Abstract
Chronic hypoxia reduces aerobic capacity (mitochondrial content) in limb skeletal muscles, and one of the causes seems to be decreased physical activity. Diaphragm and other respiratory muscles, however, may have a different pattern of adaptation as hypoxia increases the work of breathing. Thus, we hypothesized that chronic hypoxia would not reduce mitochondrial content in mouse diaphragm. Adult male C57BL/6J mice were kept in normoxia (FiO2 = 21%, control) or normobaric hypoxia (FiO2 = 10%, hypoxia) for 1, 2, and 4 wk. Mice were then killed, and the diaphragm and gastrocnemius muscles collected for analysis. In the diaphragm, cytochrome c oxidase histochemistry showed less intense staining in the hypoxia group. The total content of subunits from the electron transport chain, pyruvate dehydrogenase kinase 1 (PDK1), and voltage-dependent anion channel 1 (VDAC1) was evaluated by Western blot. These proteins decreased by 25–30% after 4 wk of hypoxia (P < 0.05 vs. control for all comparisons), matching a comparable decrease in diaphragmatic mitochondrial volume density (control 33.6 ± 5.5% vs. hypoxia 26.8 ± 6.7%, P = 0.013). Mitochondrial volume density or protein content did not change in gastrocnemius after hypoxia. Hypoxia decreased the content of peroxisome proliferator-activated receptor gamma (PPARγ) and PPARγ cofactor 1-alpha (PGC-1α) in diaphragm but not in gastrocnemius. PGC-1α mRNA levels in diaphragm were also reduced with hypoxia. BCL2/adenovirus E1B interacting protein 3 (BNIP-3) mRNA levels were upregulated after 1 and 2 wk of hypoxia in diaphragm and gastrocnemius, respectively; BNIP-3 protein content increased only in the diaphragm after 4 wk of hypoxia. Contrary to our hypothesis, these results show that chronic hypoxia decreases mitochondrial content in mouse diaphragm, despite the increase in workload. A combination of reduced mitochondrial biogenesis and increased mitophagy seems to be responsible for the decrease in mitochondrial content in the mouse diaphragm after hypoxia.
Keywords: respiratory muscles, metabolism, mitochondrial biogenesis, autophagy
chronic hypoxia (low partial pressure of oxygen, or low Po2) occurs physiologically when animals move to higher altitude. High altitude is defined as elevations above 2,500 m (8,000 ft). Approximately 140 million people permanently reside at high altitude (39), whereas many others are transiently exposed for work and leisure activities. There are well-known responses to chronic hypoxia, such as hyperventilation, higher hematocrit, and blood flow redistribution (36). However, the adaptive changes in skeletal muscle are controversial, in particular, those related to mitochondrial content.
The concept of how skeletal muscle copes with low oxygen levels was greatly influenced in the early 1960s by Reynafarje (41), who reported increased myoglobin content and mitochondrial oxidative capacity in sartorius muscles biopsies from high-altitude natives. Chronic hypoxia was also considered a stimulus to increase capillarity density (47). Years later, Banchero (1) suggested that hypoxia is not sufficient to induce angiogenesis; instead, he proposed that the combination of hypoxia and cold is responsible for the formation of new vessels. More recent studies have shown that the ratio of capillaries to fibers remains unchanged in hypoxic skeletal muscles (14, 18). Thus, the observed increase in capillary density has been attributed to reduced fiber cross-sectional area (29). Later, studies on human subjects found decreased mitochondrial enzymes activities (14, 20) and mitochondrial volume density (18, 22) after short-term and prolonged hypoxic exposure. The consensus from these studies was that chronic hypoxia reduces muscle fiber cross-sectional area, does not induce angiogenesis, and decreases mitochondrial capacity. The latter is controversial and still debated: some investigators find no change in mitochondrial enzymatic activity in response to chronic hypoxia, while others have documented changes in the expression of genes and content of proteins involved in mitochondrial metabolism (4, 10, 11, 40).
Most of the studies on skeletal muscle adaptation to hypoxia have been done in limb muscles; thus, one of the factors likely to contribute to decreased mitochondrial oxidative capacity is the inactivity that results from the drastic change in arterial Po2. While this is true for limb muscles, respiratory muscles—in particular, the diaphragm—are more active as a result of increased ventilation (9). In other words, the diaphragm and other respiratory muscles are recruited more frequently and extensively to compensate for the low ambient Po2. In consequence, the present study tested the hypothesis that chronic hypoxia does not reduce mitochondrial volume density in the mouse diaphragm compared with limb muscles. We evaluated the effect of chronic hypoxia (FiO2 = 10%) on mitochondrial mass, and the factors that would contribute to changes in mitochondrial density (i.e., mitochondrial biogenesis and autophagy) in diaphragm and gastrocnemius, a representative hind limb muscle. We also examined the effect of hypoxia on peroxisome proliferator-activated receptor γ cofactor 1 (PGC-1α) and BCL-2 /adenovirus E1B interacting protein 3 (BNIP-3). PGC-1α plays a central role in mitochondrial biogenesis; altered expression will impact the mitochondrial mass (42). On the other hand, BNIP-3 is a hypoxia-inducible gene shown to be important for mitophagy (16, 45, 50).
METHODS
Animals.
All procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee. Adult C57BL/6J male mice (12 wk old) were exposed to normobaric hypoxia (10% O2) for 2 or 4 wk. A total of 70 mice were used in the study: 22 control mice exposed to normoxia, 8 mice exposed for hypoxia for 1 wk and used only for RNA extraction, 18 mice exposed to hypoxia for 2 wk and 22 mice exposed to hypoxia for 4 wk. Mice were used because others have shown that they tolerate hypoxia well, and their adaptation to it is similar to that of humans (24). Hypoxia was induced using a Plexiglas chamber that was continuously flushed with nitrogen (Scott Gross, Lexington, KY) to maintain a FiO2 of 10%. Control mice remained in normoxic conditions. Mice were then euthanized, and the diaphragm and gastrocnemius—the hind limb muscle chosen for comparison—-were removed. For all of the experiments, costal diaphragm was dissected and removed after performing a median thoracotomy. Both gastrocnemius muscles were removed and randomly assigned for RNA extraction, histology, or biochemical analysis.
Cytochrome c oxidase histochemistry.
Diaphragm and gastrocnemius were covered with OCT-embedding medium and frozen in isopentane cooled to its freezing point in liquid nitrogen. Cross sections of the muscles (10-μm thickness) were collected and used for cytochrome c oxidase histochemistry. Slides were incubated in phosphate buffer (pH 7.4) containing cytochrome c (1 mg/ml), 4,3,3′-diaminobenzidine (0.5 mg/ml), and catalase (0.02 mg/ml). Sections were then dehydrated with ethanol and mounted in Permount. Images were obtained using a light microscope (model E600; Nikon, Melville, NY) attached to a computer-controlled digital camera (Spot RT; Diagnostic Instruments, Sterling Heights, MI).
Western blot analyses.
Muscle homogenates (30 μg of protein) were resolved electrophoretically in 10–20% SDS-polyacrylamide gels (Bio-Rad, Hercules CA) and transferred to polyvinylidene difluoride membranes (Immobilon-FL, Millipore, Billerica, MA). Equal protein loading was confirmed by Ponceau S staining. Membranes were blocked for 1 h with 1:1 dilution of Odyssey blocking buffer PBS at room temperature. Membranes were then incubated using the same buffer containing 0.2% Tween and primary antibodies at the concentration recommended by manufacturers. The monoclonal antibodies against subunits of mitochondrial respiratory complexes (all from Invitrogen, Carlsbad, CA) were a 30-kDa subunit from complex II, VIb subunit from complex IV, and α subunit from complex V. Other primary antibodies were against pyruvate dehydrogenase kinase 1 (PDK-1; Abcam, Cambridge MA) and voltage-dependent anion channel 1 (VDAC-1; Abcam), peroxisome proliferator-activated receptor gamma (PPARγ; Abcam), PGC-1α (Novus Biological, Littleton, CO), and BNIP-3 (Abcam). After washing the membranes with PBS and 0.1% Tween, we incubated them with Alexa Fluor 680-conjugated goat anti-mouse secondary antibody (1:7,500; Invitrogen) and then rewashed them with PBS and 0.1% Tween. Membranes were finally rinsed with PBS and scanned using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln NE). The density of resulting bands was quantified using National Institutes of Health Image J software.
Mitochondrial volume density.
Eight mice (4 control and 4 mice exposed to hypoxia for 4 wk) were perfused with 4% glutaraldehyde and 2% paraformaldehyde for electron microscopy. Gastrocnemius and diaphragm were then dissected and cut into pieces and fixed in glutaraldehyde (4%) and paraformaldehyde (2%) for 2 h at 4 C. Samples were then postfixed in osmium tetraoxide (1%) for 1 h, dehydrated, and embedded for further sectioning. To measure mitochondrial volume density, thin (80 nm) fiber transverse sections were stained with uranyl acetate and lead citrate and examined with a transmission electron microscope. Mitochondrial volume density (% of muscle fiber volume occupied by mitochondria) was determined using a standard point counting method (144 point grid) (19).
RNA isolation and RT-PCR.
Muscles were removed, and RNA was isolated using TRIzol (Invitrogen), according to the manufacturer's instructions. RNA was then subjected to DNAase treatment using a Turbo DNA-free kit (Applied Biosystems, Foster City, CA). Reverse transcription was performed using Superscript II RNAse H- reverse transcriptase. Primers were designed using PrimerExpress (Applied Biosystems). The evaluated genes include PPARγ coactivator 1-alpha (PGC-1α), nuclear respiratory factors (NRF) 1 and 2, mitochondrial transcription factor A (TFAM), autophagy gene 8 (ATG-8 or microtubular-associated protein 1 light chain 3α), 5 (ATG-5) and 7 (ATG-7), and BNIP-3 and BNIP-3-like protein (BNIP-3L). Analyses were performed in triplicate, using SYBR Green and 18S rRNA as the calibrator housekeeping gene. Fluorescence signal was detected using ABI PRISM 7500 Sequence Detection System. Fold-change expression related to control group was expressed using the threshold cycle method. Primer sequences are shown in table 1.
Table 1.
