Abstract
Aims
We hypothesized that subjects with a normal body mass index (BMI), but high body fat (BF) content [normal weight obesity (NWO)], have a higher prevalence of cardiometabolic dysregulation and are at higher risk for cardiovascular (CV) mortality.
Methods and results
We analysed 6171 subjects >20 years of age from the Third National Health and Nutrition Examination Survey (NHANES III) and the NHANES III mortality study, whose BMI was within the normal range (18.5–24.9 kg/m2), and who underwent a complete evaluation that included body composition assessment, blood measurements, and assessment of CV risk factors. Survival information was available for >99% of the subjects after a median follow-up of 8.8 years. We divided our sample using sex-specific tertiles of BF%. The highest tertile of BF (>23.1% in men and >33.3% in women) was labelled as NWO. When compared with the low BF group, the prevalence of metabolic syndrome in subjects with NWO was four-fold higher (16.6 vs. 4.8%, P < 0.0001). Subjects with NWO also had higher prevalence of dyslipidaemia, hypertension (men), and CV disease (women). After adjustment, women with NWO showed a significant 2.2-fold increased risk for CV mortality (HR = 2.2; 95% CI, 1.03–4.67) in comparison to the low BF group.
Conclusion
Normal weight obesity, defined as the combination of normal BMI and high BF content, is associated with a high prevalence of cardiometabolic dysregulation, metabolic syndrome, and CV risk factors. In women, NWO is independently associated with increased risk for CV mortality.
Keywords: Normal weight obesity, Body fat, Metabolic syndrome, Cardiovascular risk factor, Mortality, Cardiovascular mortality
Introduction
The prevalence of obesity in the USA has risen remarkably over the past four decades, increasing from ∼13% in the 60s, to over 30% in the most recent analyses of the National Health and Nutrition Examination Surveys (NHANES).1–3 Although the gold standard definition of obesity is considered an excess in body fat (BF),4 clinicians and epidemiologists usually rely on body mass index (BMI) as a means of defining the presence of adiposity or obesity. Body mass index has shown many advantages as a surrogate of BF, such as simplicity and reproducibility, and epidemiologic studies have shown an association between extreme values of BMI and increased mortality.5–8 However, a significant limitation of using BMI is its failure to differentiate between an elevated BF content and preserved or increased lean mass, especially in patients with a BMI <30 kg/m2.9–15
An excess in adiposity has been clearly associated with numerous comorbidies and pathophysiologic processes, including insulin resistance, altered lipid metabolism, and endothelial dysfunction.16 Therefore, the determination of adiposity by methods more accurate than BMI could have public health implications.3,17–20 We hypothesized that (i) BF, measured as a continuous variable, is associated with the prevalence of metabolic syndrome and its components in individuals with normal body weight, and that (ii) subjects who have normal body weight based on BMI and high BF content [normal weight obesity (NWO)] are at higher risk for cardiometabolic dysregulation and cardiovascular (CV) mortality when compared with normal weight subjects with low/preserved BF content.
Methods
Study design and subject selection
The NHANES III examined a representative sample of the US non-institutionalized civilian population from 1988 to 1994. It consists of a periodic survey using a stratified multistage probability sampling design to produce a generalizable health estimate of the US population. Details on design and conduct of the survey are available to the public at http://www.cdc.gov/nchs/nhanes.htm. Briefly, of a sample of 39 695 people selected for the NHANES III, 33 994 were interviewed and 30 818 submitted to an examination by a physician at a mobile examination centre which included extensive anthropometric, physiological, and laboratory testing. For this study, 14 025 adult subjects aged >20 years had bioelectrical impedance analysis to estimate body composition.21 From those, we selected subjects with blood samples and with a normal BMI (18.5–24.9 kg/m2), as defined by the US National Institutes of Health, resulting in a sample of 6171 subjects, 3042 men and 3129 women.
Anthropometric measurements and body composition analyses
All personnel performing NHANES III anthropometric and body composition measurements were previously trained and followed a strict protocol.21–24 Body weight was measured with an electronic load cell scale to the nearest 0.01 kg. Participants wore only under-shorts and disposable paper shirts, pants and foam slippers. Stature was measured to the nearest 0.1 cm using a fixed stadiometer. Participants were positioned with heels, buttocks, back, and head against the upright surface of the stadiometer with the head positioned in the Frankfort horizontal plane. Waist and hip circumference were measured by a trained examiner and determined using a measuring tape positioned at the high point of the iliac crest for the waist and at the greatest circumference of the buttocks. The measurement was made with a minimal respiration to the nearest 0.1 cm, with the tape snug but not compressing the skin.24 Body mass index was calculated as weight in kilograms divided by squared height in meters (kg/m2) and waist-to-hip ratio was calculated as waist circumference divided by hip circumference. Children younger than 12 years of age, pregnant women and subjects with pacemakers were ineligible for bioelectrical impedance analysis. All subjects were requested to avoid eating or drinking anything except water during the fasting period. There were no restrictions on physical activity or alcohol consumption before the fasting period. The prediction equations for total body water and fat free mass use resistance measured with data from RJL bioelectrical impedance analyzers (Clinton Twp, MI, USA).25 NHANES III resistance data were obtained using Valhalla impedance analyzers. Therefore, bioimpedance resistance was converted to RJL Res values (Ω) and was used to calculate BF as previously described by Chumlea et al.26 The prediction equations used to estimate lean mass are the following:
Men: Lean mass =−10.678 + 0.262 kg + 0.652 S2/Res + 0.015 Res
Women: Lean mass =−9.529 + 0.168 kg + 0.696 S2/Res + 0.016 Res
where S2/Res represent the stature squared divided by resistance (cm2/Ω). We then calculated BF % as follows:
Detailed information on the bioelectrical impedance analysis procedure is presented elsewhere.26
Laboratory measurements
