated from oxygen consumption data [0.21 mM·twitch−1, (23)]. The minor discrepancy between the values for human and animal muscle can be accounted for by differences in fiber-type distribution, as well as the lower tension cost of human fibers within a given fiber type compared with those of the rat (47).
In the present study, the cost of a twitch was related to the maximal rate of force relaxation, which was slower in the older group compared with the younger group. The mechanism of slowed force relaxation in older muscle is unclear (26), but age-related changes in muscle fiber composition likely play a role. Support for this possibility is provided by White et al. (56), who found a significant association between the rate of force relaxation following a 50-Hz tetanus and the percentage of type II fibers in the triceps surae of young and older men. Likewise, the cost of a twitch contraction in our study was inversely related to pH at the end of the contraction protocol, which could reflect differences in fiber-type composition, as type II fibers generally have higher levels of glycolytic enzymes than type I fibers (6).
Aging is associated with increased variability in muscle fiber size (34) and a general decline in type II fiber area (27, 35), leading to an increase in the volume of the muscle occupied by more economical type I fibers (7, 21, 47, 49). Hepple et al. (22) found a decrease in the proportion of less economical type IIb myosin heavy chain in the hindlimbs of senescent, but not late middle-aged, rats compared with young adult rats. Concomitantly, contractile cost was reduced in the senescent rats, but was elevated in the late middle-aged rats. The authors suggested that the changes in fiber-type composition contributed to the reduction in contractile cost, and in that case, helped to compensate for the reduced ATP-generating capacity they also found in these senescent rats. The current study is the first, to our knowledge, to extend this concept to humans; our results suggest that changes in fiber-type composition play a key role in reducing contractile cost in older men, even in the absence of a deficiency in ATP production.
Using literature values for fiber-type composition and the fiber-type difference in energy cost of contraction, we can predict the magnitude of the effect of age-related changes in muscle composition on the energy cost of contraction. According to Jakobssen et al. (27), the tibialis anterior is ∼66% type I fibers by area in young adults and ∼82% in older adults, the balance being type IIA fibers. Szentesi et al. (49) found higher actomyosin (∼3-fold) and sarcoplasmic reticulum-ATPase (∼1.4-fold) rates in human skinned type IIA fibers compared with type I fibers. From these data, we estimate a 22% difference in ATP cost between young and older muscle, solely based on the difference in fiber-type composition. This estimate is generally consistent with the observed 27% difference found in the present study.
Two additional mechanisms may contribute to lower energy cost of twitch contractions in older adults. First, some recent studies suggest that the kinetics of type I myosin are slowed in older humans (9, 24), possibly due to a posttranslational modification of the myosin molecule (24). This could reduce the actomyosin ATPase rate within the type I fibers of older muscle, contributing further to a reduced ATP cost of contraction. Second, a reduction in the number of cross-bridges formed per unit of muscle cross-sectional area would reduce the overall actomyosin ATPase rate, as well as the force produced. However, because twitch force was similar in our two groups, it seems unlikely that reduced cross-bridge cycling would explain the current results.
Similar twitch force, despite lower tetanic and MVC force in older compared with young muscle, has been reported previously by this laboratory (29) and others (2, 5, 52). This result could be due to age-related stiffening of the series elastic component, which has been reported in whole muscle (42, 51) and single fibers (41). Greater stiffness would enhance the efficiency of the transfer of mechanical energy from the contractile elements to the joint, thereby preserving twitch force in older muscle. It should be noted, however, that there is evidence that tendon stiffness declines with aging (36). Further study is needed to clarify the effects of aging on the stiffness of the entire musculotendinous unit.
Tetanic Contractions
Force-frequency relationship.
