Abstract
Asthma is characterized by airway inflammation, mucus overproduction, airway hyperreactivity, and peribronchial fibrosis. Intelectin has been shown to be increased in airway epithelium of asthmatics. However, the role of intelectin in the pathogenesis of asthma is unknown. Airway epithelial cells can secrete chemokines such as monocyte chemotactic protein (MCP)-1 and -3 that play crucial roles in asthmatic airway inflammation. We hypothesized that intelectin plays a role in allergic airway inflammation by regulating chemokine expression. In a mouse allergic asthma model, we found that mRNA expression of intelectin-2 as well as MCP-1 and -3 in mouse lung was increased very early (within 2 h) after allergen challenge. Expression of intelectin protein was localized to mucous cells in airway epithelium. Treatment of MLE12 mouse lung epithelial cells with interleukin IL-13, a critical mediator of allergic airway disease, induced expression of intelectin-1 and -2 as well as MCP-1 and -3. When IL-13-induced intelectin-1 and -2 expression was inhibited by RNA interference, IL-13-induced extracellular signal-regulated kinase 1/2 phosphorylation and MCP-1 and -3 production by MLE12 cells was inhibited. Furthermore, inhibition of intelectin expression by airway transfection with shRNA targeting intelectin-1 and -2 attenuated allergen-induced airway inflammation. We conclude that intelectin, a molecule expressed by airway epithelial cells and upregulated in asthma, is required for IL-13-induced MCP-1 and -3 production in mouse lung epithelial cells and contributes to allergic airway inflammation.
Keywords: lectin, chemokine, asthma
asthma is an increasingly common disease with a worldwide impact on health care systems (8). Asthma is characterized by T-helper type 2 (Th2) airway inflammation, mucus overproduction, airway hyperreactivity (AHR), and peribronchial fibrosis. A number of chemokines such as monocyte chemotactic protein (MCP)-1 and -3 and RANTES (regulated on activation, normal T cells expressed and secreted) play essential roles in asthmatic airway inflammation (15). These chemokines can be secreted by airway epithelial cells and alveolar macrophages, and are responsible for the recruitment of inflammatory cells from the vascular compartment to the lung (1, 6, 19, 21, 22). The Th2 cytokine IL-13, which is a critical mediator of allergic airway disease (7, 27), has been shown to induce the expression of MCP-1 and -3 in mouse lung and human bronchial epithelial cells (2, 9). The mechanism by which IL-13 induces the expression of these chemokines in asthmatic airway remains unclear.
Intelectins are IL-13-inducible lectins that are produced by epithelial cells in the airway and the intestine (10, 30). We have shown that IL-13 stimulates intelectin expression in mouse airway in vivo and in cultured human bronchial epithelial cells (30). Furthermore, intelectin expression was found to be increased in bronchial epithelial cells from asthmatic subjects (12). Recently, it has been reported that a single-nucleotide polymorphism in intelectin-1 is associated with increased asthma risk (18). These findings indicate that intelectin may play a role in the pathogenesis of asthma.
Intelectin was first reported to be expressed in small intestinal Paneth cells in mice (10). There are two intelectin genes, intelectin-1 and -2, in mouse and human. These genes are highly homologous to a Xenopus oocyte granule lectin (13, 17). The cDNA sequence for mouse intelectin-1 and -2 have 94% identity (17). Human intelectin-1 is also known as omentin (20) and as intestinal lactoferrin receptor (23) since it is found in human omental adipose tissue and can bind to human lactoferrin. Human intelectin-1 is a soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall, indicating that intelectin may play a role in immune defense against bacteria (24). Intelectin has also been implicated in clearance of intestinal nematode parasite infestations. Intelectin-1 and -2 expression is increased in small intestine of resistant BALB/c mice after nematode parasite infection, whereas intelectin-2 gene is either lacking or not increased in susceptible mouse strains (3, 17). Interestingly, Th2 inflammation is essential for clearing intestinal parasite infection (25). Since intelectin expression is increased during Th2 response in both asthma and parasite infection, we hypothesized that intelectin plays a role in asthmatic airway inflammation by regulating chemokine expression.
To test our hypothesis, we examined the kinetics of intelectin-1 and -2 and MCP-1 and -3 expression and the localization of intelectin protein in a mouse allergic asthma model. We used RNA interference to determine whether intelectin is involved in the upregulation of MCP-1, MCP-3, and RANTES after IL-13 treatment of mouse lung epithelial cells and allergen-induced airway inflammation. The results support a role for intelectin in IL-13-mediated increases in expression of the chemokines MCP-1 and -3 and allergic airway inflammation.
MATERIALS AND METHODS
Mouse allergic asthma model and airway transfection with short hairpin RNA.
All procedures and protocols were reviewed and approved by the Animal Care and Use Committee at Tongji Medical College of Huazhong University of Science and Technology. FVB/NCrl mice (Charles River Laboratories) were used for the allergic asthma model. The procedure has been described in previous studies (12). In brief, mice were sensitized by intraperitoneal administration of ovalbumin (OVA) mixed with adjuvant three times at weekly intervals (day 0, 7, 14). Control mice received adjuvant alone. Beginning 1 wk after the last injection, mice were challenged three times by intranasal administration of OVA at daily intervals (day 21, 22, 23). Control mice were challenged with PBS alone.
