Mammals and insects use different strategies to detect similar chemicals. Silbering and Benton consider possible reasons for this dichotomy, taking into account biophysical, cell biological, ecological and evolutionary influences on how information is extracted from chemosensory cues.
Keywords: olfaction, gustation, receptor, GPCR, ion channel
Abstract
Chemosensory receptors convert an enormous diversity of chemical signals from the external world into a common language of electrical activity in the brain. Mammals and insects use several families of transmembrane receptor proteins to recognize distinct classes of volatile and non-volatile chemicals that are produced by conspecifics or other environmental sources. A comparison of the signalling mechanisms of mammalian and insect receptors has revealed an unexpected functional distinction: mammals rely almost exclusively on metabotropic ligand-binding receptors, which use second messenger signalling cascades to indirectly activate ion channels, whereas insects use ionotropic receptors, which are gated directly by chemical stimuli, thereby leading to neuronal depolarization. In this review, we consider possible reasons for this dichotomy, taking into account biophysical, cell biological, ecological and evolutionary influences on how information is extracted from chemosensory cues by these animal classes.
See Glossary for abbreviations used in this article.
Glossary.
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- CNG
cyclic nucleotide-gated channel
- GPCR
G-protein-coupled receptor
- GR
gustatory receptor (invertebrates)
- GUR
gustatory receptor family (Caenorhabditis elegans)
- iGluR
ionotropic glutamate receptor
- IR
ionotropic receptor
- OR
odorant receptor
- T1R
taste receptor type 1
- T2R
taste receptor type 2
- TRC
taste receptor cell
- TRP
transient receptor potential
- V1R
vomeronasal receptor type 1
- V2R
vomeronasal receptor type 2
Introduction
Anyone who has extracted a drowning fly from a glass of wine, swatted a wasp from a pot of jam or stamped on an army of ants approaching a picnic basket will have a keen appreciation for the common attractiveness of a multitude of chemosensory cues to insects and mammals. Similarly, many poisonous compounds that are often found in plants trying to evade predation are aversive to both animal classes. Such parallels naturally reflect the basic need of organisms with a largely conserved cellular metabolism to identify nutritional food sources and avoid intoxication. Advances in our understanding of the neuroanatomical logic and physiological coding properties of insect and mammalian olfactory and gustatory systems—which are normally associated with the detection of volatile and non-volatile stimuli, respectively—have revealed important commonalities in how insects and mammals process and represent chemical signals in the brain. These similarities are suggestive either of the evolution of these chemosensory systems from those present in a common ancestral animal, or of their convergent shaping by the same selective constraints, defined by their role in mediating odour-evoked and taste-evoked behaviours (Ache & Young, 2005; Benton, 2009; Hildebrand & Shepherd, 1997; Scott, 2005; Strausfeld & Hildebrand, 1999; Su et al, 2009; Yarmolinsky et al, 2009).
However, recent investigations into the molecular mechanisms of chemosensory signalling have revealed an unexpected dichotomy: mammals (and vertebrates in general) almost exclusively use metabotropic chemosensory receptors—that is, the ligand-binding receptors indirectly activate ion channels through second messengers—whereas insects (and possibly all arthropods) might predominantly use ionotropic mechanisms, in which the primary chemosensory receptors are ligand-gated ion channels (Pellegrino & Nakagawa, 2009; Spehr & Munger, 2009; Touhara & Vosshall, 2009).
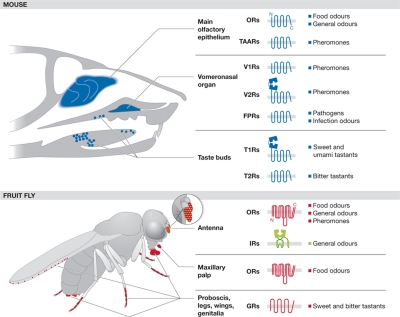
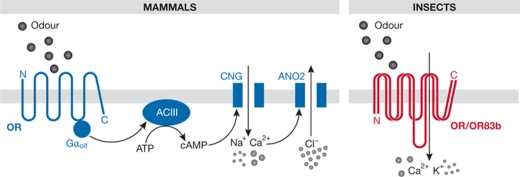
In mice, for example, five families of olfactory sensory receptors are now known: ORs and trace amine-associated receptors, which are expressed in olfactory sensory neurons (OSNs) in the main olfactory epithelium, and V1R, V2R and formyl peptide receptors, which are expressed in the vomeronasal organ—an accessory olfactory system believed to be dedicated mainly to pheromone recognition (Fig 1; Spehr & Munger, 2009; Touhara & Vosshall, 2009). These five families belong to the GPCR superfamily of seven transmembrane domain proteins that signal through the activation of heterotrimeric G proteins. Olfactory signal transduction has been best defined for ORs (Fig 2; Kleene, 2008), which are coupled to an olfactory G-protein subunit (Gαolf). Gαolf activates adenylyl cyclase III, leading to cAMP production, which binds to and opens a multisubunit CNG. The influx of Ca2+ through this channel promotes the opening of a calcium-gated chloride channel—which is probably anoctamin 2 (Stephan et al, 2009)—and the combined effect of calcium influx and chloride efflux leads to OSN depolarization. The olfactory GPCR families are not slight variants of each other, but instead represent distinct and evolutionarily ancient clades that are discernible across vertebrates (Nei et al, 2008; Shi & Zhang, 2009). Furthermore, their expression in different subsets of olfactory neurons or organs and their responsiveness to specific classes of ligand imply that they fulfil distinct functions in odour perception (Fig 1; Spehr & Munger, 2009; Su et al, 2009; Touhara & Vosshall, 2009).
