Abstract
Airway acidification has been consistently observed in airway inflammatory conditions and is known to cause cardiorespiratory symptoms that are, at least in part, mediated through the activation of bronchopulmonary C fibers and the subsequent reflexes. Protease-activated receptor-2 (PAR2) is expressed in a variety of cells in the lung and airways and is believed to play a role in airway inflammation and hyperresponsiveness. This study was carried out to investigate the effect of PAR2 activation on the acid signaling in rat bronchopulmonary C-fiber sensory neurons. Our RT-PCR results revealed the expression of mRNAs for transient receptor potential vanilloid receptor 1 (TRPV1) and four functional acid-sensing ion channel (ASIC) subunits 1a, 1b, 2a, and 3 in these sensory neurons. Preincubation of SLIGRL-NH2, a specific PAR2-activating peptide, markedly enhanced the Ca2+ transient evoked by extracellular acidification. Pretreatment with PAR2 agonists significantly potentiated both acid-evoked ASIC- and TRPV1-like whole cell inward currents. Activation of PAR2 also potentiated the excitability of these neurons to acid, but not electrical stimulation. In addition, the potentiation of acid-evoked responses was not prevented by inhibiting either PLC or PKC nor was mimicked by activation of PKC. In conclusion, activation of PAR2 modulates the acid signaling in pulmonary sensory neurons, and the interaction may play a role in the pathogenesis of airway inflammatory conditions, where airway acidification and PAR2 activation can occur simultaneously.
Keywords: acid-sensing ion channels, transient receptor potential vanilloid receptor 1, airway inflammation
expression of protease-activated receptor-2 (PAR2) has been demonstrated in a variety of cells including pulmonary sensory neurons in the lung and airways (15, 33). Mast cell tryptase, trypsin, trypsin-like proteases, and coagulation factors VIIa and Xa are considered as the endogenous agonists of PAR2 (33, 39). The elevated levels of both endogenous agonists and expression of PAR2 receptors have been reported from patients and in animals under airway-inflammatory conditions (23, 39). PAR2 is also known to be activated by certain airborne allergens such as house dust mites Der p1, p3, and p9 (3, 32, 41). Compelling evidence has indicated that activation of PAR2 by endogenous or exogenous agonists contributes to the pathogenesis of airway inflammation and airway hyperresponsiveness (4, 9, 33, 36, 37).
Local tissue acidosis frequently occurs in a variety of pathophysiological conditions such as inflammation, ischemia, hypoxia, trauma, and cancer metastases (5, 31, 34, 40). Indeed, endogenous airway acidification has been well demonstrated in various airway-inflammatory diseases (19, 24, 26). Airway exposure to endogenous or exogenous acid (such as air pollution-induced acid fogs, gastroesophageal reflux with microaspiration) is known to evoke cardiorespiratory symptoms including coughing, bronchoconstriction, dyspnea/apnea, bradycardia, and systemic hypotension (35). It has been recognized that these symptoms are at least partially mediated through the activation of bronchopulmonary C fibers and the subsequent reflex responses (24, 25, 35). However, the acid-sensing mechanism in these C-fiber afferents is still not fully understood, and its regulation, particularly under pathophysiological conditions, is largely unknown.
Acid is known to activate the acid-sensing ion channels (ASICs), which belong to the voltage-insensitive, amiloride-sensitive degenerin/epithelial Na+ channel superfamily (27, 42). Acid can also directly activate transient receptor potential vanilloid receptor 1 (TRPV1), which is abundant in various sensory neurons including vagal pulmonary sensory neurons and acts as polymodal nociceptor (8, 11, 12). Recent studies from our laboratory as well as other investigators have shown that physiologically/pathophysiologically relevant acidification indeed activates rat vagal bronchopulmonary C fibers, which is mediated through the activation of both ASICs and TRPV1 (13, 25). In the present study, we aimed to investigate whether and how the acid sensing in rat pulmonary C-fiber sensory neurons can be regulated by the activation of PAR2. The rationale and significance of this study are that airway acidification and PAR2 activation may occur simultaneously under airway-inflammatory conditions or numerous other pathophysiological conditions.
MATERIALS AND METHODS
The procedures described below were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Labeling vagal pulmonary sensory neurons with DiI.
Young Sprague-Dawley rats (4–6 wk old) were anesthetized with isoflurane inhalation (1% in O2) via a nose cone connected to a vaporizing machine (AB Bickford, Wales Center, NY). A small midline incision was made on the ventral neck skin to expose the trachea. The fluorescent tracer 3,3-dioctadecylindocarbocyanine (DiI) (0.2 mg/ml, 0.05 ml) was instilled into the lungs via a 30-gauge needle inserted into the lumen of the trachea; the incision was then closed. Animals recovered undisturbed for 7–10 days until they were euthanized for the tissue harvest and cell culture.
Isolation of nodose and jugular ganglion neurons.
