Abstract
Human primary and clonal synovial cells were incubated with glutamate receptor agonists to assess their modulating influence on glutamate receptors N-methyl-d-aspartate (NMDA) NR1 and NR2 and inflammatory cytokines to determine potential for paracrine or autocrine (neurocrine) upregulation of glutamate receptors, as has been shown for bone and chondrocytes. Clonal SW982 synoviocytes constitutively express vimentin, smooth muscle actin (SMA), and NMDA NR1 and NR2. Coincubation (6 h) with glutamate agonists NMDA (5 μM), and the NMDA NR1 glycine site activator (±)1-aminocyclopentane-cis-1,3-dicarboxylic acid (5 μM), significantly increases cellular mRNA and protein levels of glutamate receptors, as well as increasing vimentin, SMA, tumor necrosis factor-α, and RANTES (regulated on activation, normal T-cell expressed and secreted), assessed qualitatively and quantitatively with nucleotide amplification, image analysis of immunocytochemical staining, fluorescein-activated cell sorting, Western blotting, and immunoassays. Human primary synovial cells harvested from patients with arthritic conditions also constitutively expressed NMDA NR1 with increases after agonist treatment. Glutamate receptor agonist-induced increases were blocked by the noncompetitive glutamate antagonist MK-801 (8 μg/ml) and NR1 blocking antibody. Coincubation with glutamate agonists and phorbol 12-myristate 13-acetate, a protein kinase C activator, significantly enhanced mean levels of TNF-α and RANTES in SW982 cell supernatants compared with incubation with either agent alone. Increases were diminished with protein kinase inhibitor and NR1 blocking antibody. The functional activation of glutamate receptors on human synoviocytes establishes a neurogenic cell signaling link between neurotransmitter glutamate released from nerve terminals and target cells in the joint capsule. The influence of glutamate on subsequent release of cellular proinflammatory mediators in non-neural tissue for activation of downstream immune events supports a peripheral neuroimmune link in arthritis.
Keywords: neurogenic inflammation; arthritis; glutamate receptor; cytokines, protein kinase C; pain
dual sensory-efferent function is part of the neuroregulatory response of peripheral nociceptive nerve fibers after injury and in inflammatory states that mediates local vasodilation and edema (56) through release of neuropeptides such as substance P and calcitonin gene-related peptide (22). It is firmly established that excitatory amino acid neurotransmitters also contribute directly to the inflammatory cascades, joint dysfunction, and pain of arthritis by mechanisms that include peripheral nerve and spinal cord sensitization, glial release of tumor necrosis factor-α (TNF-α), and dorsal root reflexes (2, 3, 6, 21, 23, 25, 30, 31, 34, 35, 42–51, 53, 58–60, 62, 63). There is emerging support for the concept that glutamate receptors on nonneural cells locally in joint tissue are activated by pathophysiological levels of glutamate to cause increased blood flow and bone resorption (2, 7, 8, 15, 24, 29, 30, 35, 41, 42). Previous studies indicate that osteoblasts secrete glutamate and express glutamate receptors (13, 14). The present study characterizes the N-methyl-d-aspartate (NMDA) receptors on nonneuronal synoviocytes. Functional glutamate NMDA receptor channels, however, require obligatory binding of the coagonist glycine (Gly) to the Gly-binding site located on the NR1 subunit to mediate ion influx (17). Thus in these studies we combined administration of NMDA and (±)1-aminocyclopentane-cis-1,3-dicarboxylic acid (ACPD) for coactivation of NMDA receptors on synoviocytes. Response to activation of glutamate NMDA receptors is secretion of inflammatory mediators that in turn can directly activate peripheral nerve endings and the immune response (9, 18, 19, 24, 25, 56, 61).
Induction of experimental arthritis leads to increases in the excitatory amino acids (EAA) glutamate (Glu) and aspartate (Asp) in the spinal cord dorsal horn (45, 47, 48, 50, 54, 59), as well as locally in the knee joint synovial fluid (23, 25). Elevated levels of these glutamate receptor agonists are correlated with the development and time course of inflammatory swelling and blood flow changes (24, 43, 49, 52) and the hypersensitivity measureable electrophysiologically and behaviorally in rats, cats, and primates (3, 18, 21, 23, 37, 40, 43, 46, 53, 59, 62, 63). Thus glutamate is proposed as a major neurogenic factor affecting the activation state of both the central and peripheral terminals of primary afferent nerve fibers and as a potentiator of the dorsal root reflexes that enhance and prolong inflammation and nociceptive responses (29, 30, 36–38, 43, 51, 53, 54).
Elevated levels of Glu and Asp have been reported in the synovial fluid of patients with clinically active arthritis (31, 32). Correlations between synovial fluid EAA levels and synovial fluid levels of inflammatory mediators TNF-α, regulated on activation, normal T-cell expressed and secreted (RANTES), and interleukin-8 (IL-8) also have been reported in human arthropathies of rheumatoid arthritis, acute gout, and osteoarthritis (31). The addition of l-glutamate to primary synovial cell cultures harvested from patients with arthropathies increases TNF-α levels in vitro (31). The following experimental data support a neurogenic role for synovial fluid EAA with effector actions on peripheral nonneural tissue: 1) EAA levels increase over several hours in inflamed knee joints, with fluctuation ranging from normal to elevations of several fold (23, 25). The increased levels can be blocked by pretreatment with intra-articular glutamate antagonists or lidocaine. 2) Direct intra-articular injection of Glu and Asp increases blood flow to the joint and is counteracted by glutamate antagonists (23). 3) Local injection of glutamate agonists induces pain-related behaviors, including secondary heat and mechanical hyperalgesia and allodynia, and is blocked by glutamate receptor antagonists (23).
Although glutamate-mediated inflammatory and pain-related events have been an intense focus of our studies, the role of glutamate receptor activation on local cellular targets has not been directly addressed previously. The present study demonstrates glutamate NMDA receptors on human nonneuronal synoviocytes. NMDA receptors assemble from two Gly-binding NR1 subunits with two Glu-binding NR2 subunits to form functional Glu-gated excitatory receptors that when coactivated mediate plastic responses. Efficient NMDA receptor activation promoting LTP-like activation requires not only Glu but also a coagonist, Gly (17). The NMDA and ACPD used in this study are potent activators of the Glu and the Gly binding sites, respectively, on NMDA receptor subunits. Synoviocytes that line the knee joint secrete inflammatory mediators and proliferate in inflammatory states. The glutamate dl-α-amino-3-hydroxy-5-methylisoxazole-propionic acid (AMPA) receptor antagonist NBQX and the channel blocker memantine have been shown to inhibit synoviocyte proliferation (34). The present study demonstrates that specific coactivation of the glutamate receptors on the cells in vitro, i.e., in conditions independent of neural and humoral influences, results in the upregulation of specific cellular proteins, including inflammatory mediators. These results are consistent with a role for neurotransmitter Glu, glutamate nerve terminals, and their subsequent effector influences on targeted peripheral tissues in the promotion and maintenance of sensitized pain states and inflammatory events.
MATERIAL AND METHODS
Cell cultures.
The clonal cell line SW982, derived from a human synovial sarcoma, was obtained through American Type Culture Collection (HTB-93; Bethesda, MD). Immunocytochemical studies ascertained that these cells were vimentin positive, smooth muscle actin positive, and CD11b negative, consistent with the phenotype of type B synovial cell fibroblasts. In addition, the SW982 cells did not stain with anti-neuronal nuclei (neuN) antibody. The cells were maintained and grown in Leibowitz medium, 10% heat-inactivated fetal calf serum, 2 mM l-Glu, 1,000 U/ml penicillin G, and 1,000 pg/ml streptomycin (all from GIBCO, Grand Island, NY). Cells were incubated in a humidified cell incubator at 37°C with ambient CO2 and used in passages 4–21. Cells were split using 0.25% trypsin-0.02% EDTA disruption and plated in a six-well plate on glass slides at a density of 200,000 cells/well for immunocytochemistry and plated in a 24-well plate at 35,000 cells/well for immunoassays. Cells were allowed to grow to 30–50% confluency for 24 h before treatment.
Primary human synovial cell cultures were derived from synovial fluid obtained at the time of therapeutic arthrocentesis from patients diagnosed with rheumatoid arthritis or psoriatic arthritis according to the American College of Rheumatology diagnostic criteria (20) and collected under Institutional Review Board approval and guidelines. The culture protocol was slightly modified from previously established methods (9). The fluid was plated within 40 min of arthrocentesis on sterile glass coverslips in a 1:2 dilution of DMEM, 10% heat-inactivated fetal calf serum, 2 mM l-Glu, 1,000 U/ml penicillin G, and 1,000 pg/ml streptomycin overnight. For the next 2 days, nonadherent cells were removed by multiple washings with sterile PBS. The adherent cells were then incubated in DMEM buffer as described above for 14–21 days, with several washings and clearing of nonadherent cells before initiation of the experiment. The primary cell cultures were incubated at 37°C with 5% CO2. Cell viability was monitored by using a cytotoxicity detection kit for lactate dehydrogenase (LDH; Roche Diagnostics, Indianapolis, IN) (26) and by trypan blue dye exclusion. Cell viability >95% in SW982 and primary synovial cell cultures was maintained throughout the experiments (>24 h). No primary cell proliferation was noted.
Treatment of human synovial cells with cellular activators and inhibitors.
Separate and combined incubations with NMDA and ACPD (trans-ACPD; Tocris Cookson, Ellisville, MO) were used to activate glutamate receptors for these experiments. Dose-response (0, 0.1, 0.5, 5, 25, and 50 μM) and time-course experiments (0, 2, 4, 6, 12, 24 and 48 h) were performed to determine the minimal doses and incubation times for glutamate agonist activation. Increased NR1 expression was noted with both NMDA and ACPD incubations, but the maximum increase was noted at the smallest dose using 5 μM NMDA and 5 μM ACPD. Therefore, coincubations with 5 μM NMDA and 5 μM ACPD for 6 h were used for all experiments unless otherwise indicated. Primary cell lines were incubated in their regular growth media containing 5 μM NMDA and 5 μM ACPD for 6 h unless otherwise indicated. Preincubation of the SW982 cells with the potent, selective, and noncompetitive glutamate receptor antagonist MK-801 [(+)-MK-801 maleate; Tocris, Ellisville, MO] before addition of NMDA and ACPD was also done to assess specificity of the glutamate receptor agonist activation. The MK-801 dose presented is 10 μg/ml, although doses tested ranged from 0.5 to 50 μg/ml. The above-described and all other experiments were performed in triplicate conditions for a minimum of three times unless otherwise stated.
