Abstract
Neurons in the rostral raphe pallidus (RPa) play an essential role in the regulation of sympathetically mediated metabolism and thermogenesis in brown adipose tissue (BAT). The presence of serotonergic neurons in the RPa that are retrogradely labeled following pseudorabies virus injections into BAT suggests that these neurons play a role in the regulation of BAT. In urethane/chloralose-anesthetized rats, whole body cooling decreased skin (−5.7 ± 2.3°C) and core (−1.3 ± 0.2°C) temperatures and resulted in an increase in BAT sympathetic nerve activity (SNA; +1,026 ± 344% of baseline activity). Serial microinjections of the 5-hydroxytryptamine (5-HT) receptor antagonist, methysergide (1.2 nmol/site), but not saline vehicle, into the intermediolateral cell column (IML) in spinal segments T2–T5 markedly attenuated the cooling-evoked increase in BAT SNA (remaining area under the curve, AUC: 36 ± 9% of naive cooling response). Microinjections of the 5-HT1A receptor antagonist, WAY-100635 (1.2 nmol/site), or the 5-HT7 receptor antagonist, SB-269970 (1.2 nmol/site), into the T2–T5 IML also attenuated the cold-evoked increase in BAT SNA (remaining activity at peak inhibition: 47 ± 8% and 39 ± 12% of the initial cold-evoked response, respectively). The increases in BAT SNA evoked by microinjection of N-methyl-d-aspartate (NMDA) (12 pmol) or bicuculline (30 pmol) into the RPa were attenuated following microinjections of methysergide, but not saline vehicle, into the T2–T5 IML (NMDA remaining AUC, 64 ± 13% of naive response; bicuculline remaining AUC, 52 ± 5% of naive response). These results are consistent with our earlier demonstration of a potentiating effect of 5-HT within the IML on BAT SNA and indicate that activation of 5-HT1A and 5-HT7 receptors in the spinal cord contributes to increases in BAT SNA and thermogenesis.
Keywords: thermoregulation, sympathetic premotor neurons, raphe, serotonin, metabolism
several lines of evidence suggest that bulbospinal serotonergic neurons of the raphe pallidus (RPa) are involved in the regulation of the sympathetic outflow to brown adipose tissue (BAT). First, the sympathetic premotor neurons responsible for activation of BAT are located in the RPa area, an area containing many serotonergic neurons that directly innervate the spinal cord (1, 3, 9) and that are retrogradely labeled following injection of transsynaptic viral tracers into BAT (2, 4, 32). In addition, physiologically identified serotonergic neurons of the RPa are activated by cooling in the cat (19) and the rat (21). Furthermore, serotonin release in the spinal cord is increased by cold exposure (26, 27). Interestingly, mice lacking nearly all central serotonergic neurons have impaired thermogenesis in BAT and fail to maintain body temperature during cold exposure (10) although the specific role of bulbospinal serotonergic neurons in this impairment is unclear.
We recently demonstrated that exogenous application of serotonin and excitatory amino acid receptor agonists within the intermediolateral cell column (IML) can act synergistically to increase BAT sympathetic nerve activity (SNA) and thermogenesis (12, 16). Although excitatory amino acid receptor activation within the IML is necessary for the RPa-evoked increase in BAT thermogenesis (20), the role of endogenously released serotonin within the spinal cord in RPa-evoked or cooling-evoked BAT SNA has yet to be determined. Therefore, the present study was designed to determine whether endogenous activation of serotonin receptors within the spinal cord contributes to cooling-evoked and RPa-evoked increases in BAT SNA.