Lipids were measured enzymatically with the use of commercially available reagents (Cholesterol/HP, cat. no. 816302, and triglycerides/GPO, cat. no. 816370, both from Boehringer Mannheim). HDL cholesterol was measured in the clear supernatant after precipitating the other lipoproteins with heparin and MnCl2 (1.3 g/L and 0·046 mol/L, respectively) and removing excess Mn2+ by precipitation with NaHCO3. The biases (coefficients of variation) for cholesterol, triglycerides, and HDL-C averaged –0.3% (1.7%), –2.1% (3.9%), and 0.3% (3.4%), respectively. Glucose was measured using standard assay (Sigma chemical, St Louis, MO, USA), and plasma insulin was measured with the Pharmacia insulin radioimmunoassay kit (Pharmacia diagnostics, Sweden). We determined the insulin sensitivity index using the updated computer model homeostatic model assessment (HOMA2) index.27 The apoB and apoAI were measured by radial immunodiffusion in the first 8.2% of the specimens during the first 5 months of the study and by rate immunonephelometry for the remaining specimens.28 Serum leptin concentrations were measured by radioimmunoassay at Linco Research, Inc. (St Charles, MO, USA).29 C-reactive protein was measured using a modification of the Behring latex-enhanced C-reactive protein assay (Behring Diagnostics, Westwood, MA, USA), as previously described.30 Detailed methodology on laboratory procedures of NHANES III is published elsewhere.31
Normal weight obesity, metabolic syndrome, and cardiovascular risk factor definitions
Normal weight obesity was defined as subjects with a normal BMI (18.5–24.9 kg/m2) and an excess in BF%, defined by the highest sex-specific tertiles of BF% (>23.1% in men and >33.3% in women). The updated ATP-III definition of metabolic syndrome17 was met when three or more of the following criteria were present: (1) waist circumference ≥102 cm in men and ≥88 cm in women; (2) HDL <1.04 mmol/L (40 mg/dL) in men and <1.30 mmol/L (50 mg/dL) in women; (3) triglycerides ≥1.7 mmol/L (150 mg/dL) or specific treatment for this lipid abnormality; (4) systolic blood pressure≥130 mmHg or diastolic blood pressure ≥85 mmHg or treatment of previously diagnosed hypertension; and (5) fasting glucose ≥5.5 mmol/L (100 mg/dL) or previously diagnosed diabetes. Subjects were considered to have dyslipidaemia if they reported current usage of lipid medications, a self-reported diagnosis of hypercholesterolaemia, and/or HDL-cholesterol <1.04 mmol/L (40 mg/dL) in men and <1.30 mmol/L (50 mg/dL) in women, and/or triglycerides ≥1.7 mmol/L (150 mg/dL), and/or LDL-cholesterol ≥4.10 mmol/L (160 mg/dL).32 Subjects were considered to be hypertensive if they were taking antihypertensive medications or had a self-reported diagnosis of hypertension or if their systolic pressure was ≥140 mmHg or diastolic pressure was ≥90 mmHg.33 Subjects were considered to have diabetes if they reported current usage of anti-diabetic medications (insulin and oral medications), a self-reported diagnosis of diabetes and/or if their fasting morning plasma glucose was ≥7.0 mmol/L (126 mg/dL).34 Cardiovascular disease was defined as the composite of self-reported history of myocardial infarction and stroke.35 Subjects were considered as never or ever smokers (have you ever smoked more than 100 cigarettes in your life?).
Total and cardiovascular mortality assessment
NHANES III participants aged 17 years or older for whom data were available for matching were matched to the National Death Index to determine mortality status. The National Death Index was searched through 31 December 2000, for follow-up. NHANES III and the National Death Index are linked by probabilistic matching in the NHANES III mortality study. The National Center for Health Statistics conducted the linkage and created scores for potential matches. For a selected sample of NHANES III records, the Center reviewed the death certificate record to verify correct matches. Overall, 20 024 adult NHANES III participants were eligible for mortality follow-up by linkage with the National Death Index, of whom 3384 were identified as deceased. A complete description of the methodology used to link NHANES III records to the National Death Index can be found elsewhere.36 Cardiovascular deaths were defined as those with ICD-9 codes 390–398, 402, and 404–429 and ICD-10 codes I00–I09, I11, I13, and I20–I51 (NHANES III codes 53–75). Person-months of follow-up were calculated for each participant based on the end of follow-up (date of death for those assumed deceased or 31 December 2000, for those assumed alive minus the date of the NHANES III examination). Mortality and CV mortality at follow-up were ascertained for 99% of our sample.
Statistical analyses
Data for anthropometric and cardiometabolic variables were summarized by calculating means and standard errors for quantitative variables and numbers and percentages for categorical variables. We used BF as a continuous variable for the primary analysis in this study. For secondary analyses, we divided our sample of normal BMI subjects into sex-specific tertiles of BF: low BF content (<18.65% in men and <28.9% in women); medium BF content (second tertile); and high BF content, defined as NWO (>23.1% in men and >33.3% in women). We stratified all our analyses by sex based on the biologic effect of this variable on BF. All analyses were adjusted for age and race/ethnicity.
Only subjects with fasting and morning samples (n = 2127) were used for analyses of HOMA2, glucose, and metabolic syndrome. We performed log transformation to reduce the skewness of HOMA2, triglycerides, C-reactive protein, and leptin. We defined subjects as having an elevated apoB/apoAI ratio, C-reactive protein, and leptin if they were in the upper sex-specific quartile of these measurements. We calculated the prevalence and P-values for trend (adjusted for age and race) for metabolic syndrome, CV risk factors, and cardiometabolic measures between BF groups at baseline. To assess the effects of central obesity, we performed similar analyses using sex-specific tertiles of waist circumference and used the lowest tertile as the reference combining men and women. After testing the linearity of the association between BF% and metabolic syndrome, we used logistic regression models adjusted for age and race to determine if there was a dose response association between sex-specific quartiles of BF% with insulin sensitivity (lowest quartile as the reference). We applied Cox proportional hazard regression to estimate relations between BF% as a continuous variable and sex-specific tertiles of BF% with total and CV mortality for men and women (lowest tertile used as the reference). Hazard ratios were calculated after adjusting for age and race and smoking (model 1-reference), further adjustment for waist circumference (model 2), waist-to-hip ratio (model 3), and further adjustment for CV risk factors, namely dyslipidaemia, hypertension, diabetes, history of CV disease (model 4). The assumption of hazard proportionality was confirmed by examining interactions of survival time and timed-dependent variables in Cox models. Finally, to assess the generalizability of our results, we compared our selected population of subjects with normal BMI with body composition analyses and blood measurement to subjects in NHANES who did not have these measurements. A two-sided alpha of 0.05 was considered statistically significant. All analyses were weighted according to NHANES methodology and were performed using SAS version 9.1 and SUDAAN 9.0.3.