Incubation with NMDA and ACPD does not significantly increase TNF-α protein expression in the primary or SW982 cell supernatants in most cases. Therefore, in some experiments, phorbol 12-myristate, 13-acetate (PMA; Sigma Chemical, St. Louis, MO), which activates protein kinase C (PKC) and upregulates TNF-α protein expression in the cell supernatant, was added to the cell incubations to generate a predictable submaximal activation of the cultures. Dose-response experiments were performed to determine the dose that would generate a submaximal response (PMA dose range of 0.5–100 nM). In these studies, PMA was added to cell cultures at a final concentration of 25 nM, 6 h after the addition of NMDA (5 μM) and ACPD (5 μM), for a final incubation time of 24 h, unless otherwise stated. At the end of the coincubations with NMDA, ACPD, and PMA, the cell supernatants were pelleted and assayed for cellular cytokines TNF-α or RANTES, as detailed below. Cellular disruption by sonication did not increase TNF-α concentrations in the cell supernatant above that for intact cells.
Bisindolylmaleimide-1 (Bis; Calbiochem, La Jolla, CA), a specific PKC inhibitor, was prepared in sterile solution according to the manufacturer's recommendations. The Bis was added to the cell cultures (final concentration range: 0–1 μM) 1 h before the addition of NMDA and ACPD and 7 h before the addition of PMA. Dose-response curves and cell cytotoxicity assays were used to assess minimal effective dose and sustained cell viability ≥95% in the presence of the inhibitor. It was determined that final doses of Bis >500 nM affected cell viability.
Immunocytochemistry.
Immunocytochemistry was used to identify glutamate receptor subtypes present on cultured synoviocytes. Several primary antibodies were used for staining cells in culture, including 1) rabbit polyclonal anti-NMDA NR1 glutamate receptor antibody (Chemicon International, Temecula, CA; 1:50 dilution), 2) goat polyclonal NMDAζ1 IgG (Santa Cruz Biotechnology, Santa Cruz, CA; 1:50 dilution), 3) mouse monoclonal anti-glutamate receptor (NMDA R1) antibody (BD Biosciences-Pharmingen, San Diego, CA; 1:50 dilution), or 4) mouse monoclonal anti-NR1, CT IgG (Upstate Biotechnology, Lake Placid, NY; 1:50 dilution). The studies presented used COOH-terminal rabbit, COOH-terminal mouse, or NH2-terminal rabbit anti-NR1 antibodies as the primary antibody. The primary antibody was diluted in PBS with 1.0% normal goat serum (NGS; Sigma Chemical) for 48-h incubation at room temperature. The glass coverslips were then washed with 1% NGS and 0.02% Triton X-100 and then incubated for 30 min at room temperature with the appropriate secondary antibody, goat anti-rabbit IgG or rabbit anti-mouse IgG with a fluorescent Alexa tag (Alexa Fluor 568 red or Alexa Fluor 488 green; Molecular Probes, Eugene, OR; 1:100 dilution).
Additional positively staining antibody used to characterize the cells included mouse monoclonal anti-NMDA NR2 subunit antibody (Tocris Cookson; 1:100 dilution), mouse monoclonal anti-vimentin antibody (DakoCytomation, Glostrup, Denmark; 1:400 dilution), or mouse monoclonal anti-smooth muscle actin (SMA; DakoCytomation; 1:200 dilution). Matched nonspecific antibodies were used at appropriate dilutions to serve as negative antibody controls. The nonspecific negative controls included mouse monoclonal anti-CD21 monoclonal antibody (HB-135; ATCC), normal mouse IgG, normal rabbit serum, normal rabbit IgG, and NGS (Sigma Chemical). Immunochemical negative controls included wells processed in the absence of primary or secondary antibody, and these showed no staining. In all staining experiments, matched nonspecific primary antibody controls were also performed on untreated and treated controls to confirm the specificity of the specific primary antibody. The cell staining experiments with positive and negative controls were performed a minimum of three times.
After coverslips were applied with hard-set mounting medium with or without 4,6-diamidino-2-phenylindole nuclei counterstain (Vector Laboratories, Burlingame, CA), the stained cells were visualized with a Nikon microscope (E1000; Nikon, Corning, NY) equipped with a SPOT digital camera system (Nikon Instruments, Melville, NY). Photomicrographs were generated with MetaVue software (Molecular Imaging Systems, Downington, PA). Specificity of immunostaining by each primary antibody was confirmed by an absence of staining when 1) the primary antibody was deleted, 2) only matched nonspecific antibody at the same dilution was used, or 3) matched nonspecific antibody controls did not yield immunofluorescence in untreated (control) or treated cells. All immunostaining was processed at the same time with the same solutions so that valid comparisons could be made across treatment groups.
Quantification of immunostaining.
For immunochemical quantification, data for image analysis were collected using the Bioquant True Color Windows 98 image analyzer system software with an Optronics DEI 750 camera (Bioquant Image Analysis, Nashville, TN). Under the same conditions, stained sections were observed, photomicrographs taken, and stain densities quantified. Mean fluorescence density (arbitrary units, au) and areas (μm2) of whole cells were derived from two independent experiments using an unbiased sampling method. According to the manufacturer's protocol, densities and areas were measured from two sets of nine fields sampled from a standardized nine-point grid transferred to the coverslip back. Data were obtained on whole cells in the nine fields at ×40 magnification, which included >80 cells per experiment. Mean fluorescence intensity is expressed as per the protocol in arbitrary units normalized to unstained cells.
Fluorescence-activated cell sorting analysis.
Adherent SW982 cells were prepared for fluorescence-activated cell sorting (FACS) analysis using the following protocol. Cells were detached from flasks using Cell Dissociation solution (Sigma Chemical). Cells were washed three times in PBS (phosphate-buffered saline containing 0.9 mM CaCl2 and 0.5 mM MgCl2) with 1% BSA. The pelleted cells were resuspended and aldehyde-fixed using 200 μl of a mixture of methanol and 4% paraformaldehyde (1:1, vol/vol) for 5 min at room temperature. Cells were pretreated with 200 μl of PBS, with 3% NGS (Sigma Chemical) and 0.01% Triton X-100 (Sigma Chemical) for 30 min at room temperature and were then washed three times with PBS. Cells were aliquoted as 200,000 cells per condition and incubated with primary antibody (control, normal rabbit serum, or rabbit polyclonal anti-NMDA NR1 glutamate receptor antibody; Chemicon International; 1:100 dilution), 0.01% Triton X-100, and 1% NGS for 1 h at room temperature. Mouse monoclonal anti-vimentin antibody (DakoCytomation; 1:400 dilution) was used as a positive control for these experiments. The cells were washed three times with PBS and resuspended in 200 ml of PBS containing second antibody (FITC-labeled goat anti-rabbit; Molecular Probes; 1:200 dilution) or rabbit anti-mouse IgG with a fluorescent Alexa tag (Molecular Probes; 1:100 dilution) in 0.01 Triton X-100 and 1% NGS for 1 h at room temperature.
At the end of the second incubation, the cells were washed three times in PBS, and the pellet was resuspended in 50 ml of PAB (PBS buffer that is Ca2+ and Mg 2+ free with 0.01% sodium azide and 1% BSA). The cells were transferred to polystyrene tubes for FACS (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ) and titrated to a final volume of 500 ml with PBS. Fluorescence intensity was measured at 530 nM. A population of 10,000 cells was counted for each condition. The FACS measurements were repeated three times using rabbit specific and nonspecific antibodies and in another set of experiments using mouse monoclonal specific NMDA NR1 and nonspecific antibodies as controls.
Western blot analysis.
Cytosolic protein extracts were obtained using established methods (31). Protein lysates (40 μg protein/well for SW982 cell lysates and 10 μg/well for brain tissue lysate) from cytosolic cell fractions were diluted in 4× SDS loading buffer [100 ml of SDS buffer contained 3 g of Tris, 8 g of SDS, 2.5 g of DTT, 0.05 g of bromphenol blue, and 40% (vol/vol) glycerol] and loaded onto a 10% SDS-polyacrylamide denaturing gel. After electrophoresis (1–2 h at 50 mA), the gels were blotted onto nitrocellulose membranes by overnight electrophoretic transfer. Membranes were then incubated with mouse monoclonal anti-NR1 (BD Biosciences-Pharmingen; 1:1,000) or goat polyclonal anti-NR1 antibody (Santa Cruz Biotechnology; 1:1,000), mouse monoclonal anti-vimentin antibody (1:1,000), or matched nonspecific antibody controls. Reactive bands were detected by a chemiluminescent Western blot detection kit (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instructions. Staining for β-actin was used as an internal control. Band intensity was measured by densitometry with the densitometry program, using Labworks image acquisition and analysis software (UVP Bioimaging, Upland, CA), from three separate blotting experiments.
RT-PCR and real-time PCR.
Low-passage SW982 cell cultures were grown in 75-mm flasks until they reached 70–80% confluence. Cell flasks were then either untreated, as controls, or treated with 5 μM NMDA and 5 μM ACPD in cell medium and incubated for 6 h. The cells were harvested by disruption by removal of the incubation medium and extraction of total RNA using the Trizol reagent per the manufacturer's protocol (catalog no. 10296028; Invitrogen, Carlsbad, CA). The cell culture protocol for treated and untreated cells was performed in three separate experiments. First-strand cDNAs were synthesized from total RNA by RT using the SuperScript III first-strand kit (catalog no. 18080051; Invitrogen) with random hexamer primers as described by the manufacturer.
Quantitative real-time PCR analysis was performed using an Applied Biosystems 7000 sequence detection system (ABI, Foster City, CA). Each sample was analyzed in triplicate, and the means were used for statistical purposes. The thermal profiles were obtained using 2-min incubation at 50°C, followed by an initial 10-min denaturation step at 95°C and by 40 cycles of 1 min each at 60°C, plus 15 s at 95°C. Significant contamination with genomic DNA was excluded by amplifying non-reverse-transcribed RNA. The probes and primers NMDA NR1 (Hs00609557_m1) and β-actin (Hs99999903 m1) were ordered from Applied Biosystems. The threshold cycle (Ct), i.e., the cycle number at which the amount of amplified gene of interest reached a fixed threshold, was determined subsequently. Relative quantification of NMDA NR1 mRNA expression was calculated using the comparative Ct method described elsewhere (26, 33). The relative quantification value of target, normalized to an endogenous control β-actin gene and relative to a control, is expressed as 2ΔΔct (fold), where ΔCt = Ct of target gene (NMDA NR1) − Ct of endogenous control gene (β-actin), and ΔΔCt = ΔCt of samples for target gene − ΔCt of the control for the target gene. Two microliters of synthesized cDNA from each individual sample were used to amplify NMDA NR1 and β-actin, respectively.
