Abstract
Recent studies have shown that asymmetric dimethylarginine (ADMA), a nitric oxide synthase inhibitor, is increased in hypertension and chronic kidney disease. However, little is known about the effects of hypertension per se on ADMA metabolism. The purpose of this study was to test the hypothesis that ANG II-induced hypertension, in the absence of renal injury, is associated with increased oxidative stress and plasma and renal cortex ADMA levels in rats. Male Sprague-Dawley rats were treated with ANG II at 200 ng·kg−1·min−1 sc (by minipump) for 1 or 3 wk or at 400 ng·kg−1·min−1 for 6 wk. Mean arterial pressure was increased after 3 and 6 wk of ANG II; however, renal injury (proteinuria, glomerular sclerosis, and interstitial fibrosis) was only evident after 6 wk of treatment. Plasma thiobarbituric acid reactive substances concentration and renal cortex p22phox protein abundance were increased early (1 and 3 wk), but urinary excretion of isoprostane and H2O2 was only increased after 6 wk of ANG II. An increased in plasma ADMA after 6 wk of ANG II was associated with increased lung protein arginine methyltransferase-1 abundance and decreased renal cortex dimethylarginine dimethylaminohydrolase activity. No changes in renal cortex ADMA were observed. ANG II hypertension in the absence of renal injury is not associated with increased ADMA; however, when the severity and duration of the treatment were increased, plasma ADMA increased. These data suggest that elevated blood pressure alone, for up to 3 wk, in the absence of renal injury does not play an important role in the regulation of ADMA. However, the presence of renal injury and sustained hypertension for 6 wk increases ADMA levels and contributes to nitric oxide deficiency and cardiovascular disease.
Keywords: kidney, protein arginine methyltransferase-1, dimethylarginine dimethylaminohydrolase, oxidative stress
nitric oxide (NO), an important mediator of vascular tone and renal function, regulates glomerular, vascular, and tubular function in the kidney (20). Regulation of NO biosynthesis is complex, and the endogenous competitive inhibitor of NO synthase (NOS), asymmetric dimethylarginine (ADMA), is one important influence on NO production. ADMA is generated by protein arginine methyltransferase (PRMT) class 1 (PRMT-1) enzymes (1). Some ADMA is excreted in the urine, but the major route of ADMA elimination is via metabolism by dimethylarginine dimethylaminohydrolase (DDAH)-1 and DDAH-2 (28). The kidney plays an important role in the metabolism of ADMA, inasmuch as the highest density of the DDAH enzymes is found in the kidney cortex (30); however, these enzymes are also abundantly expressed in many other organs, including the liver and lung.
There is a robust correlation between ADMA levels and severe cardiovascular events and mortality (3). However, little is known about the effects of high blood pressure per se on ADMA levels. Some small clinical studies have shown a relationship between high blood pressure and high plasma ADMA concentrations (31, 39, 49), but other reports have not shown the same association (24, 37). In animal models of hypertension, such as the spontaneously hypertensive rat, some studies have implicated increased ADMA, whereas others report that the hypertension in spontaneously hypertensive rats is not ADMA-dependent (22, 23). However, when hypertension and renal disease are present, an increased plasma ADMA is observed (5, 23, 34), and our own experiments have shown that ADMA levels are increased in rat models of chronic kidney disease (CKD), even without elevations in blood pressure (44, 48).
Because the effects of high blood pressure alone on ADMA metabolism are unknown, the purpose of this study was to determine whether chronic ANG II hypertension without kidney damage is associated with increases in oxidative stress and ADMA levels. To address this issue, we examined the impact of 1 and 3 wk of ANG II infusion on systemic and renal ADMA levels, on the abundance of PRMT-1, and the abundance and activity of DDAH-1 and DDAH-2 in the renal cortex, liver, and lung. We also looked at several indexes of oxidative stress, since oxidants are reported to increase ADMA and are a feature of hypertension (30). In addition, we incorporated a group infused with high-dose ANG II for 6 wk, where sustained hypertension and CKD were evident.
MATERIALS AND METHODS
All experiments were performed using male Sprague-Dawley rats (400–500 g body wt; Harlan Laboratories) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved and monitored by the University of Florida Institutional Animal Care and Use Committee. Animals were housed under conditions of constant temperature and humidity and exposed to a 12:12-h light-dark cycle. All rats were given free access to regular rat chow and water.
Rats were randomly assigned to one of four groups: control, ANG II infusion for 1 wk, ANG II infusion for 3 wk, or high-dose ANG II infusion for 6 wk. ANG II (Sigma, St. Louis, MO) was infused via osmotic minipump for 1 or 3 wk at 200 ng·kg−1·min−1 or for 6 wk at 400 ng·kg−1·min−1. ANG II at 200 ng·kg−1·min−1 was expected to increase blood pressure with minimal renal injury over a 1- to 3-wk period, whereas ANG II at 400 ng·kg−1·min−1 would increase blood pressure and also cause proteinuria and renal structural injury when infused over a 6-wk period.