Figure 1.
The main chemosensory organs, receptors and putative ligands in the mouse and the fruit fly. The image of the mouse head was adapted from Matsunami & Amrein (2003). FPRs, formyl peptide receptors; GRs, gustatory receptors; IRs, ionotropic receptors; ORs, odorant receptors; T1Rs, taste receptors type 1; T2Rs, taste receptors type 2; TAARs, trace amine-associated receptors; V1Rs, vomeronasal receptors type 1; V2Rs, vomeronasal receptors type 2.
Figure 2.
Signalling mechanisms of mammalian and insect odorant receptors. A schematic of the molecular basis of olfactory signal transduction in the mouse and fruit fly. ACIII, type III adenylyl cyclase; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; ANO2, anoctamin 2 channel; CNG, cyclic nucleotide-gated channel;.Gαolf, olfactory G protein α-subunit; OR, odorant receptor.
Most receptors underlying mammalian taste perception are also GPCRs, including the T1R family, which recognizes sweet and umami stimuli, and the T2Rs, which underlie bitter taste detection (Chandrashekar et al, 2006; Yarmolinsky et al, 2009). These families also define distinct subclassess of GPCR, although T1Rs are distantly related to V2Rs.
Insect chemosensory receptors have been best characterized at a molecular level in the fruit fly Drosophila melanogaster (Benton, 2008; Vosshall & Stocker, 2007). These proteins localize to the ciliated endings of sensory neuron dendrites, housed in porous cuticular hairs called sensilla, that cover the external surface of chemosensory organs (Fig 1). ORs are expressed in the olfactory organs, the antenna and maxillary palp, whereas GRs are expressed predominantly in the proboscis and various other contact chemosensors on the legs, wings and genitalia (Fig 1; Stocker, 1994). Drosophila ORs and GRs are related families of polytopic transmembrane proteins that appear to be largely arthropod-specific or—in the case of ORs—insect-specific (Penalva-Arana et al, 2009; Robertson et al, 2003). Although they are predicted to contain seven transmembrane domains, they are unrelated in sequence to GPCRs; structural analysis has shown they adopt a distinct membrane topology, with intracellular amino-termini, which is probably shared by GRs (Benton et al, 2006; Lundin et al, 2007). A functional analysis of ORs has provided compelling evidence that their primary transduction mechanism is ionotropic (Fig 2), and that a complex of a ligand-binding OR and the OR co-receptor OR83b—which is essential for subcellular localization and function (Benton et al, 2006; Larsson et al, 2004)—acts as an odour-gated ion channel (Sato et al, 2008; Smart et al, 2008; Wicher et al, 2008). Although the involvement of G proteins and second messengers downstream from insect ORs has been studied intensively over the past decade, their contribution to odour sensing remains unclear, and in vivo they might have a principally modulatory role. These issues have been discussed in detail elsewhere (Benton, 2008; Nakagawa & Vosshall, 2009; Pellegrino & Nakagawa, 2009; Ronderos & Smith, 2009). Little is known about how GRs transduce signals, but their homology to ORs makes it plausible that these receptors also function as ion channels.
Recently, a third family of Drosophila chemosensory receptors—the IRs—has been identified (Benton et al, 2009). IRs were named for their homology to iGluRs, a class of ligand-gated ion channels best characterized for their role in mediating synaptic transmission. Importantly, IRs contain divergent ligand-binding domains that lack glutamate-interacting residues. The conservation of the ion channel domain in IRs suggests that these receptors signal ionotropically, although this is yet to be confirmed.
Here, we consider the potential molecular, physiological, evolutionary and ecological reasons for why mammals and insects seem to use fundamentally different chemosensory receptors, although we acknowledge that formal proof of the signalling mechanism of several mammalian and insect receptor families is still unavailable (Sidebar A). A reflection on these possibilities also leads to verifiable hypotheses to illuminate further the emerging mechanistic distinctions.