For airway transfection with shRNA, mice were divided into three groups: unsensitized mice treated with control short hairpin RNA (shRNA) plasmid and challenged with PBS (control group); OVA-sensitized and -challenged mice treated with control shRNA plasmid and challenged with OVA (OVA group); or OVA-sensitized and -challenged mice treated with intelectin shRNA plasmid and challenged with OVA (OVA + shRNA group). Twenty micrograms of control or intelectin shRNA plasmids were complexed with ExGen500 (Fermentas) and 5% glucose, and a total volume of 40 μl was instilled intranasally into an anesthetized mouse 24 h before each of the three PBS or OVA challenges. Mouse lungs were harvested 24 h after PBS or OVA challenges for bronchoalveolar lavage (BAL), quantitative RT-PCR, and histology.
Construction and screening of plasmids expressing shRNA.
PGCsi 3.0 vector (Genechem, China) was used to construct plasmids for the expression of shRNAs under the control of the human U6 promoter. A 21-nt sense sequence and the reverse complement of the same sequence (antisense) separated by a short hairpin spacer (loop) were cloned downstream of U6 promoter in the PGCsi 3.0 vector. Six thymidines were used as the termination signal. All constructs were sequenced to confirm identity. Three intelectin shRNAs designed to target different conserved sequences in mouse intelectin-1 and -2 were screened by determining the levels of intelectin transcripts after transfection of MLE12 cells with these plasmids. One of the three designed intelectin shRNAs significantly inhibited the basal level of both intelectin-1 and -2 transcripts in MLE12 cells (data not shown) and was used for all shRNA experiments presented here. The sequence of this intelectin shRNA (sense strand) was: 5′-AAGGAAAGTGTTGGACTGACATTCAAGACGTGTCAGTCCAACACTTTCCTT-3′. A control shRNA plasmid designed not to target any mouse genes was used for comparison. The sequence of control shRNA (sense strand) was: 5′-AAAAGAGGCTTGCACAGTGCATTCAAGACGTGCACTGTGCAAGCCTCTTTT-3′.
MLE12 cell culture, transfection with shRNA, IL-13, and galactose treatment.
MLE12 cells (CRL-2110, American Type Culture Collection) were cultured in DMEM/F-12 medium (Hyclone) in six-well plates and divided into three groups: cells transfected with control shRNA plasmid and cultured in the absence of IL-13 (control group); cells transfected with control shRNA plasmid and cultured in IL-13-containing medium (IL-13 group); or cells transfected with intelectin shRNA plasmid and cultured in IL-13-containing medium (IL-13 + shRNA group), n = 4 wells/group. When cells were 90% confluent, 4 μg of intelectin shRNA or control shRNA plasmid in 250 μl of Opti-MEM medium was mixed with 10 μl of Lipofectamine 2000 (Invitrogen) diluted in 240 μl of medium and added to each well. Five hours later, the media were substituted with DMEM/F-12 with or without IL-13 (20 ng/ml). Forty-eight hours later, cells were harvested for quantitative RT-PCR and Western blot, and the media were collected for ELISA. For inhibition of intelectin with galactose, galactose (Sigma) was added to the medium (final concentration 30 mM) 1 h before IL-13 treatment. For inhibition of ERK1/2 pathway, PD-98059 (Sigma) was added to the medium (final concentration 30 μM) 1 h before IL-13 treatment.
Quantitative RT-PCR.
Total RNA from mouse lungs and MLE12 cells was isolated using TRIzol (Invitrogen). First-strand cDNA synthesis was performed using PrimeScript RT reagent kit (Takara, Japan). Sybr Green real-time PCR was performed for mouse intelectin-1, intelectin-2, MCP-1, MCP-3, and RANTES using Perfect Real Time kit (Takara, Japan). The primers for intelectin-1 were 5′-TGACAATGGTCCAGCATTACC-3′ and 5′-ACGGGGTTACCTTCTGGGA-3′. The primers for intelectin-2 were 5′-GCGCTTGGGCCATAATCTGT-3′ and 5′-CGGCCAGAGGGAGAGTAATAA-3′. The sequences of the primers for MCP-1, -3, and RANTES were obtained from Primer Bank (http://pga.mgh.harvard.edu/primerbank/). Real-time PCR was performed using SYBR Premix Ex Taq polymerase (Takara, Japan) and Rotor-Gene 3000 (Corbett Research, Australia). The cycle threshold (Ct) of each mouse gene transcript was normalized to the Ct of mouse β-actin. Fold differences were determined by the 2−ΔΔCt method (14).
Western blotting.
Mouse lungs and MLE12 cells were harvested at the indicated time points, washed twice with cold PBS, and lysed in M-PER Mammalian Protein Extraction Reagent with PMSF (Pierce Biotechnology). After 10 min on ice, lysates were centrifuged at 15,000 g for 15 min to remove insoluble material. Fifty micrograms of protein samples were resolved on 10% SDS-PAGE and transferred and immunoprobed with chicken antibody against intelectin (1:500). The intelectin antibody was raised against a peptide containing a sequence that is completely conserved between mouse intelectin-1 and -2 (4). The antibody was kindly provided by Dr. Alan Pemberton (Div. of Veterinary Clinical Sciences, Univ. of Edinburgh, Roslin, UK). Antibody was detected using peroxidase-conjugated rabbit anti-chicken IgY (1:10,000, Sigma) followed by ECL Western blot detection reagent (Pierce Biotechnology). Blots were stripped and then reprobed for β-actin (1:5,000, Santa Cruz Biotechnology). Densitometry was performed using ImageJ (National Institutes of Health), and the protein level of intelectin was indexed to β-actin. For phosphorylated ERK and total ERK Western blotting, rabbit polyclonal phosphorylated ERK antibody (1:200, Santa Cruz Biotechnology) and total ERK antibody (1:200, Cell Signaling Technology) were used.
