The invention of iPSC in 2006 has been heralded as a major breakthrough. Wenbin Deng explains the enormous potential of these cells for research into human diseases and development, and discusses the technical and regulatory hurdles to be overcome for their therapeutic use.
A catalyst for disease modelling, drug discovery and regenerative therapy
Human embryonic stem cells (hESCs) have captured the interest of scientists, the public and the media because of their potential to realize the goal of regenerative medicine, to aid drug discovery and to advance our knowledge about human development and biology. However, practical applications have been slow to come, owing to technical challenges and to the intense ethical and moral debate over the destruction of embryos that has led to legislation prohibiting or seriously curtailing the generation of hESC lines in some countries. Nevertheless, on 21 January 2009, the US Food and Drug Administration (FDA) approved the first Investigational New Drug Application for a therapy based on hESCs to repair spinal cord injury, which was developed by Geron (Menlo Park, CA, USA).
Because traditional techniques to generate hESCs use surplus IVF embryos, it is not possible to create patient or disease-specific stem-cell lines—a drawback that significantly diminishes their therapeutic usability. The recent breakthrough work of ‘reprogramming' human somatic cells to become pluripotent cells has therefore energized the field; in addition, it offers basic researchers and clinical scientists the possibility to generate patient or disease-specific stem-cell lines without the legal, regulatory and ethical problems that often go hand-in-hand with hESCs. In fact, because these reprogrammed cells are so similar in developmental potential to authentic hESCs, they are able to differentiate into all possible cell types representing all three embryonic germ layers (Takahashi & Yamanaka, 2006; Takahashi et al, 2007; Yu et al, 2007; Park et al, 2008a). They have therefore been named induced pluripotent stem cells (iPSCs).
The iPSC technology could become an extremely useful tool for basic research, therapeutic developments and other applications; for example, human stem cells could have a significant impact on drug development and toxicity tests to replace and refine animal experiments and tests. In addition to allaying public concern over the use of animals in research, the use of iPSCs could address those instances in which animals have not proven appropriate and safe models to predict the efficacy and toxicity of drug candidates in humans. The most notorious example of a drug deemed safe after animal trials but with devastating effects in humans is thalidomide, which had no effect on prenatal development in rodents but which caused severe developmental defects in human children whose mothers had taken it during pregnancy to relieve morning sickness. A more recent example is the 2006 clinical trial of the TGN1412 antibody to treat multiple sclerosis, rheumatoid arthritis and leukaemia. Although previous tests in various animals, including primates, had shown no adverse effects, the drug caused catastrophic systemic organ failure in all six human volunteers who took it. Differentiated cells or tissues derived from multipotent or pluripotent stem cells could therefore improve preclinical tests for drug efficacy and toxicity and could similarly help to replace many animals in toxicity tests.
…human stem cells could have a significant impact on drug development and toxicity tests to replace and refine animal experiments and tests
Stem cells are undifferentiated cells that not only retain their capacity for self-renewal and proliferation but are also able to generate progeny that can differentiate into multiple cell types. ESCs, which are isolated from the inner cell mass of the embryo at the blastocyst stage, have the potential to differentiate into all of the cell types in the organism, hence the name pluripotent stem cells.
By contrast, somatic stem cells from tissues such as the brain generally have a limited repertoire of developmental choices and are often called multipotent; they can differentiate into a limited number of cell types that make up the tissue of origin, for example neurons, astrocytes and oligodendrocytes. Although some reports indicate that somatic stem cells might have the ability to differentiate into other lineages, others suggest that this apparent trans-differentiation could be caused by fusion with other cell types. The true extent of somatic stem-cell plasticity and the potential utility of trans-differentiated cell types are still to be determined. Yet, the difficulty in obtaining a pure population of somatic stem cells and expanding these cells to sufficient quantities while maintaining the progenitor state remain significant obstacles to their use as cell sources for therapy, drug discovery and toxicity testing.
An alternative method to create patient-specific pluripotent stem cells is somatic cell nuclear transfer (SCNT). The key step of this technology is inserting the nucleus from a somatic cell—for instance, from a patient—into an enucleated oocyte, which, after appropriate stimulation, would then develop into an embryo that is genetically identical to the person from whom the original cell was taken. However, it does not fully circumvent ethical and legal problems as it still involves the destruction of an embryo; more importantly, it turned out to have a low success rate and requires an extraordinarily large amount of human oocytes to generate just a few stem-cell lines.
