Abstract
Nitric oxide signaling has an important role in regulating pulmonary development and function. Expression of soluble guanylate cyclase (sGC) and cGMP-dependent protein kinase I (PKGI), both critical mediators of nitric oxide (NO) signaling, is diminished in the injured newborn lung through unknown mechanisms. Recent studies suggest that excessive transforming growth factor-β (TGF-β) activity inhibits injured newborn lung development. To explore mechanisms that regulate pulmonary NO signaling, we tested whether TGF-β decreases sGC and PKGI expression in the injured developing lung and pulmonary vascular smooth muscle cells (SMC). We found that chronic oxygen-induced lung injury decreased pulmonary sGCα1 and PKGI immunoreactivity in mouse pups and that exposure to a TGF-β-neutralizing antibody prevented this reduction of sGC and PKGI protein expression. In addition, TGF-β1 decreased expression of NO signaling enzymes in freshly isolated pulmonary microvascular SMC/myofibroblasts, suggesting that TGF-β has a direct role in modulating NO signaling in the pup lung. Moreover, TGF-β1 decreased sGC and PKGI expression in pulmonary artery and aortic SMC from adult rats and mice, suggesting a general role for TGF-β in modulating NO signaling in vascular SMC. Although other cytokines decrease sGC mRNA stability, TGF-β did not modulate sGCα1 or PKGIβ mRNA turnover in vascular SMC. These studies indicate for the first time that TGF-β decreases NO signaling enzyme expression in the injured developing lung and pulmonary vascular SMC. Moreover, they suggest that TGF-β-neutralizing molecules might counteract the effects of injury on NO signaling in the newborn lung.
Keywords: soluble guanylate cyclase, cGMP-dependent protein kinase I, bronchopulmonary dysplasia, hyperoxia
nitric oxide (NO) and cGMP have an important role in regulating pulmonary vascular tone and development. NO produced by nitric oxide synthase (NOS) in pulmonary endothelial cells diffuses into subjacent smooth muscle cells (SMC) where it stimulates soluble guanylate cyclase (sGC) to increase cGMP production. Although cGMP interacts with several proteins in SMC, cGMP regulates pulmonary vascular tone primarily by stimulating cGMP-dependent protein kinase I (PKGI). Cyclic GMP-activated PKGI phosphorylates several cytosolic protein targets that regulate intracellular Ca2+ levels, the calcium sensitivity of the contraction apparatus, and thin filament proteins and thereby cause vasodilatation (45). Emerging evidence suggests that cGMP also regulates the expression of genes that modulate cell phenotype (56). NO inhibits the proliferation and increases the apoptosis of pulmonary artery SMC (PASMC) primarily by increasing sGC-mediated cGMP production and cGMP-stimulated PKGI activity (15). Although the mechanisms through which PKGI regulates SMC phenotype are not well-understood, recent evidence suggests that cGMP-stimulated PKGI proteolysis yields an active kinase fragment (PKGIγ) that translocates into the nucleus where it phosphorylates transcription regulators that modulate gene expression (67). The role of NO and cGMP in regulating lung development is supported by studies in which increased NO levels promoted branching of early fetal rat lung explants while decreased levels had the opposite effect, inhibiting branching of nascent airways (84). In addition, decreased NO production inhibited pulmonary alveolar and microvascular development in the newborn mouse (30). Although studies suggest that NO nitrosylates proteins that might regulate lung function (31), a balance of available evidence indicates that NO-stimulated cGMP signaling has a critical role in modulating pulmonary development.
Injury of the developing lung decreases the expression of sGC and PKGI, which critically mediate pulmonary NO and cGMP signaling. For example, prenatal flow- and pressure-induced lung injury decreases sGC expression and activity in the lamb (5, 66, 73, 81). In addition, postnatal ventilator- and oxygen-induced injury reduces sGC levels in the pulmonary arteries of prematurely born lambs (7). Although less is known about the effects of lung injury on PKGI, hypoxia has been observed to decrease PKGI expression and activity in isolated fetal lamb pulmonary vessels (26) and PKGI protein expression in SMC obtained from fetal lamb pulmonary veins (86). Moreover, pulmonary hypertension decreases PKGI expression levels in PASMC isolated from fetal lambs (59). The part that NO and cGMP signaling plays in modulating pulmonary development is further supported by studies indicating that inhaled NO improves alveologenesis and vascular development of injured animal lungs (6, 44, 50, 60, 61, 69). It is likely that understanding the mechanisms that decrease the expression of NO signaling enzymes in the injured newborn lung will permit the identification of novel therapeutic targets for protecting or improving pulmonary development.
Transforming growth factor-β (TGF-β) also has an important function in regulating lung development (79). TGF-β is secreted by several cells in the lung in an inactive form that associates with extracellular matrix proteins. Proteolytic cleavage of this latent form releases active TGF-β, which binds to surface receptors on several cell types. TGF-β receptor binding stimulates intracellular signaling, activating both the canonical Smad-dependent pathway and other, less specific signaling pathways, to modulate expression of genes that regulate cellular phenotype and proliferation (29). Recent studies suggest that excessive pulmonary TGF-β activity has a direct role in inhibiting injured lung development. In these studies, blocking intracellular TGF-β signaling with a neutralizing antibody improved alveolar and microvascular development in newborn mice with hyperoxic lung injury (51). Moreover, decreasing TGF-β receptor activity using a dominant-negative approach inhibited hypoxia-mediated pulmonary neomuscularization (3). Although the mechanisms through which TGF-β modulates lung development are incompletely understood, it is plausible that TGF-β has a role in modulating NO signaling. Studies suggest that TGF-β decreases the expression of a cytokine-induced isoform of NOS (NOS2) in cultured endothelial cells and SMC (23, 36, 63) and might decrease sGC expression in aortic SMC (68). However, the role of TGF-β in modulating pulmonary NO signaling is unknown.
Based on the countervailing effects of TGF-β and NO signaling in pulmonary development, we hypothesized that excessive TGF-β activity modulates the expression of critical NO signaling enzymes in the injured newborn lung. We observed that expression of sGC and PKGI were decreased following 85% O2 inhalation and that exposure to a TGF-β-neutralizing antibody counteracted this effect, suggesting that excessive TGF-β signaling is involved in regulating pulmonary NO signaling following lung injury. A similar reduction of NO signaling enzyme expression was observed in SMC/myofibroblasts freshly isolated from the periphery of mouse pup lungs and in pulmonary and systemic vascular SMC obtained from adult mice and rats. These data demonstrate for the first time that TGF-β regulates the expression of critical NO signaling enzymes in the injured lung and pulmonary vascular SMC.
MATERIALS AND METHODS
Experimental design.
The effect of TGF-β neutralization on NO signaling enzyme protein expression in the injured developing lung was measured in the following manner. On embryonic days 17 and 19, pregnant C57BL/6 mice were treated with intraperitoneal injections of 0.5 ml of PBS with or without 10 mg/kg 1D11, a pan-specific TGF-β-neutralizing murine monoclonal antibody (18). In previous studies, this level of 1D11 was observed to inhibit TGF-β signaling in the lungs of mouse pups with oxygen-induced injury (51), mutant fibrillin-1 (52), or lacking Thy-1 (53). Moreover, treatment with an irrelevant, isotypic control antibody, MOPC 21, did not modulate TGF-β signaling (51). Within 12 h of birth, the pups and their mothers were exposed to air or 85% O2 in an acrylic chamber. The mothers of similarly treated mouse pups were exchanged between the O2 environments every 24 h to decrease the effects of hyperoxia exposure on them. The details of the oxygen exposure and gas measurement systems are described elsewhere (51). After 10 days, the mouse pups were killed with an intraperitoneal injection of 200 mg/kg sodium pentobarbital, and protein expression of sGCα1 and PKGI in the peripheral lung was determined using quantitative immunofluorescence. The Subcommittee for Research Animal Studies of the Massachusetts General Hospital approved the animal protocols.
The effect of TGF-β on NO signaling enzyme expression in cultured SMC was examined in the following manner. Vascular SMC were exposed to up to 20 ng/ml human recombinant TGF-β1 (240-B; R&D Systems) for up to 6 h in DMEM containing 0.1% FBS (HyClone). In freshly isolated mouse pup microvascular SMC/myofibroblasts, sGCα1 and PKGI protein expression was determined using indirect immunofluorescence; in mouse and rat pulmonary artery and aortic SMC, sGC subunit and PKGI isoform mRNA expression levels were determined using end-point RT-PCR. The effect of SMC density on the modulation of NO signaling enzyme mRNA expression by TGF-β was determined using the following approach. Two days after 1, 2, and 2.5 × 105 rat aortic SMC/cm2 were seeded onto cell culture dishes, the cells were treated with and without 10 ng/ml TGF-β1 in DMEM containing 0.1% FBS for 3 h. Subsequently, RNA was isolated from the cells, and sGCα1 and PKGIβ mRNA levels were determined using end-point RT-PCR. To test whether TGF-β modulates NO signaling enzyme mRNA stability, 1 day after 1 × 105 rat aortic SMC/cm2 were seeded onto cell culture plates, the cells were treated with 0.2% DMSO without or with 10 ng/ml TGF-β1 and/or 2 μg/ml actinomycin D (Act D) for 6 h. DMSO was used as a treatment control in these experiments because it was the diluent for Act D. Previous studies revealed that 0.2% DMSO did not decrease sGC or PKGI mRNA levels compared with media without DMSO. Six hours later, RNA was isolated from the cells, and the sGCα1 and PKGIβ mRNA levels were determined using end-point RT-PCR.
