Abstract
Pulmonary expression of granulocyte/macrophage colony-stimulating factor (GM-CSF) is critically important for normal functional maturation of alveolar macrophages. We found previously that lung GM-CSF is dramatically suppressed in mice exposed to hyperoxia. Alveolar epithelial cells (AEC) are a major source of GM-CSF in the peripheral lung, and in vivo hyperoxia resulted in greatly reduced expression of GM-CSF protein by AEC ex vivo. We now explore the mechanisms responsible for this effect, using primary cultures of murine AEC exposed to hyperoxia in vitro. Exposure of AEC to 80% oxygen/5% CO2 for 48 h did not induce overt toxicity, but resulted in significantly decreased GM-CSF protein and mRNA expression compared with cells in normoxia. Similar effects were seen when AEC were stressed with serum deprivation, an alternative inducer of oxidative stress. The effects in AEC were opposite those in a murine lung epithelial cell line (MLE-12 cells), in which hyperoxia induced GM-CSF expression. Both hyperoxia and serum deprivation resulted in increased intracellular reactive oxygen species (ROS) in AEC. Hyperoxia and serum deprivation induced significantly accelerated turnover of GM-CSF mRNA. Treatment of AEC with catalase during oxidative stress preserved GM-CSF protein and mRNA and was associated with stabilization of GM-CSF mRNA. We conclude that hyperoxia-induced suppression of AEC GM-CSF expression is a function of ROS-induced destabilization of GM-CSF mRNA. We speculate that AEC oxidative stress results in significantly impaired pulmonary innate immune defense due to effects on local GM-CSF expression in the lung.
Keywords: lung, innate immunity, growth factors, oxidative stress
the pulmonary alveolar space forms the largest surface of interaction of the body with the outside world. The pulmonary innate immune response is finely tuned to provide measured defense against aspirated and inhaled pathogens without inducing exaggerated responses that might unduly impair gas exchange. Alveolar macrophages (AM), the principal resident inflammatory cells in the alveolar space, are highly differentiated cells with distinctive functional characteristics (6). AM are mobile cells that engulf and kill microbes themselves and play an important role as sentinels, secreting early response cytokines to recruit and activate other constituents of the immune system. AM also play a crucial role in normal surfactant homeostasis, taking up and degrading surfactant phospholipids and proteins as part of normal turnover (19).
Granulocyte/macrophage colony-stimulating factor (GM-CSF) is a growth factor that is essential for normal maturation of AM. In gene-targeted mice lacking GM-CSF (GM-CSF−/− mice) (25, 32) or its receptor (24), murine AM express an immature phenotype. These animals develop pulmonary pathology identical to human pulmonary alveolar proteinosis, with excess surfactant material clogging the alveolar spaces, leading to abnormal gas exchange (12, 24). They also have greatly impaired pulmonary host defense and are significantly more susceptible than wild-type mice to infection with bacteria and fungi (22, 26). GM-CSF was originally purified from murine lung and is the product of a number of different cell types, including alveolar epithelial cells (AEC). Expression of GM-CSF exclusively by AEC is sufficient to restore normal surfactant homeostasis (18, 27, 28) and pulmonary host defense in GM-CSF−/− mice (22, 25–26, 32). Conversely, transient antibody neutralization of GM-CSF within the lung for as little as 24 h leads to impaired AM function (8). Thus, ongoing production of GM-CSF by parenchymal cells within the lung is necessary for maintenance of normal AM maturation.
In studies of the impact of mild lung injury due to hyperoxia on pulmonary host defense, we found previously that limited (4-day) exposure of mice to hyperoxia resulted in greatly increased susceptibility to lethal pneumonia with Klebsiella pneumoniae (2). This enhanced susceptibility to infection was associated with alterations in AM phenotype resembling those found in GM-CSF−/− mice. Furthermore, exposure to hyperoxia led to suppression of lung and AEC GM-CSF expression, whereas treatment of hyperoxic mice with GM-CSF restored normal AM function and bacterial clearance (1). Thus, in the setting of lung injury due to hyperoxia, suppression of pulmonary GM-CSF expression results in impaired AM function and greatly impaired pulmonary host defense. In the present work we explore the hypothesis that hyperoxia results in oxidative stress, which directly alters AEC GM-CSF expression through changes in mRNA stability.
MATERIALS AND METHODS
Animals.
Wild-type C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were housed under specific pathogen-free conditions and were monitored daily by veterinary staff. Experiments were carried out using male mice aged 6–10 wk. The animal care committee at the Salt Lake City Veterans Affairs Medical Center approved these experiments.
Isolation and purification of type II alveolar epithelial cells.
Murine type II AEC were isolated and purified using a modification of published methods (11, 23). Following anesthesia with Avertin (Sigma, St. Louis, MO) and heparinization, mice were exsanguinated and the pulmonary vasculature was perfused with saline. The trachea was cannulated and the lungs were filled with 1–2 ml of dispase (BD-Bioscience, San Jose, CA). Subsequently, low-melting point agarose (1%, 0.45 ml; Sigma) was infused via the trachea, and the lungs were placed in iced PBS for 2 min to harden the agarose. The lungs were incubated in dispase (BD-Bioscience), and parenchymal tissue was teased away from the agarose-filled airways. Lung parenchymal tissues were minced in DMEM with 0.01% DNase I (Sigma), and the resultant cellular suspension was filtered. The cells were incubated with biotinylated anti-CD32 and anti-CD45 (BD-Bioscience) followed by streptavidin-coated magnetic particles (Promega, Madison, WI) for magnetic removal of leukocytes. Mesenchymal cells were removed by overnight adherence to tissue culture-treated plastic (day 0). The nonadherent cells were plated in DMEM with penicillin/streptomycin and 10% FCS in wells coated with fibronectin (Millipore, Temecula, CA; day 1). AEC were allowed to attach for 48 h after which the cell layer was washed gently with several changes of room temperature, sterile PBS to remove nonattached cells and debris. Fresh growth media was added, and the AEC were utilized for experiments (day 3). For experiments in which AEC were deprived of serum, the medium was changed from DMEM + 10% FCS to serum-free DMEM on day 3. In each experiment, purity of the epithelial cell preparations was confirmed by vimentin staining with anti-vimentin (Santa Cruz Biotechnology, Santa Cruz, CA) to identify nonepithelial cells. Secondary staining was performed with FITC-conjugated anti-mouse IgM. Routinely, fibroblast contamination was 3–6% on day 3 after isolation. Each individual experiment was performed using a single isolation, with cells pooled from the lungs of six to eight mice.
