Abstract
Despite the associated morbidity and mortality, underlying mechanisms leading to the development of acute lung injury (ALI) remain incompletely understood. Frequently, ALI develops in the hospital, coinciding with institution of various therapies, including the use of supplemental oxygen. Although pathological evidence of hyperoxia-induced ALI in humans has yet to be proven, animal studies involving high oxygen concentration reproducibly induce ALI. The potentially injurious role of lower and presumably safer oxygen concentrations has not been well characterized in any species. We hypothesized that in the setting of a preexisting insult to the lung, the addition of moderate-range oxygen can augment lung injury. Our model of low-dose intratracheal LPS (IT LPS) followed by 60% oxygen caused a significant increase in ALI compared with LPS or oxygen alone with increased alveolar neutrophils, histological injury, and epithelial barrier permeability. In the LPS plus oxygen group, regulatory T cell number was reduced, and macrophage activation markers were increased, compared with LPS alone. Antibody-mediated depletion of neutrophils significantly abrogated the observed lung injury for all measured factors. The enhanced presence of alveolar neutrophils in the setting of LPS and oxygen is due, at least in part, to elevated chemokine gradients signaling neutrophils to the alveolar space. We believe these results strongly support an effect of lower concentrations of oxygen to augment the severity of a mild preexisting lung injury and warrants further investigation in both animals and humans.
Keywords: neutrophils, supplemental oxygen, chemokine, macrophage, regulatory T cell
acute lung injury (ALI), and its more severe form, acute respiratory distress syndrome (ARDS), are common disease entities associated with poor outcomes. Annual mortality from ALI is ∼75,000 (32), and despite extensive efforts, few interventions have produced an improvement in survival. Pulmonary inflammation predates the onset of clinically defined ALI/ARDS, and factors that can cause progression to ALI are not well defined (22). Indeed, patients frequently develop ALI after admission to the hospital (6, 22, 28), coinciding with their exposure to various therapies, including supplemental oxygen.
Hyperoxia, or exposure to oxygen tensions >70%, causes lung injury in animals, with severity and mortality rates that are species dependent (7, 15). In limited human studies, hyperoxic exposure has not produced the severity of pathology seen in other species, as identified by neutrophil alveolar infiltrates, intra-alveolar coagulation and fibrin deposition, and denudation of the alveolar epithelial basement membrane; in contrast, only mild increases in alveolar capillary permeability have been observed (5, 12, 22). However, prospective human hyperoxia studies were generally performed in individuals without preexisting lung damage or a known proinflammatory state; therefore, subjects did not have multiple risk factors that could have significantly increased the likelihood of developing ALI or ARDS (2, 5, 8, 35). In contrast, limited animal studies involving secondary exposure to oxygen have demonstrated an augmentation of pathological lung injury and deteriorating lung function (7, 20, 37). This enhanced injury is thought to be due, in part, to the propagation of underlying inflammatory pathways and inhibition of compensatory anti-inflammatory mechanisms (22). In humans, similar propagation of injury may occur such that a preexisting mild injury may lower the threshold for oxygen-induced lung damage, accelerating the development of ALI (20).
The presence of neutrophils in the alveolar space is often described as the pathological hallmark of ALI (22). However, the putative role of alveolar neutrophils in progression of lung injury has been debated and appears to vary based on the species and the model (22, 24, 29, 33). In one study of hyperoxia in rats, increased alveolar neutrophils did not exacerbate measured parameters of lung injury (29). In contrast, a study of hyperoxia in mice investigating the role of CXCR2 demonstrated that impaired recruitment of neutrophils to the alveolar space did abrogate lung injury (36). While the impact of alveolar neutrophils on lung injury in models of hyperoxia is debatable, other models have clear-cut dependence on alveolar neutrophils to promote ALI. Antibody-mediated depletion studies have demonstrated that lung injury secondary to intratracheal lipopolysaccharide (IT LPS) instillation or acid aspiration depends heavily on the accumulation of alveolar neutrophils (20, 22, 33).
In the current study, we exposed mice to 60% oxygen 12 h after administration of IT LPS for up to 4 days and observed significantly augmented lung injury. The potentiation of lung injury with exposure to supplemental oxygen was accompanied by a substantial increase in alveolar neutrophils, and antibody-mediated depletion of neutrophils significantly abrogated the oxygen-stimulated augmentation. In the LPS plus oxygen group, neutrophil chemokines were increased, macrophage activation markers were increased, and regulatory T cell numbers were decreased, contributing to an overall increase in the proinflammatory environment. We believe these findings are relevant to the pathophysiology of lung injury and may have extension to the clinical setting where oxygen remains a mainstay of treatment for patients with a variety of illnesses.
MATERIALS AND METHODS
Animals.
Six-to-eight-week-old male C57BL/6 mice were purchased from the National Cancer Institute (Bethesda, MD). All mice were housed in a specific pathogen-free facility, and experiments were conducted under protocols approved by the Johns Hopkins Animal Care and Use Committee.
Animal care and preparation.
All mice had access ad libitum to food and water. At baseline, mice were weighed and then anesthetized with intraperitoneal ketamine/acetylpromazine (150/13.5 mg/kg) before tracheal exposure or harvest. Escherichia coli LPS (0.375 μg/g; O55:B5 Sigma L2880) or sterile water (control) was instilled intratracheally into the mice via a 20-gauge one-half inch long catheter (Johnson and Johnson, New Brunswick, NJ). At specified time points after instillation, three to eight animals from various groups were anesthetized and killed by exsanguination from the inferior vena cava. The lungs were perfused free of blood with 1 ml of PBS unless otherwise specified.
Oxygen exposure.
Twelve hours after IT LPS, instilled mice were placed in customized and sealed cages with ad libitum food and water. Sixty percent oxygen was achieved with a mixture of air and medical-grade oxygen (Roberts Oxygen, Rockville, MD) at adjustable flow rates and constant pressure, with continuous measurements via an oxygen analyzer with a feedback loop to automatically adjust oxygen concentrations (model 65, www.amio2.com; Advanced Micro Instruments, Huntington Beach, CA). Oxygen exposure was uninterrupted except for ∼5 min every 2 days for cage cleaning.
