Abstract
Aims
To determine whether changes in practice, over time, are associated with altered rates of major bleeding in acute coronary syndromes (ACS).
Methods and results
Patients from the Global Registry of Acute Coronary Events were enrolled between 2000 and 2007. The main outcome measures were frequency of major bleeding, including haemorrhagic stroke, over time, after adjustment for patient characteristics, and impact of major bleeding on death and myocardial infarction. Of the 50 947 patients, 2.3% sustained a major bleed; almost half of these presented with ST-elevation ACS (44%, 513). Despite changes in antithrombotic therapy (increasing use of low molecular weight heparin, P < 0.0001), thienopyridines (P < 0.0001), and percutaneous coronary interventions (P < 0.0001), frequency of major bleeding for all ACS patients decreased (2.6 to 1.8%; P < 0.0001). Most decline was seen in ST-elevation ACS (2.9 to 2.1%, P = 0.02). The overall decline remained after adjustment for patient characteristics and treatments (P = 0.002, hazard ratio 0.94 per year, 95% confidence interval 0.91–0.98). Hospital characteristics were an independent predictor of bleeding (P < 0.0001). Patients who experienced major bleeding were at increased risk of death within 30 days from admission, even after adjustment for baseline variables.
Conclusion
Despite increasing use of more intensive therapies, there was a decline in the rate of major bleeding associated with changes in clinical practice. However, individual hospital characteristics remain an important determinant of the frequency of major bleeding.
Keywords: Acute coronary syndrome, Bleeding, Unstable angina, Myocardial infarction
Introduction
Randomized trials and observational studies have reported that patients who experience in-hospital bleeding are at higher risk of in-hospital and later death after presentation with an acute coronary syndrome (ACS).1,2 Guidelines from the European Society of Cardiology recommend that clinicians take into account the individual patient's risk of bleeding when selecting the optimal management strategy.3 However, it is unclear whether more intensive antithrombin and antiplatelet treatments and greater use of angiography and percutaneous coronary intervention (PCI), over time, may have increased the frequency of major bleeding.
By design, randomized trials exclude patients with extensive comorbidity, hence large-scale studies with unbiased sample populations are required to define bleeding risk and their temporal changes. The Global Registry of Acute Coronary Events (GRACE) is the largest multinational study of patients with ACS and has shown increasing use of invasive cardiac procedures and antithrombotic strategies over an 8-year interval (2000–2007).4 This change in practice was associated with improvements in clinical outcome, notably reductions in death and new heart failure,4 but it was unclear whether the benefits in outcome were offset by a greater frequency of bleeding.
The reported frequencies of major bleeding in large randomized trials of ACS vary substantially1,2,5–9 but inter-study comparisons are confounded by differences in inclusion criteria and varying definitions of bleeding.10 Observational studies that include the full spectrum of patients with an ACS and that apply consistent criteria for bleeding may provide more reliable estimates of the ‘real world’ frequency of bleeding and changes over time. In the GRACE study in 2003, in-hospital major bleeding rates (including haemorrhagic stroke) ranged from 2.3% in patients with unstable angina to 4.8% in those with ST-segment elevation myocardial infarction (STEMI).11 Patients with an acute myocardial infarction who had a major in-hospital bleed tended to be older than those who did not bleed, had more comorbid conditions, were more likely to undergo interventional procedures, and had a much higher in-hospital mortality rate (21% vs. 6%, P < 0.001).12
For this report from the GRACE registry, we hypothesized that changes in clinical practice over 8 years (more intensive antithrombotic and interventional therapy) would be accompanied by an increased frequency of major bleeding. In addition, we aimed to determine whether major bleeding impacted on the subsequent rate of death or myocardial infarction, and we explored whether variation in frequency of bleeding among hospitals is accounted for by differences in the risk characteristics of patients.
Methods
The design, the standardized definitions, and the data-collection and quality control methods for GRACE have been published elsewhere.13–15 GRACE is a prospective, multinational, observational, cohort study of patients with ACS. GRACE is designed to reflect an unselected population of patients with ACS, irrespective of geographic region. A total of 123 hospitals located in 14 countries in North and South America, Europe, Australia, and New Zealand have contributed data to this study.
In brief, adult patients (18 or more years) admitted with a presumptive diagnosis of ACS at participating hospitals were potentially eligible for this study. Eligibility required a clinical history of ACS accompanied by at least one of the following: electrocardiographic changes consistent with ACS, serial increases in biochemical markers of cardiac necrosis (troponin, creatinine kinase MB, creatinine phosphokinase), and documented coronary artery disease. Patients with non-cardiovascular causes for the clinical presentation, such as trauma, surgery, or aortic aneurism, were excluded. Patients were followed-up at ∼6 months by telephone, clinic visits, or through calls to their primary care physician to ascertain the occurrence of several long-term outcomes. Where required, study investigators received approval from their local hospital ethics or institutional review board for the conduct of this study.