Rats were killed after isoflurane inhalation. Nodose and jugular ganglia were extracted under a dissecting microscope and placed in ice-cold DMEM/F12 solution. Each ganglion was desheathed, cut into ∼10 pieces, placed in the combination of 0.04% type IV collagenase and 0.02% dispase II, and incubated for 80 min in 5% CO2 in air at 37°C. The ganglion suspension was centrifuged (150 g, 5 min) and supernatant aspirated. The cell pellet was then resuspended in a modified DMEM/F12 [DMEM/F12 supplemented with 10% (vol/vol) heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μM MEM nonessential amino acids] solution and gently triturated with a small bore fire-polished Pasteur pipette. The dispersed cell suspension was centrifuged (500 g, 8 min) through a layer of 15% BSA to separate the cells from the myelin debris. The pellets were resuspended in the modified DMEM/F12 solution, plated onto poly-l-lysine-coated glass coverslips, and incubated at 37°C in 5% CO2 in air. Isolated neurons were used for patch-clamp recording or RT-PCR within 48 h of culture.
Ca2+ imaging.
Intracellular Ca2+ was monitored using the fluorescent Ca2+ indicator fura-2 AM. Cells were loaded with 5 μM fura-2 AM for 30 min at 37°C. The coverslip containing cells was then mounted into a chamber (0.2 ml) placed on the stage of a Zeiss fluorescence inverted microscope equipped with a variable filter wheel (Sutter Instruments, Novato, CA) and digital CCD camera (Princeton Instruments, Trenton, NJ). The recording chamber was perfused continuously with extracellular solution (ECS; containing in mM: 136 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 0.33 NaH2PO4, 10 glucose, 10 HEPES, pH at 7.4) or the test chemicals by a gravity-fed valve control system (VC-66CS; Warner Instruments, Hamden, CT); a complete change of bath solution occurred in 6 s. Cells were allowed to deesterify for at least 30 min before the recording when the dual images (340- and 380-nm excitation, 510-nm emission) were collected and pseudocolor ratiometric images monitored by using the software Axon Imaging Workbench (Axon Instruments, Union City, CA).
Whole cell perforated patch-clamp recordings.
The recording chamber with the cultured cells was perfused continuously with the same standard ECS as that used in the Ca2+ imaging study. Whole cell perforated patch configuration (50 μg/ml gramicidin) was performed by using Axopatch 200B/pCLAMP 9.0 (Axon Instruments). The intracellular solution contained the following (in mM): 92 potassium gluconate, 40 KCl, 8 NaCl, 1 CaCl2, 0.5 MgCl2, 10 EGTA, 10 HEPES, pH at 7.2. The chemical stimulants were applied by a pressure-driven drug delivery system (ALA-VM8; ALA Scientific Instruments, Westbury, NY). The series resistance was usually in the range of 6–10 MΩ and was not compensated. The resting membrane potential was held at −70 mV in voltage-clamp mode. The experiments were performed at room temperature (∼22°C).
Both patch-clamp recordings and Ca2+-imaging analysis were performed selectively in the pulmonary sensory neurons on the basis of the following criteria: 1) labeling with DiI as indicated by fluorescence intensity; 2) cell diameter <35 μm; and 3) response to 0.75 μM capsaicin. These neurons presumably give rise to pulmonary C-fiber afferents as proposed in our recent studies (16). Although neurons from rat nodose and jugular ganglia were isolated and studied separately, data from the neurons of these two different origins were pooled for group analysis because no difference was found between responses of the neurons obtained from these two ganglia.
RT-PCR.
Cytoplasm of ∼20 individual pulmonary nodose or jugular ganglion neurons was retrieved by aspiration through a patch pipette. The pipette content was expelled into a test tube in which RT-PCR was performed by using Titan One Tube RT-PCT Kit (Roche Applied Science, Indianapolis, IN). A nested PCR was then performed by using the first PCR product as the template. Primers for both the first and nested PCR amplifications were designed to cross exon boundaries to distinguish amplified products from mRNA vs. genomic DNA. Primer sequences and predicted product sizes are summarized in Table 1. Reverse transcription was performed at 50°C for 30 min. Cycling conditions for the first PCR were as the follows: 1) initial denaturation at 94°C for 3 min; 2) 10 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 30 s, elongation at 68°C for 1 min; 3) 25 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 30 s, elongation at 68°C (45 s to 4 min), cycle elongation of 5 s for each cycle; and 4) a prolonged elongation at 68°C for 7 min. Reaction products were subsequently maintained at 4°C until they were used as templates for nested reactions.
Table 1.