MATERIALS AND METHODS
All procedures conform to the regulations detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health & Science University. All efforts were made to minimize animal suffering and to limit the number of animals used. Male Sprague-Dawley rats (Charles River, Indianapolis, IN; n = 31) weighing 250–470 g were kept on a 12-h:12-h light/dark cycle and given ad libitum access to standard rat chow and water in a colony room maintained at 22–23°C. Rats were anesthetized with isoflurane (2–3% in oxygen), instrumented with femoral arterial and venous catheters, and transitioned to urethane and chloralose anesthesia (750 mg/kg and 60 mg/kg iv, respectively) over a 10-min period. All physiological variables were digitized [Micro 1401 MKII; Cambridge Electronic Design (CED), Cambridge, UK] and recorded onto a computer hard drive for subsequent analysis (Spike 2, CED). Arterial blood pressure was recorded from the arterial catheter attached to a pressure transducer, and heart rate (HR) was derived from the arterial pressure signal. The trachea was cannulated, and the animals were ventilated (tidal volume: ∼1 ml per 100 g body wt, ∼60 cycles per min) with 100% oxygen. End-expiratory CO2 was monitored using a capnometer (model 2200; Dynatech Electro-optics, Saline, MI), and ventilation rate was adjusted to maintain resting end-expiratory CO2 in the range of 4.0–5.5%. Cooling-evoked changes in arterial pressure, HR, and end-expiratory CO2, which were small, variable, and did not differ between groups, are not reported. Neuromuscular blockade was achieved by administration of d-tubocurarine (0.5 mg iv, supplemented with 0.1 mg as needed to suppress spontaneous contractions of the diaphragm). Adequacy of anesthesia was verified before initial and supplemental neuromuscular blockade by absence of a withdrawal reflex or pressor response to foot pinch as well as by absence of a corneal reflex. Rectal (core) temperature (Tcore) was monitored using a copper-constantan thermocouple inserted 6 cm into the rectum and, except as noted, was maintained between 36.5–38.0°C with a water-perfused heating/cooling blanket and a heat lamp. The trunk skin was shaved, and a copper-constantan thermocouple was taped to the abdominal skin to monitor skin temperature (Tskin) beneath the heating/cooling blanket. Animals were placed in a stereotaxic instrument with the incisor bar positioned 4 mm below the interaural line. To stabilize the spinal cord a clamp was placed on the caudal thoracic vertebrae.
Recording BAT SNA and temperature.
The temperature of BAT (TBAT) was monitored using a thermocouple meter (TC-1000; Sable Systems International, Las Vegas, NV) with a Type T needle style microprobe thermocouple (Physitemp, Clifton, NJ) inserted into the intact, left interscapular BAT pad. Postganglionic BAT SNA was recorded under mineral oil with a bipolar hook electrode from the central cut end of a small-diameter (∼100 μm) nerve bundle isolated from the ventral surface of the right interscapular adipose pad after dividing it along the midline and reflecting it laterally. Nerve activity was filtered (1–300 Hz) and amplified (10,000–20,000×) with a Cyberamp 380 (Axon Instruments, Union City, CA). Spike 2 software (CED) was used to obtain a continuous measure (4-s bins) of BAT SNA amplitude by calculating the root mean square (RMS) amplitude of the BAT SNA (square root of the total power in the 0.1- to 20-Hz band) from the autospectra of sequential 4-s segments of BAT SNA. Control values of BAT SNA were the averages of the BAT SNA amplitudes during the 32-s periods before all treatments, when Tcore was maintained between 36.5–38.0°C. For the cooling response, the area under the curve (AUC) of the BAT SNA RMS amplitude was calculated by summing the total area under the curve over the entire cooling period (13 min). For raphe-evoked responses, the AUC was calculated by summing the total AUC from the time of injection until the RMS amplitude returned to the control level. For the cold-evoked responses, the cold-evoked increase in BAT SNA before any treatment was established as 100% and was obtained by subtracting (1) the AUC of the BAT SNA RMS amplitude during a 32-s period with Tcore maintained at 37–38°C (i.e., the nonthermally regulated SNA activity level) from (2) the AUC of the BAT SNA RMS amplitude during the 32-s period of cold exposure before microinjections into the IML. The nonthermally regulated SNA activity level was also subtracted from subsequent treatment-induced BAT SNA RMS amplitude measurements, and these were then expressed as a percentage of the initial cold-evoked increase in BAT SNA. The peak value for the BAT SNA response to a given treatment was defined as the average value during the 32-s period of maximal change in BAT SNA evoked by the treatment.
Microinjections.
Glass micropipettes (outer tip diameter, 20–30 μm) were used for all microinjections, which were given over a 5- to 10-s period using a pressure injection system (model IIe; Toohey, Fairfield, NJ). The volume of the microinjection was determined using a reticule to measure the displacement of the meniscus in the micropipette.