To enrol an unbiased sample of patients with ACS, sites were encouraged to recruit the first 10–20 consecutive eligible patients each month. Training was conducted and regular audits performed at all participating hospitals. Data were collected by trained study coordinators using standardized case report forms. Demographic characteristics, medical history, presenting symptoms, duration of pre-hospital delay, biochemical and electrocardiographic findings, treatment practices, and a variety of hospital outcome data were collected. Standardized definitions of all patient-related variables, clinical diagnoses, major bleeding, and hospital complications and outcomes were used.13 Major bleeding, including haemorrhagic stroke, was defined as life-threatening bleeding occurring in-hospital and requiring a transfusion of ≥2 U of packed red blood cells or resulting in a decrease in haematocrit of ≥10%, and/or resulting in death and/or haemorrhagic stroke and/or subdural haematoma.
Patients were diagnosed with STEMI when they had new or presumed new ST-segment elevation ≥1 mm seen in any location, or new left bundle branch block on the index or subsequent ECG with at least one positive cardiac biochemical marker of necrosis (including troponin measurements). In cases of NSTEMI at least one positive cardiac biochemical marker of necrosis without new ST-segment elevation seen on the index or subsequent ECG had to be present. Unstable angina was diagnosed when serum biochemical markers indicative of myocardial necrosis in each hospital's laboratory were within the normal range. Full definitions can be found on the GRACE website at www.outcomes.org/grace. Hospital-specific feedback regarding patient characteristics, presentation, management, and outcomes was provided to each centre on a quarterly basis in the form of written reports.
This report is based on data from 50 947 patients with an ACS who were enrolled at 123 hospitals in 14 countries between January 2000 and December 2007. Patients transferred in, patients with a discharge diagnosis not related to ACS, and patients with bleeds related to coronary artery bypass graft surgery were not included. Patients were categorized at presentation into one of three categories for ST shift: new or presumed new ST-elevation with or without ST-depression, new or presumed ST-depression alone, and no ST-wave segment change.
Statistical analysis
Baseline statistics for patients are given as frequencies and percentages for categorical data; differences between those with and without bleeds were tested using Fisher's exact test. Continuous variables are given as medians and 25th and 75th percentiles, and differences were tested using a two-sided Wilcoxon rank-sum test. Cumulative bleeds by type of ST-segment shift are presented as Kaplan–Meier plots.
For temporal trends, patients were assigned to 1 of 8 years based on date of discharge or in-hospital death. Unadjusted linear trends in in-hospital medication use, and outcomes, were assessed using a two-sided Cochran-Armitage test at α = 0.05. Post-discharge outcomes were collected at ∼6 months after discharge and unadjusted linear trends evaluated using the two-sided Cochran-Armitage test. The number of patients followed for post-discharge events for year 8 was less than half that for the previous years due to the end of patient enrolment. Data used for adjusted Cox proportional hazards regression consider event status up to day 30 from hospitalization. All statistical tests were performed at α = 0.05 using SAS software version 9.1 (SAS Institute Inc., Cary, NC, USA).
Adjusted trends in bleeding
Linear trends in major in-hospital bleeding were examined using a Cox proportional hazards model adjusting for hospital and new or presumed new ST-wave change, and the following risk factors for bleeding:12 age, sex, glomerular filtration rate (using the Modification of Diet in Renal Disease calculation), pulse, pulmonary artery catheter, intra-aortic balloon pump, catheterization, PCI, fibrinolytic therapy, medical history (bleeding, peripheral arterial disease, atrial fibrillation, hypertension, and smoking), and medications given within the first 24 h [intravenous glycoprotein IIb/IIIa inhibitors, intravenous inotropic drugs, low molecular weight heparin (LMWH), unfractionated heparin (UFH), and aspirin]. Catheterization, PCI, and fibrinolytic therapy preceding major bleeds were included as time-varying covariates. The proportional hazards assumption was checked by testing for the statistical significance of the interaction of each variable with the logarithm base 10 of the days to major bleed/haemorrhagic stroke/subdural haematoma. Statistically significant interaction terms were retained in the model.
Adjusted trends in outcomes and effect of bleeding
Linear trends in all deaths and new myocardial infarctions (occurring >24 h after presentation) up to 30 days from admission were adjusted for patient characteristics and hospital procedures using Cox proportional hazards regression. Since the date of new myocardial infarctions was available from 2003 onwards, adjusted temporal trends were examined for 2003–2007, whereas adjusted trends for 30-day death covered the years 2000–2007. Linear trends for post-discharge deaths and myocardial infarctions up to 180 days from admission were for 2000–2007 and 2003–2007, respectively.
Cox regression candidate variables for both 30-day outcomes included the GRACE risk variables for in-hospital death16 [age, Killip class (continuous variable), systolic blood pressure, pulse, serum creatinine, cardiac arrest during presentation, positive initial cardiac markers, ST-segment shift category], major bleed status, year of discharge or death, and hospital; and catheterization, PCI, and fibrinolytic status as time-varying covariates. Adjusted trends in post-discharge death and myocardial infarction for patients surviving hospitalization were similarly examined using Cox regression, the same candidate variables, and additionally, medical histories of congestive heart failure, peripheral arterial disease, and myocardial infarction. The proportional hazards assumption was checked for all models by testing for the statistical significance of the interaction of each variable with the logarithm base 10 of the days-to-event. Statistically significant interaction terms were retained in the models.