Immunohistochemistry and Alcian blue staining.
Mouse lungs were fixed for 5 min by instillation of 4% paraformaldehyde-PBS (Sigma) through a tracheal catheter at a transpulmonary pressure of 15 cmH2O, and lungs were fixed in 4% paraformaldehyde-PBS overnight. Five-micrometer-thick sections were used for immunohistochemistry with chicken antibody against intelectin (1:500) and control chicken IgY (R&D Systems). The peroxidase-conjugated rabbit anti-chicken IgY (1:750, Sigma) was used as the secondary antibody. Antibodies were detected using the DAB kit (Zhongshan Goldenbridge Biotechnology, China) as directed by the manufacturer. Lung sections were also stained with 1% Alcian blue (pH 2.5, Sigma) for detection of mucus.
Assessment of airway inflammation.
BAL fluid eosinophils were measured as previously described (12). Paraffin-embedded 5-μm lung sections were stained with H&E. The severity of peribronchial inflammation was scored by a blinded observer using the following features: 0, normal; 1, few cells; 2, a ring of inflammatory cells one cell layer deep; 3, a ring of inflammatory cells two to four cells deep; 4, a ring of inflammatory cells of >4 cells deep (16).
ELISA for MCP-1 and MCP-3.
Mouse MCP-1 and -3 concentrations in MLE12 cell culture supernatants were determined using commercially available ELISA sets (R&D Systems). ELISA was performed according to the manufacturer's instructions. All samples and standards were measured in triplicate.
Statistical analysis.
Data are presented as means ± SE. Differences between groups were assessed by one-way ANOVA analysis followed by Student-Newman-Keuls test. Statistical difference was accepted at P < 0.05.
RESULTS
Intelectin-2 and MCP-1 and -3 are rapidly upregulated after airway allergen challenge.
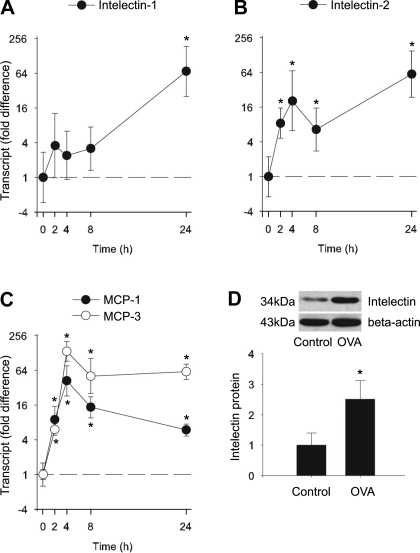
We first determined the kinetics of intelectin-1 and -2 and MCP-1 and -3 expression in mouse lung after sensitization and challenge with OVA. We measured expression of these genes in lung at 0, 2, 4, 8, and 24 h after a single OVA challenge of sensitized mice by quantitative RT-PCR and Western blot analysis. We found that intelectin-1 transcripts were increased 24 h after the OVA challenge (Fig. 1A). However, intelectin-2 transcripts were increased very early (within 2 h), and this increase was sustained at later time points (4, 8, and 24 h; Fig. 1B). Intelectin-1 and -2 mRNA remained elevated after three OVA challenges (24 h after the last of 3 daily OVA challenges, data not shown). Western blotting with an antibody that recognizes identical sequences in intelectin-1 and -2 showed that the amount of intelectin protein was significantly increased after three OVA challenges (Fig. 1D). Similar to intelectin-2 mRNA expression, MCP-1 and -3 transcripts were also increased by 2 h after a single OVA challenge. MCP-1 and -3 transcripts peaked at 4 h and remained elevated at 8 and 24 h (Fig. 1C). Our findings indicate that the expression of intelectin-1 and -2 and MCP-1 and -3 are upregulated in the lung after OVA challenge. Also of note, intelectin-2 and MCP-1 and -3 are all rapidly upregulated at a very early stage after OVA challenge.
Fig. 1.
Kinetics of intelectin-1, intelectin-2, and monocyte chemotactic protein (MCP)-1 and -3 expression in mouse lung after sensitization and challenge with ovalbumin (OVA). FVB/NCrl mice were sensitized and challenged with OVA as described in materials and methods. Lungs were harvested at 0, 2, 4, 8, 24, and 72 h after the first OVA challenge. Intelectin-1 (A), intelectin-2 (B), and MCP-1 and -3 (C) transcript expression was measured by quantitative RT-PCR. Fold difference was calculated relative to expression in control mice without OVA sensitization and challenge (time 0). Western blot (D) was performed to examine the protein level of intelectin in lungs from control mice and mice at 72 h after the first OVA challenge (24 h after the last of 3 daily challenges). Representative Western blots from 4 mice in each group are shown. Densitometry analysis of intelectin protein was normalized by β-actin. The protein level was calculated relative to control mice. Results shown are means ± SE for 4 mice at each time point. *P < 0.05 compared with control mice.
