Abstract
The bone morphogenetic protein (BMP) type 2 receptor ligand, Bmp2, is upregulated in the peripheral pulmonary vasculature during hypoxia-induced pulmonary hypertension (PH). This contrasts with the expression of Bmp4, which is expressed in respiratory epithelia throughout the lung. Unlike heterozygous null Bmp4 mice (Bmp4LacZ/+), which are protected from the development of hypoxic PH, mice that are heterozygous null for Bmp2 (Bmp2+/−) develop more severe hypoxic PH than their wild-type littermates. This is associated with reduced endothelial nitric oxide synthase (eNOS) expression and activity in the pulmonary vasculature of hypoxic Bmp2+/− but not Bmp4LacZ/+ mutant mice. Furthermore, exogenous BMP2 upregulates eNOS expression and activity in intrapulmonary artery and pulmonary endothelial cell preparations, indicating that eNOS is a target of Bmp2 signaling in the pulmonary vasculature. Together, these data demonstrate that Bmp2 and Bmp4 exert opposing roles in hypoxic PH and suggest that the protective effects of Bmp2 are mediated by increasing eNOS expression and activity in the hypoxic pulmonary vasculature.
Keywords: hypoxia, pulmonary hypertension, bone morphogenetic protein 2, bone morphogenetic protein 4, bone morphogenetic protein receptor 2, endothelial nitric oxide synthase
bulmonary hypertension (PH) encompasses a range of diseases defined by sustained elevation in pulmonary arterial pressures. All forms of PH are characterized by structural remodeling of pulmonary resistance arteries and increased pulmonary vascular tone and reactivity. Clues to the mechanisms regulating these responses have come from genetic studies in patients with a rare familial form of PH, hereditary pulmonary arterial hypertension (HPAH). The majority of patients with HPAH inherit heterozygous mutations in bone morphogenetic protein type 2 receptor, BMPR2 (17), suggesting that dysregulated BMPR2 signaling contributes to the pathogenesis of HPAH. These observations also raise the wider question as to the role of BMPR2 signaling in other more common forms of PH that are not associated with inherited BMPR2 mutations.
BMPR2 is a member of the transforming growth factor-β. family of receptors and is activated by BMP ligands, including BMP2 and BMP4 (6). Activation of these receptors leads to phosphorylation of receptor-activated Smads (Smad1, 5, and 8), resulting in their nuclear translocation and transactivation of target genes. BMPR2 is expressed in endothelial cells (ECs) and, to a lesser extent, vascular smooth muscle cells (VSMCs) in the pulmonary vasculature (11). Much of our understanding of the role of BMPR2 signaling in the pulmonary vasculature has come from genetic studies in mice. Heterozygous null Bmpr2+/− mice are viable, have a mild basal increase in pulmonary vascular resistance (1), and have increased susceptibility to PH in response to inflammatory stress or a combination of hypoxia and serotonin (16, 22, 23). Mice carrying the heterozygous hypomorphic Bmpr2ΔEx2/+ mutation have increased PH in response to chronic hypoxia alone (11). Both of the Bmpr2 mutant mice have increased agonist-induced pulmonary artery vasoconstriction (11, 16), but there are also marked defects in EC-dependent and EC-independent pulmonary vasodilatation in Bmpr2ΔEx2/+ mice (11). Furthermore, there is a reduction in hypoxia-induced endothelial nitric oxide synthase (eNOS) expression in Bmpr2ΔEx2/+ mice, suggesting that defects in EC-dependent vasodilatation result from reduced eNOS expression and activity in the pulmonary vasculature.
To explore the role of Bmpr2 signaling in regulating pulmonary vascular function in the absence of Bmpr2 mutations, we evaluated pulmonary expression of Bmpr2 ligands in a mouse model of hypoxic PH. Pulmonary Bmp2 and Bmp4 expression (but not Bmp5, Bmp6, or Bmp7) is upregulated following exposure to hypoxia (10). Loss of hypoxia-induced Bmp4 expression in heterozygous null Bmp4LacZ/+ mice is associated with reduced PH and decreased pulmonary vascular remodeling and VSMC proliferation. This suggests that Bmp4 promotes hypoxic PH by increasing VSMC proliferation and vascular remodeling. This is consistent with the observation that conditional loss of Alk3 [a type 1 Bmp receptor required for Bmpr2 signaling (6)] in VSMCs protects mice against hypoxic pulmonary vascular remodeling (8). These findings contrast with our observations in Bmpr2ΔEx2/+ mice, which show greater susceptibility to hypoxic PH (11). Furthermore, ∼30% of mice with conditional deletion of Bmpr2 in ECs spontaneously develop PH (13). This indicates that loss of Bmpr2-mediated signaling in ECs is sufficient to promote PH. Our observations in Bmp4LacZ/+ and Bmpr2ΔEx2/+ mice suggest that Bmp signaling exerts opposing effects on the pulmonary vasculature through its effects on VSMC and EC function and also raise questions as to the dominant ligand activating Bmpr2 in the PECs. Because Bmp2 is also upregulated in the hypoxic lung (10) and is a Bmpr2 ligand, we therefore hypothesized that hypoxia-induced expression of Bmp2 exerts opposing effects to Bmp4 in the hypoxic pulmonary vasculature and that Bmp2 is the dominant ligand activating Bmpr2 in PECs.
To test this hypothesis, we investigated the role of Bmp2 in hypoxic PH using mice deficient for the Bmp2 ligand (Bmp2+/− mice). We show that these mice develop more severe hypoxic PH than their wild-type littermates. Unlike Bmp4LacZ/+ mice, Bmp2+/− mice have decreased hypoxia-induced expression and activation of eNOS in the pulmonary vasculature, and treatment with BMP2 increases eNOS expression and activity in intrapulmonary artery preparations and cultured intrapulmonary endothelial cells. Taken together, these findings suggest that Bmp2 and Bmp4 exert opposing effects on the pulmonary vasculature in vivo and that the effects of Bmp2 are likely to be mediated through activation of protective, Bmpr2-dependent effects in PECs in hypoxic PH.
MATERIALS AND METHODS
Mice.
