Abstract
Pulmonary edema is mediated in part by disruption of interendothelial cell contacts. Protein tyrosine phosphatases (PTP) have been shown to affect both cell-extracellular matrix and cell-cell junctions. The SH2 domain-containing nonreceptor PTP, SHP2, is involved in intercellular signaling through direct interaction with adherens junction proteins. In this study, we examined the role of SHP2 in pulmonary endothelial barrier function. Inhibition of SHP2 promoted edema formation in rat lungs and increased monolayer permeability in cultured lung endothelial cells. In addition, pulmonary endothelial cells demonstrated a decreased level of p190RhoGAP activity following inhibition of SHP2, events that were accompanied by a concomitant increase in RhoA activity. Furthermore, immunofluorescence microscopy confirmed enhanced actin stress fiber formation and diminished interendothelial staining of adherens junction complex-associated proteins upon SHP2 inhibition. Finally, immunoprecipitation and immunoblot analyses demonstrated increased tyrosine phosphorylation of VE-cadherin, β-catenin, and p190RhoGAP proteins, as well as decreased association between p120-catenin and VE-cadherin proteins. Our findings suggest that SHP2 supports basal pulmonary endothelial barrier function by coordinating the tyrosine phosphorylation profile of VE-cadherin, β-catenin, and p190RhoGAP and the activity of RhoA, signaling molecules important in adherens junction complex integrity.
Keywords: RhoA, protein tyrosine phosphorylation
the pulmonary endothelium regulates protein and fluid extravasation at the alveolar-capillary interface. In acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), this barrier is disrupted, resulting in vascular leakage and pulmonary edema (69). Identification of the cellular components involved in the pathogenesis of these diseases is extremely important for the development of efficacious treatments.
The barrier disruption involved in ALI and ARDS is mediated, in part, by the breakdown of interendothelial cell adhesions (24, 36, 68, 69). Numerous studies support a link between increased protein tyrosine phosphorylation and vascular permeability (7, 59, 68, 75). Agonists have been shown to stimulate protein tyrosine phosphorylation in cultured endothelial monolayers, as well as in lungs in vivo; in all cases, an increase in tyrosine phosphorylation correlated with increased permeability (2, 3, 20, 27, 58, 62).
The level of protein phosphorylation within the cell is controlled by the opposing actions of the protein kinases and protein phosphatases. Protein phosphatases are divided into three groups: serine/threonine-specific protein phosphatases; dual-specificity phosphatases, which can dephosphorylate protein substrates on serine, threonine, and tyrosine residues; and the protein tyrosine phosphatases (PTP) (64).
PTP, the most abundant type of cellular phosphatase, are divided into four classes, composed of 44 isoforms; the majority belong to either the receptor-type or nonreceptor-type class (53). Several studies have shown that general PTP inhibition leads to an increase in endothelial monolayer permeability (70, 74). Several PTP isoforms, PTPμ, PTP1B, and VE-PTP, have been implicated in the regulation of endothelial paracellular permeability (48, 51, 62). In both human pulmonary artery and lung endothelial cells (EC), PTPμ expression was observed almost exclusively at cell-cell contacts, bound directly to VE-cadherin (62). PTPμ protein suppression significantly impaired endothelial barrier function, whereas overexpression resulted in decreased tyrosine phosphorylation of VE-cadherin and enhanced barrier function (62). In human umbilical vein EC, overexpression of PTP1B stabilized VE-cadherin-mediated adhesions by reducing VE-cadherin tyrosine phosphorylation, and decreased monolayer permeability, whereas suppression of PTP1B generated opposite effects (48). Similarly, suppression of VE-PTP resulted in barrier dysfunction of endothelioma cells and diminished homotypic interactions of VE-cadherin (51).
The SH2 domain-containing nonreceptor PTP, SHP2, has been shown to associate with VE-cadherin through β-catenin. Thrombin-induced loss of SHP2 from adherens junctions has correlated with increased tyrosine phosphorylation of β-catenin and γ-catenin, altering their association with α-catenin and leading to adherens junction disassembly (66). While SHP2 has been shown to associate with VE-cadherin and its adherens junction components (66, 74) and to play a role in the regulation of angiogenesis (45), a direct link between SHP2 and endothelial barrier function has not been established. This study is the first to show that SHP2 is essential for maintenance of endothelial barrier function in the pulmonary vasculature, both in cultured monolayers and in intact lungs. Additionally, the results of this study imply that inhibition of SHP2 promotes endothelial barrier dysfunction through enhanced tyrosine phosphorylation of p190RhoGAP, VE-cadherin, and β-catenin and RhoA activation, resulting in adherens junction disassembly.
MATERIALS AND METHODS
Cell lines and reagents.
Rat lung microvascular endothelial cells (LMVEC) and bovine pulmonary artery endothelial cells (PAEC) from Vec Technologies (Rensselaer, NY) were maintained in MCDB-131 (Vec Technologies) and MEM supplemented with 10% FBS (Gemini Bio-Products, West Sacramento, CA), 1% sodium pyruvate, and 1% penicillin/streptomycin/fungizone (Invitrogen, Carlsbad, CA), respectively, and used between passage 5 and passage 11. LMVEC and PAEC used throughout the study maintained the traditional endothelial cell characteristics of von Willebrand factor and VE-cadherin expression, uptake of acetylated LDL (unpublished observations), and positive staining for characteristic lectins.
SHP2 inhibitor, 8-hydroxy-7-(6-sulfonaphthalan-2-yl)diazenyl-quinoline-5-sulfonic acid (NSC-87877), was purchased from Calbiochem (San Diego, CA). Pan SHP2, VE-cadherin, p120-catenin, RhoGDI-1α, and RhoA antibodies, as well as the antibody directed against phosphorylated tyrosine (pY99), were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). Antibodies directed against p190RhoGAP-A and β-catenin were obtained from BD Transduction Laboratories (San Jose, CA). Expression of influenza hemagglutinin (HA) was detected using an antibody from Roche Applied Science (Indianapolis, IN). Texas red-conjugated and Alexa Fluor 488-conjugated phalloidin were obtained from Molecular Probes (Eugene, OR).
The prokaryotic expression vector encoding the catalytically inactive SHP2 mutant (C459S) was obtained from Addgene (Addgene plasmid 8382; Cambridge, MA), referred to as SHP2C459S. Prokaryotic expression vectors encoding the GST-RBD, GST-RhoA(Q63L), and GST-RhoGDI-1α motifs were kind gifts from Dr. John G. Collard (The Netherlands Cancer Institute, Amsterdam, The Netherlands) (55), Dr. Keith Burridge (UNC School of Medicine, Chapel Hill, NC) (50), and Dr. Allan Hall (University College, London, UK), respectively. The pGFP-C1 vector was purchased from Clontech (Mountain View, CA).