Hazard ratios (HRs) comparing patients with major bleeds/haemorrhagic stroke to patients without were computed to assess the effect of bleeding on outcomes. The effect on 30-day death differed by ST-segment shift category, whereas the effect of bleeding on 30-day myocardial infarctions and post-discharge outcomes did not.
Results
Of the 50 947 patients in the study, 2.3% (n = 1160) sustained a major bleed. Of those with a major bleed, 513 (44%) presented with ST-elevation ACS, 296 (26%) presented with ST-depression ACS, and in 351 (30%) there was no ST-deviation at presentation. Patients who bled were older than those who did not bleed and a greater proportion were women (Table 1). When compared with those without a bleed, patients who bled had higher rates of prior atrial fibrillation, congestive heart failure, diabetes, transient ischaemic attack/stroke, peripheral arterial disease, and hypertension, but lower rates of current or former smoking and hyperlipidaemia. Patients with a bleed had a higher GRACE risk score for hospital mortality and presented with higher heart rates, more heart failure (Killip classes II–IV), and more had sustained a cardiac arrest (Table 1). Patients with a bleed were more likely than those without to have undergone cardiac catheterization, PCI, or to have received fibrinolytic therapy during hospitalization (Table 1).
Table 1.
Patients’ baseline characteristics, according to bleeding status
| Patients without a bleed (n = 49 787) | Patients with a bleed (n = 1160) | P-valuea | |
|---|---|---|---|
| Ageb (years) | 67 (56–76) | 74 (65–81) | <0.0001 |
| Men (%) | 33 332 (67) | 615 (53) | <0.0001 |
| Medical history (%) | |||
| Angina | 25 583 (52) | 568 (49) | 0.10 |
| Atrial fibrillation | 3904 (7.9) | 127 (11) | <0.001 |
| Congestive heart failure | 5094 (10) | 196 (17) | <0.0001 |
| Coronary angiography | 15 800 (32) | 327 (29) | 0.014 |
| Diabetes | 12 428 (25) | 349 (30) | <0.0001 |
| Myocardial infarction | 15 073 (30) | 352 (31) | 0.92 |
| Positive stress test | 5312 (11) | 102 (8.9) | 0.043 |
| Transient ischaemic attack/stroke | 4137 (8.4) | 121 (11) | 0.010 |
| Smoker (current/former) | 28 035 (57) | 598 (52) | 0.001 |
| Peripheral arterial disease | 4516 (9.1) | 179 (16) | <0.0001 |
| Hypertension | 30 922 (63) | 790 (68) | <0.0001 |
| Hyperlipidaemia | 24 019 (49) | 515 (45) | <0.01 |
| CABG | 6265 (13) | 139 (12) | 0.62 |
| PCI | 9035 (18) | 178 (16) | 0.017 |
| Clinical presentation | |||
| Pulseb (b.p.m.) | 76 (65–90) | 82 (70–99) | <0.0001 |
| Systolic BPb (mmHg) | 140 (120–160) | 137 (115–160) | <0.0001 |
| Diastolic BPb (mmHg) | 80 (70–90) | 76 (62–89) | <0.0001 |
| Glomerular filtration rate | 71 (55–86) | 58 (41–75) | <0.0001 |
| Cardiac arrest (%) | 941 (1.9) | 43 (3.8) | <0.0001 |
| Killip class (%) | |||
| I | 40 754 (84) | 813 (71) | <0.0001 |
| II | 5762 (12) | 214 (19) | <0.0001 |
| III | 1816 (3.7) | 88 (7.7) | <0.0001 |
| IV | 445 (0.9) | 27 (2.4) | <0.0001 |
| Positive cardiac biomarkers (%) | 22 496 (46) | 628 (55) | <0.0001 |
| Initial serum creatinineb (mg/dL) | 1.0 (0.9–1.3) | 1.1 (0.9–1.5) | <0.0001 |
| Initial glucoseb (mg/dL) | 125 (103–166) | 143 (113–191) | <0.0001 |
| Fasting glucosea (mg/dL) | 104 (92–130) | 111 (95–140) | <0.0001 |
| GRACE risk scoreb | 144 (122–169) | 169 (144–192) | <0.0001 |
| Type of ACS (%) | |||
| STEMI | 17 349 (35) | 530 (46) | <0.0001 |
| NSTEMI | 16 811 (34) | 437 (38) | <0.01 |
| Unstable angina | 15 627 (31) | 193 (17) | <0.0001 |
| Type of ST shift (%) | |||
| ST-elevation | 18 242 (37) | 513 (44) | <0.0001 |
| ST-depression | 8949 (18) | 296 (26) | <0.0001 |
| No shift | 22 596 (45) | 351 (30) | <0.0001 |
| In-hospital procedures (%) | |||
| Cardiac catheterization | 29 089 (59) | 747 (65) | <0.0001 |
| PCI | 17 907 (36) | 559 (49) | <0.0001 |
| Fibrinolytic drugs | 5995 (12) | 161 (14) | <0.01 |
| CABG | 2248 (4.6) | 29 (2.5) | <0.0001 |
ACS, acute coronary syndrome; BP, blood pressure; CABG, coronary artery bypass grafting; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
aTwo-sided Fisher's exact test for binomial variables and two-sided Wilcoxon rank-sum test for continuous variables.
bMedian (interquartile range).