List of primers used for quantitative PCR
| Gene of Interest | Accession Number | Sequence | Product Size | |
|---|---|---|---|---|
| PGC-1α | AF049330.1 | Forward | AGACGGATTGCCCTCATTTG | 84 |
| Reverse | CCGTCAGGCATGGAGGAA | |||
| TFAM | NM_009360.4 | Forward | GCACCCTGCAGAGTGTTCAA | 71 |
| Reverse | CGCCCAGGCCTCTACCTT | |||
| NRF-1 | NM_010938.3 | Forward | TTCCAGTCTCTGTGGACAAAATGA | 79 |
| Reverse | CGACCTGTGGAATACTTGAGCAT | |||
| NRF-2 | NM_010902.3 | Forward | CACCAGCTCAAGGGCACAGT | 83 |
| Reverse | ACCAGGACTCACGGGAACTTCT | |||
| C-Myc | NM_010849.4 | Forward | ACGGTTCCTTCTGACAGAACTGA | 75 |
| Reverse | CCAGCCAAGGTTGTGAGGTT | |||
| PPARγ | NM_011146.3 | Forward | TGCTCAAGTATGGTGTCCATGAG | 77 |
| Reverse | TGAGATGAGGACTCCATCTTTATTCA | |||
| BNIP-3 | NM_009760 | Forward | GTTACCCACGAACCCCACTTT | 74 |
| Reverse | TGGACAGCAAGGCGAGAATC | |||
| BNIP-3L | NM_009761.3 | Forward | GCAATGCCACCAGCAGATTATA | 82 |
| Reverse | AGCATGAATGATGCAGGTGACT | |||
| ATG-5 | NM_053069.5 | Forward | ACTGCTTCGCTGAGACACACA | 68 |
| Reverse | GCTTCGGCTGCATTGCAT | |||
| ATG-7 | NM_028835.3 | Forward | CGCCAAGATCTCCTACTCCAA | 72 |
| Reverse | TTGCCACCCCCTAGACAATC | |||
| LC-3 | NM_026160.4 | Forward | GACGGCTTCCTGTACATGGTTT | 70 |
| Reverse | TGGAGTCTTACACAGCCATTGC | |||
| Housekeeping RNA | ||||
| 18S | NR_003278.1 | Forward | AGTCCCTGCCCTTTGTACACA | 69 |
| Reverse | GATCCGAGGGCCTCACTAAAC |
Statistical analysis.
Data were analyzed using t-tests when comparing only two groups: control and 4 wk of hypoxia. One-way ANOVA was used to test for differences between multiple groups. Tukey's post hoc test was used to identify pair-wise differences. Significance was considered when P ≤ 0.05.
RESULTS
All of the mice in the hypoxia group survived and completed their stay in the hypoxic environment. After 4 wk of hypoxia, mice had higher hematocrit (control, 35.0 ± 2.9% vs. hypoxia, 55.0 ± 5.3%, P = 0.006, n = 10 mice sampled per group) and lower body weight (control, 28.6 ± 1.8 g vs. hypoxia, 25.0 ± 1.5 g, P < 0.001), similar to changes described by others (6, 7). Further analysis showed that most of the body weight was lost during the 1st wk of hypoxia; afterward, both groups gained weight at the same rate (0.52 vs. 0.48 g/wk, control and hypoxia groups, respectively). In addition, gastrocnemius wet weight did not change after 4 wk of hypoxia (336.0 ± 17.8 mg and 331.8 ± 8.9 mg, control and hypoxia, respectively, P = 0.84, n = 6 muscles sampled in each group).
Hypoxia decreases mitochondrial content in the diaphragm.
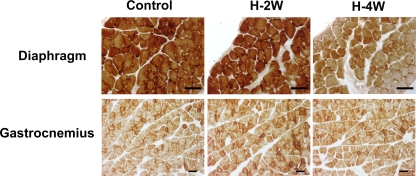
We used cytochrome c oxidase histochemistry as an initial approach to document changes in muscle mitochondrial content in response to hypoxia. After 4 wk of hypoxia, there was less intense staining in the diaphragm, which was not noted after 2 wk of hypoxia. This is suggestive of decreased mitochondrial content. There was no detectable change in the gastrocnemius at either time point (Fig. 1).
Fig. 1.
Cytochrome c oxidase histochemistry shows less intense staining in hypoxic diaphragm [hypoxia, 4 wk (H-4W) and hypoxia, 2 wk (H-2W)] compared with the normoxic controls (n = 4 in each group). No differences were observed after either 2 wk of hypoxia in diaphragm or in gastrocnemius (scale bars = 50 μm).
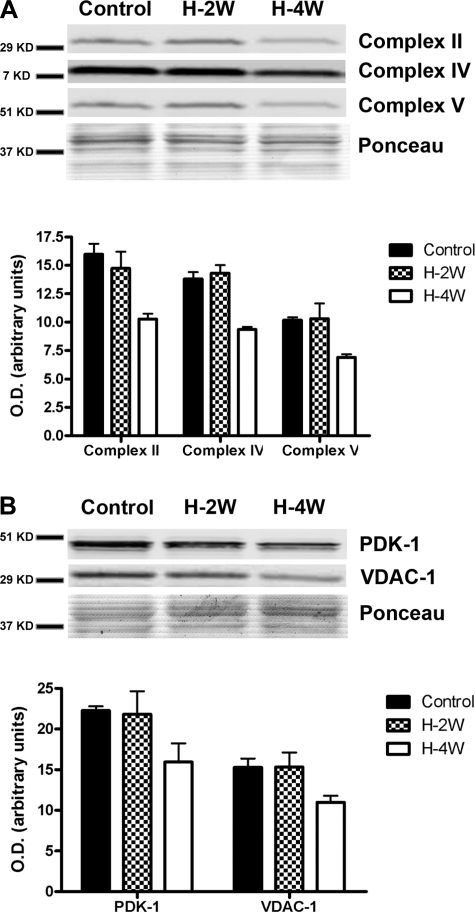
We then used Western blot analyses of whole diaphragm and gastrocnemius muscle homogenates to evaluate whether mitochondrial protein content changed after hypoxia. There was a significant decrease in the content of proteins involved in oxidative phosphorylation after 4 wk of hypoxia, but not after 2 wk (Fig. 2A). To examine whether the reduction was restricted to the respiratory complexes, other mitochondrial proteins were measured. PDK-1 and VDAC-1 contents were also reduced after 4 wk of hypoxia. No changes were noted in the gastrocnemius at either time point (data not shown).