Sidebar A | In need of answers.
Do insect ORs and GRs function exclusively as ligand-gated ion channels in vivo? Do they also couple to second messenger cascades?
How are insect chemosensory-receptor-evoked neuronal signals amplified and terminated at the molecular level?
How conserved are the molecular mechanisms of signal transduction between different vertebrate chemosensory GPCRs?
How does regulation of mammalian chemosensory signalling contribute to higher order perception of stimuli?
Do insect IRs and GRs, and mammalian taste receptors, form heteromeric complexes in vivo and, if so, what is the precise role of different subunits within these complexes?
When did insect and mammalian chemosensory receptors evolve, and what were the genetic ancestors of these different repertoires?
All in the timing?
Ionotropic and metabotropic signalling pathways are fundamentally different in their temporal properties. The former are usually faster, operating on a millisecond (ms) to sub-ms timescale, as ligand binding directly gates the ion channel. By contrast, metabotropic receptors have a longer latency, from a few tens to several hundred ms, owing to the necessity to produce second messengers and activate secondary effectors. However, as a consequence of these downstream effects, metabotropic signalling can have a much longer duration, from a few seconds to several minutes. Do such temporal distinctions occur in insect and mammalian chemosensory systems? Are they important for how chemical stimuli are detected?
Most available data concerns ORs, although comparison among studies is complicated by the use of different animal models, experimental systems and the receptors analysed. Insect ORs can produce odour-evoked currents with very short latencies (<20 ms) when expressed in heterologous cells (Sato et al, 2008), which are similar to the latencies of OR-dependent, stimulus-evoked action potentials in OSNs in vivo (<30 ms; de Bruyne et al, 1999). By contrast, electrophysiological studies of isolated mammalian OSNs, or those in an intact olfactory epithelium, have revealed much higher latencies of odour-evoked currents—from about 90 ms to several hundred ms (Firestein et al, 1993; Grosmaitre et al, 2006; Kleene, 2008; Spors et al, 2006). Metabotropic signalling cascades can achieve faster reaction times, as observed in the Drosophila visual system—in which response latency can be as short as 20 ms—but this example depends on a sophisticated scaffolding mechanism for phototransduction signalling components and might be unique (Hardie & Raghu, 2001). The rapid reactions of insect OSNs to environmental odours are certainly also assisted by peripheral morphological specializations. The most evident of these is the ‘everted' nature of the insect nose, the sensory cilia of which are separated from external odours often by less than a micrometre. By contrast, most mammalian OSNs are separated from the environment by long nasal cavities.
The reason behind the rapid responses of insects might be the dynamics of odour stimuli that these small animals experience. Volatile chemicals are released from their sources in the form of plumes, which are characterized by the alternation of odour strands of high concentration—which last from as little as 10–20 ms in a given position—with ‘clean' air gaps (Kaissling et al, 1987). To locate an odour source, flying insects sample the air frequently to determine their position in either odour strands or odourless air, while also determining the direction of the wind carrying these strands (Budick & Dickinson, 2006; Carde & Willis, 2008). In essence, insect OSNs—at least in this context—act as flux detectors rather than concentration sensors (Baker, 2009). Analyses of insect OSN responses to artificial odour plumes have demonstrated their ability to rapidly track the presence or absence of odours through changes in action potential frequency (Barrozo & Kaissling, 2002; Schuckel et al, 2009). Such fast behavioural responses require both the onset and the termination of cellular responses to the stimuli to be fast. In these cases, an ionotropic mechanism might also be advantageous, because the dissociation of an odour molecule from the receptor could lead to rapid channel closure. By contrast, the termination of metabotropic responses requires the degradation of second messenger molecules, introducing a lag between the offset of the stimulus and of the response. An efficient enzymatic inactivation of odours inside sensilla probably provides a complementary mechanism that promotes fast signal termination (Ishida & Leal, 2005).
The situation is different in mammals, in which odours are actively drawn into the nasal cavity by sniffing. This modified respiratory phase could be a means to increase stimulus concentration (Wesson et al, 2009) or to improve detection at near-threshold concentrations (Oka et al, 2009). Sniffing completely changes stimulus dynamics, possibly obviating a need for temporally sensitive OSN responsiveness. Metabotropic signalling mechanisms could in fact allow the integration of signals over time, such that increases in stimulus duration result in comparable increases in the magnitude of the neuronal response to those evoked by higher stimuli concentrations presented over a proportionally shorter time period (Firestein et al, 1993; Takeuchi & Kurahashi, 2002). This integrative capacity might enhance the sensitivity of the vertebrate olfactory system.