Bmp2+/− mice bred on a C57Bl/6 background were a gift from Stephen Harris (31). Bmp4LacZ/+ mice bred on an ICR background were a gift from Brigid Hogan (15). 5′- and 3′-Bmp2 bacterial artificial chromosome (BAC) transgenic reporter mice have been described (4). H-2Kb-tsA58 transgenic mice (Immortomouse) (14) and wild-type ICR mice were purchased from Charles River. Genotyping was performed by PCR of ear punch DNA using the following primer sets: Bmp2 wild-type allele, forward AGCATGAACCCTCATGTCTTGG (PGK-PRO) and reverse GTGACATTAGGCTGCTGTAGCA, product size 322 bp, annealing temperature 62°C; Bmp2 mutant allele, forward AGCATGAACCCTCATGTCTTGG (PGK-PRO) and reverse GAGACTAGTGAGACGTGCTACT, product size 367 bp, annealing temperature 62°C; LacZ cassette in Bmp4LacZ knock-in and the 5′- and 3′-Bmp2 BAC transgenic mice, forward GTTGCAGTGCACGGCGATACACTTGCTG and reverse GCCACTGGTGTGGGCCATAATTCATTCGC, product size 350 bp, annealing temperature 58°C; and H-2Kb-tsA58 mice, forward AGCGCTTGTGTCGCCATTGTATTC and reverse GTCACACCACAGAAGTAAGGTTCC, product size 1 Kb, annealing temperature 58°C.
Experimental PH.
Eight- to ten-week-old Bmp2+/− mice and wild-type littermate controls were exposed to normoxia or 10% normobaric oxygen for 1 or 3 wk, as described previously (10, 11). At completion, mice were anesthetized with 375 mg/kg Avertin (Sigma) and ventilated by tracheotomy, the chest was opened, and right ventricular systolic pressure (RVSP) was measured. For this, mice were ventilated using a Harvard rodent ventilator at 110 strokes/min, and cardiac pressures were measured after the chest cavity was opened. RSVP, right ventricular diastolic pressure (RVDP), left ventricular diastolic pressure, and heart rates were collected using a Digi-Med Blood Pressure Analyzer (Micro-Med), with verification by comparison with pressure tracings (Gould). RVSPs were calculated by averaging continuous recordings over 20 s. Values corresponding to heart rates <360 beats/min and RVDP >0 were excluded. After completion of the studies, mice were exsanguinated by cardiac puncture, and blood samples were anti-coagulated with EDTA and used to estimate hematocrit by centrifugation. Experimental methods were approved by the Vanderbilt University Institutional Animal Care and Use Committee.
Histological evaluation of pulmonary vascular remodeling.
Lungs were inflated and fixed in 4% formalin in PBS overnight before mounting. The percentage of muscularized peripheral vessels and VSMC proliferation in the pulmonary vasculature was assessed in a blinded fashion, as described previously (10). Vessel count per alveoli was determined after hematoxylin and eosin staining and counting peripheral vessels and alveoli.
Analysis of β-galactosidase expression in Bmp2 BAC LacZ transgenic mice.
Lungs were inflated and fixed in 0.2% gluteraldehyde in PBS with 2 mM MgCl2 and 5 mM EGTA overnight. Tissue processing and β-galactosidase staining were performed as described (2). Results were quantified by counting the number of blue cells per alveolus or vessel in cross section, excluding vessels if their longitudinal length was more than two times their width. Cell counts were obtained in peripheral vessels (20–50 μm and distal to terminal bronchioles) and larger vessels (50–200 μm associated with muscularized airways). We evaluated >300 alveoli and >30 small and >20 large vessels per mouse.
Isolation of intrapulmonary artery trees.
Intrapulmonary artery (IPA) preparations were used to evaluate eNOS expression in the pulmonary vasculature. This was performed by separating the left lung IPA tree from freshly isolated lungs under a dissecting microscope, as described previously (11). Approximately 100 μg of protein were recovered from each IPA tree, sufficient for two to three Western blots. For BMP-response studies, freshly isolated IPAs were cultured in DMEM with 10% FBS and treated with ±50 ng/ml recombinant BMP2 or BMP4 (R & D Systems) for 18 h before lysis.
Isolation and treatment of pulmonary endothelial cells.
The isolation and characterization of conditionally immortalized pulmonary endothelial cells (PECs) have been described (10). For this, we isolate PECs from the H-2Kb-tsA58 transgenic mice (Immortomice), a transgenic strain that carries a temperature-sensitive mutant of the SV40 large T antigen (14). When cultured at 33°C, the SV40 large T protein is in an active conformation, binding to p53 and allowing cellular transformation. However, under nonpermissive conditions (culture at 37°C), conformation of the gene product changes: it no longer binds p53 or immortalizes the cells. Importantly, we have characterized PECs derived from the “Immortomouse” extensively: these cells behave like differentiated ECs when grown under nonpermissive conditions (cobblestone appearance, capillary structures in three-dimensional culture, express eNOS, and >90% express CD31) (10, 11). Wild-type PECs were treated with 10 ng/ml recombinant human BMP2 (R&D Systems) under serum-free conditions for up to 4 h before lysis to evaluate protein expression by Western blot. For analysis of nitric oxide synthase (NOS) activity, PECs were cultured in basal EGM-2 MV media (Clonetics) without serum supplemented with 2.5 mM ascorbic acid (Sigma), and [3H]arginine-to-citrulline conversion was evaluated using an NOS Activity Assay Kit (Cayman). For this, PECs were exposed to 160 nM [3H]arginine for the indicated times. Cells were lysed and mixed with equilibrated resin, and [3H]citrulline eluate was collected and quantified by liquid scintillography. Pretreatment with 1 mM NG-nitro-l-arginine methyl ester (l-NAME) for 45 min served as a control for NOS-specific accumulation of [3H]citrulline. Assays were performed in triplicate, and results were expressed as counts per minute per microgram protein.
qRT-PCR.
Total RNA from snap-frozen left lung was extracted in Trizol reagent (Invitrogen) and quantified by ultraviolet spectrophotometery (NanoDrop; Thermo Scientific). Reverse transcription of 1 μg RNA by Superscript III (Invitrogen) was used to generate cDNA for analysis by RT-PCR (Biosystems 7300 Real Time PCR; Applied Biosystems) using SYBR Green (Applied Biosystems). Primers were designed to cross exon boundaries to avoid detection of contaminating genomic DNA, and included: for mouse Bmp2, forward TGTGGGCCCTCATAAAGAAGCAGA and reverse AGCAAGCTGACAGGTCAGAGAACA (product size 164 bp); mouse Bmp4, forward CCTCAAGGGAGTGGAGATTG and reverse GACTACGTTTGGCCCTTCTG (product size 102 bp); mouse eNOS, forward GGGAAAGCTGCAGGTATTTG and reverse TGATGGCTGAACGAAGATTG (product size 115 bp); and mouse β-actin, forward ACGGCCAGGTCATCACTATTG and reverse AGGGGCCGGACTCATCGTA (product size 371 bp). Changes in mRNA expression were determined by comparison of sample cycle threshold values against a standard curve generated using pooled sample cDNA. All data were normalized to β-actin mRNA and expressed as degree of change of normoxic control.
Western blots.
