Abstract
The serine protease zymogen factor X is converted to its catalytically active form factor Xa by the binary complex of factor VIIa bound to its cell surface receptor tissue factor (TF) or by the intrinsic Xase complex, which consists of active factors VIII (VIIIa), IX (IXa), factor X, and Ca2+. Factor Xa has procoagulant activity by conversion of prothrombin to thrombin and also induces signal transduction, either alone or in the ternary TF:VIIa:factor Xa coagulation initiation complex. Factor Xa cleaves and activates protease activated receptor (PAR)1 or -2, but factor Xa signaling efficiency varies among cell types. We show here that annexin 2 acts as a receptor for factor Xa on the surface of human umbilical vein endothelial cells and that annexin 2 facilitates factor Xa activation of PAR-1 but does not enhance coagulant function of factor Xa. Overexpression of TF abolishes annexin 2 dependence on factor Xa signaling and diminishes binding to cell surface annexin 2, whereas selectively abolishing TF promotes the annexin 2/factor Xa interaction. We propose that annexin 2 serves to regulate factor Xa signaling specifically in the absence of cell surface TF and may thus play physiological or pathological roles when factor Xa is generated in a TF-depleted environment.
Keywords: annexin 2, endothelial cells, factor Xa, signal transduction, tissue factor
The serine protease zymogen factor X is converted to catalytically active protease factor Xa on binding to the binary complex of the cell surface receptor tissue factor (TF) with its protease ligand factor VIIa (VIIa)1 or by the intrinsic pathway. The transient TF:VIIa:factor Xa ternary complex is the target for physiological inhibitory control by TF pathway inhibitor.2 On dissociation from TF:VIIa and association with its cofactor factor Va, factor Xa proteolytically converts prothrombin to thrombin, which in turn leads to fibrin formation, fibrin deposition, and thrombus formation (reviewed elsewhere3). The intact form of factor Xa, Xaα, can be cleaved by autoproteolysis, resulting in the excision of a 4-kDa peptide and the formation of Xaβ.4,5 Factor Xaα and Xaβ behave identically as enzymes,6 although recent work has shown that further cleavage by plasmin results in modifications that eliminate clotting and amidolytic activity.7
In addition to promoting coagulation, coagulation proteases induce signal transduction through the activation of G protein–coupled protease-activated receptors (PARs).8–12 Thrombin signaling has been extensively characterized and occurs via cell type–specific combinations of PAR-1, PAR-3, and PAR-4.13–16 TF-dependent coagulation complexes also directly activate PARs.2,9,17 TF:VIIa activates PAR-218 and the ternary TF:VIIa:factor Xa complex signals by factor Xa–dependent activation of either PAR-1 or PAR-2.8,12,19 However, factor Xa–dependent signaling independent of the TF initiation complex has also been demonstrated in certain cell types, albeit with lesser efficiency.2
In this study, we characterize a previously unrecognized cell surface factor Xa receptor function for annexin 2. The annexins are a family of phospholipid-binding membrane proteins that were originally identified as mediators of cellular responses to changes in intracellular Ca2+ levels.20 Annexin 2 dimerizes with p11 to form a heterotetramer.21 Annexin 2 binds plasminogen and stimulates its activation by tissue plasminogen activator,22 with the p11 subunit believed to be responsible for the direct interaction with tissue plasminogen activator, plasmin, and plasminogen.23 The interaction with p11 is dependent on C-terminal lysine residues, and this interaction is sensitive to inhibition by ε-amino caproic acid (EACA).24
We here demonstrate that factor Xa binds to annexin 2 specifically and annexin 2 regulates factor Xa–mediated signal transduction independent of procoagulant activity. Our findings support the conclusion that annexin 2 serves as a previously unrecognized cell surface receptor for factor Xa to enable signal transduction in the absence of cell surface TF.
Materials and Methods
Mice
Generation of annexin 2−/− and TFflox/flox mice has been described previously.25,26 TFflox/flox mice were crossed with LysM Cre mice to generate TFflox/floxLysM Cre+ mice lacking TF in myeloid cells. All mouse experiments were carried out under approved care and use protocols.