Results
Our study sample included 6171 subjects. Overall, weighted mean age±standard error was 41.3 ± 0.31 years. From the total weighted sample, 77.7% were Non-Hispanic Whites, 9.4% were Non-Hispanic Blacks, 4.1% were Mexican Americans, and 8.6% were from a different ethnicity. The sample included in this study had similar distributions for age, sex, race, and BMI in comparison with the group excluded from the analysis that had a normal BMI but did not undergo body composition and laboratory evaluations (data not shown). All data are presented in our three pre-established tertiles of BF and stratified by sex.
Cardiometabolic parameters according to body fat
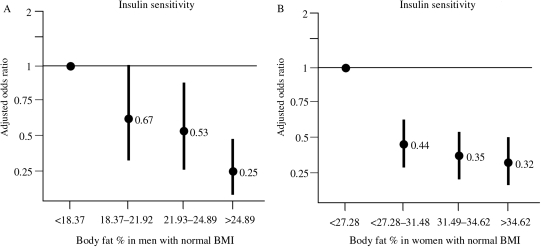
As age increased, the observed BF increased as well. After controlling for sex, age, and race, each BF percent was significantly associated with lower levels of HDL (β = −0.0008 mmol/L, P-value<0.0001) and higher levels of LDL (β = 0.027 mmol/L, P-value <0.0001), triglycerides (β=0.026 mmol/L, P-value <0.0001), apoB/A-I ratio (β = 0.011, P-value <0.0001), C-reactive protein (β=1.02 mg/dL, P-value <0.0001), and leptin (β = 1.15 ng/dL, P-value <0.0001). The results were similar when BF was categorized using sex-specific tertiles (Tables 1 and 2). In subjects with fasting morning blood samples (n = 2127), insulin sensitivity (HOMA-S) diminished progressively as BF increased (β = −2.78, P-value <0.0001, Figure 1A and B). Similar results were obtained for triglycerides (β = 1.93 mg/dL, P-value <0.0001) and insulin (β = 0.97 µg/L, P-value <0.0001), but not for fasting blood glucose (β = 0.0003 mg/dL per 1% increase of BF, P-value 0.21).
Table 1.
Anthropometric and metabolic parameters in men with a normal body mass index by body fat tertiles
| Variable (N = 3042) | BF<18.65% (N = 1011) | BF >18.65–23.15% (N = 1014) | BF >23.15 (N = 1017) | Age+race Padj-value for trend |
|---|---|---|---|---|
| Mean ± SE or number (%) | Mean ± SE or number (%) | Mean ± SE or number (%) | ||
| Age, years | 37.0 ± 0.53 | 40.1 ± 0.54‡ | 43.4 ± 0.53* | <0.0001 |
| Race | ||||
| Non-Hispanic White | 155 (76.0) | 162 (73.7) | 84 (71.7) | <0.0001 |
| Non-Hispanic Black | 119 (11.4) | 102 (10.1) | 64 (10.4)* | |
| Mexican-Americans | 82 (3.5) | 120 (5.8) | 82 (6.8) | |
| Other ethnicity | 22 (8.9) | 16 (10.1) | 18 (10.9) | |
| Body mass index, kg/m2 | 21.8 ± 0.05 | 22.7 ± 0.04* | 23.5 ± 0.04* | <0.0001 |
| Waist circumference, cm | 80.2 ± 0.20 | 84.8 ± 0.19* | 88.9 ± 0.20* | <0.0001 |
| Hip circumference, cm | 91.1 ± 0.15 | 93.2 ± 0.13* | 94.6 ± 0.13* | <0.0001 |
| Waist-to-hip ratio | 0.88 ± 0.001 | 0.91 ± 0.001* | 0.94 ± 0.001* | <0.0001 |
| Body fat, % | 14.8 ± 0.09 | 20.9 ± 0.04* | 25.8 ± 0.06* | <0.0001 |
| Body fat, kg | 10.1 ± 0.08 | 14.6 ± 0.05* | 18.5 ± 0.08* | <0.0001 |
| Lean mass, kg | 57.9 ± 0.22 | 55.4 ± 0.18* | 53.0 ± 0.18* | <0.0001 |
| Systolic blood pressure, mmHg | 119 ± 0.5 | 122 ± 0.5 | 125 ± 0.5 | 0.18 |
| Diastolic blood pressure, mmHg | 72 ± 0.4 | 74 ± 0.4§ | 76 ± 0.3* | <0.0001 |
| Low density lipoprotein, mmol/L | 2.88 ± 0.04 | 3.15 ± 0.04† | 3.43 ± 0.04* | <0.0001 |
| High density lipoprotein, mmol/L | 1.33 ± 0.01 | 1.27 ± 0.01‖ | 1.23 ± 0.01* | <0.0001 |
| Triglycerides, mmol/L | 1.11 ± 0.02 | 1.31 ± 0.03† | 1.51 ± 0.03* | <0.0001 |
| ApoB/apoAI ratio | 0.62 ± 0.009 | 0.72 ± 0.009* | 0.80 ± 0.008* | <0.0001 |
| Glucosea, mmol/L | 5.21 ± 0.04 | 5.31 ± 0.03 | 5.38 ± 0.04 | 0.39 |
| HOMA 2a | ||||
| Insulin resistance | 0.73 ± 0.015 | 0.84 ± 0.016* | 1.00 ± 0.022* | <0.0001 |
| Insulin sensitivity | 152.2 ± 2.48 | 133.3 ± 2.19* | 111.5 ± 2.54* | <0.0001 |
| β cell function | 69.6 ± 1.02 | 73.3 ± 0.92§ | 79.3 ± 1.31* | <0.0001 |
| C-reactive protein, mg/L | 2.8 ± 0.1 | 3.3 ± 0.2 | 3.7 ± 0.2§ | 0.018 |
| Leptin, μg/L | 2.21 ± 0.05 | 3.66 ± 0.18* | 4.38 ± 0.17* | <0.0001 |
aFasting morning samples.
