Abstract
Neuronal nitric oxide synthase is critically involved in the pathogenesis of acute lung injury resulting from combined burn and smoke inhalation injury. We hypothesized that 7-nitroindazole, a selective neuronal nitric oxide synthase inhibitor, blocks central molecular mechanisms involved in the pathophysiology of this double-hit insult. Twenty-five adult ewes were surgically prepared and randomly allocated to 1) an uninjured, untreated sham group (n = 7), 2) an injured control group with no treatment (n = 7), 3) an injury group treated with 7-nitroindazole from 1-h postinjury to the remainder of the 24-h study period (n = 7), or 4) a sham-operated group subjected only to 7-nitroindazole to judge the effects in health. The combination injury was associated with twofold increased activity of neuronal nitric oxide synthase and oxidative/nitrosative stress, as indicated by significant increases in plasma nitrate/nitrite concentrations, 3-nitrotyrosine (an indicator of peroxynitrite formation), and malondialdehyde lung tissue content. The presence of systemic inflammation was evidenced by twofold, sixfold, and threefold increases in poly(ADP-ribose) polymerase, IL-8, and myeloperoxidase lung tissue concentrations, respectively (each P < 0.05 vs. sham). These molecular changes were linked to tissue damage, airway obstruction, and pulmonary shunting with deteriorated gas exchange. 7-Nitroindazole blocked, or at least attenuated, all these pathological changes. Our findings suggest 1) that nitric oxide formation derived from increased neuronal nitric oxide synthase activity represents a pivotal reactive agent in the patho-physiology of combined burn and smoke inhalation injury and 2) that selective neuronal nitric oxide synthase inhibition represents a goal-directed approach to attenuate the degree of injury.
Keywords: pathogenesis, acute lung injury
combined burn and smoke inhalation injury frequently occurs in both industrial and domestic accidents and is associated with significant morbidity and mortality (29). In the past, we were able to demonstrate that the combination of burn and smoke inhalation injury is ideal to model ARDS. In fact, this model mimics human ARDS secondary to multiple trauma and airway damage. Although inhalation injury alone represents a significant clinical problem, it is not as severe as the combined injury and may not always progress to ARDS (39, 50). We have studied each injury alone and in combination and noted that the latter produces a much more significant insult, such as a higher degree in pulmonary shunting and a greater fall in PaO2/FiO2 (43).
On a molecular level, nitric oxide (NO) and reactive nitrogen species (RNS) are involved in this pathogenesis, leading to multiple organ dysfunction or even failure (30, 41). Currently, three different nitric oxide synthase (NOS) isoforms have been identified, all of which are able to produce NO under normal circumstances. In the presence of systemic inflammation and oxidative stress, however, the inducible enzyme, i.e., iNOS (NOS-2), is upregulated and produces large amounts of NO. We have previously reported that iNOS plays a critical role in the pathogenesis of acute lung injury (ALI) resulting from smoke inhalation and thermal injury (15, 44, 48), and that iNOS inhibition attenuates systemic transvascular fluid flux, pulmonary edema, and pulmonary shunting (14, 44).
In response to combined burn and smoke inhalation injury, however, only about one-half of total NOS activity is derived from iNOS, suggesting that the other half is produced by the constitutive isoforms (cNOS), namely neuronal NOS (nNOS; NOS-1) or endothelial NOS (eNOS; NOS-3) (48). The latter isoenzyme was first detected in vascular endothelial cells and plays an important regulatory role in loco-regional tissue perfusion (8, 9). In this context, it is noteworthy that activity and protein expression of eNOS are not affected by burn and smoke inhalation injuries (13, 21). Conversely, previous studies suggested that nNOS may significantly increase its activity in response to cellular stress, thus playing a critical role in the pathogenesis of ALI (13). Interestingly, nNOS inhibition in sheep with pulmonary dysfunction resulted in lower iNOS mRNA compared with untreated animals, suggesting that nNOS activation may be the trigger for iNOS induction and the inflammatory cascade (48).
While nNOS was first discovered in neuronal tissue (7), later studies provided evidence that it is also present in the airway and bronchial glands (17). The formation of NO in the lung results in the loss of hypoxic pulmonary vasoconstriction (HPV) that, in turn, leads to ventilation/perfusion mismatching and subsequently poor oxygenation. In a recent study by Westphal et al. (52), it was shown that the loss of HPV in sheep with ALI can be prevented by inhibition of nNOS-derived NO. The exact molecular mechanisms, however, remained to be determined.
We hypothesized that nNOS represents an early and central enzyme involved in inflammatory signaling leading to pulmonary damage following combined burn and smoke trauma, and that selective nNOS inhibition blocks pulmonary damage on a molecular level. The aim of the present study was, therefore, to identify the molecular biological mechanisms by which selective nNOS inhibition attenuates pulmonary dysfunction resulting from combined burn and smoke inhalation injury. These objectives were tested in an established and clinically relevant ovine model that closely mimics the pathophysiology of human fire victims (43).
MATERIALS AND METHODS
This study was approved by the Animal Care and Use Committee of the University of Texas Medical Branch. All the animals were handled according to the guidelines established by the American Physiological Society and the National Institutes of Health.
Animal Model
Surgical preparation.
Twenty-five female sheep (33 ± 1 kg) were surgically prepared for chronic study under halothane anesthesia using an established protocol (31). The right femoral artery and vein were cannulated with Silastic catheters (16 gauge, 24 inches; Intracath, Becton-Dickinson Vascular Access, Sandy, UT). In addition, a thermodilution catheter (Swan Ganz model 131 F7; Baxter, Edwards Critical-Care Division, Irvine, CA) was introduced through the right jugular vein into the pulmonary artery. Another catheter (0.062 inches ID, 0.125 inches OD; Durastic silicone tubing DT08, Allied Biomedical, Paso Robles, CA) was positioned in the left atrium for pressure monitoring. A right-sided thoracotomy at the fifth-interspace was performed, and an efferent lymphatic from the caudal mediastinal lymph node was cannulated (Silastic medical grade tubing 0.025 inches ID, 0.047 inches OD; Dow Corning, Midland, MI) by a modification of the technique of Staub et al. (45). The systemic contribution to the caudal mediastinal lymph node was ligated, and the systemic diaphragmatic lymph vessels were cauterized through a ninth-interspace thoracotomy incision. The incision sites were infiltrated with 2% lidocaine to minimize postoperative pain, and the wounds were closed. During the postoperative period, Buprenex (0.3 mg iv; Butler Animal Health Supply, Dublin, OH) was administered as needed for pain treatment. The animals were given 5–7 days to recover from the surgical procedure; meanwhile, they had free access to food and water.
Burn and smoke inhalation injury.