The expression of intelectin protein is localized to airway mucous cells.
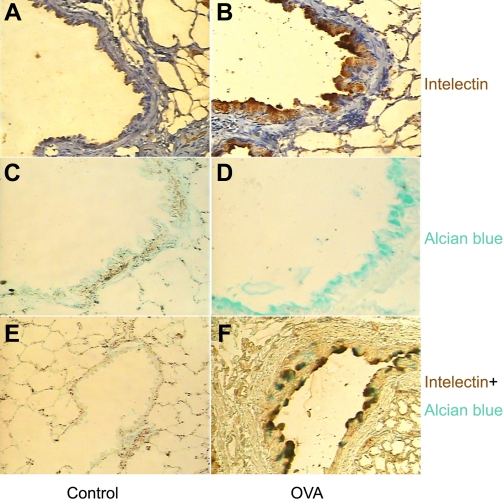
Next we determined the localization of intelectin protein in mouse lung. Intelectin has been shown to be expressed in mouse intestinal Paneth and goblet cells (10, 17) and sheep airway mucous cells (4). To examine the localization of intelectin protein in mouse OVA asthma model, we performed immunohistochemical staining using an antibody against both intelectin-1 and -2. We also did Alcian blue staining to detect mucus-containing cells in mouse lung sections. We could not detect staining for intelectin in control mouse lungs (Fig. 2, A and E). Alcian blue staining was also absent in control mouse lungs (Fig. 2, C and E). However, staining for intelectin was detected in a subset of airway epithelial cells with mucous cell morphology in the lungs from mice sensitized and challenged with OVA (Fig. 2B). Furthermore, intelectin staining was seen in the same regions stained with Alcian blue on consecutive lung sections (Fig. 2, D and F). Our data indicate that intelectin is expressed in airway mucous cells in a mouse allergic asthma model.
Fig. 2.
The expression of intelectin protein is localized to airway mucous cells in mice after sensitization and challenge with OVA. A and B: immunostaining was performed to detect the localization of intelectin protein in control mice (A) and in mice sensitized and challenged with OVA (B). C and D: Alcian blue staining was used to detect mucus-containing cells. E and F: double staining with intelectin antibody and Alcian blue. Original magnification, × 200.
Intelectin is required for IL-13-induced MCP-1 and -3 expression in mouse lung epithelial cells.
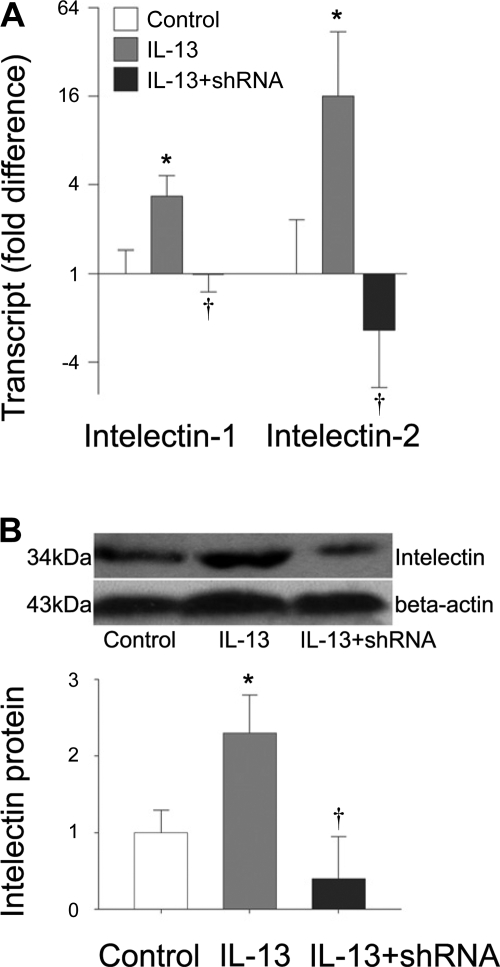
Since MCP-1 and -3 have been reported to be secreted by airway epithelial cells and intelectin is also expressed in airway epithelium, we next used a mouse lung epithelial cell line, MLE12, to examine whether intelectin is involved in the production of these chemokines. We first examined the expression of intelectin-1 and -2 in MLE12 cells after IL-13 treatment. We found that both intelectin-1 and -2 transcripts and protein were significantly increased at 48 h after IL-13 treatment (Fig. 3). After transfection with intelectin shRNA targeting conserved sequences in intelectin-1 and -2, expression of both intelectin-1 and -2 transcripts in IL-13-stimulated cells was reduced to levels similar to those seen in cells not stimulated with IL-13 (Fig. 3A). In a separate experiment, we confirmed that IL-13-induced intelectin protein expression was also inhibited after intelectin shRNA transfection by Western blot (Fig. 3B).
Fig. 3.
Transfection with intelectin shRNA inhibits IL-13-induced intelectin expression in MLE12 cells. MLE12 cells were transfected with control shRNA plasmid and cultured in the absence of IL-13 (control), transfected with control shRNA plasmid and cultured in IL-13-containing medium (IL-13), or transfected with intelectin shRNA plasmid and cultured in IL-13-containing medium (IL-13 + shRNA). Forty-eight hours after IL-13 treatment, cells were harvested for RNA extraction and Western blot. A: intelectin-1 and -2 transcript expression was measured by quantitative RT-PCR. Fold difference was calculated relative to expression in control group. B: intelectin protein was measured by Western blotting. Representative Western blots from 4 replicate wells in each group are shown. Densitometry analysis of intelectin protein was normalized by β-actin. The protein level was calculated relative to control group. Results shown are means ± SE for 4 replicate wells. *P < 0.05 compared with control group. †P < 0.05 compared with the group treated with control shRNA and IL-13.