At the end of the treatment period, all groups of rats were placed on a low-nitrate diet (custom AIN76C, MP Biomedicals, Solon, OH) for 24 h before urine collection. Then the rats were placed in metabolic cages overnight for collection of urine. At the time of death, mean arterial pressure was measured via the abdominal aorta in anesthetized rats, an aortic blood sample was collected, and the kidneys were perfused blood-free with cold PBS. The right kidney was removed and separated into cortex and medulla, sections of the liver and lung were removed, and the tissues were snap frozen in liquid nitrogen. The left kidney was then perfused with 2% paraformaldehyde-lysine-meta-periodate for 8–10 min and removed.
A subset of control and 3-wk ANG II-infused rats were implanted with indwelling catheters for the measurement of mean arterial pressure in conscious rats. All surgeries were conducted using full sterile technique, with rats under general isoflurane anesthesia (Abbott, North Chicago, IL), and buprenorphine HCl (0.05 mg/kg sc; Reckitt Benckiser Pharmaceuticals, Richmond, VA) was given for analgesia. A Tygon vascular catheter was placed in the left femoral artery, threaded under the skin by trocar, exteriorized at the back of the neck, and primed and plugged. After recovery from anesthesia, the rats were returned to individual cages, in which they moved freely and were allowed to recover for 1 wk. All catheterized rats were trained to accept handling and the activity in the laboratory. Conscious mean arterial pressures were measured via the indwelling catheter. No qualitative differences in mean arterial pressure were observed between the awake and anesthetized animals; mean arterial pressures in this subset of anesthetized rats were 111 ± 4 and 141 ± 5 mmHg for the control and 3-wk ANG II-treated groups, respectively. In the same rats, blood pressures measured in the conscious state averaged 126 ± 1 and 146 ± 6 mmHg for the control and 3-wk ANG II-treated groups, respectively. This finding validates our use of the anesthetized terminal blood pressure measurement for assessment of hypertension in all rats.
Paraffin-embedded kidneys were sectioned at a thickness of 5 μm onto Superfrost Plus slides, and kidney sections were stained using a periodic acid-Schiff stain kit (Sigma). The level of renal injury was assessed on a blinded basis on a scale of 0–5, where 1 involves <10% damage, 2 represents 11–25% damage, 3 represents 25–50% damage, 4 represents 51–75% damage, and 5 represents 76–100% damage.
Urine protein concentrations were measured by the Bradford method (Bio-Rad). For creatinine clearance measurements, plasma was prepared using the method of Tsikas et al. (42) with few modifications. Plasma (≥80 μl) was precipitated in acetonitrile at four times its volume and then centrifuged at 15,000 g for 15 min and dried under nitrogen at 45°C. The dried sample was dissolved in glass-distilled water at half of its original sample volume and then centrifuged for 10 min at 15,000 g. Urine samples were diluted 1:50 or 1:200 and then prepared using the method of Tsikas et al. Creatinine was measured by HPLC using the chromatographic method of George et al. (12). Creatinine was eluted on a 3.9 × 150 mm Waters AccQ-Tag C18 column in a 20 mmol/l potassium dihydrogen phosphate (pH 7.4) isocratic mobile phase, followed by a 60:40 buffer-acetonitrile 12-min column washout and then a 5-min reequilibration in 100% buffer (20 mmol/l potassium dihydrogen phosphate buffer, pH 7.4). Creatinine was then measured with a PerkinElmer series 200 HPLC with a series 200 UV detector. Plasma thiobarbituric acid reactive substances (TBARS) concentration was measured using the OxiTek TBARS assay kit (Zeptometrix), urinary H2O2 excretion was measured using Amplex Red (Molecular Probes), and isoprostane excretion was measured by enzyme immunoassay after pretreatment with β-glucuronidase (Oxford Biomedical).
ADMA and l-arginine levels in plasma and kidney cortex homogenates were measured using reverse-phase HPLC with the Waters AccQ-Fluor fluorescent reagent kit by an adaptation of the method of Heresztyn et al. (15). Briefly, 100 μl of plasma or cortex homogenate were mixed with 350 μl of borate buffer (pH 9). The sample was placed on an unconditioned Waters Oasis MCX column and then washed with 1 ml of borate buffer, three times with 1 ml of H2O, and then with 1 ml of methanol. The sample was eluted with 1 ml of NH4OH-H2O-methanol (10:40:50), dried under nitrogen gas, and then reconstituted with 50 μl of H2O. Recovery was 85%. The sample (50 μl) was injected on a Luna 150 × 3 mm C18 reverse-phase column (Phenomenex, Torrance, CA). A flow rate of 1.2 ml/min was used, and intensity was measured using a series 200 fluorescent detector with excitation at 250 nm and emission at 395 nm (PerkinElmer Life and Analytical Sciences, Shelton, CT). Standards contained concentrations of ADMA in the range of 0.125–8 μmol/l and l-arginine in the range of 3.12–200 μmol/l.