Amplification of NMDA NR2 A, B, C, and D subunits was performed via reverse transcriptase-PCR (RT-PCR). Total RNA was extracted from untreated SW982 and selectively amplified for NMDA NR2 A–D subunits. The amplified NMDA NR2 subunit cDNA fragments were extracted from the gel, and subunit identity was confirmed by nucleotide sequencing. The NMDA NR2 subunit fragments were amplified from designed 20-base pair primers amplified from the following nucleotide regions of the NMDA NR2 subunit nucleotide templates provided by GenBank (National Center for Biotechnology Information, Bethesda, MD) and generated by the University of Texas Medical Branch Biochemistry and Molecular Biology Core (Galveston, TX). Amplified NMDA NR2 fragment identities were confirmed by dideoxynucleotide sequencing at the UTMB Biochemistry and Molecular Biology Core. The accession numbers, primer locations, and primer sequences for each NMDA NR2 subunit are as follows: NMDA NR2A (4,745 bp), NM_001134408, nt 1953–2303, forward: gttggatacaacagaaacttagc, reverse: gatagttattccgaatgtttctc; NMDANR2B (5,941 bp), NM_000834, nt 2840–3251, forward: caccgcaaccatgaacaacacac, reverse: gtccaggggcttcttgctgatg; NMDA NR2C (4,298 bp), NM_000835, nt 1473–1932, forward: gaggtgctcttcgcggaggctgcac, reverse: atactggatacttcatgtacag; tk;3and NMDA NR2D (5,109 bp), NM_000836, nt 1631–1984, forward: aagaagatcgatggcgtctgg, reverse: ggatttcccaatggtgaaggttga.
Additional amplification regions were performed for the NMDA NR2C subunit, because amplification from nt region 1977–2379 was successful in human brain cDNA but not successful from human synoviocyte cDNA. The additional NMDA NR2C regions yielded successful amplification fragments from human brain (nt 307–747 and nt 1473–1932). Commercially obtained total brain extract (Ambion, Austin, TX) served as positive control for all subunits as shown. Reactions performed in the absence of DNA yielded no detectable bands.
Cellular cytokine and chemokine detection assays.
To assess the effects of glutamate receptor activation on the expression of cellular inflammatory mediators, we measured quantitation of cell supernatant TNF-α and RANTES. Cellular TNF-α levels were measured from cell culture supernatants using a TNF-α ELISA (R&D Systems, Minneapolis, MN). Cellular RANTES levels were measured from cell culture supernatants using a RANTES ELISA (R&D Systems). Conditions were run in triplicate, and experiments were repeated a minimum of three times; therefore, each condition represents a mean of nine samples.
Histological confirmation in rat inflamed knee joint.
The presence of glutamate receptors was confirmed in histological samples from control and inflamed knee joints harvested from rats. One knee joint of anesthetized rats was injected with complete Freund's adjuvant (CFA; 250 mg of Myobacterium butyricum, 0.01 ml in saline). After 1 wk, animals were perfused with warm saline (30 ml), followed by 4% phosphate-buffered paraformaldehyde (500 ml). Knee joints were dissected, and histological sections were cut and stained immunocytochemically for NR1 as described above or with hematoxylin and eosin.
Statistics.
Mann-Whitney U-tests were used to assess significant differences in mean values when comparing three or more groups. Student's t-tests were also used for comparisons between pairs. A P value <0.05 was considered significant. Data are means ± SE.
RESULTS
Glutamate receptor activation increases NMDA NR1 immunostaining in synoviocytes.
Glutamate NMDA NR1 subunit protein was constitutively expressed in human clonal SW982 synoviocytes. Figure 1 illustrates the immunocytochemical localization of NMDA NR1 cellular subunit protein in cultured human SW982 synoviocytes. Figure 1A shows glutamate receptor NMDA NR1 subunit in SW982 cells under baseline conditions. A 2-h coincubation with glutamate receptor agonists NMDA and ACPD markedly increased cellular staining along the nuclear rim (Fig. 1B). A 6-h coincubation with glutamate receptor agonists NMDA and ACPD markedly increased cellular staining in the perinuclear, nuclear, and cytosolic regions and increased cell size, as shown in Fig. 1C. Similar increases in NMDA NR1 subunit protein were also noted after 24-h incubations with NMDA and ACPD (Fig. 1D). Cellular viability was maintained, as determined by LDH cytotoxicity assays. Similar increases in NMDA NR1 subunit protein expression were obtained with either polyclonal or monoclonal antibodies directed against either the COOH or NH2 terminus of NMDA NR1 subunit protein. Incubation with NMDA alone or ACPD alone resulted in increases in NMDA NR1 expression, but optimal increases were noted with coincubation of both agonists. Incubation of the SW982 cells with 10 μM Asp also resulted in increased staining of NMDA NR1 subunit protein in the cytosolic and nuclear regions. No cellular staining was detected when normal rabbit serum (Fig. 1E) or matched nonspecific isotypic antibody was used as the primary antibody. A change in cellular morphology was noted in cell cultures after 24-h incubations with 5 μM NMDA and 5 μM ACPD (Fig. 1, C and D). The cells appeared larger and had expansive shapes (Fig. 1D compared with Fig. 1A). Computed areas of the clonal synoviocytes were significantly increased comparing untreated and treated cells (633.7 ± 32.3 vs. 798.9 ± 48.6 μm2, respectively, P = 0.004).
Fig. 1.
Glutamate N-methyl-d-aspartate (NMDA) NR1 receptors on human SW892 cultured synoviocytes increase after treatment with NMDA and (±)1-aminocyclopentane-cis-1,3-dicarboxylic acid (ACPD). A: glutamate NMDA NR1 receptor staining (red) is imaged in a high-power photomicrograph of control untreated synovial cells. B–D: increases in immunostaining for glutamate NMDA NR1 are evident in cells incubated concurrently with 5 μM NMDA and 5 μM ACPD for 2 (B), 6 (C), and 24 h (D). Cells shown were stained with rabbit anti-NMDA NR1 antibody (1:100). E: cells were stained with normal rabbit serum (NRS) as a nonspecific control (1:100). Dual nuclear counterstaining with 4,6-diamidino-2-phenylindole (DAPI) is shown in blue. Bar, 20 μm. F: 1 of 3 fluorescence-activated cell sorting (FACS) analyses used to assess expression of NMDA NR1 subunit protein in SW892 cultured synoviocytes treated for 6 h with 5 μM NMDA and 5 μM ACPD (except peak 3). Peak 1, with secondary antibody only; peak 2, nonspecific rabbit serum control; peak 3, untreated SW892 cultured synoviocytes with rabbit anti-NMDA NR1 antibody; peak 4, rabbit anti-NR1 antibody; peak 5, mouse monoclonal anti-vimentin antibody. Nonspecific antibody control peaks (not shown) were identical to peak 2 in both untreated and NMDA/ACPD-treated cultures. Fluorescence intensity was read at 530 nm.
Detection of NMDA NR1 FACS.
FACS was also used to demonstrate NMDA NR1 subunit protein on SW982 cells, as shown in Fig. 1F. All cell aliquots were first treated with 5 μM NMDA and 5 μM ACPD, with the exception of the untreated control (peak 3, in green). Treated cells incubated with the second antibody alone (FITC-goat anti-rabbit antibody) are shown as peak 1 (in blue). Peak 2 (in red) depicts treated cells incubated with normal rabbit serum as the matched, nonspecific antibody control. Peaks 3 and 4 (in orange) demonstrate the fluorescence intensities of protein staining for NMDA NR1. Peak 3 depicts untreated cells stained with rabbit anti-NMDA NR1 antibody, showing that fluorescence clearly increased over the nonspecific antibody control (peak 2). Peak 4 shows that when the cells were treated with NMDA and ACPD and then stained with rabbit anti-NMDA NR1 antibody, there was a slight shift in the fluorescence intensity compared with the staining of untreated cells (peak 3). Peak 5 (magenta) represents the fluorescence intensity observed in cells stained with monoclonal mouse anti-vimentin antibody after treatment with NMDA and ACPD.
The FACS experiment was also performed with mouse monoclonal antibodies. Shifts in peak intensity over nonspecific controls similar to those observed in peaks 3 and 4 were seen using mouse monoclonal anti-NR1 antibody. The fluorescence intensity peaks of untreated control cells and treated cells stained with nonspecific mouse antibody were identical to the peaks of NMDA- and ACPD-treated cells shown in peak 2.
MK-801 blocks NMDA NR1 staining increase in synoviocytes.
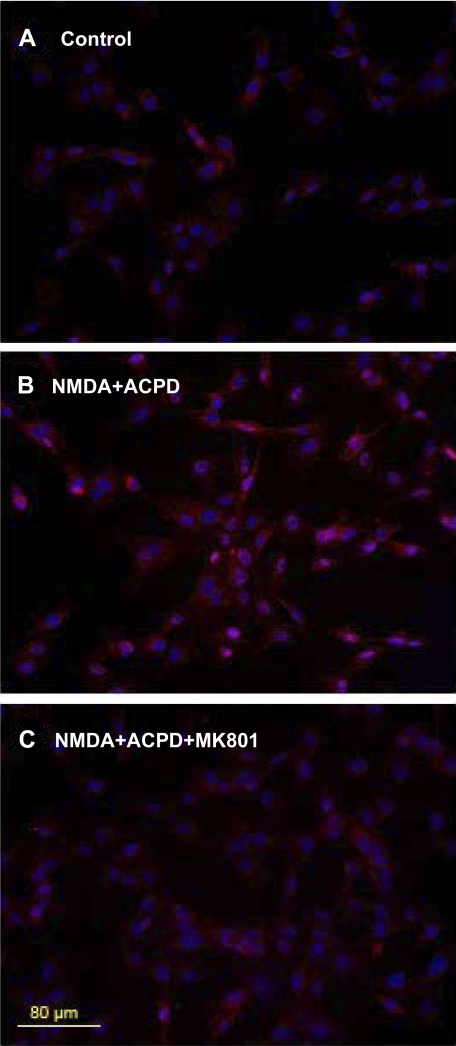
The increase in NMDA NR1 staining with NMDA and ACPD incubation was blocked by preincubation of the SW982 synoviocytes with the selective NMDA receptor antagonist MK-801. Figure 2 demonstrates NMDA NR1 staining of unstimulated SW982 cells (Fig. 2A), after 6-h incubation with 5 μM NMDA and 5 μM ACPD (Fig. 2B), and with preincubation with 10 μM MK-801 before the addition of NMDA and ACPD (Fig. 2C).
Fig. 2.