SMC isolation and culture.
Unless otherwise indicated, the SMC were cultured in DMEM containing 10% FBS, penicillin, streptomycin, and glutamine (CM, complete media). Pulmonary microvascular SMC/myofibroblasts (PmvSMC) were obtained from peripheral segments of mouse pup lung tissue using a modification of previously described methods (19, 41). After the mouse pups were killed with sodium pentobarbital, the pulmonary vasculature was flushed with PBS containing 0.1 M sodium citrate. Subsequently, ∼1-mm-thick peripheral segments of the left lungs were obtained using a dissecting microscope, minced, and then treated with 2 mg/ml collagenase (Worthington) in CM for 2–4 h at 37°C with agitation. The lung digestates were then centrifuged at 300 g for 5 min, and the pelleted cells were resuspended in CM and allowed to adhere to glass slides. One hour later, the nonattached cells were removed, and the adherent cells were exposed to CM. Subconfluent primary cultures of these cells were used in the studies within 24 h of their isolation. The CS-54 rat PASMC were generated by A. Rothman (62) and kindly provided by R. B. Pilz (University of California, San Diego). The mouse PASMC were obtained from main and first generation intrapulmonary arteries, and mouse and rat systemic SMC were obtained from thoracic aortas using collagenase and the method described by Bruce and Honaker (12). These SMC were used in the experiments before passage 8. The pulmonary microvascular endothelial cells (PmvEC) were obtained using methods described by others (16) and kindly provided by P. Yu (Massachusetts General Hospital). The PmvEC were maintained in DMEM supplemented with 20% FBS, penicillin, glutamine, 0.1 mM nonessential amino acids, 100 μg/ml heparin, and 100 μg/ml endothelial cell growth factor (47). All cells were maintained at 37°C in gas containing 21% O2 and 5% CO2.
Protein expression in the mouse pup lung.
Peripheral lung NO signaling enzyme protein expression was examined using quantitative immunofluorescence. The tracheae were cannulated, and then the lungs were fixed in situ while being distended with 3% paraformaldehyde in PBS at a transpulmonary pressure of 22 cmH2O for 24 h. Subsequently, the lungs were dissected from the body, and the left lungs were embedded in paraffin. Six-micrometer-thick sections from transverse sections of the left lungs were reacted with antibodies that identify sGCα1 (160895; Cayman) or the catalytic region of PKGI (PKGICR; KAS-PK005; Stressgen), which is shared between PKGIα and PKGIβ, followed by an alkaline phosphatase-conjugated secondary antibody and Vector Red substrate (SK-5100; Vector Laboratories). Using the systematic sampling approach described by Tschanz and Burri (72), four 0.15-mm2 nonoverlapping epifluorescent images were obtained from randomly oriented peripheral lung fields that excluded large airway and vascular structures and were masked to detail the lung parenchymal structures. To construct the violin plot histograms, the pixel luminosity and numbers in the lung images were obtained using a custom MATLAB script (MathWorks). To obtain the percent fluorescent volume density (%FVD) of sGCα1 and PKGI in each lung image, the pixel numbers representing thresholded sGC and PKGI immunoreactivity were obtained using ImageJ (58) and methods detailed by Weibel (82) and then normalized to the total pixel numbers in the lung parenchyma.
Whole lung NO signaling enzyme protein expression was determined using immunoblotting. Soluble protein extracts were obtained from pulverized frozen pup lungs using 200 mM NaCl, 10 mM Tris·HCl, pH 7.4, 5 mM EDTA supplemented with 1:100 volume protease inhibitors (P8340; Sigma). Subsequently, 60 μg of protein was resolved using SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes, and blocked using 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20. After the membranes were exposed to antibodies that recognize sGCα1 or PKGI (detailed above), the immunocomplexes were detected using peroxidase-conjugated secondary antibodies and enhanced chemiluminescence. Equal protein transfer was confirmed by reprobing the membranes with an antibody that detects α-tubulin (clone DM1A; Sigma).
TGF-β modulation and lung structure.
To determine the effect of lung injury on alveolar development, the mean linear intercept (Lm) and the percent air space volume density (%AVD) were determined using 0.15-mm2 peripheral pup lung images and previously described methods (48, 51, 70, 82). The Lm and %AVD are inversely proportional to the internal surface area and level of alveolarization of the lung, respectively. After the lung images were thresholded, filtered, and segmented using methods described by others (71), chords bisected by the walls of the peripheral airways were obtained by comparing the lung images with another image containing horizontal lines that were 33 μm apart using a bitwise logical operation. After discarding chords <8 and >250 μm long, which are associated with pulmonary capillaries and conducting airways (65), and those associated with the image margin, the average Lm was determined. The %AVD was derived using point-counting methods (82).
NO signaling enzyme protein expression in PmvSMC.
The cells were fixed with 3% paraformaldehyde and permeabilized with methanol before being exposed to 1% goat serum followed by antibodies that detect sGCα1, PKGICR, prosurfactant protein B (AB3432; Chemicon), α-smooth muscle actin (αSMA; 1A4; Sigma), or calponin (hCP; Sigma). Subsequently, the cells were reacted with secondary antibodies labeled with fluorescent molecules, and immune complexes were detected using epifluorescence microscopy. In control studies, the cells were incubated with preimmune serum or a nonspecific monoclonal antibody (MOPC 21; Sigma) before exposure to the secondary antibodies. To identify microvascular endothelial cells, the isolated cells were fixed with 4% paraformaldehyde and then exposed to Alexa 488-conjugated Griffonia simplicifolia IB4 lectin or diluent that did not contain the lectin. The IB4 lectin-bound cells were detected using epifluorescent microscopy.
Detection of TGF-β signaling by immunoblotting.
SMC were lysed in buffer containing 50 mM Tris, pH 6.8, 2% SDS, and 10% (vol/vol) glycerol. After measuring the soluble protein concentration with BCA protein assay reagent (Pierce), 0.01 volume 2-mercaptoethanol and 1% bromophenol blue were added, the samples were heated, and 20 μg of protein was resolved using SDS-PAGE and electroblotted onto charged membranes. After blocking the membranes using 0.5% nonfat dry milk in PBS containing 0.01% Tween 20, they were exposed to antibodies that detect phospho- (p-) Smad2 (3101; Cell Signaling), Smad2 (L16D3; Cell Signaling), and α-tubulin (T6199; Sigma) and then to peroxidase-labeled secondary antibodies. The immunocomplexes bound to the membranes were detected using enhanced chemiluminescence.
Measurement of mRNA expression by end-point RT-PCR.
RNA expression was determined using end-point RT-PCR, with 18S RNA expression used as an internal standard, and methods detailed by others (28). RNA was obtained from SMC using phenol and guanidine isothiocyanate reagent (TRIzol; Invitrogen), dissolved in diethyl pyrocarbonate-treated water, and quantified using an RNA-binding fluoroprobe (RiboGreen; Invitrogen) and fluorescence spectroscopy. Subsequently, cDNAs were generated using 500 ng of RNA, 250 ng of random primer, 1 mM dNTP, 40 units of RNasin, and 200 units of Moloney murine leukemia virus reverse transcriptase. Duplex PCR was performed using 0.1 volume of the cDNA, 500 nM gene-specific primers, 18S primer-competimer mix (Ambion), 5 mM dNTP, 1.1 mM MgCl2, and thermostable DNA polymerase (REDTaq; Sigma). The oligonucleotides used in this study are shown in Table 1 and are displayed in a 5′-to-3′ nucleotide orientation. Because the PKGI isoforms are generated by the alternate splicing of mRNA encoding 5′ exons, the antisense oligonucleotides for PKGIα and PKGIβ are the same. PCR with these primers yielded a single amplicon of expected size. Following initial heating of the reaction mixture for 5 min at 95°C, the samples were denatured for 1 min at 94°C, annealed for 1 min at 55°C, and extended for 3 min at 72°C. After 26 cycles, DNA synthesis was extended for 10 min at 72°C. During pilot studies, the reaction cycle numbers were optimized to obtain amplicon quantities in the linear range of PCR amplification. The PCR reaction products were resolved using a 3% (wt/vol) agarose gel. The amplicons were detected using epifluorescence following ethidium bromide staining and recorded using a digital camera. Densitometric analysis of the images was performed using ImageJ, and the gene-specific amplicon density was referenced to that of 18S.
Table 1.