Exposure to hyperoxia.
C57BL/6 mice exposed to an atmosphere of >95% oxygen die within ∼6 days. We have shown previously that lung GM-CSF expression is significantly decreased by day 3 in hyperoxia, and mice recover fully from 4 days in >95% oxygen if they are then returned to room air (1). In preliminary experiments, we found that AEC in vitro did not tolerate this intensity and duration of hyperoxic stress without irreversible injury. Therefore, to model the stress of sublethal hyperoxia in vivo using an in vitro system, we exposed AEC to an atmosphere of 80% oxygen/5% CO2 for 48 h. Cells were placed in a sealed Plexiglas chamber that was maintained at 37°C and flushed daily with a commercially available gas mixture of CO2 and oxygen, adjusted to maintain a fractional concentration of oxygen of 0.80, as measured in real time with an oxygen analyzer within the chamber (maxO2+; Maxtec, Salt Lake City, UT). Cell numbers following hyperoxia exposure were determined by counting cells that had been released from the dish with trypsin/EDTA, using a hemocytometer counter.
GM-CSF and MCP-1 protein in culture supernatants.
AEC were harvested as above and on day 3 placed in normoxia or hyperoxia in the appropriate growth media. After 48 h, fresh medium was added to the cells. After 18 h of further incubation under the experimental conditions, the culture supernatants were harvested and cellular debris was removed by centrifugation. ELISAs for GM-CSF and MCP-1 (both from R&D Systems, Minneapolis, MN) were performed immediately or on samples frozen at −80°C, with care being taken to ensure only a single cycle of freeze-thaw (as per manufacturer's instructions).
RT-PCR.
Total cellular RNA was isolated from cultured cells using RNeasy (Qiagen, Valencia, CA). First-strand cDNA was reverse transcribed from 1 μg of total RNA using a high capacity cDNA kit (ABI, Foster City, CA). GM-CSF and MCP-1 transcripts were quantified using the primer pairs previously described (1). Specific PCR products were generated from cDNA (100 ng) using Brilliant SYBR Green QRT-PCR 2-step (Stratagene, La Jolla, CA) and an Mx3000P real-time computerized cycler from Stratagene. The 2-step cycle program (Tm = 60°C) with a dissociation analysis was used as recommended by Stratagene. Appropriate controls (no template control and Rox reference dye) were included in each experiment as recommended by Stratagene. Results are expressed as fold-induction over control values after correcting for GAPDH. Each biological sample was amplified in duplicate and the average of the duplicates taken as a single value for statistical analysis. The threshold cycle from GAPDH was used as a calibrator for each individual sample to normalize the specific RNA quantitation. The value for the first biological control sample was arbitrarily set at 100%, and results are expressed as fold-change over that control value after correcting for GAPDH.
Lactate dehydrogenase release.
We measured change in release of lactate dehydrogenase (LDH; Roche, Indianapolis, IN) as a reflection of gross cellular injury. Briefly, the media were collected and spun free of debris before assay. LDH values were corrected to reflect total media volume. The cell layer was washed with PBS and then incubated in RIPA buffer (ThermoFisher, Pittsburgh, PA) for 20 min on ice. RIPA insoluble material was removed by centrifugation and LDH determined in the RIPA soluble cytoplasmic fraction. Total cytoplasmic LDH was calculated by correction for volume. Percent LDH release was used as a measure of injury = (LDH in medium/LDH medium + LDH cytoplasm) × 100.
Mitochondrial injury.
Mitochondrial injury due of oxidative stress in AEC was quantified using MitoPT (Immunochemistry Technologies, Bloomington, MN). This is a measure of early mitochondrial injury due to a collapse in the electrochemical gradient across the mitochondrial membrane, as measured by the change in the membrane potential. Under optimal culture conditions, the lipophilic MitoPT reagent enters the cell and the cationic dye JC-1 enters the mitochondria where it aggregates and fluoresces red. Under conditions that change the mitochondrial membrane potential, the MitoPT reagent cannot enter the mitochondria and remains as a green fluorescent monomer throughout the cytoplasm. A decrease in the ratio red/green fluorescence is a simple yet quantitative measure of mitochondrial injury. The assay was carried out as described in the technical brochure following the 96-well fluorescence spectroscopy staining protocol. The plate reader was set at an excitation 488 nm and emission 527 nm for green fluorescence and 590–600 nm for red fluorescence. The changes in mitochondrial potential were assessed by comparing the ratios of 590–600 nm (red)/527 nm (green) ODs. A drop in the ratio corresponds to a reduction in the number of healthy mitochondria able to maintain the negative potential necessary to concentrate the MitoPT dye in the red aggregate form (29).
Intracellular superoxide production.
Intracellular O2− production was measured by nitroblue tetrazolium (NBT) reduction as described by Rook et al. (30) and previously detailed (35).
mRNA decay studies.
