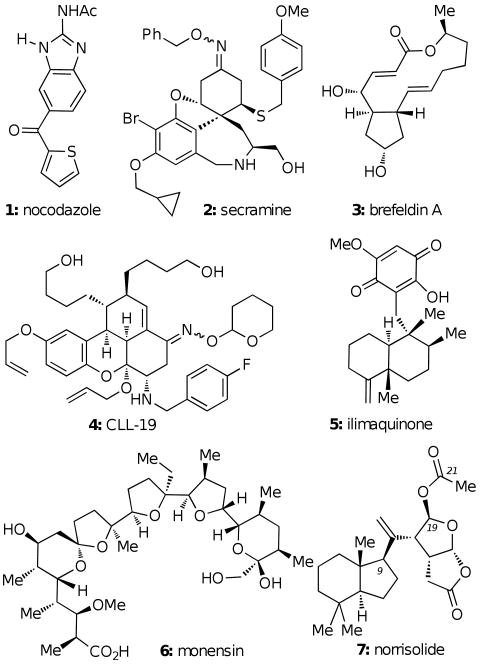
Abstract
The cellular activity of norrisolide (7), a novel Golgi-vesiculating agent, was dissected as function of its chemical structure. This natural product induces irreversible vesiculation of the Golgi membranes and blocks protein transport at the level of the Golgi. The Golgi localization and fragmentation effects of 7 depend on the presence of the perhydroindane core, while the irreversibility of fragmentation depends on the acetyl group of 7. We show that fluorescent derivatives of norrisolide are able to localize to the Golgi apparatus and represent important tools for the study of the Golgi structure and function.
Introduction
The process of protein secretion through the secretory pathway is fundamental for the survival of cells and the development of every organism. In complex organisms specialized secretory cells carry out key functions, such as the release of neurotransmitters, the release of antibodies and the secretion of digestive enzymes.1 Malfunctions of the secretory pathway can lead to a variety of diseases including asthma, Lowe's syndrome and cystic fibrosis.2 Moreover, toxins and pathogens have been shown to exploit various steps of this pathway to gain access to the cytosol where they exert their function.3
Essential to the secretory pathway is the Golgi apparatus, an organelle that consists of organized stacks of flattened membranes, referred to as cisternae. This organelle is responsible for the modification and sorting of cargo proteins.4 Within the Golgi, secretory proteins undergo complex post-translational modifications and are eventually sorted to their final destination. During protein transport, large amounts of membranes and proteins move across the Golgi complex; despite this dynamic trafficking, Golgi membranes are able to maintain their structural identity. The Golgi apparatus is therefore a dynamic structure whose organization is maintained by a balance of membrane input and output.5
Together with genetic screens6 and in vitro assays,7 pharmacological approaches based on small molecules have proven to be extremely helpful in studying the complex organization and membrane architecture of the Golgi apparatus. For example, studies with N-ethymaleimide have led to the isolation and identification of a protein, termed N-ethymaleimide sensitive factor, which is required for fusion of transport vesicles with Golgi.8 Investigations with nocodazole (1, Fig. 1) have shown that polymerization of microtubules can lead to stacking of the Golgi membrane.9 Screening of combinatorial libraries led to the identification of secramine (2), a small molecule that can block protein transport from Golgi to the plasma membrane,10 and CCL-19 (4), an agent that blocks the exit of proteins from Golgi and induces Golgi fragmentation.11
Fig. 1.
Structures of selected Golgi-disturbing agents.
Natural products can also affect the dynamics of the Golgi complex. For instance, ionophores such as monensin (6) can disrupt the pH gradient within the Golgi ultimately affecting protein transport.12 The fungal metabolite brefeldin A (3) was found to cause fusion of Golgi with endoplasmic reticulum (ER) and helped in unraveling the Golgi to ER retrograde pathway.13 The marine sesquiterpene ilimaquinone (5) was found to induce a reversible vesiculation of the Golgi and led to the identification of Protein Kinase D as a component of the secretion machinery.14
Screening of a natural products library for molecules that affect the secretory pathway led to the discovery of norrisolide (7),15 a marine diterpene that induces irreversible fragmentation of the Golgi complex.16 The chemical structure of norrisolide contains an uncommon fused γ-lactone-γ-lactol ring system pendant from a hydrophobic trans hydrindane core.15 Inspired by these observations, we sought to characterize the cellular phenotype of norrisolide and explore its Golgi activity as a function of its structure. Here we report a detailed account of these studies.
Results and Discussion
Characterization of norrisolide-induced Golgi fragmentation
At the onset of this investigation we compared the phenotypic changes induced by norrisolide to that of other known Golgi-disturbing agents. It should be noted that different known Golgi-disturbing agents have different effects on Golgi morphology. These effects can be grouped in three main phenotypes: Golgi fragmentation in discrete “ministacks” (e.g. Fig. 2b), Golgi fusion with the ER (e.g Fig. 2c and 2e), and Golgi dispersion into a cytosolic haze (e.g Fig. 2d and 2f).
Fig. 2.
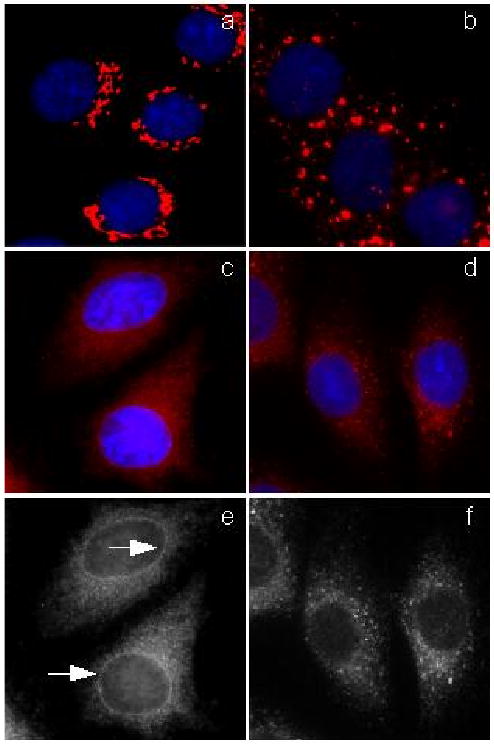
Phenotypic comparison of selected Golgi disturbing agents. NRK cells (a) were treated with the following Golgi disturbing agents: nocodazole (b), brefeldin A (c, e), ilimaquinone (d, f). Arrows indicate nuclear envelope staining. Golgi complex is shown in red, nuclei in blue.