*P-value = <0.0001.
†P-value <0.001.
‡P-value <0.01.
§P-value <0.05.
‖P-value <0.07 when compared with BF<18.65%.
Table 2.
Anthropometric and metabolic parameters in women with a normal body mass index by body fat tertiles
| Variable (N = 3129) | BF<28.9% (N = 1044) | BF >28.9–33.3% (N = 1040) | BF >33.3% (N = 1045) | Age + race Padj-value for trend |
|---|---|---|---|---|
| Mean ± SE or number (%) | Mean ± SE or number (%) | Mean ± SE or number (%) | ||
| Age, years | 38.7 ± 0.53 | 43.7 ± 0.58* | 46.7 ± 0.54* | <0.0001 |
| Race | ||||
| Non-Hispanic White | 260 (87.7) | 200 (76.2) | 108 (77.0) | <0.0001 |
| Non-Hispanic Black | 90 (6.1) | 101 (8.5)* | 54 (11.0)* | |
| Mexican-Americans | 60 (2.0) | 99 (3.4) | 75 (4.5) | |
| Other ethnicity | 18 (4.0) | 27 (11.8) | 9 (7.3) | |
| Body mass index, kg/m2 | 20.7 ± 0.04 | 22.1 ± 0.04* | 23.5 ± 0.03* | <0.0001 |
| Waist circumference, cm | 73.6 ± 0.18 | 78.3 ± 0.20* | 83.3 ± 0.20* | <0.0001 |
| Hip circumference, cm | 91.3 ± 0.14 | 94.4 ± 0.14* | 97.7 ± 0.15* | <0.0001 |
| Waist-to-hip ratio | 0.80 ± 0.001 | 0.83 ± 0.002* | 0.85 ± 0.002* | <0.0001 |
| Body fat, % | 24.9 ± 0.10 | 31.0 ± 0.04* | 35.6 ± 0.05* | <0.0001 |
| Body fat, kg | 13.7 ± 0.07 | 18.1 ± 0.06* | 22.1 ± 0.07* | <0.0001 |
| Lean mass, kg | 41.3 ± 0.13 | 40.21 ± 0.13§ | 39.9 ± 0.11* | 0.0002 |
| Systolic blood pressure, mmHg | 114 ± 0.5 | 117 ± 0.6 | 119.9 ± 0.62 | 0.22 |
| Diastolic blood pressure, mmHg | 69 ± 0.3 | 71 ± 0.3 | 72.1 ± 0.32* | <0.0001 |
| Low density lipoprotein, mmol/L | 2.79 ± 0.04 | 3.01 ± 0.04 | 3.21 ± 0.05* | <0.0001 |
| High density lipoprotein, mmol/L | 1.55 ± 0.01 | 1.5 ± 0.01§ | 1.49 ± 0. 01† | 0.0039 |
| Triglycerides, mmol/L | 0.98 ± 0.53 | 1.14 ± 0.03† | 1.54 ± 0.08* | <0.0001 |
| ApoB/apoAI ratio | 0.56 ± 0.008 | 0.64 ± 0.009† | 0.68 ± 0.008* | <0.0001 |
| Glucosea, mmol/L | 5.0 ± 0.04 | 5.11 ± 0.04 | 5.17 ± 0.04 | 0.27 |
| HOMA 2a | ||||
| Insulin resistance | 0.72 ± 0.011 | 0.87 ± 0.015* | 0.98 ± 0.027* | <0.0001 |
| Insulin sensitivity | 151.7 ± 2.09 | 127.6 ± 1.92* | 116.7 ± 2.59* | <0.0001 |
| β cell function | 78.1 ± 0.93 | 82.0 ± 1.10* | 89.0 ± 1.55* | <0.0001 |
| C-reactive protein, mg/L | 3.1 ± 0.01 | 3.2 ± 0.01§ | 3.8 ± 0.01* | 0.0001 |
| Leptin, μg/L | 6.40 ± 0.16 | 9.71 ± 0.22* | 12.3 ± 0.28* | <0.0001 |
aFasting morning samples.
*P-value ≤0.0001.
†P-value <0.001.
§P-value <0.05.
‖P-value <0.07 when compared with BF<28.9%.
Figure 1.
Risk for lower insulin sensitivity according to body fat percent quartiles (lowest quartile as the reference) in subjects with a normal body mass index. (A) Men, (B) women.
Metabolic syndrome and cardiovascular risk factors according to body fat
The prevalence of metabolic syndrome and of its individual components increased as the BF content increased in men and women (Tables 3 and 4). After adjusting for sex, age, and race/ethnicity, BF was associated with higher odds of having metabolic syndrome (OR = 1.11, 95% CI 1.09–1.14, for each percent of BF). With respect to CV risk factors, as BF increased, men had higher prevalence of dyslipidaemia and hypertension (Table 3), while in women, similar differences were observed in the prevalence of dyslipidaemia and CV disease (Table 4).
Table 3.