Studies to comparative mRNA stability were carried out immediately after exposure to hyperoxia or serum deprivation (48 h) followed by incubation with IL-1β (5 ng/ml, R&D Systems) for 3 h to maximally induce GM-CSF mRNA expression. Actinomycin D (5 μg/ml; Calbiochem-EMD, San Diego, CA) was then added to stop transcription, and relative mRNA expression was determined over time. Time 0 was collected immediately before addition of Actinomycin D. Actinomycin D was prepared as a 20X stock in DMSO immediately before use, using a single-use sealed vial to ensure full activity. Upon solubilization, the stock was diluted in prewarmed media, and 1/10th total volume (100 μl) was added to each sample to avoid volume/concentration errors. Precise time points were achieved by rapid solubilization in RNA lysis buffer (Qiagen). Samples were processed for RNA and RT-PCR as described above. Data are expressed relative to time 0 for each experimental condition.
Statistical analysis.
Data are presented as means ± SD. Statistical analysis was carried out using GraphPad Prism v4C software. Differences between two groups were compared with the unpaired Student's t-test. Two-tailed tests of significance were used. Differences between multiple groups were compared with one-way analysis of variance. Comparisons were deemed statistically significant for P values < 0.05.
RESULTS
Effect of in vitro hyperoxia on AEC GM-CSF protein expression.
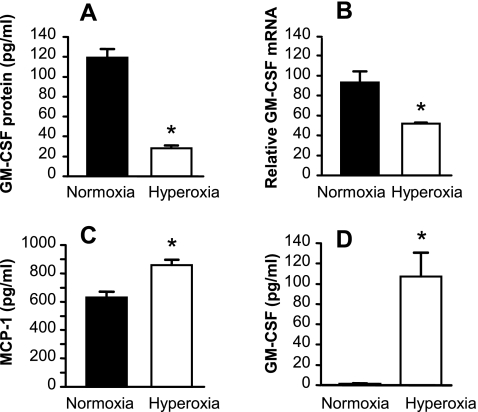
C57BL/6 mice exposed to an atmosphere of >95% oxygen die within ∼6 days. We have shown previously that lung GM-CSF expression is significantly decreased by day 3 in hyperoxia. To model the effects of in vivo hyperoxic exposure on primary AECs in vitro, AECs 3 days postisolation were exposed to 80% hyperoxia for 48 h in the presence of 10% FCS. Exposure to hyperoxia resulted in ∼70% reduction in AEC GM-CSF protein expression (Fig. 1A). The change in GM-CSF protein was a reflection of decreased GM-CSF steady-state mRNA (Fig. 1B). This reduction in GM-CSF expression was not a reflection of overt cytotoxicity; hyperoxia induced minimal, insignificant loss in cell number (2.89 × 105 ± 0.25 × 105 cells/well in normoxia vs. 2.65 × 105 ± 0.21 × 105 cells/well in hyperoxia) and no significant increase in release of LDH from the monolayers (20.22 ± 0.66% in normoxia vs. 23.29 ± 1.26% in hyperoxia; P > 0.05). Furthermore, secretion of another important macrophage-active product of AEC, MCP-1, was increased by exposure to hyperoxia (Fig. 1C). These data demonstrate that primary AEC respond to hyperoxic stress in vitro in a similar manner to that observed in the lung in vivo, following exposure to hyperoxia. Interestingly, a frequently used murine lung epithelial cell line (MLE-12 cells) responded to hyperoxic stress very differently from primary AEC, by significantly increasing GM-CSF protein release (Fig. 1D). This result underscores the value of carrying out these studies in primary cells, whose responses recapitulate those found in the lung in vivo.
Fig. 1.
Effects of exposure to hyperoxia on lung epithelial cell expression of granulocyte/macrophage colony-stimulating factor (GM-CSF) and MCP-1. Primary murine alveolar epithelial cells (AEC) (A–C) or MLE-12 cells (D) were exposed to hyperoxia (80% oxygen/5% CO2) or normoxia (21% oxygen/5% CO2) for 48 h. The culture medium was then replaced with fresh medium, and the cells returned to hyperoxic or normoxic conditions for 18 h further for determination of GM-CSF and MCP-1 protein in the culture supernatant. All data are expressed as means ± SD, n = 3, and are representative of 3 independent experiments involving separate type II cell isolations. A: GM-CSF protein in culture supernatants from AEC. *P < 0.0001. B: relative GM-CSF mRNA in primary AEC was measured by real-time PCR. *P < 0.002. C: MCP-1 protein in culture supernatants from primary AEC. *P < 0.002. D: GM-CSF protein in culture supernatants from MLE-12 cells. *P < 0.001.
AEC mitochondrial stress in the setting of hyperoxia.
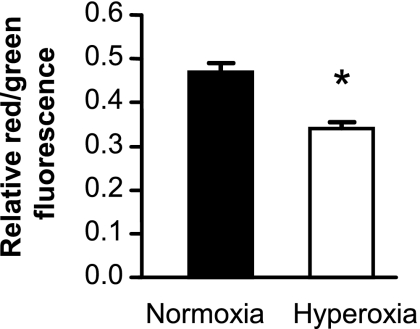
AEC exposed to hyperoxia did not display gross evidence of injury, and in fact produced increased quantities of MCP-1 (Fig. 1C). However, it is possible that hyperoxia might induce mitochondrial stress and injury, an early step in apoptosis. We measured mitochondrial injury using MitoPT, a mitochondrial permeability transition detection kit. Exposure to hyperoxia for 48 h resulted in mildly decreased red/green fluorescent ratio indicating mitochondrial stress (Fig. 2). However, we found no evidence that this level of hyperoxic stress induced apoptosis in AEC, as indicated by cell surface staining for annexin V, induction of histone-associated DNA, or cleaved caspase-3 (data not shown). Thus, exposure to 80% oxygen for 48 h appears to induce moderate stress and a change in cellular behavior without causing apoptosis or widespread cellular dysfunction.
Fig. 2.