Just before the injury, a baseline (BL) measurement was performed. Thereafter, sheep were randomly allocated to one of the three study groups: an uninjured, untreated sham group (sham, n = 7), an injured control group with no treatment (control, n = 7), or an injury group treated with 7-nitroindazole (7-NI, n = 7). In addition, four sham-operated animals were subjected to 7-nitroindazole to judge the effects in health. A tracheostomy was performed under ketamine anesthesia (Ketanest; Fort Dodge Animal Health, Fort Dodge, IA) in all animals. Thereafter, a cuffed tracheostomy tube (10-mm diameter; Shiley, Irvine, CA) was inserted into the stoma, and general anesthesia was continued using halothane as the anesthetic. In addition, a urinary bladder catheter (Dover, 12–14 Fr; Sherwood Medical, St. Louis, MO) was placed and a BL urine sample was taken. Animals allocated to the control and 7-NI groups were then subjected to a 40% total body surface area third-degree burn using a Bunsen burner and 4 × 12 breaths of cotton smoke inhalation as described previously (44). In this context, it is important to note that as the injury becomes full thickness (third-degree), it constricts. Because this area no longer has blood flow, it is blanched. Only when this was the case, the investigator moved to a new area for injury. As the edge of the burn was reached, a sheet of stainless steel was placed so the burn was within the bounds of the demarcation. It was always insured that the burn was third-degree. The latter is essential for the welfare of the animals, since third-degree burns are painless due to the destruction of the epidermis, dermis, and nerve endings in the skin (28).
Carboxyhemoglobin (COHb) levels were determined after each set of smoke inhalation and served as an indicator of injury immediately after smoke inhalation (23). In the sham groups, room air was insufflated into the tracheal tube instead of cotton smoke. At the end of the burn and smoke inhalation injury, the animals were allowed to wake up. Before awakening, the sheep received Buprenex (0.3 mg iv; Butler Animal Health Supply) to explicitly exclude any discomfort. Apart from general anesthetics not being needed, it is noteworthy that they may even have compromised hemodynamics. If there were any signs of discomfort, pain medication was provided unhesitatingly. Postinjury, Buprenex (0.3 mg iv, Butler Animal Health Supply) was given every 12 h for 2 days and subsequently as needed, depending on the judgment of the investigator.
Experimental Protocol
For this survival study, all animals were maintained on mechanical ventilation (Servo Ventilator 900C; Siemens-Elema, Solna, Sweden) throughout the entire 24-h experimental period as reported previously (49). During the first 3 h after injury, the inspiratory O2 fraction (FiO2) was maintained at 100%, and the respiratory rate was maintained at 30 breaths/min. Then, settings were adjusted according to blood gas analyses to maintain arterial O2 saturation above 90% and PaCO2 at 30 mmHg. Resuscitation was performed with lactated Ringer solution according to the Parkland formula (4 ml/kg/% burned total body surface area for 24 h) (4). However, within the first 8 h postinjury, sheep received one-half of the total fluid to avoid a drop in cardiac output.
The 7-NI groups received a continuous intravenous infusion of the specific nNOS inhibitor 7-NI (1 mg·kg−1·h−1 Sigma-Aldrich, St. Louis, MO) given from 1 h postinjury (or sham injury) to the end of the 24-h study period. To have the same fluid balance for all three groups, the amount of the 7-NI infusion was subtracted from the quantity of the Ringer solution.
Hemodynamic and Oxygenation Variables
Cardiopulmonary hemodynamics, blood gases, and acid-base balance were determined as described previously in detail (26). Vascular pressures were measured using pressure transducers (model PX-1800; Baxter, Edwards Critical-Care Division) connected to a hemodynamic monitor (model 78304A; Hewlett-Packard, Santa Clara, CA). All hemodynamic measurements were made in awake animals in the standing position. Zero calibrations were taken at the level of the olecranon joints of the front legs of the sheep. Cardiac output was measured by the thermodilution technique using a cardiac output computer (model COM-1; Baxter, Edward Critical-Care Division). A 5% dextrose solution was used as the indicator.
Blood gases and acid-base balance were measured using a blood gas analyzer (model IL 1600; Instrumentation Laboratory, Lexington, MA). Arterial and mixed venous blood gas results were corrected for the body temperature of the sheep. Oxyhemoglobin saturation and carboxyhemoglobin concentration were analyzed with a CO oximeter (model IL 482, Instrumentation Laboratory).
Lung Tissue Analyses
At the end of the 24-h study period, all animals were killed under deep ketamine anesthesia (15 mg/kg Ketanest; Fort Dodge Animal Health, Fort Dodge, IA) using 60 ml of saturated potassium chloride intravenously. Immediately thereafter, a necropsy was performed, and lung tissue samples were harvested for the subsequently described immunological, molecular biological, and histopathological investigations. Samples were either kept in formaldehyde solution or stored in cryo vials at −80°C until use.
Plasma and Serum Samples
Blood was sampled under sterile conditions at specific time points. It was then centrifuged and frozen for biochemical analysis at a later time point as described below.
Determination of NOS Activity
Total NOS activity was measured in lung tissue using a commercially available NOS activity assay kit (Cayman Chemical, Ann Arbor, MI) and evaluated as a conversion of [14C]arginine (Amersham Biosciences, Pittsburgh, PA) to [14C]citrulline as described previously (10).
Measurements of Plasma Supernatant Nitrates/Nitrites
The concentration of nitrate/nitrite (total amount of NO metabolites) in plasma was measured intermittently using a chemiluminescent NO analyzer (7020, Antek) and was recorded by dedicated software as the NO content using an established protocol (51).
Malondialdehyde Formation in Lung Homogenates
Malondialdehyde (MDA) formation was utilized to quantify the lipid peroxidation in the lung and measured as thiobarbituric acid reactive material. Lung tissue MDA levels were quantified with a commercially available assay (Northwest Life Science Specialties, Vancouver, WA). The level of lipid peroxides was expressed as MDA per milligram of protein, measured by a commercially available assay (Fluka BioChemika, Buchs, Switzerland) (41).
Western Blot Analysis for 3-Nitrotyrosine
For the determination of pulmonary 3-nitrotyrosine concentrations (3-NT), a reliable biomarker of peroxynitrate formation in vivo, homogenized tissue samples were analyzed using the Western blot technology as described previously in detail (25).
Immunohistochemistry Analysis for Poly(ADP-Ribose)
For the determination of poly(ADP-ribose) polymerase (PARP) activity, seven representative tissue sections of each animal were evaluated. Using immunohistochemistry, poly(ADP-ribose = PAR), the enzymatic product of PARP, was assessed (2). Immunohistological stainings were analyzed by densitometry as described previously (5).
Western Blotting for p65 (Rel A, Subunit of NF-κB)
For the determination of the heterodimeric transcription factor p65, tissue samples were homogenized preserving the nuclear extract that contains p65. Thereafter, the nuclear extract was processed for Western blot analysis for p65 using an established protocol (40).
ELISA for IL-8
Detection of IL-8 in the lung lymph was accomplished with a sandwich ELISA consisting of K221, a human monoclonal antibody that cross-reacts with sheep IL-8 (53) as the capture antibody and goat polyclonal anti-human IL-8 (R&D Systems, Minneapolis, MN) as the second antibody. The sandwich was detected by HRP-conjugated donkey anti-goat IgG (Jackson Laboratories). The lower detection limit for this assay is ∼20 pg/ml (38).