Materials
Factor Xa, Xaβ, Xa-EGR, Gla domainless factor Xa [Xa(-)Gla], factor Va, thrombin, and mouse monoclonal antibody against the heavy chain of factors X and Xa were from Hematologic Technologies (Essex Junction, Vt). Rabbit polyclonal antibody which binds both factors X and Xa (anti-X/Xa) was prepared as previously described.27 Plasminogen was purified as previously described.28 Angiostatin was from Calbiochem (San Diego, Calif). Mouse monoclonal antibodies against annexins 1 and 2 and p11 were from BD Biosciences (San Jose, Calif). Glutathione S-transferase–fused recombinant annexin 2, annexin 1, and p11 were from Abnova (Taipei, Taiwan). Fluorescein isothiocyanate and Texas red–conjugated immunoglobulins (IgGs) were from Vector Labs (Burlingame, Calif). Nap5 was from Corvas Inc (San Diego, Calif). EACA and other chemicals were from Sigma (St Louis, Mo), unless otherwise specified. Mouse monoclonal PAR-1 antibodies, WEDI and ATAP-2, and rabbit polyclonal PAR-2 antibody have been described previously.19,29
Mass Spectrometry
Matrix-assisted laser desorption/ionization (MALDI) mass spectrometric experiments were performed as described previously.30
Cell Culture and Adenoviral Transduction
Human umbilical vein endothelial cells (HUVECs) were from Clonetics (San Diego, Calif) and grown according to the instructions of the supplier. Ad5 serotype vectors expressing full-length human TF have been described previously in detail.11,31 HUVECs grown to 50% to 60% confluence were transduced with TF (50 particles per cell) for 3.5 hours and grown for 48 hours.
Cell Surface Binding, Immunoprecipitation, and Western Blotting
Factors X and Xa were incubated with HUVECs for 4 hours at 4°C at increasing concentrations, followed by extensive washing to remove unbound protein and quantitation via Western blotting. HUVECs were grown to confluence in 6-well plates and then incubated with factor Xa (200 nmol/L) alone or in the presence of Nap5 (1 µmol/L), hirudin (100 nmol/L), EDTA (1 mmol/L), or EACA (10 to 50 mmol/L) for 4 to 6 hours at 4°C in HBS (25 mmol/L Hepes, 0.9% saline, pH 7.5) supplemented with 5 mmol/L CaCl2 and 0.02% Tween 20. Cells were then extensively washed and solubilized with 50 mmol/L n-octyl-β-d-glucopyranoside, followed by incubation with anti-X/Xa coupled to Dynabeads (Invitrogen, Carlsbad, Calif) for 12 to 16 hours at 4°C. Beads were then extensively washed and resuspended in 2× SDS reducing buffer followed by PAGE and Western blotting with anti-X/Xa or monoclonal antibodies against annexins 1 and 2, p11, and IgG as control. Quantification was performed using Scion Image (NIH, Bethesda Md) within the linear range of the detection apparatus. Plasma membrane purification was performed using the Qproteome plasma membrane purification kit (Qiagen, Valencia, Calif). Factor Xa binding and annexin 2 coimmunoprecipitation were also assayed in thioglycollate-elicited peritoneal macrophages isolated from TFflox/floxLysM Cre+ and TFflox/flox/LysM Cre− mice.
Protein/Protein Binding Assay
Glutathione S-transferase–fused recombinant annexin 2, annexin 1, and p11 were coated on to Reacti-Bind glutathione-coated plates (Pierce, Rockford, Ill) according to the protocol of the manufacturer. Increasing concentrations of factor Xa (10 to 400 nmol/L) was incubated on the plates for up to 4 hours at 4°C in HBS supplemented with 5 mmol/L CaCl2 and 0.02% Tween 20, followed by extensive washing with ice-cold HBS to remove unbound factor Xa. The remaining annexin 2 bound factor Xa was quantitated with the chromogenic substrate Spectrozyme FXa (American Diagnostics, Greenwich, Conn). Absorbance (405 nm) was converted to factor Xa (picomoles bound) using an established standard curve.
