Summary
Background
Tissue factor (TF) is present in blood in various forms, including small membrane vesicles called microparticles (MPs). Elevated levels of these MPs appear to play a role in the pathogenesis of thrombosis in a variety of diseases, including sepsis.
Objective
Measure levels of MP TF activity and activation of coagulation in control and endotoxemic mice.
Materials and methods
MPs were prepared from plasma by centrifugation. The procoagulant activity (PCA) of MPs was measured using a two-stage chromogenic assay. We also measured levels of thrombin-antithrombin and the number of MPs.
Results
Lipopolysaccharide (LPS) increased MP PCA in wild-type mice; this PCA was significantly reduced by an anti-mouse TF antibody (1H1) but not with an anti-human TF antibody (HTF-1). Conversely, in mice expressing only human TF, MP PCA was inhibited by HTF-1 but not 1H1. MPs from wild-type mice had 6-fold higher levels of PCA using mouse factor (F)VIIa compared with human FVIIa, which is consistent with reported species-specific differences in FVIIa. Mice expressing low levels of human TF had significantly lower levels of MP TF activity and TAT than mice expressing high levels of human TF; however, there were similar levels of phosphatidylserine (PS)-positive MPs. Importantly, levels of MP TF activity in wild-type mice correlated with levels of TAT but not with PS-positive MPs in endotoxemic mice.
Conclusion
These results suggest that the levels of TF-positive MPs can be used as a biomarker for evaluating the risk of disseminated intravascular coagulation in endotoxemia.
Keywords: coagulation, endotoxemia, microparticle, tissue factor
Introduction
Tissue factor (TF) within the vessel wall plays a critical role in hemostasis by activating the clotting cascade after vessel injury [1]. TF is also present in plasma in various forms, including microparticles (MPs) and alternatively spliced TF [2,3]. MPs are small (< 1 µm) membrane vesicles generated by activated or apoptotic cells [4]. Levels of TF-positive MPs are elevated in a variety of diseases, such as sepsis, cardiovascular disease, sickle cell disease and cancer [5–11]. One study showed that TF-positive MPs from pericardial blood of cardiac surgery patients enhanced venous thrombosis in a rat model [10]. In healthy mice, hematopoietic cell-derived TF-positive MPs enhance thrombosis in cremaster muscle arterioles of mice [12]. In tumor-bearing mice, levels of human TF antigen in plasma correlated with the size of the tumor, and increased levels of tumor-derived MPs were associated with increased levels of thrombin-antithrombin (TAT) complex [13,14]. These studies strongly suggest that TF-positive MPs contribute to thrombosis in many diseases.
TF expression contributes to disseminated intravascular coagulation in a baboon model of sepsis [15]. Monocytes appear to be the major source of TF within the circulation [1,16]. Indeed, LPS stimulation of monocytes induces TF mRNA and protein expression in monocytes in a human model of endotoxemia [17,18]. Moreover, we have shown that reducing levels of TF expression in hematopoietic cells is associated with a reduction in TAT levels in endotoxemic mice [19]. TF expression by other cell types, such as endothelial cells, may also contribute to intravascular coagulation [1].
Levels of TF in human plasma can be measured using various methods, including enzyme-linked immunosorbent assays (ELISA), flow cytometry, an impedance assay and activity assays [9,20–24]. A wide range of TF antigen levels have been reported in healthy individuals, which appears to be due to the use of different anti-TF antibodies and the relatively high background associated with the commercial ELISA called IMUBIND® [25]. A commercial assay called ACTI-CHROME ® can be used to measure factor (F)Xa generation in plasma [22,26]. However, a recent study indicated that this assay did not measure TF activity in plasma because the observed FXa generation was not inhibited by active site inactivated FVIIa [27]. An alternative strategy is to isolate MPs from the plasma and assay their PCA. MPs can be collected by either capturing the MPs with a specific antibody called 1B10 [7] or by isolating the MPs by centrifugation [23,28]. In these assays, the procoagulant activity (PCA) is measured using a two-stage chromogenic activity assay in the presence and absence of an inhibitory anti-human TF antibody.
Human TF antigen and activity can be measured in plasma from mice bearing human tumors or in mice expressing human TF in place of mouse TF [13,14,19]. One study showed a human TF-dependent increase in thrombin generation in cell-free plasma from mice bearing human pancreatic tumors [14]. There have been several papers describing the measurement of PCA in mouse plasma or in isolated MPs from mice [29–31]. However, a limitation of these studies is that they did not use an anti-mouse TF antibody to determine the level of TF-dependent vs. TF-independent activity in the assays. In this study, we used an endotoxemia model to generate TF-positive MPs in various mice and compare the levels of MP TF activity with TAT complex.
Materials and methods
Materials
Lipopolysaccharide (LPS) (Escherichia coli serotype O111:B4), mouse IgG and rat IgG were obtained from Sigma-Aldrich (St Louis, MO, USA). Human FVIIa and human FX were purchased from Enzyme Research Laboratories (South Bend, IN, USA). Purified recombinant annexin V was purchased from BD Pharmingen™ (San Jose, CA, USA). Inactivated human recombinant FVIIa (FVIIai) was purchased from American Diagnostica (Stamford, CT, USA). Mouse FVIIa was kindly provided by Dr Lars Petersen (Novo Nordisk, Bagsvaerd, Denmark) [32]. Mouse FVIIa was subjected to the same tests as human FVIIa and has a comparable purity (Dr Lars Petersen, personal communication). A rat anti-mouse TF monoclonal antibody (1H1) was provided by Genentech Inc. (South San Francisco, CA, USA) [33]. A mouse anti-human TF monoclonal antibody (HTF-1) was kindly provided by Dr Ronald Bach (VAMC, Minneapolis, MN, USA) [34]. FXa substrate (Pefachrome FXa 8595) was obtained from Centerchem, Inc (Norwalk, CT, USA). Human recombinant relipidated TF (Dade Innovin) and Enzygnost TAT micro ELISA kit were purchased from Dade Behring (Marburg, Germany).