Lung Myeloperoxidase Activity
The activity of myeloperoxidase (MPO), an indicator of neutrophil accumulation, was determined directly in whole lung homogenates. MPO concentrations were evaluated on homogenized right lung with a commercially available assay. One unit of enzyme activity was defended as the amount of MPO present that caused a change in absorbance at 450 nm for 10 min. MPO activity was reported as U/g tissue (CytoStore, Calgary AB, Canada) (22).
Measurement of Tracheal Blood Flow
Tracheal blood flow was determined using colored microspheres. Approximately 5 million microspheres with a diameter of 15.0 ± 0.1 μm were injected into the left atrium at BL (preinjury), 3, 6, 12, 18, and 24 h, while reference blood was withdrawn from the femoral arterial catheter at a constant rate of 10 ml/min. The lower part of the trachea just above the carina (to which blood is supplied only by the bronchial artery) was harvested to quantify tracheal blood flow (37).
Airway Obstruction and Histopathological Score
The lower lobe of the right lung of each animal was excised and inflated with 10% formalin. Airway obstruction was quantified using a standardized protocol. Additionally, histopathological changes of the right lower lung lobe were analyzed, as described by Cox et al. (12).
Pulmonary Function
Pulmonary peak and pause pressures were measured at BL, 3, 6, 12, 18, and 24 h postinjury. In addition, the Horowitz Index (PaO2/FiO2 ratio) was determined and shunt blood flow (Qs/Qt) calculated using a standard shunt formula.
Statistical Analysis
Data are expressed as means ± SE, if not otherwise specified. GraphPad Prism was used for statistical analysis (18). After obtaining normality for all measurements using the Kolmogorov-Smirnov test for goodness-of-fit to normal distribution, a two-way ANOVA for repeated measurements with group and time as factors was applied. In case of significant group differences over time, appropriate post hoc comparisons (Bonferroni) were performed. The differences of COHb levels between groups were calculated using Student's unpaired t-test. For all statistical tests, an α-error probability of P < 0.05 was considered as statistically significant.
RESULTS
Injury and Survival
There were no differences between groups at baseline. Arterial COHb plasma levels measured immediately after the fourth set of cotton smoke insufflation were similar between the control group and the 7-NI group (76.0 ± 6.9 vs. 73.4 ± 7.7%; P = 0.8), confirming a consistent injury. In comparison, sham animals had arterial COHb concentrations of 5.9 ± 0.4%. With aggressive fluid challenge strictly following the Parkland formula, all sheep survived the 24-h study period.
NOS Enzyme Activity in Lung Tissue
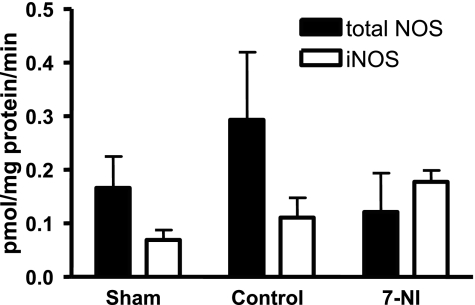
Albeit not statistically different, total NOS activity was doubled in injured control animals compared with the sham group. NOS activity of the injured group treated with 7-NI was similar to those of sham animals (sham vs. 7-NI P = 0.6, control vs. 7-NI P = 0.3; Fig. 1).
Fig. 1.
Mean total NOS enzyme activity and iNOS enzyme activity in lung tissue at death 24 h postinjury in pmol·mg protein−1·min−1 ± SE in the sham, control, and 7-nitroindazole (7-NI) groups. Within each group, the left column represents total NOS enzyme activity and the right column iNOS enzyme activity.
NOx Plasma Levels
NOx plasma levels increased significantly over time in the injured control group, peaking 12 h postinjury (16.4 ± 4.1 μmol/l). NOx plasma concentrations were significantly higher in the injured control group compared with sham animals (6.4 ± 0.9 μmol/l; P < 0.05). NOx levels of the 7-NI group did not differ from those of the sham group, but were statistically different to the control group, indicating the blockage of NO production by 7-NI (8.4 ± 1.1 μmol/l; control vs. 7-NI P < 0.05).
MDA Lung Tissue Concentration
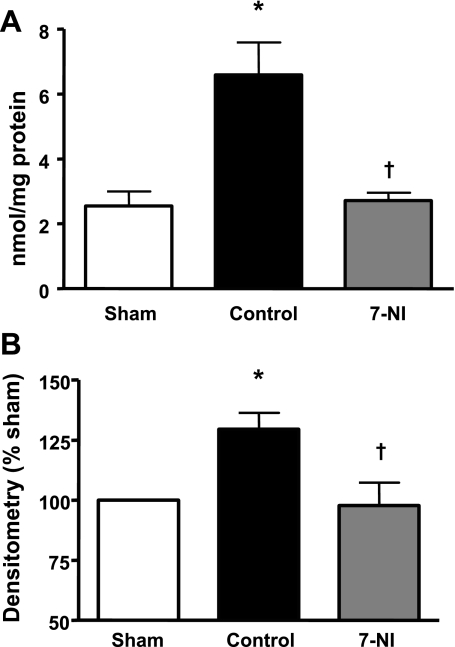
Lung tissue MDA concentration was almost threefold higher in the injured control group compared with the sham group and significantly reduced in the 7-NI group (Fig. 2A).
Fig. 2.
Malondialdehyde (MDA) (nmol/mg protein ± SE; A) and 3-nitrotyrosine (3-NT) (densitometry % sham; B) in lung tissue at death 24 h postinjury in the sham, control, and 7-NI groups. Data are expressed as means ± SE. *P < 0.05 vs. sham; †P < 0.05 vs. control.
3-NT Lung Tissue Content
The injured control group had a significantly higher lung tissue 3-NT concentration than the sham group. The pulmonary 3-NT content in the 7-NI group, however, was significantly lower than in the control group, but not statistically different from the sham group (Fig. 2B).
PARP Activation
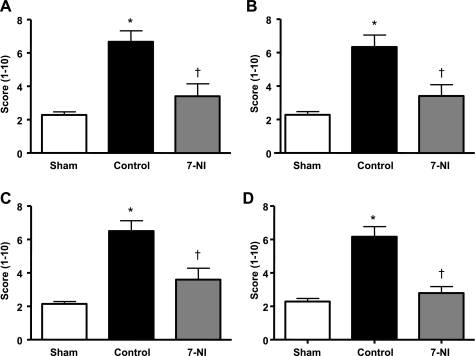
Combined burn and smoke inhalation injury was associated with a significant increase in PARP activation in airway, pulmonary glands, alveoli, and pulmonary vessels compared with the sham group. This activation was significantly reduced in animals allocated to the 7-NI group (Fig. 3).
Fig. 3.
PAR in airway (A), pulmonary glands (B), lung alveoli (C), and pulmonary vessels (D) at death 24 h postinjury, expressed in a mean score ± SE in the sham, control, and 7-NI groups. *P < 0.05 vs. sham; †P < 0.05 vs. control.