The coordinates for the RPa were 3.0 mm caudal to lambda, on the midline, and 9.6–9.8 mm ventral to the dura. Spinal microinjections were made unilaterally into the right (i.e., ipsilateral to the recorded BAT nerve) IML (0.5–0.7 mm lateral to the midline and 1.0 mm ventral to the dorsal surface) and were spaced 0.5–1.5 mm apart (7–10 microinjections) between the caudal portions of the second to the fifth thoracic spinal segments because these segments are the most heavily infected following pseudorabies viral injections into the BAT (4) and because microinjections of excitatory amino acids into these segments increase BAT SNA (12, 16).
The microinjection sites were marked by pressure microinjection of fluorescent polystyrene microspheres (FluoSpheres, F8797, F8801 or F8803; Molecular Probes, Eugene, OR) included in the injectate (1:200 dilution of FluoSpheres in the injectate). After the physiological recordings, rats were perfused (10% paraformaldehyde) transcardially, and brains and spinal cords were removed, postfixed (2–12 h), and sectioned on a microtome (60-μm coronal sections for brainstem and 60-μm horizontal or transverse sections for the spinal cord). Sections were mounted on slides, and microinjection sites were localized and photographed, as described previously (14).
Drugs and solutions.
Drugs were obtained from Sigma (St. Louis, MO), except SB-269970 hydrochloride, which was obtained from Tocris Bioscience (Ellisville, MO), and were dissolved in saline. Isoflurane was obtained from Abbott Laboratories (North Chicago, IL).
Statistics.
All statistics were performed using Systat software (version 10; Cranes Software International, Chicago, IL). Data are expressed as means ± SE. Statistical significance was assessed using two-tailed paired t-tests. Statistical results with P < 0.05 were considered significant.
Protocols.
For Protocol 1, to determine whether activation of 5-hydroxytryptamine (5-HT) receptors within the spinal cord contributes to the sympathetic activation of BAT evoked by cooling, we compared (n = 5) the cooling-evoked increase in BAT SNA before and following spinal microinjections of the broad spectrum 5-HT receptor antagonist, methysergide, into the T2–T5 IML. To control for the repeated trial design, in a separate group of rats (n = 5), the cooling-evoked increase in BAT SNA was compared before and after IML microinjections of saline vehicle.
For Protocol 2, to determine whether activation of 5-HT receptors within the IML contributes to the sympathetic activation of BAT evoked by activation of neurons within the RPa, the increases in BAT SNA following a microinjection of N-methyl-d-aspartate (NMDA; 12 pmol in 60 nl, n = 5) or bicuculline (Bic; 30 pmol in 60 nl, n = 5) into the RPa were compared before and after microinjections of methysergide (1.2 nmol in 120 nl per site). To control for this repeated trial protocol, the increase in BAT SNA following NMDA (n = 5) or Bic (n = 3) microinjection into the RPa was compared before and after similar T2–T5 IML microinjections of saline vehicle (120 nl per site). We have previously demonstrated that repeated microinjections of NMDA into the RPa produce responses of consistent magnitude (13, 15).
For Protocol 3, to determine the specific 5-HT receptor subtype within the spinal cord that contributes to the sympathetic activation of BAT evoked by cold exposure, we used a within-trial design to compare (n = 5) the cold-evoked BAT SNA before and following microinjections of the 5-HT1A receptor antagonist, WAY-100635 (1.2 nmol in 120 nl per site) or, subsequently, the 5-HT7 receptor antagonist SB-269970 (1.2 nmol in 120 nl per site), into the T2–T5 IML. BAT SNA was allowed to return to premicroinjection levels before spinal administration of the second antagonist. In a subset of these animals (n = 2), we also compared the cold-evoked BAT SNA before and following microinjections into the T2–T5 IML of the 5-HT2 antagonist, ketanserin (120 pmol in 120 nl per site). Ketanserin did not decrease the cold-evoked BAT SNA (data not shown). This cold-evoked protocol differed from the cooling-evoked protocol (Protocol 1) only in that the drug treatment effects were determined during a maintained level of cold-evoked BAT SNA rather than by observing the treatment-evoked changes in the BAT SNA responses to two consecutive cooling episodes. The former protocol affords an opportunity to assess the effects of drugs with short durations of action.
RESULTS
Microinjection of methysergide into the IML attenuated cooling-evoked sympathetic activation of BAT.