Transfection.
Transient transfection of PAEC was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Optimal overexpression of GFP and SHP2C459S was confirmed to occur in PAEC transfected with the prokaryotic expression vectors at 48 h posttransfection.
Endothelial monolayer permeability.
Changes in endothelial monolayer permeability were assessed using the electrical cell impedance sensor technique (Applied Biophysics, Troy, NY) as previously described (28). For analysis of monolayer permeability following transfection with SHP2C459S or GFP, cells were transfected in plastic tissue culture dishes, trypsinized 48 h posttransfection, and replated onto collagen-coated ECIS arrays. Cells were allowed to adhere to arrays for 5 h in a tissue culture incubator; the arrays were then placed in the ECIS chamber and allowed to equilibrate for 4 h. Thus, resistance of transfected monolayers was assessed 57 h posttransfection. For all experiments, overexpression of protein of interest was confirmed by immunoblot analysis of monolayers, post-ECIS measurements.
Immunoblot analyses.
Equivalent amounts of protein were resolved by SDS-PAGE and immunoblotted as previously described (32).
Immunoprecipitations.
Immunoprecipitations were performed using 500 μg of lysate, as detailed in Ref. 29.
Immunofluorescence analyses.
Endothelial cells were seeded onto collagen-coated glass coverslips and treated as described. The cells were washed with PBS, fixed with 4% paraformaldehyde, and rendered permeable with 0.1% Triton X-100. Following blocking in 5% normal serum, the cells were incubated with primary antibody raised against β-catenin, VE-cadherin, or HA, or with fluorescently conjugated phalloidin. Following removal of primary antibodies, cells were incubated with fluorescently conjugated secondary antibodies and then viewed and recorded at ×1,000 magnification with a Nikon Eclipse E400 fluorescence microscope interfaced with a SPOT Diagnostics Instruments digital camera.
RhoA activity assay.
RhoA activity was determined as previously detailed (30, 42). Briefly, endothelial cells were lysed in FISH buffer (10% glycerol, 50 mM Tris, pH 7.4, 100 mM NaCl, 1% Nonidet P-40, and 2 mM MgCl2). The lysates were incubated on ice for 10 min and then cleared by centrifugation at 15,000 g for 10 min at 4°C. Equivalent volumes of supernatants were incubated with 50 μg of bacterially produced GST-rhotekin binding domain (RBD) bound to glutathione sepharose beads for 2 h at 4°C. The beads were then washed with FISH buffer and resuspended in 30 μl of 2× Laemmli buffer. Protein complexes bound to the beads were resolved on 15% SDS-PAGE and then transferred to Immobilon-P membrane for immunoblot analysis using an antibody directed against RhoA. Parallel immunoblots were performed with corresponding total cell lysates, allowing for calculation of the ratio of active RhoA to total RhoA.
p190RhoGAP activity assay.
Activity of p190RhoGAP was assessed as in Fordjour (21) and Noren et al. (50). Briefly, endothelial cells were lysed in a HEPES-based buffer (50 mm HEPES, pH 7.5, 50 mm NaCl, and 1 mm MgCl2). The lysates were incubated on ice for 10 min and then cleared by centrifugation at 15,000 g for 10 min at 4°C. Equivalent volumes of supernatants were incubated with 50 μg of bacterially produced GST-RhoA(Q63L) bound to glutathione sepharose beads for 2 h at 4°C. The beads were then washed with lysis buffer and resuspended in 30 μl of 2× Laemmli buffer. Protein complexes bound to the beads were resolved on 10% SDS-PAGE and then transferred to Immobilon-P membrane for immunoblot analysis using an antibody specific for p190RhoGAP-A. Parallel immunoblots were performed with corresponding total cell lysates, allowing for calculation of the ratio of active p190RhoGAP-A to total p190RhoGAP-A.
Measurement of edema in rat lungs.
All animal experimental protocols were approved by the Providence Veterans Affairs Medical Center and Brown University Institutional Animal Care and Use Committee and comply with the Health Research Extension Act and the Public Health Service policy. For the ex vivo lung edema studies, lungs were isolated from anesthetized adult male Sprague-Dawley rats (250–500 g) and perfused as previously described (35). Filtration coefficient (kf) was determined by the rate of weight gain during the final 2 min, following an increase in Pv pressure by ∼8 cmH2O (high hydrostatic challenge) for 15 min divided by the change in capillary pressures induced by the double occlusion technique. kf was normalized to 100 grams of wet lung mass, empirically derived as 0.00472 body mass (65). After establishment of baseline kf, lungs were returned to basal pressures, and either vehicle or indicated concentration of NSC-87877 was added to the reservoir and allowed to circulate for 30 min, after which a second kf measurement was taken.
For the in vivo lung edema studies, adult male Sprague-Dawley rats were anesthetized and a catheter placed in the external jugular vein. Blood volume of the rats was calculated as detailed in Lee and Blaufox (38). The rats were then randomly given a 100 μM dose of the SHP2 inhibitor, NSC-87877, or a corresponding dose of vehicle (H2O) in 1 ml of 1× PBS through the jugular vein catheter. Five minutes after infusion of the vehicle or SHP2 inhibitor, 1.25 ml of 4% Evans blue dye (EBD)-conjugated albumin in PBS was injected at the same site. The animals were killed after an additional 45 min, the pulmonary vasculature was perfused with 3 ml of PBS via the right ventricle, and the lungs were removed and homogenized in 2 ml of formamide and incubated at 60°C overnight. The amount of dye in the lungs was then determined spectrophotometrically and extrapolated from a standard curve.
Statistical analyses.
For three or more groups, differences among the means were tested for significance in all experiments, using ANOVA with the Fisher least significance difference test. For two groups, differences among the means were tested for significance using Student's unpaired t-test or paired t-test. Significance was reached when P < 0.05. Data are presented as means ± SE; n is indicated for each set of data.
RESULTS
SHP2 inhibition disrupts the pulmonary endothelial barrier.