Presence of p65 (Rel A) in the Nuclei of Lung Parenchymal Cells
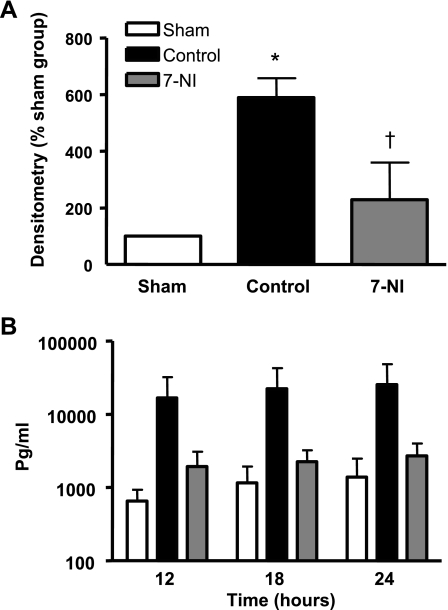
A significantly increased amount of p65, a subunit of NF-κB, was found in the control group compared with sham animals. 7-NI infusion significantly inhibited nuclear p65 levels (Fig. 4A).
Fig. 4.
A: p65 in lung tissue at death 24 h after burn and smoke inhalation injury in densitometry % sham in the sham, control, and 7-NI groups. *P < 0.05 vs. sham; †P < 0.05 vs. control. B: IL-8 (pg/ml) in lung lymph at 12, 18, and 24 h postinjury. Data are expressed as means ± SE. At each time point, the left column represents the sham group, the middle column the control, and the right column the 7-NI group.
IL-8 Protein Concentration in Lung Lymph (Transvascular Fluid)
The combination injury was linked to a three- to fourfold increase in IL-8 lung lymph concentrations between 12 and 24 h postinjury. Infusion of 7-NI markedly attenuated this response (Fig. 4B).
MPO Activity in Lung Tissue
MPO enzyme activity was significantly higher in the injured untreated control group (5.7 ± 0.7 U/g tissue) compared with animals allocated to the sham group (2.8 ± 0.2 U/g tissue; P < 0.01). In injured animals treated with 7-NI, MPO was significantly lower compared with the control group (3.9 ± 0.2 U/g tissue; P < 0.05).
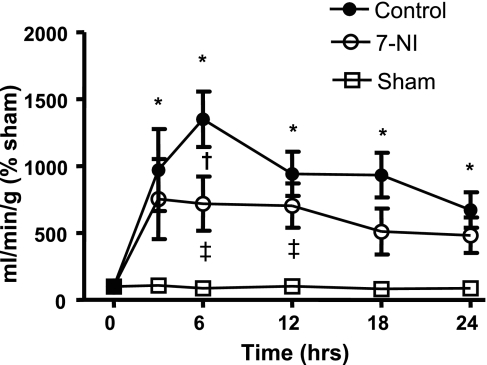
Tracheal Blood Flow
In the untreated control group, tracheal blood flow was significantly increased after burn and smoke inhalation compared with the uninjured sham group. However, this increase was significantly attenuated by 7-NI treatment (Fig. 5).
Fig. 5.
Trachea blood flow in ml/min/g % sham at 0, 3, 6, 12, 18, and 24 h after injury in the sham, control, and 7-NI groups. Data are presented as means ± SE. *P < 0.05 vs. sham; †P < 0.05 vs. control; ‡P < 0.05 vs. 7-NI.
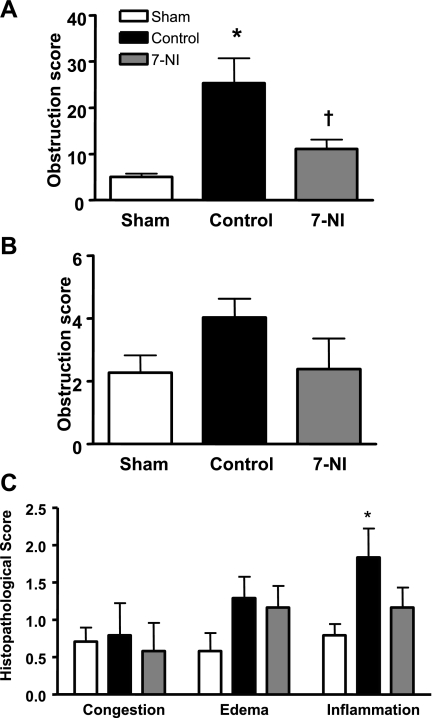
Airway Obstruction and Histopathology Score
Whereas there was little evidence of airway obstruction in the sham group, the injured control group was characterized by a fivefold and twofold obstruction of bronchi and bronchioles, respectively. Continuous iv infusion of 7-NI significantly reduced the degree of injury in bronchi. Similarly, the histopathological changes induced by the injury were alleviated by 7-NI (Fig. 6, A–C).
Fig. 6.
Obstruction score in bronchi (A) and bronchioles (B), as well as histopathological score (C), in lung tissue at death 24 h postinjury in the sham, control, and 7-NI groups. Data are expressed as means ± SE. *P < 0.05 vs. sham; †P < 0.05 vs. control.
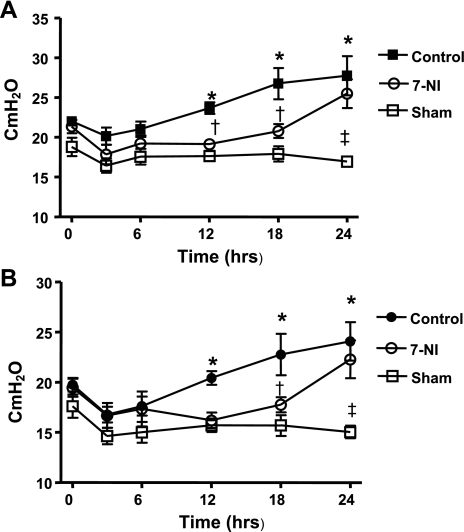
Airway Pressures
The peak and pause airway pressures were stable in sham-operated animals, but were significantly increased in the control group from 12 h postinjury until the end of the experiment. In contrast, a significant reduction of airway pressures was observed in the group treated with 7-NI compared with the control group (Fig. 7, A and B).
Fig. 7.
Peak (A) and pause (B) ventilatory pressures in cmH2O ± SE at 0, 3, 6, 12, 18, and 24 h after injury in the sham, control, and 7-NI groups. *P < 0.05 vs. sham; †P < 0.05 vs. control; ‡P < 0.05 vs. 7-NI.