Immunofluorescence
For confocal imaging, nonpermeabilized HUVECs, grown to confluence on cover slips, were incubated with factor Xa as described above but in the absence of Tween 20 to prevent permeabilization. Cells were fixed in 10% formalin in HBS for 15 minutes at 4°C, followed by incubation with rabbit anti-X/Xa (10 µg/mL) in combination with mouse monoclonal anti–annexin 2 (10 µg/mL) for 1 to 2 hours at 4°C. Cells were then washed extensively and incubated with Texas red–conjugated anti-rabbit IgG (1:200) and fluorescein isothiocyanate–conjugated anti-mouse IgG (1:200) for 1 hour at 4°C, followed by extensive washing and mounting in Vectashield with 4′,6-diamidino-2-phenylindole (Vector Labs). Cells were visualized by confocal microscopy using a Bio-Rad MRC1024 laser scanning confocal microscope followed by quantification with Image Pro Plus (Media Cybernetics, Silver Spring, Md). Control experiments were performed using secondary antibodies in the absence of primary. TF was detected in transduced cells using the 9C3 monoclonal antibody32 (5 µg/mL) directly conjugated to Texas red.
Extracellular Signal-Regulated Kinase 1/2 Phosphorylation
TF-transduced and nontransduced HUVECs were incubated in serum-free conditions (medium 199 supplemented with 2 mmol/L l-glutamine and 1 mmol/L CaCl2) for 5 hours at 37°C. Cells were then incubated with factor Xa (50 nmol/L) for 5 minutes, followed by lysis in 2× SDS reducing buffer for Western blot analysis using phosphospecific extracellular signal-regulated kinase (ERK)1/2 and non–phospho-ERK1/2 rabbit polyclonal antibodies (Cell Signaling, Danvers, Mass) as described previously. To assay the effect of annexin 2 on factor Xa–induced ERK phosphorylation, anti–annexin 2 (20 µg/mL) was preincubated for 15 minutes before the addition of factor Xa. Control experiments were performed with mouse monoclonal antibodies against PAR-1 (50 µg/mL WEDI and 25 µg/mL ATAP-2) and rabbit polyclonal antibody against PAR-2 (200 µg/mL). All experiments were performed in the presence of hirudin to exclude thrombin effects.
Thrombin Generation Assay
HUVECs grown to confluence in 12-well polystyrene plates were preincubated with anti–annexin 2 (20 µg/mL) for 15 minutes, followed by addition of factor Xa or Xaβ (50 pmol/L), factor Va (400 pmol/L), prothrombin (1.3 µmol/L) for 10 minutes at 37°C, followed by addition of S-2238 Chromogenix thrombin substrate from Diapharma (West Chester, Ohio), followed by kinetic assay at 405 nm. Absorbance was converted to units per milliliter thrombin using an established standard curve.
Cell-Based Factor Xa Generation Assay
Cells grown to confluence in 12-well polystyrene plates were incubated in the presence of 20 µg/mL anti–annexin 2 or anti–annexin 1 for up to 4 hours at 37°C. Cells were then washed twice with Hepes (10 mmol/L) buffered saline supplemented with 5 mmol/L CaCl2. Factors VIIa (10 nmol/L) and X (50 nmol/L) were then added. Reactions were stopped at selected time points by adding 100 mmol/L EDTA to the sample and the activity of the generated factor Xa in the medium was quantitated with the chromogenic substrate Spectrozyme FXa. Absorbance (405 nm) was converted to factor Xa generated (picomoles per minute) using an established standard curve.
Single-Stage Clotting Assay
Peritoneal macrophages were isolated from thioglycollate injected wild-type and annexin 2 knockout mice and allowed to adhere in 6-well polystyrene plates for 3 hours (37°C). Nonadherent cells were removed by extensive washing and the remaining, adherent macrophages incubated overnight at 37°C. Untreated and lipopolysaccharide-treated (1 µg/mL, 4 hours, 37°C) cells were washed with HBS and left intact or solubilized in 15 mmol/L n-octyl-β-d-glucopyranoside. Time to clot was measured using a START4 Coagulation Analyzer (Diagnostica Stago, Parsippany, NJ).