The results of the phenotypic comparison studies are shown in Fig. 2. Normal rat kidney (NRK) cells were chosen for these studies as they allow an optimal visualization of cellular organelles. Untreated cells (Fig. 2a) show the typical perinuclear Golgi stacks of cisternae. Application of 30 μM nocodazole for 60 minutes led to depolymerization of microtubules, which resulted in the breakdown of the Golgi apparatus to ministacks that were dispersed throughout the cytoplasm (Fig. 2b).9 Brefeldin A, on the other hand, induced fusion of Golgi membranes with the ER (Fig. 2c); after brefeldin treatment (7 μM for 60 min), Golgi staining showed a typical ER pattern, as can be deduced by the presence of Golgi markers within the rim of the nuclear envelope (Fig. 2e).10 A third group of Golgi-disturbing agents is represented by ilimaquinone (5) and norrisolide (7). As shown in Fig. 2d and 2f, treatment of NRK cells with 30 μM of 5 for 60 min induced complete Golgi vesiculation without fusion with ER membranes.
Norrisolide induced a similar phenotype to that of ilimaquinone (5) (Fig. 3b). However, in contrast to 5, the norrisolide-induced Golgi fragmentation was found to be irreversible. Specifically, at concentrations and times comparable to those of ilimaquinone, removal of norrisolide by extensive washing does not result in Golgi reassembly (Fig. 3c).16
Fig. 3.
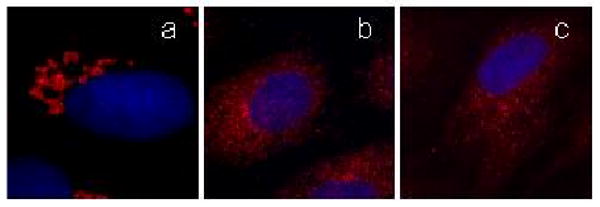
Norrisolide induces irreversible Golgi fragmentation. NRK cells plated on coverslips were treated with norrisolide for 0 (a) and 60 minutes (b). Cells were then washed and allowed to recover in fresh medium (c). Golgi complex is shown in red, nuclei in blue.
It is known that the intact microtubule (MT) cytoskeleton is required to maintain the Golgi architecture. 17 For instance, depolymerization of MT by nocodazole, leads to fragmentation of the Golgi membranes into ministacks that subsequently disperse across the cytoplasm (Fig. 4a).18 Two experiments were performed to test whether the norrisolide-induced Golgi fragmentation arises from disrupting the MT cytoskeleton. First, cells were treated with Taxol (10 μg/mL, 30 min), a compound known to stabilize the MT cytoskeleton.19 We found that norrisolide can still induce Golgi fragmentation of the Taxol-treated cells.16 Second, cells pre-treated with nocodazole (Fig. 4a) were subsequently incubated with norrisolide. We found that norrisolide could further fragment the Golgi ministacks produced by nocodazole (Fig 4b). These experiments allowed us to conclude that norrisolide induces Golgi vesiculation without affecting the MT cytoskeleton and that the intact MT cytoskeleton is not required for the norrisolide-induced Golgi fragmentation.
Fig. 4.
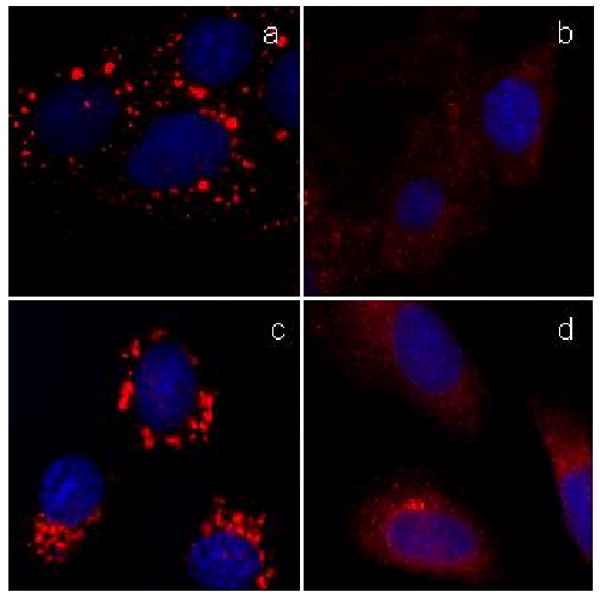
The norrisolide-induced Golgi fragmentation is independent on intact MT cytoskeleton and PKD activity. NRK cells pretreated for 60 min with nocodazole (a) were incubated with norrisolide for additional 60 min (b). NRK cells were treated for 60 min with IQ (c) or norrisolide (d) in presence of PKD inhibitor H89.
It has also been shown that the overactivation of the membrane fission process results in the complete conversion of Golgi stacks into transport vesicles.20 Specifically, ilimaquinone (5) induces Golgi vesiculation by overstimulating Protein Kinase D (PKD), a protein required for Golgi membrane fission and production of secretory vesicles (Fig. 4d). With this in mind, we tested whether the norrisolide activity proceeds via a PKD-dependent membrane fission process. NRK cells were pretreated with PKD inhibitor H-8921 (20 μM) for 30 min, then incubated with 5 (Fig. 4c) or norrisolide (Fig. 4d) for 1h. As shown in Fig. 4c, under these conditions ilimaquinone is no longer able to fragment the Golgi, since its downstream target, PKD, is no longer functional. On the other hand, H-89 does not affect norrisolide-mediated Golgi fragmentation (Fig. 4d). These results demonstrate an important difference between the mode-of-action of ilimaquinone and norrisolide. Although the changes in Golgi morphology induced by ilimaquinone and norrisolide are indistinguishable at the immunofluorescence level, at molecular level the two processes are different, as norrisolide activity is not sensitive to the PKD inhibitor H89, while ilimaquinone is.