Notably, although the latency of mammalian OSN responses is longer compared with that of insect OSNs, it does not necessarily constrain the timing of behavioural responses. A single sniff can result in highly accurate behavioural decision-making in rats in less than 200 ms (Uchida & Mainen, 2003), not much more than the fastest documented reaction time of a moth to a sex pheromone (150 ms; de Bruyne & Baker, 2008). Thus, the use of ionotropic or metabotropic mechanisms could simply be related to which aspects of olfactory information are first collected—such as the presence or absence of a stimulus, or its precise concentration—rather than how quickly an animal reacts to it.
Amplifying and adapting to odours
Although ionotropic chemosensory receptors provide an elegantly simple way to convert chemical detection into neuronal activation, the multicomponent nature of metabotropic signalling pathways could allow for a more sophisticated regulation of odour-evoked neuronal currents. Signal amplification is one broadly accepted advantage of metabotropic GPCR signalling, which is conferred by the ability of a single cell surface receptor to activate multiple G proteins, each of which can activate several downstream effectors to lead to the production of many second-messenger molecules. However, vertebrate olfactory GPCRs have a surprisingly low probability of activating even a single G protein owing to the brief odorant dwell-time (Bhandawat et al, 2005); higher affinity ligand–receptor combinations could, of course, have different properties. Although the amplification of olfactory signals might not happen at the receptor level, it clearly occurs further down the pathway, during the activation of CNG and chloride channels (Kleene, 1997). As in other contexts, signal amplification presumably increases the overall sensitivity of vertebrate chemosensory neurons.
The metabotropic olfactory receptor cascade—and probably that of other vertebrate chemosensory GPCR mechanisms—provides numerous points of regulation for the termination or adaptation of odour-evoked signals. These might act directly on the receptor, for example through phosphorylation by G-protein-coupled receptor kinases and binding of β-arrestins (Dawson et al, 1993), but essentially every known component of the cascade—Gαolf, adenylyl cyclase III, cAMP, CNG and Ca2+—has intrinsic or extrinsic mechanisms for downregulation or modulation (Kleene, 2008). Such a complex regulatory network undoubtedly shapes the dynamics of odour-evoked signals, ultimately having an impact on how these are represented and interpreted in the brain (Laurent, 2002; Spors et al, 2006).
Ionotropic insect ORs presumably cannot amplify odour-evoked signals directly, although one in vitro study indicates that the secondary activation of a metabotropic cAMP/cGMP-dependent pathway by ORs could feedback positively on the co-receptor OR83b to produce a more sustained and larger current response to a stimulus (Wicher et al, 2008). Insect sensory neurons do not seem to have a higher detection threshold than their vertebrate equivalents, however, and even single pheromone molecules are enough to elicit OSN activity in moths (Kaissling & Priesner, 1970). Such observations indicate that the coupling between insect receptors and spike generation can be extremely efficient. Signal amplification in insects could occur mainly at the first olfactory synapse, where relatively weak and variable spike trains in OSNs stimulated by low odour concentrations are transformed into amplified and temporally robust responses in second-order neurons with no appreciable delay (Bhandawat et al, 2007; Schlief & Wilson, 2007; Wilson et al, 2004).
Electrical signal modulation, such as termination and adaptation, has been reported in insect chemosensory systems (de Bruyne & Baker, 2008), but its molecular basis is not understood. However, the intracellular regulation of ion channels by a variety of second messengers—such as ions, cyclic nucleotides and lipids—is well-characterized in many other contexts (Damann et al, 2008). Thus it is possible that ionotropic insect chemosensory receptors are major targets for regulatory cascades. Clearly, much remains to be determined to fully appreciate and compare the modulatory capacities of metabotropic and ionotropic signalling pathways, and their biological significance for chemosensory perception.
‘Combinatorial coding' in receptor complexes
Historically, there has been substantial interest in determining how many types of chemosensory receptor are expressed in a particular sensory neuron—or, for the mammalian gustatory system, a non-neuronal TRC—as this has allowed the inference of its potential breadth of tuning and discriminability of ligands. However, studies of metabotropic and ionotropic receptors in other contexts have revealed many cases in which co-expressed subunits function together, rather than independently, to create heteromeric complexes with properties not exhibited by any individual subunit (Hille, 2001; Milligan, 2007). Do chemosensory GPCRs and ion channels use subunit combinations to define or expand their functional properties?
Most mammalian olfactory GPCRs are probably expressed uniquely in a given OSN, which indicates that heteromeric complex formation is unlikely (Mombaerts, 2004). Studies of ORs in heterologous systems indicate that some can form complexes with other non-olfactory GPCRs but the physiological relevance of these complexes in vivo has not been elucidated (Bush et al, 2007; Hague et al, 2004).