Data Analysis
All data are representative of a minimum of 4 experiments performed in triplicate and are shown as means±SD.
Results
Factor Xa Binds to Endothelial Cells and Coprecipitates With Annexin 2
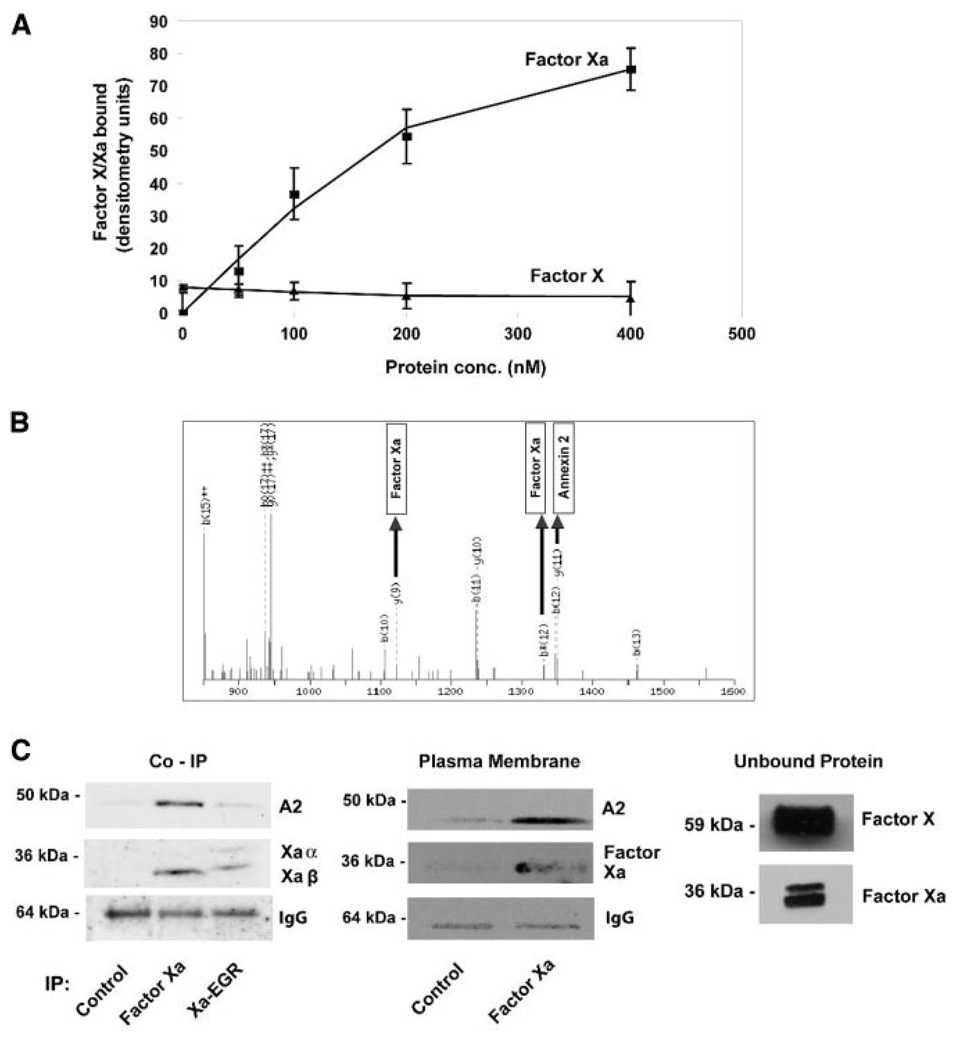
Factors X and Xa were incubated with HUVECs at 4°C to determine whether there was a unique cell surface receptor responsible for the observed binding of factor Xa. Factor Xa bound to HUVECs, whereas factor X binding was negligible (Figure 1A). To identify the putative receptor for factor Xa, factor Xa bound to HUVECs was immunoprecipitated using an anti-X/Xa antibody, followed by nonreducing SDS-PAGE, resulting in multiple bands that were excised and subjected to MALDI-TOF mass spectroscopy. Peptides corresponding to both factor Xa and immunoglobulin heavy and light chains were identified in addition to a peptide that represented annexin 2 (Figure 1B). Annexin 2 coprecipitation was confirmed by Western blotting (Figure 1C). Factor Xa immunoprecipitated from endothelial cells was primarily Xaβ (Figure 1C), but the ratio of Xaα to Xaβ ranged from ≈1:1 to 1:2 in the unbound fraction, and there was no conversion of unbound factor X to factor Xa (Figure 1C). Active site blocked factor Xa with Glu-Gly-Arg (EGR)-chloromethyl ketone exhibited reduced binding relative to active factor Xa, possibly attributable to incomplete processing to the annexin 2–binding species (note the somewhat slower mobility of the EGR-Xaβ form). Factor Xa–bound HUVECs were also subjected to plasma membrane purification, followed by immunoprecipitation which confirmed the cell surface binding of factor Xa to annexin 2 (Figure 1C).