Metabolic syndrome components, definition, and cardiovascular risk factors in men with a normal body mass index by body fat tertiles
| Variable (N = 3042) | BF <18.65% (N = 1011) | BF >18.65–23.15% (N = 1014) | BF >23.15% (N = 1017) | Age+race Padj-value for trend |
|---|---|---|---|---|
| Metabolic syndrome | N (%) | N (%) | N (%) | |
| Central obesity ATP (WC >102 cm) | 2 (0.18) | 4 (0.43) | 23 (1.95)‡ | 0.0004 |
| Central obesity by (W/H≥0.90) waist-to-hip ratio | 270 (26.71) | 347 (34.30) | 396 (39.01)† | <0.0001 |
| High triglycerides (>1.7 mmol/L) or lipid treatment | 116 (11.95) | 193 (21.02)† | 283 (31.12)* | <0.0001 |
| Low high-density lipoprotein (<1.04 mmol/L) | 149 (18.10) | 181 (21.25) | 225 (27.20)* | <0.0001 |
| High blood pressure (>130/>85 mmHg) or treatment for hypertension | 319 (26.54) | 353 (33.45) | 484 (46.84)‡ | 0.0042 |
| High fasting plasma glucosea (>5.55 mmol/L) or previously diagnosed diabetes | 169 (16.58) | 220 (21.51) | 293 (28.62)‡ | 0.0044 |
| Metabolic syndrome by ATP III criteriaa | 44 (5.28) | 75 (8.34) | 143 (15.83)* | <0.0001 |
| Cardiovascular risk factors | ||||
| Dyslipidaemia | 93 (10.62) | 136 (16.05)§ | 189 (20.44)* | <0.0001 |
| Hypertension | 212 (14.86) | 226 (19.68)§ | 342 (31.70)‖ | 0.039 |
| Diabetes | 40 (2.26) | 40 (2.07) | 50 (2.59) | 0.30 |
| Ever smokers | 561 (57.00) | 589 (59.67) | 648 (63.27) | 0.92 |
| CVD (myocardial infarction+stroke) | 46 (3.46) | 63 (4.82) | 69 (4.35) | 0.67 |
P-values adjusted for age and race.
aFasting morning samples.
*P-value = <0.0001 when compared with BF <18.65%.
†P-value <0.001 when compared with BF <18.65%.
‡P-value <0.01 when compared with BF <18.65%.
§P-value <0.05 when compared with BF <18.65%.
‖P-value <0.07 when compared with BF <18.65%.
Table 4.
Metabolic syndrome components, definition, and cardiovascular risk factors in women with a normal body mass index by body fat tertiles
| Variable (N = 3129) | BF <28.9% (N = 1044) | BF >28.9–33.3% (N = 1040) | BF >33.3% (N = 1045) | Age+race Padj-value for trend |
|---|---|---|---|---|
| Metabolic syndrome | N (%) | N (%) | N (%) | |
| Central obesity ATP III (WC >88 cm) | 21 (1.62) | 96 (7.85)* | 271 (24.22)* | <0.0001 |
| Central obesity by (W/H≥0.85) waist-to-hip ratio | 254 (24.31) | 350 (33.70)‡ | 439 (42.02)* | <0.0001 |
| High triglycerides (>1.7 mmol/L) or lipid treatment | 99 (7.67) | 159 (15.66)† | 227 (22.16)* | <0.0001 |
| Low high density lipoprotein (<1.3 mmol/L) | 256 (23.84) | 299 (28.67)‖ | 326 (31.69)‡ | 0.0024 |
| High blood pressure (>130/>85 mmHg) or treatment for hypertension | 260 (20.61) | 336 (28.46)‖ | 387 (34.11)§ | 0.049 |
| High fasting plasma glucosea (>5.55 mmol/L)or previously diagnosed diabetes | 110 (8.67) | 151 (14.10)§ | 193 (17.93)‡ | 0.0029 |
| Metabolic syndrome by ATP III criteriaa | 52 (3.38) | 103 (9.68)† | 178 (17.24)* | <0.0001 |
| Cardiovascular risk factors | ||||
| Dyslipidaemia | 166 (16.16) | 188 (18.06) | 242 (23.98)‡ | 0.0012 |
| Hypertension | 210 (15.65) | 262 (21.48) | 300 (25.97) | 0.25 |
| Diabetes | 27 (1.57) | 34 (2.29) | 49 (2.59)§ | 0.50 |
| Ever smokers | 417 (47.96) | 386 (42.59) | 412 (45.99) | 0.35 |
| CVD (myocardial infarction+stroke) | 22 (1.28) | 32 (2.11) | 42 (3.60)‖ | 0.038 |
P-values adjusted for age and race.
aFasting morning samples.
*P-value = <0.0001 when compared with BF <28.9%.
†P-value <0.001 when compared with BF <28.9%.
‡P-value <0.01 when compared with BF <28.9%.
§P-value <0.05 when compared with BF <28.9%.
‖P-value <0.07 when compared with BF <28.9%.
Total and cardiovascular mortality according to body fat
After a median follow-up of 8.83 years (interquartile range 7.25–10.33, 22 600 person-years), there were 787 deaths (34.64 deaths/1000 person-years), 470 in men (44.66 per 1000 man-years) and 317 in women (11.65 per 1000 woman-years). Of those, 337 were classified as CV deaths (14.91 CV deaths/1000 person-years), 195 in men (18.53 CV deaths/1000 man-years) and 142 in women (5.22 CV deaths/1000 woman-years).
In men and women, total and CV mortality increased as BF increased (Table 5). When BF was analysed as a continuous variable, BF was neither associated with the risk of death in men (HR 0.99, 95% CI 0.97–1.02) nor in women (HR 1.01, 95% CI 0.97–1.05). The lack of association was observed after adjusting for dyslipidaemia, hypertension, metabolic syndrome, smoking status, and waist circumference (HR in men 0.99, 95% CI 0.96–1.02; HR in women 1.01, 95% CI 0.97–1.05). Similarly, when we analysed mortality by tertiles, subjects with NWO were not at an increased risk for total mortality compared with the lowest sex-specific tertile of BF% (for men, HR = 0.90; 95% CI, 0.63–1.27 and for women HR 1.06, 95% CI 0.69–1.62, Table 5).
Table 5.