PCR primer sequences
| Receptor | Accession No. | Primers for the First PCR | Size, bp | Primers for the Nested PCR | Size, bp |
|---|---|---|---|---|---|
| ASIC1a | |||||
| Sense | NM_012892 | 5′-gctgctctcctgcaagtacc-3′ | 965 | 5′-cggtctactggcagaaaagg-3′ | 278 |
| Anti-sense | 5′-agtggtttggcattgtgtca-3′ | 5′-tgaacagtcccatctgacca-3′ | |||
| ASIC1b | |||||
| Sense | NM_001034014 | 5′-gatcttcgccaatacctcca-3′ | 982 | 5′-gttctccaggcttaccacca-3′ | 418 |
| Anti-sense | 5′-aggctctgcacactccttgt-3′ | 5′-ttcctctgtctctccccaga-3′ | |||
| ASIC2a | |||||
| Sense | NM_024154 | 5′-tcaccaagcttgacgaagtg-3′ | 703 | 5′-cagatggctgatgaaaagca-3′ | 347 |
| Anti-sense | 5′-caggcagtgatgctgtagga-3′ | 5′-gcttcgaaggatgtctcgtc-3′ | |||
| ASIC3 | |||||
| Sense | NM_173135 | 5′-ggagtatctgcccatctgga-3′ | 700 | 5′-gcaataccgcatctttggat-3′ | 301 |
| Anti-sense | 5′-aagcaggcttgctccaataa-3′ | 5′-atggagagctccttggcata-3′ | |||
| TRPV1 | |||||
| Sense | NM_031982 | 5′-agcgagttcaaagacccaga-3′ | 822 | 5′-tggaggtggcagataacaca-3′ | 349 |
| Anti-sense | 5′-gttcaagggttccacgagaa-3′ | 5′-ttaggggtctcactgctgct-3′ |
ASIC, acid-sensing ion channel; TRPV1, transient receptor potential vanilloid receptor 1.
The nested PCR was performed as the following by using AccuPrime Taq DNA Polymerase System (Invitrogen, Carlsbad, CA): an initial denaturation at 94°C for 3 min was followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 56°C for 1 min, and elongation at 68°C for 2 min. Reaction products were separated on 1% agarose gel in Tris-borate EDTA buffer and visualized with 0.5% ethidium bromide fluorescence.
Chemicals.
DiI was purchased from Invitrogen. Dispase II was obtained from Roche Applied Science. SLIGRL-NH2 (PAR2-activating peptide or PAR2-AP) and 2-furoyl-LIGRLO-NH2 were from Bachem (King of Prussia, PA). All other chemicals were obtained from Sigma Chemical (St. Louis, MO). Stock solution of capsaicin (1 mM) was prepared in a vehicle of 10% Tween 80, 10% ethanol, and 80% ECS; those of PAR2-AP (10 mM) and 2-furoyl-LIGRLO-NH2 (10 mM) were in ECS, and amiloride (500 mM), phorbol 12-myristate 13-acetate (PMA; 1 mM), U73122 (3 mM), and chelerythrine (20 mM) were in dimethyl sulfoxide. These stock solutions were divided into small aliquots and kept at −80°C. The solutions of these chemicals at desired concentrations were prepared daily by dilutions with ECS before use. No detectable effect of the vehicles of these chemical agents was found in our preliminary experiments.
Statistical analysis.
Data were analyzed by a one-way ANOVA unless mentioned otherwise. When the ANOVA showed a significant interaction, pair-wise comparisons were made with a post hoc analysis (Fisher's least-significant difference). Results were considered significant when P < 0.05. Data are means ± SE.
RESULTS
Expression of mRNAs for ASIC subunits and TRPV1 in rat vagal pulmonary sensory neurons.
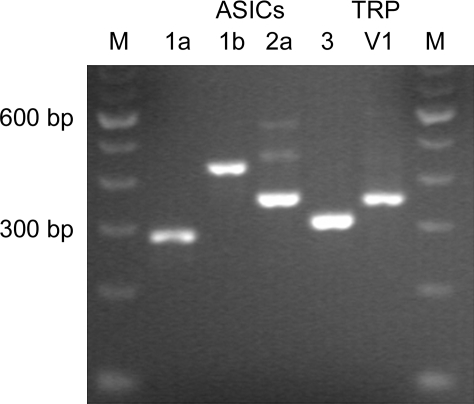
The expression of PAR2 (immunoreactivity for PAR2 protein) in rat pulmonary sensory neurons has recently been demonstrated (15). In the present study, RT-PCR and the nested PCR were carried out using the cytoplasm harvested from multiple DiI-labeled nodose and jugular ganglion neurons to determine the presence of two groups of acid-sensitive ion channels, ASICs and TRPV1. As shown in Fig. 1, our results showed that these neurons express mRNAs for TRPV1 and four functional ASIC subunits 1a, 1b, 2a, and 3. These results were confirmed in four separate trials using the neurons collected from three different animals. Control reactions that did not contain RNA template or the RT enzyme showed no amplification products (n = 4).
Fig. 1.
Detection of the presence of acid-sensing ion channels (ASICs) and transient receptor potential vanilloid receptor 1 (TRPV1) mRNAs in rat pulmonary sensory neurons using RT-PCR. Cytoplasm of 20 individual pulmonary nodose neurons was collected into single PCR tubes. One-step RT-PCR and the nested PCR were carried out to detect the presence of the transcripts for TRPV1 and ASIC subunits 1a, 1b, 2a, and 3. M, DNA molecular weight marker.
Potentiation of acid-evoked Ca2+ transient by PAR2-AP.