Preincubation with MK-801 blocks NMDA NR1 upregulation in synoviocytes. A: low-power photomicrograph of glutamate NMDA NR1 receptors (red) stained with rabbit anti-NMDA NR1 antibody on control untreated synovial cells. B: cells incubated concurrently with 5 μM NMDA and 5 μM ACPD for 6 h. C: cells preincubated for 1 h with 10 μM MK-801, a selective, noncompetitive NMDA receptor antagonist, and then incubated for 6 h with 5 μM NMDA and 5 μM ACPD. Dual nuclear counterstaining with DAPI is shown in blue. Bar, 80 μm.
Immunocytochemical localization of NMDA NR1 on primary synovial cells.
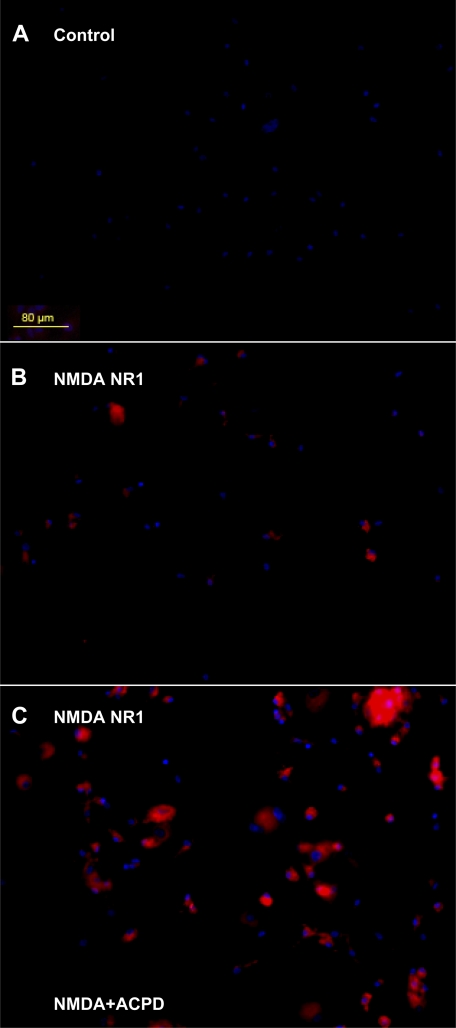
Primary synovial cell cultures were established from synovial fluid samples from patients with clinically symptomatic rheumatoid arthritis. Results for the primary synovial cultures were similar to those obtained for the clonal synoviocytes. Negligible cell staining was detected in primary synoviocyte cultures treated with nonspecific isotypic antibody controls (Fig. 3A). Staining for NMDA NR1 protein was demonstrated in the primary synovial cell cultures under unstimulated conditions (Fig. 3B). Staining for NMDA NR1 protein was increased after 6-h incubation with 5 μM NMDA and 5 μM ACPD (Fig. 3C) compared with untreated cells (Fig. 3B). A change in cellular morphology was also noted in the primary cultures after 24-h incubations with 5 μM NMDA and 5 μM ACPD compared with unstimulated primary synovial cultures (Fig. 3A), as previously noted for the clonal synovial cell cultures.
Fig. 3.
Immunocytochemical localization of glutamate NMDA NR1 on primary synovial cell cultures. Photomicrographs of cells derived from synovial fluid of a patient with rheumatoid arthritis are stained (red) immunocytochemically for glutamate NMDA NR1 receptor subunit. A: untreated synovial cells were stained with NRS as a nonspecific matched antibody control. B: immunostaining for NMDA NR1 receptor subunit is evident in synoviocyte cell cultures stained with NMDA NR1 subunit antibody. C: increases in the immunostaining for NMDA NR1 receptor subunit are evident in synoviocyte cultures stained with NMDA NR1 subunit antibody after coincubation with 5 μM NMDA and 5 μM ACPD for 6 h. Dual nuclear counterstaining with DAPI is shown in blue. Bar, 80 μm.
Glutamate receptor activation upregulates NMDA NR2 on human synoviocytes.
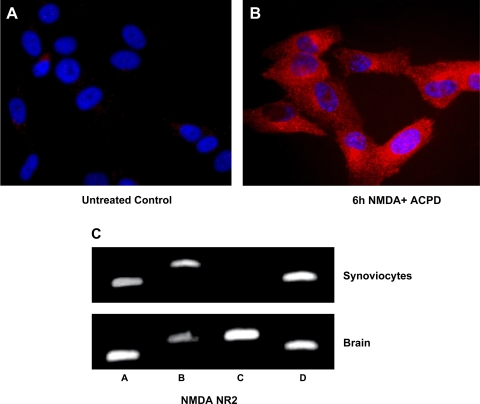
Fluorescent immunostaining of glutamate NMDA NR2 subunit protein was also constitutively expressed in human clonal SW982 synoviocytes. Glutamate NMDA ionotropic receptors exist as multiple subtypes with distinct pharmacological and biophysical properties (i.e., channel open probability) that are largely determined by the type of NR2 subunit incorporated in the heteromeric NR1/NR2 complex. These studies confirmed the presence of NMDA NR2 glutamate receptor product of the predicted size (26, 33, 64, 65) on SW982 cells detected by immunocytochemistry and RT-PCR. Figure 4A shows SW982 cells with fluorescent immunostaining demonstrating cellular glutamate receptor NMDA NR2 subunit under baseline conditions. A 6-h coincubation with glutamate receptor agonists NMDA and ACPD markedly increased cellular staining in perinuclear and cytosolic regions (Fig. 4B).
Fig. 4.
Glutamate NMDA NR2 receptor subunit expression on synoviocytes identified by immunostaining and RT-PCR. A: high-power photomicrograph of glutamate NMDA NR2 receptors (red) on control untreated clonal SW892 human synoviocytes synovial cells. B: immunostaining was increased in cells incubated concurrently with 5 μM NMDA and 5 μM ACPD for 6 h. Cells shown were stained with rabbit anti NMDA NR2 antibody. Bar, 20 μm. C: amplification of specific NMDA NR2 subunits was identified by RT-PCR in human synoviocytes. Cytosolic lysates of untreated SW982 cells (synoviocytes) and human brain (hippocampus) as control were applied to SDS-PAGE and transferred to a nitrocellulose filter. Lanes were primed for NMDA NR2 subunits A–D as shown. All 4 subunits were expressed in the lysates from human brain. NMDA NR2 subunits A, B, and D were expressed in lysates from untreated SW982 synoviocytes.
Figure 4C demonstrates the results of amplification of NMDA NR2 subunits by RT-PCR in total RNA extracts of untreated SW982 human synovial cells and a human brain preparation. NMDA NR2 subunits A–D were amplified from total RNA lysates from brain, at the appropriate size. NMDA NR2 subunits A, B, and D were amplified from SW982 synovial cell RNA extracts. NMDA NR2A, B, and D subunit amplifications yielded one product of the predicted size in both human brain and synoviocytes. NMDA NR2C was not amplified from untreated human synovial cell extracts. Three regions of the NMDA NR2C subunit (nt 307–747, nt 1473–1932, and nt 1977–2379) were amplified successfully in human brain but not in SW982 human synoviocytes.
Glutamate receptor activation increases vimentin, NR1 and NR2, CD11b, or CD21 in human synoviocytes.
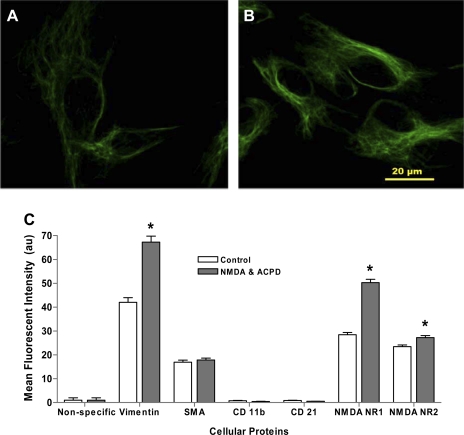
The effect of glutamate receptor activation on additional cellular activation proteins was also assessed in SW982 cultured synoviocytes. Cellular staining of intracellular protein vimentin is shown in unstimulated cultures in Fig. 5A. There was a marked increase in cytosolic staining for vimentin after a 6-h coincubation with 5 μM NMDA and 5 μM ACPD (Fig. 5B). A perinuclear distribution of vimentin was noted in some cells, but there was no increase in staining in the nuclear region. The SMA and other proteins typically upregulated by cellular activation, CD11b and CD21, were not changed after glutamate treatments. A semiquantitative protocol for immunofluorescent staining density was used to assess relative content of stained cellular synoviocyte proteins in the context of glutamate receptor activation with NMDA and ACPD in SW982 cells. Comparisons between treated and untreated pairs for each protein were performed and are shown in Fig. 5C. There was minimal immunofluorescence staining density in the nonspecific antibody controls for untreated and NMDA- and ACPD-treated cells (0.76 ± 0.13 au). The maximum fluorescence density was obtained with cells stained with the anti-vimentin antibody. The untreated cells had a mean vimentin density staining of 54.24 ± 28.12 au, which was significantly increased with glutamate receptor activation (89.34 ± 31.69 au, P < 0.01).
Fig. 5.
Vimentin expression on clonal SW982 synoviocytes. A and B: high-power photomicrographs of immunocytochemical staining for vimentin in control untreated synovial cells (A) and in cells coincubated with 5 μM NMDA and 5 μM ACPD for 6 h (B). Matched nonspecific antibody staining yielded no detectable fluorescence. Bar, 20 μm. C: bar graph showing the mean relative fluorescence densities of cellular proteins after glutamate receptor activation in SW982 cultured synoviocytes. Mean cellular protein densities were calculated from unbiased sampled fields using Bioquant software. The untreated cell culture groups are illustrated with open bars, and filled bars represent cell cultures that were coincubated for 6 h with 5 μM NMDA and 5 μM ACPD. Significant increases (*P < 0.05) in mean fluorescence densities were noted for vimentin, NMDA NR1, and NMDA NR2 staining in the NMDA- and ACPD-treated cells compared with untreated cells for each of these antibody pairs. No increases in fluorescence intensity were noted comparing control and treated cells with smooth muscle actin (SMA) staining. Negligible fluorescence densities were noted with nonspecific, anti-CD11b, or anti-CD21 antibody staining in untreated and treated synoviocyte cultures.
The mean fluorescence density detected for SMA protein remained constant comparing untreated cultures (Fig. 5C, 16.91 ± 7.81 au) with NMDA- and ACPD-treated cultures (17.85 ± 7.57 au). Mean fluorescence density scores were minimal for incubations with antibody against CD11b and CD21. No increase in stain density was obtained in coincubations, indicating these proteins were not influenced by NMDA and ACPD, as shown.