Total and cardiovascular mortality in men and women with a normal body mass index by body fat tertiles
| Men (N = 3042) | BF <18.65% | BF >18.65–23.15% | BF >23.15% |
|---|---|---|---|
| Total mortality events, n = 470 (44.66 deaths/1000 man-years) | 137 (16.23) | 143 (16.17) | 190 (75.62) |
| Hazard ratio | |||
| Model 1 | Reference | 0.87 (0.59–1.27) | 0.90 (0.63–1.27) |
| Model 2 | 0.92 (0.63–1.34) | 0.99 (0.67–1.45) | |
| Model 3 | 0.87 (0.57–1.31) | 0.86 (0.57–1.30) | |
| Cardiovascular mortality events, n = 195 (18.53 deaths/1000 man-years) | 56 (6.63) | 66 (7.46) | 73 (29.05) |
| Hazard ratio | |||
| Model 1 | Reference | 1.12 (0.65–1.91) | 1.07 (0.67–1.72) |
| Model 2 | 1.15 (0.68–1.94) | 1.14 (0.72–1.79) | |
| Model 3 | 1.06 (0.58–1.94) | 1.09 (0.61–1.96) | |
| Model 4 | 1.09 (0.62–1.92) | 1.17 (0.69–1.98) | |
| Women (N = 3129) | BF <28.9% | BF >28.9–33.3% | BF >33.3% |
| Total mortality events, n = 317 (11.65 deaths/1000 woman-years) | 97 (4.57) | 102 (11.66) | 118 (12.49) |
| Hazard ratio | |||
| Model 1 | Reference | 0.92 (0.61–1.41) | 1.06 (0.69–1.62) |
| Model 2 | 0.95 (0.62–1.45) | 1.11 (0.71–1.75) | |
| Model 3 | 0.91 (0.58–1.41) | 1.04 (0.66–1.63) | |
| Cardiovascular mortality events, n = 142 (5.22 deaths/1000 woman-years) | 40 (4.44) | 46 (5.25) | 56 (5.92) |
| Hazard ratio | |||
| Model 1 | Reference | 1.20 (0.65–2.22) | 1.84 (1.02–3.32) |
| Model 2 | 1.21 (0.64–2.30) | 1.88 (1.00–3.60) | |
| Model 3 | 1.26 (0.66–2.40) | 1.92 (1.06–3.47) | |
| Model 4 | 1.39 (0.67–2.90) | 2.20 (1.03–4.67) | |
Model 1: Adjusted for age, race, and smoking status.
Model 2: Adjusted age, race, smoking status, and waist circumference.
Model 3: Adjusted age, race, dyslipidaemia, hypertension, impaired fasting glucose, smoking, and waist-to-hip ratio.
Model 4: Adjusted for age, race, smoking status, waist circumference, dyslipidaemia, hypertension, diabetes, and CV disease.
Interestingly, BF was associated with an increased risk for CV mortality in women (HR 1.06 per each percent of BF, 95% CI 1.01–1.12) and the association was stronger after adjusting for dyslipidaemia, hypertension, metabolic syndrome, smoking status, and waist circumference (HR 1.07, 95% CI 1.01–1.14). A similar association was found when we analysed CV mortality in women using BF tertiles; NWO women were at significantly higher risk for total CV mortality (HR 1.84, 95% CI 1.02–3.32). This association prevailed even after further adjustment (HR 2.2, 95% CI 1.03–4.67, Table 5). In men, BF was neither associated with CV mortality as a continuous variable (adjusted HR 0.99, 95% CI 0.95–1.04) nor as sex-adjusted tertiles (adjusted HR for NWO in men 1.07, 95% CI 0.67–1.72 when compared with the lowest tertile).
Impact of central obesity on cardiovascular risk and mortality
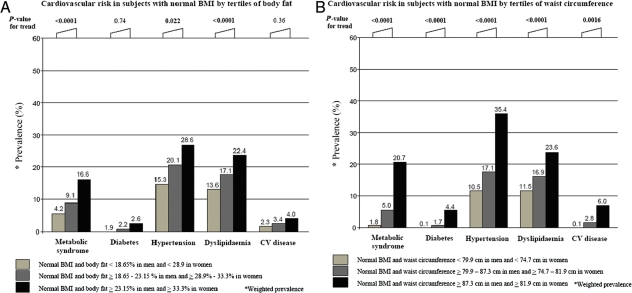
Figure 2A and B shows that sex-specific tertiles of waist circumferences were associated similarly to CV risk as sex-specific tertiles of BF%, suggesting that waist circumference can also stratify the risk for cardiometabolic dysregulation within subjects with a normal BMI. However, in contrast with NWO, the highest sex-specific tertile of waist circumference was not associated with an increased risk for CV mortality in women (HR = 1.42, 95% CI 0.69–2.94). Furthermore, the association between NWO and CV mortality in women was independent of waist circumference, as demonstrated in multivariate models including waist circumference as a covariate (Table 5). Additionally, waist circumference and waist-to-hip ratio were not significantly associated with a higher risk for total mortality in men or women.
Figure 2.
Comparison of metabolic syndrome components and definition (ATP-III) in subjects with a normal body mass index by sex-specific tertiles of body fat (A) and by sex-specific tertiles of waist circumferences (B).
Discussion
Metabolically obese normal weight subjects have been described since the late 1990s.37 These subjects have been characterized as having blunted insulin sensitivity and low lean mass despite having a normal BMI, characteristics similar to subjects with NWO described in our study. The condition has been previously defined but its prevalence has never been studied in the general population. Results from our study suggest that NWO might be a key factor in the emerging worldwide epidemic of obesity, metabolic syndrome, diabetes, and coronary artery disease.
Our study shows that NWO is significantly associated with cardiometabolic dysregulation and a high prevalence of metabolic syndrome, which is in fact, similar to the prevalence of metabolic syndrome described in overweight subjects.38 Additionally, this is the first study showing that in women, NWO is independently associated with an increased risk for CV mortality. These findings provide important insights into understanding obesity—subjects who would otherwise considered non-obese, based on a normal BMI, may actually have an excess in BF, and therefore be at high risk for cardiometabolic dysregulation and CV mortality. A normal BMI therefore does not necessarily imply protection from consequences of increased BF.