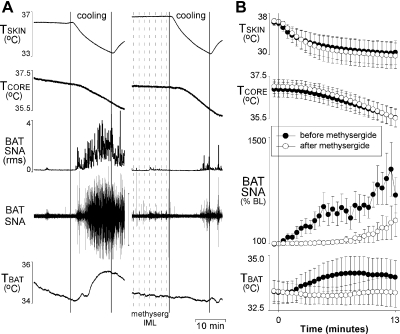
To test the hypothesis that activation of 5-HT receptors within the spinal cord contributes to BAT SNA, the BAT SNA response to skin and core cooling was determined before and after T2–T5 IML microinjections of methysergide. In naive, urethane/chloralose anesthetized rats with Tcore maintained between 36.5°C and 38.0°C, BAT SNA was low and exhibited few bursting discharges (Fig. 1). Perfusion of the water blanket with cold water (∼5°C) resulted in an immediate, robust, and progressive decrease in Tskin, a fall in Tcore, and an increase in BAT SNA (Fig. 1). Compared with the robust increase in BAT SNA in the naive trial, the increase in BAT SNA was markedly delayed and significantly attenuated following microinjections of methysergide into the IML (Fig. 1). Following methysergide into the IML, the AUC of the BAT SNA response to cooling was reduced to 36 ± 9% of the untreated response, and the peak of the BAT SNA response was also significantly attenuated (+1,026 ± 344% of baseline for the untreated response vs. +318 ± 218% of baseline following methysergide). Neither the decrease in Tskin (−5.7 ± 2.3°C vs. −6.7 ± 2.4°C) nor that in Tcore (−1.3 ± 0.2°C vs. −1.5 ± 0.2°C) differed between the naive trials and the trials following microinjections of methysergide into the IML. Despite marked falls in Tcore, TBAT, measured contralateral to the microinjections into the IML, was not significantly decreased during cooling in either the naive cooling trial or the cooling trial following microinjections of methysergide into the IML. The change in TBAT during cooling was not significantly different between trials (0.7 ± 0.3°C in the naive trial vs. −0.1 ± 0.3°C following methysergide into the IML, P = 0.06; Fig. 1).
Fig. 1.
Microinjection of methysergide into the second thoracic through the fifth thoracic segments of the intermediolateral cell column (T2–T5 IML) attenuates the increase in sympathetic nerve activity (SNA) to brown adipose tissue (BAT) evoked by whole body cooling. Representative example (A) and group data (B) (means ± SE, n = 5) in which each point is the 30-s average of the variable values. ●, data before microinjections into the T2–T5 IML; ○, data following microinjection of methysergide into the T2–T5 IML. BL, baseline; Tskin, skin temperature; Tcore, core temperature; TBAT, BAT temperature. The cooling-evoked increase in BAT SNA after methysergide is significantly smaller than the naïve response (P < 0.05, paired t-test of area under the curve).
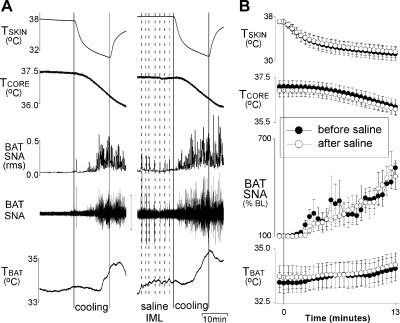
Microinjections of saline vehicle into the IML did not attenuate the cooling-evoked increase in BAT SNA.
The evoked increase in BAT SNA in the cooling trial following microinjections of saline vehicle into the IML did not differ from that in the naive trial (Fig. 2). The AUC of the increase in BAT SNA following saline was 100 ± 13% of the naive trial, and the peak increase in BAT SNA was +421 ± 45% of baseline for the naive response compared with +421 ± 101% of baseline for the response following saline. The change in TBAT evoked by cooling was variable and not significantly different between trials (0.7 ± 0.3°C in the naive trial vs. 0.6 ± 0.4°C following saline into the IML; Fig. 2). The decrease in Tskin was similar in the cooling trials before and after microinjections of saline into the IML (−5.7 ± 1.0°C in the naive trial vs. −5.4 ± 0.9°C following saline into the IML). Likewise, the decrease in Tcore did not differ between the cooling trials (−0.9 ± 0.1°C in the naive trial vs. −0.8 ± 0.1°C following saline into the IML) (Fig. 2).
Fig. 2.
Microinjection of saline into T2–T5 IML did not attenuate the increase in BAT SNA evoked by whole body cooling. A: representative example. B: group data (means ± SE, n = 6). ●, data before microinjections into the T2–T5 IML; ○, data following microinjection of saline into the T2–T5 IML. RMS, root mean square.