Oligonucleotides used in study
| Gene | Oligo | Sequence |
|---|---|---|
| sGCα1 | Sense | 5′ -caa gga caa att gtg caa gc-3′ |
| Antisense | 5′ -cca gca aac act gat cca ga-3′ | |
| sGCβ1 | Sense | 5′ -gat acg aca atg tga cca tcc tc-3′ |
| Antisense | 5′ -cca atc aca cct gtc acc ac-3′ | |
| PKGIα | Sense | 5′ -atg agc gaa ctg gag gaa ga-3′ |
| Antisense | 5′ -ctt gat gat gca gct gtc-3′ | |
| PKGIβ | Sense | 5′ -gct gga gtt gga tca gaa gg-3′ |
| Antisense | 5′ -ctt gat gat gca gct gtc c-3′ |
sGC, soluble guanylate cyclase.
Data analysis and presentation and statistical methods.
During the capturing, processing, and analysis of the images, the investigator was unaware of the lung treatment group. The data are presented as means ± SD and were compared using a factorial model of ANOVA. When significant differences were detected, a Scheffé test was used post hoc. Significance was determined at P < 0.05. The luminosity histogram and quartile data were depicted using box plot density tracings (violin plots; Ref. 32).
RESULTS
TGF-β decreases expression of NO signaling enzymes in the injured developing lung.
We (51) previously observed that 1D11, a monoclonal pan-specific TGF-β-neutralizing antibody, inhibits excessive TGF-β signaling in the injured newborn mouse lung. Therefore, to determine the role of TGF-β signaling in modulating NO signaling enzyme expression in vivo, we tested whether 1D11 modulates sGCα1 and PKGI expression in the injured lung. We examined the protein expression of sGCα1 because, in contrast to sGCα2, it is abundantly expressed in the lung and is a critical mediator of pulmonary NO signaling (75). PKGIα, PKGIβ, and PKGIγ are all expressed in pulmonary cells (37, 67); thus protein levels of all three PKGI isoforms were detected simultaneously using an antibody that recognizes their conserved COOH-terminal catalytic domain.
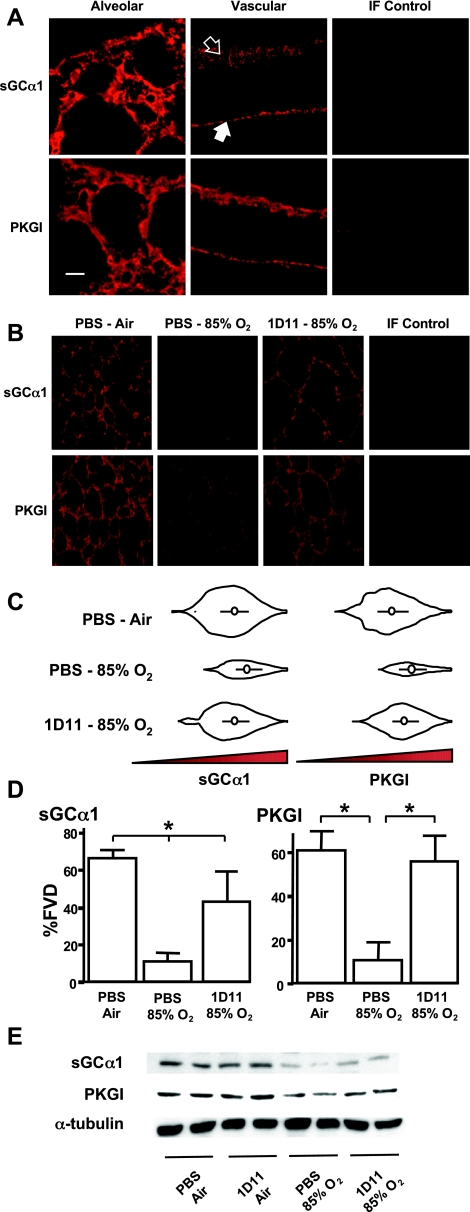
NO signaling enzyme protein expression was detected in the interstitium of the pup lung. sGCα1 and PKGI immunoreactivity were observed within the walls of the peripheral lung and in distal and proximal blood vessels (Fig. 1A). Immunoreactivity was not observed in endothelial cells or in lung specimens not exposed to the NO signaling enzyme-detecting antibodies. A similar pattern of sGC immunoreactivity in the developing lung has been observed by others (4). Although the tissue distribution of PKGI immunoreactivity in the newborn lung is less characterized, PKGI protein expression previously has been observed in freshly isolated SMC and myofibroblasts from peripheral pulmonary arteries and veins of fetal and newborn lambs (25, 26, 59, 86). Inhalation of 85% O2 decreased sGCα1 and PKGI protein expression in the peripheral mouse pup lung (Fig. 1B). Although the modulation of NO signaling enzyme expression in the injured mouse lung has not been reported previously, a similar decrease in sGCα1 and PKGI immunoreactivity has been observed in the injured lungs or hypoxic SMC and pulmonary vessels of lambs (7, 59, 73). The decrease we observed in NO signaling enzyme levels appeared to be limited to the peripheral lung; no obvious change in sGCα1 and PKGI immunoreactivity was observed in the proximal airway and vascular structures of the injured pup lungs. Similar to previous observations (51, 54, 80), chronic exposure to 85% O2 appeared to decrease secondary septation in the mouse pup lung (Fig. 1B). Breathing 85% O2 was associated with a ∼100% increase in Lm (PBS-air 55 ± 5 μm vs. PBS-85% O2 114 ± 22 μm; n = 6 each group; P < 0.05) and 23% increase in %AVD (PBS-air 74 ± 3% vs. PBS-85% O2 90 ± 3%; n = 6 each group; P < 0.05) in the peripheral lung compared with the air-exposed lung.
Fig. 1.
Transforming growth factor-β (TGF-β) neutralization increases soluble guanylate cyclase (sGC) and PKGI protein expression in the injured newborn lung. sGCα1 and PKGI immunoreactivity were analyzed using distension-fixed lungs from mouse pups exposed to air or 85% O2 and PBS or a TGF-β-neutralizing antibody (1D11), as indicated. A: sGCα1 and PKGI immunoreactivity was observed in cells in walls of the alveoli and pulmonary arteries (open arrow) and veins (closed arrow) of the developing mouse pup lung. Laser scanning confocal micrographs of air-breathing mouse pup lungs are shown. Immunofluorescent (IF) control lungs were exposed to nonimmune IgG instead of the specific antibodies. Bar = 10 μm. B: sGCα1 and PKGI immunoreactivity was increased in the hyperoxic lung exposed to TGF-β-neutralizing antibodies. Representative IF images of mouse pup lungs are shown. C: objective image analysis reveals that sGCα1 and PKGI immunoreactivity levels in the peripheral lung are greatly decreased by 85% O2, but expression is increased to nearly control levels with TGF-β neutralization. Violin plots of the immunoreactive signal luminosity are shown; abscissas represent the luminosity, and ordinates show the pixel frequency; white circles indicate the median values, and the black bars delineate the 1st and 3rd quartiles. D: percent fluorescent volume density (%FVD) of thresholded epifluorescent signals in the lung parenchyma. Data are means ± SD; n = 6 each group. *P < 0.05. E: immunoblotting also revealed that sGCα1 and PKGI protein expression was improved in hyperoxic pup lungs exposed to TGF-β-neutralizing antibodies. Membranes with 60 μg of pup lung protein were probed with antibodies detecting sGCα1 and PKGI to examine NO signaling enzyme protein expression and α-tubulin to confirm equal protein transfer.
Treatment with a TGF-β-neutralizing antibody partially prevented the decrease in NO signaling enzyme levels in the oxygen-injured lung. Exposure to a level of 1D11 that previously was shown to inhibit TGF-β signaling in the injured lung (51) increased the quantity and density of sGCα1 and PKGI immunoreactivity in the 85% O2-treated pup lung (Fig. 1C and D). Luminosity histogram violin plots revealed that 1D11 exposure increased the quantity of the sGCα1 and PKGI immunoreactivity in the periphery of O2-injured lungs compared with the PBS-treated control. Moreover, 1D11 treatment increased the volume density of sGCα1 and PKGI immunoreactive signals in the peripheral parenchyma of the oxygen-injured pup lungs. In the 85% O2-exposed lungs, 1D11 treatment increased the %FVD of sGCα1 immunoreactivity nearly 4-fold and that of PKGI ∼5-fold compared with the injured lungs treated with PBS. The TGF-β-neutralizing antibody did not modulate NO signaling enzyme expression in the control lungs; treatment with 1D11 did not change the %FVD in the air-exposed pup lungs (%FVD PBS vs. 1D11 in air-exposed lungs: sGCα1, 33 ± 2 vs. 38 ± 5; PKGI, 50 ± 12 vs. 57 ± 4; n = 4; P > 0.05). Moreover, in previous studies, we (51) observed that the protective effect of 1D11 in the injured developing lung is due to its TGF-β-neutralizing activities; an isotypic control antibody, MOPC 21, did not neutralize TGF-β-activity in the O2-injured mouse pup lung or improve injured lung development. A similar modulation of sGC and PKGI protein expression by hyperoxia and TGF-β-neutralizing antibodies was observed in whole pup lung homogenates using immunoblotting (Fig. 1E). These studies suggest for the first time that excessive TGF-β activity in the injured developing lung decreases levels of critical NO signaling enzymes.