The mean fluorescence density obtained for NMDA NR1 protein (Fig. 5C) was significantly increased as a result of glutamate receptor activation with NMDA and ACPD (50.28 ± 1.45 au) compared with untreated cultures (28.45 ± 0.91 au, P < 0.01). The differences in mean fluorescence density for protein staining are consistent with the differences in protein expression noted visually in immunofluorescent images and intensity measurements illustrated in Figs. 1 and 3 and with Western blot analysis (Fig. 6, see below). The mean fluorescence density of NMDA NR2 was less than the density obtained for cellular staining of NMDA NR1 but did show significant increases in comparisons between untreated cultures and coincubations with 5 μM NMDA and 5 μM ACPD (23.44 ± 0.73 and 27.25 ± 0.82 au, respectively, P < 0.01).
Fig. 6.
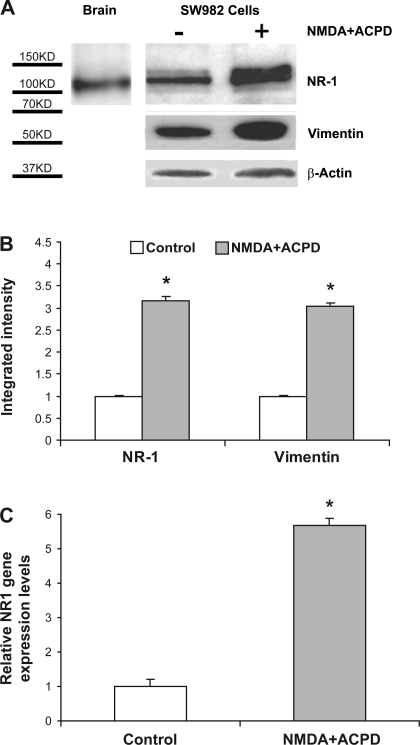
NMDA NR1 and vimentin expression by Western blot and real-time PCR analysis of clonal SW982 synoviocytes. A: cytosolic lysates were applied to SDS-PAGE and transferred to a nitrocellulose filter. Top row: human brain (hippocampus) was used as a positive control for NMDA NR1 subunit protein, at 116 kDa (left lane). Untreated SW982 human synovial cell lysate was stained with mouse monoclonal anti-NMDA NR1 antibody (middle lane). SW982 human synovial cell lysate was coincubated with 5 μM NMDA and 5 μM ACPD for 6 h and stained with mouse monoclonal anti-NMDA NR1 antibody(right lane). Middle row: the blot was stripped, and the lanes were then stained with mouse monoclonal anti-vimentin antibody. Middle lane, untreated cells. Right lane: cells were coincubated with 5 μM NMDA and 5 μM ACPD for 6 h. Bottom row: All lanes were also stained for β-actin as an internal control. Left lane was exposed for 10 s, and all other lanes were exposed for 30 s. Similar results were obtained on Western blot studies using a mouse monoclonal antibody specific for NMDA NR1 (shown in A) or a rabbit polyclonal antibody. B: corrected (normalized) densitometry determinations of Western blot staining for NMDA- and ACPD-treated cells showed a 3-fold increase in NMDA NR1 subunit protein and a 3.1-fold increase in vimentin protein compared with untreated cells (control, *P < 0.01). C: the mean relative NMDA NR1 gene expression level determined by real-time PCR was increased in NMDA- and ACPD-stimulated synoviocytes (5.8-fold) compared with untreated cells (control, *P < 0.01).
Measurement of NMDA NR1 and vimentin upregulation by glutamate receptor agonists with Western blot analysis and real-time PCR.
Cytosolic protein lysates from untreated and NMDA- and ACPD-treated SW982 cells were employed to assess NMDA NR1 and vimentin upregulation by Western blot analysis as shown in Fig. 6A. The left lane shows a NMDA NR1 protein band at an apparent molecular mass of 116 kDa, using human brain hippocampus as a positive control for the NMDA NR1 antibody. The middle lane shows that NMDA NR1 protein from SW982 human synoviocytes isolated with a similar electrophoretic mobility, with an apparent molecular mass of 116–120 kDa. The increase in NMDA NR1 protein after glutamate receptor activation observed with immunostaining was also reflected as an upregulation of NR1 protein, demonstrated by densitometric determinations of the Western blot analysis. The right lane illustrates NMDA NR1 upregulation with a mean 3.3-fold increase in band density for soluble cell preparations incubated for 6 h with 5 μM NMDA and 5 μM ACPD. Incubation of the soluble preparation with anti-vimentin antibody yielded a 67-kDa band in the control synoviocytes (middle lane, second row) and a mean 3.1-fold increase in vimentin after NMDA and ACPD coincubation (right lane, second row) compared with lysates from untreated cells. The β-actin band density is also shown as an internal gel loading control for all conditions. Incubation of the soluble preps with nonspecific goat IgG control did not yield NR1 bands in untreated control lysate or NMDA- and ACPD-treated lysate lanes (not shown). Figure 6B demonstrates the corrected band density increases between control and NMDA- and ACPD-treated cells for NMDA NR1 and vimentin, as noted above.
In addition, total mRNA was extracted from SW982 human synoviocytes, and real-time PCR was performed to amplify NMDA NR1 cDNA around the putative nuclear localization sequence (nt 1890–2581), confirming the identity as NMDA NR1 compared with established sequences derived from human neural tissue obtained from GenBank (accession no. NM_0077327) (23, 59, 61). Figure 6C demonstrates that the mean relative NMDA NR1 gene expression level was significantly increased in NMDA- and ACPD-stimulated clonal synoviocytes (5.8-fold, compared with control cells, P < 0.01). Further characterization of NR2 is not included.
Cellular TNF-α levels are enhanced with NMDA and ACPD coincubation and blocked by pretreatment with anti-NMDA NR1 antibody.
To simulate conditions of inflammation in the synovial cultures, cells were incubated with PMA (25 nM). The PMA-induced TNF-α production in synoviocyte cultures is enhanced by coincubation with glutamate receptor agonists. Figure 7A demonstrates the enhancing effects of coincubation of glutamate receptor agonists NMDA and ACPD and PMA in SW982 cells. The TNF-α production after incubation with PMA alone are shown as open bars for each dose trial, and coincubations of PMA with NMDA and ACPD are illustrated using filled bars for each dose.
Fig. 7.
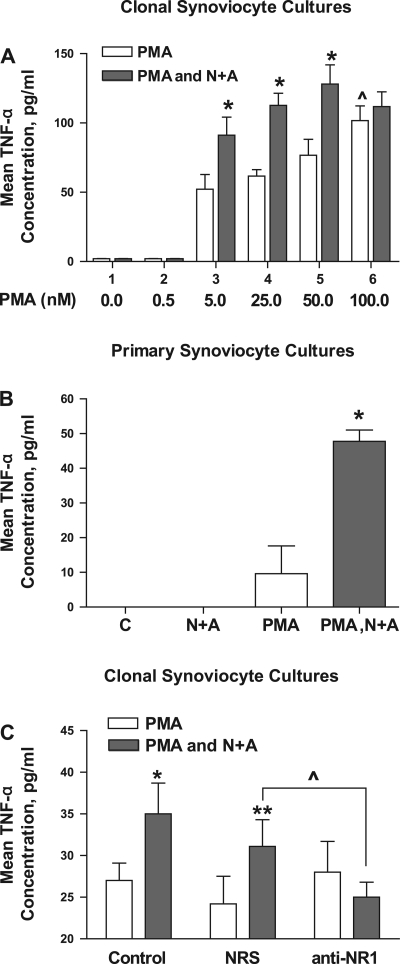
Mean tumor necrosis factor-α (TNF-α) levels increase in clonal and primary synovial cell cultures after treatment with glutamate receptor agonists measured by immunoassay. A: dose response for increasing doses of PMA with a fixed dose of 5 μM NMDA and 5 μM ACPD (N+A). Coincubation of SW982 human synoviocytes with PMA and N+A resulted in a significant increase in mean TNF-α concentrations compared with cell cultures incubated with PMA alone, demonstrating an enhancement of PMA activation by glutamate receptor agonists. *P < 0.05, PMA alone vs. PMA and N+A. ^P < 0.05, 100 nM PMA vs. 25 nM PMA. Each condition represents the mean of 9 samples. B: coincubation of primary synovial cell cultures derived from the synovial fluid of a patient with active psoriatic arthritis with 25 nM PMA, 5 μM NMDA, and 5 μM ACPD resulted in a significant increase (4.3-fold) in mean TNF-α concentrations compared with cell cultures incubated with PMA alone. *P < 0.05, PMA alone vs. PMA and N+A. Untreated cells and cells treated with 5 μM NMDA and 5 μM ACPD did not demonstrate increases in TNF-α levels in the culture supernatant. C: coincubation of SW892 human synoviocytes with 25 nM PMA, 5 μM NMDA, and 5 μM ACPD resulted in a significant increase in mean TNF-α concentrations compared with cell cultures incubated with PMA alone (*P < 0.05 compared with PMA alone). Preincubation of the cells with NRS did not diminish the enhancement seen with PMA, NMDA, and ACPD incubations (**P < 0.01 compared with PMA alone). However, preincubation with specific NMDA NR1 antibody blocked the increase in mean TNF-α levels seen with coincubation of SW892 synoviocytes with PMA and NMDA and ACPD (^P < 0.05 comparing cells preincubated with NRS to cells preincubated with NMDA NR1 antibody).
Incubation with 5 (group 3), 25 (group 4), or 50 nM PMA alone (group 5) resulted in a significant 25- to 38-fold increase in TNF-α production over baseline levels (52.16 ± 10.69, 61.71 ± 4.64, and 76.74 ± 11.44 pg/ml, respectively, P < 0.01). Incubation with 100 nM PMA (group 6) resulted in a significant increase in TNF-α levels (101.66 ± 10.61 pg/ml) compared with 5, 25, or 50 nM PMA (P = 0.04 for 50 vs. 100 nM PMA).
Combined incubation of 5, 25, or 50 nM PMA with 5 μM NMDA and 5 μM ACPD resulted in further significant increases in mean TNF-α protein release into the media (91.13 ± 13.06, 112.64 ± 8.74, and 128.11 ± 13.81 pg/ml, respectively, P < 0.05) compared with incubation with PMA alone. The addition of the glutamate receptor agonists to 25 nM PMA significantly enhanced increases in the TNF-α levels, comparable to the levels seen with the incubation of 100 nM PMA alone. Coincubation of 100 nM PMA and glutamate receptor agonists did not increase mean TNF-α levels (111.18 ± 10.31 pg/ml) over incubation with 100 nM PMA alone (101.66 ± 10.61 pg/ml). Since higher doses resulted in cell toxicity, the plateau effect attained with the 50 and 100 nM doses of PMA were a maximal effect.