Mouse endotoxemia model
All studies were approved by the University of North Carolina-Chapel Hill Animal Care and Use Committee and comply with National Institute of Health guidelines. Wild-type mice (C57BL/6J) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Human chromosome vector (HTF) mice (~100% of wild-type TF levels except in the heart) and low TF mice (~1% of wild-type TF mice) were used in this study [35,36]. Mice (2–3 months old) were injected intraperitoneally with either LPS (7.5 mg kg−1) or saline. Six hours after LPS injection, whole blood was collected from the inferior vena cava into sodium citrate (final concentration: 0.38%). Mouse plasma was prepared by centrifugation at 4000 g for 15 min. Plasma was divided into 50-µL aliquots and frozen at −80 °C.
MP TF activity assay
50 µL of mouse plasma was diluted with 1 mL of HBSA (137 mm NaCl, 5.38 mm KCl, 5.55 mm glucose, 10 mm HEPES, 0.1% bovine serum albumin, pH 7.5) and MPs were pelleted at 20 000 g for 30 min at 4 °C, washed once with 1 mL of HBSA and re-suspended in 100 µL HBSA. Samples (50 µL each) were then incubated with the rat inhibitory anti-mouse TF antibody (1H1) (100 µg mL−1), the mouse anti-human TF antibody (HTF-1) (10 µg mL−1) or IgG controls for 15 min at room temperature. Human FVIIai and annexin V were used in some experiments. Next, 50µL of HBSA containing 10 nm human FVIIa, 300 nm FX and 10 mm CaCl2 was added to the sample and incubated for 2 h at 37 °C in a 96-well plate. Mouse FVIIa was used in some experiments. FXa generation was stopped by the addition of 25 µL of 25 mm EDTA buffer. Finally, 25 µL of the chromogenic substrate Pefachrome FXa 8595 (4 mm) was added and the mixture incubated at 37 °C for 15 min. Absorbance at 405 nm was measured using a VERSAmax microplate reader (Molecular Devices). The PCA of the sample was calculated by reference to a standard curve generated using recombinant human relipidated TF (0–14 pg mL−1). The TF-dependent PCA generation (pg mL−1) was determined by subtracting the amount of PCA generated in the presence of blocking antibodies from the amount of total PCA generated in the presence of the IgG controls.
Measurement of MPs
Phosphatidlyserine (PS)-positive MPs in mouse plasma were detected using a Zymuphen MP-activity kit (Hyphen BioMed, Neuville-sur-Oise, France) and expressed as PS equivalents (nm).
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Differences between different groups were analyzed using a one-tailed heteroscedastic Student’s t-test and a one-way anova. A probability value P < 0.05 was considered statistically significant. Correlation between MP TF activity and TAT was analyzed using Spearman’s rho and correlation was significant at the 0.01 level (two-tailed).
Results
Measurement of MP TF activity in plasma from control and endotoxemic wild-type mice
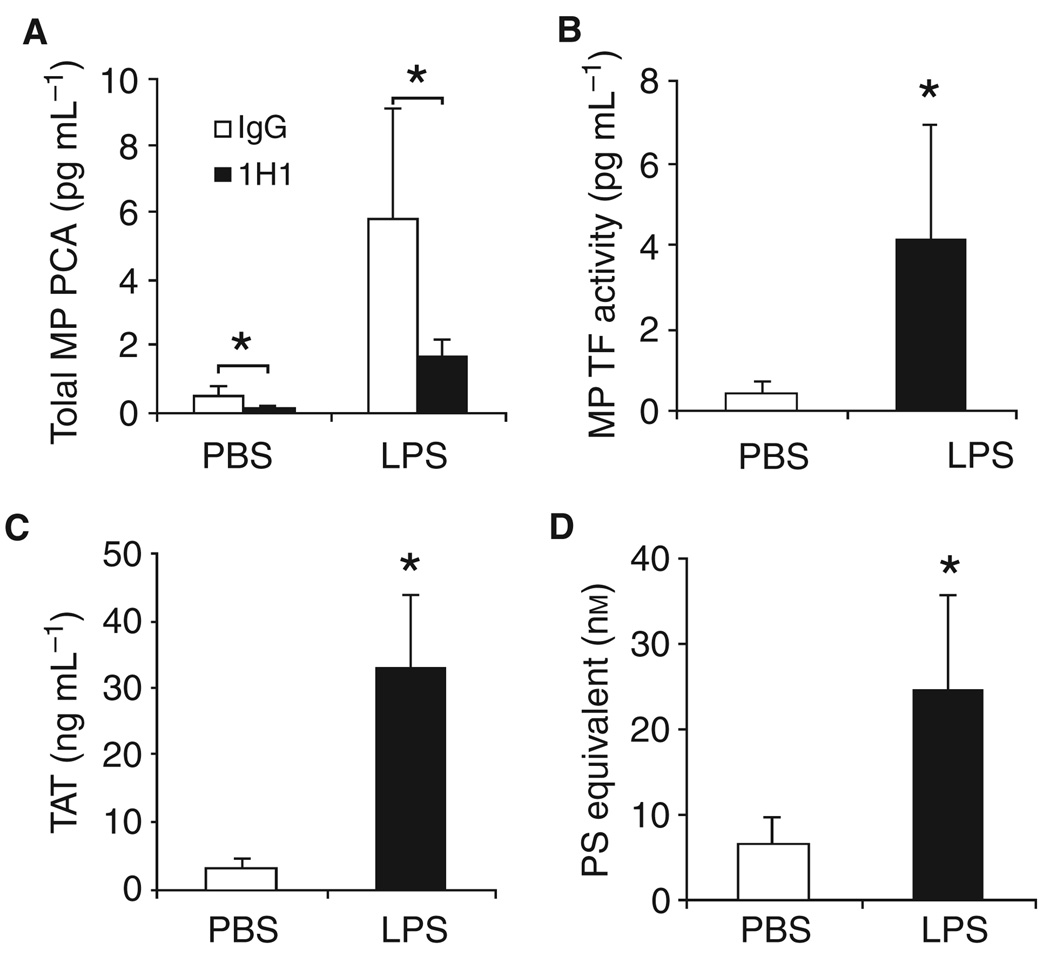
We chose a mouse endotoxemia model in which LPS (7.5 mg kg−1) was administered via a single intraperitoneal injection. In the first series of experiments, MP PCA was measured using mouse FVIIa and human FX in the presence of either the anti-mouse TF antibody 1H1 or rat IgG. We observed a low baseline of MP PCA in plasma from PBS-treated mice that was significantly reduced by 1H1 (Fig. 1A). Administration of LPS increased the level of MP PCA, which was significantly reduced by 1H1 (Fig. 1A). Endotoxemic mice had increased levels of MP TF activity compared with controls (Fig. 1B). Furthermore, we found increased levels of TAT and PS-positive MPs in the plasma from LPS-treated mice compared with control mice (Fig. 1C,D).