Western blots were performed in whole lung, IPA, and cell lysates using rabbit anti-phospho-Smad1/5/8 and anti-phospho Ser239 vasodilator stimulated phosphoprotein (VASP) (Cell Signaling), rabbit anti-vascular endothelial growth factor receptor (VEGFR) 2 (Santa Cruz), mouse anti-eNOS (BD) and anti-β-actin (Sigma), and goat anti-vascular endothelial (VE)-cadherin (Santa Cruz). These were detected with the respective, species specific, horseradish peroxidase-conjugated secondary antibodies (Jackson Laboratories and KPL). Results were quantified by densitometry normalized to β-actin.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism 5 with two-tailed t-test for pairwise comparisons and one-way ANOVA for multiple between-group comparisons. The minimal level of significance was set at P < 0.05. For multiple between-group analyses, significant data are indicated if one-way ANOVA reaches the minimal level of significance and pairwise, post hoc comparison between all possible group combinations demonstrates the same level of significance (P < 0.05) for the indicated group comparisons after Bonferroni correction for multiple comparisons.
RESULTS
Impaired Bmp2 signaling in hypoxic Bmp2+/− mutant mice.
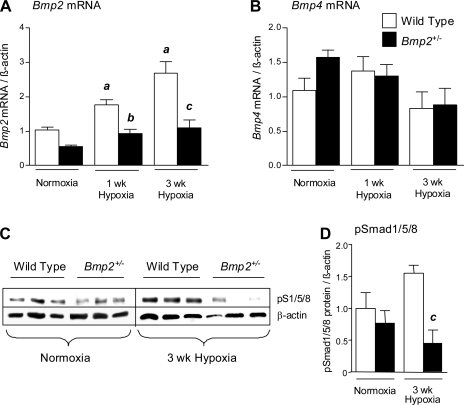
We used heterozygous null Bmp2+/− mice to examine the functional role of Bmp2 in hypoxic PH. Homozygous null Bmp2−/− mutation is embryonic lethal, but mice carrying the heterozygous null Bmp2+/− mutation are phenotypically normal (31). Pulmonary Bmp2 and pSmad1/5/8 expression are upregulated following exposure to prolonged hypoxia in wild-type mice (10). However, both responses are significantly blunted in heterozygous null Bmp2+/− mice after exposure to hypoxia (Fig. 1, A, C, and D). There is, however, no compensatory increase in Bmp4 mRNA expression in Bmp2+/− mice after 1 or 3 wk of hypoxia (Fig. 1B). Loss of Bmp2-dependent signaling in hypoxic Bmp2+/− mice indicates that they provide a useful model to study the role of Bmp2 in hypoxic PH.
Fig. 1.
Bone morphogenetic protein (BMP) signaling in Bmp2+/− heterozygous null mice. A and B: RT-PCR for Bmp2 (A) and Bmp4 (B) mRNA expression in wild-type and Bmp2+/− mouse lungs under normoxia, (wild type: n = 8; Bmp2+/−: n = 6), 1 wk of hypoxia (wild type: n = 6; Bmp2+/−: n = 6), or 3 wk of hypoxia (wild type: n = 5; Bmp2+/−: n = 5). C and D: whole lung pSmad1/5/8 in wild-type and Bmp2+/− mice detected by Western blot (C), quantified by densitometry (D). Data are expressed as means ± SE for wild-type mice (open bars) and Bmp2+/− mice (filled bars). ANOVA with Bonferroni posttest, P < 0.05 vs. wild-type normoxia (a), wild-type 1 wk hypoxia (b), and wild-type 3 wk hypoxia (c).
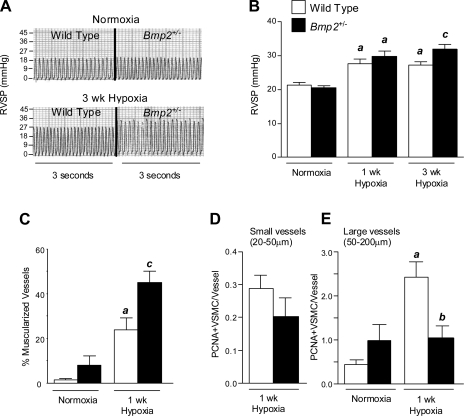
Increased hypoxic PH in Bmp2+/− mice.
The role of Bmp2 in hypoxia was investigated by exposing wild-type and Bmp2+/− mice to hypoxia (10% normobaric oxygen for up to 3 wk). Bmp2+/− mice have higher RVSP after 3 wk of hypoxia compared with wild-type littermates (Fig. 2, A and B and Table 1). RV mass was also increased in Bmp2+/− vs. wild-type mice after 3 wk of hypoxia (mean ± SE RV/LV + septal weight, 0.471 ± 0.046 vs. 0.397 ± 0.032, respectively) but these differences failed to reach statistical significance (Table 1). Increased RVSP in Bmp2+/− mice is not the result of left ventricular failure, since no elevation in LVEDP was observed (J. W. Lowery, unpublished observations), and did not result from differences in polycythemic responses to hypoxia between genotypes (Table. 1).
Fig. 2.
Hypoxic pulmonary hypertension (PH) responses in Bmp2+/− mice. A: representative right ventricular (RV) pressure tracings from wild-type and Bmp2+/− mice exposed to normoxia or 3 wk hypoxia. B: RV systolic pressure (RVSP) in wild-type and Bmp2+/− mice exposed to normoxia or 3 wk hypoxia. Mouse nos./group are indicated in Table 1. C: analysis of peripheral vessel muscularization in wild-type and Bmp2+/− mice maintained under normoxic conditions or after 3 wk hypoxia. D: vascular smooth muscle cell (VSMC) proliferation in small, muscularized peripheral vessels (20–50 μm) after 1 wk of hypoxia. Because there are few small, peripheral muscularized vessels in normoxic lungs, studies were performed by comparing genotypes exposed to hypoxia only. E: VSMC proliferation in larger airway-associated arteries (50–200 μm) from mice maintained under normoxia or after 1 wk of hypoxia. Nos. analyzed are indicated in Table 2. Data are expressed as means ± SE for wild-type mice (open bars) and Bmp2+/− mice (filled bars). ANOVA with Bonferroni posttest, P < 0.05 vs. wild-type normoxia (a), wild-type 1 wk hypoxia (b), and wild-type 3 wk hypoxia (c).
Table 1.