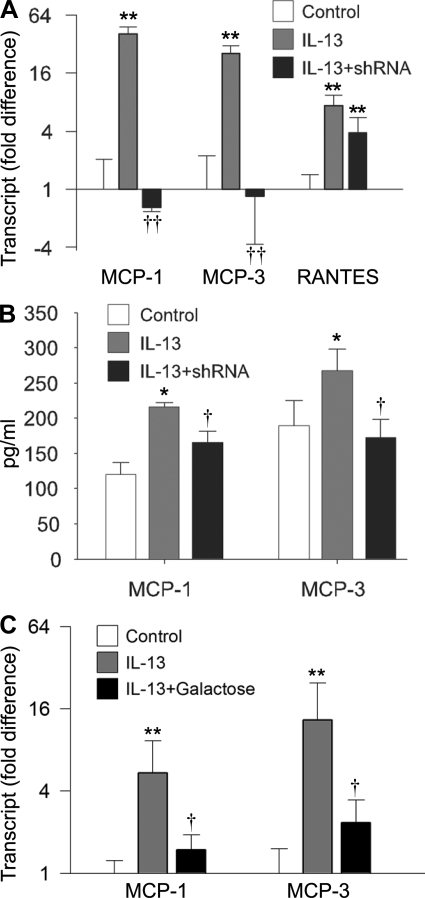
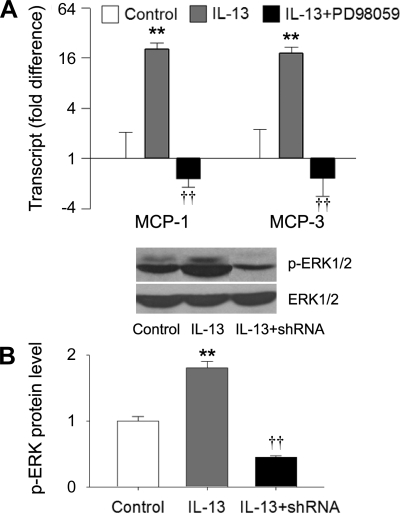
We used the MLE12 system to determine whether intelectin plays a role in IL-13-induced chemokine expression. Using quantitative RT-PCR, we found that the transcript levels of MCP-1, MCP-3, and RANTES were significantly increased following 48 h of IL-13 treatment (Fig. 4A). Using ELISA, we confirmed that the concentrations of MCP-1 and -3 in the media from the IL-13 group were significantly higher than the control group (Fig. 4B). Remarkably, the IL-13-induced MCP-1 and -3 mRNA expression was completely prevented in the cells transfected with intelectin shRNA (Fig. 4A). In contrast, mRNA encoding another chemokine, RANTES, was still induced by IL-13 in intelectin shRNA-treated cells (Fig. 4A), indicating that some aspects of the IL-13 response were not completely intelectin dependent. MCP-1 and -3 ELISAs indicated that IL-13-induced MCP-1 and MCP-3 protein production was also inhibited by intelectin shRNA (Fig. 4B). Pretreatment of MLE12 cells with galactose, which binds intelectin and can be used as an inhibitor of intelectin (24), also inhibited IL-13-induced MCP-1 and -3 mRNA expression (Fig. 4C). These data indicate that intelectin is required for IL-13-induced MCP-1 and -3 expression in mouse lung epithelial cells.
Fig. 4.
Intelectin is required for IL-13-induced MCP-1 and -3 expression in MLE12 cells. MLE12 cells were transfected with control shRNA plasmid and cultured in the absence of IL-13 (control), transfected with control shRNA plasmid and cultured in IL-13-containing medium (IL-13), or transfected with intelectin shRNA plasmid and cultured in IL-13-containing medium (IL-13 + shRNA). Forty-eight hours after IL-13 treatment, cells were harvested for quantitative RT-PCR, and the media was collected for ELISA. A: gene transcript expression was measured by quantitative RT-PCR. Fold difference was calculated relative to expression in control group. B: ELISA for MCP-1 and -3. C: galactose was added into medium 1 h before IL-13 treatment. MCP-1 and -3 transcript expression was measured by quantitative RT-PCR. Fold difference was calculated relative to expression in control group. Results are shown as means ± SE for 4 replicate wells. *P < 0.05, **P < 0.01 compared with control group. †P < 0.05, ††P < 0.01 compared with the group treated with IL-13 and control shRNA or galactose.
IL-13-induced MCP-1 and -3 expression is mediated by the ERK1/2 pathway, and intelectin is required for the IL-13-induced ERK1/2 activation.