Fig. 1.
Lipopolysaccharide (LPS) increases levels of microparticles (MP), MP procoagulant activity (PCA) and TAT in wild-type mice. Wild-type C57/B6J mice were given an intraperitoneal injection of either phosphate-buffered saline (PBS) or LPS and blood was collected after 6 h. (A) Total MP PCA was measured using mouse factor (F)VIIa and human FX in the presence of rat IgG (white bars) or 1H1 (black bars). (B) MP tissue factor (TF) activity was calculated by subtraction of the MP PCA generated in the presence of 1H1 from the total MP PCA generated in the presence of IgG control. (C) Levels of TAT in the plasma from mice treated with PBS (white bar) or LPS treated (black bar). (D) The number of PS-positive MPs in plasma from mice treated with PBS (white bar) or LPS (right bar) is shown. All results are shown as mean ± SD, n = 4–6 mice per group. Asterisks indicate statistically significant differences between the groups.
Measurement of MP PCA in wild-type mice using mouse FVIIa vs. human FVIIa
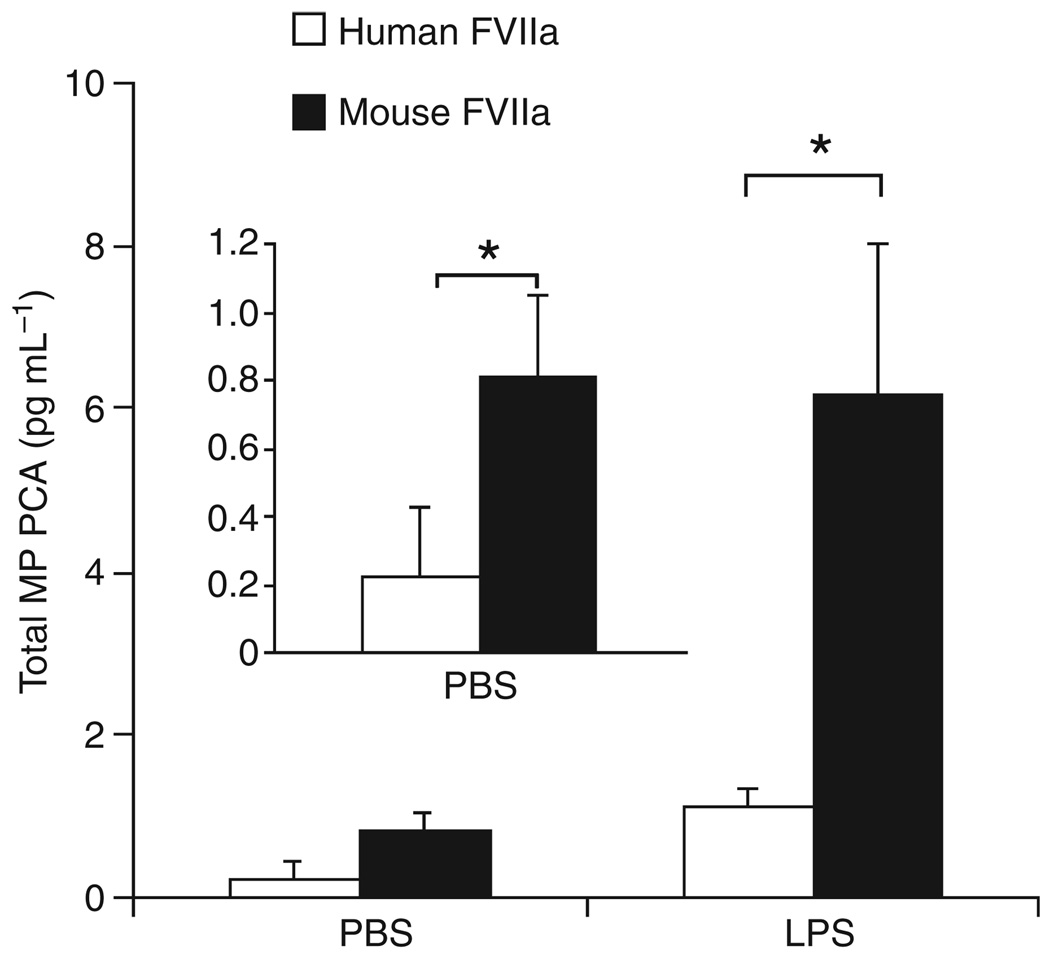
We measured the level of PCA in MPs from wild-type mice using either mouse FVIIa or human FVIIa. As expected, we detected significantly higher levels of MP PCA in control and LPS-treated mice using mouse FVIIa compared with human FVIIa (Fig. 2). Use of mouse FVIIa was associated with a 6-fold increase in MP PCA.
Fig. 2.
Measurement of microparticle (MP) procoagulant activity (PCA) in wild-type mice using mouse or human factor (F)VIIa. Total MP PCA in plasma from wild-type mice treated with either phosphate-buffered saline (PBS) control (left bars) or lipopolysaccharide (LPS) (right bars) was measured using either human FVIIa (white bars) or mouse FVIIa (black bars) and human FX. The inset is an enhanced plot of the PBS control. Data are shown as mean ± SD (n = 5). Asterisks indicate a statistically significant difference between the MP PCA observed with human FVIIa.