Neutrophil depletion.
Mice were administered intraperitoneal (IP) injections of anti-Gr-1 (RB6-8C5 clone) antibody [Ab; 250 μg·mouse−1·day−1 (BD Pharmingen, 553123) or isotype Ab (Rat IgG2b, κ) on day −2 and day 0 (at the time of LPS injection)].
Arterial blood gas.
At the time of harvest, animals were anesthetized with intraperitoneal pentobarbital (120 mg/kg). A midline neck incision exposed the trachea to facilitate endotracheal intubation with a 20-gauge catheter, and the animals were subjected to mechanical ventilation with room air (Harvard Apparatus, Boston, MA) at 7 ml/kg. The respiratory rate was 160 breaths/min, and the dead space was adjusted to maintain arterial pH between 7.35 and 7.45. After catheterization of the right carotid artery, mean arterial pressure was continuously monitored (Cardiomax-III) and recorded (Columbus Instruments, Columbus, OH) and was ∼80 mmHg in all mice. Mice were ventilated with room air for 15 min before blood gas sampling. After discarding a 100-μl aliquot of blood, 200–300 μl of arterial blood was collected and analyzed by an automated blood gas analyzer (Instrumentation Laboratories, Lexington, MA).
Analysis of bronchoalveolar lavage fluid.
Bronchoalveolar lavage fluid (BALF) was obtained by cannulating the trachea with a 20-gauge catheter. The right lung was lavaged with two aliquots (0.7 ml) of PBS without calcium; total returns after lavage were 0.8–1.2 ml/mouse. BALF was centrifuged (600 g, 10 min at 4°C), and cell-free supernatants were stored at −80°C. The cell pellet was diluted in PBS, and the total cell number was counted with a hemocytometer after staining with trypan blue. Differential cell counts were done on cytocentrifuge preparations (Cytospin 3; Shandon Scientific, Cheshire, UK) stained with Diff-Quik stain (Baxter Diagnostics, McGaw Park, IL). Cell populations were determined by counting 300 cells/sample, and a percentage was calculated based on a minimum of three mice per group. Total protein was measured in the cell-free supernatant using the Lowry method (21).
Lung morphology.
Mouse lungs (n = 3–5/group) were inflated under a pressure of 25 cmH2O with 1% of low melting agarose (Invitrogen, Carlsbad, CA) for histological evaluation by hematoxylin and eosin staining.
Histology scoring system.
Three to four sections of lung were evaluated per mouse at both high- and low-power views (n = 3–5 mice/condition per time point). Scoring was based on three categories, each assigned a score of 0–4 based on percentage of the tissue affected (0 = 0%, 1 = 1–25% affected, 2 = 26–50%, 3 = 51–75%, and 4 = 76–100%). Categories included interstitial changes (interstitial or interalveolar septal thickening), inflammation (intra-alveolar neutrophilic infiltrate), and consolidation (a combination of both cellular debris and fibrin-filling alveolar space). Scoring was done by a lung pathologist (M. M. Fraig) who was masked to the injury groups.
Lung gravimetrics.
Non-PBS-perfused left lungs from animals (n = 3–5) at day 4 were extracted and weighed in pre-tared tubes for wet lung weight compared with baseline body weight.
BALF and ex vivo supernatants for cytokines/chemokines/albumin.
BAL cell-free supernatants were analyzed with ELISA in duplicate and cell culture supernatants in triplicate for TNFα, activated TGF-β, LIX, MIP-2, and KC (R&D Systems, Minneapolis, MN) and for albumin (Alpha Diagnostic International, San Antonio, TX).
Flow cytometry.
BAL cells were first incubated with Fc Block-2.4G2 (BD PharMingen) antibody to block Fcg III/II receptors before staining with specific antibodies: anti-CD14-FITC (11-0141-82), anti-CD11c-Ax700 (56-0114), anti-IA/IE-PE (12-5321), ant-TLR2-PacBlue (57-9024) (Ebiosience, San Diego, CA); and biotin anti-CD40- (553789) coupled to PE-Texas Red, anti-Ly6g-FITC (553126), anti-CD80-PerCpCy5.5 (560526) (BD Pharmingen, San Diego, CA), with appropriate conjugating isotype controls. The PE Annexin V apoptosis detection kit (BD Pharmingen) was used to identify Annexin V-positive neutrophils. Granulocytes and monocytes were first gated using forward and side scatter. Cell fluorescence was analyzed using a FACSCalibur or FACSAria (Becton Dickinson, San Jose, CA) cytometer. Data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
Ex vivo alveolar macrophage and alveolar epithelial cell line cultures.
After anesthesia, alveolar fluid and cells from unexposed mice were obtained via lavage with 4 × 1.0 ml of PBS with EDTA aliquots. Macrophages (2.5 × 104) were plated in 96-well plates in serum-containing media (SCM). After allowing >2 h for adherence, media were removed and cells were washed with PBS, followed by exposure to LPS in serum-free media (100 ng/ml) or media alone. After 3 h of exposure, either 21% oxygen or an increase to 60% oxygen was administered for certain plates/cells. Supernatants were collected at 4, 8, and 24 h after LPS exposure. Additionally, 1.0 × 105 murine type II alveolar epithelial cells (American Type Culture Collection CRL-2110, MLE-12 cells) were grown in 37°C in 21% O2/5% CO2 in SCM in 12-well plates, and following overnight adherence, were exposed to LPS and oxygen as for macrophages above, followed by harvest at 24 h.
Lung homogenates in single cell suspension.
After BAL, lungs were minced and incubated at 37°C in an enzyme cocktail of RPMI containing 2.4 mg/ml of collagenase I and 20 μg/ml DNase (Invitrogen) and then mashed through a 70-μm nylon cell strainer (BD Falcon), followed by RBC lysis.
Measurement of intracellular reactive oxygen species.
BAL cells were separated for ROS measurement before flow cytometry and resuspended in 1 ml of PBS+ (PBS containing 0.5 mM MgCl2, 0.7 mM CaCl2 + 0.1% glucose) containing 10 μM 2′,7′-dichlorofluorescin diacetate (EMD Chemicals, Gibbstown, NJ) and then incubated in the dark at 37°C for 20 min. After washing with 1 ml of PBS+, cells were processed as in flow cytometry.