Red blood cell transfusion was undertaken in 57% of all major bleeds. The median number of red blood cell units transfused within the first 24 h after a bleed was 2.0 (interquartile range 1–2). The median total number of red blood cell units transfused after a bleed was 2.0 (interquartile range 2–3).
Trends in the management of acute coronary syndrome
Table 2 shows temporal trends in the medical management of all ACS patients since 2000. The use of antithrombotic therapies changed, with a fall in the use of UFH and increasing use of LMWH for all types of ACS (P < 0.0001 for linear trend). For all types of ACS, the use of a thienopyridine within 24 h of presentation also increased (P < 0.0001 for linear trend). Overall use of glycoprotein IIb/IIIa inhibitors among all ACS patients did not change over time. Among patients with ST-elevation, use of GP IIb/IIIa inhibitors decreased for those who underwent any PCI, including primary PCI (P < 0.0001 for linear trend) (Table 2).
Table 2.
Temporal trends in the medical management of (within first 24 h) and selected interventions for ACS since 2000
| Treatment | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | P-value |
|---|---|---|---|---|---|---|---|---|---|
| All ACS (n) | 7050 | 7068 | 7343 | 7477 | 7014 | 5831 | 5276 | 3888 | |
| Aspirin (%) | 89 | 90 | 90 | 88 | 89 | 91 | 91 | 91 | <0.002 |
| GP IIb/IIIa inhibitor (%) | 15 | 17 | 20 | 20 | 16 | 18 | 19 | 15 | 0.95 |
| LMWH (%) | 37 | 41 | 50 | 55 | 55 | 56 | 56 | 63 | <0.0001 |
| Unfractionated heparin (%) | 48 | 44 | 39 | 34 | 31 | 31 | 32 | 24 | <0.0001 |
| Thienopyridine (%) | 14 | 22 | 35 | 41 | 46 | 56 | 59 | 65 | <0.0001 |
| ST-elevation (n) | 1405 | 2635 | 2677 | 2856 | 2597 | 2059 | 1769 | 1268 | |
| GP IIb/IIIa inhibitora with PCI (%) | |||||||||
| Primary PCI | 64 | 75 | 73 | 65 | 49 | 56 | 53 | 46 | <0.0001 |
| Other PCI | 25 | 28 | 32 | 30 | 21 | 17 | 21 | 18 | <0.0001 |
| Any PCI | 42 | 50 | 54 | 50 | 39 | 41 | 44 | 38 | <0.0001 |
| No PCI | 6.2 | 5.6 | 5.7 | 7.3 | 5.6 | 6.1 | 5.8 | 4.8 | 0.68 |
ACS, acute coronary syndrome; GP, glycoprotein; LMWH, low molecular weight heparin; PCI, percutaneous coronary intervention.
aDenominator is the number of patients in each PCI category.
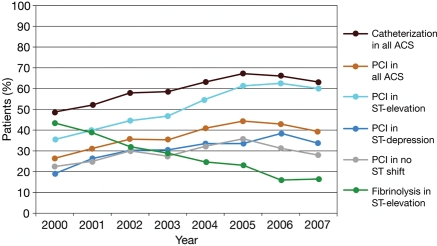
Figure 1 shows temporal trends in the use of selected interventions and fibrinolysis for ACS since 2000. Use of PCI has increased, particularly for patients with ST-segment elevation ACS (P < 0.0001 for linear trend), whereas the use of fibrinolytic drugs in this patient group has decreased (P < 0.0001 for linear trend).
Figure 1.
Temporal trends in cardiac procedures in all patients with an ACS, in patients with ST-segment elevation, ST-segment depression, or no ST shift, and fibrinolysis in patients with ST-elevation ACS.
Temporal trends in major bleeding
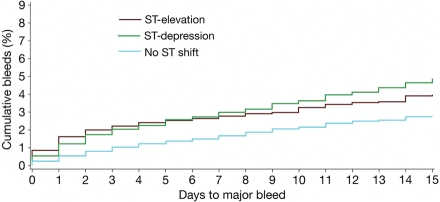
In the overall ACS population, the rates of bleeding decreased over the study period (Table 3). This decline was also observed in the population with ST-depression and in those with no ST shift. Kaplan–Meier cumulative rates of bleeding from hospital admission up to 15 days are shown in Figure 2. Patients with ST-segment elevation were at greatest risk of ‘early’ bleeding (admission to 6 days). The rate of major bleeding increased steadily for patients with ST-segment depression, exceeding that in the ST-elevation group after 6 days. Individuals with neither ST-elevation nor depression remained at lowest risk of major bleeding.