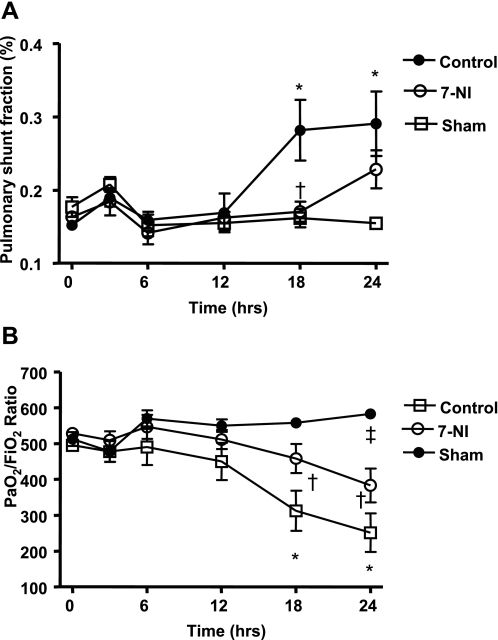
Pulmonary Function
In this study, Qs/Qt was significantly increased in the control group from 18 h after injury and was accompanied by a reciprocal decrease in PaO2/FiO2 ratio compared with sham animals. 7-NI limited pulmonary shunting and the drop in PaO2/FiO2 ratio noticed in the control group (Fig. 8, A and B).
Fig. 8.
Pulmonary shunt fraction (Qs/Qt) (A) and PaO2/FiO2 ratio (B) at 0, 3, 6, 12, 18, and 24 h postinjury in the sham, control, and 7-NI groups. Data are expressed as means ± SE. *P < 0.05 vs. sham; †P < 0.05 vs. control; ‡P < 0.05 vs. 7-NI.
Hemodynamic Variables
Hemodynamic variables were similar among groups, except for an increase in mean pulmonary artery pressure in control animals at 24 h postinjury (P < 0.05 vs. sham) and a transient increase in CI in the sham group (P < 0.05 vs. control and 7-NI at 3 h postinjury). Notably, none of the hemodynamic variables of the sham group treated with 7-NI were significantly different from the untreated sham group (Table 1).
Table 1.
Hemodynamic variables of the systemic and pulmonary circulation of the sham, control, and 7-NI groups
| Baseline | 3 h | 6 h | 12 h | 18 h | 24 h | |
|---|---|---|---|---|---|---|
| Mean arterial pressure, mmHg | ||||||
| Sham | 108 ± 3 | 112 ± 4 | 114 ± 4 | 112 ± 3 | 104 ± 2 | 105 ± 2 |
| B/S Control | 93 ± 3 | 98 ± 5 | 99 ± 4 | 99 ± 5 | 98 ± 6 | 96 ± 5 |
| 7-NI | 101 ± 5 | 112 ± 6 | 111 ± 5 | 108 ± 5 | 102 ± 5 | 103 ± 7 |
| Sham 7-NI | 105 ± 5 | 105 ± 6 | 106 ± 3 | 104 ± 5 | 104 ± 3 | 105 ± 5 |
| Mean pulmonary artery pressure, mmHg | ||||||
| Sham | 20 ± 0.7 | 23 ± 0.3 | 24 ± 0.2 | 24 ± 0.5 | 24 ± 0.3 | 23 ± 0.8 |
| B/S Control | 20 ± 0.6 | 23 ± 0.4 | 24 ± 0.8 | 25 ± 0.8 | 27 ± 1.8 | 28 ± 2.1† |
| 7-NI | 20 ± 0.8 | 24 ± 1.3 | 27 ± 0.8 | 28 ± 1.6 | 27 ± 1.6 | 27 ± 1.3 |
| Sham 7-NI | 20 ± 1.0 | 25 ± 1.7 | 26 ± 1.1 | 27 ± 1.3 | 24 ± 2.3 | 25 ± 1.1 |
| Systemic vascular resistance index (dynes·s·cm−5m2) | ||||||
| Sham | 1,279 ± 34 | 1,173 ± 63 | 1,287 ± 71 | 1,376 ± 82 | 1,320 ± 56 | 1,294 ± 33 |
| B/S Control | 1,100 ± 43 | 1,380 ± 114 | 1,241 ± 117 | 1,290 ± 175 | 1,134 ± 93 | 1,203 ± 98 |
| 7-NI | 1,294 ± 121 | 1,536 ± 183 | 1,305 ± 92 | 1,319 ± 102 | 1,285 ± 134 | 1,404 ± 271 |
| Sham 7-NI | 1,293 ± 39 | 1,143 ± 79 | 1,321 ± 42 | 1,384 ± 45 | 1,409 ± 50 | 1,363 ± 105 |
| Pulmonary vascular resistance index (dynes·s·cm−5m2) | ||||||
| Sham | 99 ± 6 | 94 ± 5 | 120 ± 3 | 125 ± 9 | 139 ± 9 | 118 ± 7 |
| B/S Control | 136 ± 16 | 140 ± 13 | 150 ± 16 | 178 ± 32 | 180 ± 37 | 204 ± 39† |
| 7-NI | 133 ± 11 | 157 ± 10 | 147 ± 7 | 166 ± 21 | 171 ± 34 | 179 ± 37 |
| Sham 7-NI | 105 ± 11 | 128 ± 28 | 143 ± 14 | 182 ± 8 | 149 ± 57 | 168 ± 39 |
| Cardiac index (l/min/m2) | ||||||
| Sham | 6 ± 0.1 | 7 ± 0.2*‡ | 7 ± 0.2 | 6 ± 0.3 | 6 ± 0.2 | 6 ± 0.1 |
| B/S Control | 6 ± 0.2 | 5 ± 0.3 | 6 ± 0.4 | 6 ± 0.6 | 7 ± 0.7 | 6 ± 0.5 |
| 7-NI | 6 ± 0.4 | 5 ± 0.4 | 6 ± 0.4 | 6 ± 0.5 | 6 ± 0.6 | 6 ± 0.8 |
| Sham 7-NI | 6.1 ± 0.1 | 6.8 ± 0.3 | 5.9 ± 0.1 | 5.4 ± 0.2 | 5.3 ± 0.3 | 5.4 ± 0.2 |
Data are expressed as means ± SE;
P < 0.05 vs. sham;
P < 0.05 vs. control;
P < 0.05 vs. 7-NI.
DISCUSSION
The present study investigated the molecular biological effects of nNOS inhibition in the common setting of combined burn and smoke inhalation injury using an established and clinically relevant ovine model of ALI (14, 41). The major findings were that nNOS-derived NO was identified as a precursor of RNS, subsequently activating PARP and contributing to inflammatory signaling via the NF-κB pathway. This study clearly shows that nNOS activation plays an early and central role in the pathophysiology of ALI resulting from combined burn and smoke inhalation injury. Postinjury infusion of the selective nNOS inhibitor 7-NI inhibited the inflammatory cascade, as demonstrated by a 40% and 30% decrease in IL-8 and MPO lung tissue concentrations compared with the injured control group. These molecular changes were linked to significant attenuation of pulmonary dysfunction typically seen in our large animal model of combined burn and smoke inhalation injury (43). As such, pulmonary tissue damage and airway obstruction were significantly attenuated and associated with less pulmonary shunting and improved gas exchange vs. untreated controls.