Effect of exposure to hyperoxia on AEC mitochondrial stress. AEC were exposed to normoxia or hyperoxia for 48 h. Mitochondrial stress was measured as the ratio of red/green fluorescence in the MitoPT assay as described in materials and methods. Data are expressed as means ± SD, n = 3. *P < 0.001.
Oxidative stress induced by hyperoxia and serum deprivation.
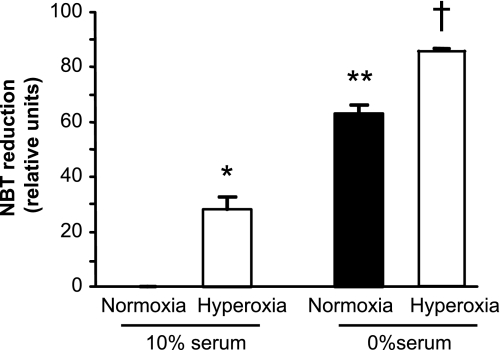
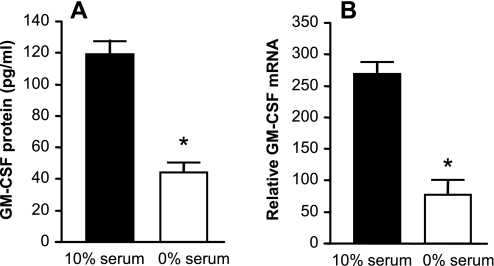
A major mechanism by which exposure to hyperoxia may influence AEC behavior is by inducing oxidative stress. We confirmed that exposure of AEC to hyperoxia for 48 h resulted in significant oxidative stress, as demonstrated by increased intracellular superoxide production in the NBT reduction assay (Fig. 3). It has been shown previously that serum deprivation may also increase production of ROS and lead to oxidative stress (4, 20, 33). We found that serum deprivation increased intracellular AEC ROS (Fig. 3). Similarly, serum deprivation alone (in normoxia) suppressed AEC GM-CSF protein and mRNA expression compared with cells maintained in 10% FCS and normoxia (Fig. 4), without causing frank toxicity, as indicated by change in LDH release from baseline (data not shown). These data provide further support for the relationship between oxidative stress and GM-CSF expression in AEC.
Fig. 3.
Effects of hyperoxia and serum deprivation on generation of ROS in primary murine AEC. AEC were exposed to normoxia (black bar) or hyperoxia (white bars) for 48 h in the presence of control medium (with 10% serum) or serum-free medium. ROS were then determined using the nitroblue tetrazolium (NBT) reduction assay as described in materials and methods. Data are expressed as means ± SD, n = 5. *P < 0.05 vs. normoxia/10% serum; P < 0.01 vs. normoxia/0% serum; and P < 0.001 vs. hyperoxia/0% serum. **P < 0.001 vs. normoxia/10% serum; and P < 0.01 vs. hyperoxia/10% serum. †P < 0.001 vs. both normoxia/10% serum and hyperoxia/10% serum.
Fig. 4.
Effect of serum deprivation on GM-CSF expression by AEC. Primary murine AEC were placed in culture as described in materials and methods. On day 3 the medium was changed to control medium (10% serum) or medium without serum (0% serum). The cells were maintained in normoxia throughout. GM-CSF protein (A) and steady-state mRNA (B) were measured after 48 h. Data are expressed as means ± SD, n = 3. *P < 0.0002.
Effects of oxidative stress on GM-CSF mRNA turnover.
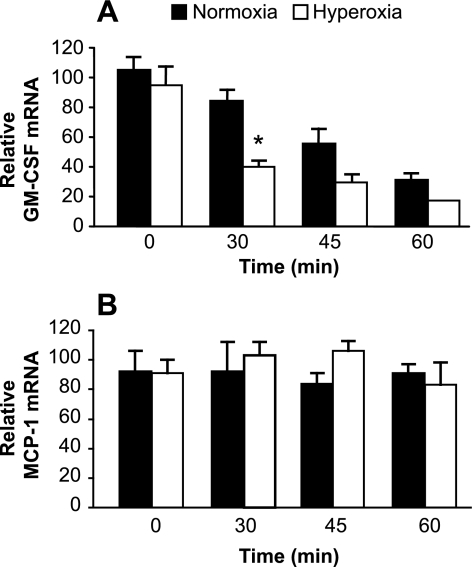
The reduction in GM-CSF protein production associated with oxidative stress was mirrored by a similar reduction in steady-state GM-CSF mRNA expression in both hyperoxia and serum deprivation (Figs. 1B and 4B). Changes in gene expression may be a consequence of changes in mRNA decay, in addition to or instead of change in rates of transcription. The mRNA for GM-CSF contains AU-rich elements (ARE) in the 3′-untranslated region that can facilitate rapid degradation (31). These ARE provide a mechanism by which gene expression may be regulated at the level of mRNA stability. Mechanisms regulating mRNA decay remain poorly understood but are known to involve redox-sensitive pathways (10, 15). We hypothesized that the hyperoxia-induced reduction in GM-CSF expression in primary AECs involves an increased rate of decay of GM-CSF mRNA. As shown in Fig. 5A, exposure to hyperoxia induced a more rapid loss of GM-CSF mRNA expression compared with control cells in normoxia. Thirty minutes after translation was blocked by the addition of Actinomycin D, relative GM-CSF mRNA had decreased significantly more in cells subjected to 80% oxygen for 48 h compared with AEC in normoxia. (Fig. 5A). In contrast, MCP-1 transcripts were relatively stable in normoxia, without major change in mRNA turnover in response to hyperoxia (Fig. 5B). Thus oxidative stress leads to changes in GM-CSF expression by AEC at least in part through changes in specific mRNA stability.
Fig. 5.