Hypoxic PH responses in Bmp2± mice
| Normoxia |
1 Wk Hypoxia |
3 Wk Hypoxia |
||||
|---|---|---|---|---|---|---|
| Wild type | Bmp 2+/− | Wild type | Bmp 2+/− | Wild type | Bmp2+/− | |
| RVSP, mmHg | 21.26 ± 0.822 (18) | 20.49 ± 0.69 (17) | 27.66 ± 1.41* (6) | 29.8 ± 1.47* (6) | 27.29 ± 0.86* (16) | 31.95 ± 1.40*† (8) |
| RV/LV + S, mg/mg | 0.313 ± 0.020 (14) | 0.309 ± 0.011 (11) | 0.363 ± 0.028 (6) | 0.330 ± 0.020 (7) | 0.397 ± 0.032 (19) | 0.471 ± 0.046 (9) |
| Hematocrit, % | 42.75 ± 1.57 (8) | 43.00 ± 1.81 (5) | ND | ND | 56.27 ± 3.27* (11) | 60.11 ± 358* |
Data expressed as means ± SE; no. of mice is in parentheses. PH, pulmonary hypertension; Bmp, bone morphogenetic protein; RVSP, right ventricle systolic pressure; RV, right ventricle; LV, left ventricle; S, septum. Shown are data for RVSP, RV weight (RV/LV + septal ratio), and hematocrit in wild-type and Bmp2+/− mice exposed to normoxia or 1 or 3 wk hypoxia. ND, no data recorded.
ANOVA with Bonferroni posttest, P < 0.05 vs. wild-type normoxia and
vs. wild-type 3 wk hypoxia.
In addition to increased RVSP, Bmp2+/− mice show a marked increase in peripheral vessel muscularization after exposure to hypoxia for 3 wk compared with their wild-type littermates (Fig. 2C and Table 2). Increased vessel remodeling is not associated with significant differences in alveolar vascular density in wild-type and Bmp2+/− mice under normoxic or hypoxic conditions (Table 2), suggesting that aberrant remodeling and PH responses to hypoxia do not result from a developmental defect in small vessel patterning in the lung. Because previous studies indicate that proliferative effects of hypoxia occur early in the development of hypoxic PH (18, 21), we went on to evaluate pulmonary VSMC proliferation after 1 wk of hypoxia in both small peripheral muscularized vessels (20–50 μm diameter) and larger muscularized, airway-associated vessels (50–200 μm). There are very few α-smooth muscle actin positive, small peripheral muscularized vessels in normoxic lungs of either wild-type or Bmp2+/− mice (L. Anderson, unpublished observations). For this reason, we were unable to evaluate basal, normoxic VSMC proliferation rates in these vessels. Studies were therefore performed by comparing genotypes exposed to hypoxia only. VSMC proliferation in these small muscularized peripheral vessels is reduced in hypoxic Bmp2+/− mouse lungs, although these differences are not significantly different from VSMC proliferation rates in wild-type littermates (Fig. 2D and Table 2). Basal VSMC proliferation in larger muscularized vessels is slightly increased in Bmp2+/− vs. wild-type mice under normoxic conditions, but this is not statistically significant. There is, however, a significant reduction in VSMC proliferation in larger muscularized pulmonary arterioles from Bmp2+/− mice compared with wild-type littermates after 1 wk exposure to hypoxia (Fig. 2D and Table 2). Taken together, these findings indicate that Bmp2+/− mice have increased susceptibility to hypoxic PH. This is associated with increased muscularization of the peripheral pulmonary vasculature that occurs without evidence of increased VSMC proliferation.
Table 2.
Hypoxia-induced vascular remodeling and proliferation in Bmp2+/− mice
| Normoxia |
1 Wk Hypoxia |
3 Wk Hypoxia |
||||
|---|---|---|---|---|---|---|
| Wild type | Bmp2+/− | Wild type | Bmp2+/− | Wild type | Bmp2+/− | |
| Peripheral muscularization, % | 1.33 ± 0.49 (6) | 8.00 ± 4.03 (5) | ND | ND | 23.66 ± 5.57* (6) | 45.00 ± 4.92*† (5) |
| Proximal VSMC proliferation, PCNA+/vessel | 0.43 ± 0.11 (5) | 0.97 ± 0.36 (5) | 2.43 ± 0.34* (5) | 1.04 ± 0.25‡ (6) | ND | ND |
| Peripheral VSMC proliferation, PCNA+/vessel | ND | ND | 0.28 ± 0.03 (5) | 0.20 ± 0.05 (7) | ND | ND |
| Peripheral vessel density, vessel/100 alveoli | 3.68 ± 0.61 (6) | 3.73 ± 0.31 (5) | ND | ND | 3.59 ± 0.36 (6) | 3.31 ± 0.29 (5) |
Data are expressed as means ± SE; no. of mice is in parentheses. VSMC, vascular smooth muscle cell; PCNA, proliferating cell nuclear antigen. Shown are data for peripheral pulmonary vessel muscularization, VSMC proliferation in small muscularized peripheral and larger proximal vessels, and pulmonary vessel-to-alveolar ratios in wild-type and Bmp2+/− mice exposed to normoxia or 1 or 3 wk hypoxia. ND, no data recorded.
ANOVA with Bonferroni posttest, P < 0.05 vs. wild-type normoxia,
wild-type 1 wk hypoxia, and
wild-type 3 wk hypoxia.
Localization of Bmp2 in the hypoxic lung.
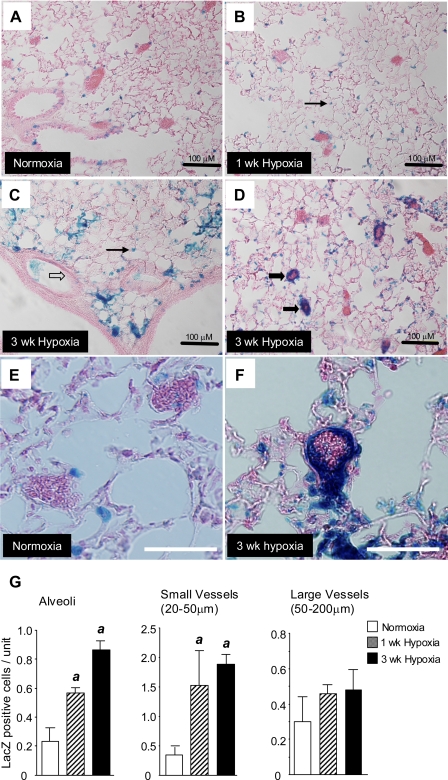
To determine the mechanism by which Bmp2 regulates pulmonary vascular responses to chronic hypoxia, we first evaluated the cellular localization of Bmp2 in hypoxic lungs. We were unsuccessful in detecting Bmp2 protein expression by immunohistochemical analysis using commercially available antibodies (L. Anderson and D. B. Frank, unpublished observations). Therefore, to determine which cells are expressing Bmp2, we evaluated LacZ expression (β-galactosidase activity) in the lungs of two Bmp2 BAC LacZ reporter mice (4). The 5′-Bmp2 BAC LacZ and 3′-Bmp2 BAC LacZ mice encompass most of the 5′ and 3′ cis-acting regulatory sequences for Bmp2. The 5′-Bmp2 BAC reporter expresses LacZ in the embryonic lung, whereas the 3′-Bmp2 BAC reporter does not (4). Consistent with these findings, there are only occasional (<1% of the total cells) intra-alveolar β-galactosidase positive cells after 3 wk of hypoxia in the 3′-Bmp2 BAC reporter mouse lung (L. Anderson, unpublished observations). In contrast, there is abundant expression of LacZ in the adult lungs of 5′-Bmp2 BAC LacZ mice (Fig. 3, A–F), indicating that pulmonary regulation of Bmp2 in the adult is dependent on cis-acting elements in the 5′-Bmp2 BAC reporter. With the use of these mice, we show that Bmp2 LacZ is expressed at low levels in alveolar and bronchial epithelium and in a small number of vessels under normoxic conditions and that there is a step-wise increase in LacZ-positive cells in the lung after exposure to hypoxia for 1 and 3 wk (Fig. 3G). Although there is increased expression in LacZ-positive cells in alveoli, there is a marked increase in Bmp2 LacZ-expressing cells in small, peripheral pulmonary vessels following 1 and 3 wk of hypoxia but no change the number of LacZ positive cells in larger vessels. Higher-magnification images of LacZ positive cells in small peripheral pulmonary vessels indicate that the majority of these cells are located in the inner wall of these peripheral vessels under hypoxic conditions (Fig. 3F). This suggests that ECs are the main site of Bmp2 expression in hypoxic peripheral pulmonary vasculature. These findings contrast with our observations using Bmp4 LacZ knock-in reporter mice (10). Bmp4 LacZ is also widely expressed in bronchial and alveolar epithelium, but, unlike the 5′-Bmp2 LacZ reporter, we do not see increased Bmp4 LacZ expression in these small peripheral vessels.