By contrast, mammalian gustatory GPCRs are one of the most striking examples of GPCR heteromerization, which has an impact on their functional properties (Chandrashekar et al, 2006; Yarmolinsky et al, 2009). Sweet-sensing TRCs express two T1R family members: T1R2 and T1R3, which interact physically in vitro and are both necessary—and together sufficient—to mediate responses to sweet tastants in vivo (Nelson et al, 2001). A distinct population of TRCs dedicated to umami taste also express T1R3 in combination with T1R1; T1R1 can also form a complex with T1R3 in vitro and both receptors are necessary—and together sufficient—to mediate umami responses in vivo (Nelson et al, 2002). Thus, the exchange of one GPCR subunit—that is, T1R1 for T1R2—can radically alter substrate selectivity. However, T1R3 is not simply a ‘silent' partner, as the ligand-binding domains of both T1R2 and T1R3 can interact with sugars in vitro (Nie et al, 2005), and T1R3 can alone mediate responses to a high concentration of sugars in vivo (Zhao et al, 2003).
T2R bitter taste receptors exhibit the opposite extreme to olfactory receptors: the entire repertoire is potentially co-expressed in every TRC (Chandrashekar et al, 2006; Yarmolinsky et al, 2009). Different T2Rs recognize different bitter ligands and there is no evidence that they function in a combinatorial fashion. Thus, co-expression might confer a broad response profile on each individual bitter-sensing TRC.
The many examples of receptor co-expression for both olfactory and gustatory ionotropic insect receptors hint at the importance of combinations in sensory function. The best-studied case is that of OR83b, a highly conserved member of the repertoire that seems to be co-expressed with all other ORs (Benton et al, 2006; Larsson et al, 2004). OR83b forms a heteromeric complex with ligand-binding ORs and is essential to target them to the sensory cilia (Benton et al, 2006). OR83b might also form an integral part of the ion channel pore (Wicher et al, 2008). Assigning central cellular and signalling functions to a single member of the repertoire might allow ligand-specific ORs greater flexibility to evolve new odour specificities without compromising the signal transduction function of the OR–OR83b heteromer.
The dependence of insect sugar-sensing and bitter-sensing neurons on taste receptor combinations is probably more complex than that of their counterparts in mammals, as both types of sensory neuron express several (∼5–10) different GRs, and loss-of-function genetic studies have demonstrated the requirement for up to three different receptors in mediating responses to specific tastants (Montell, 2009). Moreover, no in vivo reconstitution of taste receptors has been reported, suggesting that a functional receptor complex incorporates additional GRs.
An initial expression map of IRs revealed that some neurons might express 2–5 receptors, which is consistent with at least some IRs acting in heteromeric complexes (Benton et al, 2009). This would be analogous to the function of iGluRs in many variants of heterotetrameric complexes, the precise subunit composition of which is crucial in defining transport properties, ligand specificity, permeability and desensitization dynamics (Mayer & Armstrong, 2004). Could subunit-dependent properties also be relevant to insect chemosensory receptor complexes? Varied receptor-dependent temporal dynamics of insect OSN responses have been reported both in IR-expressing and OR-expressing neurons (de Bruyne et al, 2001; Hallem et al, 2004; Yao et al, 2005). Perhaps the heterogeneity in insect chemosensory receptor complexes compensates for the lack of downstream signalling components, to define neuron-specific dynamic properties of ligand-evoked responses.
In conclusion, although subunit combinations might be used by both GPCR and ionotropic chemosensory receptors, there seems to be greater potential for ion channel subunits to combine in functionally distinctive ways.
A just-so story?
Beyond mechanistic explanations, the dichotomy in signalling strategies could reflect a mere chance of evolution. Chemosensory repertoires can rapidly expand and diversify, as shown by the dramatic differences that exist between even closely related species (Nei et al, 2008). Therefore, the crucial event in determining the class of receptor that provides chemosensory abilities is probably the initial selection of a founding chemosensory receptor gene. Both GPCRs and ion channels are ancient protein families that were present before the divergence of animals. Thus, the genetic substrates to make this ‘choice' of chemosensory signalling mechanism were certainly available to the ancestors of insects and mammals. As insects seem to be the exception of animal chemosensory transduction, we will consider the origins of their receptor repertoires.
The IRs could have evolved very early, as iGluR/IR-like genes are present across animal, plant and prokaryotic genomes (Chiu et al, 1999). In bacteria, it is probable that iGluRs have at least an analogous role to IRs in peripheral chemosensing of amino acids (Chen et al, 1999). Whether the ancestral function of animal iGluRs was in synaptic transmission and Drosophila evolved divergent members to fulfil roles in environmental chemical sensing, or if ancestral chemosensing iGluRs/IRs were progressively lost and/or specialized in mammals as GPCRs became predominant in providing their olfactory and gustatory needs, is unknown. Comparative genomics and expression analysis of iGluRs/IRs across metazoans could distinguish between these possibilities.