The sequence of reagent additions in the cell culture incubations was assessed and found to be an important variable. Coincubation with 5 μM NMDA and 5 μM ACPD for an initial 6 h before addition of 25 nM PMA to the incubation for the last 18 h (24-h total incubation) consistently yielded 1.76- to 2.1-fold increases in mean TNF-α levels compared with incubation with PMA alone. Coincubation of both reagents for 24 h yielded the same results. If the sequence of reagent addition was reversed so that PMA was added first (for either 6 or 18 h) and then glutamate receptor agonists were added, no significant increases in mean TNF-α levels were seen compared with incubations with PMA alone. A similar and significant enhancement of TNF-α concentrations was obtained from synovial cultures coincubated with lipopolysaccharide (LPS) and NMDA and APCD.
In addition, a significant enhancing effect of PMA, NMDA, and ACPD incubations was demonstrated in primary synovial cultures derived from a patient with active psoriatic arthritis, as shown in Fig. 7B. Coincubations of 25 nM PMA with 5 μM NMDA and 5 μM ACPD in primary cell cultures resulted in a significant (4.5-fold) increase in mean TNF-α levels in the culture supernatant compared with levels seen with incubations using 25 nM PMA alone (47.75 ± 3.25 vs. 9.6 ± 8.0 pg/ml, respectively, P < 0.04). The mean TNF-α concentration shown for the primary synovial cultures from arthritis patients is lower than that for the clonal synoviocytes. The number of primary cells available in the samples was considerably fewer than in the clonal cell plates. Since primary cultures grow slowly and were not passaged, exact cell count was not repeated before the experiment. The count on the initial plating would not be reliable because of cell survival issues common in any primary culture transition. We estimate that the cell number was at least lower by a log value for the primary cultures.
Figure 7C demonstrates the blocking effect of anti-NMDA NR1 antibody on the enhancement of TNF-α concentrations after coincubation of PMA with NMDA and ACPD in SW982 cultures. In these experiments, TNF-α levels generated with 25 nM PMA were used as controls. TNF-α levels were not increased in the following incubation conditions used as controls: 5 μM NMDA and 5 μM ACPD alone (not shown), 25 nM PMA with no antibody preincubation (control), or preincubation with normal rabbit serum (NRS) or anti-NMDA NR1 followed by 25 nM PMA. Preincubation of the cells with a matched nonspecific antibody control (NRS) resulted in a similar and significant increase in mean TNF-α levels with PMA, NMDA, and ACPD (1.43-fold, 60.91 ± 4.40 pg/ml, P < 0.01) compared with cells incubated with PMA, NMDA, and ACPD alone. However, preincubation with anti-NMDA NR1 antibody directed against the NH2 terminus resulted in a specific and significant blunting of the enhancing effects of TNF-α concentration ratios seen with coincubations of PMA, NMDA, and ACPD (0.8-fold). The above studies with MK-801 and blocking NMDA NR1 antibody provide evidence that added NMDA and ACPD are acting on the cellular glutamate receptors to effect or enhance cellular activation.
PMA- and glutamate agonist-mediated TNF-α and RANTES increases are reduced by PKC inhibition.
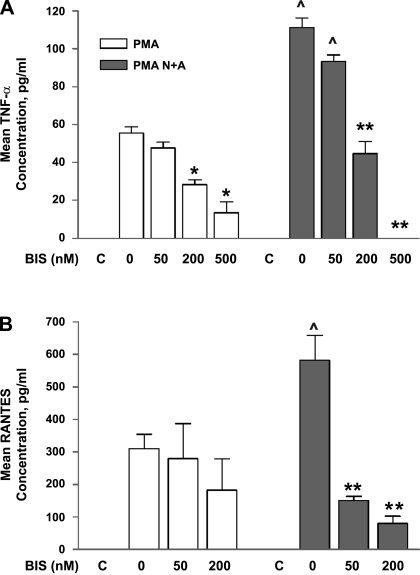
Figure 8A demonstrates that the mean TNF-α increases seen in SW982 synovial cells treated with PMA or incubated with PMA and glutamate receptor agonists can be diminished in a dose-dependent manner by preincubation with the PKC inhibitor Bis. In these experiments, Bis was added to the cell cultures 1 h before the addition of NMDA and ACPD. The addition of 200 nM Bis significantly diminished the increases in mean TNF-α levels seen with 25 nM PMA (55.52 ± 3.42 vs. 47.75 ± 2.97 pg/ml, P < 0.04). Preincubation with 500 nM Bis significantly reduced the PMA-induced mean TNF-α levels by 51% (28.48 ± 2.38 pg/ml, P < 0.01), indicating involvement of PKC signaling.
Fig. 8.
Preincubation of synoviocytes with bisindolylmaleimide-1 (Bis), a potent protein kinase C (PKC) inhibitor, markedly decreases mean TNF-α and RANTES concentrations. A: SW982 cells preincubated with Bis had significant reductions in supernatant TNF-α levels compared with levels generated with PMA alone or PMA and N+A incubations. *P < 0.05, Bis-treated cells compared with cells not treated with Bis. **P < 0.01 compared with cells preincubated with NMDA NRS. ^P < 0.05, cells coincubated with PMA and N+A compared with cells treated with PMA alone. The degree of inhibition was dose dependent. The control lanes (C) depict baseline levels obtained in the absence of PMA or in the absence of PMA but coincubated with NMDA and ACPD. B: in independent experiments, preincubation of SW982 synoviocyte cultures with Bis markedly decreased the mean RANTES levels generated with PMA alone or with PMA and NMDA+ACPD incubations. **P < 0.01, Bis-treated cells compared with cells not treated with Bis. ^P < 0.05, cells coincubated with PMA and NMDA+ACPD compared with cells treated with PMA alone.
Coincubation of 25 nM PMA with 5 μM NMDA and 5 μM ACPD resulted in a significant twofold increase in mean TNF-α concentrations (Fig. 8A, 111.26 ± 4.99 pg/ml, P < 0.03) compared with PMA alone. Preincubation with Bis diminished the expected increases in mean TNF-α concentrations seen with incubations with PMA and the glutamate receptor agonists NMDA and ACPD, again in a dose-dependent manner. Preincubation with 200 nM Bis significantly diminished the predicted increase in mean TNF-α protein concentrations seen with coincubation with PMA and glutamate receptor agonist by 40% (44.69 ± 6.42 pg/ml, P < 0.01). Preincubation with 500 nM Bis completely abrogated the predicted increase in mean TNF-α protein concentrations seen with coincubation with PMA and glutamate receptor agonists (P < 0.01).
Figure 8B demonstrates mean RANTES concentrations in SW982 human synoviocyte cultures with incubation of 25 nM PMA alone, 25 nM PMA with 5 μM NMDA and 5 μM ACPD coincubation, and coincubations pretreated with Bis. Significant increases in mean RANTES concentrations were seen in cultures treated with PMA (310.23 ± 44.04 pg/ml) compared with cells not treated with PMA (<30 pg/ml, P < 0.01).
Significant PKC-mediated increases in mean RANTES concentrations were also seen in cell cultures coincubated with PMA, NMDA, and ACPD (582.07 ± 76 pg/ml, P < 0.01) compared with RANTES from control cells and compared with PMA-treated cells (P < 0.03). Preincubation with 50 or 200 nM Bis significantly diminished the mean RANTES concentrations expected after coincubation with PMA, NMDA, and ACPD (151.72 ± 12 pg/ml, P < 0.01 and 79.65 ± 22.64 pg/ml, P < 0.01), respectively, compared with PMA-, NMDA-, and ACPD-treated cells.
Histological confirmation in rat inflamed knee joint.
The presence of glutamate NMDA NR1 receptors was confirmed in histological samples from control and CFA-inflamed knee joints harvested from rats (Fig. 9). In uninflamed knee joints, the thin outer layer of synoviocytes (Fig. 9A) is devoid of NR1 staining (Fig. 9B). The outer synovial layer is highly proliferated in inflamed knee joints when observed histologically (Fig. 9C). The synoviocytes in inflamed knee joints have abundant staining for NMDA NR1 (Fig. 9D).
Fig. 9.
Normal and inflamed rat knee joints: histological and immunocytochemical staining for NMDA NR1. Microphotographs of knee joint capsules from naive rats (A and B) and from rats 1 wk after induction of arthritis with complete Freund's adjuvant (CFA) (C and D). Note the thin synovial cell layer at the edge of the tissue section in the naive animal with no apparent NMDA N1 staining (arrows). The proliferation of synoviocytes in animals with inflamed knee joints is evident histologically (C) and with NMDA NR1 staining throughout most of the section in D (arrows). A and C, hematoxylin and eosin stain; B and D, NMDA NR1 immunohistochemical stain.
DISCUSSION
Glutamate receptor activation of synovial cells.
On the basis of histological evidence of NMDA NR1 in rat synovial lining as well as the correlation between human synovial fluid glutamate and aspartate content and increased synovial fluid chemokine concentrations (31), we predicted that 1) human synovial cells would express glutamate receptors, and 2) the release of EAA in the joint would activate glutamate receptors on synoviocytes to ultimately modulate or compound the arthritic processes of pain and inflammation.
The present study demonstrates that both NMDA receptor NR1 and NR2 subunits that are required for functional activation are present on cultured human synovial cells. Activation of the synoviocyte glutamate receptors with specific agonists of the NMDA and glycine activation sites increased the inflammatory mediators, TNF-α and RANTES, that can further influence cytokine and chemokine release. The findings reported in this study provide additional evidence of a significant role for neurotransmitter glutamate in neurogenic inflammation through activation of its receptors present on nonneuronal peripheral target cells in the knee joint. This delineates a novel and important pathological mechanism in arthritis.
Calcium mobilization responses to high concentrations of glutamate have been reported previously, indicating the potential for initiation of functional cellular responses (5). Incubation of cultured synoviocytes with the specific glutamate receptor agonists NMDA and ACPD (glycine site) in the present report resulted in significantly increased cellular detection of NMDA NR1 and NR2 subunits that was blocked by the noncompetitive glutamate NMDA antagonist MK-801. An increase in NMDA NR1 protein expression was also seen after synoviocyte cultures were incubated with the glutamate NMDA receptor agonist aspartate.
Increases in NMDA NR1 subunit in synoviocytes were seen in both cytosolic and nuclear compartments after glutamate receptor activation, consistent with previous reports identifying nuclear localization sequences in the NR1 subunit (26, 28). In the previous reports, transfection of neurons with selected glutamate receptor subunits to produce nuclear localization was found to be lethal to the cells. However, in our study, glutamate receptor agonists increased nuclear localization of NMDA NR1 and mediated increased TNF-α and RANTES production in synoviocytes but did not increase cell mortality, as determined by LDH release and trypan blue dye exclusion, up to 24 h of incubation, which we routinely monitored. Glutamate receptor activation with NMDA and ACPD also increased intracellular expression of vimentin. Vimentin is an intracellular protein in fibroblasts involved in early cellular activation that may play a role in intracellular transport (1). Other fibroblast proteins that are frequently upregulated on cellular activation under certain conditions, α-SMA, CD11b, and CD21, were not increased. Upregulation of vimentin stresses two points: 1) that vimentin is a downstream consequence of glutamate receptor activation, and 2) that the glutamate activation events are selectively targeted to specific functions and not just globally noxious to the cells.