We then tested whether norrisolide can affect protein secretion. For this experiment we used a temperature-sensitive variant of the vesicular stomatitis virus (VSV)–G protein. This protein is commonly used as it allows one to follow its transport through the secretory pathway in discrete steps by temperature blocks. For our experiments we used the tsO45 strain of VSV-G-GFP, which has a thermosensitive mutation causing it to unfold in the ER at the nonpermissive temperature of 39.5 °C. 22 In addition, the green fluorescent protein (GFP) is fused with VSV-G to allow its visualization within the cells. After shifting the cells to 20 °C, VSV-G reaches the Golgi apparatus. At such temperature, protein transport is arrested at the level of the Golgi. When the temperature is raised to 32 °C, VSV-G finally reaches the plasma membrane.
We followed the transport of VSV-G from the ER to the Golgi and to the plasma membrane in control cells (Figs. 5a and 5c) and norrisolide-treated cells (Figs 5b and 5d). Cells transfected with VSV-G were incubated at 40 °C to arrest VSV-G in the ER. The cells were then incubated with DMSO (control) or norrisolide for 1h and then shifted to 20 °C, to allow VSV-G to exit the ER and reach the Golgi. In both DMSO (Fig. 5a) and norrisolide (Fig. 5b) treated cells, VSV-G was able to reach the Golgi apparatus, indicating that 7 does not affect protein transport from ER to Golgi. It is important to note that although Golgi structure is affected by norrisolide (Fig. 5b), the ability of the fragmented Golgi to receive protein cargo is not impaired (Fig. 5b: VSV-G, in green, enters norrisolide-induced Golgi fragments, in red). To measure the arrival of VSV-G at the plasma membrane, after arresting VSV-G in Golgi, cells were incubated with DMSO (control) or norrisolide, then shifted at permissive temperature (32 °C). As shown in Fig. 5, while in DMSO-treated cells VSV-G reached the plasma membrane (Fig. 5c), in norrisolide-treated cells VSV-G (green) was permanently trapped in the Golgi vesicles (red) induced by 7 (Fig. 5d).
Fig. 5.
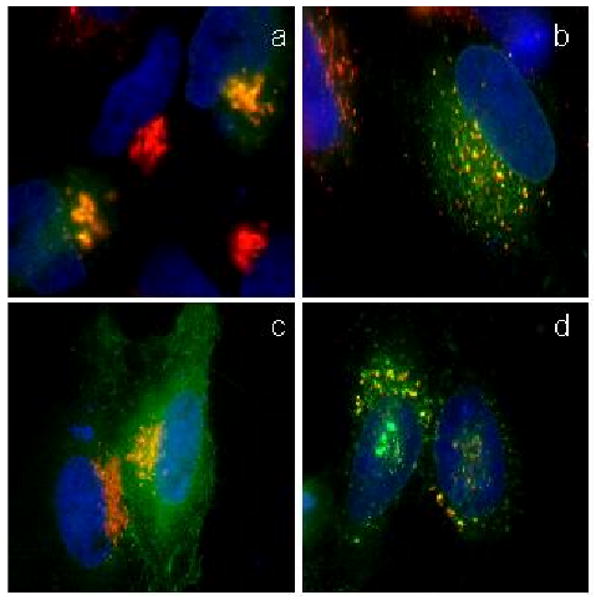
Protein transport in norrisolide-treated cells. Cells transfected with VSV-G (green) were incubated at 40 °C, then shifted to 20 °C (a, b) and 32 °C (c, d) in presence of DMSO (a, c) and norrisolide (b, d). VSV-G localizes in Golgi (a,b) and at the plasma membrane (c,d). Golgi complex is shown in red, VSV-G in green. Nuclei are in blue. Colocalization between Golgi and VSV-G is in yellow
Based on the above results, we can make the following conclusions regarding the cellular characterization of norrisolide-induced Golgi fragmentation: (a) norrisolide affects the Golgi structure in a direct manner that is independent of the MT cytoskeleton architecture; (b) the norrisolide-induced Golgi fragmentation is independent of PKD, a protein previously shown to be involved in Golgi membrane fission and required for ilimaquinone-dependent Golgi vesiculation; (c) while norrisolide-induced Golgi fragments are able to receive protein cargo, they are not competent for the delivery of cargo molecules to their final destination. The latter effect results in a norrisolide-induced post Golgi block in protein transport.
Structure-function studies of norrisolide
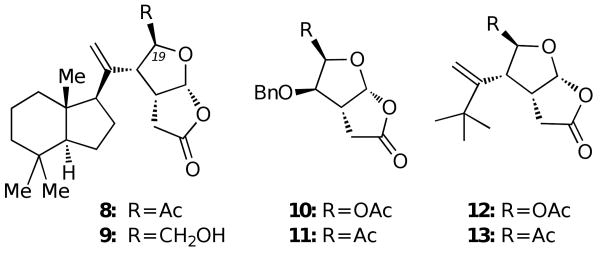
To evaluate the biological activity of norrisolide as a function of its structure we studied the effect of analogues 8-13 (Fig. 6) on the Golgi complex.16 Compounds 8 and 9 are synthetic precursors of norrisolide 23 and contain the entire carbon framework of the natural product but lack the acetoxyl functionality. On the other hand, compounds 10-13 contain a variably functionalized γ-lactol-γ-lactone ring system of the natural product but lack the perhydroindane core. 24
Fig. 6.
Synthetic analogues of norrisolide
As shown in Fig. 7, compound 8 exhibited partial Golgi vesiculation without inducing any microtubule disassembly (Fig. 7b). However, in contrast with norrisolide (see Fig. 3), the fragmentation was reversible and after a standard washing protocol the cells recovered completely (Fig. 7c). Similar results were obtained with 9. In contrast, NRK cells treated with compounds 10-13 were indistinguishable from control untreated cells, suggesting that these analogues have no effect on Golgi membranes.