Protein abundances were detected using Western blotting, as previously described (51). Briefly, samples (200 μg of kidney cortex, 150 μg of liver, and 75 μg of lung) were loaded on a 12% polyacrylamide gel and separated by electrophoresis. Membranes were incubated overnight with specific antibodies: goat anti-DDAH-1 antibody (1:250 dilution), goat anti-DDAH-2 antibody (1:100 dilution), and goat anti-p22phox antibody (1:50 dilution) from Santa Cruz Biotechnology (Santa Cruz, CA) and rabbit anti-PRMT-1 antibody (1:2,000 dilution) from Upstate Biotechnology (Lake Placid, NY). The membranes were then incubated with corresponding secondary antibodies: donkey anti-goat antibody (1:2,000 dilution; Santa Cruz Biotechnology) and goat anti-rabbit antibody (1:3,000 dilution; Bio-Rad, Hercules, CA). Bands of interest were visualized using enhanced chemiluminescence reagent and quantified by densitometry (VersaDoc imaging system and Quantity One Analysis software, Bio-Rad) as integrated optical density (IOD) after subtraction of background. IOD was factored for Ponceau Red staining (Sigma) to correct for any variations in total protein loading and for an internal standard, and protein abundance is represented as IOD/Ponceau Red/standard.
DDAH activity was measured by a colorimetric assay that measures the rate of citrulline production, as previously described (43). Kidney cortex, liver, and lung were homogenized in sodium phosphate buffer. Tissue homogenate was preincubated with urease for 15 min, and then 100 μl of homogenate containing 2 mg of protein were incubated with 1 mmol/l ADMA for 45 min at 37°C. After deproteinization, the supernatant was incubated with the color mixture at 60°C for 110 min. The absorbance was measured on a Tecan Safire optical system at 466 nm. DDAH activity is represented as micromoles of citrulline formation per gram of protein per minute.
Values are means ± SE. Parametric data were analyzed by one-way ANOVA using Prism 4 software (Graph Pad Software, San Diego, CA). Histological (nonparametric) data were analyzed by Kruskal-Wallis test. P < 0.05 was considered statistically significant.
RESULTS
Blood pressure, renal injury, and urinary nitrate/nitrite (NOx) excretion.
After 1 wk of ANG II treatment, there was a nonsignificant tendency for blood pressure to increase. No changes were observed in renal injury markers or NOx excretion. ANG II at 200 ng·kg−1·min−1 for 3 wk and at 400 ng·kg−1·min−1 for 6 wk raised blood pressure in a dose-dependent manner (Table 1) and lowered 24-h NOx excretion. Proteinuria, glomerular sclerosis, and interstitial fibrosis were significant only in the 6-wk group.
Table 1.
Mean arterial pressure, urinary protein excretion, glomerular sclerosis and interstitial fibrosis, and NOx excretion in control ANG II-treated rats
| Treatment | Mean Arterial Pressure, mmHg | Urinary Protein Excretion, mg·day−1·100 g−1 | Glomerular Sclerosis Score | Interstitial Fibrosis Score | Urinary NOx Excretion, μmol/day |
|---|---|---|---|---|---|
| Control | 104 ± 3 | 7.7 ± 2.0 | 0.2 ± 0.1 | 0.3 ± 0.1 | 4.1 ± 0.7 |
| ANG II | |||||
| 1 wk | 125 ± 12 | 4.2 ± 0.7 | 0.4 ± 0.2 | 0.8 ± 0.2 | 2.4 ± 0.8 |
| 3 wk | 148 ± 5* | 17.5 ± 6.9 | 0.4 ± 0.2 | 0.4 ± 0.2 | 2.0 ± 1.1* |
| 6 wk | 161 ± 5*† | 61.6 ± 6.0*† | 1.0 ± 0.0*† | 2.1 ± 0.2*† | 2.0 ± 0.3* |
Values are means ± SE (n =5–11). NOx, nitrate/nitrite.
P < 0.05 vs. control.
P < 0.05 vs. ANG II 1 wk or ANG II 3 wk.
Oxidative stress measures.
As shown in Table 2, ANG II hypertension was associated with increases in markers of oxidative stress, although these were variable among the treatments. Treatment with ANG II for 1 and 3 wk at 200 ng·kg−1·min−1 increased plasma TBARS concentration but did not affect urinary measures of oxidative stress (H2O2 or isoprostane excretion). Treatment with ANG II for 1 wk increased the abundance of the NADPH oxidase subunit p22phox in the kidney cortex. Although there was a tendency for the p22phox abundance to be elevated in the rats treated for 3 wk, this did not reach statistical significance.
Table 2.
Oxidative stress measures
| Treatment | Urinary H2O2 Excretion, nmol/day | Urinary Isoprostane Excretion, ng/day | Plasma TBARS, nmol/l | p22phox Abundance, IOD/Ponceau/Std |
|---|---|---|---|---|
| Control | 93 ± 16 | 56 ± 4 | 15 ± 2 | 1.0 ± 0.1 |
| ANG II | ||||
| 1 wk | 86 ± 13 | 48 ± 7 | 31 ± 1* | 1.3 ± 0.02* |
| 3 wk | 113 ± 26 | 61 ± 10 | 33 ± 5* | 1.7 ± 0.5 |
| 6 wk | 328 ± 82*† | 114 ± 8*† | 19 ± 2† | 0.9 ± 0.1 |
Values are means ± SE (n =5–11). TBARS, thiobarbituric acid reactive substances. Integrated optical density (IOD) was factored for Ponceau Red staining and for an internal standard (Std).
P < 0.05 vs. control.
P < 0.05 vs. ANG II 1 wk or ANG II 3 wk.