Supporting our current observations, recent studies have reported the presence of several metabolic abnormalities in women with normal BMI with a medium-to-high BF content. De Lorenzo et al.39 reported that 28 women with high BF (>30%) had a significantly lower resting metabolic rate and oxygen consumption, when compared with 20 women with normal BMI and no excess in BF (<30%). Furthermore, in a similar group of women (n = 20), De Lorenzo et al.40 noted that plasma levels of several inflammatory biomarkers, including interleukins, and C-reactive protein were significantly higher in women with a normal BMI but high BF content, supporting the concept that subjects with NWO may be predisposed to develop metabolic syndrome and CV disease.
Because bioimpedance does not give information about fat distribution, we explored the impact of central obesity in our results by performing analyses using sex-specific tertiles of waist circumference. Interestingly, an increased waist circumference (>87 cm in men and >82 cm in women) was similarly associated with CV risk as were sex-specific tertiles of BF% (Figure 2A and B). This has important clinical implications because devices for measuring BF are not widely available in clinical practice. In contrast, waist circumference can be easily and inexpensively measured. However, it is important to note that an increased waist circumference was not related to higher CV mortality as was BF content in subjects with NWO and only 2% of men had central obesity according to the ATP-III criterion. Thus, while central deposition of fat may play a crucial role in cardiometabolic abnormalities,41–43 it does not fully account for the higher risk for CV mortality noted in subjects with NWO. Furthermore, we found that the higher CV mortality noted in subjects with NWO remained significant, even after adjustment for central obesity. Finally, the association between NWO and CV mortality persisted in women, even after adjusting for CV risk factors, several of which could be considered intermediate mechanisms linking NWO and mortality.
Our study has several potential limitations. First, we used an arbitrary cut-off for BF% based on tertiles to define NWO. The harmful effects of an excess in BF very likely follow a continuum rather than a specific threshold for acquiring clinically significant cardiometabolic disturbances. Unfortunately, neither the World Health Organization nor any major scientific society involved in the study of obesity has defined a normal value for BF%. We believe that the use of tertiles to classify those with a relatively high BF% is more valid than using an arbitrary cut-off not previously validated. Second, misclassification could have occurred in this study, as subjects could have had changes in their body composition during the follow-up period. However, this concern is true for most epidemiologic studies that only use baseline information on the exposure variable, including those evaluating BMI. Third, bioelectrical impedance underestimates upper-body obesity, especially in athletes and elderly patients.44 Other more accurate methods to estimate BF%, such as hydrostatic weighing or dual energy X-ray absorptiometry, may be preferable to estimate body composition.45 Nevertheless, bioelectrical impedance's acceptable accuracy, simplicity, lack of radiation, and relatively low cost make it a practical and feasible alternative for measuring body fatness, especially in large populations.46,47 Fourth, there were relatively few CV events at follow-up in our sample, limiting the statistical power to assess the relationship between NWO and mortality. The low rate of events may have occurred because our sample of normal weight subjects comprised a relatively healthy, young group, with a mean age of just over 40 years. Finally, due to the cross-sectional nature of our analyses linking BF content to cardiometabolic dysregulation, we cannot establish causality or directionality between these two factors. However, numerous studies in different settings have shown that increases in adiposity worsen most cardiometabolic measures, while adiposity reduction has been related to improvements in most cardiometabolic markers.14,16 Furthermore, there is strong evidence showing an association between metabolic syndrome and CV mortality, supporting the notion that NWO may increase CV mortality by increasing cardiometabolic dysregulation.
Implications
Based on the latest US census and obesity prevalence data, we estimate that NWO is present in ∼30 million Americans, many of whom may be unaware of their heightened cardiometabolic risk despite their normal BMI. Because self awareness of a condition is the initial step in behavioural modification and incorporation of therapeutic lifestyle changes, it might be relevant to incorporate BF measurement in the regular physical exam, using simple methods to diagnose NWO in clinical practice. The cardiometabolic dysregulation found in subjects with NWO, such as insulin resistance, altered lipid profile, and metabolic syndrome are potentially remediable if appropriately treated with diet, exercise, and possibly pharmacological therapies.
Conclusions
Normal weight obesity is associated with significant cardiometabolic dysregulation, including metabolic syndrome and CV risk factors. Furthermore, NWO appears to be associated independently with increased CV mortality in women. Screening for adiposity in subjects with a normal BMI could better identify those at higher risk for cardiometabolic disturbances and CV mortality.
Funding
A.R.-C. is supported by a Postdoctoral Fellowship from the American Heart Association. V.K.S. is supported by NIH grants HL-65176, HL-70302, HL-73211, and M01-RR00585. J.S.-J. is partially supported by faculty funds from the Board of Post-Graduate Education of the Karolinska Institute (KID Award) and by the European Foundation for the Study of Diabetes through a Research Fellowship. F.L.-J. is a recipient of a Clinical Scientist Development Award from the American Heart Association. A.R.-C., V.K.S., and F.L.-J. are recipients of a grant obtained by Select Research Ltd for separate work related to the topic of this paper.
Conflict of interest: A.R.-C., F.L.-J. and V.K.S. are advisors for Select Research. V.K.S. also served as a consultant for ResMed, Respironics, GlaxoSmithKline, Sepracor, and Cardiac Concepts; he has received research grants from the ResMed Foundation, the Respironics Sleep and Respiratory Research Foundation, Sorin, Inc., and works with Mayo Health Solutions and iLife on intellectual property related to sleep and to obesity.
Acknowledgements
All authors have read and approved submission of the manuscript and the mention of their names.