Effects of hyperoxia on stability of AEC mRNA for GM-CSF and MCP-1. AEC were placed in culture in hyperoxia or normoxia for 48 h as described in materials and methods. The AEC were then stimulated with IL-1β for 3 h before the addition of Actinomycin D (5 μg/ml) to stop transcription. A: relative mRNA for GM-CSF was measured at time points from 0 to 60 min. Black bars represent cells in normoxia; white bars represent cells in hyperoxia. Data are presented as means ± SE of triplicate samples. *P < 0.01 vs. normoxia at the same time point. The experiment is representative of 3 independent experiments. B: relative mRNA for MCP-1 was measured at time points from 0 to 60 min. Black bars represent cells in normoxia; white bars represent cells in hyperoxia. Data are presented as means ± SE of triplicate samples. There were no significant differences (P > 0.05) for comparisons between normoxia and hyperoxia at any time point. The experiment is representative of 3 independent experiments.
Effects of catalase on hyperoxia-induced reduction in GM-CSF expression by AEC.
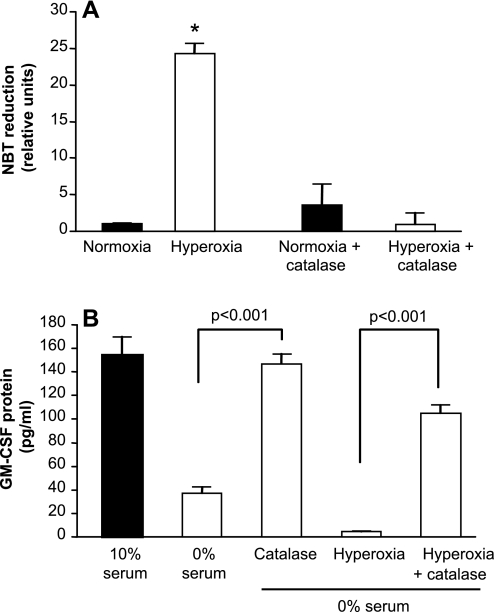
Having determined that GM-CSF production by AEC is responsive to oxidative stress, we next examined the ability of an antioxidant to protect against suppression of GM-CSF in response to stress due to serum deprivation and hyperoxia. Addition of PEG-catalase (1,000 U/ml) to AEC at the time of exposure to hyperoxia was sufficient to prevent the induction of intracellular ROS (Fig. 6A). Catalase completely reversed the reduction in GM-CSF protein secretion due to serum deprivation and largely restored GM-CSF release in AECs subjected to the dual stress of hyperoxia and serum deprivation (Fig. 6B). Interestingly, treatment with catalase also augmented GM-CSF production in unstressed AEC maintained in normoxia in 10% FBS (154 ± 16 pg/ml control vs. 353 ± 12 pg/ml with PEG-catalase). PEG-catalase treatment did not appear to be toxic to the cells, as LDH release did not increase in response to this treatment. This effect was relatively specific for PEG-catalase; neither PEG-superoxide dismutase, DMSO, nor N-acetylcysteine was protective of AEC GM-CSF expression in the face of oxidative stress.
Fig. 6.
Effects of catalase on AEC oxidative stress and GM-CSF expression following exposure to hyperoxia. Primary murine AEC were treated with PEG-catalase (1,000 U/ml) during exposure to hyperoxia or serum deprivation. Control AEC were maintained in 10% serum in normoxia. Data are expressed as means ± SD. The experiments shown are representative of 3 independent experiments. A: AEC ROS were measured as NBT reduction; n = 5. *P < 0.001 vs. each of the other conditions. There was no significant difference between normoxia, normoxia + catalase, and hyperoxia + catalase. B: GM-CSF protein was measured in culture supernatants. Data are expressed as means ± SD. P < 0.001 comparing catalase vs. no catalase, n = 3.
Effect of catalase on AEC GM-CSF mRNA stability.
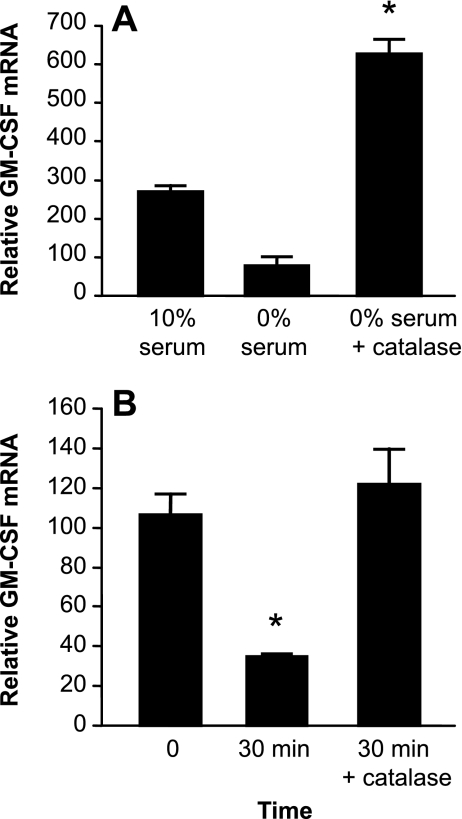
We next confirmed that the protective effects of PEG-catalase treatment on GM-CSF expression in the setting of oxidative stress due to serum deprivation was a reflection of increased relative expression of GM-CSF mRNA (Fig. 7A). Furthermore, this effect on GM-CSF mRNA expression was associated with stabilization of GM-CSF transcripts. As anticipated, under serum-free conditions, GM-CSF mRNA had decayed significantly within 30 min after the addition of Actinomycin D (Fig. 7B). Addition of PEG-catalase to AEC in 0% serum 24 h before Actinomycin D was sufficient to totally inhibit GM-CSF mRNA decay for 30 min after inhibition of transcription, even under these serum-free conditions (Fig. 7B). Thus, the addition of catalase to cells cultured in the absence of serum resulted in alleviation of oxidative stress, stabilization of GM-CSF mRNA, increased steady-state GM-CSF mRNA expression, and increased release of GM-CSF protein.