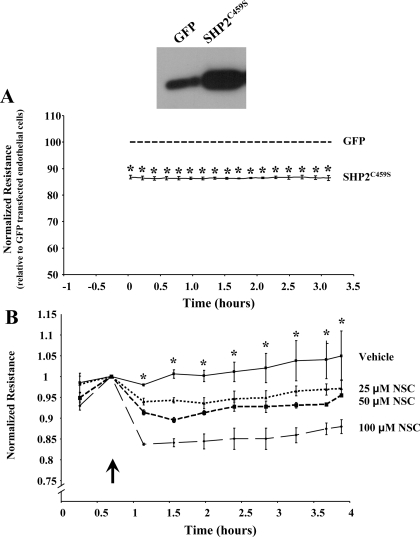
The current study further investigated the role of SHP2 in endothelial cell function, focusing on its role in regulating monolayer permeability. In the first set of experiments, we tested the effects of SHP2 inhibition on barrier function of pulmonary endothelial monolayers. Equivalent numbers of PAEC were transiently transfected with eukaryotic vectors encoding a catalytically inactive form of SHP2, C459S (referred to as SHP2C459S) or GFP, as a control. In the absence of any edemagenic agents, we noted that the resistance across the monolayers overexpressing SHP2C459S was significantly decreased compared with those endothelial cells transfected with GFP (Fig. 1A), with an average difference in resistance between GFP and SHP2C459S transfected endothelial monolayers of ∼100 ± 10 ohms (Supplemental Fig. S1; Supplemental data for this article is available online at the AJP-Lung web site.). Similarly, treatment of endothelial cells with the SHP2 chemical inhibitor NSC-87877 demonstrated significant increases in monolayer permeability in a dose-dependent manner (Fig. 1B). Because chelation of divalent cations is known to disrupt adherens junction complexes (10) and promote endothelial barrier dysfunction (23), we next compared the degree of change in resistance across the endothelial monolayers exposed to the SHP2 inhibitor with that of endothelial monolayers exposed to EGTA. We noted that the maximum change in resistance of endothelial cell monolayers exposed to the SHP2 chemical inhibitor increased in a stepwise fashion upon increasing doses of NSC-87877 (Supplemental Fig. S2). In addition, at the greatest concentration of NSC-87877 used, the maximum drop in resistance across these monolayers was less than those in which the adherens junctions were disrupted by the ion chelating agent (100 μM NSC-87877, 131.5 ± 5.3 ohms; 1.5 mM EGTA, 257.74 ± 12.2 ohms).
Fig. 1.
SHP2 inhibition causes endothelial barrier dysfunction. A: changes in resistance across monolayers were measured after transient transfection of equivalent numbers of pulmonary artery endothelial cells (PAEC) with GFP (dashed line) or SHP2C459S (solid line) for 48 h. Data are presented as means ± SE of the resistance across the SHP2C459S monolayers normalized to the resistance across the GFP-transfected monolayers. Means ± SE; n = 3; *P < 0.0001 vs. GFP. A representative immunoblot probing for SHP2 is shown. B: changes in resistance across PAEC monolayers were measured following exposure to vehicle or NSC-87877. Arrow indicates addition of treatment. Means ± SE; n = 4; *P < 0.05 vs. vehicle for all doses.
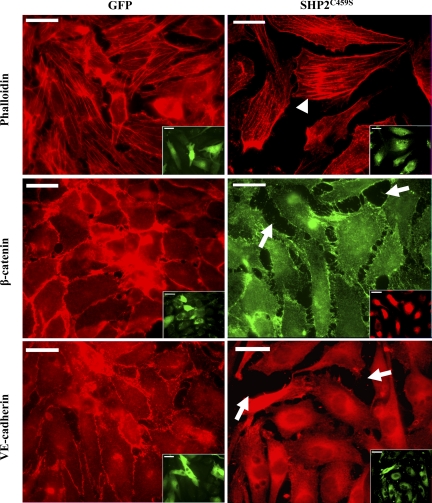
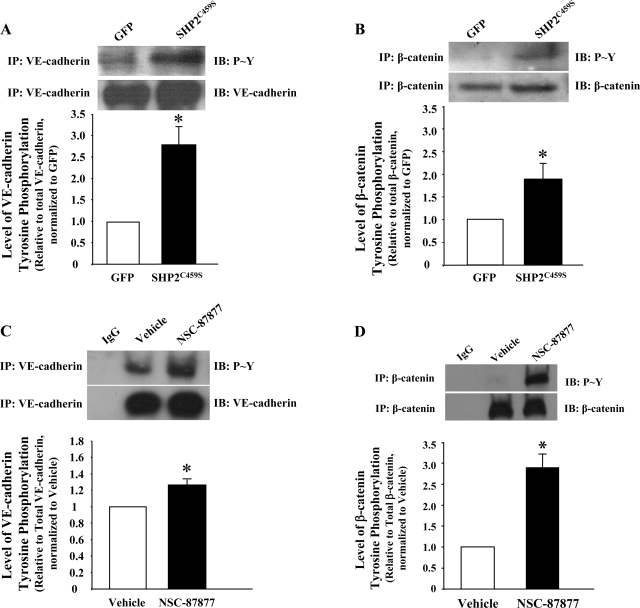
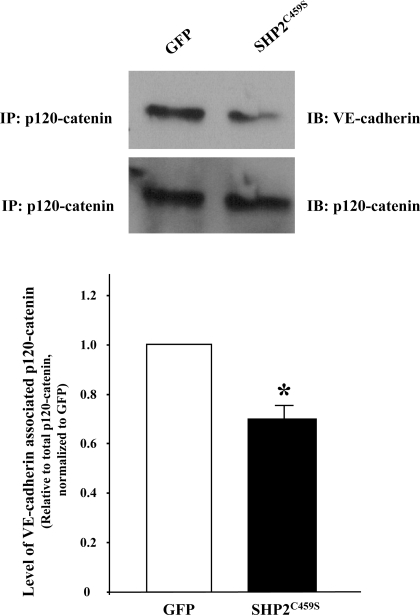
Immunofluorescence microscopy analyses correlated the observed barrier dysfunction upon SHP2C459S overexpression with enhanced level of interendothelial cell gapping and disruption of adherens junctions, as determined by diminished VE-cadherin and β-catenin staining, as well as increased stress fiber formation (Fig. 2). Again, similar results were noted in NSC-87877-treated endothelial monolayers (Supplemental Fig. S3). While total levels of VE-cadherin and β-catenin did not differ significantly between the transfected endothelial cells, both overexpression of the catalytically inactive SHP2 (Fig. 3, A and B) or treatment with NSC-87877 (Fig. 3, C and D) resulted in a significant increase in tyrosine phosphorylation of both VE-cadherin and β-catenin. In addition, endothelial monolayers overexpressing SHP2C459S demonstrated a reduced association between p120-catenin and VE-cadherin, relative to GFP overexpressing endothelial cells (Fig. 4). These results suggest that SHP2 regulates endothelial barrier function by maintaining adherens junction integrity through the suppression of tyrosine phosphorylation of adherens junction proteins, thus preserving the protein-protein interactions and complex formation.