Conversely, high-dose (400 ng·kg−1·min−1) ANG II treatment for 6 wk increased urinary H2O2 and isoprostane excretion but did not change plasma TBARS concentration or renal cortex p22phox abundance.
ADMA and l-arginine concentrations.
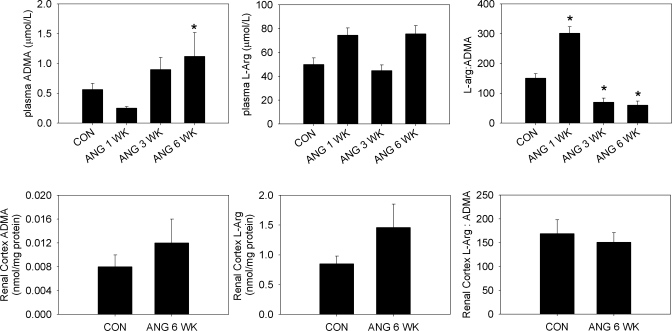
In the rats infused with ANG II for 1 wk, a slight increase in plasma l-arginine levels was coupled with a slight decline in plasma ADMA levels (Fig. 1). Although neither of these changes reached statistical significance, there was a significant increase in the l-arginine-to-ADMA ratio. After 3 wk of ANG II treatment, the opposite was true, with nonsignificant declines in l-arginine and increases in ADMA, resulting in a significant reduction in the l-arginine-to-ADMA ratio. The 6-wk high-dose ANG II treatment increased plasma ADMA almost threefold, also resulting in a fall in the l-arginine-to-ADMA ratio. Because of the changes in plasma levels of ADMA, we also examined the ADMA and l-arginine levels in the cortex of the rats treated with the high-dose ANG II. Despite the increased plasma levels of ADMA at 6 wk of ANG II treatment, there were no changes in l-arginine or ADMA concentrations in the kidney cortex.
Fig. 1.
Top: plasma concentrations of asymmetric dimethylarginine (ADMA) and l-arginine (B) as measured by HPLC and the ratio of l-arginine to ADMA in plasma from control (CON) rats and rats treated with ANG II (n = 5–11). Bottom: renal cortex concentrations of ADMA and l-arginine as measured by HPLC in tissue homogenates and the ratio of l-arginine to ADMA in renal cortex from rats treated with high-dose ANG II for 6 wk and control rats (n = 6). *P < 0.05 vs. CON.
Abundance and activity of ADMA regulatory enzymes.
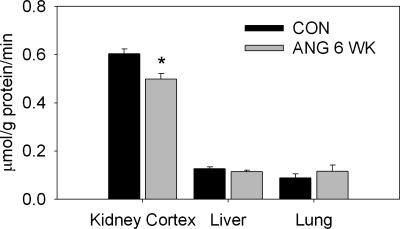
We also evaluated the protein abundance of the enzymes that regulate ADMA (PRMT-1, DDAH-1, and DDAH-2) by Western blot (Table 3). We found that 1 wk of treatment with ANG II raised renal cortical abundance of DDAH-1 but did not affect abundance of DDAH-1 in liver or lung or abundance of DDAH-2 or PRMT-1 in any organ. No changes were observed in protein abundance with 3 wk of ANG II treatment. Liver and lung from the 3-wk ANG II infusion groups were not available for Western blot analysis. Although the 6 wk of high-dose ANG II treatment did not affect the quantity of the DDAH enzymes, PRMT-1 abundance was significantly elevated in the lung of this group of rats. Because plasma ADMA levels were elevated in rats treated with high-dose ANG II for 6 wk, we also evaluated the activity of DDAH in these animals. DDAH activity, as measured by the production of citrulline, was significantly reduced in the kidney cortex of rats treated with 400 ng·kg−1·min−1 ANG II for 6 wk (Fig. 2). No changes in enzyme activity were observed in the liver or lung.
Table 3.
Abundance of ADMA regulatory enzymes
| Treatment | PRMT-1 | DDAH-1 | DDAH-2 |
|---|---|---|---|
| Renal cortex | |||
| Control | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| ANG II | |||
| 1 wk | 1.2 ± 0.1 | 1.6 ± 0.2* | 0.9 ± 0.1 |
| 3 wk | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.2 |
| 6 wk | 1.1 ± 0.1 | 0.7 ± 0.1 | 1.1 ± 0.1 |
| Lung | |||
| Control | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| ANG II | |||
| 1 wk | 1.4 ± 0.2 | 1.0 ± 0.2 | 1.2 ± 0.2 |
| 6 wk | 1.7 ± 0.4* | 0.9 ± 0.1 | 1.2 ± 0.6 |
| Liver | |||
| Control | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| ANG II | |||
| 1 wk | 1.2 ± 0.3 | 0.7 ± 0.1 | 1.0 ± 0.1 |
| 6 wk | 1.3 ± 0.1 | 0.8 ± 0.2 | 1.1 ± 0.1 |
Values are mean ± SE (n =5–8), expressed as IOD/Ponceau/Std (see Table 2 footnote). PRMT-1, protein arginine methyltransferase-1; DDAH-1 and DDAH-2, dimethylarginine dimethylaminohydrolase-1 and -2.
P < 0.05 vs. control.
Fig. 2.