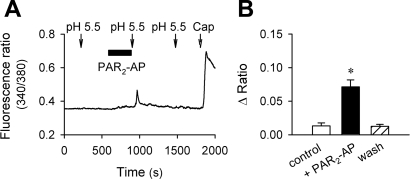
A 5-min perfusion of PAR2-AP (100 μM), a short synthetic peptide that mimics the sequence of tethered ligand of PAR2 and selectively and specifically activates the receptor (33), caused a small but detectable elevation of intracellular Ca2+ in rat pulmonary sensory neurons (e.g., Fig. 2A). Application of acidic solution (pH 5.5, 40 s) evoked only a mild and reversible (or no) Ca2+ transient in these neurons. However, immediately after the PAR2-AP pretreatment, the peak response of the pH 5.5-evoked Ca2+ transient was significantly potentiated (P < 0.05; n = 26); the augmented response returned to the control level after a 10-min washout (Fig. 2).
Fig. 2.
Potentiation of acid-evoked Ca2+ transient by protease-activated receptor-2-activating peptide (PAR2-AP) in rat pulmonary sensory neurons. A: Ca2+ transients evoked by extracellular acidification (pH 5.5, 40 s) before and after pretreatment with PAR2-AP (SLIGRL-NH2; 100 μM, 5 min). The neuronal sensitivity to capsaicin (Cap; 0.5 μM, 40 s) was tested at the end of experimental run. B: group data showing the enhancement of acid-evoked Ca2+ transient by PAR2-AP pretreatment. *Significantly different from the control response before PAR2-AP (P < 0.05, n = 26).
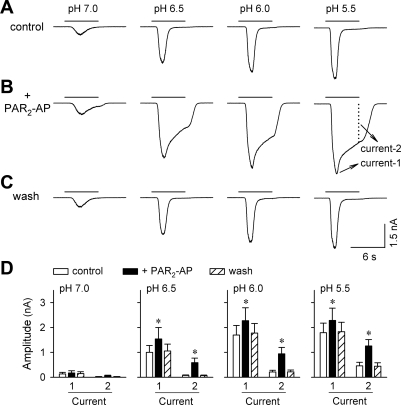
Potentiation of acid-evoked inward current by activation of PAR2.
As what we have reported recently (13), a rapid drop of extracellular pH from pH 7.4 evoked an inward, amiloride-sensitive ASIC current in rat pulmonary sensory neurons voltage clamped at −70 mV. As shown in Fig. 3, pretreatment with PAR2-AP (100 μM, 2 min) induced a marked increase in the peak amplitude of ASIC-like current evoked by acid. The peak currents (Current 1 in Fig. 3) were significantly increased by 49.6 ± 8.2%, 34.9 ± 6.3%, and 30.8 ± 7.4% for pH 6.5, 6.0, and 5.5, respectively (P < 0.05, n = 17). Similar to the peak current, the sustained component (Current 2 in Fig. 3) of the ASIC-like current, measured at the end of a 6-s acid application, was also dramatically increased after PAR2-AP pretreatment (Fig. 3). The potentiation for both the peak and sustained components of ASIC-like current was readily reversible after a 5-min washout.
Fig. 3.
Effect of PAR2-AP on the acid-evoked ASIC-like inward current in rat pulmonary sensory neurons. A–C: inward currents evoked by acid (pH 7.0, 6.5, 6.0, and 5.5; 6 s each) before, immediately after pretreatment with PAR2-AP (100 μM, 2 min), and after 5-min washout, respectively. In all panels, Current 1 is defined as the peak of transient ASIC-like inward current, and Current 2 is measured as the amplitude of sustained inward current when the acid challenge was terminated. D: group data showing that both the peak (Current 1) and sustained (Current 2) components of acid-evoked current were significantly enhanced after PAR2-AP pretreatment. *Significantly different from the corresponding control (P < 0.05, n = 17).
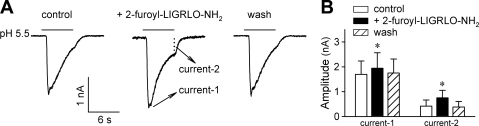
We next tested 2-furoyl-LIGRLO-NH2, a recently reported selective and potent agonist for PAR2 (21). As shown in Fig. 4, pretreatment with 2-furoyl-LIGRLO-NH2 (100 μM, 2 min) mimicked the effect of PAR2-AP. The peak and sustained components of ASIC-like current evoked by pH 5.5 were potentiated by 15.4 ± 5.6% and 180.9 ± 78.4%, respectively, after 2-furoyl-LIGRLO-NH2 pretreatment (P < 0.05, n = 7).
Fig. 4.
Pretreatment with 2-furoyl-LIGRLO-NH2 mimicked the effect of PAR2-AP. A: inward current evoked by pH 5.5 (6 s) before and after pretreatment with 2-furoyl-LIGRLO-NH2 (100 μM, 2 min), a selective PAR2 agonist. The acid-evoked current was evaluated by the amplitudes of its peak (Current 1) and sustained (Current 2) components. B: group data showing that both the peak and sustained components of ASIC-like current were significantly increased after 2-furoyl-LIGRLO-NH2. *Significantly different from the corresponding control (P < 0.05, n = 7).