References
- 1.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. J Am Med Assoc. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, Narayan KM, Williamson DF. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 4.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. 1995 World Health Organ Tech Rep Ser. [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 7.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjonneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, May AM, Bueno-de-Mesquita HB, van Duijnhoven FJ, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quiros JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 8.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 11.Franzosi MG. Should we continue to use BMI as a cardiovascular risk factor? Lancet. 2006;368:624–625. doi: 10.1016/S0140-6736(06)69222-2. [DOI] [PubMed] [Google Scholar]
- 12.Romero-Corral A, Somers VK, Sierra-Johnson J, Jensen MD, Thomas RJ, Squires RW, Allison TG, Korinek J, Lopez-Jimenez F. Diagnostic performance of body mass index to detect obesity in patients with coronary artery disease. Eur Heart J. 2007;28:2087–2093. doi: 10.1093/eurheartj/ehm243. [DOI] [PubMed] [Google Scholar]
- 13.Poirier P. Adiposity and cardiovascular disease: are we using the right definition of obesity? Eur Heart J. 2007;28:2047–2048. doi: 10.1093/eurheartj/ehm321. [DOI] [PubMed] [Google Scholar]
- 14.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Jimenez F. Speakable and unspeakable facts about BMI and mortality. Lancet. 2009;373:1055–1056. doi: 10.1016/S0140-6736(09)60628-0. [DOI] [PubMed] [Google Scholar]
- 16.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM. Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25:2243–2244. doi: 10.1161/01.ATV.0000189155.75833.c7. [DOI] [PubMed] [Google Scholar]
- 18.Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6:97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 19.Trakas K, Lawrence K, Shear NH. Utilization of health care resources by obese Canadians. CMAJ. 1999;160:1457–1462. [PMC free article] [PubMed] [Google Scholar]
- 20.Zizza C, Herring AH, Stevens J, Popkin BM. Length of hospital stays among obese individuals. Am J Public Health. 2004;94:1587–1591. doi: 10.2105/ajph.94.9.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plan and operation of the third National Health Health and Nutrition Examination Survey, 1988–1994. 1994 [Google Scholar]
- 22.Kuczmarski RJ. Bioelectrical impedance analysis measurements as part of a national nutrition survey. Am J Clin Nutr. 1996;64(Suppl. 3):453S–458S. doi: 10.1093/ajcn/64.3.453S. [DOI] [PubMed] [Google Scholar]
- 23.National Center for Health Statistics. UDoHaH Services; 1996. NHANES III Anthropometric Procedure Video, Stock no. 017-022-01355. [Google Scholar]
- 24.The Third National Health and Nutrition Examination Survey (NHANES III, 1988-1994); 1996 [Google Scholar]
- 25.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 26.Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, Lukaski HC, Friedl K, Hubbard VS. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–1609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- 27.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 28.Sierra-Johnson J, Somers VK, Kuniyoshi FH, Garza CA, Isley WL, Gami AS, Lopez-Jimenez F. Comparison of apolipoprotein-B/apolipoprotein-AI in subjects with versus without the metabolic syndrome. Am J Cardiol. 2006;98:1369–1373. doi: 10.1016/j.amjcard.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Ruhl CE, Everhart JE. Leptin concentrations in the United States: relations with demographic and anthropometric measures. Am J Clin Nutr. 2001;74:295–301. doi: 10.1093/ajcn/74.3.295. [DOI] [PubMed] [Google Scholar]
- 30.Wong ND, Pio J, Valencia R, Thakal G. Distribution of C-reactive protein and its relation to risk factors and coronary heart disease risk estimation in the National Health and Nutrition Examination Survey (NHANES) III. Prev Cardiol. 2001;4:109–114. doi: 10.1111/j.1520-037x.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- 31.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 32.NHANES III Reference Manuals. 1994 Available from: http://www.cdc.gov/nchs/about/major/nhanes/NHANESIII_Reference_Manuals.htm. (8 December 2008) [Google Scholar]
- 33.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 34.Rayburn WF. Diagnosis and classification of diabetes mellitus: highlights from the American Diabetes Association. J Reprod Med. 1997;42:585–586. [PubMed] [Google Scholar]
- 35.Ninomiya JK, L'Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- 36.Matching Methodology for NHANES III. Available from: http://www.cdc.gov/nchs/data/datalinkage/matching_methodology_nhanes3_final.pdf. (23 November 2007) [Google Scholar]
- 37.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 38.De Lorenzo A, Deurenberg P, Pietrantuono M, Di Daniele N, Cervelli V, Andreoli A. How fat is obese? Acta Diabetol. 2003;40(Suppl. 1):S254–S257. doi: 10.1007/s00592-003-0079-x. [DOI] [PubMed] [Google Scholar]
- 39.De Lorenzo A, Martinoli R, Vaia F, Di Renzo L. Normal weight obese (NWO) women: an evaluation of a candidate new syndrome. Nutr Metab Cardiovasc Dis. 2006;16:513–523. doi: 10.1016/j.numecd.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 40.De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L. Normal-weight obese syndrome: early inflammation? Am J Clin Nutr. 2007;85:40–45. doi: 10.1093/ajcn/85.1.40. [DOI] [PubMed] [Google Scholar]
- 41.Votruba SB, Jensen MD. Regional fat deposition as a factor in FFA metabolism. Annu Rev Nutr. 2007;27:149–163. doi: 10.1146/annurev.nutr.27.061406.093754. [DOI] [PubMed] [Google Scholar]
- 42.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28:850–856. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 43.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 44.Snijder MB, Kuyf BE, Deurenberg P. Effect of body build on the validity of predicted body fat from body mass index and bioelectrical impedance. Ann Nutr Metab. 1999;43:277–285. doi: 10.1159/000012795. [DOI] [PubMed] [Google Scholar]
- 45.Fogelholm M, van Marken Lichtenbelt W. Comparison of body composition methods: a literature analysis. Eur J Clin Nutr. 1997;51:495–503. doi: 10.1038/sj.ejcn.1600448. [DOI] [PubMed] [Google Scholar]
- 46.Khaodhiar L, Blackburn GL. Results of expert meetings: obesity and cardiovascular disease. Obesity assessment. Am Heart J. 2001;142:1095–1101. doi: 10.1067/mhj.2001.119420. [DOI] [PubMed] [Google Scholar]
- 47.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]