Dimethylarginine dimethylaminohydrolase (DDAH) enzyme activity (μmol citrulline·g protein−1·min−1) in homogenates of kidney cortex, liver, and lung from control rats and rats treated with high-dose ANG II for 6 wk (n = 8–9). *P < 0.05 vs. CON.
DISCUSSION
The main new findings of this study are that treatment with a high dose (400 ng·kg−1·min−1) of ANG II for 6 wk, which induced significant renal injury along with marked hypertension, leads to increased plasma ADMA levels. This increased plasma ADMA is associated with elevated PRMT-1 protein abundance in the lung and reduced DDAH enzyme activity (but no change in DDAH-1 or DDAH-2 protein abundance) in the kidney cortex. When a lower dose (200 ng·kg−1·min−1) of ANG II was infused over a shorter duration (1–3 wk), however, circulating levels of ADMA were unchanged and kidney damage was absent. We measured several indexes of oxidative stress, which gave variable results at the three stages of chronic ANG II administration.
Previous studies demonstrated that chronic ANG II hypertension is associated with increased oxidative stress (6, 11, 33). In the present study, we measured indexes of lipid peroxidation (plasma TBARS and urinary isoprostane excretion) and H2O2 excretion as indexes of oxidative stress. Urinary measures of oxidative stress were elevated only in the rats that exhibited significant renal injury. However, we cannot determine whether the increased urinary H2O2 or isoprostane originated in the kidney or is a reflection of exacerbation of the systemic production of oxidative species. Plasma TBARS (generally considered a relatively insensitive marker of systemic lipid peroxidation) were elevated at 1 and 3 wk but returned to control levels by 6 wk, when kidney damage was apparent. Although they are widely used and accepted, these indirect measures of reactive oxygen species production do not reveal the sites of reactive oxygen species production or action and have significant limitations.
As a direct measure of oxidative stress in the renal cortex, we quantitated the NADPH oxidase subunit p22phox, which is a critical component of the NADH/NADPH oxidase system and is the predominant source of superoxide in the renal cortex (13, 25). Vascular gene expression of p22phox increases during the first 5 days of ANG II hypertension (11, 46) but falls at 8–14 days of ANG II treatment, even though blood pressure remains elevated (11). Recently, Chabrashvili et al. (6) also showed that 1 wk of ANG II infusion (200 ng·kg−1·min−1) increased p22phox mRNA in kidney cortex. This finding agrees with our observation that an early increase in renal p22phox protein abundance returned to control levels after 3 and 6 wk of ANG II infusion. The variability in the responses observed in these different measures of oxidative stress is quite confusing, and this is in just one model of hypertension! As Vaziri (47) points out, tissue and cellular production of oxidants is very complex and can involve a number of possible pathways. Furthermore, oxidative stress begets inflammation, which establishes a vicious cycle (47); therefore, it is not surprising that the specific markers of oxidative stress vary during the evolution of hypertension and CKD.
The main goal of this study was to investigate the relationship between ANG II-induced hypertension and ADMA regulation. Our observation that plasma and kidney cortex ADMA levels are unchanged in ANG II-induced hypertension at 1 or 3 wk is contrary to our original hypothesis. We and others predicted that ADMA levels would increase secondary to ANG II-induced oxidative stress (6, 11, 33, 41). Indeed, the rise in blood pressure in response to ANG II infusion (0.7 mg·kg−1·day−1) can be abrogated by treatment with liposome-entrapped SOD (21). The ADMA regulatory enzymes PRMT-1, DDAH-1, and DDAH-2 are redox sensitive, with increasing levels of oxidative stress causing upregulation of PRMT gene expression and downregulation of DDAH activity (41). However, in the present study, the only change that we observed in PRMT-1 and DDAH-1 and DDAH-2 protein abundance at 1 and 3 wk of ANG II administration was an increase in renal DDAH protein at week 1, which was associated with a rise in the l-arginine-to-ADMA ratio. As discussed above, there were variable changes in the different markers of oxidative stress at these time points. Our study demonstrates that chronic infusion of ANG II at 200 ng·kg−1·min−1 for up to 3 wk is not sufficient to increase circulating or kidney cortex ADMA levels in the rat, despite increased blood pressure.
With 6 wk of high-dose (400 ng·kg−1·min−1) ANG II, we did observe an increase in plasma ADMA levels and a fall in the l-arginine-to-ADMA ratio but no change in renal cortex ADMA content. This was associated with increased PRMT-1 abundance in the lung, but not in the liver or kidney cortex. Bulau et al. (4) showed that the lung is a major source of circulating ADMA; therefore, this increase in PRMT-1 expression likely contributes to the increased plasma ADMA after 6 wk of high-dose ANG II. Although no changes were observed in DDAH-1 or DDAH-2 protein abundance in any tissue, we found that activity of DDAH was reduced in the kidney cortex of rats treated with high-dose ANG II for 6 wk. Among the tissues we studied, we also observed the highest enzyme activity in the kidney cortex, consistent with previous work that showed that the kidney plays a crucial role in the regulation of circulating ADMA levels in the rat (27). This decline in renal cortex DDAH activity is also likely to have contributed to the increase in plasma ADMA concentration in this group; however, the mechanisms responsible for the decline in DDAH activity remain unknown. The lack of concordance between enzyme activity and abundance underscores the need to better understand the posttranslational regulation of DDAH.