Considering that there are more than 1 million fire victims in the U.S. alone (16) and that such injury is associated with an unacceptably high morbidity and mortality, both further understanding of the underlying pathophysiology and the evaluation of innovative treatment strategies are essential to improve outcome in the future (34, 42). In this context, it is noteworthy that the ovine model used in the present study creates an inflammatory response that is similar to the one noted in burn patients (11). Another important feature of this model is that the hemodynamic stability allows for invasive cardiovascular monitoring for many hours. Evidently, the ovine animal model has substantially contributed to the investigation of combined burn and smoke inhalation injury (19, 24, 26, 27, 36). For this study, we have chosen a 24-h period, since our aim was to identify the molecular mechanisms resulting within 1 day after combined burn and smoke inhalation injury. Since we hypothesized that nNOS-derived NO plays a key role in the pathogenesis of pulmonary dysfunction in this model, it was an additional objective to determine the value of selective nNOS inhibition when started early in the course of the disease.
There are several molecular mechanisms involved in the pathophysiology of ALI that are of specific interest. In the present study, we measured NOS enzyme activity and noticed that in injured, untreated controls, total NOS activity including nNOS, was almost doubled compared with both sham animals and the injury group treated with 7-NI. Although the raise in total NOS activity was not statistically different among groups, the total NOS level almost doubled in the injured control group compared with the sham group. Notably, total NOS in the 7-NI group was similar to, or even lower than, that of sham animals. One reason for the lack of significance in the NOS data could be that the measurement of NOS at 24 h postinjury was already too late in the time course and that the peak of total NOS activity including nNOS was already missed. Since the peak plasma concentration of nitrate/nitrite occurred at 12 h, total NOS activity could have been at its maximum before.
As previously reported (15, 52) the IC50 for iNOS inhibition is not reached in sheep treated with 7-NI in a dose of 1 mg·kg−1·h−1. In this regard, it is also important to note that arterial blood pressure and systemic vascular resistance remained unchanged in response to 7-NI infusion (data shown in Table 1), thus making in vivo inhibition of eNOS and iNOS unlikely (6). 7-NI was also given to healthy, uninjured sham animals, and all of the hemodynamic variables obtained were not different from the untreated sham group. This finding is in line with the assumption that the nNOS inhibitor used for this study caused no obvious adverse effects (Table 1).
In the present study, administration of 7-NI inhibited not only nitrate/nitrite production, but also the formation of RNS. It is of note that excessively produced NO typically reacts with ROS, such as superoxide, deriving from NADPH oxidase and neutrophils. By degradation of polyunsaturated lipids, ROS in turn contribute to MDA formation, a reactive aldehyde causative for cellular stress (55). In this context, it is another interesting finding of the present study that MDA was markedly increased in control animals compared with the sham and the 7-NI group.
Superoxide may also react with NO, thereby leading to peroxynitrite production, a potent oxidant and key mediator of NO-mediated tissue injury (46). When excessive amounts of NO and subsequently ONOO− are present, nitrosylation of the amino acid tyrosine occurs in the cell plasma, reflecting cellular and tissue damage by ONOO− (55). In the current study, we were able to quantify 3-NT lung tissue content, the in vivo biomarker of ONOO− production (35). In fact, the injury was associated with a significantly higher nitrosylation of the amino acid tyrosine compared with the sham group. Notably, this effect was entirely blocked in the 7-NI group.
ONOO− has been identified as a potent contributor to DNA single-strand breaks (47). DNA damage, in turn, is typically associated with upregulation of PARP, trying to reverse, or at least limit, cellular damage on the protein and DNA level (3). To test PARP activity, the enzymatic product of PARP, i.e., PAR, was determined in the present study. The immunohistological stains showed a twofold increased PAR in the control group vs. sham animals. This effect was completely reversed by 7-NI infusion. PARP, however, may be considered a “suicide enzyme” because its activation typically contributes to cellular ATP depletion, thereby promoting cell death (20). In this regard, we have previously shown that inhibition of PARP activity attenuates pulmonary dysfunction in ovine ALI (41). Interestingly, PARP inhibition also resulted in reduced iNOS mRNA expression and plasma NOx levels, suggesting that PARP may be responsible for activation of iNOS and formation of ROS/RNS (30).
PARP is also involved in inflammatory signaling via the NF-κB pathway (1, 54). Notably, NF-κB plays a key role in the production of cytokines and chemokines, such as IL-8. In the present study, Western blotting for the subunit p65 (also referred to as Rel A) was fivefold increased in the control group vs. sham and was markedly reduced in sheep treated with 7-NI. We also noticed a 10-fold increase in IL-8 concentrations in pulmonary lymph in the untreated injury group compared with uninjured controls. Importantly, IL-8 concentrations were markedly attenuated in sheep subjected to 7-NI infusion. The fact that high IL-8 concentrations are typically present in the bronchoalveolar lavage fluid and lung edema fluid of patients with ALI/ARDS suggests that IL-8 is critically involved in the migration of activated neutrophils towards the lesion (32). These activated neutrophils release degradative metabolites, such as MPO and ROS/RNS, to cause inflammatory tissue injury (33). In our study, MPO was measured as a marker of neutrophil accumulation in lung tissue at death. Whereas MPO levels were markedly elevated in the control group compared with sham animals, it was significantly blocked in the 7-NI group.
Excessive NO production also contributes to increased tracheal blood flow, the main source of obstructive tracheobronchial cast formation. In the setting of burn and smoke injury, this obstructive pulmonary material typically leaks across the damaged microvascular barrier, thereby deteriorating gas exchange (16). In this study, the injured control group showed significantly elevated tracheal blood flow, as well as airway obstruction, compared with sham animals. 7-NI treatment prevented these consequences by attenuating the increase in tracheal blood flow.
In the burn and smoke control group, bronchial occlusion was associated with elevated pulmonary peak and pause pressures. In addition, pulmonary shunting was more pronounced in the injured control group compared with sham animals. The increase in shunt blood flow, in turn, was inversely correlated with PaO2/FiO2 ratio. All those pathophysiological changes were markedly reduced by 7-NI treatment.
This study has some limitations that we would like to acknowledge. Since this experiment was conducted in large animals, it remains undetermined whether or not early nNOS inhibition is likewise efficient in patients suffering from ALI secondary to burn and smoke inhalation. Because we aimed to investigate the molecular mechanisms of ALI in the early phase of burn and smoke inhalation injury, this study was limited to a 24-h period. Future studies should determine whether the impressive effects resulting from selective nNOS inhibition also translate into an overall reduction in mortality. Given that early 7-NI infusion is associated with a survival benefit, clinical trials are warranted.
In summary, this is the first study elucidating the molecular mechanisms by which increased nNOS activity contributes to pulmonary dysfunction in sheep with ALI. Our findings provide strong evidence that nNOS plays a central role in the pathophysiology of combined burn and smoke inhalation injury. In view of the current findings, it appears that selective nNOS inhibition represents a useful approach to attenuate the degree of pulmonary injury without obvious adverse effects.