Since ERK1/2 signaling pathway has been implicated in IL-13-induced MCP-1 expression in bronchial epithelial cells (9), we examined whether ERK1/2 pathway is involved in IL-13-induced MCP-1 and -3 expression in MLE12 cells. We found that PD-98059, an inhibitor of ERK1/2, blocked IL-13-induced MCP-1 and -3 expression in MLE12 cells (Fig. 5A). Furthermore, IL-13 stimulation increased ERK1/2 phosphorylation, and intelectin shRNA transfection inhibited IL-13-induced ERK1/2 phosphorylation (Fig. 5B). Signal transducer and activator of transcription 6 (Stat6) is believed to be a critical signaling molecule mediating the biological effects of IL-13 (11, 29). We found that intelectin shRNA transfection did not inhibit IL-13-induced Stat6 phosphorylation (data not shown). These data indicate that the ERK1/2 pathway mediates IL-13-induced MCP-1 and -3 expression, and that intelectin expression is required for IL-13-induced ERK1/2 activation in mouse lung epithelial cells. Therefore, intelectin may participate in IL-13-induced MCP-1 and -3 expression, at least in part, through contributing to ERK1/2 activation.
Fig. 5.
IL-13-induced MCP-1 and -3 expression is mediated by ERK signaling, and intelectin is required for IL-13-induced ERK activation. MLE12 cells were treated with IL-13 or both PD-98059 and IL-13. Forty-eight hours after treatment, cells were harvested for quantitative RT-PCR and Western blotting. A: MCP-1 and -3 transcript expression was measured by quantitative RT-PCR. Fold difference was calculated relative to expression in control group. B: phosphorylated ERK1/2 and total ERK1/2 protein were measured by Western blotting. Densitometry analysis of phosphorylated ERK1/2 was normalized by total ERK1/2. The protein level was calculated relative to control group. Results shown are means ± SE for 3 replicate wells. **P < 0.01 compared with control group. ††P < 0.01 compared with the group treated with IL-13.
Inhibition of intelectin expression attenuates allergic airway inflammation.
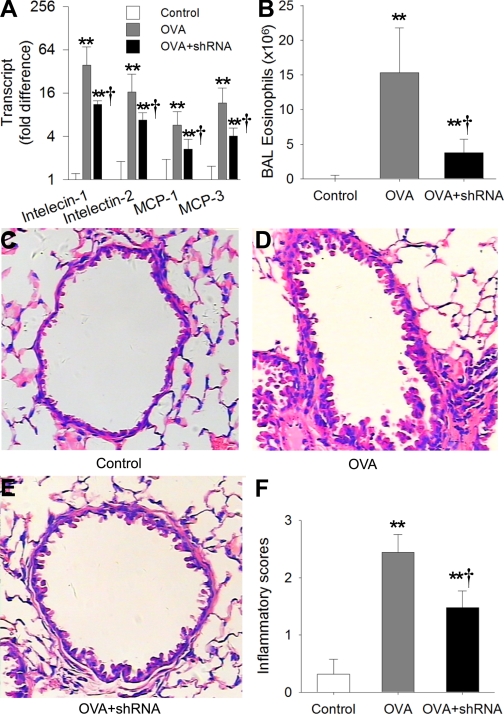
Since intelectin participates in the expression of chemokines MCP-1 and -3, we asked whether intelectin is involved in in vivo allergic airway inflammation. To address this question, mouse airway was transfected with intelectin shRNA 24 h before each OVA challenge. We found that intelectin shRNA transfection inhibited the increase of intelectin-1 and -2 transcript levels after three OVA challenges by 72% and 59%, respectively, when compared with OVA-challenged mice transfected with control plasmid (Fig. 6A). Intelectin shRNA transfection also inhibited the increase of MCP-1 and -3 transcript levels (Fig. 6A). The number of eosinophils in BAL fluid from OVA-challenged mice was decreased by 76% after intelectin shRNA transfection when compared with control shRNA transfection (Fig. 6B). Histological analysis revealed that inflammatory cell infiltration around conducting airways was ameliorated after intelectin shRNA transfection (Fig. 6, C, D, and E). Evaluation of airway inflammation with inflammation score also confirmed that transfection of airway with intelectin shRNA inhibited inflammatory cell infiltration around airways (Fig. 6F). These data indicate that intelectin is involved in allergic airway inflammation.
Fig. 6.
Inhibition of intelectin expression attenuates OVA-induced airway inflammation. Unsensitized mice were transfected with control shRNA plasmid 24 h before each PBS challenge (control). OVA-sensitized mouse was transfected with control shRNA plasmid 24 h before each OVA challenge (OVA) or transfected with intelectin shRNA plasmid before each OVA challenge (OVA + shRNA). Mouse lungs were harvested 24 h after OVA challenges. Intelectin-1, intelectin-2, and MCP-1 and -3 transcript expression in mouse lung was measured by quantitative RT-PCR (A). Fold difference was calculated relative to expression in control group. BAL fluid was collected, and eosinophils were counted (B). Lung sections were stained with H&E (C, D, E), and lung inflammation scores (F) were calculated as described in materials and methods. Results shown are means ± SE for 4–6 mice in each group. **P < 0.01 compared with control group, †P < 0.05 compared with OVA group. Original magnification, ×200.
DISCUSSION
Chemokines such as MCP-1 and -3 are responsible for the migration of inflammatory cells and play crucial roles in asthmatic airway inflammation (6, 22). In the present study, we demonstrate that intelectin-2 and MCP-1 and -3 are upregulated very early after allergen challenge in the lung in a mouse allergic asthma model. The expression of intelectin protein is localized to mouse airway mucous cells. For the first time, we show that intelectin is required for IL-13-induced ERK1/2 activation and MCP-1 and -3 expression in mouse lung epithelial cells, and that inhibition of intelectin expression attenuates allergic airway inflammation.