Use of species-specific anti-TF antibodies to measure TF activity in MP from mice expressing mouse or human TF
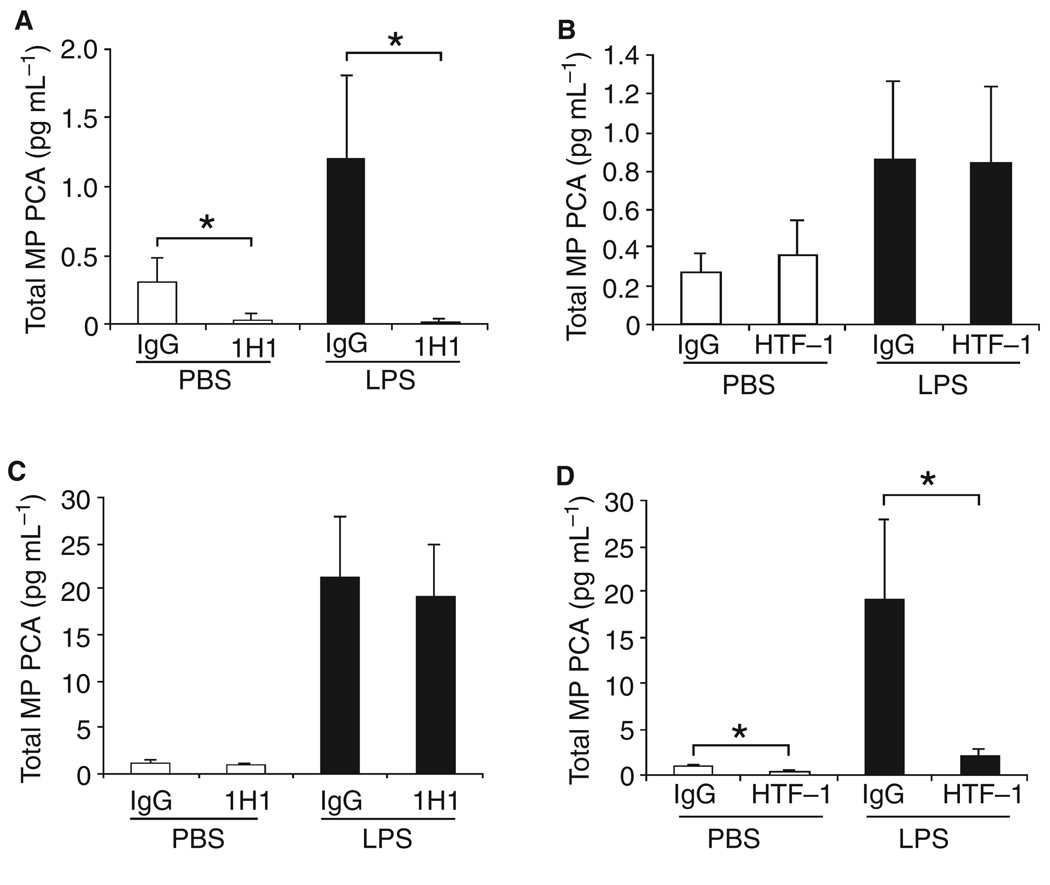
We used species-specific inhibitory antibodies against either mouse TF (1H1) or human TF (HTF-1) to distinguish between mouse and human TF activity in MPs from wild-type mice and mice expressing human TF (HTF mice). Human FVIIa was used in these studies. The PCA of MPs from wild-type mice with or without LPS was significantly reduced by 1H1 but not by HTF-1, indicating that the PCA was due to mouse TF (Fig. 3A,B). Conversely, the PCA of MPs from HTF mice with or without LPS was inhibited by HTF-1 but not 1H1, indicating that the PCA was due to human TF (Fig. 3C,D). These results indicate that species-specific antibodies can be used to measure mouse and human TF-dependent PCA in MPs derived from genetically modified mice.
Fig. 3.
Species-specific inhibition of microparticle (MP) procoagulant activity (PCA) from wild-type and human TF (HTF) mice. (A and B) Total MP PCA in plasma from wild-type mice treated with either phosphate-buffered saline (PBS) control (left bars) or lipopolysaccharide (LPS) (right bars) was measured using human factor (F)VIIa and human FX in the presence of 1H1 or isotype IgG or HTF-1 or isotype IgG. Data are shown as mean ± SD (n = 6–7). (C and D) Total MP PCA in plasma from HTF mice treated with either PBS control (left bars) or LPS (right bars) was measured using human FVIIa and human FX in the presence of 1H1 or isotype IgG or HTF-1 or isotype IgG. Data are shown as mean ± SD (n = 4). Asterisks indicate statistically significant differences between the groups.