Statistical analyses.
Differences between groups at each time point were assessed by the Student's two-tailed unpaired t-test and the Mann-Whitney test for chemokine and cytokine data. Figures were generated using SigmaPlot (SigmaPlot 11.0, Systat software), and data were expressed as means ± SE where applicable. Statistical difference was accepted at P < 0.05.
RESULTS
Moderate oxygen augments LPS-induced lung injury.
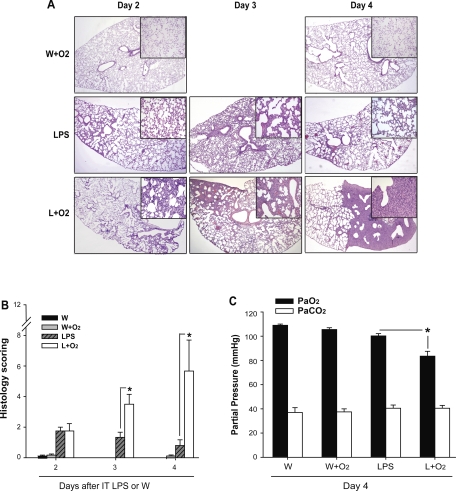
To examine the effects of moderate oxygen on LPS-induced pulmonary responses, we exposed mice to LPS, supplemental oxygen (12 h after LPS), or the combination as noted (Fig. 1). Compared with mice receiving LPS alone or oxygen alone, mice receiving LPS plus oxygen had increased histological evidence of lung injury at days 3 and 4 (Fig. 1A), with greater interstitial thickening, and cellular infiltration in the interstitium and alveolar compartments. Mice that received IT H2O alone (control) had no morphological evidence of injury (not shown). Morphological differences were validated by a masked pathologist who scored lung sections (Fig. 1B). Compared with treatment with LPS alone, lung injury scores were significantly increased at days 3 and 4 in mice that received supplemental oxygen after LPS (Fig. 1B).
Fig. 1.
Supplemental oxygen augments LPS-induced lung injury. A: representative histology sections are shown at low- (2×) and high- (20×) power for days 2, 3, and 4 after IT water (W) + supplemental oxygen (W+O2) or IT LPS + supplemental oxygen. B: a semiquantitative assessment of morphological changes based on percent of involved lung with pathological interstitial changes, inflammation, and consolidation (n = 3–5/group, *P < 0.05 vs. LPS group at each time point). C: day 4 arterial blood gases were obtained via cannulation of the carotid artery in mice ventilated for 15 min at FiO2 = 0.21 (n = 3–4/group, *P < 0.05).
To correlate the observed morphological differences to a physiological parameter, we measured arterial partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2) at day 4 after injury (Fig. 1C). Room air PaO2 was reduced 25% on day 4 in the LPS and oxygen group, but was not reduced in other groups. PaCO2 was not different between groups. These results demonstrate that exposure to 60% oxygen after IT LPS can significantly augment lung injury and produce relative hypoxemia.
IT LPS plus 60% oxygen increases lung endothelial and epithelial permeability.
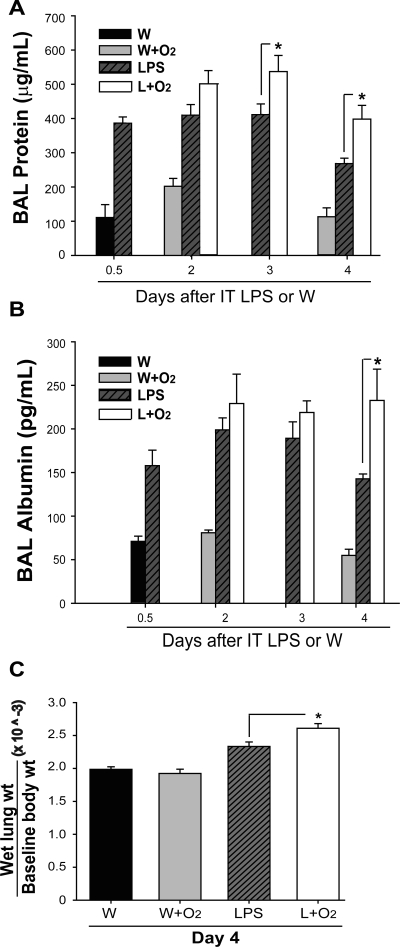
To further characterize the response in these mice, we measured alveolar protein and albumin at intervals after LPS or LPS plus oxygen (Fig. 2). In mice receiving LPS alone, BAL protein (Fig. 2A) and albumin (Fig. 2B) were increased as early as 12 h (day 0.5), remained elevated at days 2 and 3, and declined by day 4. BAL protein and albumin was similar in mice receiving supplemental oxygen after LPS compared with the LPS alone group at early time points; however, days 3 and 4 BAL protein as well as day 4 albumin were significantly increased in mice receiving LPS plus oxygen compared with LPS alone. The exposure of mice to moderate oxygen alone did not produce an increase in alveolar protein or albumin over control at any point. Similarly, exposure of LPS-treated mice to 27% oxygen did not further increase BAL protein (Supplemental Fig. S1A; Supplemental data for this article is available online at the AJP-Lung web site.).
Fig. 2.
Supplemental oxygen increases LPS-induced permeability changes. Bronchoalveolar lavage (BAL) total protein (A) and albumin (B) were analyzed from the cell-free BAL fluid at intervals after IT LPS or IT W (n = 3–6/group, *P < 0.05). C: ratio of lung wet weight (g) to baseline body weight (g) was measured at day 4 after IT W or IT LPS with or without the addition of oxygen (n = 3–5/group, *P < 0.05).
As a marker of lung water accumulation (Fig. 2C), the ratio of wet lung weight to baseline body weight at day 4 was greatest in the IT LPS + O2 group, ∼25% greater than control, and significantly increased over mice treated with LPS alone. Overall, the addition of 60% oxygen to LPS augments lung endothelial and epithelial permeability compared with oxygen or LPS alone.
The BAL cell profile is altered after IT LPS and moderate oxygen.