Fig. 7.
Effect of catalase on GM-CSF mRNA expression and stability during oxidative stress. Primary murine AEC were exposed to serum deprivation with or without the addition of PEG-catalase (1,000 U/ml). Additional control cells were cultured in 10% serum. A: relative GM-CSF mRNA expression in cells exposed to serum deprivation. Data are expressed as means ± SE, n = 3. *P < 0.001 vs. 0% serum. B: after 24 h of oxidative stress, AEC were stimulated with IL-1β for 3 h before the addition of Actinomycin D (5 μg/ml) to stop transcription. Relative GM-CSF mRNA was determined by real-time PCR at 0 and 30 min. Data are expressed as means ± SE, n = 4. *P < 0.01.
DISCUSSION
We have shown previously that expression of GM-CSF in the lung is suppressed during exposure to hyperoxia (1). In the present study, we now show that expression of GM-CSF by AEC in primary culture is also suppressed by exposure to hyperoxia in vitro, or by serum deprivation, and that this effect is not a consequence of simple cytotoxicity. Both hyperoxia and serum deprivation induce significant oxidative stress that can be reversed by treatment with the potent antioxidant catalase. Similarly, PEG-catalase treatment restores GM-CSF expression in the face of hyperoxia or serum deprivation. Finally, we found that changes in mRNA stability are a major mechanism by which AEC GM-CSF expression is suppressed in the setting of oxidative stress.
Considerable evidence indicates that GM-CSF plays a critical role in homeostasis and innate immunity in the lung. Gene-targeted mice lacking GM-CSF (12) or its receptor (24) develop a pathological picture of pulmonary alveolar proteinosis due to impaired turnover of surfactant by AM. In addition to defects in surfactant turnover, AM from GM-CSF−/− mice demonstrate major abnormalities in host defense function, including decreased phagocytosis and killing of microbes, absent cell surface expression of Toll-like receptors (TLR)-2 and -4 (responsible for recognition of gram+ and gram− pathogens, respectively), and greatly decreased expression of TNF, IL-6, and leukotriene B4 in response to inflammatory stimulation (25, 32). These functional defects are associated with diminished expression of the important myeloid transcription factor, PU.1 (32). As might be anticipated in light of the critical role of AM in pulmonary innate immunity, GM-CSF−/− mice are more susceptible than wild-type mice to pneumonia with a variety of pathogens, including bacteria and fungi (3, 22, 26). Expression of GM-CSF exclusively in the lung, or administration of GM-CSF to the lung, is sufficient both to reverse the picture of alveolar proteinosis and to restore normal host defense function. Humans with primary pulmonary alveolar proteinosis have now been shown to have high titers of neutralizing antibodies to GM-CSF (37). AM from these individuals demonstrate functional characteristics similar to those of AM from GM-CSF−/− mice (37).
Intratracheal administration of neutralizing anti-GM-CSF antibodies in mice is sufficient to induce loss of normal AM functional characteristics (8). Interestingly, we have found that relatively mild lung injury induced by short-term (sublethal) exposure to hyperoxia suppressed lung GM-CSF expression in vivo, leading to impaired AM function and increased susceptibility to lethal pneumonia due to K. pneumoniae (1). Alveolar macrophage function and lung bacterial clearance were restored by treatment with recombinant GM-CSF during hyperoxia. Thus, continuous GM-CSF is required to maintain the normal mature AM phenotype, and GM-CSF expression in the lung is susceptible to short-term changes in the setting of lung injury.
Oxidative stress is a common feature in a number of pathological processes leading to lung injury. Inflammation driven by neutrophils or mononuclear phagocytes, ischemia-reperfusion injury, toxic inhalations (ozone, cigarette smoke, sulfur dioxide), and high concentrations of oxygen all induce significant oxidative stress in the lung (7, 9, 21). Although oxygen therapy is an essential component of supportive care for many patients, breathing high concentrations of oxygen may lead to lung injury, impaired barrier function of the alveolar wall, and death (16). Because exposure of mice to >95% oxygen causes a reproducible lung injury that recapitulates features of human ARDS, hyperoxia is an important and biologically relevant model for the study of acute lung injury.
We have used a model of sublethal lung injury induced by limited exposure to hyperoxia to examine the mechanisms by which pulmonary innate immunity might be altered in the setting of lung injury. AEC are an important source of GM-CSF in the lung. We found that sublethal hyperoxia significantly suppressed GM-CSF mRNA expression both measured in lung homogenates and assessed in cells in the alveolar wall (1). Furthermore, GM-CSF protein expression ex vivo was diminished in AEC isolated from mice exposed to hyperoxia in vivo. Interestingly, these cells did not demonstrate signs of overt injury and expressed increased levels of MCP-1 compared with similarly isolated cells from mice in normoxia. When normal murine AEC in primary culture are exposed to an atmosphere of 80% oxygen for 48 h, they experience significant stress, as indicated by measures of mitochondrial stress and oxidative stress, without gross toxicity (as measured by changes in cell number or LDH release). However, GM-CSF expression was significantly suppressed. Thus, this concentration of oxygen altered GM-CSF expression without inducing major injury to these cells, suggesting that this exposure provides an appropriate model of the stress associated with a limited exposure to hyperoxia in vivo from which mice can recover fully if returned to room air. Thus, we have now extended our previous work and determined that at least one component of the hyperoxia-induced decrease in lung GM-CSF expression involves direct effects of hyperoxia on AEC.