Inhibition of MP PCA using human FVIIai and annexin V
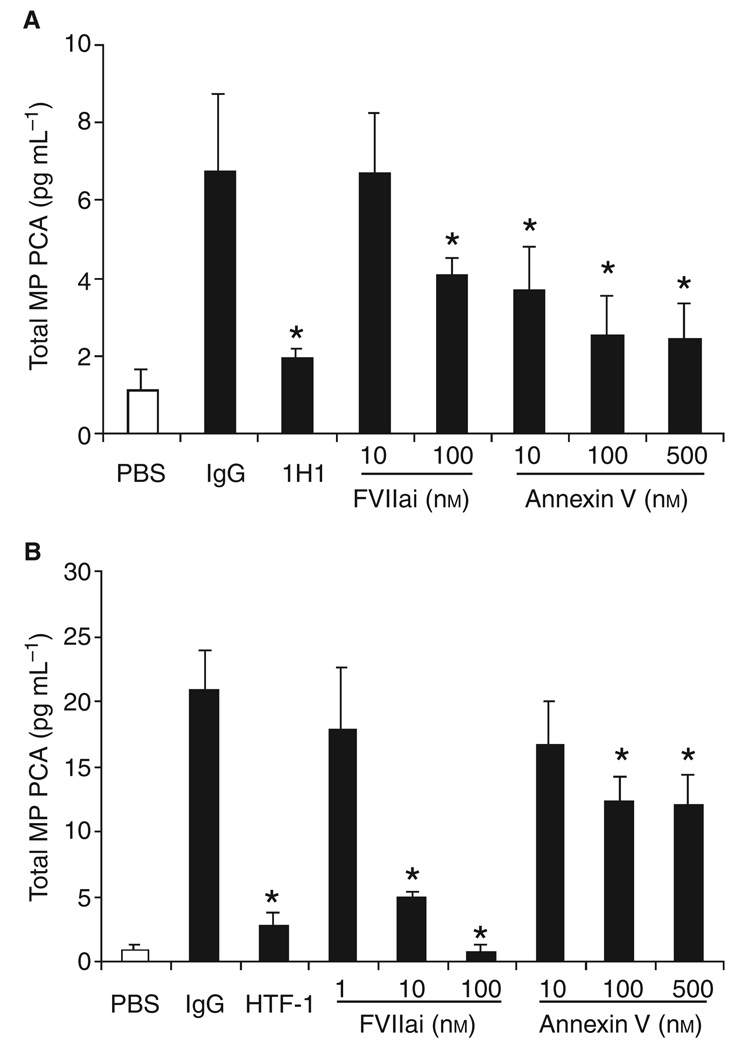
We determined the effect of human FVIIai on the MP PCA isolated from wild-type and HTF mice. As expected, human FVIIai was a more potent inhibitor of PCA of MPs from HTF mice compared with the PCA of MPs from wild-type mice (Fig. 4A,B). We also examined the effect of annexin V on MP PCA. Annexin V has been shown to reduce the activity of relipidated TF by binding to the anionic phospholipid PS [37]. We found that annexin V partially inhibited the PCA of MPs from both wild-type and HTF mice.
Fig. 4.
Effect of human factor (F)VIIai and annexin V on microparticle (MP) procoagulant activity (PCA) from wild-type and human TF (HTF) mice. (A) Total PCA in MPs isolated from plasma of wild-type mice treated with either phosphate-buffered saline (PBS) control (white bar) or lipopolysaccharide (LPS) (black bars) was measured using mouse FVIIa and human FX in the presence of 1H1, isotype IgG, human FVIIai or annexin V. (B) Total MP PCA from plasma of HTF mice treated with either PBS control (white bar) or LPS (black bars) was measured using human FVIIa and human FX in the presence of HTF-1, isotype IgG, human FVIIai or annexin V. Data are shown as mean ± SD (n = 4). Asterisks indicate statistically significant differences relative to the IgG control.
Levels of MP TF activity correlate with levels of TAT in endotoxemic mice
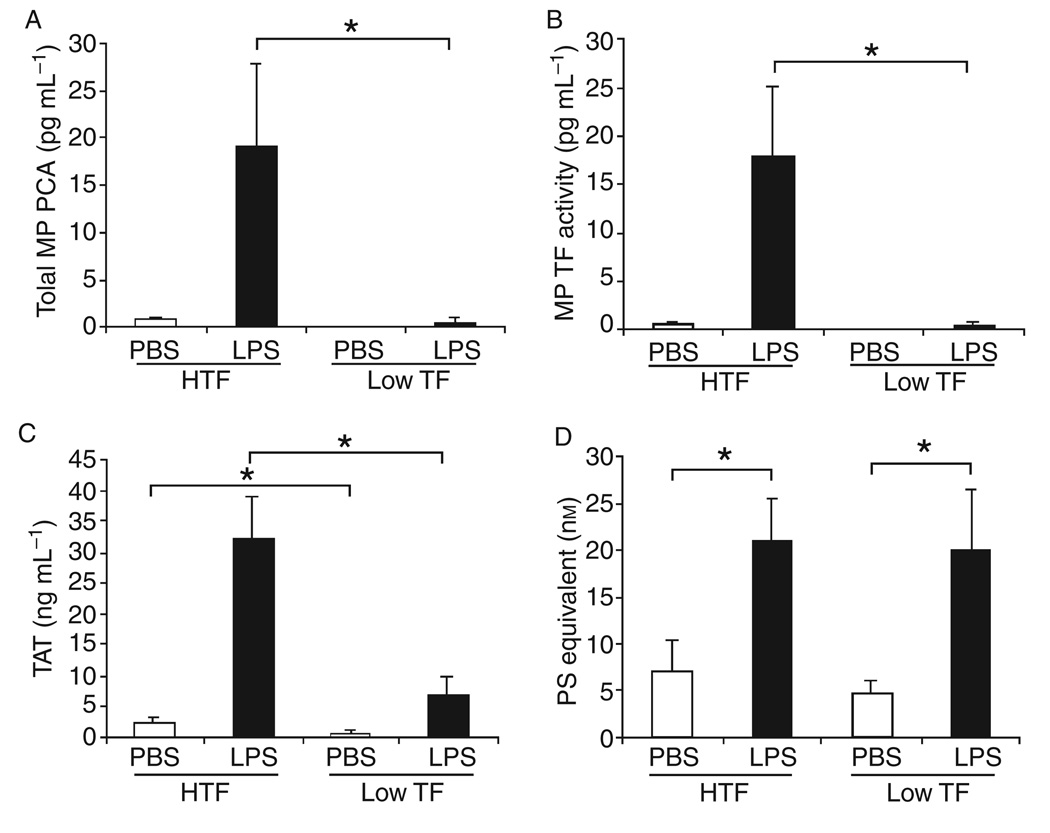
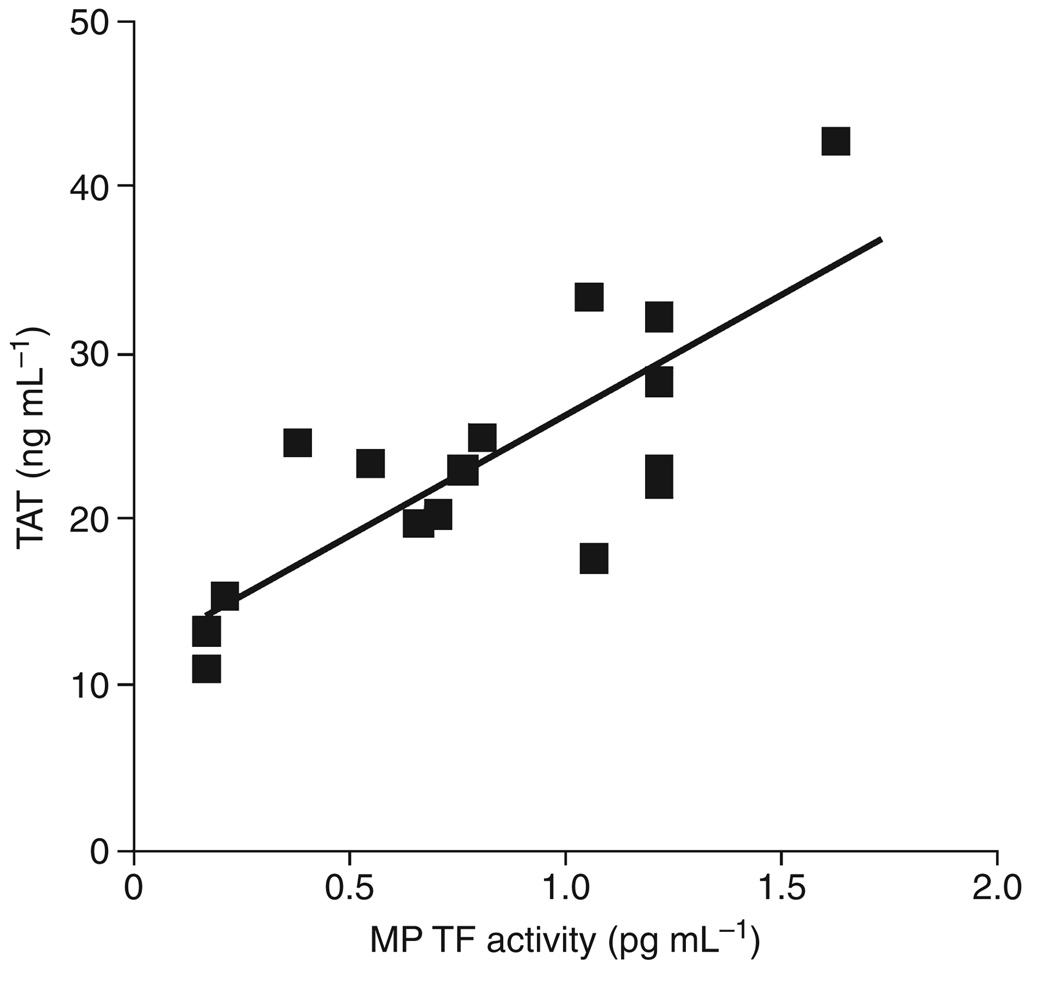
We and others have used plasma TAT levels to measure the activation of coagulation in mice [19,38]. First, we analyzed the levels of MP PCA, MP TF activity and TAT in mice expressing high (HTF) and low (low TF) levels of human TF. Low TF mice expressed significantly lower levels of MP PCA, MP TF activity and TAT compared with HTF mice (Fig. 5A–C). However, low TF and HTF mice had similar levels of PS-positive MPs (Fig. 5D). Second, we compared the levels of MP TF activity and TAT in wild-type mice. Importantly, we found that there was a strong correlation between MP TF activity and TAT levels in endotoxemic wild-type mice (Fig. 6). No correlation was found between PS-positive MPs and TAT (data not shown). Levels of MP TF activity also correlated with TAT in mice expressing human TF (data not shown). These results indicate that the presence of TF-positive MPs is a better predictive marker of activation of the clotting cascade than PS-positive MPs.