The dissimilarity of insect ORs and GRs to known classes of ion channel prevents definitive conclusions of their evolutionary history, but the existence of a few distant relatives in the nematode worm Caenorhabditis elegans—the GURs (Robertson et al, 2003)—indicates that they were present in the common ancestor of ecdysozoans. However, whether GURs also function in chemosensation is unclear (Edwards et al, 2008; Moresco & Koelle, 2004), and this organism has an enormous number of chemosensory GPCRs (Thomas & Robertson, 2008). C. elegans has an atypical chemosensory system organization, with dozens of receptor genes co-expressed in individual sensory neurons, which couple to common signal transduction cascades (Bargmann, 2006). Perhaps such organization, in which different receptor proteins are competing for space in the limited ciliary membranes, demands the amplification mechanisms offered by metabotropic signalling to produce sensitive responses. Thus, although nematode ancestors potentially had the choice of GPCRs and insect-like chemosensory receptors, the former class ultimately fulfilled the requirements for chemical sensing by their particular nervous systems.
Finally, we note that the independent appearance of two fundamentally different chemosensory ion channel families in insects—GR/ORs and IRs—at potentially different times in evolution, argues against the emergence of the observed dichotomy by ‘chance', and rather points towards the specific mechanistic advantages of the ionotropic signalling mechanism for insect chemosensation.
Closing remarks
Although we have highlighted the distinctions between insects and mammals, there is evidence for some conserved chemosensory receptors, such as members of the ionotropic TRP channel repertoire, which have been variously implicated in the perception of sour, pungent or spicy chemical stimuli in mice and Drosophila (Damann et al, 2008), and guanylyl cyclases, which mediate detection of various environmental gases in these animal classes through the cGMP second messenger (Luo et al, 2009). The existence of such parallels is consistent with the significant molecular conservation observed in other sensory modalities—such as opsins in the visual system (Fernald, 2006)—and central neuronal communication mechanisms (Ryan & Grant, 2009), and reinforces the need to consider why the fly on your banana smells and tastes it—at least mechanistically—quite differently from you.

Ana Florencia Silbering

Richard Benton
Acknowledgments
We thank Giovanni Galizia, Carolina Gomez-Diaz, Yael Grosjean, Sophie Martin, Takao Nakagawa, Maurizio Pellegrino, Pavan Ramdya and Haiqing Zhao for comments on the manuscript. Research in R.B.'s laboratory is supported by the University of Lausanne, a European Research Council Starting Independent Researcher Grant and the Swiss National Science Foundation. We apologize to colleagues for being unable to cite all relevant primary literature owing to space constraints.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ache BW, Young JM (2005) Olfaction: diverse species, conserved principles. Neuron 48: 417–430 [DOI] [PubMed] [Google Scholar]
- Baker TC (2009) Representations of odor plume flux are accentuated deep within the moth brain. J Biol 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI (2006) Chemosensation in C. elegans. WormBook Oct 25: 1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrozo RB, Kaissling KE (2002) Repetitive stimulation of olfactory receptor cells in female silkmoths Bombyx mori L. J Insect Physiol 48: 825–834 [DOI] [PubMed] [Google Scholar]
- Benton R (2008) Chemical sensing in Drosophila. Curr Opin Neurobiol 18: 357–363 [DOI] [PubMed] [Google Scholar]
- Benton R (2009) Eppendorf winner: evolution and revolution in odor detection. Science 326: 382–383 [DOI] [PubMed] [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB (2006) Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol 4: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB (2009) Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136: 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandawat V, Reisert J, Yau KW (2005) Elementary response of olfactory receptor neurons to odorants. Science 308: 1931–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI (2007) Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci 10: 1474–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budick SA, Dickinson MH (2006) Free-flight responses of Drosophila melanogaster to attractive odors. J Exp Biol 209: 3001–3017 [DOI] [PubMed] [Google Scholar]
- Bush CF, Jones SV, Lyle AN, Minneman KP, Ressler KJ, Hall RA (2007) Specificity of olfactory receptor interactions with other G protein-coupled receptors. J Biol Chem 282: 19042–19051 [DOI] [PubMed] [Google Scholar]
- Carde RT, Willis MA (2008) Navigational strategies used by insects to find distant, wind-borne sources of odor. J Chem Ecol 34: 854–866 [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS (2006) The receptors and cells for mammalian taste. Nature 444: 288–294 [DOI] [PubMed] [Google Scholar]
- Chen GQ, Cui C, Mayer ML, Gouaux E (1999) Functional characterization of a potassium-selective prokaryotic glutamate receptor. Nature 402: 817–821 [DOI] [PubMed] [Google Scholar]