In the present study, the improvement in NO signaling enzyme expression in 1D11-treated lung was associated with increased alveolarization in the oxygen-injured lung, as previously reported (51). Treatment of the 85% O2-exposed lung with 1D11 decreased Lm by 49% (1D11-85% O2, 85 ± 15 μm; n = 6; P < 0.05) and %AVD by 69% (79 ± 3%; n = 6; P < 0.05) compared with O2-injured lung treated with PBS.
TGF-β decreases sGC and PKGI expression in SMC isolated from peripheral developing mouse pup lungs.
One possible explanation for the results detailed above is that excessive TGF-β in the injured newborn lung decreases the expression of NO signaling enzymes indirectly by inhibiting alveolar and vascular development or by affecting cells that are transiting the lung. Therefore, we evaluated whether TGF-β directly decreases sGC and PKGI expression in freshly isolated cells from the mouse pup lung interstitium. PmvSMC were used for these studies because they have been observed to express NO signaling enzymes (7, 26).
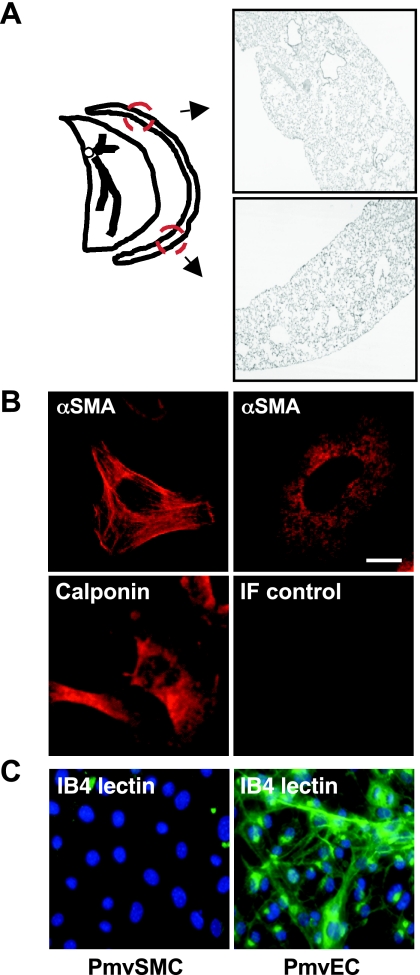
Previous reports indicate that pulmonary vascular SMC/myofibroblasts and pericytes can be isolated from collagenase-digested lung tissue in the presence of serum (19). To obtain these cells from the microvasculature, we applied this technique to peripheral mouse pup lung sections. As shown in Fig. 2, partial enzyme-mediated digestion of peripheral pup lung tissue permits the isolation of intraacinar cells from the peripheral lung that exhibit a SMC phenotype. Two prominent phenotypes of cells were isolated. The majority of cells had the appearance of SMC/myofibroblasts: spindly shape, ragged borders with numerous pseudopodia, and αSMA immunoreactivity in a stress fiber-like pattern (Fig. 2B). There were also occasional clumps of cells with a less well-defined shape and a lower intensity of αSMA immunoreactivity in a reticular pattern; these cells had a phenotype that is similar to pericytes, defined by others (19, 35). In additional studies, the pericytes that we isolated from the peripheral pup lung did not exhibit contact-mediated growth inhibition when they were allowed to continue to proliferate in the clumps. The differential appearance and αSMA immunoreactivity of these two populations of cells was similar to those of SMC and pericytes isolated from the adult rat lung reported by Johnson and coworkers (35). Moreover, the variation in mouse pup SMC phenotype and SMC marker reactivity that we observed is in agreement with studies by others demonstrating distinct pulmonary fibroblast populations in the developing lung (9, 24). The isolated PmvSMC also interacted with antibodies that detect calponin. Moreover, as shown in Fig. 2C, the PmvSMC did not exhibit the characteristic IB4 lectin binding seen in endothelial cells (40, 49). No ciliated epithelial cells were observed among the isolated PmvSMC, and none reacted with antibodies that recognize prosurfactant protein B, which is characteristic of type II alveolar epithelial cells (data not shown).
Fig. 2.
Interstitial lung cells with smooth muscle cell (SMC) lineage isolated from the periphery of mouse pup lungs. A: a ∼1-mm-thick section of peripheral mouse pup lung tissue, containing terminal airway and microvascular structures as shown here in representative lung areas mapped by the arrows, was digested with collagenase, and cells were grown in the presence of serum on cell culture slides. B: nearly all cells isolated using this method exhibited α-smooth muscle cell actin (αSMA) and calponin immunoreactivity (as indicated; red). Cells exhibiting SMC/myofibroblast phenotype had an elongated shape with pseudopodia and strong αSMA immunoreactivity along prominent stress fibers; others exhibited less αSMA reactivity and organization, which is observed in pericytes. IF control cells were exposed to MOPC 21, a nonspecific monoclonal murine antibody (IgG1). Bar = 10 μm. C: in contrast with adult mouse microvascular endothelial cells (PmvEC), the peripheral mouse pup lung SMC/myofibroblasts (PmvSMC) did not exhibit Griffonia simplicifolia IB4 lectin reactivity (green). Nuclei were identified with a DNA-binding fluorescent molecule (DAPI).
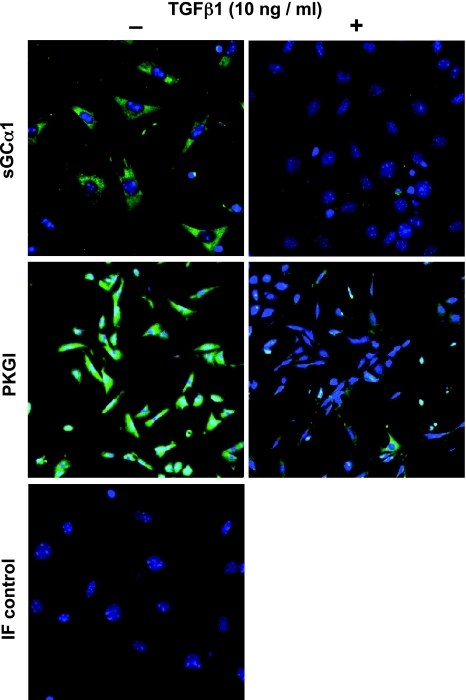
sGC and PKGI protein expression was observed in the freshly isolated mouse pup PmvSMC (Fig. 3). Although NO signaling enzyme immunoreactivity has not been reported in mouse pup pulmonary microvascular cells, others have detected sGC and PKGI protein expression in the vascular SMC of the lungs of other newborn animals (6, 7, 46, 59, 73). TGF-β1 treatment of the isolated mouse pup PmvSMC decreased immunoreactivity for both sGCα1 and PKGI (Fig. 3). In contrast with the uniform decrease in sGCα1 immunoreactivity associated with TGF-β1-treatment, the decrease in PKGI expression was more apparent in some cells than others. Along with the effects of the TGF-β-neutralizing antibody described above, these data suggest that the decrease in sGC and PKGI expression in injured developing lung is due at least in part to a direct effect of TGF-β on NO signaling enzyme expression in PmvSMC.
Fig. 3.
TGF-β1 decreases sGCα1 and PKGI immunoreactivity in peripheral mouse pup lung SMC. Shown are laser scanning confocal epifluorescent images of freshly isolated mouse pup lung PmvSMC treated with 10 ng/ml TGF-β1 or vehicle for 6 h, fixed, and reacted with antibodies to sGCα1 and PKGI and fluorescent secondary antibodies. IF control cells were exposed to preimmune serum instead of the specific antibodies; cell nuclei were detected with DAPI. Images are representative of 3 independent studies with similar results.
TGF-β decreases sGC subunit and PKGI isoform mRNA expression in pulmonary SMC.