An important feature of the present work is the use of primary murine AEC. Our isolation procedure provides highly enriched populations of type II AEC. When cultured under standard conditions, these cells lose type II cell characteristics, such as expression of surfactant protein C, and begin to express characteristics associated with the type I cell phenotype in vivo, such as expression of T1α and aquaporin 5 (unpublished observations, Reynolds PR, and Ref. 23). Although a number of cell lines derived from the lungs of humans, mice, and rats have been used in studies providing important insights into alveolar epithelial biology, these cell lines do not always recapitulate specific AEC characteristics of interest. In contrast to primary murine AEC, MLE-12 cells exposed to hyperoxia were induced to express increased GM-CSF protein. Thus, when exposed to hyperoxia in vitro, primary AEC more closely model the expression of GM-CSF in the lung of the hyperoxia-exposed mouse.
The relative contributions of type I and type II AEC to alveolar GM-CSF have not yet been determined. Similarly, it is not clear whether oxidant-dependent suppression of GM-CSF expression is a feature common to all AEC, or is a specific characteristic of type I or type II cells. Previous studies have suggested that type II AEC may be more resistant to oxidative stress than are type I cells, due to relatively increased expression of antioxidant enzymes (34). Limited expression of antioxidant defenses in type I AEC might render these cells particularly susceptible to oxygen-induced suppression of GM-CSF expression. The reasons for differences in response to hyperoxia of primary AEC compared with MLE-12 cells are not yet clear. It is possible that the immortalized cell line, generated by transgenic expression of the large T antigen of simian virus 40, simply fails to recapitulate the characteristics of normal AEC as a function of this process. Alternatively, MLE-12 cells may differ from our primary AEC with respect to GM-CSF expression because they represent a different subset of alveolar epithelial cells. In contrast to primary AEC that have been in culture for 3–4 days on tissue culture plastic, MLE-12 cells in culture continue to express significant SP-C mRNA and more limited amounts of T1α mRNA (data not shown). This observation suggests that they may continue to express type II cell features, with more limited expression of features of the type I cell phenotype compared with primary AEC. Studies to explore the relative vulnerability of GM-CSF in these different AEC types are ongoing.
Suppression of GM-CSF expression following exposure to oxidative stress was associated with decreased GM-CSF mRNA expression, which was at least in part a function of significantly accelerated mRNA turnover. This mechanism is consistent with prior studies in other cells demonstrating that GM-CSF transcripts are intrinsically unstable and may be rapidly degraded, limiting production of the protein product. This rapid turnover is attributable to ARE in the 3′-untranslated region of the transcript (31). Thus, stabilization of GM-CSF mRNA has often been found to be a crucial regulatory step in expression of GM-CSF protein. Studies in T cells (38), eosinophils (13, 14), and airway smooth muscle cells (36) have demonstrated major roles for changes in mRNA stability in the regulation of GM-CSF expression. Studies in A549 cells, a human cell line derived from a bronchoalveolar cell carcinoma that expresses some characteristics of type II AEC, found that expression of GM-CSF was controlled by changes in mRNA stability (5). Consistent with our findings, these investigators found that treatment with antioxidants stabilized GM-CSF mRNA, leading to increased expression. We have extended this observation, demonstrating in primary AEC that hyperoxia leads to more rapid GM-CSF mRNA degradation. This change is a consequence of oxidative stress and may be reversed by treatment with PEG-catalase.
These data have important implications for pulmonary host defense in the setting of acute lung injury. They demonstrate that oxidative stress due to either the precipitating cause of lung injury or treatment with high concentrations of supplemental oxygen leads to increased susceptibility to health care-associated pneumonia due to AM dysfunction attributable to decreased AEC expression of GM-CSF. They also may provide a mechanism to explain why individuals with diminished lung antioxidant defenses, including alcoholics (17), would have impaired pulmonary host defense and increased susceptibility to pneumonia. Previously, there has been considerable enthusiasm for the use of antioxidants intended to reduce the extent of lung injury. Our work suggests that antioxidants might also have a role in supporting pulmonary innate immunity in the acutely injured lung. Furthermore, it suggests that the particular choice of antioxidant might be critical for this effect.
GRANTS
This work was supported by grants (to R. Paine III) from the Department of Veterans Affairs (VA Merit Review) and National Heart, Lung, and Blood Institute (Grant HL-64558).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Susan Morris and Michael Mendez for helpful discussions in the initial phases of this work and to Diana Lim for assistance with graphics.