Glutamate receptor activation enhances TNF-α and RANTES expression in synovial cells.
Coincubation of SW982 synovial cells with glutamate receptor agonists that activate both the NMDA and the glycine (ACPD) activation sites and with PMA to simulate an inflammatory state resulted in significant enhancement of mean TNF-α and RANTES levels in the supernatant. Coincubation with glutamate receptor agonists markedly decreased the amount of PMA needed to produce the same response. The enhancement effect could be blocked by preincubation with anti-NMDA NR1 antibody directed against the NH2 terminus (blocking antibody) or by pretreatment with the PKC inhibitor Bis. Higher suppressive doses of Bis are required if TNF-α levels have been enhanced by coincubation with PMA and glutamate receptor agonists. Preincubation with the specific PKA inhibitor KT5720 mildly decreased TNF-α expression in synoviocytes coincubated with PMA and NMDA and ACPD (not shown). This suggests that glutamate activation of the NMDA binding site acting through a PKC-mediated pathway can activate synoviocyte production of TNF-α. Intracellular signaling pathways activated by proinflammatory cytokines in synoviocytes isolated from human synovial tissues from patients with rheumatoid arthritis and osteoarthritis have been well delineated in other in vitro studies (10–12, 55). Activation of signaling pathways involving p38 are proposed as important signaling events in proinflammatory pathways in joint tissue (12, 16).
In related previous studies, addition of l-glutamate to primary cultures of synovial cells harvested from patients with rheumatoid arthritis or acute gout increased concentrations of TNF-α released into the culture supernatants (31). The levels released by the isolated cells were equivalent to levels of TNF-α we have measured in patient synovial fluid. Significant correlations also have been noted for synovial fluid glutamate and aspartate content with increased synovial fluid chemokine concentrations of RANTES, macrophage inflammatory protein-1α (MIP-1α), and IL-8 (31). In another report by Flood et al. (5), glutamate-induced increases in IL-6 levels were measured in cultures from rheumatoid arthritis patients that could be blocked with NBQX (2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione), a glutamate AMPA receptor antagonist. These studies provide evidence that synoviocyte glutamate receptor activation acts as a priming mechanism to potentiate production of the inflammatory mediators TNF-α, RANTES, and IL-6, and perhaps MIP-1α and IL-8. The importance of priming first with the glutamate agonists for enhanced TNF-α release with submaximal dosing of PMA demonstrates that glutamate receptor activation in nonneuronal cells acts to potentiate the activation mechanisms through selective signaling pathways in a manner not that different from long-term potentiation mechanisms in nervous tissues.
Role of glutamate receptor activation in peripheral sensitization using nonneural cellular targets.
These data and other data in the literature demonstrate several glutamate receptor-driven consequences in peripheral nonneuronal cells in inflammatory states. The results presented are consistent with a role for the synoviocytes as another example of a nonneural tissue targeted by peripherally released neurotransmitter glutamate. Targeting synoviocytes would initiate inflammatory responses of synoviocytes such as release of TNF-α (31). Thus further data have been generated for a role for peripheral nerve terminals in signaling and mediating responses to local injury and inflammatory events in the joint (22–25, 31, 34, 37, 57–59, 62, 63).
Upregulation of its own receptor population, as well as inflammatory mediators TNF-α and RANTES, suggests that glutamate receptors are responsible for a neurogenic feedforward paracrine event targeting peripheral receptors on local cells that can perpetuate inflammation and impact pain states. This has been previously suggested as a mechanism to explain the discovery of glutamate receptors and GLAST, a glutamate transporter on osteocytes and glutamate receptor regulation in bone resorption in pathological states (2, 7, 8, 15, 21, 24, 29, 30, 35, 41, 42). It is known that TNF-α can directly activate peripheral nerves (18, 61) and can increase inflammation by stimulating fibroblast proliferation (60) and increasing production of cytokines, chemokines, and enzymes that promote joint destruction (56).
The data directly support the hypothesis of glutamate-driven neurogenic influences on arthritis proposed originally by Westlund and Sluka (49) involving peripheral and central sensitization through regenerative feedforward mechanisms. In subsequent studies, data were provided showing that 1) peripheral tissue damage releases glutamate and other neurotransmitter amino acids in the spinal cord, 2) dorsal horn sensitization mechanisms ensue, creating reverse sensory nerve firing directed toward the periphery, 3) neurotransmitter substances are release into the periphery, and 4) enhanced peripheral inflammation and nociceptive responses interpreted as pain continue in a vicious feedforward cycle (3, 22, 25, 31, 32, 36–38, 40, 43–54, 57–59, 61–63). In fact, it has been shown that joint inflammation is significantly reduced in experimental arthritis and in patients with cut peripheral nerves (19, 44). Lidocaine injected into the joint to block nerve transmission also reduces glutamate release in the joint (23). Specific glutamate NMDA receptor antagonists applied to the spinal cord, intraperitoneally, and, of more relevance, directly into the joint, reduce pronociceptive behavior induced by inflammatory models (39, 25). Downstream consequences of glutamate also have been shown by administering antagonists to the joint to reduce blood flow (24).
Previous studies have addressed the role of glutamate receptor activation in other nonneuronal joint components, including osteoblasts (2, 13–15, 21, 29, 35, 41, 42). Activation of glutamate receptors on nonneural target cells has important cellular signaling functions in chemotaxic, bone remodeling, and inflammatory events (7, 8, 24, 29, 30, 35, 41, 42). Activation of cultured rat osteoblasts and osteoclasts with NMDA receptor agonists has been shown to increase osteoclast resorptive foci in vitro (2). Glutamate receptor antagonists decreased bone resorptive foci, suggesting a functional role for glutamate receptor agonist activation in osteoclasts during osteoporosis. Thus functional activation of glutamate receptors on bone has been shown to contribute to remodeling and destructive bone resorption in inflamed tissue.
In the case of arthritis, the etiology of the inflammatory cascades in arthritis initiation and persistence are multifactorial and presumably start in the joint. The release of neurotransmitters into the joint capsule can occur as a consequence of damage, noxious or infectious stimuli in the joint tissue, which promotes the establishment of dorsal root reflexes. In some diseases, such as rheumatoid arthritis and viral arthropathies, there may be common end points in which arthritic persistence (perhaps in part driven by self-regenerative neurogenic mechanisms, such as dorsal root reflexes) can continue, presumably long after the initial insult has abated. Thus, in human acute and chronic arthropathies, the elevated levels of synovial fluid EAA (31, 32) from damaged cellular tissue and EAA shown experimentally to be derived from the nerve terminals (25) could activate glutamate receptors expressed locally on synoviocytes to enhance cellular activation, inflammation, and pain states. Diminution of joint edema and allodynic behaviors by 50% or more with glutamate receptor blockade in the spinal cord, afferent nerve lesion, or intra-articular glutamate antagonists provides support for the role of neurotransmitter glutamate release in significantly impacting inflammatory events occurring in the joint (19, 23, 37, 44, 49).
Activation of glutamate receptors on human synoviocytes in the periphery is another important neurogenic component of the pathological event sequence occurring in inflammatory conditions. Glutamate receptor activation is an important factor that results in the upregulation of specific cellular proteins, including inflammatory mediators and its own receptors as shown in the present study, and as a synovial cell proliferation factor producing the synovial lining hyperplasia present in inflamed joints (34). Characterization of peripheral nonneural glutamate receptors and subsequent intracellular activation pathways in synovial cells may provide novel strategies to significantly decrease neurogenic inflammation in arthritis, either as a monotherapy or to complement currently available therapeutic agents.