There is considerable variability in the literature on the impact of ANG II on ADMA levels. In clinical studies, Ito et al. (17) showed that an angiotensin-converting enzyme inhibitor (perindopril) and an AT1 receptor antagonist (losartan), but not a β-blocker (bisoprolol), reduced serum ADMA in patients with essential hypertension, even though all three treatments reduced blood pressure to a similar extent. In addition, Delles et al. (8) showed a significant reduction of ADMA levels by an angiotensin-converting enzyme inhibitor (enalapril) and an AT1 receptor antagonist (eprosartan) in mildly hypertensive men. However, Tomiyama et al. (45) reported that the AT1 receptor antagonist valsartan had no impact on plasma ADMA in a group of patients with essential hypertension, whereas telmisartan lowered plasma ADMA in the same patients. This was attributed to the additional peroxisome proliferator-activated receptor-γ agonist action of telmisartan, rather than to angiotensin inhibition (35, 45).
In cultured endothelial cells, incubation with ANG II for 24 h increased ADMA levels in association with increased PRMT expression and decreased DDAH activity (7). In contrast, after incubation with ANG II for 18 h, whole kidney slices from normal rats showed increased DDAH-2 protein but no change in DDAH-1 (29). There are also conflicting reports on the effects of administration of exogenous ANG II on ADMA in vivo. Whereas Hasegawa and colleagues (14) showed that 2 wk of ANG II infusion in mice resulted in a 100% increase in plasma ADMA, Jacobi et al. (19) found no effect of 4 wk of ANG II infusion on plasma ADMA levels. These studies did not examine the effects of ANG II infusion on tissue levels of ADMA. However, Jacobi et al. reported increased DDAH-2 gene expression in the kidney of mice treated with ANG II (with no change in DDAH-1), which could offset the impact of increased PRMT-1 expression and explain the lack of rise in plasma ADMA in their study. In the present study, we found that 1 and 3 wk of ANG II infusion resulted in no change in plasma or renal cortex ADMA levels. In a preliminary report by Wang et al. (50), 2 wk of ANG II infusion at the same dose used here (200 ng·kg−1·min−1) was also without effect on plasma ADMA levels in rats but did result in increased ADMA concentrations in small mesenteric arteries.
One important finding in the present study is that plasma ADMA was not elevated at 1 and 3 wk of ANG II infusion, where there was little kidney damage. Thus ANG II-induced hypertension alone, in the absence of kidney damage, does not inevitably lead to increased ADMA levels. However, after 6 wk of high-dose ANG II infusion, we did observe an increase in ADMA and significant renal injury (proteinuria, glomerular sclerosis, and interstitial fibrosis). This is consistent with previous reports of increased plasma ADMA in animal models of hypertension associated with renal disease (5, 23, 34). In addition, we previously reported elevations in plasma ADMA in the 5/6 nephrectomy (44) and chronic glomerulonephritis models of progressive CKD (48) at 15–20 wk after induction of injury. Interestingly, there was little or no hypertension in these CKD models. Our findings are also consistent with clinical data that have shown increased ADMA in the plasma of some patients with CKD and all patients with end-stage renal disease (for review see Ref. 2).
ADMA is one of many factors that determines NO bioavailability. In addition, ANG II has been shown to modulate the NO system, and the vasoconstriction elicited by ANG II is modulated by NO. Activation of the renin-angiotensin system can decrease or increase NO bioavailability. In vitro experiments showed that inhibition of NOS potentiates the vasoconstrictor effects of ANG II in the isolated perfused kidney (36), in the in vitro perfused juxtamedullary microcirculation preparation (16), and in isolated perfused afferent arterioles (18). ANG II has been shown to stimulate NOS via activation of the AT1 and AT2 receptors (38, 40). In the rat, renal excretion of NOx was increased during short-term (30–90 min) ANG II infusions but was unchanged during prolonged (5–6 days) ANG II infusion, suggesting that NO synthesis increases during acute, but not chronic, ANG II infusion (9). Mollnau et al. (26) showed that although ANG II infusion increased endothelial NOS mRNA and protein levels, NO production was reduced, possibly due to an “uncoupling” of the enzyme, and Ramseyer and Garvin (32) showed that ANG II decreased endothelial NOS expression in the renal medullary thick ascending limb. In addition, AT1 receptor activation stimulates superoxide production, which can scavenge NO to form peroxynitrite, thereby decreasing NO bioavailability (10). In the present study, we found that chronic ANG II infusion for 3 or 6 wk reduced urinary excretion of NOx an indirect measure of NO production. Because of the complex relationships between ANG II and NO production, we cannot determine the precise mechanisms by which NO production is reduced, except that the increased plasma ADMA likely contributes in the 6-wk model.