Fig. 2.
Overexpression of catalytically inactive SHP2 promotes stress fiber formation and adherens junction disassembly. Equivalent numbers of PAEC were transfected with GFP or SHP2C459S for 48 h. Endothelial cells were fixed and immunofluorescently stained for filamentous actin with phalloidin and for β-catenin or VE-cadherin with protein-directed antibodies. Insets are the same endothelial cells viewed for overexpression of GFP (left) or overexpression of SHP2C459S by costaining with anti-HA antibody (right). Arrows indicate intercellular gapping; arrowheads denote increased stress fiber formation. Scale bars, 20 μM; n = 3.
Fig. 3.
Inhibition of SHP2 leads to increased tyrosine phosphorylation of adherens junction proteins. Equivalent numbers of PAEC were transfected with GFP or SHP2C459S for 48 h (A and B) or confluent monolayers of lung microvascular endothelial cells (LMVEC) were incubated with 100 μM NSC-87877 for 3 h (C and D). Equal amounts of endothelial cell lysate were immunoprecipitated with antibodies directed against VE-cadherin (A and C) or β-catenin (B and D), and precipitates were immunoblotted for phosphorylated tyrosine. The immunoblots were stripped and reprobed for the immunoprecipitated protein. The data are presented as means ± SE of densitometric values of tyrosine phosphorylated adherens junction protein relative to total adherens junction protein, normalized to GFP (A and B) or vehicle (C and D). N = 3; *P < 0.05 vs. GFP or vehicle, respectively.
Fig. 4.
Overexpression of catalytically inactive SHP2 leads to reduced association of p120-catenin and VE-cadherin. Equivalent numbers of PAEC were transfected with GFP or SHP2C459S for 48 h. Equivalent amounts of endothelial cell lysate were immunoprecipitated with an antibody directed against p120-catenin, and precipitates were immunoblotted for VE-cadherin. The immunoblots were then stripped and reprobed for p120-catenin. N = 3; *P < 0.05 vs. GFP.
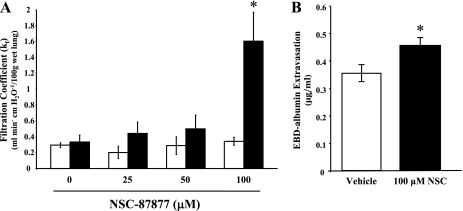
Next, to determine if our in vitro observations correlated with a role for SHP2 in regulating endothelial barrier function in the lung, we assayed the effects of NSC-87877 on edema formation in the lung, using both ex vivo and in vivo models (35). Lung filtration coefficients (kf) were determined in isolated, perfused rat lungs at baseline and following exposure to vehicle or increasing doses of NSC-87877. At 100 μM NSC-87877, we observed an approximately fourfold increase in kf compared with baseline, whereas vehicle had no significant effect (Fig. 5A). Additionally, lung edema formation, as assessed by measuring the level of EBD-conjugated albumin extravasation into the lung, demonstrated a significant level of edema formation in the lungs of rats administered 100 μM NSC-87877 compared with the lungs of animals administered vehicle (Fig. 5B). These findings support a role for SHP2 in maintaining endothelial barrier function within the pulmonary vasculature and suggest that a change in SHP2 activity could contribute to formation of lung edema.
Fig. 5.
Pulmonary edema formation results on SHP2 inhibition. A: filtration coefficients (kf) were determined in isolated, perfused rat lungs at baseline (open bars) and following a 30-min exposure to vehicle (0 μM) or indicated concentration of NSC-87877 (solid bars). Values are means ± SE; n = 3–6; *P < 0.05 vs. vehicle. B: anesthetized rats were given a bolus of vehicle (PBS; open bar) or 100 μM NSC-87877 (solid bar). The rats were then given 4% Evans blue dye (EBD)-conjugated albumin after 5 min and were euthanized after an additional 45 min. The lungs were harvested, and the amount of extravasated albumin in the lungs was determined spectrophotometrically. Values are means ± SE; n = 9–10; *P < 0.05 vs. vehicle.
SHP2 inhibition inversely affects the activities of RhoA and p190RhoGAP.
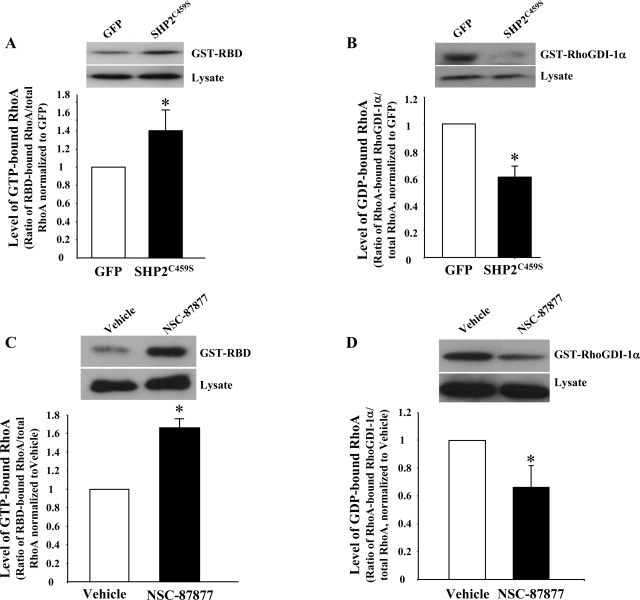
Since RhoA activity is closely associated with endothelial barrier function through regulation of adherens junctions and stress fibers (1, 12, 43, 54, 71, 73), we next assayed its activity in endothelial cells overexpressing catalytically inactive SHP2, relative to GFP overexpression, as well as in those cells treated with NSC-87877 vs. vehicle. Affinity precipitation assays revealed that molecular inhibition of SHP2 caused a significant increase in RhoA activity compared with GFP overexpressing endothelial cells (Fig. 6A). Parallel experiments similarly noted enhanced RhoA activity in NSC-87877-treated endothelial cells relative to vehicle-treated endothelial cells (Fig. 6C). Similarly, we noted a significantly lower amount of GDI-1α-bound RhoA in endothelial cells overexpressing SHP2C459S, relative to GFP (Fig. 6B), as well as in those incubated with NSC-87877 (Fig. 6D), indicating a reduced level of GDP-bound, inactive RhoA in these cells.
Fig. 6.