Fig. 7.

Effect of norrisolide analogue 8 on Golgi vesiculation. NRK cells were treated with 8 for 60 min (a) and allowed to recover for 60 min (b). Golgi complex is shown in red. Nuclei are in blue.
Evaluation of the above data indicates that the perhydroindane core of norrisolide is essential for norrisolide activity and appears to function as the target recognition element. This could explain why compounds 10-13, lacking the perhydroindane core, have no effect on the Golgi membranes, while analogues 8-9 induce a visible although reversible fragmentation. In turn, the irreversible Golgi fragmentation observed with norrisolide may be due to a covalent modification of a target protein, presumably due to the highly electrophilic nature of the C21 acetyl group (norrisolide numbering). In other words, norrisolide may act as a selective acetylating reagent. Selective binding is therefore achieved by the perhydroindane core of norrisolide, which brings the lactol-lactone side chain in close proximity to a nucleophile, which can then undergo acetylation.
Intracellular localization studies of norrisolide
To further characterize the biological effect of norrisolide we examined the intracellular localization of norrisolide-derived fluorescent probes. The fluorophore coumarin was coupled to a norrisolide precursor that contains the entire carbon framework of the natural product, to the perhydroindane core and to the lactol-lactone side chain respectively, to generate probes 14, 15 and 16 (Fig. 8). 25
Fig. 8.
Norrisolide-based fluorescent probes
To assess the biological activity, NRK cells were incubated with probes 14-16 for 60 min, then processed for immunofluorescence to reveal Golgi structure. These probes had comparable stability with norrisolide in cells. While 15 had no activity (Fig. 9a), both 14 and 16 induced complete Golgi fragmentation in 60 min (results with 14 are shown in Fig. 9b). When live cells were imaged after 10 min incubation with 14 and 16, both probes showed a perinuclear localization identical to a typical Golgi staining (Fig. 9c). To confirm that 14 and 16 indeed localized to the Golgi apparatus, NRK cells were fixed, labeled with a Golgi specific antibody (Fig. 9d), and incubated with the fluorescent probes and imaged by light microscopy. Compound 15 showed no specific localization (data not shown), while compounds 14 and 16 both co-localized with Golgi markers (results with 14 are shown in Fig. 9e and 9f). To evaluate whether these compounds bind the same receptor as norrisolide, we performed the following competition experiment: NRK cells were fixed and incubated with analogue 14 in the presence of cell medium (Fig. 9g), DMSO (Fig. 9h) or norrisolide (Fig. 9i). We found that norrisolide (7) can displace 14 from the Golgi complex, as shown by the loss of green color from the Golgi (due to the coumarin fluorescence) in Fig. 9i. Similar competition experiments showed that norrisolide could displace 16 from its target, indicating that compounds 7, 14 and 16 share the same cellular receptor. These results indicate that 7 induces phenotypic changes in Golgi morphology by binding directly to the Golgi complex and further support the notion that the perhydroindane core of 7 is necessary and sufficient for binding.
Fig. 9.

Fluorescent analogues of norrisolide induce Golgi fragmentation and localize on Golgi. NRK cells were treated with 15 (a) and 14 (b) for 60 min. Cells were treated with 14 for 10 min and imaged in live conditions (c). Fixed cells were labeled with a Golgi-specific antibody (d) then incubated with 14 (e); yellow color in (f) indicates colocalization of probe 14 and the antibody. Competition experiment: fixed NRK cells were incubated with 14 in presence of cell medium (g), DMSO (h) or 7 (i). Golgi complex is shown in red. Coumarin fluorescence is shown in green. Nuclei are in blue.
Design of norrisolide-based irreversible Golgi vesiculating agents
The above findings suggest that the C19 acetoxy group of norrisolide plays an essential role in the irreversibility of the fragmentation either by stabilizing the binding or by creating a covalent bond with its target protein. Inspired by a study that identified epoxides as suitable functionalities for protein labeling, we replaced the entire side chain of 7 with a bis-epoxide motif to form adduct 18. Compound 17 was synthesized in a similar manner and used as a control.25
Fig. 11 summarizes the effect of probes 17-20 on the Golgi membranes. Compounds 17 and 18 induced extensive Golgi vesiculation, as shown in Fig. 11a and Fig 11c respectively. However, while the fragmentation induced by 17 was completely reversible (Fig. 11b), the Golgi fragmentation by 18 persisted even after washing (Fig. 11d). This level of irreversibility was the highest we had achieved with a norrisolide derivative. Similarly, iodinated analogue 19 and alkyne analogue 20 induced irreversible Golgi fragmentation in more than 90% of cells.26 These findings indicate that it is possible to functionalize further the aromatic ring of probe 18 without affecting the cellular phenotype of norrisolide.
Fig. 11.
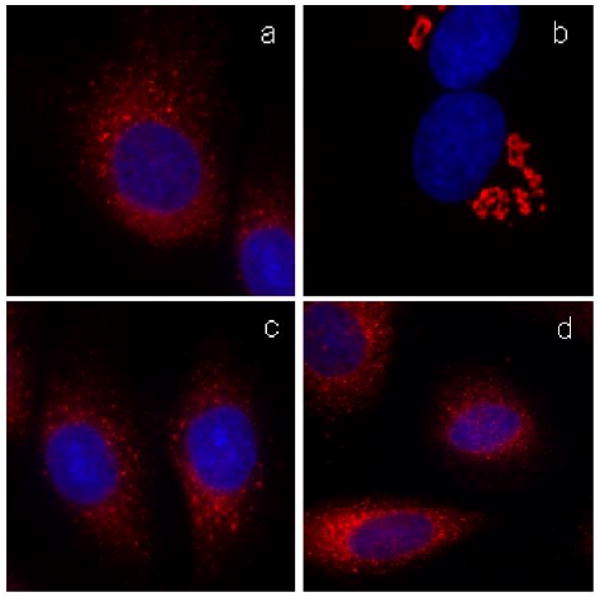
Effect of probes 17 and 18 on Golgi. NRK cells were treated with the probe 17 for 60 min (a) and then allowed to recover for additional 60 min (b). Cells were treated with 18 for 60 min (c) and then allowed to recover (b). Golgi complex is shown in red. Nuclei are in blue.