The increase in BAT SNA evoked from the RPa was attenuated by prior microinjections of methysergide, but not saline, into the T2–T5 IML.
The increase in BAT SNA evoked by microinjection of NMDA into the RPa was significantly smaller following microinjections of methysergide into the IML compared with that elicited in the naive trial (AUC, 64 ± 13% of the naive trial; peak response, +796 ± 144% baseline after methysergide into the IML vs. +1,293 ± 307% baseline in the naive trial; Fig. 3, A and B). Similarly, the increase in BAT SNA evoked by microinjection of Bic into the RPa was significantly smaller following microinjections of methysergide into the IML compared with the naive trial (AUC, 52 ± 5% of the naive trial; peak response, +1,559 ± 660% baseline after methysergide into the IML vs. +2,378 ± 690% baseline in the naive trial; Fig. 4, A and B). In contrast, the increase in BAT SNA evoked by microinjection of NMDA or Bic into the RPa did not differ between the naive trial and that following microinjections of saline vehicle into the IML [for NMDA: AUC following saline was 84 ± 11% of the naive trial (P = 0.12) and peak BAT SNA response was +1,113 ± 383% baseline vs. +1,049 ± 441% baseline (P = 0.75), Fig. 3, C and D; for Bic: AUC following the saline was 86 ± 3% of the naive trial (P = 0.1) and peak BAT SNA response was +1,301 ± 480% baseline vs. +1,201 ± 197% baseline (P = 0.64), data not shown]. The TBAT measured contralateral to the microinjections in the IML did not differ between trials (Figs. 3 and 4). The HR response also did not differ between trials (Figs. 3 and 4). Tcore and Tskin were stable and did not differ between trials (data not shown).
Fig. 3.
The increases in SNA to BAT evoked by microinjection of N-methyl-d-aspartate (NMDA) into the raphe pallidus (RPa) is attenuated by prior microinjection of methysergide (A and B) but not saline vehicle (C and D), into the T2–T5 IML. Representative examples (A and C) and group data (B and D) (means ± SE, n = 5) in which each point is the 30-s average of the variable values. ●, data before microinjection into the T2–T5 IML. ○, data following microinjection of methysergide or saline into the T2–T5 IML. HR, heart rate. *P < 0.05, paired t-test between responses before and after microinjection of methysergide into T2–T5 IML.
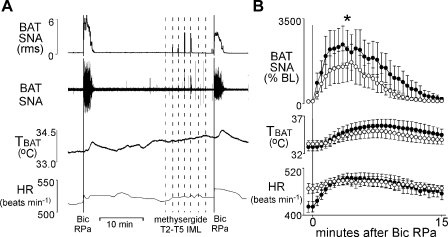
Fig. 4.
The increase in SNA to BAT evoked by microinjection of bicuculline (Bic) into the RPa is attenuated by prior microinjection of methysergide into T2–T5 IML. Representative example (A) and group data (B) (means ± SE, n = 5) in which each point is the 30-s average of the variable values. ●, data before microinjection into the T2–T5 IML; ○, data following microinjection of methysergide into the T2–T5 IML. *P < 0.05, paired t-test between responses before and after microinjection of methysergide into T2–T5 IML.
Microinjections of WAY-100635 or SB-269970 into the IML attenuated cold-evoked sympathetic activation of BAT.
To determine which 5-HT receptor subtypes within the spinal cord contribute to BAT SNA, we determined the effect of T2–T5 IML microinjections of WAY-100635 or SB-269970 on the cold-evoked increase in BAT SNA. In urethane/chloralose-anesthetized rats with Tcore maintained between 34.5°C and 36.0°C, BAT SNA was characterized by large and frequent burst discharges (Fig. 5A). Following WAY-100635 into the IML, the AUC of the BAT SNA response to the cold stimulus was significantly reduced to 47 ± 8% of the initial cold-evoked BAT SNA response; this transient attenuation recovered to the initial cold-evoked level within 20 min (Fig. 5, A and B). TBAT was not affected by the microinjections of WAY-100635 into the IML (P = 0.266). Tcore decreased slightly from 35.6 ± 0.3 to 35.4 ± 0.3 (P = 0.02). HR increased gradually following microinjection of WAY-100635 into the IML and by 20 min postinjection was significantly elevated (P = 0.004, Fig. 5).