Inhibition of SHP2 correlates with increased RhoA activity. Lysates from PAEC transfected with SHP2C459S or GFP (A and B) or from LMVEC monolayers treated with 100 μM NSC-87877 for 3 h (C and D) were incubated with GST-RBD (A and C) or GST-RhoGDI-1α (B and D) conjugated to agarose beads, and precipitates, in parallel with total lysates, were immunoblotted for RhoA. Data are presented as means ± SE; n = 3. *P < 0.05 vs. GFP or vehicle, respectively.
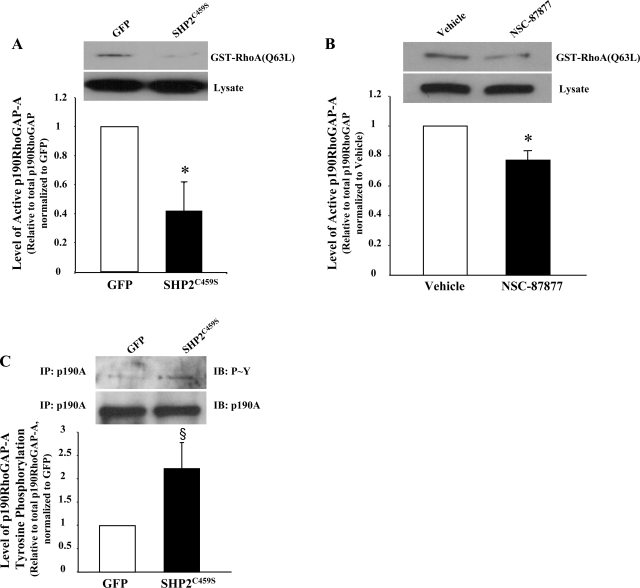
RhoA activity is regulated by the action of the GTPase-activating protein p190RhoGAP (57), thus, we next examined the activity of p190RhoGAP, as we have done previously (21). Endothelial cells overexpressing SHP2C459S demonstrated a significant reduction in the activity of p190RhoGAP (Fig. 7A). Similar results were noted in endothelial cells treated with NSC-87877 compared with vehicle (Fig. 7B). Because p190RhoGAP tyrosine phosphorylation is thought to regulate its activity, we next assayed this endpoint. We found that expression of SHP2C459S protein led to an increase in tyrosine phosphorylation of p190RhoGAP (Fig. 7C). These data, in conjunction with our observed decreases in barrier function and disruption of adherens junctions following SHP2 inhibition, suggest that SHP2 acts upstream of RhoA in the pulmonary vasculature, serving to suppress its activity under normal physiological conditions, possibly through modulation of its negative regulator, p190RhoGAP.
Fig. 7.
Inhibition of SHP2 correlates with decreased p190RhoGAP activity and increased tyrosine phosphorylation of p190RhoGAP. PAEC were transfected with SHP2C459S or GFP (A and C) or confluent LMVEC monolayers were incubated with 100 μM NSC-87877 for 3 h (B). A and B: equivalent amounts of lysate were incubated with GST-RhoA(Q63L) conjugated to agarose beads, and precipitates, in parallel with total lysates, were immunoblotted for p190RhoGAP. Equivalent amounts of lysates were immunoprecipitated with anti-p190RhoGAP antibody, and the precipitates were immunoblotted for phosphorylated tyrosine (C). Membranes were then stripped and reprobed for total p190RhoGAP. Data are presented as means ± SE; n = 3. *P < 0.05 vs. GFP or vehicle, respectively; §P < 0.08 vs. GFP.
DISCUSSION
We demonstrate, for the first time, a functional role for SHP2 in the regulation of pulmonary vasculature, with SHP2 inhibition causing edema in rat lungs and barrier dysfunction in cultured pulmonary endothelial cell monolayers. Intercellular gapping occurred in endothelial cells in which SHP2 was inhibited, marked by disruption of adherens junctions, effects that were accompanied by an increase in tyrosine phosphorylation of VE-cadherin, and β-catenin, as well as p190RhoGAP, and dissolution of protein-protein interactions between p120-catenin and VE-cadherin. SHP2 inhibition also increased RhoA activity and attenuated p190RhoGAP activity. Thus, our findings suggest that SHP2 activity is important in regulating the pulmonary endothelial barrier by coordinating the tyrosine phosphorylation profile and subsequent activities of key signaling molecules that serve to stabilize adherens junctions, including VE-cadherin, β-catenin, and p190RhoGAP and RhoA.
The vascular endothelial barrier is maintained through a balance of cell-cell and cell-extracellular matrix interactions (26, 44, 47, 61). Tight junctions and adherens junctions function to regulate paracellular transport, and, as such, are essential in the regulation of edema formation (26, 44). Numerous studies have been aimed at deciphering how the structures of both tight junctions and adherens junctions are altered in response to various agents (2, 3, 20, 27, 34, 39, 58). The findings of many studies implicate that the phosphorylation state of the protein components regulate junction integrity by affecting the stability of the intercellular protein interactions (67). Our findings that increased tyrosine phosphorylation of VE-cadherin and β-catenin correlated with disruption of adherens junction integrity and overall endothelial barrier dysfunction in the setting of SHP2 inhibition corroborate earlier studies that assayed the effects of edematogenic agents on endothelial monolayer permeability (2, 3, 7, 18, 20, 27, 58, 66, 74).
PTP activity has been shown to be crucial in maintaining endothelial monolayer barrier function. Using general PTP inhibitors, several groups have shown that PTP inhibition promoted the disruption of cell-cell contacts, as well as a breakdown of cell-extracellular matrix junctions, effects that were accompanied by a concomitant decrease in endothelial barrier function (16, 25, 41, 60, 74, 76). In addition to VE-cadherin, an increase in the tyrosine phosphorylation levels of various tight junction and focal adhesion components has been shown to play a key role in regulating endothelial monolayer permeability (41, 60, 76). While our data suggest that SHP2 modulates vascular permeability in the lung through regulation of VE-cadherin, β-catenin, and p190RhoGAP tyrosine phosphorylation, we cannot discount the possibility that SHP2 also acts on other adherens junction components. Indeed, adherens junction stability is known to be regulated in part by p120-catenin. Disruption of the interaction between p120-catenin and VE-cadherin has been associated with the endocytosis of both proteins and the activation of RhoA (17). Thus, while we show SHP2 inhibition promotes disruption of p120-catenin/VE-cadherin protein-protein interactions, further work is needed to determine if these changes were a result of altered phosphorylation of either or both adherens junction proteins.