Design of norrisolide-based trifunctional probes
The above results suggest that the hydrocarbon-containing perhydroindane core of norrisolide binds to a receptor on the Golgi membranes and induces their reversible vesiculation. When this core is decorated with electrophilic residues, like the C19 acetoxy group (norrisolide numbering), the vesiculation becomes irreversible. To further explore this concept, we sought to synthesize and study trifunctional norrisolide probes that would contain the following groups: (a) the perhydroindane core for Golgi localization, (b) a crosslinking reagent for covalent binding to its target and, (c) a tag for subsequent protein purification and isolation.
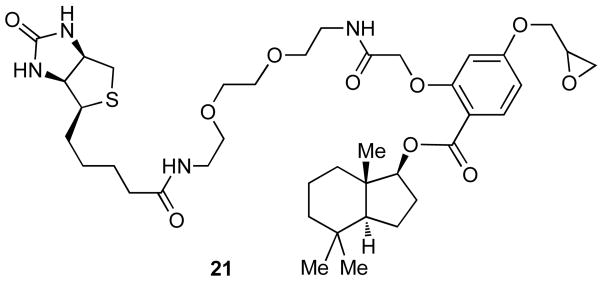
An example of this design, that combines the perhydroindane motif with an epoxide unit and biotin, is shown with probe 21 (Fig. 12). Incubation of NRK cells with 21 induced less than 10% of Golgi fragmentation in the entire cell population (Fig. 13a). Moreover, this effect was fully reversible after cell washing (Fig. 13b). The extent of Golgi fragmentation was clearly below the level observed with norrisolide or probes 17-20 that, under identical conditions, led to complete and irreversible vesiculation in more than 90% of the cell population.26
Fig. 12.
A norrisolide-based trifunctional probe 21 that combines the perhydroindane core with an epoxide and a biotin motif.
Fig. 13.
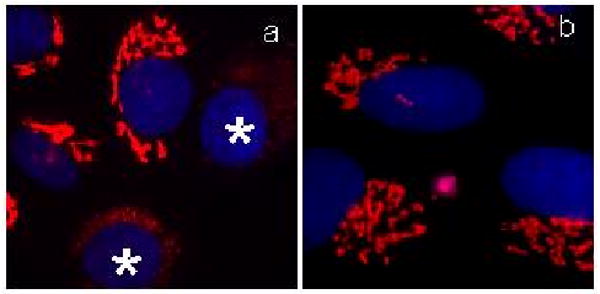
Effect of probe 21 NRK on Golgi. Cells were treated with 21 for 60 min (a); then washed and incubated in fresh medium for 60 min (b). Asterisks indicate cells with fragmented Golgi. Golgi is shown in red; nuclei in blue.
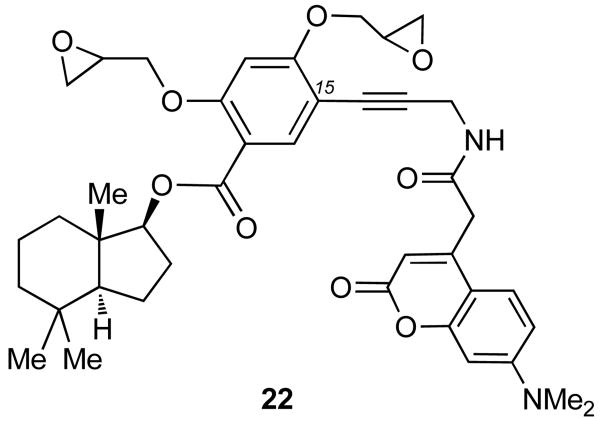
We hypothesized that the low extent of Golgi vesiculation induced by compound 21 is due to the presence of the biotin tag. It is possible that the steric hindrance caused by biotin in combination with its intrinsic affinity for cellular biotin receptors leaves insufficient amounts of these probes on the Golgi membranes thus dramatically reducing their effect on the Golgi complex. In other words, the biotin tag could interfere with the ability of the perhydroindane motif to localize these probes to the Golgi. 27 This analysis led us to reconsider our overall strategy and replace the biotin tag with a fluorescent probe. As the fluorophore, we selected the 7-dimethyl aminocoumarin-4-acetic acid (DMACA), since this probe displays desirable photophysical properties,28 and shows negligible interference in cellular assays. Moreover, this probe has been used in combination with a monoclonal antibody (mAb) for immunoaffinity fluorescence studies.29 We also felt that maintaining the bis epoxide motif would ensure the irreversible Golgi vesiculation, as observed with probes 18-20. With this in mind, we synthesized trifunctional probe 22, by linking DMACA to 19 (fig. 14).
Fig. 14.
A norrisolide-based trifunctional probe that combines the perhydroindane core with two epoxides and a DMACA fluorophore.
As predicted, probe 22 localized efficiently to the Golgi complex in living cells (Fig. 15b) and induced complete Golgi vesiculation after a 60 min incubation period (Fig. 15c). However, the Golgi vesiculation proved to be reversible (Fig. 15d). We also performed competition experiments, which showed that the fluorescent staining of probe 22 could be displaced from the Golgi after incubation with an excess of norrisolide (data not shown), indicating that the binding of 22 to its target was not covalent. Similar results were obtained with derivatives of 22 in which the triple bond was partially or fully reduced, suggesting that the flexibility of the linker connecting the norrisolide core with the DMACA fluorophore does not contribute to the loss of irreversibility of the Golgi vesiculation.
Fig. 15.