Fig. 5.
Levels of microparticle (MP) procoagulant activity (PCA), MP tissue factor (TF) activity, TAT and MPs in human TF (HTF) and Low TF Mice. Total MP PCA (A) and MP TF activity (B) in plasma from HTF and low mice treated with either PBS control (white bars) or LPS (black bars) was measured using human FVIIa and human FX. TF activity in the MPs was determined using HTF-1. Data are shown as mean ± SD (n = 4–5). (C) Levels of TAT in the plasma from HTF (left bars) and low TF (right bars) mice treated with phosphate-buffered saline (PBS) (white bars) or lipopolysaccharide (LPS)-treated (black bars). (D) Number of PS-positive MPs in plasma from HTF mice (left bars) and low TF mice (right bars). All results are shown as mean ± SD, (n = 4–6). Asterisks indicate statistically significant differences between the groups.
Fig. 6.
Levels of microparticle (MP) tissue factor (TF) activity correlate with levels of TAT. A significant linear correlation was observed between levels of MP TF activity and TAT in endotoxemic wild-type mice (R = 0.78, P < 0.01, n = 16).
Discussion
TF-bearing MPs appear to play a role in thrombotic complication associated with a variety of diseases, including sepsis and cancer [5–11]. As mice are the most common laboratory animals used for in vivo experiments, we developed an assay to measure levels of circulating TF-positive MPs in mice. We found that administration of LPS increased the level of MP TF activity in wild-type mice. In addition, MP TF activity was significantly higher in endotoxemic mice expressing high levels of human TF compared with mice expressing low levels of human TF. However, the number of circulating PS-positive MPs was the same in these two groups of mice. Importantly, we observed a significant linear correlation between MP TF activity and TAT in endotoxemic wild-type mice (R = 0.78, P < 0.01, n = 16). A previous study showed a similar correlation between levels of human TF antigen and TAT in plasma from mice bearing human pancreatic tumors [14]. These data strongly suggest that MP TF activates coagulation in mouse models of endotoxemia and cancer.