Fig. 5.
Microinjection of 5-HT1A or 5-HT7 receptor subtype-selective antagonists into the T2–T5 IML attenuates the SNA to BAT evoked by cold exposure. A: representative example. B and C: group data (means ± SE, n = 5) in which each point is the 30-s average of the variable values for WAY-100635 (B) and SB-269970 (C). Time zero represents the time at which the first microinjection of the antagonist was made into the IML. *P < 0.05, paired t-test of value at the maximal change compared with the initial value before microinjections into the IML.
Following SB-269970 into the T2–T5 IML, the AUC of BAT SNA evoked by the cold stimulus was significantly reduced to 39 ± 8% of the initial cold-evoked BAT SNA response (Fig. 5, A and C). In addition, following microinjection of SB-269970, TBAT did not decrease significantly (P = 0.11), whereas Tcore and Tskin decreased slightly (35.1 ± 0.2 to 34.9 ± 0.2, P = 0.009, and 33.8 ± 0.3 to 33.7 ± 0.3, P < 0.001, respectively). HR was decreased by microinjection of SB-269970 into the T2–T5 IML (442 ± 17 beats/min to 391 ± 20 beats/min; P = 0.033, Fig. 5).
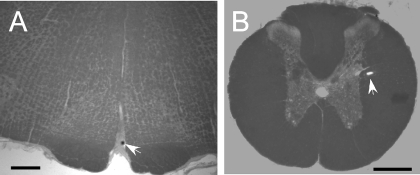
Figure 6 provides representative examples of the locations of the microinjection sites in the RPa and the IML. Microinjections targeting the RPa were located within 200 μm of the RPa at the rostrocaudal level of the caudal pole of the facial nucleus (∼bregma −11.6 mm; Ref. 28). All microinjections targeting the IML were located within 200 μm of the IML.
Fig. 6.
Representative injection sites in the rostral RPa (A), coronal section taken ∼11.6 mm caudal to bregma, and the IML at the upper thoracic level (B). Arrows indicate bead deposits marking injection sites. Scale bars = 0.5 mm.
DISCUSSION
Blockade of serotonin receptors within the spinal cord markedly attenuated the cooling-evoked increase in BAT SNA, providing direct evidence that endogenous activation of serotonin receptors within the IML of the spinal cord plays an important role in physiologically evoked increases in BAT SNA. In addition, blockade of serotonin receptors within the spinal cord attenuated the increase in BAT SNA evoked by activation of neurons within the RPa, the principal site of the sympathetic premotor neurons controlling BAT thermogenesis.
One interpretation of our observation that neither cooling-evoked nor RPa-evoked activation of BAT SNA was completely prevented by blockade of 5-HT receptors within the IML is that nonserotonergic receptors in the spinal cord also contribute to these activations of BAT sympathetic preganglionic neurons. In this regard, activation of NMDA receptors within the IML can increase BAT SNA (12, 16), and activation of glutamate receptors within the spinal cord is necessary for the increase in TBAT evoked by blockade of GABAA receptors within the RPa (20). Thus, although not directly tested, it seems likely that spinal glutamate receptor activation could have contributed to the residual BAT SNA activation following blockade of spinal serotonin receptors in the present experiments. Consistent with this hypothesis, serotonin agonists in the IML potentiate the NMDA-evoked increases in BAT SNA (12, 16). Together with the present results, these observations are consistent with contributions from both glutamate and serotonin receptor activation within the spinal cord to increases in BAT SNA and BAT thermogenesis. Whether both glutamate and serotonin receptors in the IML are always activated simultaneously during stimulations of BAT SNA remains unknown.
The present data demonstrate that serotonin receptor activation within the spinal cord is important for increasing BAT SNA during cooling. Consistent with this observation, Hodges et al. (10) have demonstrated that mice lacking serotonergic neurons have impaired BAT thermogenic responses. However, by using the difference between back temperature and interscapular temperature as an index of BAT thermogenesis, without regard to the underlying Tcore, their analysis may have underestimated the role of 5-HT in BAT thermogenesis during mild cold exposures. Because mice lacking serotonergic neurons would have an impaired ability to maintain Tcore during cold exposure, at a given ambient temperature below the thermoneutral range, the Tcore of mice lacking serotonergic neurons would be lower than that of wild-type mice, providing a stronger stimulus to increase BAT thermogenesis. If this is the case, the finding during cold exposure at an ambient temperature of 10°C of a similar BAT thermogenesis in mice lacking serotonergic neurons compared with wild-type mice would be interpreted as an impaired response because the stimulus to increase BAT thermogenesis is larger in the mice lacking serotonergic neurons.