Fig. 3.
LacZ expression in 5′-Bmp2 BAC reporter mice. A–F: β-galactosidase staining of lung tissue sections from 5′-Bmp2 BAC LacZ reporter mice under normoxia (A), 1 wk of hypoxia (B), or 3 wk of hypoxia (C and D). Higher-power images of LacZ-expressing cells under normoxic conditions (E) and after 3 wk of hypoxia (F). LacZ staining is indicated in alveoli (thin black arrows), small vessels (<50 μm and distal to terminal bronchioles, thick black arrows), and larger vessels (50–200 μm associated with muscularized airways, clear block arrows). Black scale bars = 100 μm (A–D); white scale bars = 50 μm (E and F). G: quantification of LacZ-expressing cells. LacZ positive cells were counted and expressed as stained cells/unit. Data are expressed as means ± SE (n = 5/group). ANOVA with Bonferroni posttest, P < 0.05 vs. normoxic controls (a).
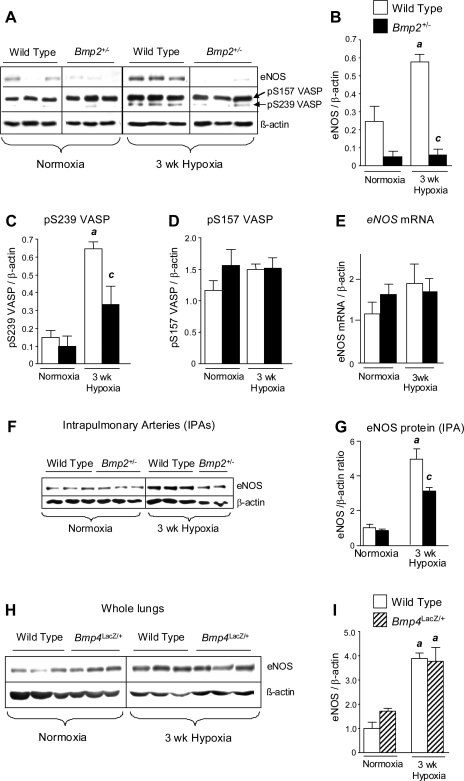
Decreased pulmonary eNOS in Bmp2+/− but not Bmp4LacZ/+ mice.
Because abnormalities in the eNOS pathway play an important role in regulating pulmonary vascular responses in PH (5) and because we have detected abnormalities in hypoxic induction of eNOS in mice carrying a heterozygous Bmpr2 mutation (11), we evaluated the regulation of pulmonary eNOS in hypoxic Bmp2+/− mice. Although chronic hypoxia increases pulmonary eNOS protein expression in wild-type mice, hypoxia-induced eNOS expression is reduced in Bmp2+/− mouse lungs (Fig. 4, A and B). Pulmonary eNOS expression is also reduced in normoxic Bmp2+/− mice compared with wild-type littermates, but this difference is not statistically significant. There are no differences in pulmonary eNOS mRNA expression in hypoxic wild-type or Bmp2+/− mice, suggesting that the associated changes in eNOS protein expression are regulated at a posttranscriptional level (Fig. 4E). Decreased pulmonary eNOS in Bmp2+/− mice is associated with a reduction in hypoxia-induced pSer239 VASP expression (Fig. 4, A and C). VASP is phosphorylated at Ser239 by cGMP-dependent protein kinases (PKGs) (3). Because PKGs are downstream mediators of NO signaling, analysis of pSer239 VASP provides a sensitive indicator of defective NOS activity in vivo (20). The pSer239 VASP antibodies also recognize a higher MW band corresponding to pSer157 VASP, a target of cAMP-dependent protein kinases (3). There is no significant change in pSer157 VASP expression between wild-type and Bmp2+/− mice under normoxic or hypoxic conditions (Fig. 4, A and D). This suggests that alterations in pSer239 VASP expression reflect differences in pulmonary PKG activity and that reduced eNOS expression is associated with reduced pulmonary NOS activity in hypoxic Bmp2+/− mice.
Fig. 4.
Endothelial nitric oxide synthase (eNOS) expression in Bmp2+/− and Bmp4LacZ/+ mouse lungs. A–D: Western blot and quantification of eNOS (A and B), phosphor-S239 VASP (A and C), and phospho-S157 VASP (A and D) in whole lung lysates from wild-type and Bmp2+/− mice exposed to normoxia or 3 wk hypoxia. E: pulmonary eNOS mRNA expression assessed by RT-PCR under normoxic conditions (wild type: n = 8; Bmp2+/−: n = 6) or after 3 wk hypoxia (wild type: n = 5; Bmp2+/−: n = 5). F and G: Western blot and quantification for eNOS expression in intrapulmonary artery preparations (IPAs) isolated from normoxic or 3 wk hypoxic wild-type and Bmp2+/− mice. H and I: eNOS protein expression and quantification in wild-type and Bmp4LacZ/+ lungs. Data are expressed as means ± SE for wild-type (open bars), Bmp2+/− (filled bars), and Bmp4LacZ/+ (hatched bars) mice. ANOVA, with Bonferroni posttest, P < 0.05 vs. wild-type normoxia (a) and wild-type 3 wk hypoxia (c).