Previous studies measuring PCA in mouse plasma or in isolated MPs from wild-type mice used a two-stage assay with human FVIIa and human FX [29,31]. However, these studies could not distinguish between TF-dependent and TF-independent activity because an inhibitory anti-mouse TF antibody was not used. To date, a major limitation in measuring mouse TF activity has been the lack of an inhibitory anti-mouse TF antibody. Importantly, the rat anti-mouse TF antibody called 1H1 was shown to inhibit mouse TF activity [33]. It has been used in two studies in mice to show that TF plays a role in inflammation in a colitis model and in antiphospholipid antibody-induced fetal loss [39,40]. We found that 1H1 and HTF-1 inhibited the majority of the PCA of MP in endotoxemic wild-type mice and HTF mice. The residual PCA could be due to either incomplete blocking of TF activity or low levels of TF-independent PCA. As expected, human FVIIai was a more potent inhibitor of the PCA of MPs isolated from endotoxemic HTF mice than the PCA of MPs from wild-type mice. Annexin V also partially inhibited the PCA of MPs from both wild-type and HTF mice, suggesting that PS contributed to the PCA.
Petersen et al. [32] reported that the association of human FVIIa to mouse TF is about three orders of magnitude weaker than the association to human TF, whereas mouse FVIIa binds with high affinity to both mouse and human TF. This result has led to the notion that human FVIIa cannot be used to measure the PCA of mouse TF. However, FX activation by 10 nm of mouse FVIIa on mouse TF-expressing cells was only 2-fold higher that that observed with 10 nm of human FVIIa [32]. In our study, we found that human FVIIa generated 6-fold less FXa with mouse TF-positive MPs than the level of FXa generated with mouse FVIIa. Our data indicate that human FVIIa can be used to measure MP TF activity in mouse plasma, albeit with a slightly reduced sensitivity compared with mouse FVIIa.
In conclusion, measurement of the PCA of MPs should be performed in the presence and absence of an inhibitory anti-mouse antibody to distinguish TF-dependent vs. TF-independent PCA. We found that levels of MP TF activity but not PS-positive MPs correlated with TAT, which indicates that TF-positive MPs are a better biomarker for thrombosis risk than total MPs in a mouse model of endotoxemia.
Acknowledgements
We would like to thank N. Key, M. Monroe and A. Wolberg for critical reading of the manuscript and funding from the National Institutes of Health and the University of North Carolina at Chapel Hill Cancer Research Fund.
Footnotes
Addendum
J. Wang performed the experiments, analyzed data and co-wrote the manuscript; D. Manly analyzed data and co-wrote the manuscript; D. Kirchhofer contributed to experimental design; R. Pawlinski contributed to experimental design; N. Mackman conceived the research, analyzed data, and co-wrote the manuscript.
Disclosure and Conflict of Interests
The authors state they have no conflict of interest.
References
- 1.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 2.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nature Med. 2003;9:458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 3.Bruggemann LW, Drijfhout JW, Reitsma PH, Spek CA. Alternatively spliced tissue factor in mice: induction by Streptococcus pneumoniae. J Thromb Haemost. 2006;4:918–920. doi: 10.1111/j.1538-7836.2006.01870.x. [DOI] [PubMed] [Google Scholar]
- 4.Morel O, Toti F, Hugel B, Bakouboula B, Camoin-Jau L, Dignat-George F, Freyssinet JM. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–2604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- 5.Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, Maquelin KN, Roozendaal KJ, Jansen PGM, ten Have K, Eijsman L, Hack CE, Sturk A. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation. 1997;96:3534–3541. doi: 10.1161/01.cir.96.10.3534. [DOI] [PubMed] [Google Scholar]
- 6.Diamant M, Nieuwland R, Pablo RF, Sturk A, Smit JW, Radder JK. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106:2442–2447. doi: 10.1161/01.cir.0000036596.59665.c6. [DOI] [PubMed] [Google Scholar]
- 7.Aras O, Shet A, Bach RR, Hysjulien JL, Slungaard A, Hebbel RP, Escolar G, Jilma B, Key NS. Induction of microparticle-and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–4553. doi: 10.1182/blood-2003-03-0713. [DOI] [PubMed] [Google Scholar]
- 8.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 9.Hron G, Kollars M, Weber H, Sagaster V, Quehenberger P, Eichinger S, Kyrle PA, Weltermann A. Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost. 2007;97:119–123. [PubMed] [Google Scholar]
- 10.Biro E, Sturk-Maquelin KN, Vogel GM, Meuleman DG, Smit MJ, Hack CE, Sturk A, Nieuwland R. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561–2568. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 11.Mallat Z, Hugel B, Ohan J, Leseche G, Freyssinet JM, Tedgui A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation. 1999;99:348–353. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]
- 12.Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004;104:3190–3197. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- 13.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–1741. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 14.Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost. 2008;6:1517–1524. doi: 10.1111/j.1538-7836.2008.02987.x. [DOI] [PubMed] [Google Scholar]
- 15.Taylor FB, Chang ACK, Peer GT, Mather T, Blick K, Catlett R, Lockhart MS, Esmon CT. DEGR-factor Xa blocks disseminated intravascular coagulation initiated by Escherichia coli without preventing shock or organ damage. Blood. 1991;78:364–368. [PubMed] [Google Scholar]
- 16.Osterud B, Bjorklid E. Sources of tissue factor. Semin Thromb Hemost. 2006;32:11–23. doi: 10.1055/s-2006-933336. [DOI] [PubMed] [Google Scholar]