The 5-HT antagonist, methysergide, was chosen for use in some of the present experiments because of its long duration of action; it is effective in preventing the serotonin-induced potentiation of BAT SNA for at least 30 min following injection into the IML (16). Blockade of 5-HT1 and/or 5-HT7 receptors within the spinal cord is likely to be responsible for the methysergide-induced attenuation of the cooling and RPa-evoked increases in BAT SNA observed in the present study, as indicated by the observation that selective 5-HT1A and 5-HT7 receptor antagonists attenuated the cold-evoked increase in BAT SNA and given the previous observation that the pharmacological profile of the potentiating effects of serotonin within the spinal cord on BAT SNA most closely matches that of spinal 5-HT1A and 5-HT7 receptors (12). Although systemically administered 5-HT2 receptor agonists and antagonists can influence thermogenesis in BAT (23, 24), it seems likely that these effects are mediated outside the IML because microinjections of 5-HT2 receptor agonists and antagonists directly into the IML have no effect on BAT SNA (12). In contrast, 5-HT2 receptor activation within the spinal cord plays an important role in eliciting cutaneous vasoconstriction (25) to regulate heat loss. Together, these results suggest that different complements of serotonin receptor subtypes play prominent roles within the spinal sympathetic circuits controlling the cutaneous vasculature and those determining BAT thermogenesis, highlighting the differential control of the sympathetic outflows to these distinct tissues.
Because of the large injection volumes (120 nl), as well as the number of microinjections (up to 10), the functional site of action of the 5-HT antagonists in the present study cannot be unequivocally localized to the IML. However, our finding that all of the microinjection sites within the spinal cord were located within or in close proximity to the IML and the localization of 5-HT1A/5-HT7 and 5-HT2 receptors within the IML (5, 7, 17, 18, 29) provide strong support for the IML as the site at which the 5-HT antagonists act to attenuate the BAT SNA. In addition, the observation that methysergide was effective in attenuating RPa-evoked responses suggests that the sympathetic outflow to BAT is affected by serotonergic receptor blockade downstream of the premotor neurons, presumably within the IML.
In the present study, blockade of 5-HT receptors in the IML did not diminish the increase in HR evoked by stimulation of the RPa. Similarly, the increase in HR evoked by microinjection of NMDA into the rostral thoracic IML was not potentiated by prior microinjection of 5-HT even though microinjection of NMDA into the IML in doses that are subthreshold for evoking an increase in HR can be brought to threshold by prior microinjection of 5-HT into the IML (16). We interpret these data to suggest that 5-HT receptor activation can act synergistically with excitatory amino acid receptor activation to increase HR but that excitatory amino acid receptor activation alone is capable of driving a maximal HR response.
Perspectives and significance.
The physiological role of BAT in adult humans has been largely ignored attributable to in large part the prevalent but erroneous view that adult humans do not have significant depots of BAT. However, adult humans clearly possess BAT (6, 22, 30, 31), and furthermore its activity is inversely correlated with body mass index and adiposity (6, 30). This intriguing inverse correlation between BAT activity and indices of obesity is reminiscent of the observation that elimination of BAT in rodents results in a propensity for weight gain (8). Regardless of whether the observed decreased activation of BAT is causally related to weight gain in humans, the large increases in the metabolism that occur during activation of BAT represent an attractive therapeutic target in the treatment of overweight and obesity.
Drugs that affect serotonergic systems, such as fenfluramine, have been used as weight-reducing agents. Both reductions in food intake and increases in metabolism likely contribute to the weight-reducing effects of serotonergic agents (11); however, remarkably little is known about the neural pathways underlying 5-HT-mediated increases in BAT energy expenditure. The present study demonstrates that activation of 5-HT receptors within the spinal cord contributes to sympathetic outflow to BAT under physiological conditions such as cold exposure and suggests a potential site of action for the metabolic effects of serotonergic agents capable of producing weight loss. A more complete understanding of the neural circuits regulating BAT thermogenesis should provide a foundation for developing improved therapeutic approaches to obesity.