To examine the regulation of eNOS expression in the pulmonary vasculature of Bmp2+/− mice, we isolated whole IPA trees from wild-type and Bmp2+/− mice exposed to normoxia or 3 wk of hypoxia: eNOS protein expression is reduced significantly in IPAs of hypoxic Bmp2+/− vs. wild-type IPAs (Fig. 4, F and G). These findings are consistent with our observations in whole lungs and indicate that there is a defect in hypoxia-induced eNOS expression in the pulmonary vasculature of Bmp2+/− mice.
Finally, because heterozygous null Bmp4LacZ/+ mice are protected from the development of hypoxic PH (10), we went on to evaluate the regulation of eNOS in hypoxic Bmp4LacZ/+ mouse lungs. Unlike Bmp2+/− mice, hypoxic regulation of eNOS in Bmp4LacZ/+ mouse lungs is preserved (Fig. 4, H and I). These findings indicate that Bmp2, and not Bmp4, regulates eNOS expression in the hypoxic lung in vivo.
Bmp2 regulates eNOS expression in the pulmonary vasculature.
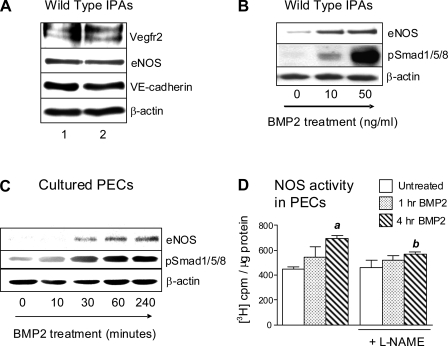
To explore the regulation of eNOS by Bmp2, we developed a novel assay in which isolated IPA trees were treated ex vivo with recombinant BMP2. IPA preparations are viable and express EC markers eNOS, VE-cadherin, and Vegfr2 after 18 h of culture (Fig. 5A). There is a dose-dependent increase of eNOS and pSmad1/5/8 expression in wild-type IPAs following treatment with BMP2 (Fig. 5B). To explore BMP2-dependent regulation of eNOS further, we evaluated BMP2-dependent responses in cultured PECs from wild-type mice. Similar to IPAs, PECs show a rapid induction of eNOS and pSmad1/5/8 in response to BMP2 (Fig. 5C). To determine the functional significance of these changes, we evaluated NOS activity by assessing [3H]arginine-to-[3H]citrulline conversion in PECs. We detect low basal NOS activity in cultured PECs (no change in [3H]arginine conversion after treatment with l-NAME), indicating that the assay lacks sensitivity to detect basal NOS activity in these cells. However, NOS activity is increased significantly following 4 h of treatment with BMP2 and is inhibited by treatment with l-NAME, indicating that this response reflects BMP2-induced changes in NOS activity (Fig. 5D). Therefore, BMP2 increases eNOS expression and NOS activity in PECs.
Fig. 5.
Regulation of eNOS by Bmp2. A: expression of endothelial cell (EC) markers vascular endothelial growth factor receptor (Vegfr) 2, eNOS, and VE-cadherin assessed by Western blot in wild-type IPAs after culture for 18 h. Lanes 1 and 2 are from two separate IPA preparations. B: eNOS and phospho-Smad1/5/8 in wild-type IPAs treated with BMP2 for 18 h. C: Western blot for eNOS and pSmad1/5/8 in pulmonary endothelial cells (PECs) treated with 10 ng/ml BMP2 over 4 h. D: BMP2-mediated effects on NOS activity in PECs assessed using a radiolabeled arginine conversion assay in PECs treated with 10 ng/ml of BMP2 for 1 or 4 h ±1 mM NG-nitro-l-arginine methyl ester (l-NAME). Data are expressed as means ± SE. The assay was performed in triplicate and repeated with similar results. Results from one experiment are shown. ANOVA with Bonferroni posttest, P < 0.05 vs. untreated control (a) and 4 h BMP2 without l-NAME (b).
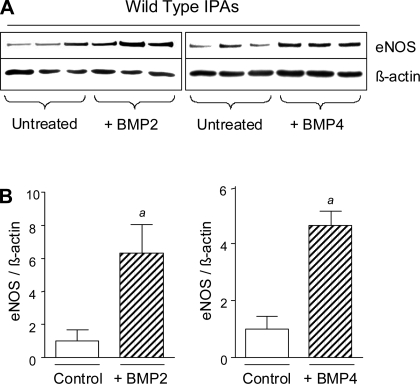
Our studies suggest that reduced eNOS expression in hypoxic Bmp2+/− mice results from decreased expression of Bmp2 in the hypoxic lung. However, Bmp4LacZ/+ mice show a normal eNOS response to chronic hypoxia (Fig. 4, H and I), indicating that Bmp2 and Bmp4 have different effects on hypoxic regulation of eNOS in vivo. To evaluate this further, we compared eNOS responses to exogenous BMP2 and BMP4 treatment in isolated pulmonary vessels. BMP2 and BMP4 induce a similar increase in eNOS expression in wild-type IPAs (Fig. 6, A and B). These findings indicate that the different effects of Bmp2 and Bmp4 in the hypoxic regulation of eNOS in vivo are unlikely to result from differences in direct ligand-dependent cellular responses. Based on our analyses of Bmp2 and Bmp4 expression domains in the hypoxic lung, we propose that the differential effects of these ligands on vascular function and eNOS expression in vivo result from their distinct cellular expression domains in the hypoxic lung.
Fig. 6.
Regulation of eNOS by BMP2 and BMP4 in isolated IPA preparations. A: Western blot for eNOS expression in IPAs cultured ±50 ng/ml BMP2 or BMP4 for 18 h before lysis. B: quantification of eNOS blots by densitometry normalized to β-actin. Data are expressed as means ± SE. t-Test, P < 0.05 vs. untreated controls (a).
DISCUSSION
The majority of patients with HPAH inherit heterozygous BMPR2 mutations (17), indicating that dysregulated BMPR2 signaling contributes to the development of PH. However, these studies also suggest that BMPR2 signaling may play a role in regulating the pulmonary vasculature in patients with more common forms of PH not associated with inherited BMPR2 mutations. To address this, we have explored the mechanisms by which two of the Bmpr2 ligands that are expressed in the adult mouse lung, Bmp2 and Bmp4, modulate pulmonary vascular function and remodeling in hypoxic PH.