- 17.Gregory SA, Morrissey JH, Edgington TS. Regulation of tissue factor gene expression in the monocyte procoagulant response to endotoxin. Mol Cell Biol. 1989;9:2752–2755. doi: 10.1128/mcb.9.6.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franco RF, de Jonge E, Dekkers PEP, Timmerman JJ, Spek CA, van Deventer SJH, van Deursen P, van Kerkhoff L, van Gemen B, ten Cate H, van der Poll T, Reitsma PH. The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: relationship with activation of coagulation. Blood. 2000;96:554–559. [PubMed] [Google Scholar]
- 19.Pawlinski R, Pedersen B, Schabbauer G, Tencati M, Holscher T, Boisvert W, Andrade-Gordon P, Frank RD, Mackman N. Role of tissue factor and protease activated receptors in a mouse model of endotoxemia. Blood. 2004;103:1342–1347. doi: 10.1182/blood-2003-09-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soejima H, Ogawa H, Yasue H, Kaikita K, Nishiyama K, Misumi K, Takazoe K, Miyao Y, Yoshimura M, Kugiyama K, Nakamura S, Tsuji I, Kumeda K. Heightened tissue factor associated with tissue factor pathway inhibitor and prognosis in patients with unstable angina. Circulation. 1999;99:2908–2913. doi: 10.1161/01.cir.99.22.2908. [DOI] [PubMed] [Google Scholar]
- 21.Misumi K, Ogawa H, Yasue H, Soejima H, Suefuji H, Nishiyama K, Takazoe K, Kugiyama K, Tsuji I, Kumeda K, Nakamura S. Comparison of plasma tissue factor levels in unstable and stable angina pectoris. Am J Cardiol. 1998;81:22–26. doi: 10.1016/s0002-9149(97)00801-1. [DOI] [PubMed] [Google Scholar]
- 22.El-Ghoroury EA, El-Din HG, Abdel-Kader M, Ragab S. Study of factor VII, tissue factor pathway inhibitor and monocyte tissue factor in noninsulin-dependent diabetes mellitus. Blood Coagul Fibrinolysis. 2008;19:7–13. doi: 10.1097/01.mbc.0000304148.26525.da. [DOI] [PubMed] [Google Scholar]
- 23.Tesselaar ME, Romijn FP, van der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 24.Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit Rey Oncol Hematol. 2007;62:126–136. doi: 10.1016/j.critrevonc.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Parhami-Seren B, Butenas S, Krudysz-Ambio J, Mann KG. Immunologic quantitation of tissue factor. J Thromb Haemost. 2006;4:1747–1755. doi: 10.1111/j.1538-7836.2006.02000.x. [DOI] [PubMed] [Google Scholar]
- 26.Krupinski J, Turu MM, Font MA, Catena E, Slevin M, Morchon S, Rubio F, Badimon L, Martinez-Gonzalez J. Blood-borne tissue factor activity predicts major cerebrovascular events in patients undergoing carotid endarterectomy: results from a 1-year follow-up study. Cerebrovasc Dis. 2008;25:32–39. doi: 10.1159/000111497. [DOI] [PubMed] [Google Scholar]
- 27.Bogdanov VY, Cimmino G, Tardos JG, Tunstead JR, Badimon JJ. Assessment of plasma tissue factor activity in patients presenting with coronary artery disease: limitations of a commercial assay. J Thromb Haemost. 2009 Feb 13; doi: 10.1111/j.1538-7836.2009.03315.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, Mackman N, Taubman MB. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–1985. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hrachovinova I, Cambien B, Hafezi-Moghadam A, Kappelmayer J, Camphausen RT, Widom A, Xia L, Kazazian HH, Jr, Schaub RG, McEver RP, Wagner DD. Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A. Nature Med. 2003;9:1020–1025. doi: 10.1038/nm899. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh M, Wang H, Youxi A, Romero E, Luyendyk J, Peters M, Mackman N, Dey S, Hla T. COX-2 suppresses tissue factor expression via endocannabinoid-directed PPARdelta activation. J Exp Med. 2007;204:204–209. doi: 10.1084/jem.20070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Miller C, Swarthout RF, Rao M, Mackman N, Taubman MB. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2008;113:705–713. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen LC, Norby PL, Branner S, Sorensen BB, Elm T, Stennicke HR, Persson E, Bjorn SE. Characterization of recombinant murine factor VIIa and recombinant murine tissue factor: a human-murine species compatibility study. Thromb Res. 2005;116:75–85. doi: 10.1016/j.thromres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Kirchhofer D, Moran P, Bullens S, Peale F, Bunting S. A monoclonal antibody that inhibits mouse tissue factor function. J Thromb Haemost. 2005;3:1098–1099. doi: 10.1111/j.1538-7836.2005.01253.x. [DOI] [PubMed] [Google Scholar]
- 34.Carson SD, Ross SE, Bach R, Guha A. An inhibitory monoclonal antibody against human tissue factor. Blood. 1987;70:490–493. [PubMed] [Google Scholar]
- 35.Parry GC, Erlich JH, Carmeliet P, Luther T, Mackman N. Low levels of tissue factor are compatible with development and hemostasis in mice. J Clin Invest. 1998;101:560–569. doi: 10.1172/JCI814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pawlinski R, Tencati M, Holscher T, Pedersen B, Voet T, Tilley RE, Marynen P, Mackman N. Role of cardiac myocyte tissue factor in heart hemostasis. J Thromb Haemost. 2007;5:1693–1700. doi: 10.1111/j.1538-7836.2007.02649.x. [DOI] [PubMed] [Google Scholar]
- 37.Wolberg AS, Monroe DM, Roberts HR, Hoffman MR. Tissue factor de-encryption: ionophore treatment induces changes in tissue factor activity by phosphatidylserine-dependent and -independent mechanisms. Blood Coagul Fibrinolysis. 1999;10:201–210. [PubMed] [Google Scholar]
- 38.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, Castellino FJ, Mackman N, Griffin JH, Weiher H. Exdotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anthoni C, Russell J, Wood KC, Stokes KY, Vowinkel T, Kirchhofer D, Granger DN. Tissue factor: a mediator of inflammatory cell recruitment, tissue injury and thrombus formation in experimental colitis. J Exp Med. 2007;204:1595–1601. doi: 10.1084/jem.20062354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redecha P, Tilley R, Tencati M, Salmon JE, Kirchhofer D, Mackman N, Girardi G. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110:2423–2431. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]