GRANTS
This work was supported by NIH grants NS40987 (S. Morrison), DK57838 (S. Morrison), DK065401 (C. Madden), and DK082558 (C. Madden).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We are grateful to Joseph Rathner for constructive comments on this article.
REFERENCES
- 1.Allen GV, Cechetto DF. Serotoninergic and nonserotoninergic neurons in the medullary raphe system have axon collateral projections to autonomic and somatic cell groups in the medulla and spinal cord. J Comp Neurol 350: 357–366, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R1569–R1578, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Bowker RM, Westlund KN, Coulter JD. Origins of serotonergic projections to the spinal cord in rat: an immunocytochemical-retrograde transport study. Brain Res 226: 187–199, 1981 [DOI] [PubMed] [Google Scholar]
- 4.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460: 303–326, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol 409: 187–209, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doly S, Madeira A, Fischer J, Brisorgueil MJ, Daval G, Bernard R, Verge D, Conrath M. The 5-HT2A receptor is widely distributed in the rat spinal cord and mainly localized at the plasma membrane of postsynaptic neurons. J Comp Neurol 472: 496–511, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Hamann A, Flier JS, Lowell BB. Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology 137: 21–29, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Helke CJ, Capuano S, Tran N, Zhuo H. Immunocytochemical studies of the 5-HT(1A) receptor in ventral medullary neurons that project to the intermediolateral cell column and contain serotonin or tyrosine hydroxylase immunoreactivity. J Comp Neurol 379: 261–270, 1997 [PubMed] [Google Scholar]
- 10.Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levitsky DA, Troiano R. Metabolic consequences of fenfluramine for the control of body weight. Am J Clin Nutr 55: 167S–172S, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Madden CJ, Morrison SF. Brown adipose tissue sympathetic nerve activity is potentiated by activation of 5-hydroxytryptamine (5-HT)1A/5-HT7 receptors in the rat spinal cord.. Neuropharmacology 54: 487–496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience 122: 5–15, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol 566: 559–573, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R831–R843, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol 577: 525–537, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeshima T, Ito R, Hamada S, Senzaki K, Hamaguchi-Hamada K, Shutoh F, Okado N. The cellular localization of 5-HT2A receptors in the spinal cord and spinal ganglia of the adult rat. Brain Res 797: 118–124, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Marlier L, Teilhac JR, Cerruti C, Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res 550: 15–23, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Martin-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience 98: 301–309, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci 24: 5370–5380, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nason MW, Jr, Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. J Neurosci 26: 1190–1198, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Ootsuka Y, Blessing WW. Thermogenesis in brown adipose tissue: increase by 5-HT2A receptor activation and decrease by 5-HT1A receptor activation in conscious rats. Neurosci Lett 395: 170–174, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Ootsuka Y, Blessing WW, Nalivaiko E. Selective blockade of 5-HT2A receptors attenuates the increased temperature response in brown adipose tissue to restraint stress in rats. Stress 11: 125–133, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Ootsuka Y, Nalivaiko E, Blessing WW. Spinal 5-HT2A receptors regulate cutaneous sympathetic vasomotor outflow in rabbits and rats; relevance for cutaneous vasoconstriction elicited by MDMA (3,4-methylenedioxymethamphetamine, “Ecstasy”) and its reversal by clozapine. Brain Res 1014: 34–44, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Passerin AM, Bellush LL, Henley WN. Activation of bulbospinal serotonergic neurons during cold exposure. Can J Physiol Pharmacol 77: 250–258, 1999 [PubMed] [Google Scholar]
- 27.Passerin AM, Henley WN. Activation of spinal cord serotonergic neurons accompanies cold-induced sympathoexcitation. Can J Physiol Pharmacol 72: 884–892, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates Sydney, Australia: Academic, 1986 [Google Scholar]
- 29.Thor KB, Nickolaus S, Helke CJ. Autoradiographic localization of 5-hydroxytryptamine1A, 5-hydroxytryptamine1B and 5-hydroxytryptamine1C/2 binding sites in the rat spinal cord. Neuroscience 55: 235–252, 1993 [DOI] [PubMed] [Google Scholar]
- 30.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Yoshida K, Nakamura K, Matsumura K, Kanosue K, Konig M, Thiel HJ, Boldogkoi Z, Toth I, Roth J, Gerstberger R, Hubschle T. Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur J Neurosci 18: 1848–1860, 2003. [DOI] [PubMed] [Google Scholar]