Bmp4 is expressed widely in bronchial and alveolar epithelium and to a lesser extent PECs, and is upregulated by chronic hypoxia; Bmp4 levels do not increase with hypoxia in Bmp4LacZ/+ mice, and these mice are protected from the development of hypoxic PH (10). Because Bmpr2ΔEx2/+ mice develop more severe hypoxic PH (11), these data suggest that dominant effects of the Bmpr2 mutation do not result from loss of Bmp4-dependent responses in the pulmonary vasculature. This led us to explore whether Bmp2, which is also upregulated in the hypoxic lung (10), is the predominant intrapulmonary Bmp ligand responsible for activating these Bmpr2-dependent responses in the pulmonary vasculature. We now show that Bmp2 levels do not increase with hypoxia in heterozygous null Bmp2+/− mice and that Bmp2+/− mice develop more severe hypoxic PH. Furthermore, like Bmpr2ΔEx2/+ mice, there is a marked reduction in hypoxia-induced eNOS expression in the pulmonary vasculature of Bmp2+/− mice. This is associated with a reduction in phospho-Ser239 VASP expression, a sensitive indicator of defective NO/cGMP signaling in the vasculature (20), suggesting that there is a decrease in pulmonary NOS activity in hypoxic Bmp2+/− mice. This indicates that eNOS deficiency is a common feature in hypoxic Bmp2+/− and Bmpr2ΔEx2/+ mice. Furthermore, although it has been suggested that other intrapulmonary Bmp ligands (including Bmp6 and Bmp7) and/or circulating Bmp ligands (including Bmp9) may activate Bmpr2 in the pulmonary vasculature (27), our studies suggest that effects of Bmpr2 mutations result to a large part from loss of Bmp2 responses in the vasculature.
We performed these studies on Bmp2+/− mice maintained on a pure C57Bl/6 background. In contrast, our earlier studies on Bmp4LacZ/+ mice were performed in mice maintained on an outbred ICR background (10). This was necessary, since previous studies had shown that Bmp4+/− mice have significant developmental abnormalities on a C57Bl/6 background (7, 29). A notable difference between our studies on C57Bl/6 vs. ICR background strains is that hypoxic induction of pulmonary Bmp4 mRNA expression in ICR mice is lost in wild-type C57Bl/6 mice (Fig. 1B). However, there was no difference in hypoxic Bmp4 induction between wild-type and Bmp2+/− mutant C57Bl/6 mice, indicating that this difference in Bmp4 responsiveness does not account for the notable difference in hypoxic PH and vascular remodeling that we have observed in Bmp2+/− vs. wild-type C57Bl/6 mice. Furthermore, our studies show that wild-type ICR and C57Bl/6 mice show a similar response to hypoxia (increased RSVP, pulmonary vascular remodeling and proliferation, and enhanced eNOS expression). This indicates that the two background stains have similar pulmonary vascular responses to chronic hypoxia and suggests that the profound differences in pulmonary vascular responses in Bmp2+/− and Bmp4LacZ/+ mice are unlikely to result from strain differences between the mutant lines alone.
Increased PH in Bmp2+/− mice is associated with increased peripheral muscularization, a marker of pulmonary vascular remodeling. This is not associated with an increase in pulmonary VSMC proliferation in hypoxic Bmp2+/− mice. VSMC proliferation in larger muscularized vessels is actually decreased in Bmp2+/− mice after 1 wk of hypoxia. These findings were unexpected, since hypoxia-induced peripheral vessel muscularization is usually associated with increased VSMC proliferation (18). This expansion of peripheral muscularized vessels does not result from decreased VSMC apoptosis in hypoxic Bmp2+/− vs. wild-type mice, since we were unable to detect pulmonary VSMC apoptosis in wild-type mice exposed to 10% oxygen by TdT-dUTP nick end labeling assay or Cleaved Caspase 3 staining (Ref. 10 and D. B. Frank, unpublished observations). Therefore, although exogenous BMP2 promotes growth inhibition and induces apoptosis in cultured VSMCs (10, 19, 26, 30, 32), our findings indicate that these responses do not play significant roles in promoting enhanced vascular remodeling in hypoxic pulmonary vasculature of Bmp2+/− mice in vivo. It has been proposed that peripheral vascular remodeling in hypoxia results from increased migration of differentiated pulmonary VSMCs from more proximal sites and/or increased differentiation of nonmuscularized peripheral vessel pericytes into VSMCs (18). Exogenous BMP2 (and BMP4) increase migration of cultured pulmonary VSMCs (10). On this basis, reduced expression of Bmp2 would be expected to reduce, not increase, the degree of hypoxic peripheral vascular muscularization in Bmp2+/− mice. Therefore, our studies suggest that increased hypoxic peripheral muscularization in Bmp2+/− mice is most likely to result from increased vascular pericyte differentiation in VSMCs. The mechanism by which this occurs is unknown.
The role of eNOS in maintaining low pulmonary vascular resistance has been demonstrated in a number of studies. Mice that are either heterozygous or homozygous deficient for eNOS have increased hypoxic PH and pulmonary vascular tone (9, 24, 25). Therefore, our observation that eNOS expression and activity are reduced in the pulmonary vasculature of Bmp2+/− and Bmpr2ΔEx2/+ mutant mice suggests that eNOS deficiency could play a role in exacerbating hypoxic PH in these mice. Other mechanisms may contribute to these abnormal PH responses. For example, Bmpr2 mutant mice have increased pulmonary arterial vasoconstrictor responses (11, 16), suggesting that abnormal Bmpr2 signaling results in increased VSMC contractility. Furthermore, transgenic overexpression of a dominant negative Bmpr2 construct in VSMCs induces spontaneous PH in mice (28) and promotes expression of inflammatory mediators, including interleukin-6 (12). This suggests that defective Bmpr2 signaling in VSMCs also exacerbates PH by promoting a local inflammatory response in the pulmonary vasculature. However, our findings are also consistent with the observation that there is impaired EC-dependent vasodilatation of preconstricted intrapulmonary arteries from Bmpr2ΔEx2/+ mice (11) and suggest that eNOS deficiency contributes to the development of more severe hypoxic PH in Bmp2 and Bmpr2 mutant mice.