Despite similar twitch forces, peak tetanic force was 25% lower in older muscle. Furthermore, the maximal RFR was slower and the half-relaxation time longer in older muscle compared with young muscle. This is consistent with previous studies of the dorsiflexors (40, 53) and quadriceps (1) muscles and may be due to age-related changes in fiber-type composition. Slowed force relaxation contributed to the leftward shift in the force-frequency relationship, which is consistent with previous reports in the dorsiflexors (40), quadriceps (1, 46), and triceps surae (10). As a result of this leftward shift, the f50 was significantly lower for old muscle compared with young muscle in our groups.
Force and metabolites.
Force production during the 25-Hz protocol was similar in young and old muscle. In contrast, force output was higher in the young group than the old group during stimulation at the f50 due to potentiation of the unfused tetanus in the young. This result is consistent with observations of greater force potentiation in muscle composed of relatively more type II fibers (16). During both the 25-Hz and f50 protocols, young muscle exhibited a greater fall in PCr and greater acidosis than old muscle, which is consistent with previous results from sustained (30) and intermittent (31) voluntary contractions in young and older muscle.
ATP cost of tetanic contractions.
We hypothesized that the leftward shift in the force-frequency relationship would contribute to reduced ATP cost of contraction in older men, as fewer pulses would be required to produce similar relative force. Fewer pulses could reduce the overall ATP requirements, as there would be fewer action potentials to tax the ATPases responsible for ion transport. These noncontractile processes are thought to contribute up to 42% of the total cost of a contraction (54), and reducing them could have a significant impact on total energy cost. This concept is relevant to voluntary contractions, as motor unit discharge rates during MVCs are lower in older adults (5, 43).
Although the age-related difference in energy cost was larger during stimulation at f50 than at 25 Hz, the interpretation of these data are complicated by the force potentiation observed only in the young men during the contraction at f50. The younger group produced significantly greater relative force than the older group, which contributed to the higher energy cost in the young men. Indeed, the age-related difference in both force and cost increased by a factor of 2 in the f50 protocol compared with the 25-Hz protocol. Assuming a linear relationship between force output and ATP cost (7), the similar magnitude increase in force and cost suggests that the shift in the force-frequency relationship and the lower stimulation frequency in the older adults during the f50 protocol had a negligible effect on the total costs of contraction. This conclusion is consistent with in vitro experiments showing that the sarcoplasmic reticulum-ATPase, which accounts for the a large proportion of noncontractile costs, is saturated at low force levels [pCa2+ corresponding to ∼30% of maximal force; (48)]. If this is the case in vivo, the ∼4-Hz difference in stimulation frequency between the groups would not be expected to affect noncontractile costs.
Impact of fatigue.
Young men had greater fatigue than old during the 25-Hz contraction, which is consistent with several studies demonstrating greater fatigue resistance in older adults during voluntary (4, 25, 29) and stimulated contractions under some conditions (1). Differences across groups in the degree of fatigue during the protocol could have affected our results. However, the age-related difference in ATP cost was apparent at time = 24 s, before the age-related difference in fatigue was detectable. Since force output, relative to peak, was similar in the young and older men at this time-point, it is unlikely that greater fatigability in the young men affects our conclusions to a significant extent.
Fatigue was not different between the two groups during stimulation at f50. This contrasts with the results of Allman and Rice (1), who found enhanced fatigue resistance in older quadriceps muscle, compared with young muscle, during stimulation at a normalized frequency (60% of force-frequency curve), but similar fatigue in young and older muscle during stimulation at 14.3 Hz. These contrasting results may be explained by the potentiation in the young muscle during stimulation at f50 in the current study, which may have counteracted fatigue in this group. Differences in stimulation parameters and muscle group between these two studies may also contribute to their differing results.
Pathways of ATP production.
In contrast with previous studies of maximal voluntary dorsiflexion contractions, which showed that older muscle relies relatively more on oxidative phosphorylation for ATP production than younger muscle (30), we did not detect an age-related difference in the relative contribution of ATPOX, ATPCK, or ATPGLY to total ATP production during the 25-Hz protocol. However, differences in ATP pathway flux were observed during stimulation at f50. At this lower frequency, older muscle generated relatively more of its ATP from oxidative phosphorylation and the creatine kinase reaction, while younger muscle depended more on glycolysis, as expected from the studies of voluntary contractions.