We found that expression of intelectin-2 mRNA as well as MCP-1 and -3 mRNAs were increased very early (within 2 h) in mouse lung after allergen challenge. Consistent with this, we previously found that intelectin mRNA expression was increased in mouse lungs and human bronchial epithelial cells rapidly (within 2 h) after IL-13 treatment (30). The early upregulation of MCP-1 expression after OVA challenge was suggested to be an upstream signal in asthmatic inflammatory process and may be involved in the regulation of expression of other chemokines. In support of this, administration of anti-MCP-1 neutralizing antibody during early stages of OVA treatment significantly reduced the number of monocytes and eosinophils and abolished T lymphocyte accumulation in the lung and decreased AHR by 70% after OVA challenge in a mouse asthma model (6). MCP-3 also plays a significant role in allergen-induced eosinophilic inflammation of airway since pretreatment of mice with an anti-MCP-3 antibody significantly inhibited OVA-induced airway inflammation and BAL eosinophilia (22).
The airway epithelial cells play important roles in asthmatic airway inflammation by producing chemokines such as MCP-1, MCP-3, and RANTES (9, 19, 21). In this study, we show that intelectin expression is localized to mouse airway mucous cells. Our finding is consistent with a previous study showing that intelectin is expressed in sheep airway mucous cells after IL-4 treatment (4). Intelectin has also been shown to be expressed in mouse intestinal Paneth cells and goblet cells (10, 17, 28).
Since intelectin and the chemokines are produced by airway epithelial cells, we used MLE12 cells, a mouse lung epithelial cell line, to investigate the role of intelectin in IL-13-induced chemokine expression. IL-13, a Th2 cytokine, is a critical mediator of allergic airway disease (7, 27). IL-13 treatment increased MCP-1, -3, and RANTES expression in MLE12 cells. IL-13 also stimulated intelectin-1 and -2 expression in MLE12 cells. Using RNA interference and galactose, an inhibitor of intelectin, we showed that intelectin is required for IL-13-induced MCP-1 and -3 expression at both mRNA and protein levels. To our knowledge, this is the first study showing that intelectin mediates IL-13-induced upregulation of MCP-1 and -3.
Since the ERK1/2 pathway has been previously implicated in regulating IL-13-induced MCP-1 production in bronchial epithelial cells (9), we examined the possibility that intelectin might participate in IL-13-induced MCP-1 and -3 expression via effects on ERK1/2 activation. Consistent with this possibility, we found that ERK phosphorylation was inhibited by intelectin RNA interference and that ERK activity was required for IL-13-induced MCP-1 and -3 expression in MLE12 cells. Stat6 is a critical signaling molecule mediating the biological effects of IL-13 (11, 29). IL-13 has been shown to induce MCP-1 expression in a Stat-6 dependent manner in human umbilical vein endothelial cells (5). However, inhibition of intelectin expression did not inhibit IL-13-induced Stat6 activation. The mechanism by which intelectin contributes to ERK pathway activation is not yet known.
Furthermore, we demonstrate that inhibition of intelectin expression by RNA interference attenuates OVA-induced allergic airway inflammation. After airway transfection with intelectin shRNA, the increase of intelectin-1 and -2 transcript levels after OVA challenges was inhibited by 72% and 59%, respectively, and the increase of MCP-1 and -3 was also inhibited. The number of eosinophils was decreased by 76% in BAL fluid retrieved from OVA-challenged mice after intelectin shRNA transfection. Meanwhile, inflammatory cell infiltration around conducting airway of OVA-challenged mice was ameliorated. Our findings indicate that intelectin, a molecule produced by airway epithelial cells, plays an important role in allergic airway inflammation. A recent study shows that there is no difference in the numbers of eosinophils and basophils in lungs among intelectin-1 and intelectin-2 transgenic mice and littermate control (26), indicating that overexpression of intelectin-1 or intelectin-2 alone is not sufficient for airway inflammation, and suggesting that intelectin works together with other allergen-induced mediators to induce eosinophilic inflammation.
In summary, our findings indicate that intelectin is expressed in allergic airway epithelium. Intelectin plays a critical role in the production of MCP-1 and -3 by airway epithelial cells and participates in allergen-induced airway inflammation. Since MCP-1 and -3 are essential chemokines involved in the Th2 inflammation in asthma and intestinal parasite infection, intelectin could be a candidate target in the treatment of these disorders. Further investigations of the therapeutic utility of intelectin intervention in animal models of asthma or intestinal parasite infection are warranted.
GRANTS
This work was supported by grants from the National Natural Science Foundation of China (30770941, 30700244, and 30400193), Natural Science Foundation of Hubei Province (2004ABA232), and National Heart, Lung, and Blood Institute Grant 5R01-HL-085089.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Alan Pemberton (Div. of Veterinary Clinical Sciences, Univ. of Edinburgh, Roslin, UK) for kindly providing intelectin antibody.