Figure 1.
Factor Xa binding to HUVECs, spectroscopic analysis, and confirmation of factor Xa binding to annexin 2. A, Increasing concentrations of factor X and Xa were bound to HUVECs followed by quantitation of bound protein by Western blotting. B, Tryptic peptide mixture of ≈46-kDa protein band as analyzed by MALDI mass spectrometer with bands corresponding to factor Xa and annexin 2 highlighted. C, Left, Western blotting with antibodies against annexin 2 (A2) (top gels), factor X/Xa (middle gels), and IgG control (bottom gels) of HUVECs immunoprecipitated with: anti-X/Xa alone, in the presence of factor Xa, and in the presence of catalytically inactive factor Xa–EGR. Middle, Western blotting with antibodies against A2, factor X/Xa and IgG control of plasma membrane purified HUVECs immunoprecipitated with anti-X/Xa alone or in the presence of factor Xa. Right, Unbound factors X and Xa.
Annexin 2 Is a Factor Xa Receptor on the Endothelial Cell Surface
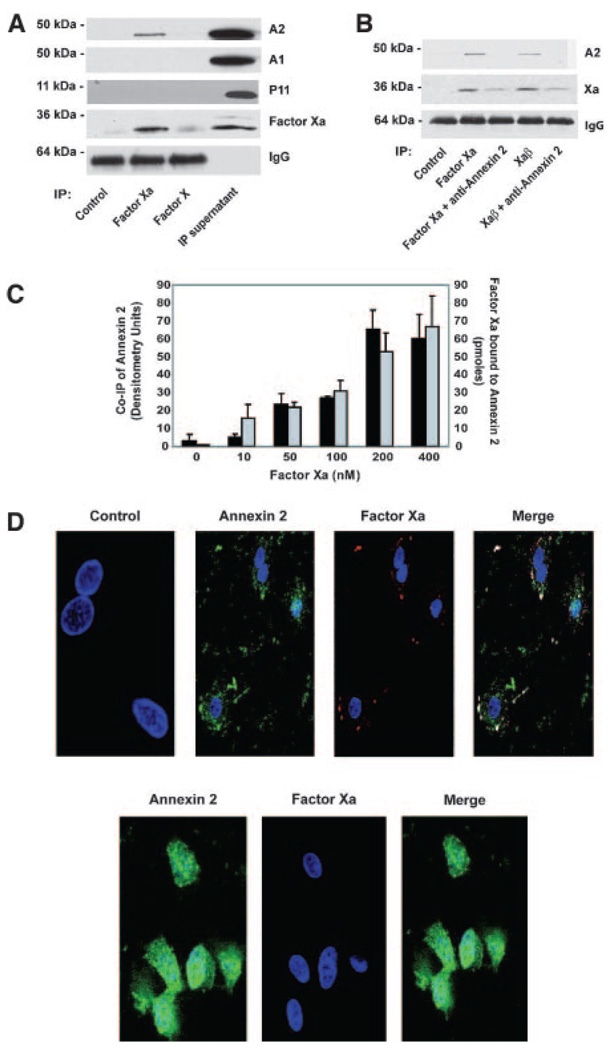
To determine whether coimmunoprecipitation of annexin 2 with factor Xa was specific, we demonstrated that only annexin 2, but not annexin 1 or p11, which are similarly expressed by HUVECs (note the nonprecipitated supernatant in Figure 2A), was detectable in factor Xa immunoprecipitates (Figure 2A). In addition, the small amount of factor X that typically bound the HUVEC surface, probably through direct binding to phospholipids, did not coprecipitate with annexin 2 (Figure 2A). Pretreatment of cells with anti–annexin 2 diminished immunoprecipitation of factor Xa from cell lysates, indicating inhibition of binding of factor Xa and Xaβ to cells. Annexin 2 in the anti–factor Xa immunoprecipitation was concordantly reduced (Figure 2B). To determine whether coimmunoprecipitation of annexin 2 followed the saturation curve of factor Xa binding to HUVECs, increasing concentrations of factor Xa were incubated with HUVECs, washed, and analyzed by immunoprecipitation and quantitation using Western blotting. Figure 2C shows that at 200 nmol/L factor Xa coimmunoprecipitation of annexin 2 was maximal and half saturation was at ≈50 nmol/L, analogous to what was observed for factor Xa binding. Direct binding of factor Xa to recombinant annexin 2 coated onto 96-well plates produced analogous results (Figure 2C). No factor Xa binding was observed on plates coated with recombinant Annexin 1 or p11.