The basis for the discrepancies between the stimulated protocols is not clear at this time. While it may be that this comparison is complicated by the force potentiation of the young muscle during f50 stimulation, this explanation seems unlikely, on the basis of previous work in which differences in relative force, in which older muscle produced relatively more force than young, did not mitigate the greater reliance on oxidative phosphorylation in the old during voluntary contractions (31). That is, if higher relative force results in greater reliance on glycolysis, we would not have observed higher reliance on oxidative metabolism in the old in that previous study. Rather, in the current study, it seems reasonable to anticipate that interactions between muscle activation patterns and age-related changes in intrinsic muscle properties may explain the discrepancy between the 25 Hz and f50 protocols. Clearly, the importance of bioenergetics in muscle performance and fatigue resistance indicates that further studies are needed to clarify these potential interactions in the aging neuromuscular system.
Perspectives and Significance
In humans, as well as other animals, the ability to perform repeated muscle contractions is critical to activities of daily living and requires a balance between the rates of ATP consumption and regeneration. The understanding of how muscle bioenergetics may change in old age is an emerging area of investigation. To address existing knowledge gaps, we examined the effects of old age on factors that determine the rates of ATP consumption (ATP cost) and regeneration (ATP flux) during contractions in vivo. The results provide the first evidence in human skeletal muscle of a lower ATP cost of contractions. The physiological and functional consequences of this difference remain to be determined.
In conclusion, we provide novel evidence that the energetic cost of isometric twitch and 25-Hz tetanic contractions are lower in vivo in older men than younger men under conditions of similar force output. The slowing of force relaxation and leftward shift of the force-frequency curve suggest that these results may be due, in part, to an age-related shift in fiber-type composition. During stimulation at f50, the age-related difference in ATP cost of contraction increased markedly. However, this increase in cost can be accounted for by the increased force output in the younger men as a result of force potentiation, suggesting that the leftward shift in the force-frequency relationship and lower frequency of stimulation in the older group had little impact on the total energy cost of contraction. Lower ATP cost of contraction may contribute to the lower metabolic perturbation and enhanced fatigue resistance that has been reported in older muscle.
GRANTS
This work was funded by National Institutes of Health R01 AG21094 and K02 AG023582.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors thank the participants, Doug Befroy, D. Phil, for technical assistance, and the members of the Muscle Physiology Laboratory for their comments on this manuscript.
REFERENCES
- 1. Allman BL, Rice CL. An age-related shift in the force-frequency relationship affects quadriceps fatigability in old adults. J Appl Physiol 96: 1026–1032, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Baudry S, Klass M, Duchateau J. Postactivation potentiation influences differently the nonlinear summation of contractions in young and elderly adults. J Appl Physiol 98: 1243–1250, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Blei ML, Conley KE, Kushmerick MJ. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol 465: 203–222, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung LH, Callahan DM, Kent-Braun JA. Age-related resistance to skeletal muscle fatigue is preserved during ischemia. J Appl Physiol 103: 1628–1635, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol 87: 843–852, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Costill DL, Daniels J, Evans W, Fink W, Krahenbuhl G, Saltin B. Skeletal muscle enzymes and fiber composition in male and female track athletes. J Appl Physiol 40: 149–154, 1976 [DOI] [PubMed] [Google Scholar]
- 7. Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol 79: 147–166, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crowther GJ, Carey MF, Kemper WF, Conley KE. Control of glycolysis in contracting skeletal muscle. I. Turning it on. Am J Physiol Endocrinol Metab 282: E67–E73, 2002 [DOI] [PubMed] [Google Scholar]