Recent advances to ‘reprogramme' differentiated somatic cells from mice and humans towards an undifferentiated state that closely resembles that of ESCs—turning back the clock in somatic cells without using embryos or oocytes—have therefore been heralded as a major breakthrough and could usher in a new era of science and medicine. In addition to their potential role in drug discovery and testing, iPSCs could be used to restore organ function after disease or injury by replacing damaged cells with healthy ones, or even to reconstruct entire tissues. They also offer a powerful tool for understanding a variety of diseases such as cancer, Alzheimer or multiple sclerosis, as well as the possibility to develop new therapeutic approaches based on their use. iPSCs, which are generated from the somatic cells of individual patients, could provide an ideal cell source for transplantation as the cells would avoid the problem of graft rejection by the patient's immune system and the need for immune suppression.
Recent advances to ‘reprogramme' differentiated somatic cells […] towards an undifferentiated state that closely resembles that of ESCs […] could usher in a new era of science and medicine
However, there are major obstacles that are preventing the development of iPSCs for therapy and drug discovery: we need to develop safe and efficient approaches to generate iPSCs from human patients and strategies to decrease the risk of tumours that result from the introduction of undifferentiated iPSCs.
Several methods have been used thus far to produce iPSCs, most of which involve the transfer of genes into the target cell. In 2006, Takahashi and colleagues (Takahashi & Yamanaka, 2006; Takahashi et al, 2007) were the first to report the generation of iPSCs through the simultaneous overexpression of the transcription factors Oct4, Sox2, Klf4 and c-Myc. Yu et al (2007) also reported in vitro reprogramming of human fibroblasts to iPSCs through viral overexpression of Oct4, Sox2, Nanog and Lin28. Last year, Carey et al (2009) reported a 10-fold more efficient generation of iPSCs by using a single polycistronic vector to transfer the reprogramming factors. To address cancer risk and other safety concerns, several other approaches have been devised to generate iPSCs, including non-integrating adenoviral apsproaches (Stadtfeld et al, 2008; Okita et al, 2008), the piggyBac transposon system, which, after inducing pluripotency, removes the transgenes from established iPSC lines (Woltjen et al, 2009; Kaji et al, 2009), the Cre/loxP recombination system (Soldner et al, 2009) and non-integrating ‘episomal' vectors to create iPSCs free of vector and transgene DNA (Yu et al, 2009).
All of these methods, however, are based on the transfer of foreign DNA into the target cell. To overcome the safety concerns raised by these methods, protein-based methods have been successfully developed and implemented (Zhou et al, 2009; Kim et al, 2009), although so far there have been no reports of methods that involve only chemical factors. However, much has been made of achievements in enhancing the efficiency and success rate of DNA or protein-based methods by using various chemical factors (Xu et al, 2008) and attempts are under way to conduct large-scale screening to identify additional chemicals that are capable of inducing pluripotency, as purely chemically reprogrammed iPSCs would be of tremendous clinical value.
So far, there have been no reports of RNA-based protocols to generate iPSCs, although a recent paper (Sul et al, 2009) described a method of “transcriptome-induced phenotype remodeling (TIPeR)” for cellular phenotype conversion, which might represent a new, ‘holistic' approach of cellular reprogramming. This method transfers the entire regulatory components from a target cell to a donor cell and achieves cellular reprogramming by manipulating ‘whole systems' rather than a small set of master genes. Given the problem of the oncogenic risk and other concerns associated with current DNA-based methods of generating iPSCs, this RNA-based technology might alleviate many safety concerns.
In addition to finding safer and more efficient methods to generate iPSCs, researchers will also need a better understanding of the logic behind cellular reprogramming. To unravel the black box of how cells differentiate and dedifferentiate, it might be useful to think of a cell as having reached a distinct molecular state that involves gene regulation, epigenetic modifications and cell physiology. If cell states are the phenotypic representations of molecular steady-states of a dynamic genome, how is it possible to move from one steady state to another during lineage switching or reprogramming into a pluripotent state? Understanding the sequence of changes involved in phenotypic reprogramming could help us to decipher the underlying molecular logic that regulates cellular development and reprogramming.
Understanding the sequence of changes involved in phenotypic reprogramming could help us to decipher […] cellular development and reprogramming
This in turn could help us to develop sophisticated applications whereby any cell can be turned into another for the purpose of tissue-specific regenerative processes and therapeutics. A recent example is the in vivo direct reprogramming of mature pancreatic exocrine cells into pancreas-specific β-cells (Zhou et al, 2008). Engineering somatic cells to switch to specific lineages of interest without passing through a stem-cell stage would be a highly promising approach for tissue-specific repair and regeneration after disease or injury.
iPSCs can now be generated from adult somatic cells with a few defined factors, but the rate at which differentiated cells can be successfully reprogrammed is low, suggesting the existence of ‘barriers' that limit the efficiency of reprogramming. Recent studies have shown that the inactivation or inhibition of p53—the ‘guardian of the genome'—can increase the efficiency of iPSC generation (Krizhanovsky & Lowe, 2009; Deng & Xu, 2009). These findings indicate that reprogramming somatic cells into iPSCs could come at the expense of genome stability and other mechanisms that control tumour progression, although the demonstration that iPSCs can grow into healthy, cancer-free, full-term live mice seems to mollify some of these fears (Zhao et al, 2009; Kang et al, 2009; Boland et al, 2009). Nevertheless, the question remains as to whether the reprogrammed iPSCs might exhibit compromised genome integrity in the absence of p53 and thus run the risk of becoming malignant. Disabling these roadblocks would greatly improve reprogramming efficiency, but at what cost? A challenge on the road to safer and more efficient reprogramming is to strike a delicate balance between reprogramming efficiency and cancer risk.