Evidence for the role of select PTP isoforms in regulating monolayer permeability is limited. VE-PTP was shown to associate with VE-cadherin through an extracellular domain and functioned to decrease its tyrosine phosphorylation (49, 51). Additional studies demonstrated a role for VE-PTP in regulating endothelial monolayer permeability, possibly through maintaining VE-cadherin homotypic interactions and cell-cell integrity (51). More recently, the isoforms PTPμ, PTP1B, and SHP2 were found to play a role in regulating the tyrosine phosphorylation level of adherens junction components, primarily through VE-cadherin (48, 62, 66). Ukropec et al. (66) found decreased SHP2 localization to interendothelial junctions following thrombin treatment, an event that coincided with disruption of VE-cadherin-mediated complexes and a significant increase in the tyrosine phosphorylation level of its associated catenins in human umbilical vein endothelial cells. While all of the PTP isoforms referenced above have been shown to bind to adherens junction-associated proteins, all but SHP2 have been shown to affect endothelial monolayer permeability. A functional in vivo role for any of these PTP has not been established. The results of the current study are the first to demonstrate a functional role for SHP2 in maintaining pulmonary endothelial barrier function both in vitro and in vivo. The dose of NSC-87877 used in the in vivo experiments is significantly higher than the IC50 values established in vitro. Thus it is possible that in addition to attenuating SHP2 activity, the NSC-87877 compound may also be affecting the activities of other PTP, such as SHP1 or PTP1B (15). However, in vitro experiments using a molecular inhibitor of SHP2 support a crucial role for this PTP in regulating endothelial barrier function.
Studies have demonstrated an intimate role for the small Rho GTPases, RhoA, Rac1, and cdc42, in modulating endothelial monolayer permeability (11, 72), through the regulation of intracellular stress fiber formation and cell-cell and cell-extracellular matrix interactions (11, 13, 63, 71). However, the regulation of these GTPases by PTP in the endothelium is not known. We found that chemical or molecular inhibition of SHP2 led to a significant increase in RhoA activity that correlated with disruption of adherens junctions and stress fiber formation. Similar to our findings, RhoA activation was elevated in SHP2-null cardiomyocytes as well as in fibroblasts overexpressing SHP2 catalytic mutants (37, 56). However, data are lacking regarding the effect(s) of SHP2 on RhoA signaling within the pulmonary endothelium.
In settings of edemagenic endothelial barrier dysfunction, RhoA activity is increased, leading to stress fiber formation, cellular contraction, and intercellular gapping (19, 46, 72). Several studies have demonstrated that inhibition of RhoA or its downstream signaling molecule, Rho kinase, attenuated agonist-induced endothelial monolayer permeability through maintenance of adherens junctions and diminished stress fiber formation (1, 12, 43, 54, 71, 73). A dynamic interplay between the actin cytoskeleton and adherens junction proteins has been proposed, whereby cortical actin stabilizes adherens junctions, whereas formation of stress fibers leads to junction disruption (19, 46). It has also been suggested that RhoA may modulate adherens junction stability through phosphorylation by Rho kinase (71). Thus, it is possible that SHP2 modulates adherens junction formation both directly, through its interaction with VE-cadherin, as well as indirectly, through modulation of RhoA activation. Future experiments will further investigate the role of the small Rho GTPases in SHP2-mediated pulmonary endothelial barrier function.
p190RhoGAP has been shown to regulate RhoA activity (4) and to be important in restoration of endothelial barrier function (33). In several studies, p190RhoGAP tyrosine phosphorylation has been inversely correlated with increased activity (5, 6, 8, 14, 33, 50, 52). While Noren et al. (50) demonstrated that increased p190RhoGAP activity coincided with enhanced tyrosine phosphorylation, the majority of studies have not directly examined the enzymatic activity of p190RhoGAP, but instead rely on RhoA activity as an indirect barometer of p190RhoGAP activity (5, 6, 8, 14, 33, 50, 52). We provide evidence that SHP2 inhibition attenuated the activity of p190RhoGAP in pulmonary endothelial cells, an effect that correlated with increased p190RhoGAP tyrosine phosphorylation. Additional work is needed to establish the role of tyrosine phosphorylation in regulating p190RhoGAP activity.
Whether p190RhoGAP tyrosine dephosphorylation by SHP2 directly or indirectly affects the GAP activity is not known. Bregeon and colleagues (9) suggest that p190RhoGAP activity is regulated by an additional signaling molecule, which is also a substrate for SHP2. Thus, it is possible that in the setting of SHP2 inhibition, p120-catenin affects adherens junction stability via p190RhoGAP.
It is likely that the phosphorylation profile of tyrosine, serine, and threonine residues of p190RhoGAP differentially regulates its activity. Indeed, a recent study indicated that phosphorylation of select p190RhoGAP serine and threonine residues dictated its phospholipid preference, hence GAP activity (21, 40). While we have previously established a role for PKCδ as a binding partner of p190RhoGAP and as an important positive regulator of the pulmonary endothelium barrier function (31), we recently have shown that PKCδ regulates monolayer permeability independently of p190RhoGAP (21).
One important finding of our study is that inhibition of SHP2 promotes lung endothelial barrier dysfunction in vivo and in vitro, suggesting that constitutive activation of SHP2 is crucial in maintaining endothelial monolayer permeability. In addition, we speculate that inhibition of SHP2 in settings of edematogenic agents promotes a cascade of signals resulting in adherens junction disruption
In conclusion, our study supports a novel role for SHP2 in maintaining basal barrier function in the pulmonary vasculature. This study is the first to present a functional link between SHP2 activity and adherens junction integrity in regulating pulmonary barrier function. Further analysis of SHP2 and its effects on p120-catenin, VE-cadherin, p190RhoGAP, and Rho GTPases will assist in more clearly deciphering the signaling pathway(s) responsible for maintaining pulmonary endothelial barrier function.
GRANTS
This work was supported with VA Merit Review and National Heart, Lung, and Blood Institute Grant HL-67795 to E. O. Harrington. NRSA 1F32 HL-091664-01A1 and 5T32HL-094300 grants supported K. L. Grinnell.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
This material is the result of work supported with resources from and the use of facilities at the Providence VA Medical Center.