- Chiu J, DeSalle R, Lam HM, Meisel L, Coruzzi G (1999) Molecular evolution of glutamate receptors: a primitive signaling mechanism that existed before plants and animals diverged. Mol Biol Evol 16: 826–838 [DOI] [PubMed] [Google Scholar]
- Damann N, Voets T, Nilius B (2008) TRPs in our senses. Curr Biol 18: R880–889 [DOI] [PubMed] [Google Scholar]
- Dawson TM, Arriza JL, Jaworsky DE, Borisy FF, Attramadal H, Lefkowitz RJ, Ronnett GV (1993) β-adrenergic receptor kinase-2 and β-arrestin-2 as mediators of odorant-induced desensitization. Science 259: 825–829 [DOI] [PubMed] [Google Scholar]
- de Bruyne M, Baker TC (2008) Odor detection in insects: volatile codes. J Chem Ecol 34: 882–897 [DOI] [PubMed] [Google Scholar]
- de Bruyne M, Clyne PJ, Carlson JR (1999) Odor coding in a model olfactory organ: the Drosophila maxillary palp. J Neurosci 19: 4520–4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyne M, Foster K, Carlson JR (2001) Odor coding in the Drosophila antenna. Neuron 30: 537H–552H [DOI] [PubMed] [Google Scholar]
- Edwards SL, Charlie NK, Milfort MC, Brown BS, Gravlin CN, Knecht JE, Miller KG (2008) A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol 6: e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald RD (2006) Casting a genetic light on the evolution of eyes. Science 313: 1914–1918 [DOI] [PubMed] [Google Scholar]
- Firestein S, Picco C, Menini A (1993) The relation between stimulus and response in olfactory receptor cells of the tiger salamander. J Physiol 468: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmaitre X, Vassalli A, Mombaerts P, Shepherd GM, Ma M (2006) Odorant responses of olfactory sensory neurons expressing the odorant receptor MOR23: a patch clamp analysis in gene-targeted mice. Proc Natl Acad Sci USA 103: 1970–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague C, Uberti MA, Chen Z, Bush CF, Jones SV, Ressler KJ, Hall RA, Minneman KP (2004) Olfactory receptor surface expression is driven by association with the β2-adrenergic receptor. Proc Natl Acad Sci USA 101: 13672–13676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR (2004) The molecular basis of odor coding in the Drosophila antenna. Cell 117: 965–979 [DOI] [PubMed] [Google Scholar]
- Hardie RC, Raghu P (2001) Visual transduction in Drosophila. Nature 413: 186–193 [DOI] [PubMed] [Google Scholar]
- Hildebrand JG, Shepherd GM (1997) Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci 20: 595–631 [DOI] [PubMed] [Google Scholar]
- Hille B (2001) Ion Channels of Excitable Membranes 3rd edn. Sunderland, MA, USA: Sinauer [Google Scholar]
- Ishida Y, Leal WS (2005) Rapid inactivation of a moth pheromone. Proc Natl Acad Sci USA 102: 14075–14079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaissling KE, Priesner E (1970) Smell threshold of the silkworm. Naturwissenschaften 57: 23–28 [DOI] [PubMed] [Google Scholar]
- Kaissling KE, Zack Strausfeld C, Rumbo ER (1987) Adaptation processes in insect olfactory receptors. Mechanisms and behavioral significance. Ann NY Acad Sci 510: 104–112 [DOI] [PubMed] [Google Scholar]
- Kleene SJ (1997) High-gain, low-noise amplification in olfactory transduction. Biophys J 73: 1110–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene SJ (2008) The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem Senses 33: 839–859 [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB (2004) Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43: 703–714 [DOI] [PubMed] [Google Scholar]
- Laurent G (2002) Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci 3: 884–895 [DOI] [PubMed] [Google Scholar]
- Lundin C, Kall L, Kreher SA, Kapp K, Sonnhammer EL, Carlson JR, Heijne G, Nilsson I (2007) Membrane topology of the Drosophila OR83b odorant receptor. FEBS Lett 581: 5601–5604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Sun L, Hu J (2009) Neural detection of gases—carbon dioxide, oxygen—in vertebrates and invertebrates. Curr Opin Neurobiol 19: 354–361 [DOI] [PubMed] [Google Scholar]
- Matsunami H, Amrein H (2003) Taste and pheromone perception in mammals and flies. Genome Biol 4: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N (2004) Structure and function of glutamate receptor ion channels. Annu Rev Physiol 66: 161–181 [DOI] [PubMed] [Google Scholar]
- Milligan G (2007) G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim Biophys Acta 1768: 825–835 [DOI] [PubMed] [Google Scholar]
- Mombaerts P (2004) Odorant receptor gene choice in olfactory sensory neurons: the one receptor–one neuron hypothesis revisited. Curr Opin Neurobiol 14: 31–36 [DOI] [PubMed] [Google Scholar]
- Montell C (2009) A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol 19: 345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moresco JJ, Koelle MR (2004) Activation of EGL-47, a Galphao-coupled receptor, inhibits function of hermaphrodite-specific motor neurons to regulate Caenorhabditis elegans egg-laying behavior. J Neurosci 24: 8522–8530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Vosshall LB (2009) Controversy and consensus: noncanonical signaling mechanisms in the insect olfactory system. Curr Opin Neurobiol 19: 284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Niimura Y, Nozawa M (2008) The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat Rev Genet 9: 951–963 [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS (2001) Mammalian sweet taste receptors. Cell 106: 381–390 [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS (2002) An amino-acid taste receptor. Nature 416: 199–202 [DOI] [PubMed] [Google Scholar]
- Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD (2005) Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol 15: 1948–1952 [DOI] [PubMed] [Google Scholar]
- Oka Y, Takai Y, Touhara K (2009) Nasal airflow rate affects the sensitivity and pattern of glomerular odorant responses in the mouse olfactory bulb. J Neurosci 29: 12070–12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M, Nakagawa T (2009) Smelling the difference: controversial ideas in insect olfaction. J Exp Biol 212: 1973–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penalva-Arana DC, Lynch M, Robertson HM (2009) The chemoreceptor genes of the waterflea Daphnia pulex: many Grs but no Ors. BMC Evol Biol 9: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM, Warr CG, Carlson JR (2003) Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci USA 100: 14537–14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronderos DS, Smith DP (2009) Diverse signaling mechanisms mediate volatile odorant detection in Drosophila. Fly (Austin) 3: 290–297 [DOI] [PubMed] [Google Scholar]
- Ryan TJ, Grant SG (2009) The origin and evolution of synapses. Nat Rev Neurosci 10: 701–712 [DOI] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K (2008) Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452: 1002–1006 [DOI] [PubMed] [Google Scholar]
- Schlief ML, Wilson RI (2007) Olfactory processing and behavior downstream from highly selective receptor neurons. Nat Neurosci 10: 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckel J, Torkkeli PH, French AS (2009) Two interacting olfactory transduction mechanisms have linked polarities and dynamics in Drosophila melanogaster antennal basiconic sensilla neurons. J Neurophysiol 102: 214–223 [DOI] [PubMed] [Google Scholar]
- Scott K (2005) Taste recognition: food for thought. Neuron 48: 455–464 [DOI] [PubMed] [Google Scholar]
- Shi P, Zhang J (2009) Extraordinary diversity of chemosensory receptor gene repertoires among vertebrates. Results Probl Cell Differ 47: 1–23 [DOI] [PubMed] [Google Scholar]
- Smart R, Kiely A, Beale M, Vargas E, Carraher C, Kralicek AV, Christie DL, Chen C, Newcomb RD, Warr CG (2008) Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem Mol Biol 38: 770–780 [DOI] [PubMed] [Google Scholar]
- Spehr M, Munger SD (2009) Olfactory receptors: G protein-coupled receptors and beyond. J Neurochem 109: 1570–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spors H, Wachowiak M, Cohen LB, Friedrich RW (2006) Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci 26: 1247–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AB, Shum EY, Hirsh S, Cygnar KD, Reisert J, Zhao H (2009) ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci USA 106: 11776–11781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF (1994) The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res 275: 3–26 [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Hildebrand JG (1999) Olfactory systems: common design, uncommon origins? Curr Opin Neurobiol 9: 634–639 [DOI] [PubMed] [Google Scholar]
- Su CY, Menuz K, Carlson JR (2009) Olfactory perception: receptors, cells, and circuits. Cell 139: 45–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Kurahashi T (2002) Photolysis of caged cyclic AMP in the ciliary cytoplasm of the newt olfactory receptor cell. J Physiol 541: 825–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JH, Robertson HM (2008) The Caenorhabditis chemoreceptor gene families. BMC Biol 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touhara K, Vosshall LB (2009) Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol 71: 307–332 [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF (2003) Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci 6: 1224–1229 [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF (2007) Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci 30: 505–533 [DOI] [PubMed] [Google Scholar]
- Wesson DW, Verhagen JV, Wachowiak M (2009) Why sniff fast? The relationship between sniff frequency, odor discrimination, and receptor neuron activation in the rat. J Neurophysiol 101: 1089–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS (2008) Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452: 1007–1011 [DOI] [PubMed] [Google Scholar]
- Wilson RI, Turner GC, Laurent G (2004) Transformation of olfactory representations in the Drosophila antennal lobe. Science 303: 366–370 [DOI] [PubMed] [Google Scholar]
- Yao CA, Ignell R, Carlson JR (2005) Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J Neurosci 25: 8359–8367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJ (2009) Common sense about taste: from mammals to insects. Cell 139: 234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS (2003) The receptors for mammalian sweet and umami taste. Cell 115: 255–266 [DOI] [PubMed] [Google Scholar]