Fig. 2.
Representative Western blots and densitometries of respiratory complexes (A) and selected mitochondrial proteins (B) in diaphragm and gastrocnemius muscles collected from mice after normoxia (control) or H-2W or H-4W. The content of all of the proteins was significantly reduced after 4 wk of hypoxia (P < 0.05, n = 8 in each group). Values are given as means ± SE.
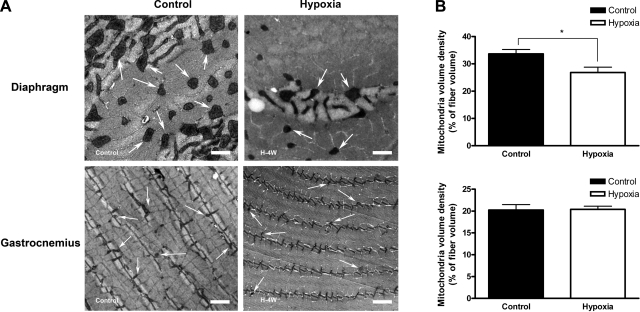
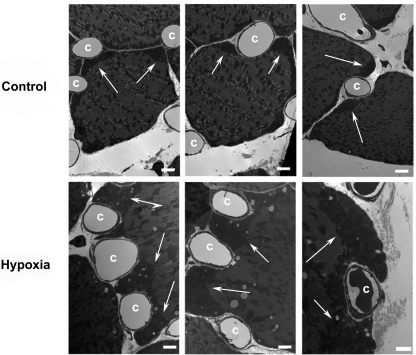
To directly quantify the effect of hypoxia on mitochondrial content, we used electron microscopy and stereology to determine mitochondrial volume density (% of muscle fiber volume occupied by mitochondria). As shown in Fig. 3, 4 wk of hypoxia decreased the mitochondrial volume density in diaphragm muscle fibers (20.5% reduction after hypoxia compared with controls, P = 0.013), but not in gastrocnemius. Interestingly, we observed that mitochondria clustered preferentially underneath the sarcolemma in diaphragm muscle fibers, in particular, around the capillaries (Fig. 4). This phenomenon was not noticed in the gastrocnemius.
Fig. 3.
A: mitochondria volume density decreased in the diaphragm but not in gastrocnemius after 4 wk of hypoxia. Representative electron micrographs of diaphragm (top: scale bar = 1 μm) and gastrocnemius (bottom: scale bar = 2 μm). Some of the mitochondria are labeled with white arrows. B: there is a noticeable reduction in mitochondrial density after 4 wk of hypoxia in diaphragm (*P = 0.013, n = 4 in each group), but no change in the gastrocnemius muscle. Values are given as means ± SE.
Fig. 4.
Electron micrographs showing abundant subsarcolemmal mitochondria in diaphragm muscle fibers after hypoxia (arrows, scale bar = 2 μm). Top: typical distribution of mitochondria and capillaries (c) in control mice. After 4 wk of hypoxia, large clusters of mitochondria (arrows) are observed under the sarcolemma that projects and encircles adjacent capillaries (bottom).
Hypoxia decreases mitochondrial biogenesis and triggers autophagy.
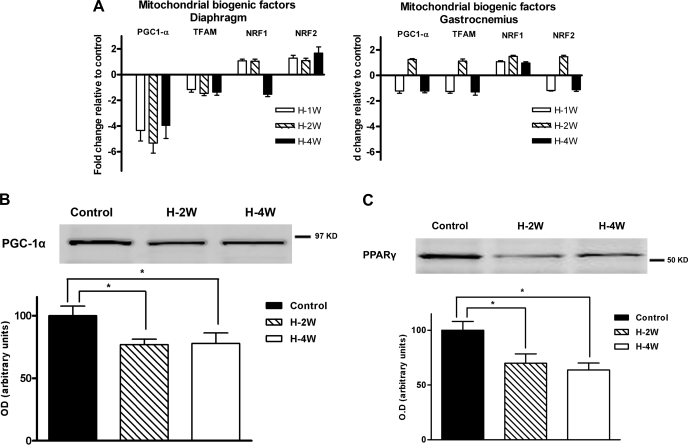
Decreased mitochondrial biogenesis could explain the loss of mitochondrial volume density in the diaphragm after chronic hypoxia. We screened the expression of mitochondrial biogenic factors by quantitative RT-PCR. PGC-1α mRNA abundance was decreased in diaphragm starting at 1 wk of hypoxia compared with the control group (Fig. 5A, P values of 0.001, 0.003, and <0.001 after 1, 2, and 4 wk of hypoxia, respectively). PGC-1α protein content in the diaphragm was also reduced from 2 wk of hypoxia (Fig. 5B, P = 0.04). There was no change in either mRNA abundance or protein content of PGC-1α in gastrocnemius. We also measured PPARγ since it influences the expression of PGC-1α. PPARγ protein content decreased in the diaphragm after 2 and 4 wk of hypoxia compared with control (30.1% and 36.4% respectively, Fig. 5C) and did not change in gastrocnemius.