REFERENCES
- 1.Adamson RH, Curry FE, Adamson G, Liu B, Jiang Y, Aktories K, Barth H, Daigeler A, Golenhofen N, Ness W, Drenckhahn D. Rho and rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice. J Physiol 539: 295–303, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriopoulou P, Navarro P, Zanetti A, Lampugnani MG, Dejana E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler Thromb Vasc Biol 19: 2286–2297, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Angelini DJ, Hyun SW, Grigoryev DN, Garg P, Gong P, Singh IS, Passaniti A, Hasday JD, Goldblum SE. TNF-α increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Physiol Lung Cell Mol Physiol 291: L1232–L1245, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell 12: 2711–2720, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol 10: 719–722, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bernards A, Settleman J. GAPs in growth factor signalling. Growth Factors 23: 143–149, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bogatcheva NV, Garcia JG, Verin AD. Role of tyrosine kinase signaling in endothelial cell barrier regulation. Vascul Pharmacol 39: 201–212, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Bouton AH, Kanner SB, Vines RR, Wang HC, Gibbs JB, Parsons JT. Transformation by pp60src or stimulation of cells with epidermal growth factor induces the stable association of tyrosine-phosphorylated cellular proteins with GTPase-activating protein. Mol Cell Biol 11: 945–953, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bregeon J, Loirand G, Pacaud P, Rolli-Derkinderen M. Angiotensin II induces RhoA activation through SHP2-dependent dephosphorylation of the RhoGAP p190A in vascular smooth muscle cells. Am J Physiol Cell Physiol 297: C1062–C1070, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Breviario F, Caveda L, Corada M, Martin-Padura I, Navarro P, Golay J, Introna M, Gulino D, Lampugnani MG, Dejana E. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler Thromb Vasc Biol 15: 1229–1239, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Broman MT, Mehta D, Malik AB. Cdc42 regulates the restoration of endothelial adherens junctions and permeability. Trends Cardiovasc Med 17: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Carbajal JM, Schaeffer RC. RhoA inactivation enhances endothelial barrier function. Am J Physiol Cell Physiol 277: C955–C964, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Carbajal JM, Schaeffer RC., Jr RhoA inactivation enhances endothelial barrier function. Am J Physiol Cell Physiol 277: C955–C964, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Chang JH, Gill S, Settleman J, Parsons SJ. c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J Cell Biol 130: 355–368, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Sung SS, Yip ML, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J. Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol 70: 562–570, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Chen WL, Lin CT, Lo HF, Lee JW, Tu IH, Hu FR. The role of protein tyrosine phosphorylation in the cell-cell interactions, junctional permeability and cell cycle control in post-confluent bovine corneal endothelial cells. Exp Eye Res 85: 259–269, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Chiasson CM, Wittich KB, Vincent PA, Faundez V, Kowalczyk AP. p120-catenin inhibits VE-cadherin internalization through a Rho-independent mechanism. Mol Biol Cell 20: 1970–1980, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csortos C, Kolosova I, Verin AD. Regulation of vascular endothelial cell barrier function and cytoskeleton structure by protein phosphatases of the PPP family. Am J Physiol Lung Cell Mol Physiol 293: L843–L854, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487–1500, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci 111: 1853–1865, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Fordjour AK. PKC delta influences p190 phosphorylation and activity: events independent of PKC delta-mediated regulation of endothelial cell stress fiber and focal adhesion formation and barrier function. Biochim Biophys Acta 1790: 1179–1190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Kouklis P, Xu N, Minshall RD, Sandoval R, Vogel SM, Malik AB. Reversibility of increased microvessel permeability in response to VE-cadherin disassembly. Am J Physiol Lung Cell Mol Physiol 279: L1218–L1225, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Garcia JG, Lazar V, Gilbert-McClain LI, Gallagher PJ, Verin AD. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol 16: 489–494, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Garcia JG, Schaphorst KL, Verin AD, Vepa S, Patterson CE, Natarajan V. Diperoxovanadate alters endothelial cell focal contacts and barrier function: role of tyrosine phosphorylation. J Appl Physiol 89: 2333–2343, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R, Frei K. Molecular and cellular permeability control at the blood-brain barrier. Brain Res Brain Res Rev 36: 258–264, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Goldblum SE, Young BA, Wang P, Murphy-Ullrich JE. Thrombospondin-1 induces tyrosine phosphorylation of adherens junction proteins and regulates an endothelial paracellular pathway. Mol Biol Cell 10: 1537–1551, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrington EO, Brunelle JL, Shannon CJ, Kim ES, Mennella K, Rounds S. Role of protein kinase C isoforms in rat epididymal microvascular endothelial barrier function. Am J Respir Cell Mol Biol 28: 626–636, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Harrington EO, Loffler J, Nelson PR, Kent KC, Simons M, Ware JA. Enhancement of migration by protein kinase Calpha and inhibition of proliferation and cell cycle progression by protein kinase Cdelta in capillary endothelial cells. J Biol Chem 272: 7390–7397, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Harrington EO, Newton J, Morin N, Rounds S. Barrier dysfunction and RhoA activation are blunted by homocysteine and adenosine in pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 287: L1091–L1097, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Harrington EO, Shannon CJ, Morin N, Rowlett H, Murphy C, Lu Q. PKCdelta regulates endothelial basal barrier function through modulation of RhoA GTPase activity. Exp Cell Res 308: 407–421, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Harrington EO, Smeglin A, Newton J, Ballard G, Rounds S. Protein tyrosine phosphatase-dependent proteolysis of focal adhesion complexes in endothelial cell apoptosis. Am J Physiol Lung Cell Mol Physiol 280: L342–L353, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J Biol Chem 281: 2296–2305, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Kevil CG, Okayama N, Alexander JS. H2O2-mediated permeability II: importance of tyrosine phosphatase and kinase activity. Am J Physiol Cell Physiol 281: C1940–C1947, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Klinger JR, Murray JD, Casserly B, Alvarez DF, King JA, AN SS, Choudhary G, Owusu-Sarfo AN, Warburton R, Harrington EO. Rottlerin causes pulmonary edema in vivo: a possible role for PKCdelta. J Appl Physiol 103: 2084–2094, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Kolosova IA, Mirzapoiazova T, Moreno-Vinasco L, Sammani S, Garcia JG, Verin AD. Protective effect of purinergic agonist ATPgammaS against acute lung injury. Am J Physiol Lung Cell Mol Physiol 294: L319–L324, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Kontaridis MI, Eminaga S, Fornaro M, Zito CI, Sordella R, Settleman J, Bennett AM. SHP-2 positively regulates myogenesis by coupling to the Rho GTPase signaling pathway. Mol Cell Biol 24: 5340–5352, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med 25: 72–76, 1985 [PubMed] [Google Scholar]