GRANTS
This work was supported by National Institutes of Health Grants NS 32778 (K. N. Westlund), R21 AR48371 (K. N. Westlund and T. A. McNearney), and P01 NS11255 (K. N. Westlund, T. A, McNearney, and G. Taglialatela) and by the Charles A. Dana Foundation (T. A. McNearney and K. N. Westlund).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Behr TM, Spes CH, Pongratz DE, Weiss M, Meiser B, Uberfuhr P, Theisen K, Angermann CE. Adult human cardiomyocytes coexpress vimentin and Ki67 in heart transplant rejection and in dilated cardiomyopathy. J Heart Lung Transplant 17: 795–800, 1998 [PubMed] [Google Scholar]
- 2.Chenu C, Serre CM, Raynal C, Burt-Pichat B, Delmas PD. Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone 22: 295–299, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Dougherty PM, Sluka KA, Sorkin LS, Westlund KN, Willis WD. Neural changes in acute arthritis in monkeys. I. Parallel enhancement of responses of spinothalamic tract neurons to mechanical stimulation and excitatory amino acids. Brain Res Brain Res Rev 17: 1–13, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Ehlers MD, Fung ET, O'Brien RJ, Huganir RL. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci 18: 720–730, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flood S, Parri R, Williams A, Duance V, Mason D. Modulation of interleukin-6 and matrix metalloproteinase 2 expression in human fibroblast-like synoviocytes by functional ionotropic glutamate receptors. Arthritis Rheum 56: 2523–2534, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Gallo V, Ghiani CA. Glutamate receptors in glia: new cells, new inputs and new functions. Trends Pharmacol Sci 21: 252–258, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Gu Y, Genever PG, Skerry TM, Publicover SJ. The NMDA type glutamate receptors expressed by primary rat osteoblasts have the same electrophysiological characteristics as neuronal receptors. Calcif Tissue Int 70: 194–203, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Gu Y, Publicover SJ. Expression of functional metabotropic glutamate receptors in primary cultured rat osteoblasts. Cross-talk with N-methyl-d-aspartate receptors. J Biol Chem 275: 34252–34259, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Hachicha M, Rathanaswami P, Schall TJ, McColl SR. Production of monocyte chemotactic protein-1 in human type B synoviocytes. Synergistic effect of tumor necrosis factor alpha and interferon-gamma. Arthritis Rheum 36: 26–34, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Hammaker D, Sweeney S, Firestein GS. Signal transduction networks in rheumatoid arthritis. Ann Rheum Dis 62, Suppl 2: ii86–ii89, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammaker DR, Boyle DL, Chabaud-Riou M, Firestein GS. Regulation of c-Jun N-terminal kinase by MEKK-2 and mitogen-activated protein kinase kinase kinases in rheumatoid arthritis. J Immunol 172: 1612–1618, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest 108: 73–81, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinoi E, Fujimori S, Yoneda Y. Modulation of cellular differentiation by N-methyl-d-aspartate receptors in osteoblasts. FASEB J 17: 1532–1534, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hinoi E, Fujimori S, Takarada T, Taniura H, Yoneda Y. Facilitation of glutamate release by ionotropic glutamate receptors in osteoblasts. Biochem Biophys Res Commun 297: 452–458, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Huggett JF, Mustafa A, O'Neal L, Mason DJ. The glutamate transporter GLAST-1 (EAAT-1) is expressed in the plasma membrane of osteocytes and is responsive to extracellular glutamate concentration. Biochem Soc Trans 30: 890–893, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Inoue T, Hammaker D, Boyle DL, Firestein GS. Regulation of p38 MAPK by MAPK kinases 3 and 6 in fibroblast-like synoviocytes. J Immunol 174: 4301–4306, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325: 529–531, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain 85: 145–151, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Kane D, Lockhart JC, Balint PV, Mann C, Ferrell WR, McInnes IB. Protective effect of sensory denervation in inflammatory arthritis (evidence of regulatory neuroimmune pathways in the arthritic joint). Ann Rheum Dis 64: 325–327, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klippel JH. Primer of the Rheumatic Diseases. Atlanta, GA: Arthritis Foundation, 2001. [Google Scholar]
- 21.Laketic-Ljubojevic I, Suva LJ, Maathuis FJ, Sanders D, Skerry TM. Functional characterization of N-methyl-d-aspartic acid-gated channels in bone cells. Bone 25: 631–637, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Lam FY, Ferrell WR. Effects of interactions of naturally occurring neuropeptides on blood flow in the rat knee joint. Br J Pharmacol 108: 694–699, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawand NB, McNearney T, Westlund KN. Amino acid release into the knee joint: key role in nociception and inflammation. Pain 86: 69–74, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Lawand NB, Reddig WJ, Cashin AE, Westlund KN, Willis WD. NMDA receptors and associated signaling pathways: a role in knee joint blood flow regulation. Eur J Pharmacol 499: 155–161, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Lawand NB, Willis WD, Westlund KN. Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur J Pharmacol 324: 169–177, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Liva KL, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Macdonald NJ, Delderfield SM, Zhang W, Taglialatela G. Tumour necrosis factor-alpha- vs. growth factor deprivation-promoted cell death: distinct converging pathways. Aging Cell 2: 245–256, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Marsh DR, Holmes KD, Dekaban GA, Weaver LC. Distribution of an NMDA receptor: GFP fusion protein in sensory neurons is altered by a C-terminal construct. J Neurochem 77: 23–33, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Mason DJ. The role of glutamate transporters in bone cell signalling. J Musculoskelet Neuronal Interact 4: 128–131, 2004 [PubMed] [Google Scholar]
- 30.Mason DJ, Suva LJ, Genever PG, Patton AJ, Steuckle S, Hillam RA, Skerry TM. Mechanically regulated expression of a neural glutamate transporter in bone: a role for excitatory amino acids as osteotropic agents? Bone 20: 199–205, 1997 [DOI] [PubMed] [Google Scholar]
- 31.McNearney T, Baethge BA, Cao S, Alam R, Lisse JR, Westlund KN. Excitatory amino acids, TNF-alpha, and chemokine levels in synovial fluids of patients with active arthropathies. Clin Exp Immunol 137: 621–627, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNearney T, Speegle D, Lawand N, Lisse J, Westlund KN. Excitatory amino acid profiles of synovial fluid from patients with arthritis. J Rheumatol 27: 739–745, 2000 [PMC free article] [PubMed] [Google Scholar]
- 33.Nash NR, Heilman CJ, Rees HD, Levey AI. Cloning and localization of exon 5-containing isoforms of the NMDAR1 subunit in human and rat brains. J Neurochem 69: 485–493, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Parada-Turska J, Rzeski W, Majdan M, Kandefer-Szerszeń M, Turski WA. Effect of glutamate receptor antagonists and antirheumatic drugs on proliferation of synoviocytes in vitro. Eur J Pharmacol 535: 95–97, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Patton AJ, Genever PG, Birch MA, Suva LJ, Skerry TM. Expression of an N-methyl-d-aspartate-type receptor by human and rat osteoblasts and osteoclasts suggests a novel glutamate signaling pathway in bone. Bone 22: 645–649, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Rees H, Sluka KA, Lu Y, Westlund KN, Willis WD. Dorsal root reflexes in articular afferents occur bilaterally in a chronic model of arthritis in rats. J Neurophysiol 76: 4190–4193, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Rees H, Sluka KA, Westlund KN, Willis WD. Do dorsal root reflexes augment peripheral inflammation? Neuroreport 5: 821–824, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Rees H, Sluka KA, Westlund KN, Willis WD. The role of glutamate and GABA receptors in the generation of dorsal root reflexes by acute arthritis in the anaesthetized rat. J Physiol 484: 437–445, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren K, Hylden JLK, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain 50: 331–344, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Schaible HG, Schmidt RF, Willis WD. Enhancement of the responses of ascending tract cells in the cat spinal cord by acute inflammation of the knee joint. Exp Brain Res 66: 489–499, 1987 [DOI] [PubMed] [Google Scholar]
- 41.Skerry TM. Identification of novel signaling pathways during functional adaptation of the skeleton to mechanical loading: the role of glutamate as a paracrine signaling agent in the skeleton. J Bone Miner Metab 17: 66–70, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Skerry TM, Genever PG. Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci 22: 174–181, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Sluka KA, Jordan HH, Westlund KN. Reduction in joint swelling and hyperalgesia following post-treatment with a non-NMDA glutamate receptor antagonist. Pain 59: 95–100, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Sluka KA, Lawand NB, Westlund KN. Joint inflammation is reduced by dorsal rhizotomy and not by sympathectomy or spinal cord transection. Ann Rheum Dis 53: 309–314, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sluka KA, Dougherty PM, Sorkin LS, Willis WD, Westlund KN. Neural changes in acute arthritis in monkeys. III. Changes in substance P, calcitonin gene-related peptide and glutamate in the dorsal horn of the spinal cord. Brain Res Brain Res Rev 17: 29–38, 1992 [DOI] [PubMed] [Google Scholar]
- 46.Sluka KA, Rees H, Westlund KN, Willis WD. Fiber types contributing to dorsal root reflexes induced by joint inflammation in cats and monkeys. J Neurophysiol 74: 981–989, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Sluka KA, Westlund KN. An experimental arthritis in rats: dorsal horn aspartate and glutamate increases. Neurosci Lett 145: 141–144, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Sluka KA, Westlund KN. An experimental arthritis model in rats: the effects of NMDA and non-NMDA antagonists on aspartate and glutamate release in the dorsal horn. Neurosci Lett 149: 99–102, 1993 [DOI] [PubMed] [Google Scholar]
- 49.Sluka KA, Westlund KN. Centrally administered non-NMDA but not NMDA receptor antagonists block peripheral knee joint inflammation. Pain 55: 217–225, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Sluka KA, Westlund KN. Spinal cord amino acid release and content in an arthritis model: the effects of pretreatment with non-NMDA, NMDA, and NK1 receptor antagonists. Brain Res 627: 89–103, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Sluka KA, Westlund-High KN. Ch 26, Neurologic Regulation of Inflammation, In: Kelley's Textbook of Rheumatology (8th ed.), edited by Firestein GS, Budd RC, Harris ED, Jr, McInnes IB, Ruddy S, Sergent JS. Philadelphia, PA: Saunders Elsevier, 2009, vol. I, p. 411–419 [Google Scholar]
- 52.Sluka KA, Willis WD, Westlund KN. Inflammation-induced release of excitatory amino acids is prevented by spinal administration of a GABAA but not by a GABAB receptor antagonist in rats. J Pharmacol Exp Ther 271: 76–82, 1994 [PubMed] [Google Scholar]
- 53.Sluka KA, Willis WD, Westlund KN. The role of dorsal root reflexes to peripheral inflammation. Pain Forum 4: 141–149, 1995 [Google Scholar]
- 54.Sorkin LS, Westlund KN, Sluka KA, Dougherty PM, Willis WD. Neural changes in acute arthritis in monkeys. IV. Time-course of amino acid release into the lumbar dorsal horn. Brain Res Brain Res Rev 17: 39–50, 1992 [DOI] [PubMed] [Google Scholar]
- 55.Sundarrajan M, Boyle DL, Chabaud-Riou M, Hammaker D, Firestein GS. Expression of the MAPK kinases MKK-4 and MKK-7 in rheumatoid arthritis and their role as key regulators of JNK. Arthritis Rheum 48: 2450–2460, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol 36: 372–378, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Szolcsanyi J. Antidromic vasodilatation and neurogenic inflammation. Agents Actions 23: 4–11, 1988. [DOI] [PubMed] [Google Scholar]
- 58.Westlund Ch KN. The dorsal horn and hyperalgesia. In: Handbook of Clinical Neurology. Pain. (3rd ed.), edited by Cervero F, Staehelin Jensen T. New York: Elsevier, 2006, vol. 81, p. 103–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willis WD, Sluka KA, Rees H, Westlund KN. A contribution of dorsal root reflexes to peripheral inflammation. In: Presynaptic Inhibition and Neural Control, edited by Rudomin P, Romo R, Mendell LM. New York: Oxford, 1998, p. 407–423 [Google Scholar]
- 60.Youn J, Kim HY, Park JH, Hwang SH, Lee SY, Cho CS, Lee SK. Regulation of TNF-alpha-mediated hyperplasia through TNF receptors, TRAFs, and NF-kappaB in synoviocytes obtained from patients with rheumatoid arthritis. Immunol Lett 83: 85–93, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Zelenka M, Schaefers M, Sommer C. Intraneural injection of interleukin-1b and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain 116: 257–263, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Lu Y, Chen Y, Westlund KN. Group I metabotropic glutamate receptor antagonists block secondary thermal hyperalgesia in rats with knee joint inflammation. J Pharmacol Exp Ther 300: 149–156, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Zhang LP, Chen Y, Clark BP, Sher E, Westlund KN. The role of type 1 metabotropic glutamate receptors in the generation of dorsal root reflexes induced by arthritis or the spinal cord infusion of 4-aminopyridine in the anesthetized rat. J Pain 1: 151–161, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmer M, Fink TM, Franke Y, Lichter P, Spiess J. Cloning and structure of the gene encoding the human N-methyl-d-aspartate receptor (NMDAR1). Gene 159: 219–223, 1995 [DOI] [PubMed] [Google Scholar]
- 65.Zukin RS, Bennett MV. Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci 18: 306–313, 1995. [DOI] [PubMed] [Google Scholar]