Fig. 5.
A: Quantitative RT-PCR of mitochondrial biogenic factors. There was a decreased expression of PGC-1α in diaphragm after 1, 2, and 4 wk of hypoxia (H) compared with the control normoxia levels. No change was observed in gastrocnemius. There was no difference in any of the other biogenic factors evaluated (i.e., TFAM, NRF1, or NRF2) in diaphragm or gastrocnemius (n = 6 in each group). Western blot analysis of PGC-1α (B) and PPARγ (C) revealed decreased protein contents in diaphragm after 2 and 4 wk of hypoxia (H-2W and H-4W respectively) compared with control (n = 6 in each group). Values are expressed as means ± SE; *P < 0.05 for indicated comparisons.
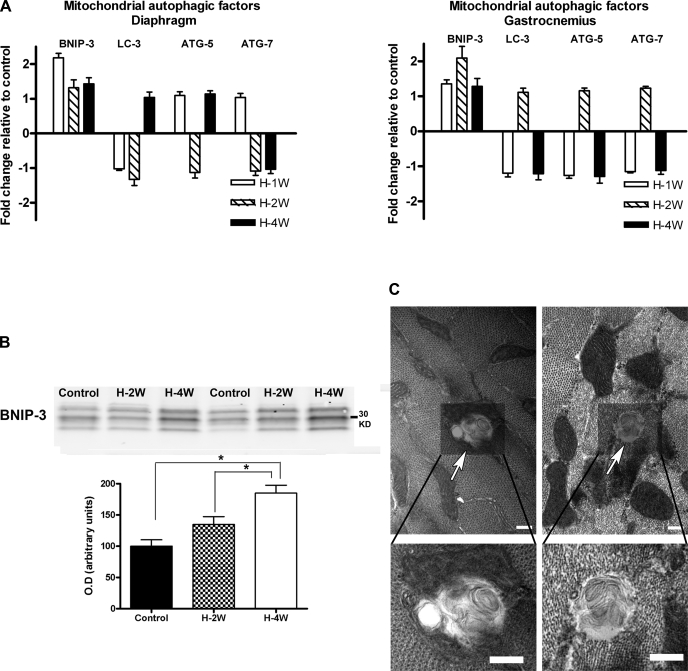
Another cellular process that could explain the loss of mitochondria in the diaphragm during chronic hypoxia is autophagy. We measured the expression of several mitochondrial autophagic factors in diaphragm and gastrocnemius samples at different time points from the start of hypoxia. As shown in Fig. 6A, BNIP-3 mRNA abundance increased after 1 wk of hypoxia in the diaphragm (P = 0.001) and after 2 wk in gastrocnemius (P = 0.02). Western blot analysis showed increased BNIP-3 content in diaphragm, but not in gastrocnemius, after 4 wk of hypoxia (Fig. 6B, P < 0.001). BNIP-3 migrates oddly in SDS PAGE, showing bands at 30 kDa and at 60 kDa that correspond to monomers and dimers, respectively (8). The 30-kDa band is presented in Fig. 6B. The presence of this marker for autophagy correlated with the observation of mitophagic vesicles by electron microscopy in diaphragm fibers from the hypoxia group (Fig. 6C). These structures were rarely observed in gastrocnemius from the hypoxia group and were completely absent in either muscle from the control group.
Fig. 6.
BNIP-3 expression was higher after 1 wk of hypoxia (H-1W) in diaphragm (A) and after 2 wk of hypoxia (H-2W) in gastrocnemius (B). LC-3, ATG-5, and ATG-7 mRNA levels were unchanged in either diaphragm or gastrocnemius. B: BNIP-3 protein content in diaphragm was increased after 4 wk of hypoxia of hypoxia (H-4W) compared with control (C) and 2 wk-hypoxia (H-2W) groups (*P < 0.05). No change in BNIP-3 protein content was observed in gastrocnemius. C: Electron micrographs of diaphragm fibers from mice after 4 wk of hypoxia. There were vesicles consistent with autophagosomes (top: arrows). Higher magnification views of these vesicles are shown in the lower panels (scale bar = 0.2 μm for all the images). Values are given as means ± SE (n = 6 in each group).
DISCUSSION
The goal of this study was to contrast the adaptive responses of limb and respiratory muscles to chronic hypoxia. Our hypothesis was that chronic hypoxia would not change mitochondrial volume density and function in the mouse diaphragm compared with limb muscles. Instead, the results demonstrate that mitochondrial content, measured histochemically, by protein content and stereology, actually decreases in the diaphragm, while the gastrocnemius remains relatively unaffected. It was particularly surprising that 4 wk of hypoxia did not increase the mitochondrial content in the mouse diaphragm, despite the increased work of breathing. We did find that the mitochondria shifted position and localized preferentially in the subsarcolemmal region, especially where the diaphragm muscle fibers were closer to capillaries. The results also suggest that the change in mitochondrial volume density in the mouse diaphragm is due to a combination of decreased biogenesis and increased autophagy.
Mitochondrial biogenesis and hypoxia.