REFERENCES
- 1.Alam R, York J, Boyars M, Stafford S, Grant JA, Lee J, Forsythe P, Sim T, Ida N. Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am J Respir Crit Care Med 153: 1398–1404, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Cho SJ, Kang MJ, Homer RJ, Kang HR, Zhang X, Lee PJ, Elias JA, Lee CG. Role of early growth response-1 (Egr-1) in interleukin-13-induced inflammation and remodeling. J Biol Chem 281: 8161–8168, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Datta R, deSchoolmeester ML, Hedeler C, Paton NW, Brass AM, Else KJ. Identification of novel genes in intestinal tissue that are regulated after infection with an intestinal nematode parasite. Infect Immun 73: 4025–4033, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.French AT, Bethune JA, Knight PA, McNeilly TN, Wattegedera S, Rhind S, Miller HR, Pemberton AD. The expression of intelectin in sheep goblet cells and upregulation by interleukin-4. Vet Immunol Immunopathol 120: 41–46, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Goebeler M, Schnarr B, Toksoy A, Kunz M, Brocker EB, Duschl A, Gillitzer R. Interleukin-13 selectively induces monocyte chemoattractant protein-1 synthesis and secretion by human endothelial cells. Involvement of IL-4R alpha and Stat6 phosphorylation. Immunology 91: 450–457, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TN, Proudfoot A, Martinez AC, Dorf M, Bjerke T, Coyle AJ, Gutierrez-Ramos JC. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med 188: 157–167, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282: 2261–2263, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartert TV, Peebles RS., Jr Epidemiology of asthma: the year in review. Curr Opin Pulm Med 6: 4–9, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Ip WK, Wong CK, Lam CW. Interleukin (IL)-4 and IL-13 up-regulate monocyte chemoattractant protein-1 expression in human bronchial epithelial cells: involvement of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2 and Janus kinase-2 but not c-Jun NH2-terminal kinase 1/2 signalling pathways. Clin Exp Immunol 145: 162–172, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komiya T, Tanigawa Y, Hirohashi S. Cloning of the novel gene intelectin, which is expressed in intestinal paneth cells in mice. Biochem Biophys Res Commun 251: 759–762, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 8: 885–889, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Kuperman DA, Lewis CC, Woodruff PG, Rodriguez MW, Yang YH, Dolganov GM, Fahy JV, Erle DJ. Dissecting asthma using focused transgenic modeling and functional genomics. J Allergy Clin Immunol 116: 305–311, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Lee JK, Schnee J, Pang M, Wolfert M, Baum LG, Moremen KW, Pierce M. Human homologs of the Xenopus oocyte cortical granule lectin XL35. Glycobiology 11: 65–73, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta CT) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Lukacs NW. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol 1: 108–116, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Myou S, Leff AR, Myo S, Boetticher E, Tong J, Meliton AY, Liu J, Munoz NM, Zhu X. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med 198: 1573–1582, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pemberton AD, Knight PA, Gamble J, Colledge WH, Lee JK, Pierce M, Miller HR. Innate BALB/c enteric epithelial responses to Trichinella spiralis: inducible expression of a novel goblet cell lectin, intelectin-2, and its natural deletion in C57BL/10 mice. J Immunol 173: 1894–1901, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Pemberton AD, Rose-Zerilli MJ, Holloway JW, Gray RD, Holgate ST. A single-nucleotide polymorphism in intelectin 1 is associated with increased asthma risk. J Allergy Clin Immunol 122: 1033–1034, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Powell N, Humbert M, Durham SR, Assoufi B, Kay AB, Corrigan CJ. Increased expression of mRNA encoding RANTES and MCP-3 in the bronchial mucosa in atopic asthma. Eur Respir J 9: 2454–2460, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Schaffler A, Neumeier M, Herfarth H, Furst A, Scholmerich J, Buchler C. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim Biophys Acta 1732: 96–102, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Sousa AR, Lane SJ, Nakhosteen JA, Yoshimura T, Lee TH, Poston RN. Increased expression of the monocyte chemoattractant protein-1 in bronchial tissue from asthmatic subjects. Am J Respir Cell Mol Biol 10: 142–147, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Stafford S, Li H, Forsythe PA, Ryan M, Bravo R, Alam R. Monocyte chemotactic protein-3 (MCP-3)/fibroblast-induced cytokine (FIC) in eosinophilic inflammation of the airways and the inhibitory effects of an anti-MCP-3/FIC antibody. J Immunol 158: 4953–4960, 1997 [PubMed] [Google Scholar]
- 23.Suzuki YA, Shin K, Lonnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry 40: 15771–15779, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Tsuji S, Uehori J, Matsumoto M, Suzuki Y, Matsuhisa A, Toyoshima K, Seya T. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J Biol Chem 276: 23456–23463, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8: 255–264, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Voehringer D, Stanley SA, Cox JS, Completo GC, Lowary TL, Locksley RM. Nippostrongylus brasiliensis: identification of intelectin-1 and -2 as Stat6-dependent genes expressed in lung and intestine during infection. Exp Parasitol 116: 458–466, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 282: 2258–2261, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Wrackmeyer U, Hansen GH, Seya T, Danielsen EM. Intelectin: a novel lipid raft-associated protein in the enterocyte brush border. Biochemistry 45: 9188–9197, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Yang M, Hogan SP, Henry PJ, Matthaei KI, McKenzie AN, Young IG, Rothenberg ME, Foster PS. Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-alpha chain and STAT-6 independently of IL-5 and eotaxin. Am J Respir Cell Mol Biol 25: 522–530, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, Erle DJ. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol 36: 244–253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]