Figure 2.
Xa binding to annexin 2 is specific. A, Immunoprecipitation of factors X or Xa bound to HUVECs, followed by Western blotting with anti–annexin 2 (A2), anti–annexin 1 (A1), anti-p11, anti-X/Xa, and IgG control. Nonimmunoprecipitated supernatant was used as an additional control. B, Western blotting with antibodies against annexin 2 (top), factor X/Xa (middle), and IgG control (bottom) of HUVECs immunoprecipitated with: anti-X/Xa alone, in the presence of factor Xa, in the presence of factor Xa and anti–annexin 2, in the presence of Xaβ, and in the presence of Xaβ and anti–annexin 2. C, Quantitation of factor Xa/annexin 2 coprecipitation (black) and factor Xa directly bound to plates coated with recombinant annexin 2 (gray). D, Immunofluorescence staining of factor Xa bound to HUVECs, followed by staining with the following antibodies: anti–annexin 2 and anti-X/Xa; images represent annexin 2 (left), factor Xa (middle), and merge (right). E, Cells preblocked with anti–annexin 2 followed by staining against annexin 2 (left) and factor Xa (middle) demonstrates no colocalization (right).
Immunofluorescence staining of HUVECs demonstrates colocalization of factor Xa with annexin 2 on the cell surface (Figure 2D). Control experiments demonstrated that secondary antibodies alone do not bind the cell surface or colocalize with one another (Figure 2D). Preincubation of cells with anti–annexin 2 eliminates factor Xa colocalization with annexin 2 (Figure 2E). Quantitation of the colocalization between annexin 2 and factor Xa indicates that >90% of the factor Xa detected on the endothelial cell surface colocalizes with annexin 2. However, <15% of total cell surface annexin 2 colocalizes with factor Xa, indicating that the majority of cell surface annexin 2 is available for interaction with other known ligands and is not limited to interaction with factor Xa.