Mitochondrial biogenesis (and the resulting increase in aerobic capacity) is initiated by different pathways, many of which rely on PGC-1α. This transcription cofactor is highly expressed in tissues with elevated metabolic rate such as brain, kidney, heart, and skeletal muscle (28). Increased PGC-1α stimulates the transcription of NRFs, followed by TFAM and nuclear-encoded mitochondrial proteins of the oxidative phosphorylation (42). On the other hand, downregulation of PGC-1α expression is associated with diminished mitochondrial biogenesis (25). In the particular case of chronic hypoxia, PGC-1α expression may be altered by a number of potential mechanisms. c-Myc is a transcription factor involved in mitochondrial biogenesis (26); it also activates PGC-1β, a member of the PGC-1 family that shares functions with PGC-1α (49). The inhibition of c-myc activity by hypoxia-inducible factor (HIF) decreases mitochondrial biogenesis in cancer cells and could play a role in chronic hypoxia as well (49). We found no change in c-myc expression after 4 wk of hypoxia in either diaphragm or gastrocnemius (data not shown). Hypoxia could also reduce the expression of PPARγ, the binding partner of PGC-1α (27, 48, 51). In turn, decreased PPARγ would also diminish PGC-1α expression (17). In view of that, we evaluated PPARγ protein content and found that it was decreased after 2 and 4 wk of hypoxia in diaphragm. Changes in PGC-1α and PPARγ content follow the same pattern (reduced after 2 and 4 wk of hypoxia), suggesting that PPARγ may have a role in PGC-1α expression under hypoxic conditions. The reduction in PGC-1α mRNA and protein content may be sufficient to decrease mitochondria biogenesis in diaphragm. It should be noted that other investigators have shown increased PGC-1α expression in mouse brain after 6 and 24 h of hypoxia (15) and in sheep fetal adipose tissue after 3 mo of high-altitude hypoxia (37). The apparent discrepancy with our findings may be due to effects of acute hypoxia (up to 24 h) and tissue specificity on PGC-1a expression. Thus, in the mouse brain study, the increase in PGC-1α expression after 48 h of hypoxia is not different from the control group. Acute hypoxia could result in a decrease in food intake (44), and this would be the stimulus to increase PGC-1α expression, because fasting increases the expression of PGC-1 (23). On the other hand, the increased PGC-1α expression in hypoxic fetal sheep adipocytes may be tissue specific and related to role of the nonshivering thermogenesis of brown fat in newborns (38). Considering the changes in expression of mitochondrial biogenic factors and the reduction in mitochondrial content, it seems that the active phase of diaphragm remodeling occurs somewhere between 2 and 4 wk of hypoxia exposure. The decrease in mitochondrial volume density in the diaphragm following hypoxia was accompanied by a redistribution of the mitochondria to the subsarcolemmal compartment, in particular, close to capillaries (Fig. 4). This seems an adaptive response intended to decrease the diffusion distance from capillary to mitochondria and increase the efficiency of oxygen transfer, as previously proposed (30).
Mitochondrial autophagy and hypoxia.
The half-life of mitochondria is about 2 wk (35) and depends on the balance between biogenesis and degradation (autophagy). Autophagy, or “mitophagy” when dealing specifically with mitochondria, is a process where cytoplasm and organelles are encapsulated in a double-membrane vesicle. Then, the outer vesicular membrane fuses with lysosomes, and its contents are degraded and recycled (33). Eleven autophagic genes (ATG genes) responsible for the induction, nucleation, expansion, and fusion of autophagic vesicles have been identified in mammals (46). The ATG gene beclin-1 (or ATG-6) is a BH3-only protein and essential in the early steps of autophagy. BH3-only proteins are a subgroup of the BCL-2 family and are called that because they share with BCL-2 proteins a short domain (9 to 16 amino acids the BH3 domain); this domain can subsequently bind to a specific pocket of the BCL-2 proteins (21). Beclin-1 can be inhibited by the interaction of the BH-3 domain with BCL-2 or BCL-XL (32). BNIP-3, another BH3-only protein, also binds BCL-2 and prevents its interaction with beclin-1 (31). Thus, beclin-1 is no longer inactive and induces autophagy. BNIP-3 is a hypoxia-inducible gene (45) and is preferentially translocated to the mitochondria inducing mitophagy in cultured fibroblast and cardiac myocytes (16, 50). In rodent skeletal muscle, BNIP-3 is upregulated after 44 h of hypoxia (2). In this study, BNIP-3 mRNA content increased after 1 and 2 wk of hypoxia in diaphragm and gastrocnemius respectively, and BNIP-3 protein content increased only in diaphragm after 4 wk of hypoxia. Interestingly, BNIP-3L had the same pattern of expression in diaphragm and gastrocnemius: mRNA levels were higher in both muscles after 1 and 2 wk of hypoxia, respectively. BNIP-3L is also a hypoxia-inducible gene and have been recently identified as an autophagic factor (3). The presence of increased hypoxia-induced mitophagy in diaphragm is also demonstrated by the presence of many autophagic vesicles in the hypoxic diaphragm (Fig. 6C).
Increased mitophagy and reduced mitochondrial biogenesis have been suggested as adaptive mechanisms to acute hypoxia. Cell culture experiments have shown that up to 48 h of hypoxia can reduce mitochondrial respiration and mitochondrial mass, the later because of increased mitophagy (50). Acute hypoxia also affects mitochondrial biogenesis in renal cancer cells (49). We found that chronic hypoxia, at least in the diaphragm, affects mitochondrial biogenesis and autophagy. PGC-1α downregulation would probably decrease biogenesis, whereas upregulation of BNIP-3 would account for increased mitophagy. Interestingly, no changes in mitochondrial mass were observed in gastrocnemius. This difference between the diaphragm and gastrocnemius may reflect their workloads. The diaphragm increases its activity during hypoxia. However, its mitochondrial content decreases (Fig. 2), a seemingly paradoxical change that is accompanied by a redistribution of mitochondria to the periphery. These consequences combine to reduce the diffusion distance from the vascular compartment to the mitochondria. Another adaptive mechanism would be to increase the capillary to fiber interface, which has been considered a critical factor for oxygen diffusion (34). Gastrocnemius, on the other hand, does not need all these adaptations given that these muscles are less active during the exposure to hypoxia.