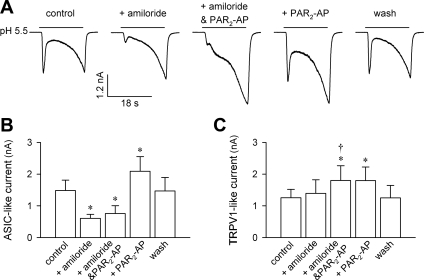
In addition to the ASIC-like current, extracellular acidification is also known to evoke a delayed and sustained TRPV1-mediated current in rat pulmonary sensory neurons (13). In a group of 10 neurons, we isolated the acid-evoked TRPV1-like current by using the prolonged acid application (≥18 s) and blockade of ASIC current by amiloride (300 μM, 2 min). As shown in Fig. 5, pretreatment with PAR2-AP (100 μM, 2 min) significantly enhanced the pH 5.5-evoked TRPV1-like current in these sensory neurons. The potentiation was clearly present whether it was with (comparing between amiloride + PAR2-AP and amiloride alone: Δ = 42.7 ± 8.6%; P < 0.05, n = 10) or without (comparing between PAR2-AP and control: Δ = 33.9 ± 6.9%; P < 0.05, n = 10) amiloride blockade, and the difference between them was not significant (P > 0.05, n = 10; paired t-test). The potentiation of acid-evoked TRPV1-like current by PAR2-AP was reversible after a 10-min washout (Fig. 5).
Fig. 5.
Potentiation of both acid-evoked ASIC-like and TRPV1-like currents by PAR2-AP in rat pulmonary sensory neurons. A: acid (pH 5.5, 18 s)-evoked ASIC-like (first peak) and TRPV1-like (second peak) inward currents at control, after pretreatment with amiloride (ASIC inhibitor; 300 μM, 2 min), amiloride + PAR2-AP (300 μM and 100 μM, respectively; 2 min), and PAR2-AP (100 μM, 2 min) alone, and after 10-min washout. B and C: group data showing the peak amplitudes of pH 5.5-evoked ASIC-like and TRPV1-like inward currents, respectively, after different pretreatments as shown in A. *Significantly different from the control response (P < 0.05, n = 10). †Significant difference between pretreatments with amiloride + PAR2-AP and amiloride alone (P < 0.05, n = 10).
Upregulation of neuronal excitability to acid stimulation by PAR2-AP.
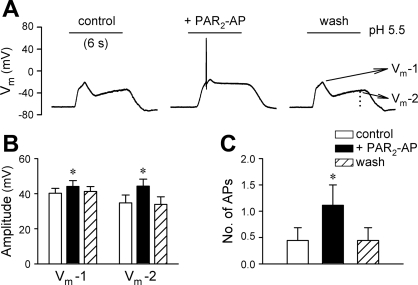
This series of experiments were carried out in current-clamp recordings to examine whether activation of PAR2 altered the excitability of rat pulmonary sensory neurons to acid stimulation. As shown in Fig. 6, pretreatment with PAR2-AP (100 μM, 2 min) significantly increased the amplitudes of both peak (an increase of 10.9 ± 3.8%; P < 0.05, n = 9) and sustained (an increase of 38.8 ± 5.5%; P < 0.05, n = 9) membrane depolarization evoked by acid application (pH 5.5, 6 s). In addition, the number of acid-evoked action potentials was significantly increased from 0.4 ± 0.2 at control to 1.1 ± 0.4 after PAR2-AP (P < 0.05, n = 9). The increases in both depolarization and action potential firing returned to control after 5-min washout.
Fig. 6.
Pretreatment with PAR2-AP increased the excitability of rat pulmonary sensory neurons to acid stimulation. A: acid (pH 5.5, 6 s)-evoked changes in membrane potential (Vm) at control, after pretreatment with PAR2-AP (100 μM, 2 min), and after 5-min washout. Vm-1 and Vm-2 refer to the values of membrane potential at the first peak and at the termination of acid challenge, respectively. B: effect of PAR2-AP on the pH 5.5-evoked membrane potential changes. C: effect of PAR2-AP on the number of pH 5.5-evoked action potentials. *Significantly different from the corresponding control (P < 0.05, n = 9).
In a separate group of six neurons, however, pretreatment with PAR2-AP (100 μM, 2 min) did not show any significant effect on either depolarization or action potential firing evoked by an above-threshold depolarizing current injection (20–500 pA, 485 ms); for example, the electrical stimulation evoked an average of 1.2 ± 0.2 action potentials both before and after PAR2-AP pretreatment (P > 0.05, n = 6).
Effect of PAR2-AP on acid signaling is not mediated through activation of PKC.
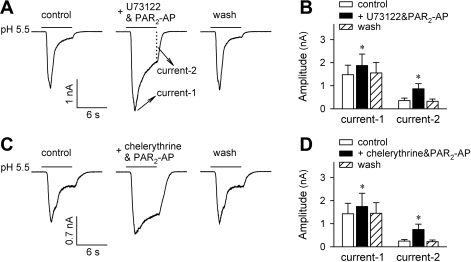
In a number of different cell systems, PAR2 has been known to be coupled to PKC activation via Gq/11 protein and the phosphatidylinositol pathway (2, 10). We have recently demonstrated that, in rat pulmonary sensory neurons, this PLC-PKC transduction cascade is indeed responsible for the potentiation of capsaicin-activated, TRPV1-mediated whole cell and single-channel responses (14, 15). In the present study, however, preincubation with neither U73122 (1 μM, 4 min), a PLC inhibitor, nor chelerythrine (10 μM, 4 min), a PKC inhibitor, prevented the potentiation of acid-evoked either ASIC-like (Fig. 7) or TRPV1-like (n = 4, not shown) inward current by PAR2-AP (100 μM, 2 min). For example, the pH 5.5-evoked peak ASIC-like current was increased by 33.3 ± 8.1% (P < 0.05, n = 7) and 21.7 ± 5.4% (P < 0.05, n = 6), and the sustained component of ASIC-like current was increased by 153.8 ± 46.8% (P < 0.05, n = 7) and 176.5 ± 48.7% (P < 0.05, n = 6) after PAR2-AP in the presence of U73122 and chelerythrine, respectively (Fig. 7).