A challenge on the road to safer and more efficient reprogramming is to strike a delicate balance between reprogramming efficiency and cancer risk
In addition, iPSCs and hESCs also express high telomerase activity to sustain self-renewal and proliferation. hESC and iPSCs can therefore become immortal and cancerous; in fact, both have the ability to form teratoma—a special kind of cancer that makes cell types from all germ layers—when transplanted into immune-compromised mice. Thus, rigorous removal of remaining pluripotent cells is a significant challenge for future attempts to create ESC- or iPSC-derived tissue and cell transplants.
Despite the genetic similarities between humans and mice, physiological differences invariably affect pathologies: a genetic defect that produces a disorder in humans does not necessarily cause the same symptoms in mice. Human cell cultures are therefore an essential complement to animal models. Researchers have been growing human somatic cells in the laboratory for years in order to mimic various diseases, but the available techniques had significant shortcomings. Cells taken directly from patients typically have a limited lifespan when grown in the laboratory, which limits the studies for which they can be used. As a result, researchers often turn to modified, immortal cells, but such alterations inevitably change the physiology of the cells and can adulterate results.
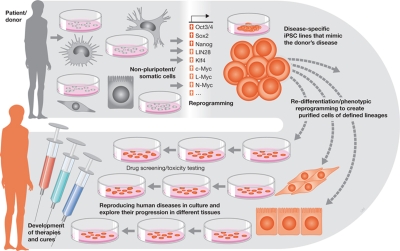
With iPSC technology, it is now possible to produce disease-specific stem cells that carry the genome of the donor and mimic human disease more reliably than do animal models. The ability to dedifferentiate patient-specific cells back to stem cells and to redifferentiate these cells into specific lineages will allow scientists to create in vitro models of diseases (Fig 1). Such a cellular U-turn might help to generate cell lines from many different patients to study the nature and complexity of disease. To reverse-engineer human disease would improve our understanding of the pathogenesis of diseases and could help to develop treatments. The most immediate application of disease-specific stem cells will be to reproduce human diseases in culture and to explore their progression in different tissues. The technique will also enable researchers to compare how the same disease varies among patients. Moreover, these cells will also offer a proving ground for drug screening and toxicity testing. Over the longer term, the technique might eventually allow us to develop therapies using a patient's own cells: reengineering the cells to correct disease-causing defects before reintroducing them into the body.
The most immediate application of disease-specific stem cells will be to reproduce human diseases in culture and to explore their progression in different tissues
Figure 1.
Cellular U-turn-based iPSC applications. Non-pluripotent/somatic cells obtained from a patient/donor can be reprogrammed by using defined factors to generate disease-specific iPSC lines that mimic the donor's disease. They can then be subjected to re-differentiation or phenotypic reprogramming to create purified cells of defined lineages for use in drug screening and toxicity testing, to reproduce human diseases in culture and explore their progression in different tissues, or for the development of therapies and cures.
Indeed, recent studies have shown the initial success of 20 different disease-specific iPSC lines (Park et al, 2008b) and patient-derived iPSCs for amyotrophic lateral sclerosis (Dimos et al, 2008), spinal muscular atrophy (Ebert et al, 2008), Parkinson disease (Soldner et al, 2009), Fanconi anaemia (Raya et al, 2009) and familial dysautonomia (Lee et al, 2009)—convincing proof-of-concept for the use of iPSC technology.
The iPSC technology is also an exploratory tool and represents a platform for a variety of basic and applied investigations. For instance, our understanding of the regulation of stem cells has been centred primarily on the nucleus and little is known about the role of mitochondria in regulating stem-cell behaviour. With iPSC technology it is feasible to investigate the variability of mitochondrial function in different cell lines. This can be achieved by reprogramming somatic cells from healthy individuals from different age groups or people with inherited mitochondrial diseases to evaluate how the state of mitochondrial structure and function influences the ability of iPSCs to generate multiple, distinct cell lineages. As mitochondrial fusion and fission dynamics can drive the cell cycle, alterations to this machinery might also be crucial for hESC and/or iPSC pluripotency. This could enhance our ability to manipulate hESC and iPSCs for designing efficient cell-based therapies and to understand mitochondrial function in development and disease. Although primarily exploratory in nature, the new perspectives and approaches to study human mitochondrial function by a reverse engineering approach through the generation of iPSCs would probably lead to important results with both scientific and medical implications.