GRANTS
This study was supported by Shriners of North America Grants SHCG-8450 and 8954 and National Institute of General Medical Sciences Grants GM-060688 and GM-066312.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank the laboratory team for expert technical assistance during the experiment. D. L. Traber is the Charles Robert Allen Professor of Anesthesiology, Univ. of Texas Medical Branch, Galveston, TX.
REFERENCES
- 1.Adaikalakoteswari A, Rema M, Mohan V, Balasubramanyam M. Oxidative DNA damage and augmentation of poly(ADP-ribose) polymerase/nuclear factor-kappa B signaling in patients with type 2 diabetes and microangiopathy. Int J Biochem Cell Biol 39: 1673–1684, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bakondi E, Bai P, Szabo EE, Hunyadi J, Gergely P, Szabo C, Virag L. Detection of poly(ADP-ribose) polymerase activation in oxidatively stressed cells and tissues using biotinylated NAD substrate. J Histochem Cytochem 50: 91–98, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Barth E, Radermacher P, Szabo C. The world according to poly(ADP-ribose) polymerase (PARP)–update 2006. Intensive Care Med 32: 1470–1474, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Baxter CR, Shires T. Physiological response to crystalloid resuscitation of severe burns. Ann NY Acad Sci 150: 874–894, 1968 [DOI] [PubMed] [Google Scholar]
- 5.Beller CJ, Radovits T, Kosse J, Gero D, Szabo C, Szabo G. Activation of the peroxynitrite-poly(adenosine diphosphate-ribose) polymerase pathway during neointima proliferation: a new target to prevent restenosis after endarterectomy. J Vasc Surg 43: 824–830, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Bland-Ward PA, Moore PK. 7-Nitro indazole derivatives are potent inhibitors of brain, endothelium and inducible isoforms of nitric oxide synthase. Life Sci 57: PL131–PL135, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347: 768–770, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee A, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul Pharmacol 49: 134–140, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Medhora M, Falck, Pritchard KA, Jr, Jacobs ER. Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 291: L378–L385, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Cho HJ, Xie QW, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med 176: 599–604, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox RA, Burke AS, Oliveras G, Enkhbaatar P, Traber LD, Zwischenberger JB, Jeschke MG, Schmalstieg FC, Herndon DN, Traber DL, Hawkins HK. Acute bronchial obstruction in sheep: histopathology and gland cytokine expression. Exp Lung Res 31: 819–837, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Cox RA, Burke AS, Soejima K, Murakami K, Katahira J, Traber LD, Herndon DN, Schmalstieg FC, Traber DL, Hawkins HK. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol 29: 295–302, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Demchenko IT, Atochin DN, Gutsaeva DR, Godfrey RR, Huang PL, Piantadosi CA, Allen BW. Contributions of nitric oxide synthase isoforms to pulmonary oxygen toxicity, local vs. mediated effects. Am J Physiol Lung Cell Mol Physiol 294: L984–L990, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, Traber L, Phillips G, Parkinson J, Salsbury JR, Biondo N, Schmalstieg F, Burke A, Cox R, Hawkins H, Herndon D, Traber D. Inducible nitric oxide synthase dimerization inhibitor prevents cardiovascular and renal morbidity in sheep with combined burn and smoke inhalation injury. Am J Physiol Heart Circ Physiol 285: H2430–H2436, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Enkhbaatar P, Murakami K, Traber LD, Cox R, Parkinson JF, Westphal M, Esechie A, Morita N, Maybauer MO, Maybauer DM, Burke AS, Schmalstieg FC, Hawkins HK, Herndon DN, Traber DL. The inhibition of inducible nitric oxide synthase in ovine sepsis model. Shock 25: 522–527, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Enkhbaatar P, Traber DL. Pathophysiology of acute lung injury in combined burn and smoke inhalation injury. Clin Sci (Lond) 107: 137–143, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Fischer A, Hoffmann B. Nitric oxide synthase in neurons and nerve fibers of lower airways and in vagal sensory ganglia of man. Correlation with neuropeptides. Am J Respir Crit Care Med 154: 209–216, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Hamahata A, Enkhbaatar P, Kraft ER, Lange M, Leonard SW, Traber MG, Cox RA, Schmalstieg FC, Hawkins HK, Whorton EB, Horvath EM, Szabo C, Traber LD, Herndon DN, Traber DL. gamma-Tocopherol nebulization by a lipid aerosolization device improves pulmonary function in sheep with burn and smoke inhalation injury. Free Radic Biol Med 45: 425–433, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamahata A, Enkhbaatar P, Sakurai H, Nozaki M, Traber DL. Effect of ablated bronchial blood flow on survival rate and pulmonary function after burn and smoke inhalation in sheep. Burns 35: 802–810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hegedus C, Lakatos P, Olah G, Toth BI, Gergely S, Szabo E, Biro T, Szabo C, Virag L. Protein kinase C protects from DNA damage-induced necrotic cell death by inhibiting poly(ADP-ribose) polymerase-1. FEBS Lett 582: 1672–1678, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiroi Y, Guo Z, Li Y, Beggs AH, Liao JK. Dynamic regulation of endothelial NOS mediated by competitive interaction with alpha-actinin-4 and calmodulin. FASEB J 22: 1450–1457, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollingsworth JW, Chen BJ, Brass DM, Berman K, Gunn MD, Cook DN, Schwartz DA. The critical role of hematopoietic cells in lipopolysaccharide-induced airway inflammation. Am J Respir Crit Care Med 171: 806–813, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kimura R, Traber LD, Herndon DN, Linares HA, Lubbesmeyer HJ, Traber DL. Increasing duration of smoke exposure induces more severe lung injury in sheep. J Appl Physiol 64: 1107–1113, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Lange M, Enkhbaatar P, Traber DL, Cox RA, Jacob S, Mathew BP, Hamahata A, Traber LD, Herndon DN, Hawkins HK. Role of calcitonin gene-related peptide (CGRP) in ovine burn and smoke inhalation injury. J Appl Physiol 107: 176–184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maybauer MO, Maybauer DM, Fraser JF, Traber LD, Westphal M, Enkhbaatar P, Cox RA, Huda R, Hawkins HK, Morita N, Murakami K, Mizutani A, Herndon DN, Traber DL. Recombinant human activated protein C improves pulmonary function in ovine acute lung injury resulting from smoke inhalation and sepsis. Crit Care Med 34: 2432–2438, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Maybauer MO, Maybauer DM, Traber LD, Westphal M, Enkhbaatar P, Morita N, Jodoin JM, Heggers JP, Herndon DN, Traber DL. Gentamicin improves hemodynamics in ovine septic shock after smoke inhalation injury. Shock 24: 226–231, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Maybauer MO, Rehberg S, Traber DL, Herndon DN, Maybauer DM. [Pathophysiology of acute lung injury in severe burn and smoke inhalation injury]. Anaesthesist 58: 805–812, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Meyer WJ, Patterson DR, Jaco M, Woodson L, Thomas C. Management of pain and other discomforts in burned patients. In: Total Burn Care, edited by Herndon D. Philadelphia, PA: Saunders Elsevier, 2007, p. 797–818. [Google Scholar]