It has been suggested that the adaptation of skeletal muscle to hypoxia includes changes in fiber type and size (12). Constantly active muscles (such as diaphragm) show increased capillary density after chronic hypoxia, compared with less active limb muscles (12). Our findings support the idea that adaptation to hypoxia differs depending on the activity level of the muscle under study. In other words, the combination of hypoxia and muscle activity may be required to reduce intramuscular Po2 to low enough levels to stabilize HIF-1α (13). HIF is a heterodimer composed of α- and β-subunits that induces the expression of a broad range of genes in response to low levels of oxygen (43).Thus, low oxygen tissue levels would activate HIF, leading to mitophagy and inhibition of mitochondrial biogenesis (13). This sequence of events does not discount the possibility that HIF-independent pathways, such as increased in AMP kinase activity, may also be important for skeletal muscle adaptation to chronic hypoxia (5).
While we have not seen any changes in gastrocnemius, some studies in humans have documented the decrease in mitochondrial oxidative capacity in leg muscles (14, 18, 20, 22). We have to be cautious when interpreting these findings since they were performed at extreme levels of hypoxia combined with exercise. The discrepancy between those results and ours may reside in the fact that we did not include a formal exercise training protocol. Therefore, it is possible that the combination of hypoxia with endurance training decreases mitochondrial mass in the limb muscles of mice.
Perspectives and Significance
In summary, we found that chronic hypoxia is associated with a reduction in mitochondrial volume density in diaphragm. Lower levels of PGC-1α seem to be responsible for reduced mitochondrial biogenesis. Increased mitophagy may also occur because of hypoxia-induced BNIP-3 expression. Both mechanisms (reduced biogenesis and increased mitophagy) may combine to decrease mitochondrial mass in the mouse diaphragm after chronic hypoxia. It is still necessary to assess whether activity level or other unknown factors are responsible for the different pattern of adaptation between gastrocnemius and diaphragm. Nevertheless, these findings point out that skeletal muscle adaptation to hypoxia cannot be generalized when only one type of muscle is analyzed.
GRANTS
This study was supported by a National Institutes of Health/National Institute of General Medical Sciences fellowship to J. L. Gamboa (F31GM846552).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We would like to acknowledge Miguel Andrade for his assistance with data collection.
REFERENCES
- 1.Banchero N. Cardiovascular responses to chronic hypoxia. Annu Rev Physiol 49: 465–476, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Band M, Joel A, Hernandez A, Avivi A. Hypoxia-induced BNIP3 expression and mitophagy: in vivo comparison of the rat and the hypoxia-tolerant mole rat, Spalax ehrenbergi. FASEB J 23: 2327–2335, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Hypoxia-induced autophagy is mediated through HIF-induction of BNIP3 and BNIP3L via their BH3-domains. Mol Cell Biol 29: 2570–2581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigard AX, Brunet A, Guezennec CY, Monod H. Skeletal muscle changes after endurance training at high altitude. J Appl Physiol 71: 2114–2121, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Breen E, Tang K, Olfert M, Knapp A, Wagner P. Skeletal muscle capillarity during hypoxia: VEGF and its activation. High Alt Med Biol 9: 158–166, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Caceda R, Gamboa JL, Boero JA, Monge C, Arregui A. Energetic metabolism in mouse cerebral cortex during chronic hypoxia. Neurosci Lett 301: 171–174, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Chavez JC, Pichiule P, Boero J, Arregui A. Reduced mitochondrial respiration in mouse cerebral cortex during chronic hypoxia. Neurosci Lett 193: 169–172, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Chen G, Ray R, Dubik D, Shi L, Cizeau J, Bleackley RC, Saxena S, Gietz RD, Greenberg AH. The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. J Exp Med 186: 1975–1983, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiodi H. Respiratory adaptations to chronic high altitude hypoxia. J Appl Physiol 10: 81–87, 1957 [DOI] [PubMed] [Google Scholar]
- 10.Dapp C, Gassmann M, Hoppeler H, Fluck M. Hypoxia-induced gene activity in disused oxidative muscle. Adv Exp Med Biol 588: 171–188, 2006 [DOI] [PubMed] [Google Scholar]
- 11.De Palma S, Ripamonti M, Vigano A, Moriggi M, Capitanio D, Samaja M, Milano G, Cerretelli P, Wait R and, Gelfi C. Metabolic modulation induced by chronic hypoxia in rats using a comparative proteomic analysis of skeletal muscle tissue. J Proteome Res 6: 1974–1984, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Deveci D, Marshall JM, Egginton S. Relationship between capillary angiogenesis, fiber type, and fiber size in chronic systemic hypoxia. Am J Physiol Heart Circ Physiol 281: H241–H252, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Flueck M. Plasticity of the muscle proteome to exercise at altitude. High Alt Med Biol 10: 183–193, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Green HJ, Sutton JR, Cymerman A, Young PM, Houston CS. Operation Everest II: adaptations in human skeletal muscle. J Appl Physiol 66: 2454–2461, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Gutsaeva DR, Carraway MS, Suliman HB, Demchenko IT, Shitara H, Yonekawa H, Piantadosi CA. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci 28: 2015–2024, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ 14: 146–157, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hondares E, Mora O, Yubero P, Rodriguez de la CM, Iglesias R, Giralt M, Villarroya F. Thiazolidinediones and rexinoids induce peroxisome proliferator-activated receptor-coactivator (PGC)-1α gene transcription: an autoregulatory loop controls PGC-1alpha expression in adipocytes via peroxisome proliferator-activated receptor-gamma coactivation. Endocrinology 147: 2829–2838, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hoppeler H, Kleinert E, Schlegel C, Claassen H, Howald H, Kayar SR, Cerretelli P. Morphological adaptations of human skeletal muscle to chronic hypoxia. Int J Sports Med 11Suppl 1: S3–S9, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Hoppeler H, Mathieu O, Krauer R, Claassen H, Armstrong RB, Weibel ER. Design of the mammalian respiratory system. VI Distribution of mitochondria and capillaries in various muscles. Respir Physiol 44: 87–111, 1981 [DOI] [PubMed] [Google Scholar]