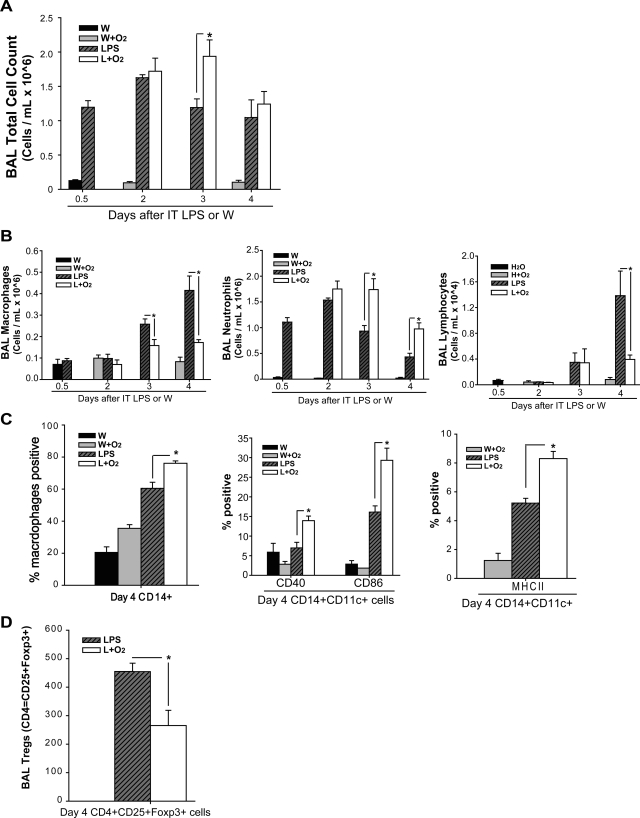
To assess the effect of LPS ± oxygen on BAL cell number, we measured total and differential alveolar cell counts at intervals. BAL total cell count was increased in LPS-treated mice at all time points (Fig. 3A). Mice receiving LPS plus O2 had total cell counts similar to LPS alone at days 2 and 4, but had increased total cells at day 3. In contrast, mice receiving H2O or oxygen alone did not have increased cellular infiltration into the alveolar space. Exposure to LPS plus moderate oxygen increased BAL neutrophils approximately twofold at days 3 and 4 compared with LPS alone; BAL neutrophils increased 50-fold compared with oxygen alone (Fig. 3B, left). The increase in alveolar neutrophils correlates temporally with other parameters of lung injury. In contrast to results with LPS plus 60% oxygen, exposure of LPS-treated mice to 27% oxygen did not further increase BAL total cell or neutrophil counts (Supplemental Fig. S1, B and C). Neutrophil ROS production was not different (mean DCF intensity per neutrophil) between LPS and LPS plus oxygen groups; both were increased compared with control (not shown). While alveolar neutrophils increased after LPS and oxygen, when we evaluated lung homogenates in single cell suspension for the presence of interstitial and intravascular neutrophils via flow cytometry, there was no difference between LPS and oxygen compared with LPS alone groups (not shown).
Fig. 3.
Supplemental oxygen alters LPS-induced BAL cell profiles. BAL total cell count (A) and BAL differential cell count (B) were obtained at defined time points (n = 5–7 for each group, *P < 0.05). C: among monocyte-gated cells, CD14+ cells are labeled via flow cytometry; subsequently, among CD14+CD11c+ alveolar macrophages, flow cytometry was used to further define the presence of costimulatory molecules CD40, CD86, and MHC class II (IA/IE) in macrophages at day 4 after IT LPS or water (n = 3, *P < 0.05). D: among CD3+ lymphocytes, the number of CD4+CD25+Foxp3+ Tregs is identified at day 4 after IT LPS (n = 4, *P < 0.05).
In contrast to the increase in neutrophils, day 3 and 4 alveolar macrophages (Fig. 3B) and day 4 alveolar lymphocytes (Fig. 3B) were significantly decreased in the LPS plus oxygen group compared with LPS alone. Flow cytometry of alveolar monocytes demonstrated increased expression of CD14+, and among alveolar macrophages (CD14+ and CD11c+) at day 4, costimulatory molecules CD40, CD86, and MHC class II (IA/IE) were significantly upregulated on macrophages exposed to LPS and oxygen compared with macrophages exposed to LPS alone (Fig. 3C). In contrast, there were no differences in expression of TLR2 or CD80 on macrophages exposed to LPS and oxygen compared with macrophages exposed to LPS alone (not shown). The BAL macrophage number decreased with LPS and oxygen at day 4; however, the macrophages present were phenotypically more activated, as reflected in the increased expression of costimulatory molecules. Analysis of lymphocyte populations revealed a 40–50% decrease in alveolar CD4+CD25+Foxp3 Tregs in the mice exposed to LPS and oxygen compared with those exposed to LPS alone (Fig. 3D).
LPS and oxygen augments proinflammatory cytokines in the alveolar space.
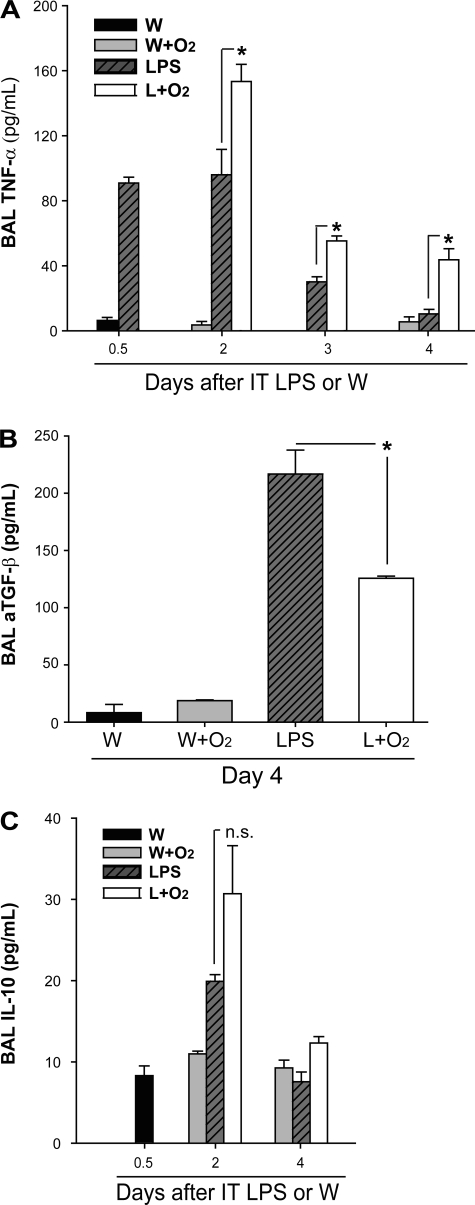
To better characterize the inflammatory response and to begin to understand potential mediators of the observed differences between groups, we measured BAL cytokines (Fig. 4). BAL TNFα (Fig. 4A), a proinflammatory cytokine, was elevated at days 2 and 3 in mice that received LPS alone, and decreased significantly by day 4. Comparatively, mice that received supplemental oxygen 12 h after LPS had higher levels of TNF-α than LPS alone at all times. Activated TGF-β, a potentially anti-inflammatory cytokine, was elevated at day 4 in the LPS alone group compared with mice that received LPS and supplemental oxygen (Fig. 4B). In contrast, IL-10 was not different between groups at any time (Fig. 4C).