Fig. 7.
Inhibition of PLC and PKC did not prevent the potentiating effect of PAR2-AP. A: acid (pH 5.5, 6 s)-evoked inward current at control, after pretreatment with U73122 (a PLC inhibitor; 1 μM, 4 min) + PAR2-AP (100 μM, 2 min), and after 5-min washout. B: group data showing that PAR2-AP potentiated both the acid-evoked transient and sustained inward currents in the presence of U73122. *Significantly different from the corresponding control (P < 0.05, n = 7). C: acid (pH 5.5, 6 s)-evoked inward current at control, after pretreatment with chelerythrine (a PKC inhibitor; 10 μM, 4 min) + PAR2-AP (100 μM, 2 min), and after 5-min washout. D: group data showing that PAR2-AP potentiated both the acid-evoked transient and sustained inward currents in the presence of chelerythrine. *Significantly different from the corresponding control (P < 0.05, n = 6).
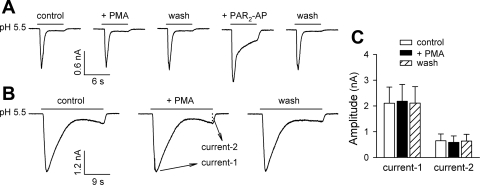
To further confirm that the activation of PKC and subsequent ion channel phosphorylation are not responsible for the potentiation of acid-evoked whole cell responses by PAR2 activation, we examined the effect of PMA, a PKC activator, on the acid-evoked inward currents. As shown in Fig. 8, pretreatment with PMA (0.1 μM, 2 min) did not significantly affect either ASIC-like (e.g., Fig. 8A) or TRPV1-like (e.g., Fig. 8B) inward currents in rat pulmonary sensory neurons (P > 0.05, n = 12).
Fig. 8.
Lack of potentiating effect of phorbol 12-myristate 13-acetate (PMA) on the acid-evoked inward current in rat pulmonary sensory neurons. A: pretreatment with PMA (0.1 μM, 2 min), a PKC activator, did not affect the acid (pH 5.5, 6 s)-evoked ASIC-like inward current, whereas the latter was markedly potentiated by PAR2-AP pretreatment (100 μM, 2 min) in the same neuron. B: pretreatment with PMA (0.1 μM, 2 min) failed to potentiate either ASIC-like or TRPV1-like inward currents evoked by a prolonged acid application (pH 5.5, 22 s). A and B were recorded from 2 different neurons. C: group data showing that PMA did not significantly affect the acid-evoked inward current (n = 12). The acid-evoked whole cell responses were evaluated as the amplitudes of transient peak current (Current 1) and sustained current at the termination of acid challenge (Current 2), regardless of the phenotypes of inward currents as shown in A and B.
DISCUSSION
Recent studies have shown that acid stimulates bronchopulmonary C fibers through the activation of two major types of receptors, ASICs and TRPV1 (13, 25). The expression and function of ASICs have recently been investigated in both peripheral sensory and central neurons (1, 42). To date, four genes encoding six ASIC subunits have been cloned in mammals, namely ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4 (27). Four of the six subunits of ASICs, 1a, 1b, 2a, and 3, can form functional homomeric channels that are activated directly by external protons (45). Neither ASIC2b nor ASIC4 can form functional homomeric proton-gated channel (17). Like other ligand-gated ion channels, functional ASICs can be formed by homomultimers as well as heteromultimers (6, 42). Different homomeric and heteromeric ASICs are known to have distinct pH sensitivity, ion selectivity, and channel kinetics (17). It has been reported that, in heterologous expression systems, the pH of half-maximal activation of these channels differs: i.e., pH 6.2, 5.9, 4.4, and 6.5 for ASIC1a, ASIC1b, ASIC2a, and ASIC3, respectively (45). Whereas ASIC1a, ASIC1b, and ASIC2a display transient activation characteristics, ASIC3 responds to acid stimulation biphasically, with a quick desensitizing and a late sustaining current (17). In the present study, our RT-PCR results showed that all four functional ASIC subunits (1a, 1b, 2a, and 3) are expressed in rat pulmonary sensory neurons (Fig. 1). However, we cannot speculate on the specific ASIC subunit combination(s) in an individual neuron only on the basis of the phenotypes of acid-evoked inward currents because 1) multi-cell instead of single-cell RT-PCR was performed in our study and 2) the native ASICs expressed in these pulmonary sensory neurons, like that in dorsal root ganglion neurons (1, 6), are most likely heteromultimeric. Nevertheless, our results showed that all ASIC-like inward currents, albeit showing distinct phenotypes at control, were significantly enhanced by selective PAR2 agonists (e.g., Figs. 3–5).