- 20.Howald H, Pette D, Simoneau JA, Uber A, Hoppeler H, Cerretelli P. Effect of chronic hypoxia on muscle enzyme activities. Int J Sports Med 11Suppl 1: S10–S14, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Huang DC, Strasser A. BH3-only proteins-essential initiators of apoptotic cell death. Cell 103: 839–842, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Kayser B, Hoppeler H, Claassen H, Cerretelli P. Muscle structure and performance capacity of Himalayan Sherpas. J Appl Physiol 70: 1938–1942, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor γ coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leon-Velarde F, Whittembury J, Monge C. Oxidative phosphorylation of liver mitochondria from mice acclimatized to hypobaric hypoxia. Int J Biometeorol 30: 283–289, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1α deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3: e101, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O'Donnell KA, Kim JW, Yustein JT, Lee LA, Dang CV. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol 25: 6225–6234, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Kimura H, Hirota K, Sugimoto H, Kimura N, Takahashi N, Fujii H, Yoshida H. Hypoxia reduces the expression and anti-inflammatory effects of peroxisome proliferator-activated receptor-gamma in human proximal renal tubular cells. Nephrol Dial Transplant 22: 1041–1051, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ 30: 145–151, 2006 [DOI] [PubMed] [Google Scholar]
- 29.MacDougall JD, Green HJ, Sutton JR, Coates G, Cymerman A, Young P, Houston CS. Operation Everest II: structural adaptations in skeletal muscle in response to extreme simulated altitude. Acta Physiol Scand 142: 421–427, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Mainwood GW, Rakusan K. A model for intracellular energy transport. Can J Physiol Pharmacol 60: 98–102, 1982 [DOI] [PubMed] [Google Scholar]
- 31.Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, Geneste O, Kroemer G. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L). Autophagy 3: 374–376, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Maiuri MC, Le TG, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 26: 2527–2539, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8: 741–752, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Mathieu-Costello O. Muscle adaptation to altitude: tissue capillarity and capacity for aerobic metabolism. High Alt Med Biol 2: 413–425, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem 246: 2425–2429, 1971 [PubMed] [Google Scholar]
- 36.Monge C, Leon-Velarde F. Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol Rev 71: 1135–1172, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Myers DA, Hanson K, Mlynarczyk M, Kaushal KM, Ducsay CA. Long-term hypoxia modulates expression of key genes regulating adipose function in the late-gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol 294: R1312–R1318, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev 64: 1–64, 1984 [DOI] [PubMed] [Google Scholar]
- 39.Niermeyer S, Zamudio S, Moore The People LG. High Altitude: An Exploration of Human Adaptation, edited by Hornbein TF, Schoene RB. New York: Marcel Dekker, 2001, p. 43–100 [Google Scholar]
- 40.Pickett CB, Cascarano J, Wilson MA. Acute and chronic hypoxia in rats. I. Effect on organismic respiration, mitochondrial protein mass in liver and succinic dehydrogenase activity in liver, kidney and heart. J Exp Zool 210: 49–57, 1979 [DOI] [PubMed] [Google Scholar]
- 41.Reynafarje B. Myoglobin content and enzymatic activity of muscle and altitude adaptation. J Appl Physiol 17: 301–305, 1962 [DOI] [PubMed] [Google Scholar]
- 42.Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol 574: 33–39, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med 7: 345–350, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Simler N, Malgoyre A, Koulmann N, Alonso A, Peinnequin A, Bigard AX. Hypoxic stimulus alters hypothalamic AMP-activated protein kinase phosphorylation concomitant to hypophagia. J Appl Physiol 102: 2135–2141, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic iduction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res 61: 6669–6673, 2001 [PubMed] [Google Scholar]
- 46.Thorburn A. Apoptosis and autophagy: regulatory connections between two supposedly different processes. Apoptosis 13: 1–9, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valdivia E. Total capillary bed in striated muscles of guinea pigs native to the Peruvian mountains. Am J Physiol 194: 585–589, 1958 [DOI] [PubMed] [Google Scholar]
- 48.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR γ 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell 2: 331–341, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11: 407–420, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283: 10892–10903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Zhou S, Lechpammer S, Greenberger JS, Glowacki J. Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires transforming growth factor-β/Smad3 signaling. J Biol Chem 280: 22688–22696, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]