Table 3.
Raw bleeding rates by year, and unadjusted and risk-adjusted hazard ratios
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | P-value for linear trend, unadjusted | Hazard ratioa*, unadjusted (95% CI) | Hazard ratioa, adjustedb (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All ACS | |||||||||||
| % | 2.6 | 2.7 | 2.5 | 2.2 | 2.5 | 1.9 | 1.7 | 1.8 | <0.0001 | 0.96 (0.93–0.98) | 0.94 (0.91–0.98) |
| Number with bleed | 181 | 193 | 181 | 162 | 174 | 110 | 88 | 71 | |||
| ST-elevation | |||||||||||
| % | 2.9 | 3.4 | 2.9 | 2.4 | 2.8 | 2.5 | 2.4 | 2.1 | 0.02 | 0.97 (0.93–1.01) | 0.98 (0.93–1.0) |
| Number with bleed | 74 | 93 | 80 | 71 | 73 | 52 | 43 | 27 | |||
| ST-depression | |||||||||||
| % | 3.3 | 2.9 | 4.0 | 3.3 | 3.8 | 2.3 | 2.8 | 2.4 | 0.19 | 0.98 (0.93–1.04) | 0.93 (0.86–1.0) |
| Number with bleed | 46 | 38 | 53 | 47 | 48 | 24 | 25 | 15 | |||
| No ST shift | |||||||||||
| % | 2.0 | 2.0 | 1.5 | 1.4 | 1.7 | 1.3 | 0.8 | 1.5 | <0.001 | 0.93 (0.89–0.98) | 0.91 (0.85–0.97) |
| Number with bleed | 61 | 62 | 48 | 44 | 53 | 34 | 20 | 29 | |||
Major bleeding was classified as contributing to death in 12% of major bleeds; classified as clinically overt within a critical organ (intracranial, retroperitoneal, intraocular, adrenal, spinal, pericardial) in 16%; was clinically overt, leading to a transfusion of two or more units of packed red blood cells in 49%; was associated with a fall in haemoglobin of 2 g/dL or more in 58% (GRACE bleeding substudy).
aPer year.
bAdjusted for patient characteristics, medications, and interventions.
Figure 2.
Kaplan–Meier plot of major bleeds and/or haemorrhagic stroke and/or subdural haematoma from hospital admission up to 15 days in patients with ST-segment elevation, ST-segment depression, or neither.
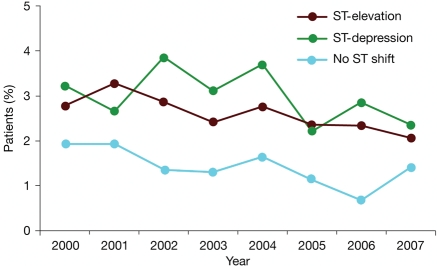
Unadjusted temporal trends in the rates of major bleeding since 2000, according to type of ST-segment shift, are shown in Figure 3. Among all ACS patients, major bleeding decreased from 2.6% to 1.8% (P < 0.0001) (Table 3). Among patients with ST-segment elevation ACS, major bleeding decreased from 2.9% to 2.1% (P = 0.02), whereas there was no change among patients with ST-segment depression ACS. Among patients with no ST-segment shift, major bleeding decreased from 2.0 to 1.5% (P < 0.001).
Figure 3.
Temporal trends in the rates of major bleeding and/or haemorrhagic stroke and/or subdural haematoma, in patients with ST-segment elevation, ST-segment depression, or neither.
After Cox proportional hazards adjustment for patient characteristics, medications, and interventions, the downward temporal trend in bleeding remained for all ACS (HR 0.94 per year, 95% CI 0.91–0.98). This decline was also observed in patients with ST-depression (HR 0.93, 95% CI 0.86–1.0) and in those with no ST shift (HR 0.91, 95% CI 0.85–0.97).
Do hospital characteristics influence the frequency of bleeding?
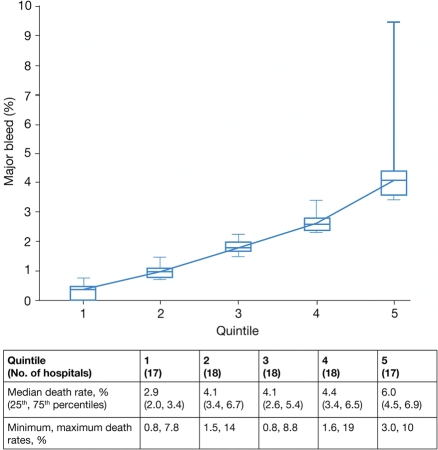
When hospitals were divided into quintiles according to median rates of major bleeds, the rate ranged from 0.4% to 4.1%, representing a 10-fold difference between the highest and lowest quintiles (Figure 4). Despite adjustment for patient characteristics, treatments, and interventions, individual hospitals remained an independent predictor of bleeding (P < 0.0001).