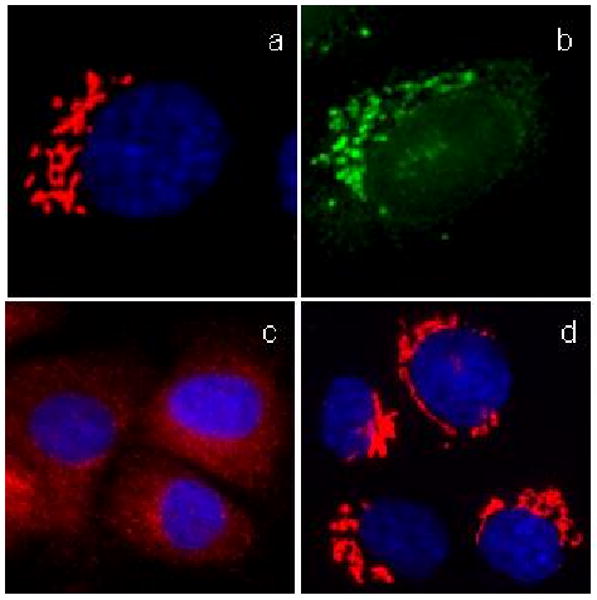
Effect of probe 22 on Golgi. Fixed NRK cells were labeled with Golgi-specific antibody (a); live cells were incubated with 22 for 10 min (b). Cells were treated with 22 for 60 min (c) and then allowed to recover for 60 min (d). Golgi complex is shown in red. DMACA fluorescence is shown in green. Nuclei are in blue.
The reversible Golgi vesiculation, induced by 22, was interesting, as compared to the irreversible vesiculation obtained with probes 18-20. One possible explanation is that the steric hindrance imposed by the added coumarin at the C15 center of 22 impairs the covalent interaction of the epoxide units with the norrisolide receptor. In contrast, smaller groups attached at C15, such as the alkyne motif of 20 or the iodide functionality of 19, do not affect significantly this interaction leading to presumed covalent binding. Alkyne-containing probe 20 is particularly interesting since it could be used to identify the cellular target of norrisolide under Click conditions.30,31 Alternatively, probe 19, armed with a radioactive isotope 125I at C15, could be used to isolate the protein target via radiochromatography.32
Conclusion
In conclusion, we report here a detailed study of norrisolide-induced Golgi vesiculation. We found that this natural product fragments Golgi membranes in an irreversible manner without affecting the microtubule cytoskeleton. This effect is distinctly different from that observed for ilimaquinone, a structurally related natural product that induces reversible Golgi fragmentation that is dependent on the activation of protein kinase D. Our studies also suggest that norrisolide-induced Golgi fragments are able to receive secretory proteins from the ER but they cannot deliver them to the plasma membrane, indicating that this compound specifically blocks protein transport at the level of the Golgi apparatus. Structure-activity studies indicate that the perhydroindane motif of norrisolide functions as the target recognition element, while the C19 acetoxy group is essential for the irreversibility of Golgi fragmentation. This group can be replaced with other electrophilic functionalities without significant loss of activity. Through the use of fluorescent derivatives of norrisolide, we were able to demonstrate that this compound binds directly to Golgi membranes, and that the perhydroindane core is necessary and sufficient for this binding. Moreover, the identification of norrisolide-based probes, such as 19 and 20, that reproduce the cellular phenotype of the natural product and are functionalized with appropriate tags, could be used for the isolation of its cellular target. In general, these studies attest to the significance of norrisolide and related designed analogues as biological tools for the evaluation of Golgi dynamics and protein trafficking.
Experimental
General Chemical Techniques
Compounds 1, 3 and taxol were purchased from Sigma-Aldrich, 5 was purchased from Alexis Biochemicals and H-89 was purchased from BIOMOL Research Laboratories. Norrisolide was synthesized according to reference 23. All other chemical reagents were commercially obtained (Aldrich, Sigma Acros) at highest commercial quality and used without further purification except where noted. The synthesis of compounds 8-13 is described in references 16 and 23. The synthesis of compounds 14-18 is described in reference 25. The synthesis of compounds 19 and 21 is described in reference 26. Compounds 20 and 22 were synthesized by standard Pd(0)-catalyzed derivatization of iodinated compound 19.
Cell culture and transfection
NRK cells were grown in complete medium (alpha MEM -GIBCO- supplemented with 10% fetal calf serum, 2 mM L-glutamine and 25 mM Hepes pH 7.4), at 37°C in a 7% CO2 incubator. For immunofluorescence experiments, the cells were plated on 12 mm glass coverslips coated with Pronectin F (Sigma) and grown in complete medium (500 μL per coverslip).
The cells were transfected with VSV-G-GFP using FuGene 6 (Roche) following the manufacturer's recommendations.
Reagents
Stock solution (25 mg/ml) of norrisolide analogues were made in DMSO and stored at −20 °C. The working concentration of the compounds was 30 μM for each coverslip and cells were incubated at 37 °C for 60 min. An equal volume of DMSO was used as a negative control for each compound. For the crosslinking reaction, cells were irradiated for 15 min with UV light using a UVL-21 hand lamp (1mW/cm2 at 366 nm) placed at 5 cm distance from the cells. To test the irreversibility of Golgi fragmentation, cells were washed 4 times with phosphate-buffered saline (PBS) (150 mM NaCl, 1.8 mM NaH2PO4, 8.4 mM Na2HPO4) and then incubated in fresh complete medium at 37 °C for 90 minutes. BFA, nocodazole and Taxol were used respectively at 7 μM, 30 μM and 10 μg/mL. H-89 was used at 20 μM.
Immunofluorescence microscopy
For fluorescent labeling, cells were incubated in blocking buffer (PBS containing 2.5% fetal bovine serum and 0.1% Triton X-100) for 30 min at room temperature. The cells were then incubated for 1h at room temperature in primary antibody diluted in blocking buffer. Rabbit Mannosidase II antibody (1:2000) (a gift from Dr. Kelly Moreman, Vanderbilt University, TN) was used to visualize Golgi apparatus. The cells were then washed three times with PBS and incubated with secondary antibody, diluted in blocking buffer, for 1h at room temperature. Alexa Fluor 594 goat anti rabbit (1:500) from Molecular Probes was used. Cells were washed three times with PBS containing Hoescht (1:100,000) (H33342, Molecular Probes) to stain DNA. Coverslips were then mounted onto glass slides and visualized using a Nikon micophot-FXA fluorescence microscope at 60× magnification.