Bmp2 and Bmp4 have 92% amino acid sequence identity and are essentially indistinguishable in most in vitro functional assays. However, our studies suggest that Bmp2 and Bmp4 have opposing effects in hypoxic PH in vivo. Furthermore, there is a marked reduction in hypoxia-induced eNOS expression in Bmp2+/− mice, whereas eNOS expression is unaffected in Bmp4LacZ/+ mouse lungs. As outlined above, eNOS deficiency could account for the differences in PH responses to chronic hypoxia in the two Bmp mutant mouse lines. However, our studies also show that eNOS expression is induced equally after treating cultured IPA preparations with exogenous BMP2 and BMP4. This suggests that the opposing effects of these ligands in vivo do not result from differences in direct cellular responses to Bmp2 and Bmp4. An alternative explanation is that vascular target cells respond differently to these ligands in vivo because Bmp2 and Bmp4 are expressed in distinct cellular domains in the lung. Our findings using Bmp2 BAC transgenic reporter mice to identify cellular expression domains of Bmp2 in the hypoxic lung support this hypothesis. Together with our earlier observations using Bmp4 LacZ knock-in mice to evaluate Bmp4 localization in the hypoxic lung (10), these findings indicate that Bmp2 and Bmp4 are expressed in overlapping expression domains in the alveolar epithelium but that Bmp2 is more strongly expressed than Bmp4 in small peripheral vessels in the hypoxic lung. Therefore, reduced expression of the two Bmp ligands in Bmp2+/− vs. Bmp4LacZ/+ mice may have different effects on vascular function and eNOS expression as a result of differences in local Bmp concentrations in and around the hypoxic pulmonary vasculature.
In summary, these studies demonstrate for the first time the opposing roles of Bmp2 and Bmp4 ligands in hypoxic PH. We show that eNOS deficiency is a common feature in hypoxic Bmp2+/− and Bmpr2ΔEx2/+ mice and suggest that Bmp2 is the predominant ligand mediating Bmpr2-dependent effects in hypoxic PH. Because the functional effects of Bmp2 and Bmp4 ligands are indistinguishable in vitro, these findings underscore the importance of studies that evaluate the functional role of the Bmp pathway in vivo. These studies shed new light on the mechanisms by which aberrant BMP signaling associated with BMPR2 mutations may contribute to the pathogenesis of HPAH as well as other more common forms of PH not associated with inherited BMPR2 mutations.
GRANTS
This work was funded primarily by a Philip Morris External Research Grant. L. Anderson is the recipient of an American Heart Association Postdoctoral Fellowship. J. Lowery is funded by the National Heart, Lung, and Blood Institute T32HL-007751 Vascular Biology Training Grant.
DISCLOSURES
None
ACKNOWLEDGMENTS
We thank Stephen Harris and Brigid Hogan for mouse mutants and the Vanderbilt University Mouse Metabolic Phenotyping Center for assistance with physiology measurements. We thank Scott Baldwin for access to microscopes.
REFERENCES
- 1.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L1241–L1247, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol 313: 234–245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem 269: 14509–14517, 1994 [PubMed] [Google Scholar]
- 4.Chandler RL, Chandler KJ, McFarland KA, Mortlock DP. Bmp2 transcription in osteoblast progenitors is regulated by a distant 3′ enhancer located 156.3 kilobases from the promoter. Mol Cell Biol 27: 2934–2951, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol 27: 1877–1885, 2007 [DOI] [PubMed] [Google Scholar]
- 6.de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev 15: 1–11, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL. Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol 188: 235–247, 1997 [DOI] [PubMed] [Google Scholar]
- 8.El-Bizri N, Wang L, Merklinger SL, Guignabert C, Desai T, Urashima T, Sheikh AY, Knutsen RH, Mecham RP, Mishina Y, Rabinovitch M. Smooth muscle protein 22alpha-mediated patchy deletion of Bmpr1a impairs cardiac contractility but protects against pulmonary vascular remodeling. Circ Res 102: 380–388, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagan KA, Fouty BW, Tyler RC, Morris KG, Jr, Hepler LK, Sato K, LeCras TD, Abman SH, Weinberger HD, Huang PL, McMurtry IF, Rodman DM. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest 103: 291–299, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank DB, Abtahi A, Yamaguchi DJ, Manning S, Shyr Y, Pozzi A, Baldwin HS, Johnson JE, de Caestecker MP. Bone morphogenetic protein 4 promotes pulmonary vascular remodeling in hypoxic pulmonary hypertension. Circ Res 97: 496–504, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Frank DB, Lowery J, Anderson L, Brink M, Reese J, de Caestecker M. Increased susceptibility to hypoxic pulmonary hypertension in Bmpr2 mutant mice is associated with endothelial dysfunction in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 294: L98–L109, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Hagen M, Fagan K, Steudel W, Carr M, Lane K, Rodman DM, West J. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol 292: L1473–L1479, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Hong KH, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD, Oh SP. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 118: 722–730, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA 88: 5096–5100, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13: 424–436, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res 98: 818–827, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips JA, 3rd, Newman J, Williams D, Galie N, Manes A, McNeil K, Yacoub M, Mikhail G, Rogers P, Corris P, Humbert M, Donnai D, Martensson G, Tranebjaerg L, Loyd JE, Trembath RC, Nichols WC. BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet 68: 92–102, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyrick BO, Perkett EA. The sequence of cellular and hemodynamic changes of chronic pulmonary hypertension induced by hypoxia and other stimuli. Am Rev Respir Dis 140: 1486–1489, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation 104: 790–795, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, Munzel T. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ Res 87: 999–1005, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Quinlan TR, Li D, Laubach VE, Shesely EG, Zhou N, Johns RA. eNOS-deficient mice show reduced pulmonary vascular proliferation and remodeling to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 279: L641–L650, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Song Y, Coleman L, Shi J, Beppu H, Sato K, Walsh K, Loscalzo J, Zhang YY. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am J Physiol Heart Circ Physiol 295: H677–H690, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Y, Jones JE, Beppu H, Keaney JF, Jr, Loscalzo J, Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation 112: 553–562, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steudel W, Ichinose F, Huang PL, Hurford WE, Jones RC, Bevan JA, Fishman MC, Zapol WM. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res 81: 34–41, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Steudel W, Scherrer-Crosbie M, Bloch KD, Weimann J, Huang PL, Jones RC, Picard MH, Zapol WM. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest 101: 2468–2477, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda M, Otsuka F, Nakamura K, Inagaki K, Suzuki J, Miura D, Fujio H, Matsubara H, Date H, Ohe T, Makino H. Characterization of the bone morphogenetic protein (BMP) system in human pulmonary arterial smooth muscle cells isolated from a sporadic case of primary pulmonary hypertension: roles of BMP type IB receptor (activin receptor-like kinase-6) in the mitotic action. Endocrinology 145: 4344–4354, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Upton PD, Morrell NW. TGF-beta and BMPR-II pharmacology–implications for pulmonary vascular diseases. Curr Opin Pharmacol 9: 274–280, 2009 [DOI] [PubMed] [Google Scholar]
- 28.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 94: 1109–1114, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9: 2105–2116, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 96: 1053–1063, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122: 2977–2986, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Fantozzi I, Tigno DD, Yi ES, Platoshyn O, Thistlethwaite PA, Kriett JM, Yung G, Rubin LJ, Yuan JX. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 285: L740–L754, 2003. [DOI] [PubMed] [Google Scholar]