Fig. 4.
Supplemental oxygen modifies LPS-induced cytokine profiles. The proinflammatory cytokine TNFα (A) and the anti-inflammatory cytokines TGF-β (B) and IL-10 (C) were measured by ELISA at various time points after IT LPS or IT W (n = 3–6/group, *P < 0.05).
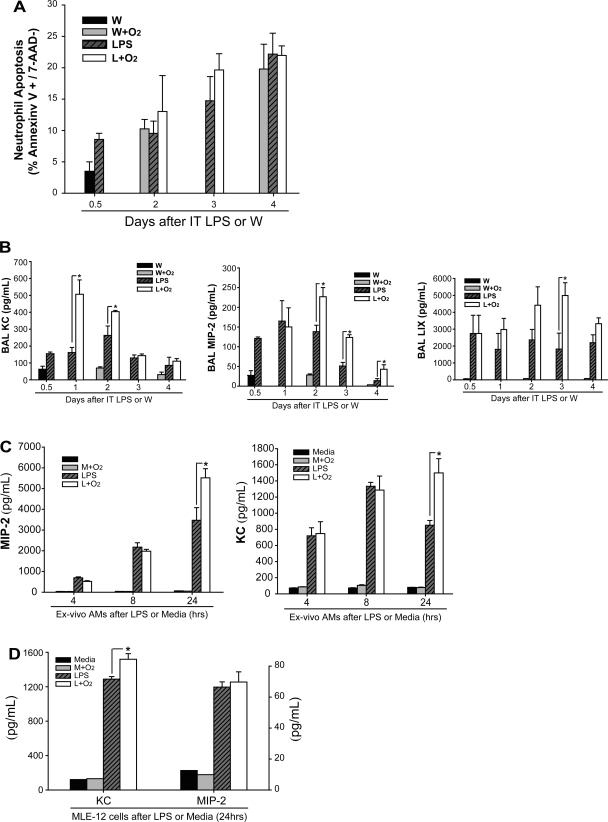
The increase in alveolar neutrophils results from enhanced recruitment.
The abundance of alveolar neutrophils is determined by the balance between recruitment and clearance. We assessed differences in neutrophil apoptosis by flow cytometry and found that the percentage of apoptotic neutrophils increased similarly in all groups over time (LPS alone, LPS + oxygen, and oxygen alone) (Fig. 5A), indicating that differences in neutrophil apoptosis do not contribute prominently to altered neutrophil numbers.
Fig. 5.
Supplemental oxygen increases neutrophil chemokines in BAL fluid or media. A: apoptosis of alveolar neutrophils was assessed via FACS using Annexin V and 7-AAD staining at various time points. B: neutrophil-recruiting chemokines including MIP-2, KC, and LIX were assessed at intervals after IT LPS or IT W in mice receiving either 21% or 60% oxygen (n = 3–6/group, *P < 0.05). C: naïve alveolar macrophages were exposed to LPS or media ex vivo followed 3 h later by 21% or 60% oxygen, and MIP-2 and KC were measured in the supernatant at 4, 8, and 24 h (n = 5–6/group, *P < 0.05). D: MLE-12 cells, a murine alveolar epithelial cell line, were exposed to LPS or media similar to above followed 3 h later by 21 or 60% oxygen, and MIP-2 and KC were measured in the supernatant at 24 h (n = 4/group, *P < 0.05).
To assess recruitment, we measured the abundance of BAL neutrophil chemokines, which signal neutrophils to migrate into sites of inflammation. In ALI models, macrophage inflammatory protein-2 (MIP-2), keratinocyte-derived chemokine (KC; also called CXCL1 chemokine), and LPS-induced chemokine (LIX) are the most potent known neutrophil-recruiting chemokines in mice (9, 31). All three were elevated in mice receiving LPS plus oxygen compared with all other groups, although the temporal profiles for each were distinct (Fig. 5B). For example, BAL KC is more than twofold increased at day 1 after LPS, after only 12 h of oxygen exposure, but fell to baseline levels by days 3 and 4. In contrast, BAL MIP-2 is persistently increased in the mice receiving LPS and oxygen starting at day 2 and continuing through day 4, a later peak than BAL KC. Therefore, at days 3 and 4, BAL MIP-2 secretion is increased in the LPS and oxygen group despite the reduced number of alveolar macrophages. Intracellular and surface expression of CXCR2, the common receptor for MIP-2, KC, and LIX on alveolar neutrophils, was not different between groups receiving LPS alone, oxygen alone, or LPS and oxygen (not shown). Overall, these results demonstrate a significant and sustained upregulation of BAL neutrophil chemokine secretion when mice are exposed to LPS and supplemental oxygen. In contrast, exposure of LPS-treated mice to 27% oxygen did not further increase BAL chemokines MIP-2, KC, or LIX (Supplemental Fig. S1D).