- 29.Mlcak RP, Suman OE, Herndon DN. Respiratory management of inhalation injury. Burns 33: 2–13, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Murakami K, Enkhbaatar P, Shimoda K, Cox RA, Burke AS, Hawkins HK, Traber LD, Schmalstieg FC, Salzman AL, Mabley JG, Komjati K, Pacher P, Zsengeller Z, Szabo C, Traber DL. Inhibition of poly (ADP-ribose) polymerase attenuates acute lung injury in an ovine model of sepsis. Shock 21: 126–133, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Murakami K, Enkhbaatar P, Yu YM, Traber LD, Cox RA, Hawkins HK, Tompkins RG, Herndon D, Traber DL. l-Arginine attenuates acute lung injury after smoke inhalation and burn injury in sheep. Shock 28: 477–483, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Nys M, Deby-Dupont G, Habraken Y, Legrand-Poels S, Kohnen S, Ledoux D, Canivet JL, Damas P, Lamy M. Bronchoalveolar lavage fluids of ventilated patients with acute lung injury activate NF-kappaB in alveolar epithelial cell line: role of reactive oxygen/nitrogen species and cytokines. Nitric Oxide 9: 33–43, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Nys M, Deby-Dupont G, Habraken Y, Legrand-Poels S, Ledoux D, Canivet JL, Damas P, Lamy M. Bronchoalveolar lavage fluids of patients with lung injury activate the transcription factor nuclear factor-kappaB in an alveolar cell line. Clin Sci (Lond) 103: 577–585, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Pruitt BA, Wolf SE, Mason AD. Epidemiological, demographic, and outcome characteristics of burn injury. In: Total Burn Care (3rd ed.), edited by Herndon D. Philadelphia, PA: Saunders Elsevier, 2007, p. 14–32 [Google Scholar]
- 35.Rawlingson A, Greenacre SA, Brain SD. Generation of peroxynitrite in localised, moderate temperature burns. Burns 26: 223–227, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Sakurai H, Soejima K, Schmalstieg FC, Nozaki M, Traber DL. Inhibition of lung permeability changes after burn and smoke inhalation by an anti-interleukin-8 antibody in sheep. Surg Today 39: 399–406, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Schenarts PJ, Bone HG, Traber LD, Traber DL. Effect of severe smoke inhalation injury on systemic microvascular blood flow in sheep. Shock 6: 201–205, 1996 [PubMed] [Google Scholar]
- 38.Schmalstieg FC, Chow J, Savage C, Rudloff HE, Palkowetz KH, Zwischenberger JB. Interleukin-8, aquaporin-1, and inducible nitric oxide synthase in smoke and burn injured sheep treated with percutaneous carbon dioxide removal. ASAIO J 47: 365–371, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Schwela DH, Goldammer JG, Morawska LH, Simpson O. Health guidelines for vegetation fire events. Guideline Document Singapore: Published on behalf of UNEP, WHO & WMO, 1999 [Google Scholar]
- 40.Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterology 129: 1518–1532, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Shimoda K, Murakami K, Enkhbaatar P, Traber LD, Cox RA, Hawkins HK, Schmalstieg FC, Komjati K, Mabley JG, Szabo C, Salzman AL, Traber DL. Effect of poly(ADP ribose) synthetase inhibition on burn and smoke inhalation injury in sheep. Am J Physiol Lung Cell Mol Physiol 285: L240–L249, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg 205: 82–87, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soejima K, Schmalstieg FC, Sakurai H, Traber LD, Traber DL. Pathophysiological analysis of combined burn and smoke inhalation injuries in sheep. Am J Physiol Lung Cell Mol Physiol 280: L1233–L1241, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Soejima K, Traber LD, Schmalstieg FC, Hawkins H, Jodoin JM, Szabo C, Szabo E, Virag L, Salzman A, Traber DL. Role of nitric oxide in vascular permeability after combined burns and smoke inhalation injury. Am J Respir Crit Care Med 163: 745–752, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Staub NC, Bland RD, Brigham KL, Demling R, Erdmann AJ, 3rd, Woolverton WC. Preparation of chronic lung lymph fistulas in sheep. J Surg Res 19: 315–320, 1975 [DOI] [PubMed] [Google Scholar]
- 46.Szabo C. Poly(ADP-ribose) polymerase activation by reactive nitrogen species–relevance for the pathogenesis of inflammation. Nitric Oxide 14: 169–179, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Szabo C, Zingarelli B, O'Connor M, Salzman AL. DNA strand breakage, activation of poly (ADP-ribose) synthetase, and cellular energy depletion are involved in the cytotoxicity of macrophages and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad Sci USA 93: 1753–1758, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Traber DL, Hawkins HK, Enkhbaatar P, Cox RA, Schmalstieg FC, Zwischenberger JB, Traber LD. The role of the bronchial circulation in the acute lung injury resulting from burn and smoke inhalation. Pulm Pharmacol Ther 20: 163–166, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Traber MG, Shimoda K, Murakami K, Leonard SW, Enkhbaatar P, Traber LD, Traber DL. Burn and smoke inhalation injury in sheep depletes vitamin E: kinetic studies using deuterated tocopherols. Free Radic Biol Med 42: 1421–1429, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss SM, Lakshminarayan S. Acute inhalation injury. Clin Chest Med 15: 103–116, 1994 [PubMed] [Google Scholar]
- 51.Westphal M, Cox RA, Traber LD, Morita N, Enkhbaatar P, Schmalstieg FC, Hawkins HK, Maybauer DM, Maybauer MO, Murakami K, Burke AS, Westphal-Varghese BB, Rudloff HE, Salsbury JR, Jodoin JM, Lee S, Traber DL. Combined burn and smoke inhalation injury impairs ovine hypoxic pulmonary vasoconstriction. Crit Care Med 34: 1428–1436, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Westphal M, Enkhbaatar P, Schmalstieg FC, Kulp GA, Traber LD, Morita N, Cox RA, Hawkins HK, Westphal-Varghese BB, Rudloff HE, Maybauer DM, Maybauer MO, Burke AS, Murakami K, Saunders F, Horvath EM, Szabo C, Traber DL. Neuronal nitric oxide synthase inhibition attenuates cardiopulmonary dysfunctions after combined burn and smoke inhalation injury in sheep. Crit Care Med 36: 1196–1204, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Yang XD, Corvalan JR, Wang P, Roy CM, Davis CG. Fully human anti-interleukin-8 monoclonal antibodies: potential therapeutics for the treatment of inflammatory disease states. J Leukoc Biol 66: 401–410, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Zerfaoui M, Suzuki Y, Naura AS, Hans CP, Nichols C, Boulares AH. Nuclear translocation of p65 NF-kappaB is sufficient for VCAM-1, but not ICAM-1, expression in TNF-stimulated smooth muscle cells: differential requirement for PARP-1 expression and interaction. Cell Signal 20: 186–194, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109: 817–826, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]