Golgi membranes fragmentation assay
Cells plated on coverslips were treated with norrisolide or analogues at the indicated concentrations. To control cells was added the same volume of DMSO as negative control. After 1h incubation at 37 °C, the cells were either processed for immunofluorescence microscopy, or washed four times with phosphate-buffered saline (PBS) (150 mM NaCl, 1.8 mM NaH2PO4, 8.4 mM Na2HPO4) and then incubated in fresh complete medium at 37 °C for 90 min, then processed for immunofluorescence microscopy.
VSV-G transport assay
cells were transfected with ts045VSV-G-GFP and cultured at 40 °C for 20 h. 100 mg/ml of cycloheximide was then added before a 2 h incubation at 20 °C. To follow the transport to the plasma membrane, the cells were then shifted at 32 °C for 1 h.
Golgi membrane localization assay
The cells were first fixed with 2% formaldehyde for 90 sec. This mild fixation induces a minimal perturbation of cellular proteins, allowing the binding between the fluorescent derivatives and the cellular receptor. Cells were processed for immunofluorescence microscopy as described above. Before the coverslips were mounted onto glass slides, the fluorescent compounds were added at 25 μM for 30 min (Fig. 3d, 3e, 3f).
Competition experiments
Norrisolide was added after compound 14 at 100 μM for 30 min. Coverslips were then mounted onto glass slides and visualized using a Nikon micophot-FXA fluorescence microscope at 60× magnification. The intracellular visualization of compound 14 was accomplished using excitation at 370 nm and emission at 460 nm.
Supplementary Material
Fig. 10.
Norrisolide-based probes 17-20
Acknowledgments
Financial support by the NIH (GM 081484 to EAT and GM 46224 to VM) is gratefully acknowledged.
Footnotes
Supplementary data: Supplementary data associated with this article can be found, in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.For selected reviews on the secretory pathway see: Pelham HR. Cell Struct Funct. 1996;5:413–419. doi: 10.1247/csf.21.413.Rothman JE, Orci L. Nature. 1992;355:409–415. doi: 10.1038/355409a0.Pelham HR, Munro S. Cell. 1993;75:603–605. doi: 10.1016/0092-8674(93)90479-A.Corda D, Hidalgo Carcedo C, Bonazzi M, Luini A, Spanò S. Cell Mol Life Sci. 2002;59:1819–1832. doi: 10.1007/PL00012508.Luini A, Mironov AA, De Matteis MA, Griffiths G. Med Secoli. 2007;19:29–54.Lowe M, Nakamura N, Warren G. Trends Cell Biol. 1998;8:40–44. doi: 10.1016/s0962-8924(97)01189-6.
- 2.For selected reports on this topic see: Fahy JV. Chest. 2002;122:320S–326S. doi: 10.1378/chest.122.6_suppl.320s.Suchy SF, Olivos-Glander IM, Nussabaum RL. Hum Mol Genet. 1995;4:2245–2250. doi: 10.1093/hmg/4.12.2245.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O'Riordan CR, Smith AE. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8.
- 3.For selected reports on this topic see: Spooner RA, Smith DC, Easton AJ, Roberts LM, Lord JM. Virol J. 2006;3:26. doi: 10.1186/1743-422X-3-26.Sandvig K, van Deurs B. Annu Rev Cell Dev Biol. 2002;18:1–24. doi: 10.1146/annurev.cellbio.18.011502.142107.Ludwig JW, Richards ML. Curr Top Med Chem. 2006;6:165–178. doi: 10.2174/156802606775270314.
- 4.For selected reports on this topic see: Roth J. Chem Rev. 2002;102:285–303. doi: 10.1021/cr000423j.Arnold SM, Kaufman RJ. New Compreh Biochem. 2003;38:411–432.van Vliet C, Thomas EC, Merino-Trigo A, Teasdale RD, Gleeson PA. Progr Biophys & Mol Biol. 2003;83:1–45. doi: 10.1016/s0079-6107(03)00019-1.Schulein R. Rev Physiol Biochem & Pharmacol. 2004;151:45–91. doi: 10.1007/s10254-004-0022-8.Sifers RN. Science. 2003;299:1330–1331. doi: 10.1126/science.1082718.
- 5.For selected reports on this topic see: Warren G, Malhotra V. Curr Opin Cell Biol. 1998;10:493–498. doi: 10.1016/s0955-0674(98)80064-1.Bard F, Malhotra V. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126.