Although iPSCs share many features with ESCs, they might not be identical (Chin et al, 2009). Furthermore, the generation of iPSCs is still not efficient and raises a number of safety concerns as discussed above. Thus, although iPSCs have enormous potential to substitute hESCs on many fronts and to generate genetically diverse and patient-specific pluripotent stem-cell populations, more research is needed to realize this potential.
Looking ahead, the rapid development of iPSC technology will surely enable the creation of banks of stem-cell lines with standardized protocols and methods for consistent differentiation of stem cells into stable homogeneous populations suitable for drug testing in high-throughput platforms. Further ahead, stem-cell-based therapeutic approaches are an attractive option for many currently incurable diseases.
However, there are also regulatory issues regarding hESC and iPSC safety for clinical use. Current protocols for directed differentiation of hESCs and/or iPSCs into many specific cell types are still stochastic with low efficiency, and the cells have not been proven suitable for clinical use. There is a pressing need to develop better, more efficient and specific methods for generating purified cells of defined lineages. Another preclinical hurdle, although by no means the only one, is scaling-up the production of clinical-grade cells by using good manufacturing practice protocols and testing their efficacy and safety in animal models.
There is a pressing need to develop better, more efficient and specific methods for generating purified cells of defined lineages
Human stem cells hold great promise for the future, but their implementation in the clinic is fraught with biological pitfalls and safety concerns. Addressing the remaining questions concerning their development and use will require more basic research into the development of directed differentiation protocols, animal modelling and testing in rodents and non-human primates, transplantation and immunology, safety and toxicology, quality control and assurance, and the regulation of cell products. It will require the efforts, expertise, resources and technologies of numerous research areas to move iPSC technology from the laboratory to preclinical applications and testing, all the way to clinical trials and eventual use to improve the lives of many for whom the current prognosis is poor.

Wenbin Deng
Acknowledgments
The author thanks Dr David Pleasure for his critical comments. The work was supported in part by grants from the National Institutes of Health (R01 NS059043 and R01 ES015988), National Multiple Sclerosis Society and Shriners Hospitals for Children.
Footnotes
The author declares that he has no conflict of interest.
References
- Boland MJ, Hazen JL, Nazor KL, Rodriguez AR, Gifford W, Martin G, Kupriyanov S, Baldwin KK (2009) Adult mice generated from induced pluripotent stem cells. Nature 461: 91–94 [DOI] [PubMed] [Google Scholar]
- Carey BW, Markoulaki S, Hanna J, Saha K, Gao Q, Mitalipova M, Jaenisch R (2009) Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc Natl Acad Sci USA 106: 157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MH et al. (2009) Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5: 111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Xu Y (2009) Genome integrity: linking pluripotency and tumorgenicity. Trends Genet 25: 425–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos JT et al. (2008) Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321: 1218–1221 [DOI] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN (2008) Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457: 277–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K (2009) Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 458: 771–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Wang J, Zhang Y, Kou Z, Gao S (2009) iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell 5: 135–138 [DOI] [PubMed] [Google Scholar]
- Kim D et al. (2009) Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 4: 472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Lowe SW (2009) Stem cells: the promises and perils of p53. Nature 460: 1085–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G et al. (2009) Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461: 402–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S (2008) Generation of mouse induced pluripotent stem cells without viral vectors. Science 322: 949–953 [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ (2008a) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451: 141–146 [DOI] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ (2008b) Disease-specific induced pluripotent stem cells. Cell 134: 877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya A et al. (2009) Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature 460: 53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner F et al. (2009) Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136: 964–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K (2008) Induced pluripotent stem cells generated without viral integration. Science 322: 945–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul JY et al. (2009) Transcriptome transfer produces a predictable cellular phenotype. Proc Natl Acad Sci USA 106: 7624–7629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872 [DOI] [PubMed] [Google Scholar]
- Woltjen K et al. (2009) piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458: 766–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Shi Y, Ding S (2008) A chemical approach to stem-cell biology and regenerative medicine. Nature 453: 338–344 [DOI] [PubMed] [Google Scholar]
- Yu J et al. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920 [DOI] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA (2009) Human induced pluripotent stem cells free of vector and transgene sequences. Science 324: 797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XY et al. (2009) iPS cells produce viable mice through tetraploid complementation. Nature 461: 86–90 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA (2008) In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455: 627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H et al. (2009) Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4: 381–384 [DOI] [PMC free article] [PubMed] [Google Scholar]