In a number of different cells and tissues, PAR2 has been shown to be coupled to Gq/11, resulting in activation of PLC and generation of 1,4,5-inositol trisphosphate and diacylglycerol, which would lead to mobilizing intracellular Ca2+ and activating PKC (2, 10). Our recent studies have demonstrated that the PLC-PKC signaling pathway is indeed responsible for the potentiation of capsaicin-activated and TRPV1-mediated responses in both whole-cell and single-channel recordings in rat pulmonary sensory neurons (14, 15). However, in the present study, our results showed that 1) the potentiating effect of PAR2 agonists on both ASIC-like and TRPV1-like whole-cell responses lasted less than 10 min (Figs. 3–5); 2) inhibition of PLC or PKC did not prevent the sensitizing effect of PAR2-AP on acid-evoked inward currents (Fig. 7); and 3) activation of PKC with PMA failed to mimic the potentiating effect of PAR2 agonists on the acid responses (Fig. 8). These findings are in distinct disparity with numerous PAR2-induced and G protein-mediated long-lasting cell signaling, as recently reported by our laboratory as well as many other investigators (2, 10, 14, 15). Therefore, we believe that the PLC-PKC intracellular cascade is not involved in the PAR2-induced potentiation of acid signaling in rat pulmonary sensory neurons.
The mechanism underlying the enhancement of acid-evoked ASIC channel responses by PAR2 agonists is not known. In contrast with TRPV1, which is modulated by numerous proinflammatory mediators (12, 29), ASICs seem to be unresponsive to many of the same mediators. Among the known interactions, FMRFamide-related peptide (44), arachidonic acid (38), and nitric oxide (7) have been shown to enhance the ASIC activity in dorsal root ganglion neurons as well as in heterologous cells. All three above mentioned mediators are believed to modulate ASICs via a direct mechanism, and an interaction with the extracellular loop of ASIC subunits was suggested to be involved in the modulation of ASIC activity by nitric oxide (7). Interestingly, certain serine proteases (e.g., furin, prostasin, channel-activating protease 2, and kallikrein) have been shown to activate epithelial Na+ channel, which belongs to the same family as ASICs, by cleaving channel subunits at specific sites within their extracellular domains (18, 22). In the present study, our results suggest an involvement of a direct interaction between PAR2 agonists and ASICs, which presumably leads to certain conformational changes and therefore affects the proton gating of ASIC channels. However, channel cleavage is unlikely the cause of the functional modification of ASICs observed in our study, given that the potentiation of ASIC responses was readily reversible upon washout of PAR2 agonists (Figs. 2–6).
TRPV1 is another ion channel that can be directly activated by acid. Results from our present study showed that the acid-evoked TRPV1-like current was also significantly enhanced after PAR2-AP pretreatment. Unlike the PLC-PKC-mediated long-lasting sensitizing effect of PAR2-AP on capsaicin-evoked whole cell and single-channel TRPV1 activities (14, 15), the potentiation of acid-evoked TRPV1-like current is short lasting and does not appear to be PKC dependent (e.g., Figs. 5 and 8). The mechanism underlying the distinct potentiating effect of PAR2-AP on capsaicin- and acid-evoked TRPV1 responses is not clear. Interestingly, a recent study from our laboratory has demonstrated that eosinophil-derived cationic proteins such as major basic protein can also exert distinctly different effects on these two TRPV1 agonists, a potentiating effect on only capsaicin- but not acid-evoked TRPV1 current (16). Although how acid and capsaicin activate TRPV1 is not yet fully understood, it is believed that these two agonists exert their actions on TRPV1 from opposite sides of the membrane, intracellularly for capsaicin and extracellularly for proton (11, 20, 43). TRPV1, when tested in terms of capsaicin activation, has been known as a target of phosphorylation by numerous proinflammatory mediators, cytokines, and growth factors (12, 29). In the present study, the potentiating effect of PAR2-AP on acid-evoked TRPV1 responses, however, appears most likely resulting from a direct interaction with the TRPV1 channel because of its short-lasting and PKC-independent feature.
It is known that vagal bronchopulmonary C-fiber sensory nerves play an important role in regulating cardiopulmonary functions under various pathophysiological conditions (11, 28, 30). Endogenous airway acidification has been well demonstrated in various airway inflammatory diseases such as asthma and chronic obstructive pulmonary disease (19, 24, 26). For example, it has been reported that the mean pH of exhaled breath condensate is over two log orders lower in patients with acute asthma (5.23 ± 0.21) than in control subjects (7.65 ± 0.20) and normalizes after corticosteroid therapy (19). In addition, pulmonary interstitial acidosis develops when the production of CO2 is exceedingly high and/or the elimination of CO2 from the lungs is hindered. Such changes can occur under both physiological (e.g., severe excise) and pathophysiological (e.g., chronic obstructive pulmonary disease) conditions (35). Airway exposure to either endogenous or exogenous acid is known to evoke cardiorespiratory symptoms that are at least in part mediated through the activation of bronchopulmonary C fibers and the subsequent reflex responses (25, 35). In the present study, our results showed that PAR2 activation significantly increased the excitability of pulmonary sensory neurons in response to the same acid stimulation, which can lead to greater reflex responses and therefore contribute to more pronounced cardiorespiratory symptoms such as coughing, dyspnea, and bronchoconstriction. The potentiation of acid signaling by PAR2 agonists in the airway sensory neurons should be important and relevant to pathophysiological conditions such as airway inflammatory diseases in which acidification and PAR2 activation may occur simultaneously.