To determine whether macrophages could be a source of alveolar chemokines that contribute to the augmentation of lung injury by supplemental oxygen, we harvested naïve alveolar macrophages and exposed them to media or LPS (100 ng/ml) followed 3 h later by continued room air (21% oxygen) or 60% oxygen for a total incubation period of 4, 8, or 24 h from the time of LPS or media (Fig. 5C). At 4 or 8 h after LPS (1 or 5 h of oxygen, respectively), there was a similar increase in secretion of MIP-2 and KC in wells treated with LPS or LPS and 60% oxygen. Twenty-four hours after LPS (21 h of oxygen), the LPS plus oxygen group had a 50% increase in MIP-2 and nearly twofold increase in KC secretion compared with LPS alone, consistent with our in vivo results. Murine lung epithelial cells (MLE-12 cells) augmented secretion of KC when exposed to LPS and oxygen compared with LPS alone; MIP-2 increased similarly in both LPS and LPS plus oxygen groups (Fig. 5D). Ex vivo alveolar macrophages and MLE-12 cells did not secrete significant quantities of LIX under any condition (not shown). This finding indicates that in cultured cells, supplemental oxygen can augment LPS-induced macrophage production of neutrophil chemokines MIP-2 and KC, and alveolar epithelial cell production of KC.
Neutrophil depletion abrogates the LPS plus oxygen augmentation of lung injury.
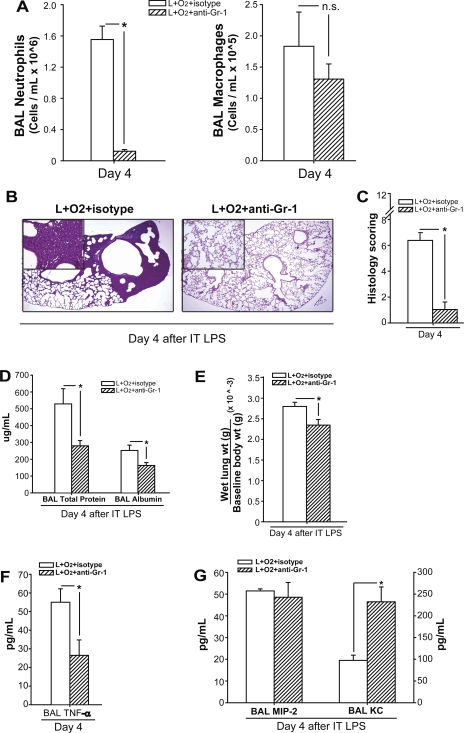
To assess the contribution of alveolar neutrophils to the augmentation of lung injury, we depleted neutrophils with anti-Gr-1 (RB6-8C5 clone) antibodies and reexamined the response to LPS and oxygen (Fig. 6). To confirm specific depletion of neutrophils, we counted BAL neutrophils and macrophages at day 4 after IT LPS plus 60% oxygen. Compared with isotype antibody-treated mice, mice that received LPS plus oxygen plus anti-Gr-1 antibody had a 90% reduction in alveolar neutrophils (Fig. 6A). Comparable neutrophil depletion was seen in the circulation (not shown). Anti-Gr-1 antibody treatment had no affect on macrophage numbers (Fig. 6A).
Fig. 6.
Neutrophil depletion abrogates the augmentation of LPS-induced lung injury by supplemental oxygen. Mice received LPS and supplemental oxygen as described, as well as concomitant treatment with neutrophil-depleting Gr-1 or isotype antibodies. Injury parameters were assessed at day 4. A: BAL neutrophils and macrophages were quantified (n = 3–5/group, *P < 0.05). B: representative lung histology sections from each group, stained with H&E. C: lung injury scores as described for each group; n = 3–5 mice/group; *P < 0.05. BAL protein and albumin (D) and wet lung to body weight (E) were in each group (n = 3–5/group, *P < 0.05). TNFα (F) and chemokines MIP-2 and KC (G) were assessed in BAL fluid of mice from each group (n = 3–5/group; *P < 0.05).
Neutrophil depletion abrogated lung injury in mice receiving LPS plus oxygen, as seen in representative histological sections (Fig. 6B) and verified by scoring (Fig. 6C), and improved room air PaO2 to 104 ± 6.2 mmHg, comparable to control, LPS alone, or oxygen alone mice. Day 4 BAL protein and BAL albumin (Fig. 6D), as well as wet lung weight to baseline body weight (Fig. 6E), were all significantly reduced after depletion of neutrophils, consistent with an improvement in alveolar-capillary barrier function.
Day 4 BAL cytokines and chemokines were assessed after neutrophil depletion as well. Consistent with other parameters of inflammation and lung injury, BAL TNF-α was significantly reduced in the LPS plus oxygen plus anti-Gr-1 antibody-treated mice (Fig. 6F). Neutrophil depletion did not alter BAL MIP-2; however, BAL KC was significantly increased (Fig. 6G). These results demonstrate that antibody-mediated neutrophil depletion significantly reduced inflammation and injury following exposure to LPS plus supplemental oxygen.
DISCUSSION
The purpose of this study was to explore the effects of supplemental oxygen on LPS-induced ALI. Our principal finding was that moderate levels of oxygen exacerbated LPS-induced lung injury as assessed by morphology, gas exchange, and barrier integrity. Second, we have shown that oxygen-induced augmentation of lung injury is caused by an influx of neutrophils in the alveolar space. Third, our data indicate that the neutrophil influx is likely related to increased secretion of neutrophil-recruiting chemokines. Last, alveolar macrophage and lymphocyte numbers, including Tregs, were significantly decreased in mice that received LPS plus oxygen compared with mice that received LPS alone; however, macrophage activation was increased, consistent with sustained inflammation.