Figure 4.
Hospitals divided into quintiles according to percentage of in-hospital bleeds (figure; data show medians, minimum and maximum values; 17 or 18 hospitals per quintile) and in-hospital death (table). All data are derived from hospitals that provided bleeding status in ≥100 patients.
Impact of major bleeding on mortality and (re)infarction
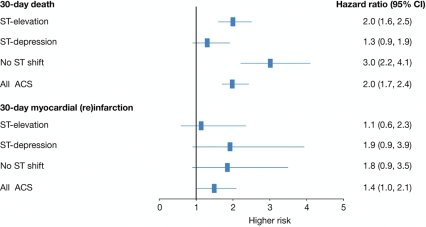
In the overall ACS population, patients who experienced a major bleed were at increased risk of 30-day death (HR 2.0, 95% CI 1.7–2.4; Figure 5). This finding was most pronounced among patients with no ST-segment shift, with the HR increasing three-fold even after adjustment for baseline variables and interventions (catheterization, PCI, fibrinolytics) (HR 3.0, 95% CI 2.2–4.1).
Figure 5.
Risk of 30-day death (2000–2007; n = 45 406 patients with an acute coronary syndrome) and 30-day myocardial (re)infarction (2003–2007; n = 26 126), adjusted for GRACE risk variables and treatment interventions (catheterization, PCI, fibrinolytics): patients with vs. without a major bleed and/or haemorrhagic stroke and/or subdural haematoma.
In patients who experienced a major bleed, there was an association with (re)infarction (HR 2.0, 95% CI 1.4–2.8, P = 0.0001). However, 10 of the 724 bleeds occurred after the infarction. The association between bleeding and subsequent (re)infarction showed a trend for increased hazard (HR 1.4, 95% CI 1.0–2.1; Figure 4).
Discussion
These data, from the largest multinational observational study of patients presenting to hospital for an ACS, show that despite increasing use of more aggressive interventional and pharmacological therapies, there has been a statistically significant downward trend in the rates of major bleeding (P < 0.0001) between 2000 and 2007. The decline in bleeding mirrors that for death and heart failure reported in an earlier study from the GRACE registry.4
Although it may have been expected that the frequency of major bleeding would have increased over time on account of more potent antithrombotic therapies and antithrombotic combinations (substitution of LMWH for UFH, greater use of thienopyridines and GP IIb/IIIa inhibitors) and greater use of cardiac catheterization and PCI, in fact the converse was found. These findings suggest that other changes in clinical practice have contributed to the reduction in bleeding frequency (e.g. improved instrumentation for cardiac catheterization, smaller calibre catheters, increasing use of radial access, possibly improved accuracy of dosing of parenteral antithrombotics, physician awareness of bleeding, reduction in the use of glycoprotein IIb/IIIa inhibitors, and changing thresholds for transfusion).
There have been changes in the use of radial access procedures, especially in some regions and some countries, but these changes are not sufficient to account for the observed decline in bleeding frequency. The use of radial access was low, for almost all countries, during the time period reported in this study. For example, according to the ACC-NCDR registry in the USA, in the fourth quarter of 2008 the national rate of radial procedures in the USA was only 1.6%. As similar declines in bleeding were observed in the USA compared with other countries, it is unlikely that radial access can account for the major part of the decline in bleeding. Physician awareness of bleeding has increased over the study period and this may have contributed to a decline in the threshold for transfusion.
We have demonstrated previously that the risk profile of the GRACE ACS population has not declined over time (it remained unchanged for ST-elevation ACS and increased for ST-depression ACS),4 and the changes in bleeding in this report take account of the risk score over time. For patients presenting with ST-elevation, there was a downward trend in the frequency of bleeding (P < 0.0001); for those presenting with ST-depression there was no change in bleeding frequency; and in those without ST-deviation there was a downward trend (P = 0.06).
Nevertheless, when major bleeding occurred it was associated with a subsequent risk of death and of myocardial infarction, and this risk is of similar magnitude to that seen in the previous clinical trials.5,17,18 The data also show that major bleeding is associated with a three-fold increase in the risk of death in patients with no ST shift and a two-fold increase in those with ST-elevation. An association was seen between bleeding and reinfarction, with a two-fold increase in the risk of 30-day myocardial (re)infarction. However, 10 of 724 bleeds occurred after the infarction, resulting in a 1.4-fold hazard (95% CI 1.0–2.1) of bleeding with subsequent infarction.
To explore whether hospital characteristics may be associated with variations in the frequency of bleeding, all models were adjusted for individual hospitals as well as baseline patient characteristics. Even after adjustment for patient characteristics, treatments, and interventions, the overall P-value for hospitals was still statistically significant (P < 0.0001). Hospitals were also divided into quintiles according to bleeding frequency. The median unadjusted rate of bleeding among hospitals in the highest quintile was ∼10 times the median rate among hospitals in the lowest quintile (Figure 4). These findings suggest that hospital-dependent variables may have an important impact upon the risk of major bleeding. Individual hospital variables are likely to be the consequence of differences in practice patterns, and potentially amenable to change.