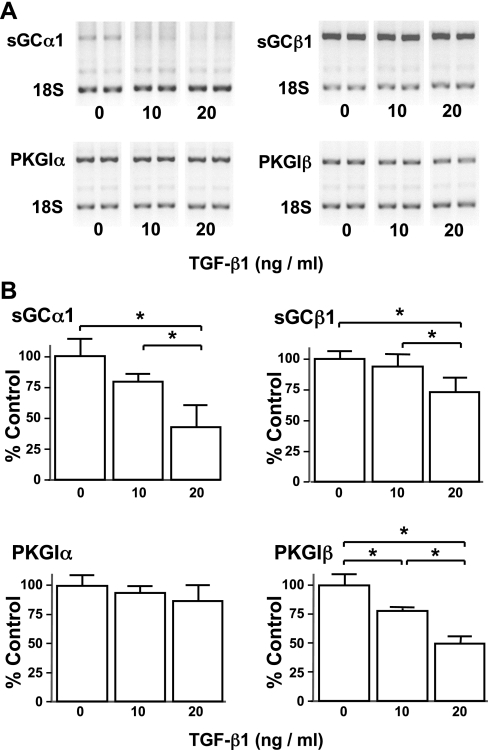
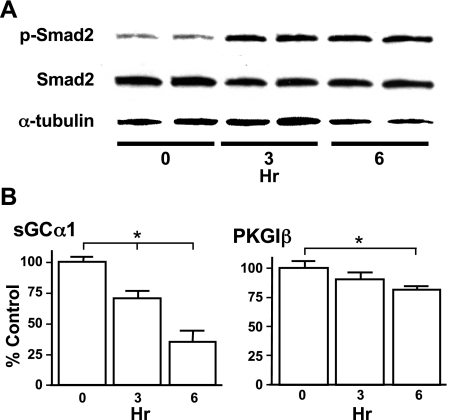
We next examined whether TGF-β decreases NO signaling enzyme mRNA expression in pulmonary vascular SMC. The number of cells obtained from the peripheral mouse pup lung would not permit quantification of mRNA expression, so for these studies we utilized CS-54 cells, a rat PASMC line that expresses sGC and PKGI (33). TGF-β1 decreased sGC and PKGI mRNA expression in CS-54 cells (Fig. 4). Although a concentration of 20 ng/ml decreased sGCα1, sGCβ1, and PKGIβ mRNA expression in these cells, PKGIα mRNA was not altered. We next examined the effect of TGF-β1 treatment over time on Smad signaling and sGCα1 and PKGIβ mRNA expression in CS-54 PASMC. TGF-β1-induced Smad2 phosphorylation (p-Smad2) reached maximum levels in CS-54 PASMC by 3 h of treatment and remained elevated at 6 h (Fig. 5A). TGF-β1 exposure did not affect the total levels of Smad2. TGF-β1 decreased sGCα1 and PKGIβ mRNA expression within 3 h, and the mRNA levels of these genes continued to decline with longer exposure to TGF-β1 (Fig. 5B). Up to 6 h of TGF-β1 exposure had no effect on the level of PKGIα mRNA (data not shown). These studies indicate that TGF-β exposure is associated with increased canonical TGF-β signaling and decreased expression of NO signaling enzyme mRNA in PASMC.
Fig. 4.
TGF-β modulates NO signaling enzyme mRNA levels in a rat pulmonary artery SMC (PASMC) line in a dose-dependent manner. Rat CS-54 PASMC were treated with the indicated levels of TGF-β1 for 3 h, and NO signaling enzyme mRNA expression was examined using end-point RT-PCR. A: TGF-β1 decreased sGC subunit and PKGIβ mRNA expression. Representative fluorescent images of ethidium bromide-stained amplicons from RT-PCR are displayed. B: densitometric analysis revealed that TGF-β1 treatment decreased sGC subunit and PKGIβ mRNA levels. Target RNA levels were referenced to 18S RNA levels as described in materials and methods. Data are means ± SD, n = 4 per group. Data shown are representative of 3 independent experiments. *P < 0.05.
Fig. 5.
TGF-β modulates Smad2 phosphorylation and expression of NO signaling enzymes in CS-54 PASMC. Rat CS-54 PASMC were treated with 10 ng/ml TGF-β1 for the indicated times. A: within 3 h of TGF-β1 exposure, phospho- (p-) Smad2 levels increased in the CS-54 PASMC. Consistent total Smad2 and α-tubulin levels revealed that the TGF-β-mediated increase in p-Smad2 immunoreactivity was not due to differences in Smad2 expression or protein loading levels. The protein levels were determined using specific antibodies and immunoblotting. B: TGF-β1 exposure decreased sGCα1 and PKGIβ mRNA levels (compared with 18S) in CS-54 PASMC as detected by end-point RT-PCR. Data are means ± SD, n = 4 per group. Results shown are representative of 3 independent experiments. *P < 0.05.
TGF-β decreases NO signaling enzyme mRNA expression in vascular SMC from a variety of sources.
A previous study suggested that TGF-β decreases PKGI mRNA levels in rat aortic SMC (68). To confirm this observation and to examine whether TGF-β modulates NO signaling enzyme expression in PASMC and systemic vascular SMC from other animals, we tested whether TGF-β1 decreases sGC subunit and PKGI isoform mRNA levels in adult mouse pulmonary and aortic SMC (Fig. 6) and rat aortic SMC (Figs. 7 and 8). As shown in Fig. 6, TGF-β1 decreased sGC subunit and PKGI isoform mRNA expression in murine PASMC and aortic SMC within 6 h. Similar to what was observed in the rat CS-54 PASMC, TGF-β1 did not decrease PKGIα mRNA levels in the adult mouse PASMC. However, TGF-β decreased PKGIα mRNA levels in the murine aortic SMC. These data suggest that TGF-β differentially regulates PKGI mRNA splicing in PASMC. Together, these results support the contention that TGF-β has a direct role in modulating NO signaling enzymes in vascular SMC.
Fig. 6.
TGF-β stimulates Smad-dependent gene expression and decreases sGC and PKGI mRNA expression in murine vascular SMC. The indicated mouse vascular SMC were treated with 10 ng/ml TGF-β1, and the levels of mRNA encoding NO signaling enzymes were determined using end-point RT-PCR. TGF-β decreased sGCα1, sGCβ1, and PKGIβ mRNA levels (compared with 18S) in murine SMC from PASMC or aorta (AoSMC) within 3 h of exposure. Cells were exposed to 10 ng/ml TGF-β1 for the indicated times. Data are means ± SD; n = 4 per group. Results shown are representative of 3 independent experiments. *P < 0.05.
Fig. 7.
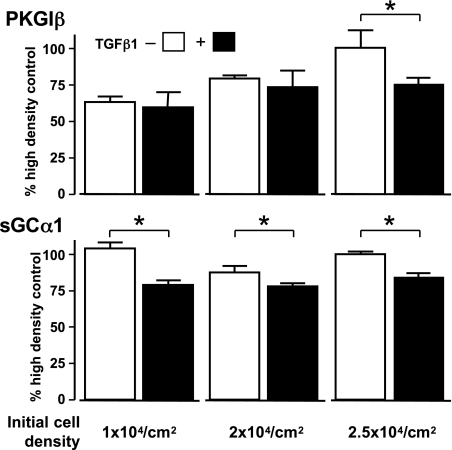
Cell density modulates the regulation of PKGIβ mRNA levels by TGF-β. Early passage rat AoSMC were seeded on cell culture plates at indicated densities. After 48 h of culture, cells were treated without or with 10 ng/ml TGF-β1 (open and closed boxes, respectively) for 6 h. PKGIβ and sGCα1 mRNA levels, relative to 18S RNA levels, were determined using end-point RT-PCR. Although the decrease in sGCα1 mRNA levels caused by TGF-β1 treatment was not affected by cell density, the effect of TGF-β1 on PKGIβ mRNA levels was seen only at higher SMC densities. Data are means ± SD, indexed to the relative mRNA levels obtained in the high-density cells without TGF-β treatment, and typical of 3 independent experiments; n = 6 per group; *P < 0.05.
Fig. 8.
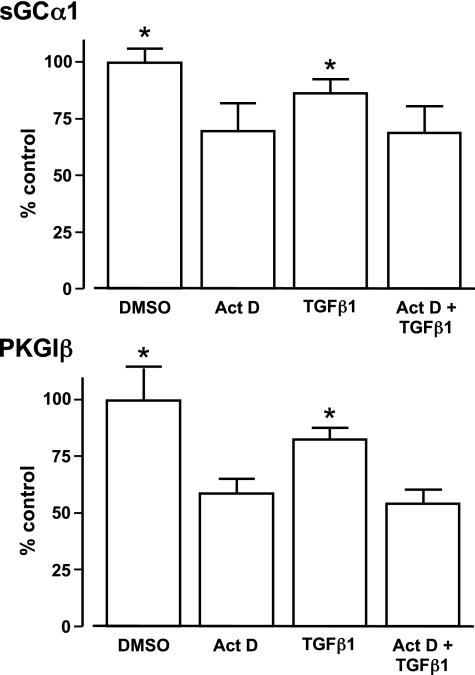
TGF-β does not regulate stability of sGCα1 and PKGIβ mRNAs. Rat AoSMC were treated as indicated with or without 10 ng/ml TGF-β1 and 10 μM actinomycin D (Act D) for 6 h. sGCα1 and PKGIβ mRNA levels were determined using end-point RT-PCR. Results are relative to the mean mRNA values observed in the cells treated with media containing vehicle (DMSO) alone. There was no significant difference between cells treated with media with or without DMSO (data not shown). The decrease in sGCα1 and PKGIβ mRNA levels observed in the SMC treated with Act D was not further exacerbated with TGF-β1 treatment. Data are means ± SD and typical of 3 independent experiments; n = 9 per group; *P < 0.05 vs. other treatments.
SMC PKGI mRNA levels are modulated by TGF-β in a cell density-dependent manner.