ALI can develop from a variety of initiating stimuli (8, 32), but progression of initial lung inflammation to ALI or ARDS often occurs after patients are in the hospital (1, 6). Only a subset of individuals with lung inflammation progress to ALI/ARDS, and the factors that determine that progression are not well understood. Oxygen toxicity has been described, and evaluated, primarily in animal models in the context of very high concentrations of supplemental oxygen, usually >90%; the role of oxygen toxicity in human ARDS remains uncertain (5, 7, 15, 22). We believe that the combination of intratracheal LPS and moderate oxygen is a model of lung injury with potential clinical relevance. We chose to administer a low dose of LPS to specifically test whether supplemental oxygen could worsen mild lung injury. With higher LPS doses (1–5 μg/g mice), initial lung injury is more severe, potentially confounding interpretation of oxygen-induced augmentation. We found that the addition of moderate levels of supplemental oxygen to low-dose LPS significantly increased murine lung injury. Similar oxygen-induced augmentation may occur in susceptible individuals with underlying lung inflammation (20). Our results are consistent with the limited number of prior studies in this area suggesting moderate levels of supplemental oxygen can alter inflammatory responses in the lung, including models of acid aspiration and multi-toxin-induced lung injury (7, 20, 37).
Exposure to moderate supplemental oxygen following LPS significantly increased alveolar neutrophils. Neutrophil migration to the alveolar space in the context of inflammatory responses may be beneficial, for example, in the setting of bacterial infection, where an appropriate neutrophil-mediated innate immune response is one of the primary determinants of outcome. However, excessive or unregulated responses can lead to host tissue damage (9, 26, 38). Therefore, the ability to balance the destructive potential of neutrophils with their critical role in antibacterial defense likely determines the severity and outcome of injury (9). We demonstrated that neutrophil depletion abrogated the increase in lung injury caused by addition of supplemental oxygen following LPS, clearly implicating neutrophils in the pathogenesis of injury.
The increase in alveolar neutrophils is due, at least in part, to an enhanced neutrophil chemokine gradient. Evidence that secretion of chemokines is normally tightly regulated stems from studies demonstrating de novo chemokine synthesis in response to inflammatory stimuli (13, 16). Greater and more sustained elevation of chemokines, as we observed in mice receiving LPS plus supplemental oxygen, can lead to persistence of inflammation with eventual tissue injury (14). Alveolar neutrophil expression of CXCR2 was not different between groups, indicating that differences in migration to the alveolar space primarily result from changes in chemokine levels, not changes in receptor abundance. It remains possible that CXCR2 expression on circulating neutrophils may be increased in mice exposed to LPS and oxygen compared with LPS or oxygen alone.
The differences in timing and duration of expression for each chemokine after LPS and oxygen suggest a dynamic cascade of events that increases alveolar neutrophils and worsens lung injury. Antibody-mediated chemokine depletion studies of one or all of these chemokines may reveal differences in functional roles for each of the chemokines. We also observed that the abundance of chemokines in the BAL could vary depending on the presence or absence of alveolar neutrophils. Neutrophil depletion did not alter MIP-2. However, KC levels were significantly increased in neutrophil-depleted mice, indicating a potential feedback loop between neutrophils and a subset of chemokines; although mechanisms underlying this regulation are largely unknown, prior work in rats suggested that MIP-2 acts within the alveolar milieu and KC acts systemically to recruit neutrophils to the alveolar space (30). Additionally, KC appears to be derived from both macrophages and epithelial cells based on in vitro analysis, suggesting the possibility that neutrophil recruitment is stimulated by multiple cell types. Although further work is clearly necessary, it is clear that interaction of macrophages and epithelial cells may play a role in propagating injury, in part due to the increased chemokine gradient.
In addition to enhanced neutrophil recruitment, other mechanisms may contribute to the augmentation of LPS-induced lung injury by supplemental oxygen. Alveolar neutrophil apoptosis was not different between the groups; however, changes in macrophage clearance function, as has been described in the setting of hyperoxic exposure (3, 4), could also contribute to differences in alveolar neutrophil number. A key feature of animal studies involving hyperoxia-induced ALI is impaired epithelial barrier function (22). Barrier disruption may contribute to the increase in alveolar neutrophils by facilitating migration into the alveolar space. In addition, TNFα can diffuse from alveolar macrophages to stimulate proinflammatory cytokine release from alveolar epithelial cells, amplifying the inflammatory milieu in the alveolus (34). Jeyaseelan et al. (19) demonstrated that AECs are a primary source of LIX after LPS induction in rodents and speculate that resident cell-myeloid cell cross-talk may be influential in propagation of inflammation.
Exposure to supplemental oxygen after LPS created a more strongly proinflammatory alveolar environment. TNFα levels were increased in mice receiving LPS plus oxygen compared with mice receiving LPS alone. In addition, although the total number of alveolar macrophages was decreased after LPS and oxygen compared with LPS alone, there was a twofold increase in the percentage of macrophages expressing costimulatory molecules CD40 and CD86, as well as an increase in MHC II, indicating a more classically activated profile (M1) (25) for alveolar macrophages in the LPS plus oxygen mice compared with LPS alone. The requirement of CD40-CD86 costimulatory molecule engagement for neutrophil-mediated activation of macrophages has been described in a murine tuberculosis model (18). CD40 KO mice appear to be protected from LPS-induced ALI with decreased alveolar macrophage secretion of MIP-2 (17), and inhibition of CD40 and CD86 was protective in a cecal ligation and puncture sepsis model (27). Together, these findings highlight the potential importance of macrophage CD40-CD86 costimulatory molecule activation in propagation of inflammation, and similar involvement could occur in our LPS and oxygen model. Maus et al. (23) have shown that in the alveolar space there is a relatively quick turnover from resident alveolar macrophages to systemic inflammatory monocytes that migrate into the alveolar space after LPS, and macrophages may play a central role in regulation of inflammation in that context. Neutrophil depletion with anti-Gr-1 antibody did not alter the number of alveolar macrophages in our studies, in contrast to results described by Daley et al. (11) in a wound model. In addition, in our studies lymphocyte numbers were decreased in mice receiving LPS plus oxygen. Recently, we described that regulatory T cells are essential to achieve normal resolution of IT LPS-induced lung injury (10). Interestingly, the addition of oxygen to LPS suppressed alveolar space Treg numbers at day 4 after LPS. This may, at least in part, explain the lower levels of TGF-β in the LPS and oxygen group at this time point. Further manipulation of Tregs may reveal an important contribution to resolution of injury in our model.