- 39.Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, Kim KY. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res 68: 231–238, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Levay M, Settleman J, Ligeti E. Regulation of the substrate preference of p190RhoGAP by PKC-mediated phosphorylation of a phospholipid binding site. Biochemistry 48: 8615–8623, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lohmann C, Krischke M, Wegener J, Galla HJ. Tyrosine phosphatase inhibition induces loss of blood-brain barrier integrity by matrix metalloproteinase-dependent and -independent pathways. Brain Res 995: 184–196, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Lu Q, Harrington EO, Hai CM, Newton J, Garber M, Hirase T, Rounds S. Isoprenylcysteine carboxyl methyltransferase modulates endothelial monolayer permeability: involvement of RhoA carboxyl methylation. Circ Res 94: 306–315, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Lu Q, Harrington EO, Jackson H, Morin N, Shannon C, Rounds S. Transforming growth factor-beta1-induced endothelial barrier dysfunction involves Smad2-dependent p38 activation and subsequent RhoA activation. J Appl Physiol 101: 375–384, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 267: L223–L241, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Mannell H, Hellwig N, Gloe T, Plank C, Sohn HY, Groesser L, Walzog B, Pohl U, Krotz F. Inhibition of the tyrosine phosphatase SHP-2 suppresses angiogenesis in vitro and in vivo. J Vasc Res 45: 153–163, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol 60: 121–142, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Nakamura Y, Patrushev N, Inomata H, Mehta D, Urao N, Kim HW, Razvi M, Kini V, Mahadev K, Goldstein BJ, McKinney R, Fukai T, Ushio-Fukai M. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res 102: 1182–1191, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J 21: 4885–4895, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noren NK, Arthur WT, Burridge K. Cadherin engagement inhibits RhoA via p190RhoGAP. J Biol Chem 278: 13615–13618, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Nottebaum AF, Cagna G, Winderlich M, Gamp AC, Linnepe R, Polaschegg C, Filippova K, Lyck R, Engelhardt B, Kamenyeva O, Bixel MG, Butz S, Vestweber D. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med 205: 2929–2945, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roof RW, Haskell MD, Dukes BD, Sherman N, Kinter M, Parsons SJ. Phosphotyrosine (p-Tyr)-dependent and -independent mechanisms of p190 RhoGAP-p120 RasGAP interaction: Tyr 1105 of p190, a substrate for c-Src, is the sole p-Tyr mediator of complex formation. Mol Cell Biol 18: 7052–7063, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salton SR. Teaching resources. Protein phosphatases. Sci STKE 2005: tr8, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Sanchez T, Skoura A, Wu M, Casserly B, Harrington E, Hla T. Induction of vascular permeaility by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol 27: 1312–1318, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Sander EE, van Delft S, ten Klooster JP, Reid T, van der Kammen RA, Michiels F, Collard JG. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol 143: 1385–1398, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schoenwaelder SM, Petch LA, Williamson D, Shen R, Feng GS, Burridge K. The protein tyrosine phosphatase Shp-2 regulates RhoA activity. Curr Biol 10: 1523–1526, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Settleman J, Albright CF, Foster LC, Weinberg RA. Association between GTPase activators for Rho and Ras families. Nature 359: 153–154, 1992 [DOI] [PubMed] [Google Scholar]
- 58.Shasby DM, Ries DR, Shasby SS, Winter MC. Histamine stimulates phosphorylation of adherens junction proteins and alters their link to vimentin. Am J Physiol Lung Cell Mol Physiol 282: L1330–L1338, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Shi S, Verin AD, Schaphorst KL, Gilbert-McClain LI, Patterson CE, Irwin RP, Natarajan V, Garcia JG. Role of tyrosine phosphorylation in thrombin-induced endothelial cell contraction and barrier function. Endothelium 6: 153–171, 1998 [DOI] [PubMed] [Google Scholar]
- 60.Staddon JM, Herrenknecht K, Smales C, Rubin LL. Evidence that tyrosine phosphorylation may increase tight junction permeability. J Cell Sci 108: 609–619, 1995 [DOI] [PubMed] [Google Scholar]
- 61.Stevenson BR, Keon BH. The tight junction: morphology to molecules. Annu Rev Cell Dev Biol 14: 89–109, 1998 [DOI] [PubMed] [Google Scholar]
- 62.Sui XF, Kiser TD, Hyun SW, Angelini DJ, Del Vecchio RL, Young BA, Hasday JD, Romer LH, Passaniti A, Tonks NK, Goldblum SE. Receptor protein tyrosine phosphatase micro regulates the paracellular pathway in human lung microvascular endothelia. Am J Pathol 166: 1247–1258, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. FASEB J 22: 1829–1838, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Tiganis T, Bennett AM. Protein tyrosine phosphatase function: the substrate perspective. Biochem J 402: 1–15, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uhlig S, Wollin L. An improved setup for the isolated perfused rat lung. J Pharmacol Toxicol Methods 31: 85–94, 1994 [DOI] [PubMed] [Google Scholar]
- 66.Ukropec JA, Hollinger MK, Salva SM, Woolkalis MJ. SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J Biol Chem 275: 5983–5986, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Verin AD. Tyrosine phosphorylation and endothelial cell barrier regulation. Am J Pathol 166: 955–957, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verin AD, Lazar V, Torry RJ, Labarrere CA, Patterson CE, Garcia JG. Expression of a novel high molecular-weight myosin light chain kinase in endothelium. Am J Respir Cell Mol Biol 19: 758–766, 1998 [DOI] [PubMed] [Google Scholar]
- 69.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 27: 337–349, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Wilasrusmee C, Shah G, Kittur S, Halverson A, Bruch D, Kittur D. Signal transduction pathway in endothelial dysfunction. Surg Infect 5: 9–14, 2004 [DOI] [PubMed] [Google Scholar]
- 71.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 114: 1343–1355, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol 39: 187–199, 2002 [DOI] [PubMed] [Google Scholar]
- 73.Wojciak-Stothard B, Tsang L, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 288: L749–L760, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Young BA, Sui X, Kiser TD, Hyun SW, Wang P, Sakarya S, Angelini DJ, Schaphorst KL, Hasday JD, Cross AS, Romer LH, Passaniti A, Goldblum SE. Protein tyrosine phosphatase activity regulates endothelial cell-cell interactions, the paracellular pathway, and capillary tube stability. Am J Physiol Lung Cell Mol Physiol 285: L63–L75, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol 39: 213–223, 2002 [DOI] [PubMed] [Google Scholar]
- 76.Yuan Y, Meng FY, Huang Q, Hawker J, Wu HM. Tyrosine phosphorylation of paxillin/pp125FAK and microvascular endothelial barrier function. Am J Physiol Heart Circ Physiol 275: H84–H93, 1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.