- 6.Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, Guizzunti G, Hu Y, Wendler F, Dasgupta R, Perrimon N, Malhotra V. Nature. 2006;439:604–607. doi: 10.1038/nature04377. [DOI] [PubMed] [Google Scholar]
- 7.Balch WE, Dunphy WG, Braell WA, Rothman JE. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- 8.Balch WE, Glick BS, Rothman JE. Cell. 1984;39:525–536. doi: 10.1016/0092-8674(84)90459-8. [DOI] [PubMed] [Google Scholar]; Malhotra V, Orci L, Glick BS, Block MR, Rothman JE. Cell. 1988;54:221–227. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- 9.Thyberg J, Moskalewski S. Exp Cell Res. 1985;159:1–16. doi: 10.1016/s0014-4827(85)80032-x. [DOI] [PubMed] [Google Scholar]; Turner JR, Tartakoff AM. J Cell Biol. 1989;109:2081–2088. doi: 10.1083/jcb.109.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelish HE, Peterson JR, Salvarezza SB, Rodriguez-Boulan E, Chen JL, Stammes M, Macia E, Feng Y, Shair MD, Kirchhausen T. Nature Chem Biol. 2006;2:1, 39–46. doi: 10.1038/nchembio751. [DOI] [PubMed] [Google Scholar]; Pelish HE, Westwood NJ, Feng Y, Kirchhausen T, Shair MD. J Am Chem Soc. 2001;123:6740–6741. doi: 10.1021/ja016093h. [DOI] [PubMed] [Google Scholar]
- 11.Goess BC, Hannoush RN, Chan LK, Kirchhausen T, Shair MD. J Am Chem Soc. 2006;126:5391–5403. doi: 10.1021/ja056338g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tartakoff AM, Vassalli P. J Exp Med. 1977;146:1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelham HR. Cell. 1991;67:449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]; Schiaky N, Presley J, Smith C, Zaal KJM, Cole N, Moreira JE, Terasaki M, Siggia E, Lippincott-Schwartz J. J Cell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takizawa PA, Yucel JK, Veit B, Faulkner JD, Deernick T, Soto G, Ellisman M, Malhotra V. Cell. 1993;73:1079–1090. doi: 10.1016/0092-8674(93)90638-7. [DOI] [PubMed] [Google Scholar]; Jamora C, Takizawa PA, Zaarour RF, Denesvre C, Faulkner DJ, Malhotra V. Cell. 1999;91:617–626. doi: 10.1016/s0092-8674(00)80449-3. [DOI] [PubMed] [Google Scholar]; Bossard C, Bresson D, Polishchuk RS, Malhotra V. J Cell Biol. 2007;179:1123–1131. doi: 10.1083/jcb.200703166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochlowski JE, Faulkner DJ, Matsumoto J, Clardy J. J Org Chem. 1983;48:1141–1142. [Google Scholar]
- 16.Brady TP, Wallace EK, Kim SH, Guizunnti G, Malhotra V, Theodorakis EA. Bioorg Med Chem Lett. 2004;14:5035–5039. doi: 10.1016/j.bmcl.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Storrie B, Yang W. Biochim Biophys Acta. 1998;1404:127–137. doi: 10.1016/s0167-4889(98)00053-6. [DOI] [PubMed] [Google Scholar]
- 18.Thyberg J, Moskalewski S. Exp Cell Res. 1999;246:263–279. doi: 10.1006/excr.1998.4326. [DOI] [PubMed] [Google Scholar]
- 19.Wehland J, Henkart M, Klausner R, Sandoval IV. PNAS USA. 1983;80:4286–4290. doi: 10.1073/pnas.80.14.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V. Cell. 1999;98:59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- 21.Johannes FJ, Prestle J, Dieterich S, Oberhagemann P, Link G, Pfizenmaier K. Eur J Biochem. 1995;227:303–307. doi: 10.1111/j.1432-1033.1995.tb20389.x. [DOI] [PubMed] [Google Scholar]
- 22.Ward TH, Lippincott-Schwartz J. Meth Biochem Anal. 2006;47:305–337. doi: 10.1002/0471739499.ch14. [DOI] [PubMed] [Google Scholar]
- 23.Brady TP, Kim SH, Wen K, Theodorakis EA. Angew Chem Int Ed. 2004;43:739–742. doi: 10.1002/anie.200352868. [DOI] [PubMed] [Google Scholar]; Brady TP, Kim SH, Wen K, Kim C, Theodorakis EA. Chemistry Eur J. 2005;11:7175–7190. doi: 10.1002/chem.200500513. [DOI] [PubMed] [Google Scholar]
- 24.Kim C, Hoang R, Theodorakis EA. Org Lett. 1999;1:1295–1297. [Google Scholar]
- 25.Guizzunti G, Brady TP, Malhotra V, Theodorakis EA. J Am Chem Soc. 2006;128:4190–4191. doi: 10.1021/ja058259a. [DOI] [PubMed] [Google Scholar]
- 26.Guizzunti G, Brady TP, Malhotra V, Theodorakis EA. Bioorg & Med Chem Lett. 2007;17:320–325. doi: 10.1016/j.bmcl.2006.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkeby S, Moe D, Bøg-Hansen TC, van Noorden CJ. Histochem. 1993;100:415–421. doi: 10.1007/BF00267821. [DOI] [PubMed] [Google Scholar]
- 28.Song HY, Ngai MH, Song ZY, MacAry PA, Hobley J, Lear MJ. Org & Biomol Chem. 2009;7:3400–3406. doi: 10.1039/b904060a. [DOI] [PubMed] [Google Scholar]; Jarzeba W, Walker GC, Johnson AE, Kahlow MA, Barbara P. J Phys Chem. 1988;92:7039–7041. [Google Scholar]; Trenor SR, Shultz AR, Love BJ, Long TE. Chem Rev. 2004;104:3059–3077. doi: 10.1021/cr030037c. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez AD, Lear MJ, La Clair JJ. J Am Chem Soc. 2008;130:7256–7258. doi: 10.1021/ja7114019. [DOI] [PubMed] [Google Scholar]; Hughes CC, MacMillan JB, Gaudencio SP, Fenical W, La Clair JJ. Angew Chem Int Ed. 2009;48:728–732. doi: 10.1002/anie.200804107. [DOI] [PMC free article] [PubMed] [Google Scholar]; Alexander MD, Burkart MD, Leonard MS, Portonovo P, Liang B, Ding X, Joullie MM, Gulledge BM, Aggen JB, Chamberlin RA, Sandler J, Fenical W, Cui J, Gharpure SJ, Polosukhin A, Zhang HR, Evans PA, Richardson AD, Harper MK, Ireland CM, Vong BG, Brady TP, Theodorakis EA, La Clair JJ. ChemBioChem. 2006;7:409–416. doi: 10.1002/cbic.200500466. [DOI] [PubMed] [Google Scholar]
- 30.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; Lutz JF. Angew Chem Int Ed. 2007;46:1018–1025. doi: 10.1002/anie.200604050. [DOI] [PubMed] [Google Scholar]
- 31.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J Am Chem Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]; Speers AE, Adam GC, Cravatt BF. J Am Chem Soc. 2003;125:4686–4687. doi: 10.1021/ja034490h. [DOI] [PubMed] [Google Scholar]
- 32.Sun P, Wang GX, Furuta K, Suzuki M. Bioorg & Med Chem Lett. 2006;16:2433–2436. doi: 10.1016/j.bmcl.2006.01.083. [DOI] [PubMed] [Google Scholar]; Hashimoto M, Kato Y, Hatanaka Y. Tetrahedron Lett. 2006;47:3391–3394. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.