REFERENCES
- 1.Baleeiro CE, Christensen PJ, Morris SB, Mendez MP, Wilcoxen SE, Paine R. GM-CSF and the impaired pulmonary innate immune response following hyperoxic stress. Am J Physiol Lung Cell Mol Physiol 291: L1246–L1255, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Baleeiro CEO, Wilcoxen SE, Morris SB, Standiford TJ, Paine R., III Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol 171: 955–963, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Ballinger MN, Paine R, 3rd, Serezani CH, Aronoff DM, Choi ES, Standiford TJ, Toews GB, Moore BB. Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa. Am J Respir Cell Mol Biol 34: 766–774, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellomo F, Piccoli C, Cocco T, Scacco S, Papa F, Gaballo A, Boffoli D, Signorile A, D'Aprile A, Scrima R, Sardanelli AM, Capitanio N, Papa S. Regulation by the cAMP cascade of oxygen free radical balance in mammalian cells. Antioxid Redox Signal 8: 495–502, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bergmann M, Barnes PJ, Newton R. Molecular regulation of granulocyte macrophage colony-stimulating factor in human lung epithelial cells by interleukin (IL)-1beta, IL-4, and IL-13 involves both transcriptional and post-transcriptional mechanisms. Am J Respir Cell Mol Biol 22: 582–589, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Bezdicek P, Crystal R. Pulmonary macrophages. In: The Lung: Scientific Foundations, edited by Crystal R, West J, Weibel E, Barnes P. Philadelphia, PA: Lippincott-Raven, 1997, p. 859–875 [Google Scholar]
- 7.Bhalla DK, Hirata F, Rishi AK, Gairola CG. Cigarette smoke, inflammation, and lung injury: a mechanistic perspective. J Toxicol Environ Health B Crit Rev 12: 45–64, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Bozinovski S, Jones J, Beavitt SJ, Cook AD, Hamilton JA, Anderson GP. Innate immune responses to LPS in mouse lung are suppressed and reversed by neutralization of GM-CSF via repression of TLR-4. Am J Physiol Lung Cell Mol Physiol 286: L877–L885, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J 11: 745–757, 1998 [PubMed] [Google Scholar]
- 10.Clark A, Dean J, Tudor C, Saklatvala J. Post-transcriptional gene regulation by MAP kinases via AU-rich elements. Front Biosci 14: 847–871, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol 14: 309–315, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, Mulligan RC. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 264: 713–716, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Esnault S, Malter JS. Extracellular signal-regulated kinase mediates granulocyte-macrophage colony-stimulating factor messenger RNA stabilization in tumor necrosis factor-alpha plus fibronectin-activated peripheral blood eosinophils. Blood 99: 4048–4052, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Esnault S, Malter JS. Hyaluronic acid or TNF-α plus fibronectin triggers granulocyte macrophage-colony-stimulating factor mRNA stabilization in eosinophils yet engages differential intracellular pathways and mRNA binding proteins. J Immunol 171: 6780–6787, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Fillebeen C, Pantopoulos K. Redox control of iron regulatory proteins. Redox Rep 7: 15–22, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Folz R, Piantadosi C, Crapo J. Oxygen toxicity. In: The Lung: Scientific Foundations, edited by Crystal R, West J, Barnes P, Weibel E. Philadelphia, PA: Lippincott-Raven, 1997, p. 2713–2722 [Google Scholar]
- 17.Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest 101: 761–768, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huffman JA, Hull WM, Dranoff G, Mulligan RC, Whitsett JA. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J Clin Invest 97: 649–655, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikegami M, Jobe AH, Huffman Reed JA, Whitsett JA. Surfactant metabolic consequences of overexpression of GM-CSF in the epithelium of GM-CSF-deficient mice. Am J Physiol Lung Cell Mol Physiol 273: L709–L714, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Kang SI, Choi HW, Kim IY. Redox-mediated modification of PLZF by SUMO-1 and ubiquitin. Biochem Biophys Res Commun 369: 1209–1214, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest 122: 314S–320S, 2002 [DOI] [PubMed] [Google Scholar]
- 22.LeVine AM, Reed JA, Kurak KE, Cianciolo E, Whitsett JA. GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J Clin Invest 103: 563–569, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendez MP, Morris SB, Wilcoxen S, Greeson E, Moore B, Paine R., III Shedding of soluble ICAM-1 into the alveolar space in murine models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 290: L962–L970, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Nishinakamura R, Nakayama N, Hirabayashi Y, Inoue T, Aud D, McNeil T, Azuma S, Yoshida S, Toyoda Y, Arai K, Miyajima A, Murray R. Mice deficient for the IL-3/GM-CSF/IL-5 beta c receptor exhibit lung pathology and impaired immune response, while beta IL3 receptor-deficient mice are normal. Immunity 2: 211–222, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Paine R, 3rd, Morris SB, Jin H, Wilcoxen SE, Phare SM, Moore BB, Coffey MJ, Toews GB. Impaired functional activity of alveolar macrophages from GM-CSF-deficient mice. Am J Physiol Lung Cell Mol Physiol 281: L1210–L1218, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Paine R, 3rd, Preston AM, Wilcoxen S, Jin H, Siu BB, Morris SB, Reed JA, Ross G, Whitsett JA, Beck JM. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. J Immunol 164: 2602–2609, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Reed J, Ikegami M, Cianciolo E, Lu W, Cho P, Hull W, Jobe A, Whitsett J. Aerosolized GM-CSF ameliorates pulmonary alveolar proteinosis in GM-CSF-deficient mice. Am J Physiol Lung Cell Mol Physiol 276: L556–L563, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Reed J, Whitsett J. Granulocyte-macrophage colony-stimulating factor and pulmonary surfactant homeostasis. Proc Assoc Am Phys 110: 321–332, 1998 [PubMed] [Google Scholar]
- 29.Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 30: 4480–4486, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Rook GA, Steele J, Umar S, Dockrell HM. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by gamma-interferon. J Immunol Methods 82: 161–167, 1985 [DOI] [PubMed] [Google Scholar]
- 31.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 46: 659–667, 1986 [DOI] [PubMed] [Google Scholar]
- 32.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 15: 557–567, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Shigemura K, Sung SY, Kubo H, Arnold RS, Fujisawa M, Gotoh A, Zhau HE, Chung LW. Reactive oxygen species mediate androgen receptor- and serum starvation-elicited downstream signaling of ADAM9 expression in human prostate cancer cells. Prostate 67: 722–731, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Simon RH, Edwards JA, Reza MM, Kunkel RG. Injury of rat pulmonary alveolar epithelial cells by H2O2: dependence on phenotype and catalase. Am J Physiol Lung Cell Mol Physiol 260: L318–L325, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Tran T, Fernandes DJ, Schuliga M, Harris T, Landells L, Stewart AG. Stimulus-dependent glucocorticoid-resistance of GM-CSF production in human cultured airway smooth muscle. Br J Pharmacol 145: 123–131, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med 349: 2527–2539, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Wang JG, Collinge M, Ramgolam V, Ayalon O, Fan XC, Pardi R, Bender JR. LFA-1-dependent HuR nuclear export and cytokine mRNA stabilization in T cell activation. J Immunol 176: 2105–2113, 2006. [DOI] [PubMed] [Google Scholar]