Strengths and limitations
GRACE employed standard definitions for outcome events including myocardial infarction and major bleeding (rather than centre- or clinician-dependent criteria), so changes in bleeding frequency do not represent changes in diagnostic criteria or diagnostic threshold for bleeding. The rates of major bleeding are of similar magnitude to those reported for large-scale trials of ACS patients5,9,19,20 but lower than those reported in trials that include haematoma in the definition of major bleeding.17 Our definition of haemorrhagic stroke includes subdural haematoma. GRACE is a non-randomized, observational study; in common with other observational studies, it may be subject to potential biases, including the collection of non-randomized data, missing or incomplete information, and potential confounding by drug indication or other unmeasured covariates. Such confounding would be of particular relevance if non-randomized treatments were compared, but is not relevant to the time-dependent analyses. GRACE included a large systematic patient cohort and the group comparisons were determined by the presence or absence of ST-deviation at presentation, rather than treatment-dependent variables. Outcomes were not analysed by final hospital discharge diagnosis (e.g. myocardial infarction or no myocardial infarction) as such outcome events may have evolved during the hospital stay. GRACE is more representative of clinical practice than a trial population as those with co-morbidity or advanced age were not excluded and this study is likely to reflect the frequency of bleeding over time in an unselected population of patients presenting with ACS.
Funding
GRACE is funded by a grant from sanofi-aventis (Paris, France) to the Centre for Outcomes Research, University of Massachusetts Medical School (Worcester, MA, USA). Sanofi-aventis had no involvement in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the paper for publication. The design, conduct, and interpretation of GRACE are undertaken by an independent steering committee. K.A.A.F. is funded by the British Heart Foundation. Funding to pay the Open Access publication charges for this article was provided by the British Heart Foundation through the BHF Chair Award to K.A.A.F.
Conflict of interest: K.A.A.F. has received research grant funding from the British Heart Foundation, Medical Research Council, and the Wellcome Trust. He has received research grants and lecture fees from sanofi-aventis, GlaxoSmithKline, and Bristol-Myers Squibb. Ph. G.S. has received a research grant from sanofi-aventis; serves on the speakers’ bureau of Boehringer-Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Nycomed, Medtronic, sanofi-aventis, Servier, and The Medicines Company; and serves as a consultant or on the advisory board of Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Endotis, GlaxoSmithKline, Medtronic, Merck Sharp & Dohme, Nycomed, sanofi-aventis, Servier, and The Medicines Company. K.A.E. has received research grants from Biosite, Bristol-Myers Squibb, Blue Cross Blue Shield of Michigan, Hewlett Foundation, Mardigian Fund, Pfizer, sanofi-aventis, and the Varbedian Fund and is a consultant/advisory board member for NIH NHLBI, Pfizer, sanofi-aventis, Robert Wood Johnson Foundation. Á.A. receives research funding from sanofi-aventis, Population Health Research Institute, and Boehringer Ingelheim. C.B.G. has received research grants from Alexion, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, decode Genetics, Genentech, GlaxoSmithKline, Novartis, Procter & Gamble, sanofi-aventis, The Medicines Company, INO Therapeutics, Medicure, and Procter & Gamble and is a consultant/advisory board member for Alexion, Astra Zeneca, GlaxoSmithKline, INO Therapeutics, Medicure, Novartis, Procter & Gamble, sanofi-aventis, and The Medicines Company. G.M. receives research grants from sanofi-aventis, Schering, Lilly, MSD, and Pfizer. S.G.G. receives research grant support and/or speaker/consulting honoraria from Astra Zeneca, Bayer, Biovail, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Guidant, Hoffman La-Roche, Johnson & Johnson, Key Schering/Schering Plough, Merck Frosst, Pfizer, sanofi-aventis, and The Medicines Company. J.M.G. has received research grants from sanofi-aventis and The Medicines Company. K.A.E. receives research grants from Biosite, Bristol-Myers Squibb, Blue Cross Blue Shield of Michigan, Hewlett Foundation, Mardigian Fund, Pfizer, sanofi-aventis, and the Varbedian Fund and is a consultant/advisory board member for NIH NHLBI, Pfizer, sanofi-aventis, and the Robert Wood Johnson Foundation. A.L.Q. has no conflicts to disclose.
Acknowledgements
We thank and express our gratitude to the physicians and nurses participating in GRACE. Sophie Rushton-Smith, PhD, provided editorial support for the manuscript and was funded by sanofi-aventis through the GRACE registry.