Factors that modify progression from mild injury to clinical development of ALI/ARDS are not well understood. Our findings extend the limited body of evidence suggesting that moderate levels of oxygen can augment milder forms of lung injury. Although the effects of moderate concentrations of oxygen remain unclear in humans, this could be an important exacerbating and potentially modifiable factor for development of ALI in susceptible hosts.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-089346 and the Johns Hopkins Bayview Scholars Program (L. S. King) and Grants HL-80105 and 5P50-HL-084945 (V. Y. Polotsky).
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Christian Reinke and Vladimir Savransky for excellent technical support in development and trouble shooting of the oxygen delivery system, and Eric Chau for assistance with cell culture and in vitro experiments.
REFERENCES
- 1.Irish Critical Care Trials Group Acute lung injury, and the acute respiratory distress syndrome in Ireland: a prospective audit of epidemiology and management. Crit Care 12: R30, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altemeier WA, Sinclair SE. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care 13: 73–78, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Baleeiro CE, Christensen PJ, Morris SB, Mendez MP, Wilcoxen SE, Paine R., 3rd GM-CSF and the impaired pulmonary innate immune response following hyperoxic stress. Am J Physiol Lung Cell Mol Physiol 291: L1246–L1255, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Baleeiro CE, Wilcoxen SE, Morris SB, Standiford TJ, Paine R Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol 171: 955–963, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Barber RE, Hamilton WK. Oxygen toxicity in man. A prospective study in patients with irreversible brain damage. N Engl J Med 283: 1478–1484, 1970 [DOI] [PubMed] [Google Scholar]
- 6.Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 30: 51–61, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Coalson JJ, King RJ, Winter VT, Prihoda TJ, Anzueto AR, Peters JI, Johanson WG., Jr O2− and pneumonia-induced lung injury. I. Pathological and morphometric studies. J Appl Physiol 67: 346–356, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Connelly KG, Repine JE. Markers for predicting the development of acute respiratory distress syndrome. Annu Rev Med 48: 429–445, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun 77: 568–575, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 119: 2898–2913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol 83: 64–70, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Davis WB, Rennard SI, Bitterman PB, Crystal RG. Pulmonary oxygen toxicity. Early reversible changes in human alveolar structures induced by hyperoxia. N Engl J Med 309: 878–883, 1983 [DOI] [PubMed] [Google Scholar]
- 13.De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol 180: 4308–4315, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Fan J, Heller NM, Gorospe M, Atasoy U, Stellato C. The role of post-transcriptional regulation in chemokine gene expression in inflammation and allergy. Eur Respir J 26: 933–947, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Frank L, Bucher JR, Roberts RJ. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol 45: 699–704, 1978 [DOI] [PubMed] [Google Scholar]
- 16.Hamilton TA, Novotny M, Datta S, Mandal P, Hartupee J, Tebo J, Li X. Chemokine and chemoattractant receptor expression: post-transcriptional regulation. J Leukoc Biol 82: 213–219, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto N, Kawabe T, Imaizumi K, Hara T, Okamoto M, Kojima K, Shimokata K, Hasegawa Y. CD40 plays a crucial role in lipopolysaccharide-induced acute lung injury. Am J Respir Cell Mol Biol 30: 808–815, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Hoshino Y, Hoshino S, Gold JA, Raju B, Prabhakar S, Pine R, Rom WN, Nakata K, Weiden M. Mechanisms of polymorphonuclear neutrophil-mediated induction of HIV-1 replication in macrophages during pulmonary tuberculosis. J Infect Dis 195: 1303–1310, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol 32: 531–539, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight PR, Kurek C, Davidson BA, Nader ND, Patel A, Sokolowski J, Notter RH, Holm BA. Acid aspiration increases sensitivity to increased ambient oxygen concentrations. Am J Physiol Lung Cell Mol Physiol 278: L1240–L1247, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 22.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, Seeger W, Welte T, Lohmeyer J. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol 35: 227–235, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Moores HK, Beehler CJ, Hanley ME, Shanley PF, Stevens EE, Repine JE, Terada LS. Xanthine oxidase promotes neutrophil sequestration but not injury in hyperoxic lungs. J Appl Physiol 76: 941–945, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Mosser DM. The many faces of macrophage activation. J Leukoc Biol 73: 209–212, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Nathan C. Points of control in inflammation. Nature 420: 846–852, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Nolan A, Weiden M, Kelly A, Hoshino Y, Hoshino S, Mehta N, Gold JA. CD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsis. Am J Respir Crit Care Med 177: 301–308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg 144: 124–130, 1982 [DOI] [PubMed] [Google Scholar]
- 29.Perkowski S, Scherpereel A, Murciano JC, Arguiri E, Solomides CC, Albelda SM, Muzykantov V, Christofidou-Solomidou M. Dissociation between alveolar transmigration of neutrophils and lung injury in hyperoxia. Am J Physiol Lung Cell Mol Physiol 291: L1050–L1058, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Quinton LJ, Nelson S, Zhang P, Boe DM, Happel KI, Pan W, Bagby GJ. Selective transport of cytokine-induced neutrophil chemoattractant from the lung to the blood facilitates pulmonary neutrophil recruitment. Am J Physiol Lung Cell Mol Physiol 286: L465–L472, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Reutershan J, Ley K. Bench-to-bedside review: acute respiratory distress syndrome – how neutrophils migrate into the lung. Crit Care 8: 453–461, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Segal BH, Davidson BA, Hutson AD, Russo TA, Holm BA, Mullan B, Habitzruther M, Holland SM, Knight PR., 3rd Acid aspiration-induced lung inflammation and injury are exacerbated in NADPH oxidase-deficient mice. Am J Physiol Lung Cell Mol Physiol 292: L760–L768, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-α during pulmonary ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 293: L105–L113, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Singer MM, Wright F, Stanley LK, Roe BB, Hamilton WK. Oxygen toxicity in man. A prospective study in patients after open-heart surgery. N Engl J Med 283: 1473–1478, 1970 [DOI] [PubMed] [Google Scholar]
- 36.Sue RD, Belperio JA, Burdick MD, Murray LA, Xue YY, Dy MC, Kwon JJ, Keane MP, Strieter RM. CXCR2 is critical to hyperoxia-induced lung injury. J Immunol 172: 3860–3868, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, Lukashev D, Bittmann I, Sitkovsky MV. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol 3: e174, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol 40: 519–535, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.