Previous studies indicate that intercellular contact increases PKGI expression in vascular SMC. For example, increased SMC density and cell-cell contact have been noted to augment PKGI expression in SMC (17, 43) by inhibiting RhoA and augmenting Rac1 signaling (85). The effect of SMC density on sGC expression has not been reported. To test how SMC density regulates the inhibition of PKGI expression by TGF-β, early passage rat aortic SMC were seeded on cell culture plates at different densities, and the effect of TGF-β1 on PKGIβ mRNA levels was examined. Pilot studies determined that rat aortic SMC become confluent 2 days after they are seeded at 2.5 × 104 cells/cm2. As shown in Fig. 7, although PKGIβ expression increased with SMC density, the inhibitory effect of TGF-β1 on PKGIβ mRNA expression was not observed until the SMC had become confluent. In contrast, SMC density had no effect on sGCα1 levels or their modulation by TGF-β1. These results suggest that TGF-β regulates PKGI and sGC mRNA expression in part through different mechanisms.
Stability of sGC and PKGI mRNAs in SMC is unaffected by TGF-β.
In previous studies, TGF-β decreased the expression of several genes by destabilizing their mRNA (20). In addition, sGC mRNA turnover in SMC has been observed to increase in response to other cytokines (22). Therefore, we examined whether TGF-β reduces the sGC and PKGI mRNA levels in vascular SMC by decreasing their mRNA stability. In these experiments, we used 10 μM Act D because our preliminary studies revealed that this concentration inhibited transcription of β-actin mRNA and 18S RNA in SMC (data not shown). We observed that treatment with TGF-β1 or Act D independently decreased sGCα1 and PKGIβ mRNA levels (Fig. 8). However, exposure of the SMC to Act D and TGF-β1 together did not further decrease the mRNA levels of these NO signaling enzymes. These results suggest that TGF-β does not destabilize sGC or PKGI mRNAs and that TGF-β regulates the mRNA expression of these NO signaling enzymes through mechanisms that are different from those used by other cytokines.
DISCUSSION
Through unknown mechanisms, NO signaling and cGMP signaling are downregulated in the injured fetal and newborn lung. Here, we report for the first time that TGF-β decreases the expression of critical enzymes required for NO and cGMP activity in the injured developing lung. The partial rescue of severe depression of sGCα1 and PKGI protein expression observed in 85% O2-exposed mouse pup lungs treated with a TGF-β-neutralizing antibody suggests that excessive TGF-β signaling plays an important role in undermining NO and cGMP signaling in the injured lung. As evidenced by the decrease in sGCα1 and PKGI protein expression in TGF-β1-treated PmvSMC, TGF-β acts through direct mechanisms to reduce the expression of these NO signaling enzymes. Moreover, the decrease in sGC and PKGI mRNA expression in TGF-β1-treated PASMC and aortic SMC from mice and rats supports a general role for TGF-β in modulating NO signaling in vascular SMC. These data link TGF-β with NO and cGMP signaling in the injured lung and confirm the importance of cytokines in mediating newborn lung injury.
Emerging evidence suggests that excessive TGF-β signaling impedes alveolar and microvascular development in the injured lung. For example, studies have documented increased TGF-β levels in the pulmonary effluents of premature infants with oxygen- and ventilator-induced lung injury, often followed by the development of bronchopulmonary dysplasia (38, 42). TGF-β signaling is also increased in models of newborn lung injury (2, 13, 39, 78, 83), and overexpression of active TGF-β causes pathological changes in the newborn lung that are similar to those observed in premature infants with chronic lung disease (27, 76). However, the data supporting the role of TGF-β in newborn lung disease are not consistent. For example, other studies found no elevation of TGF-β in the tracheal aspirates (14) or serum (74) of premature babies with lung injury. Similarly, in some studies using models of newborn lung disease, TGF-β levels were unchanged or decreased (8, 57). Nevertheless, on balance, these studies indirectly suggest a relationship between TGF-β and newborn lung disease.
Recent studies using TGF-β inhibitors indicate that TGF-β directly modulates lung development. For example, TGF-β-neutralizing antibodies inhibited the increased pulmonary TGF-β signaling and decreased alveolar development observed in mouse pups with inactive fibrillin-1 and a Marfan syndrome pulmonary phenotype (52) and in Thy-1 null mouse pups (51). In addition, inhibition of receptor-mediated TGF-β signaling reduced the precocious muscularization of peripheral pulmonary arteries in chronically hypoxic mouse pups (3), which exhibit many aspects of the pulmonary vascular disease observed in some infants and children that died with congenital heart disease. Moreover, exposure to a pan-specific TGF-β-neutralizing antibody improved pulmonary alveolarization and microvascular development in hyperoxic mouse pups that otherwise exhibit decreased lung development consistent with bronchopulmonary dysplasia (51). The mechanisms through which the inhibition of excessive TGF-β signaling improves injured lung development are incompletely understood. However, the results detailed here, along with studies indicating that NO signaling has a cardinal role in modulating lung development, suggest that the protective mechanism of TGF-β neutralization might include enhancing expression of NO signaling enzymes. To understand more clearly the role of TGF-β-regulated sGC and PKGI expression on the maturation of the injured lung, it will be useful to develop models that are resistant to the downregulation of NO signaling by TGF-β.
The results presented here support previous observations indicating that TGF-β plays a role in regulating NO and cGMP signaling in cells. TGF-β has been observed to modulate the expression of NOS isoforms in various vascular cell types. For example, studies revealed that TGF-β increases NOS3 expression and NO production in bovine aortic endothelial cells in culture (34). In addition, through Smad3-dependent mechanisms, TGF-β appears to decrease interleukin-1β-mediated upregulation of NOS2 expression and NO production in rat pulmonary artery and aortic SMC (21, 23, 55, 63). It is possible that TGF-β mediates NOS expression in the injured newborn lung. However, in contrast with the increased NOS3 expression observed in TGF-β-treated bovine aortic endothelial cells, decreased NOS3 expression is observed in the lungs of fetal lambs with flow-induced pulmonary vascular disease (5, 64, 77) and in premature lambs ventilated with oxygen (46). Decreased NOS1 and NOS3 expression is also reported in fetal baboons with oxygen- and ventilator-induced lung injury (1). The role of TGF-β in regulating sGC and PKGI expression is relatively unknown. However, in one study, TGF-β1 decreased PKGI mRNA levels within 6 h of exposure (68). In the present studies, TGF-β decreased sGC and PKGI protein expression in freshly isolated peripheral lung SMC and mRNA expression in PASMC as well as aortic SMC. Together, these studies suggest that TGF-β modulates downstream NO signaling enzymes in vivo and in vitro.
Previous experiments showing a decrease in sGC and PKGI expression levels in response to cytokines or NO donors suggest coordinate regulation of sGC and PKGI in vascular SMC (10, 11, 22). Similarly, we observed that TGF-β decreases the expression of both sGC and PKGI in vascular SMC. Because sGC-produced cGMP appears to modulate many intracellular processes primarily by stimulating PKGI (15), the coordinated regulation of sGC and PKGI expression by cytokines, such as TGF-β, suggests an efficient control mechanism for NO/cGMP signaling in SMC. Unlike previous results showing that excessive NO/cGMP reduces sGC expression by decreasing sGC mRNA stability, in our studies, TGF-β did not modulate sGC or PKGI mRNA turnover. The observation that TGF-β decreases PKGI mRNA expression in a SMC density-dependent manner but that its effect on sGC mRNA levels is density-independent raises the possibility that TGF-β regulates these transcripts through different mechanisms. In addition, TGF-β affected PKGI isoform mRNA levels differently: TGF-β consistently decreased mRNA levels of PKGIβ but not PKGIα. This suggests that TGF-β might regulate the alternate splicing of PKGI mRNA in SMC.
In summary, we found that TGF-β signaling modulates NO signaling enzyme expression in the injured developing lung and in pulmonary vascular SMC. These observations suggest that TGF-β-neutralizing therapies might counteract the negative effect of hyperoxia on newborn lung development and vascular function. These results also provide the rationale for examining the molecular mechanisms through which TGF-β signaling regulates NO signaling enzyme expression.
GRANTS
This work was supported by National Institutes of Health Grants 5-T32-GM-07592 (to P. R. Bachiller) and 1-R01-HL-080316 (to J. D. Roberts, Jr.) and INO Therapeutics/Ikaria.
DISCLOSURES
Genzyme kindly provided the pan-specific TGF-β-neutralizing antibody used in this study.
ACKNOWLEDGMENTS
We thank Katherine H. Cornog for help in the lung image analysis, Renate Pilz and Abraham Rothman for providing the CS-54 cells, and Paul Yu for kindly supplying the adult mouse microvascular endothelial cells.
Portions of this study have been presented at the Pediatric Academic Societies Meeting, Baltimore, MD, on May 2, 2009.
Present address of H. Nakanishi: Maternal & Perinatal Center, Tokyo Women's Medical University, 8-1, Kawada-cho, Shinjuku-ku, Tokyo, 162-8666 Japan.