GRANTS
This study is supported by American Heart Association Scientist Development Grant 0835320N and National Institute of Allergy and Infectious Diseases Grant AI076714.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Michelle E. Lim and Ruei-Lung Lin for skillful technical assistance.
REFERENCES
- 1.Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci USA 99: 2326–2331, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci 24: 4300–4312, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, Stewart GA. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol 169: 4572–4578, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Barrios VE, Jarosinski MA, Wright CD. Proteinase-activated receptor-2 mediates hyperresponsiveness in isolated guinea pig bronchi. Biochem Pharmacol 66: 519–525, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Benson CJ, Sutherland SP. Toward an understanding of the molecules that sense myocardial ischemia. Ann NY Acad Sci 940: 96–109, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadiou H, Studer M, Jones NG, Smith ES, Ballard A, McMahon SB, McNaughton PA. Modulation of acid-sensing ion channel activity by nitric oxide. J Neurosci 27: 13251–13260, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24: 487–517, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chambers LS, Black JL, Poronnik P, Johnson PR. Functional effects of protease-activated receptor-2 stimulation on human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 281: L1369–L1378, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci 24: 4293–4299, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher JT. The TRPV1 ion channel: implications for respiratory sensation and dyspnea. Respir Physiol Neurobiol 167: 45–52, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol 533: 207–214, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Gu Q, Lee LY. Characterization of acid-signaling in rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 291: L58–L65, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu Q, Lee LY. Hypersensitivity of pulmonary chemosensitive neurons induced by activation of protease-activated receptor-2 in rats. J Physiol 574: 867–876, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu Q, Lee LY. Effect of PAR2 activation on single TRPV1 channel activities in rat vagal pulmonary sensory neurons. Exp Physiol 94: 928–936, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu Q, Lim ME, Gleich GJ, Lee LY. Mechanisms of eosinophil major basic protein-evoked hyperexcitability of vagal pulmonary chemosensitive neurons. Am J Physiol Lung Cell Mol Physiol 296: L453–L461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem 279: 11006–11015, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med 161: 694–699, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 108: 421–430, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Kawabata A, Oono Y, Yonezawa D, Hiramatsu K, Inoi N, Sekiguchi F, Honjo M, Hirofuchi M, Kanke T, Ishiwata H. 2-Furoyl-LIGRL-NH2, a potent agonist for proteinase-activated receptor-2, as a gastric mucosal cytoprotective agent in mice. Br J Pharmacol 144: 212–219, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, Thompson PJ. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol 108: 797–803, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Kodric M, Shah AN, Fabbri LM, Confalonieri M. An investigation of airway acidification in asthma using induced sputum: a study of feasibility and correlation. Am J Respir Crit Care Med 175: 905–910, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kollarik M, Ru F, Undem BJ. Acid-sensitive vagal sensory pathways and cough. Pulm Pharmacol Ther 20: 402–411, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S. pH in expired breath condensate of patients with inflammatory airways diseases. Am J Respir Crit Care Med 165: 1364–1370, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci 26: 477–483, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Lee LY. Respiratory sensations evoked by activation of bronchopulmonary C-fibers. Respir Physiol Neurobiol 167: 26–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr Opin Pharmacol 9: 243–249, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nat Rev Cancer 2: 201–209, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Ramachandran R, Hollenberg MD. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol 153: S263–S282, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol 114: 997–1008, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Reeh PW, Steen KH. Tissue acidosis in nociception and pain. Prog Brain Res 113: 143–151, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol 113: 610–619, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Ricciardolo FL, Steinhoff M, Amadesi S, Guerrini R, Tognetto M, Trevisani M, Creminon C, Bertrand C, Bunnett NW, Fabbri LM, Salvadori S, Geppetti P. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am J Respir Crit Care Med 161: 1672–1680, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, Gater PR, Geppetti P, Bertrand C, Stevens ME. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol 169: 5315–5321, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Smith ES, Cadiou H, McNaughton PA. Arachidonic acid potentiates acid-sensing ion channels in rat sensory neurons by a direct action. Neuroscience 145: 686–698, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Sokolova E, Reiser G. A novel therapeutic target in various lung diseases: airway proteases and protease-activated receptors. Pharmacol Ther 115: 70–83, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci 12: 86–95, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol 167: 1014–1021, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Waldmann R, Lazdunski M. H(+)-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol 8: 418–424, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Welch JM, Simon SA, Reinhart PH. The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc Natl Acad Sci USA 97: 13889–13894, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ. ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J Neurophysiol 89: 2459–2465, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118: 687–698, 2004. [DOI] [PubMed] [Google Scholar]