- 9. D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552: 499–511, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies CT, White MJ. Contractile properties of elderly human triceps surae. Gerontology 29: 19–25, 1983 [DOI] [PubMed] [Google Scholar]
- 11. Ditor DS, Hicks AL. The effect of age and gender on the relative fatigability of the human adductor pollicis muscle. Can J Physiol Pharmacol 78: 781–790, 2000 [PubMed] [Google Scholar]
- 12. Edwards RH, Young A, Hosking GP, Jones DA. Human skeletal muscle function: description of tests and normal values. Clin Sci Mol Med 52: 283–290, 1977 [DOI] [PubMed] [Google Scholar]
- 13. Foley JM, Meyer RA. Energy cost of twitch and tetanic contractions of rat muscle estimated in situ by gated 31P NMR. NMR Biomed 6: 32–38, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Golding EM, Teague WE, Jr, Dobson GP. Adjustment of K′ to varying pH and pMg for the creatine kinase, adenylate kinase, and ATP hydrolysis equilibria permitting quantitative bioenergetic assessment. J Exp Biol 198: 1775–1782, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Grassi B. Delayed metabolic activation of oxidative phosphorylation in skeletal muscle at exercise onset. Med Sci Sports Exerc 37: 1567–1573, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Hamada T, Sale DG, MacDougall JD, Tarnopolsky MA. Postactivation potentiation, fiber type, and twitch contraction time in human knee extensor muscles. J Appl Physiol 88: 2131–2137, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Harkema SJ, Adams GR, Meyer RA. Acidosis has no effect on the ATP cost of contraction in cat fast- and slow-twitch skeletal muscles. Am J Physiol Cell Physiol 272: C485–C490, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33: 109–120, 1974 [PubMed] [Google Scholar]
- 19. Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol 86: 2013–2018, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Haseler LJ, Lin A, Hoff J, Richardson RS. Oxygen availability and PCr recovery rate in untrained human calf muscle: evidence of metabolic limitation in normoxia. Am J Physiol Regul Integr Comp Physiol 293: R2046–R2051, 2007 [DOI] [PubMed] [Google Scholar]
- 21. He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C. ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J 79: 945–961, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hepple RT, Hagen JL, Krause DJ, Baker DJ. Skeletal muscle aging in F344BN F1-hybrid rats. II. Improved contractile economy in senescence helps compensate for reduced ATP-generating capacity. J Gerontol A Biol Sci Med Sci 59: 1111–1119, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Hood DA, Gorski J, Terjung RL. Oxygen cost of twitch and tetanic isometric contractions of rat skeletal muscle. Am J Physiol Endocrinol Metab 250: E449–E456, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Hook P, Sriramoju V, Larsson L. Effects of aging on actin sliding speed on myosin from single skeletal muscle cells of mice, rats, and humans. Am J Physiol Cell Physiol 280: C782–C788, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Hunter SK, Critchlow A, Enoka RM. Muscle endurance is greater for old men compared with strength-matched young men. J Appl Physiol 99: 890–897, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Hunter SK, Thompson MW, Ruell PA, Harmer AR, Thom JM, Gwinn TH, Adams RD. Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol 86: 1858–1865, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Jakobsson A, Borg K, Edstrom L. Fiber-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Acta Neuropathol (Berl) 80: 459–468, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q 10: 43–63, 1994 [PubMed] [Google Scholar]
- 29. Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol 93: 1813–1823, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol 99: 1736–1744, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol 583: 1093–1105, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lanza IR, Russ DW, Kent-Braun JA. Age-related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks. J Appl Physiol 97: 967–975, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem 254: 6528–6537, 1979 [PubMed] [Google Scholar]
- 34. Lexell J, Taylor CC. Variability in muscle fibre areas in whole human quadriceps muscle: effects of increasing age. J Anat 174: 239–249, 1991 [PMC free article] [PubMed] [Google Scholar]