REFERENCES
- 1.Afshar S, Gibson LL, Yuhanna IS, Sherman TS, Kerecman JD, Grubb PH, Yoder BA, McCurnin DC, Shaul PW. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol 284: L749–L758, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, Oparil S, Chen YF. Transforming growth factor-β signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 295: L86–L95, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrends S, Kempfert J, Mietens A, Koglin M, Scholz H, Middendorff R. Developmental changes of nitric oxide-sensitive guanylyl cyclase expression in pulmonary arteries. Biochem Biophys Res Commun 283: 883–887, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Black SM, Johengen MJ, Soifer SJ. Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs. Pediatr Res 44: 821–830, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Bland RD, Albertine KH, Carlton DP, MacRitchie AJ. Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. Am J Respir Crit Care Med 172: 899–906, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bland RD, Ling CY, Albertine KH, Carlton DP, MacRitchie AJ, Day RW, Dahl MJ. Pulmonary vascular dysfunction in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 285: L76–L85, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Botney MD, Parks WC, Crouch EC, Stenmark K, Mecham RP. Transforming growth factor-beta 1 is decreased in remodeling hypertensive bovine pulmonary arteries. J Clin Invest 89: 1629–1635, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brody JS, Kaplan NB. Proliferation of alveolar interstitial cells during postnatal lung growth. Evidence for two distinct populations of pulmonary fibroblasts. Am Rev Respir Dis 127: 763–770, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Browner NC, Dey NB, Bloch KD, Lincoln TM. Regulation of cGMP-dependent protein kinase expression by soluble guanylyl cyclase in vascular smooth muscle cells. J Biol Chem 279: 46631–46636, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Browner NC, Sellak H, Lincoln TM. Downregulation of cGMP-dependent protein kinase expression by inflammatory cytokines in vascular smooth muscle cells. Am J Physiol Cell Physiol 287: C88–C96, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Bruce MC, Honaker CE. Transcriptional regulation of tropoelastin expression in rat lung fibroblasts: changes with age and hyperoxia. Am J Physiol Lung Cell Mol Physiol 274: L940–L950, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Buckley S, Warburton D. Dynamics of metalloproteinase-2 and -9, TGF-β, and uPA activities during normoxic vs. hyperoxic alveolarization. Am J Physiol Lung Cell Mol Physiol 283: L747–L754, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Buron E, Garrote JA, Arranz E, Oyaguez P, Fernandez Calvo JL, Blanco Quiros A. Markers of pulmonary inflammation in tracheobronchial fluid of premature infants with respiratory distress syndrome. Allergol Immunopathol (Madr) 27: 11–17, 1999 [PubMed] [Google Scholar]
- 15.Chiche JD, Schlutsmeyer SM, Bloch DB, de la Monte SM, Roberts JD, Jr, Filippov G, Janssens SP, Rosenzweig A, Bloch KD. Adenovirus-mediated gene transfer of cGMP-dependent protein kinase increases the sensitivity of cultured vascular smooth muscle cells to the antiproliferative and pro-apoptotic effects of nitric oxide/cGMP. J Biol Chem 273: 34263–34271, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Chittenden TW, Claes F, Lanahan AA, Autiero M, Palac RT, Tkachenko EV, Elfenbein A, Ruiz de Almodovar C, Dedkov E, Tomanek R, Li W, Westmore M, Singh JP, Horowitz A, Mulligan-Kehoe MJ, Moodie KL, Zhuang ZW, Carmeliet P, Simons M. Selective regulation of arterial branching morphogenesis by synectin. Dev Cell 10: 783–795, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Cornwell TL, Soff GA, Traynor AE, Lincoln TM. Regulation of the expression of cyclic GMP-dependent protein kinase by cell density in vascular smooth muscle cells. J Vasc Res 31: 330–337, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L. Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol 142: 1536–1541, 1989 [PubMed] [Google Scholar]
- 19.Davies P, Smith BT, Maddalo FB, Langleben D, Tobias D, Fujiwara K, Reid L. Characteristics of lung pericytes in culture including their growth inhibition by endothelial substrate. Microvasc Res 33: 300–314, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Dibrov A, Kashour T, Amara FM. The role of transforming growth factor beta signaling in messenger RNA stability. Growth Factors 24: 1–11, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Feinberg MW, Watanabe M, Lebedeva MA, Depina AS, Hanai J, Mammoto T, Frederick JP, Wang XF, Sukhatme VP, Jain MK. Transforming growth factor-beta1 inhibition of vascular smooth muscle cell activation is mediated via Smad3. J Biol Chem 279: 16388–16393, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Filippov G, Bloch DB, Bloch KD. Nitric oxide decreases stability of mRNAs encoding soluble guanylate cyclase subunits in rat pulmonary artery smooth muscle cells. J Clin Invest 100: 942–948, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finder J, Stark WW, Jr, Nakayama DK, Geller D, Wasserloos K, Pitt BR, Davies P. TGF-β regulates production of NO in pulmonary artery smooth muscle cells by inhibiting expression of NOS. Am J Physiol Lung Cell Mol Physiol 268: L862–L867, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Frid MG, Moiseeva EP, Stenmark KR. Multiple phenotypically distinct smooth muscle cell populations exist in the adult and developing bovine pulmonary arterial media in vivo. Circ Res 75: 669–681, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Gao Y, Dhanakoti S, Tolsa JF, Raj JU. Role of protein kinase G in nitric oxide- and cGMP-induced relaxation of newborn ovine pulmonary veins. J Appl Physiol 87: 993–998, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Dhanakoti S, Trevino EM, Sander FC, Portugal AM, Raj JU. Effect of oxygen on cyclic GMP-dependent protein kinase-mediated relaxation in ovine fetal pulmonary arteries and veins. Am J Physiol Lung Cell Mol Physiol 285: L611–L618, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Gauldie J, Galt T, Bonniaud P, Robbins C, Kelly M, Warburton D. Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol 163: 2575–2584, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goidin D, Mamessier A, Staquet MJ, Schmitt D, Berthier-Vergnes O. Ribosomal 18S RNA prevails over glyceraldehyde-3-phosphate dehydrogenase and beta-actin genes as internal standard for quantitative comparison of mRNA levels in invasive and noninvasive human melanoma cell subpopulations. Anal Biochem 295: 17–21, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Goumans MJ, Liu Z, ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res 19: 116–127, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Han RN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, Stewart DJ. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: a model of alveolar capillary dysplasia? Circ Res 94: 1115–1123, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Hintze JL, Nelson RD. Violin plots: a box plot-density trace synergism. Am Stat 52: 181–184, 1998 [Google Scholar]
- 33.Idriss SD, Gudi T, Casteel DE, Kharitonov VG, Pilz RB, Boss GR. Nitric oxide regulation of gene transcription via soluble guanylate cyclase and type I cGMP-dependent protein kinase. J Biol Chem 274: 9489–9493, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Inoue N, Venema RC, Sayegh HS, Ohara Y, Murphy TJ, Harrison DG. Molecular regulation of the bovine endothelial cell nitric oxide synthase by transforming growth factor-beta 1. Arterioscler Thromb Vasc Biol 15: 1255–1261, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Johnson BA, Lowenstein CJ, Schwarz MA, Nakayama DK, Pitt BR, Davies P. Culture of pulmonary microvascular smooth muscle cells from intraacinar arteries of the rat: characterization and inducible production of nitric oxide. Am J Respir Cell Mol Biol 10: 604–612, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Kanno K, Hirata Y, Imai T, Iwashina M, Marumo F. Regulation of inducible nitric oxide synthase gene by interleukin-1β in rat vascular endothelial cells. Am J Physiol Heart Circ Physiol 267: H2318–H2324, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Keilbach A, Ruth P, Hofmann F. Detection of cGMP dependent protein kinase isozymes by specific antibodies. Eur J Biochem 208: 467–473, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Kotecha S. Cytokines in chronic lung disease of prematurity. Eur J Pediatr 155, Suppl 2: S14–S17, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Kunzmann S, Speer CP, Jobe AH, Kramer BW. Antenatal inflammation induced TGF-β1 but suppressed CTGF in preterm lungs. Am J Physiol Lung Cell Mol Physiol 292: L223–L231, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Laitinen L. Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. Histochem J 19: 225–234, 1987 [DOI] [PubMed] [Google Scholar]
- 41.Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, Lungarella G, Brody A, Friedman M. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol 275: L365–L371, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Lecart C, Cayabyab R, Buckley S, Morrison J, Kwong KY, Warburton D, Ramanathan R, Jones CA, Minoo P. Bioactive transforming growth factor-beta in the lungs of extremely low birthweight neonates predicts the need for home oxygen supplementation. Biol Neonate 77: 217–223, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Lin G, Chow S, Lin J, Wang G, Lue TF, Lin CS. Effect of cell passage and density on protein kinase G expression and activation in vascular smooth muscle cells. J Cell Biochem 92: 104–112, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res 58: 22–29, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol 91: 1421–1430, 2001 [DOI] [PubMed] [Google Scholar]