References
- 1.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 2.Rao SV, O'Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, Mahaffey KW, Califf RM, Harrington RA. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–1206. doi: 10.1016/j.amjcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 3.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, Fox KA, Hasdai D, Ohman EM, Wallentin L, Wijns W, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Kristensen SD, Widimsky P, McGregor K, Sechtem U, Tendera M, Hellemans I, Gomez JL, Silber S, Funck-Brentano C, Andreotti F, Benzer W, Bertrand M, Betriu A, Desutter J, Falk V, Ortiz AF, Gitt A, Hasin Y, Huber K, Kornowski R, Lopez-Sendon J, Morais J, Nordrehaug JE, Steg PG, Thygesen K, Tubaro M, Turpie AG, Verheugt F, Windecker S. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: The Task Force for the Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of the European Society of Cardiology. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 4.Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA, Jr, Granger CB, Flather MD, Budaj A, Quill A, Gore JM. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. J Am Med Assoc. 2007;297:1892–1900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L, Joyner C, Fox KA. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006;354:1464–1476. doi: 10.1056/NEJMoa055443. [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–2216. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson JJ, Califf RM, Antman EM, Cohen M, Grines CL, Goodman S, Kereiakes DJ, Langer A, Mahaffey KW, Nessel CC, Armstrong PW, Avezum A, Aylward P, Becker RC, Biasucci L, Borzak S, Col J, Frey MJ, Fry E, Gulba DC, Guneri S, Gurfinkel E, Harrington R, Hochman JS, Kleiman NS, Leon MB, Lopez-Sendon JL, Pepine CJ, Ruzyllo W, Steinhubl SR, Teirstein PS, Toro-Figueroa L, White H. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. J Am Med Assoc. 2004;292:45–54. doi: 10.1001/jama.292.1.45. [DOI] [PubMed] [Google Scholar]
- 8.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 10.Steg PG, Lopez-Sendon J, Lopez de Sa E, Goodman SG, Gore JM, Anderson FA, Jr, Himbert D, Allegrone J, Van de Werf F. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. 2007;167:68–73. doi: 10.1001/archinte.167.1.68. [DOI] [PubMed] [Google Scholar]
- 11.Moscucci M, Fox KA, Cannon CP, Klein W, Lopez-Sendon J, Montalescot G, White K, Goldberg RJ. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE) Eur Heart J. 2003;24:1815–1823. doi: 10.1016/s0195-668x(03)00485-8. [DOI] [PubMed] [Google Scholar]
- 12.Spencer FA, Moscucci M, Granger CB, Gore JM, Goldberg RJ, Steg PG, Goodman SG, Budaj A, FitzGerald G, Fox KA. Does comorbidity account for the excess mortality in patients with major bleeding in acute myocardial infarction? Circulation. 2007;116:2793–2801. doi: 10.1161/CIRCULATIONAHA.107.694273. [DOI] [PubMed] [Google Scholar]
- 13.The GRACE Investigators. Rationale and design of the GRACE (Global Registry of Acute Coronary Events) Project: a multinational registry of patients hospitalized with acute coronary syndromes. Am Heart J. 2001;141:190–199. doi: 10.1067/mhj.2001.112404. [DOI] [PubMed] [Google Scholar]
- 14.Eagle KA, Goodman SG, Avezum A, Budaj A, Sullivan CM, Lopez-Sendon J. Practice variation and missed opportunities for reperfusion in ST-segment-elevation myocardial infarction: findings from the Global Registry of Acute Coronary Events (GRACE) Lancet. 2002;359:373–377. doi: 10.1016/S0140-6736(02)07595-5. [DOI] [PubMed] [Google Scholar]
- 15.Steg PG, Goldberg RJ, Gore JM, Fox KA, Eagle KA, Flather MD, Sadiq I, Kasper R, Rushton-Mellor SK, Anderson FA. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE) Am J Cardiol. 2002;90:358–363. doi: 10.1016/s0002-9149(02)02489-x. [DOI] [PubMed] [Google Scholar]
- 16.Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, Fox KA. Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Arch Intern Med. 2003;163:2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 17.Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW, King SB, III, Ohman EM, Stone GW. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49:1362–1368. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Mehta SR, Granger CB, Eikelboom JW, Bassand JP, Wallentin L, Faxon DP, Peters RJ, Budaj A, Afzal R, Chrolavicius S, Fox KA, Yusuf S. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol. 2007;50:1742–1751. doi: 10.1016/j.jacc.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L, Joyner C, Fox KA. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. J Am Med Assoc. 2006;295:1519–1530. doi: 10.1001/jama.295.13.joc60038. [DOI] [PubMed] [Google Scholar]
- 20.Antman EM, Morrow DA, McCabe CH, Jiang F, White HD, Fox KA, Sharma D, Chew P, Braunwald E. Enoxaparin versus unfractionated heparin as antithrombin therapy in patients receiving fibrinolysis for ST-elevation myocardial infarction. Design and rationale for the Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment-Thrombolysis In Myocardial Infarction study 25 (ExTRACT-TIMI 25) Am Heart J. 2005;149:217–226. doi: 10.1016/j.ahj.2004.08.038. [DOI] [PubMed] [Google Scholar]