Perspectives and Significance
Experimental hypertension induced by ANG II in the absence of renal injury is not associated with increased circulating ADMA levels or ADMA concentrations in the renal cortex. When the severity and duration of the treatment were increased, plasma ADMA increased, suggesting that renal injury plays an important role in the regulation of ADMA production or metabolism and implying a mechanism by which CKD contributes to NO deficiency and cardiovascular disease. It will be important to evaluate the mechanisms by which ADMA and its regulatory enzymes are modified in other forms of hypertension with and without kidney disease in future animal studies. In addition, clinical studies are needed to determine the factors involved in the elevation of ADMA levels that are observed in human disease. This might provide insights for the development of specific therapies that target ADMA-induced NO inhibition and endothelial dysfunction. It is also important for us to critically evaluate the impact of oxidative stress on ADMA levels, because the effects of oxidant stress may differ on the basis of the type and localization of the oxidant. As indicated in this study, there is a changing pattern of oxidative stress during the evolution of chronic ANG II-induced hypertension. Our studies also highlight the importance of going beyond measures of DDAH enzyme abundance to assess enzyme activity, inasmuch as the regulation of enzyme activity is much more complex than simply the regulation of gene or protein expression.
GRANTS
This work was supported by the American Heart Association (Postdoctoral Fellowship to J. M. Sasser), the National Heart, Lung, and Blood Institute Multidisciplinary Training Program in Hypertension (Grant T32 HL-083810), and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-56843 (to C. Baylis).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Harold Snellen for expert technical assistance.
REFERENCES
- 1.Anthony S, Leiper J, Vallance P. Endogenous production of nitric oxide synthase inhibitors. Vasc Med 10: S3–S9, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol 294: F1–F9, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Böger RH. Asymmetric dimethylarginine (ADMA) and cardiovascular disease: insights from prospective clinical trials. Vasc Med 10: S19–S25, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bulau P, Zakrzewicz D, Kitowska K, Leiper J, Gunther A, Grimminger F, Eickelberg O. Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. Am J Physiol Lung Cell Mol Physiol 292: L18–L24, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Carlström M, Brown RD, Edlund J, Sällström J, Larsson E, Teerlink T, Palm F, Wåhlin N, Persson AE. Role of nitric oxide deficiency in the development of hypertension in hydronephrotic animals. Am J Physiol Renal Physiol 294: F362–F370, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol 285: R117–R124, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chen MF, Xie XM, Yang TL, Wang YJ, Zhang XH, Luo BL, Li YJ. Role of asymmetric dimethylarginine in inflammatory reactions by angiotensin II. J Vasc Res 44: 391–402, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Delles C, Schneider MP, John S, Gekle M, Schmieder RE. Angiotensin converting enzyme inhibition and angiotensin II AT1-receptor blockade reduce the levels of asymmetrical NG,NG-dimethylarginine in human essential hypertension. Am J Hypertens 15: 590–593, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Deng X, Welch WJ, Wilcox CS. Role of nitric oxide in short-term and prolonged effects of angiotensin II on renal hemodynamics. Hypertension 27: 1173–1179, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Förstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med 5: 338–349, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q 4th, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res 80: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 12.George SK, Dipu MT, Mehra UR, Singh P, Verma AK, Ramgaokar JS. Improved HPLC method for the simultaneous determination of allantoin, uric acid and creatinine in cattle urine. J Chromatogr B Analyt Technol Biomed Life Sci 832: 134–137, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa K, Wakino S, Tatematsu S, Yoshioka K, Homma K, Sugano N, Kimoto M, Hayashi K, Itoh H. Role of asymmetric dimethylarginine in vascular injury in transgenic mice overexpressing dimethylarginine dimethylaminohydrolase 2. Circ Res 101: e2–e10, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Heresztyn T, Worthley MI, Horowitz JD. Determination of l-arginine and NG,NG- and NG,NG′-dimethyl-l-arginine in plasma by liquid chromatography as AccQ-Fluor fluorescent derivatives. J Chromatogr B Analyt Technol Biomed Life Sci 805: 325–329, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Ikenaga H, Fallet RW, Carmines PK. Basal nitric oxide production curtails arteriolar vasoconstrictor response to ANG II in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F365–F373, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Ito A, Egashira K, Narishige T, Muramatsu K, Takeshita A. Renin-angiotensin system is involved in the mechanism of increased serum asymmetric dimethylarginine in essential hypertension. Jpn Circ J 65: 775–778, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Ito S, Johnson CS, Carretero OA. Modulation of angiotensin II-induced vasoconstriction by endothelium-derived relaxing factor in the isolated microperfused rabbit afferent arteriole. J Clin Invest 87: 1656–1663, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobi J, Maas R, Cordasic N, Koch K, Schmieder RE, Böger RH, Hilgers KF. Role of asymmetric dimethylarginine for angiotensin II-induced target organ damage in mice. Am J Physiol Heart Circ Physiol 294: H1058–H1066, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol Renal Physiol 272: F561–F578, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Laursen J, Rajagopalan S, Galis Z, Tarpey M, Freeman B, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 95: 588–595, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Li D, Xia K, Li NS, Luo D, Wang S, Jiang DJ, Deng HW, Li YJ. Reduction of asymmetric dimethylarginine involved in the cardioprotective effect of losartan in spontaneously hypertensive rats. Can J Physiol Pharmacol 85: 783–789, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Matsuoka H, Itoh S, Kimoto M, Kohno K, Tamai O, Wada Y, Yasukawa H, Iwami G, Okuda S, Imaizumi T. Asymmetrical dimethylarginine, an endogenous nitric oxide synthase inhibitor, in experimental hypertension. Hypertension 29: 242–247, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Meinitzer A, Seelhorst U, Wellnitz B, Halwachs-Baumann G, Boehm BO, Winkelmann BR, Marz W. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health Study). Clin Chem 53: 273–283, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension 47: 238–244, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Mollnau H, Wendt M, Szöcs K, Lassègue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Förstermann U, Meinertz T, Griendling K, Münzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90: E58–E65, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Nijveldt RJ, Teerlink T, van Guldener C, Prins HA, van Lambalgen AA, Stehouwer CDA, Rauwerda JA, van Leeuwen PAM. Handling of asymmetrical dimethylarginine and symmetrical dimethylarginine by the rat kidney under basal conditions and during endotoxaemia. Nephrol Dial Tranplant 18: 2542–2550, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Ogawa T, Kimoto M, Sasaoka K. Occurrence of a new enzyme catalyzing the direct conversion of NG,NG-dimethyl-l-arginine to l-citrulline in rats. Biochem Biophys Res Commun 148: 671–677, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Onozato ML, Tojo A, Leiper J, Fujita T, Palm F, Wilcox CS. Expression of DDAH and PRMT isoforms in the diabetic rat kidney; effects of angiotensin II receptor blocker. Diabetes 57: 172–180, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol 293: H3227–H3245, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, Tripepi G, Sesti G, Zoccali C. Asymmetric dimethylarginine, l-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol 46: 518–523, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Ramseyer VD, Garvin JL. Angiotensin II decreases nitric oxide synthase 3 expression via nitric oxide and superoxide in the thick ascending limb. Hypertension 53: 313–318, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol 284: R893–R912, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Sato J, Masuda H, Tamaoki S, Hamasaki H, Ishizaka K, Matsubara O, Azuma H. Endogenous asymmetrical dimethylarginine and hypertension associated with puromycin nephrosis in the rat. Br J Pharmacol 125: 469–476, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scalera F, Martens-Lobenhoffer J, Bukowska A, Lendeckel U, Täger M, Bode-Böger SM. Effect of telmisartan on nitric oxide-asymmetrical dimethylarginine system: role of angiotensin II type 1 receptor-γ and peroxisome proliferator activated receptor-γ signaling during endothelial aging. Hypertension 51: 696–703, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Schnackenberg CG, Wilkins FC, Granger JP. Role of nitric oxide in modulating the vasoconstrictor actions of angiotensin II in preglomerular and postglomerular vessels in dogs. Hypertension 26: 1024–1029, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, Jachmann N, Post F, Peetz D, Bickel C, Cambien F, Tiret L, Munzel T. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the atherogene study. Circ Res 97: e53–e59, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest 100: 264–269, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Surdacki A, Nowicki M, Sandmann J, Tsikas D, Boeger RH, Bode- Boeger SM, Kruszelnicka-Kwiatkowska O, Kokot F, Dubiel JS, Froelich JC. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J Cardiovasc Pharmacol 33: 652–658, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Suzuki H, Eguchi K, Ohtsu H, Higuchi S, Dhobale S, Frank GD, Motley ED, Eguchi S. Activation of endothelial nitric oxide synthase by the angiotensin II type 1 receptor. Endocrinology 147: 5914–5920, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Sydow K, Münzel T. ADMA and oxidative stress. Atheroscler Suppl 4: 41–51, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Tsikas D, Wolf A, Frölich JC. Simplified HPLC method for urinary and circulating creatinine. Clin Chem 50: 201–203, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Tain YL, Baylis C. Determination of dimethylarginine dimethylaminohydrolase activity in the kidney. Kidney Int 72: 886–886, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tain YL, Freshour G, Dikalova A, Griendling K, Baylis C. Vitamin E reduces glomerulosclerosis, restores renal neuronal NOS, and suppresses oxidative stress in the 5/6 nephrectomized rat. Am J Physiol Renal Physiol 292: F1404–F1410, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Tomiyama H, Yamada J, Koji Y, Shiina K, Yoshida M, Yamashina A. Effect of telmisartan on forearm postischemic hyperemia and serum asymmetric dimethylarginine levels. Am J Hypertens 20: 1305–1311, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Ushio-Fukai M, Zafari AM, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem 271: 23317–23321, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Vaziri ND. Causal link between oxidative stress, inflammation, and hypertension. Iran J Kidney Dis 2: 1–10, 2008 [PubMed] [Google Scholar]
- 48.Wagner L, Riggleman A, Erdely A, Couser W, Baylis C. Reduced nitric oxide synthase activity in rats with chronic renal disease due to glomerulonephritis. Kidney Int 62: 532–536, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, Strandgaard S, Iversen JS, Wilcox CS. Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am J Physiol Regul Integr Comp Physiol 296: R195–R200, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D, Wang X, Luo Z, Artigiano F, Jose PA, Teerlink T, Wilcox CS. Impaired endothelial relaxation, enhanced endothelial contraction and microvascular ROS and ADMA in angiotensin II infused rat (Abstract). Hypertension 54: e126, 2009 [Google Scholar]
- 51.Xiao S, Erdely A, Wagner L, Baylis C. Uremic levels of BUN do not cause nitric oxide deficiency in rats with normal renal function. Am J Physiol Renal Physiol 280: F996–F1000, 2001. [DOI] [PubMed] [Google Scholar]