- 46.MacRitchie AN, Albertine KH, Sun J, Lei PS, Jensen SC, Freestone AA, Clair PM, Dahl MJ, Godfrey EA, Carlton DP, Bland RD. Reduced endothelial nitric oxide synthase in lungs of chronically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol 281: L1011–L1020, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Magee JC, Stone AE, Oldham KT, Guice KS. Isolation, culture, and characterization of rat lung microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 267: L433–L441, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Massaro D, Teich N, Massaro GD. Postnatal development of pulmonary alveoli: modulation in rats by thyroid hormones. Am J Physiol Regul Integr Comp Physiol 250: R51–R55, 1986 [DOI] [PubMed] [Google Scholar]
- 49.Mattsson G, Carlsson PO, Olausson K, Jansson L. Histological markers for endothelial cells in endogenous and transplanted rodent pancreatic islets. Pancreatology 2: 155–162, 2002 [DOI] [PubMed] [Google Scholar]
- 50.McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, Kerecman JD, Albertine KH, Winter VT, Coalson JJ, Crapo JD, Grubb PH, Shaul PW. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol 288: L450–L459, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Nakanishi H, Sugiura T, Streisand JB, Lonning SM, Roberts JD., Jr TGF-β-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol 293: L151–L161, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Nicola T, Hagood JS, James ML, Macewen MW, Williams TA, Hewitt MM, Schwiebert L, Bulger A, Oparil S, Chen YF, Ambalavanan N. Loss of Thy-1 inhibits alveolar development in the newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 296: L738–L750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obara H, Pappas CT, Northway WH, Jr, Bensch KG. Comparison of the effect of two and six week exposure to 80% and 100% oxygen on the lung of the newborn mouse: a quantitative SEM and TEM correlative study. Int J Radiat Oncol Biol Phys 11: 285–298, 1985 [DOI] [PubMed] [Google Scholar]
- 55.Perrella MA, Yoshizumi M, Fen Z, Tsai JC, Hsieh CM, Kourembanas S, Lee ME. Transforming growth factor-beta 1, but not dexamethasone, down-regulates nitric-oxide synthase mRNA after its induction by interleukin-1 beta in rat smooth muscle cells. J Biol Chem 269: 14595–14600, 1994 [PubMed] [Google Scholar]
- 56.Pilz RB, Broderick KE. Role of cyclic GMP in gene regulation. Front Biosci 10: 1239–1268, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Qian L, Liu H, Yu W, Wang X, Sun Z, Wang W, Zhu L, Sun B. Effects of positive end-expiratory pressure, inhaled nitric oxide and surfactant on expression of proinflammatory cytokines and growth factors in preterm piglet lungs. Pediatr Res 64: 17–23, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Rasband WS. Image J Bethesda, MD: National Institutes of Health, 1997–2007 http://rsb.info.nih.gov/ij/ [13 Oct. 2007]. [Google Scholar]
- 59.Resnik E, Herron J, Keck M, Sukovich D, Linden B, Cornfield DN. Chronic intrauterine pulmonary hypertension selectively modifies pulmonary artery smooth muscle cell gene expression. Am J Physiol Lung Cell Mol Physiol 290: L426–L433, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Roberts JD, Jr, Chiche JD, Weimann J, Steudel W, Zapol WM, Bloch KD. Nitric oxide inhalation decreases pulmonary artery remodeling in the injured lungs of rat pups. Circ Res 87: 140–145, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Roberts JD, Jr, Roberts CT, Jones RC, Zapol WM, Bloch KD. Continuous nitric oxide inhalation reduces pulmonary arterial structural changes, right ventricular hypertrophy, and growth retardation in the hypoxic newborn rat. Circ Res 76: 215–222, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Rothman A, Kulik TJ, Taubman MB, Berk BC, Smith CW, Nadal-Ginard B. Development and characterization of a cloned rat pulmonary arterial smooth muscle cell line that maintains differentiated properties through multiple subcultures. Circulation 86: 1977–1986, 1992 [DOI] [PubMed] [Google Scholar]
- 63.Schini VB, Durante W, Elizondo E, Scott-Burden T, Junquero DC, Schafer AI, Vanhoutte PM. The induction of nitric oxide synthase activity is inhibited by TGF-beta 1, PDGFAB and PDGFBB in vascular smooth muscle cells. Eur J Pharmacol 216: 379–383, 1992 [DOI] [PubMed] [Google Scholar]
- 64.Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC., 3rd Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 272: L1005–L1012, 1997 [DOI] [PubMed] [Google Scholar]
- 65.Soutiere SE, Tankersley CG, Mitzner W. Differences in alveolar size in inbred mouse strains. Respir Physiol Neurobiol 140: 283–291, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Steinhorn RH, Russell JA, Morin FC., 3rd Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol Heart Circ Physiol 268: H1483–H1489, 1995 [DOI] [PubMed] [Google Scholar]
- 67.Sugiura T, Nakanishi H, Roberts JD., Jr Proteolytic processing of cGMP-dependent protein kinase I mediates nuclear cGMP signaling in vascular smooth muscle cells. Circ Res 103: 53–60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tamura N, Itoh H, Ogawa Y, Nakagawa O, Harada M, Chun TH, Suga S, Yoshimasa T, Nakao K. cDNA cloning and gene expression of human type Ialpha cGMP-dependent protein kinase. Hypertension 27: 552–557, 1996 [DOI] [PubMed] [Google Scholar]
- 69.ter Horst SA, Walther FJ, Poorthuis BJ, Hiemstra PS, Wagenaar GT. Inhaled nitric oxide attenuates pulmonary inflammation and fibrin deposition and prolongs survival in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 293: L35–L44, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Tomkeieff SI. Linear intercepts, areas, and volumes. Nature 155: 24 (correction on p. 107), 1945 [Google Scholar]
- 71.Tschanz SA, Burri PH. A new approach to detect structural differences in lung parenchyma using digital image analysis. Exp Lung Res 28: 457–471, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Tschanz SA, Burri PH. Postnatal lung development and its impairment by glucocorticoids. Pediatr Pulmonol Suppl 16: 247–249, 1997 [DOI] [PubMed] [Google Scholar]
- 73.Tzao C, Nickerson PA, Russell JA, Gugino SF, Steinhorn RH. Pulmonary hypertension alters soluble guanylate cyclase activity and expression in pulmonary arteries isolated from fetal lambs. Pediatr Pulmonol 31: 97–105, 2001 [DOI] [PubMed] [Google Scholar]
- 74.Vento G, Capoluongo E, Matassa PG, Concolino P, Vendettuoli V, Vaccarella C, Frezza S, Zuppi C, Romagnoli C, Ameglio F. Serum levels of seven cytokines in premature ventilated newborns: correlations with old and new forms of bronchopulmonary dysplasia. Intensive Care Med 32: 723–730, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Vermeersch P, Buys E, Pokreisz P, Marsboom G, Ichinose F, Sips P, Pellens M, Gillijns H, Swinnen M, Graveline A, Collen D, Dewerchin M, Brouckaert P, Bloch KD, Janssens S. Soluble guanylate cyclase-alpha1 deficiency selectively inhibits the pulmonary vasodilator response to nitric oxide and increases the pulmonary vascular remodeling response to chronic hypoxia. Circulation 116: 936–943, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol 31: 650–656, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Villamor E, Le Cras TD, Horan MP, Halbower AC, Tuder RM, Abman SH. Chronic intrauterine pulmonary hypertension impairs endothelial nitric oxide synthase in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 272: L1013–L1020, 1997 [DOI] [PubMed] [Google Scholar]
- 78.Viscardi RM, Atamas SP, Luzina IG, Hasday JD, He JR, Sime PJ, Coalson JJ, Yoder BA. Antenatal Ureaplasma urealyticum respiratory tract infection stimulates proinflammatory, profibrotic responses in the preterm baboon lung. Pediatr Res 60: 141–146, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev 92: 55–81, 2000 [DOI] [PubMed] [Google Scholar]
- 80.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol Lung Cell Mol Physiol 275: L110–L117, 1998 [DOI] [PubMed] [Google Scholar]
- 81.Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, Black SM. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 289: L660–L666, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weibel ER. Volume density measurements. In: Stereological Methods London: Academic Press, 1979, p. 26–27 [Google Scholar]
- 83.Wu S, Capasso L, Lessa A, Peng J, Kasisomayajula K, Rodriguez M, Suguihara C, Bancalari E. High tidal volume ventilation activates Smad2 and upregulates expression of connective tissue growth factor in newborn rat lung. Pediatr Res 63: 245–250, 2008 [DOI] [PubMed] [Google Scholar]
- 84.Young SL, Evans K, Eu JP. Nitric oxide modulates branching morphogenesis in fetal rat lung explants. Am J Physiol Lung Cell Mol Physiol 282: L379–L385, 2002 [DOI] [PubMed] [Google Scholar]
- 85.Zeng Y, Zhuang S, Gloddek J, Tseng CC, Boss GR, Pilz RB. Regulation of cGMP-dependent protein kinase expression by Rho and Kruppel-like transcription factor-4. J Biol Chem 281: 16951–16961, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Zhou W, Dasgupta C, Negash S, Raj JU. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 292: L1459–L1466, 2007. [DOI] [PubMed] [Google Scholar]