- 35. Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Magnusson SP, Narici MV, Maganaris CN, Kjaer M. Human tendon behaviour and adaptation, in vivo. J Physiol 586: 71–81, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McDermott DJ, Stekiel WJ, Barboriak JJ, Kloth LC, Smith JJ. Effect of age on hemodynamic and metabolic response to static exercise. J Appl Physiol 37: 923–926, 1974 [DOI] [PubMed] [Google Scholar]
- 38. Meyer RA. Linear dependence of muscle phosphocreatine kinetics on total creatine content. Am J Physiol Cell Physiol 257: C1149–C1157, 1989. [DOI] [PubMed] [Google Scholar]
- 39. Miller RG. Dynamic properties of partially denervated muscle. Annals Neurol 6: 51/55, 1979 [DOI] [PubMed] [Google Scholar]
- 40. Ng AV, Kent-Braun JA. Slowed muscle contractile properties are not associated with a decreased EMG/force relationship in older humans. J Gerontol A Biol Sci Med Sci 54: B452–B458, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci 62: 375–381, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Ochala J, Lambertz D, Pousson M, Goubel F, Hoecke JV. Changes in mechanical properties of human plantar flexor muscles in ageing. Exp Gerontol 39: 349–358, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Rubinstein S, Kamen G. Decreases in motor unit firing rate during sustained maximal-effort contractions in young and older adults. J Electromyogr Kinesiol 15: 536–543, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Russ DW, Kent-Braun JA. Sex differences in human skeletal muscle fatigue are eliminated under ischemic conditions. J Appl Physiol 94: 2414–2422, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Shen J, Rycyna RE, Rothman DL. Improvements on an in vivo automatic shimming method [FASTERMAP]. Magn Reson Med 38: 834–839, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Stevens JE, Binder-Macleod S, Snyder-Mackler L. Characterization of the human quadriceps muscle in active elders. Arch Phys Med Rehabil 82: 973–978, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Stienen GJ, Kiers JL, Bottinelli R, Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. J Physiol 493: 299–307, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stienen GJ, Zaremba R, Elzinga G. ATP utilization for calcium uptake and force production in skinned muscle fibres of Xenopus laevis. J Physiol 482: 109–122, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Szentesi P, Zaremba R, van Mechelen W, Stienen GJ. ATP utilization for calcium uptake and force production in different types of human skeletal muscle fibres. J Physiol 531: 393–403, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taylor DJ, Styles P, Matthews PM, Arnold DA, Gadian DG, Bore P, Radda GK. Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med 3: 44–54, 1986 [DOI] [PubMed] [Google Scholar]
- 51. Valour D, Pousson M. Compliance changes of the series elastic component of elbow flexor muscles with age in humans. Pflügers Arch 445: 721–727, 2003 [DOI] [PubMed] [Google Scholar]
- 52. van Schaik CS, Hicks AL, McCartney N. An evaluation of the length-tension relationship in elderly human ankle dorsiflexors. J Gerontol 49: B121–B127, 1994 [DOI] [PubMed] [Google Scholar]
- 53. Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol 61: 361–367, 1986 [DOI] [PubMed] [Google Scholar]
- 54. Walsh B, Howlett RA, Stary CM, Kindig CA, Hogan MC. Measurement of activation energy and oxidative phosphorylation onset kinetics in isolated muscle fibers in the absence of cross-bridge cycling. Am J Physiol Regul Integr Comp Physiol 290: R1707–R1713, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Walter G, Vandenborne K, Elliott M, Leigh JS. In vivo ATP synthesis rates in single human muscles during high intensity exercise. J Physiol 519: 901–910, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. White MJ, Harridge SDR, Carrington CA, Goodman M, Cummins P. The relationship between isometric contractile characteristics and isomyosin composition of the young and elderly human tricep surae (Abstract). J Physiol 475: 27P 1994 [Google Scholar]
- 57. Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil S, Carlier PG, Richardson RS. Multiparametric NMR-based assessment of skeletal muscle perfusion and metabolism during exercise in elderly persons: preliminary findings. J Gerontol A Biol Sci Med Sci 64: 968–974, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]