Annexin 2 Binding of Factor Xa Is Dependent on the Gla Domain but Independent of Lysine Residues
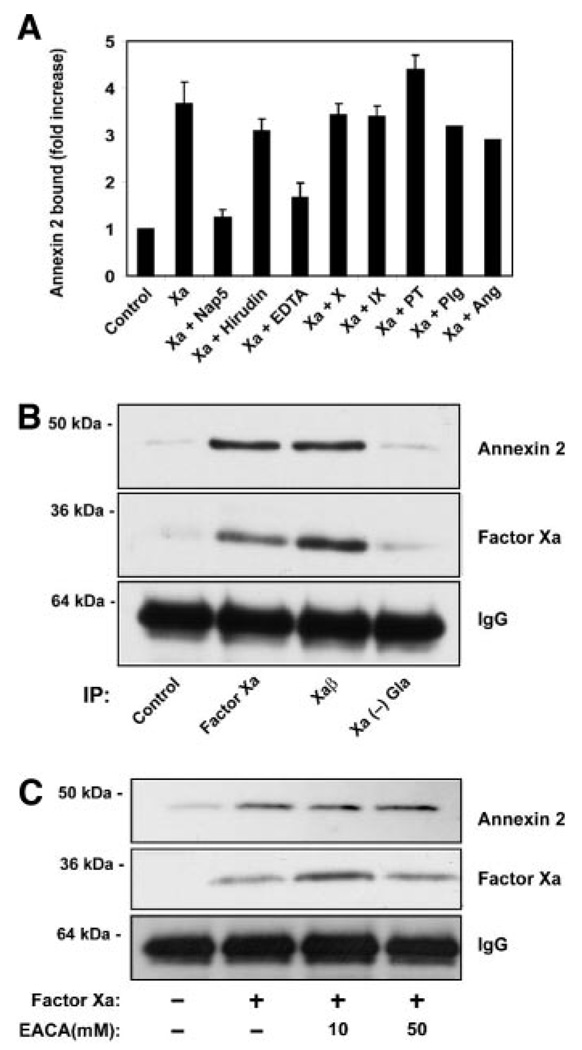
Experiments with active site blocked factor Xa indicated that processing to a specific Xaβ form is necessary for binding to annexin 2. Blocking factor Xa autoproteolysis with nematode anticoagulant peptide Nap533 also blocked factor Xa binding and annexin 2 coimmunoprecipitation (Figure 3A). Thrombin has been demonstrated to upregulate cell surface expression of annexin 2. Thrombin inhibition by hirudin did not reduce factor Xa binding to annexin 2 (Figure 3A), excluding that factor Xa activity indirectly through thrombin upregulates annexin 2 expression or contributes to the processing of factor Xa to Xaβ. The binding of factor Xa to annexin 2 is calcium-dependent because EDTA abolishes the interaction (Figure 3A). Factors X and Xa are among several proteins that contain carbohydrate-rich Gla domains that are capable of mediating protein–protein interactions.34 Binding studies performed in the presence of 20-fold molar excess of several Gla proteins (factor X, factor IX, and prothrombin) did not compete with factor Xa cell surface binding and annexin 2 coimmunoprecipitation (Figure 3A). Gla domain–deleted factor Xa showed significantly reduced binding relative to factor Xa (Figure 3B). Furthermore, adding homogenous Xaβ resulted in binding comparable to factor Xa, further confirming that Xaβ is the relevant binding partner for annexin 2 (Figure 3B). Because carboxyl-terminal proteolysis may produce carboxyl-terminal lysine residues, we tested whether factor Xa binding to annexin 2 is sensitive to EACA (Figure 3C). No reduction in coprecipitation was observed, demonstrating that factor Xa binding to annexin 2 is lysine-independent and probably does not involve p11. In addition, plasminogen and angiostatin, ligands that have been demonstrated to bind the annexin 2/p11 heterotetramer via lysine residues, do not compete with factor Xa binding to annexin 2 (Figure 3A).
Figure 3.
Factor Xa binds to annexin 2 on autolytic cleavage and exposure of C-terminal lysine residues on Xaβ. A, Immunoprecipitation and quantitation of factor Xa binding to annexin 2 on HUVECs in the presence of Nap5, the thrombin inhibitor hirudin, EDTA, and 20-fold molar excess of the Gla-binding proteins factor X, factor IX, and prothrombin, and 20-fold molar excess of plasminogen (Plg) and angiostatin (Ang). B, Immunoprecipitation of factor Xa, Xaβ, and factor Xa(-)Gla bound to cells, followed by Western blotting with anti–annexin 2, anti-X/Xa, and IgG control. C, Immunoprecipitation of factor Xa bound to HUVECs in the presence of EACA, followed by Western blotting with anti–annexin 2, anti-X/Xa, and IgG control.
Annexin 2 Regulates Factor Xa–Dependent Signal Transduction but Not Factor Xa Procoagulant Function
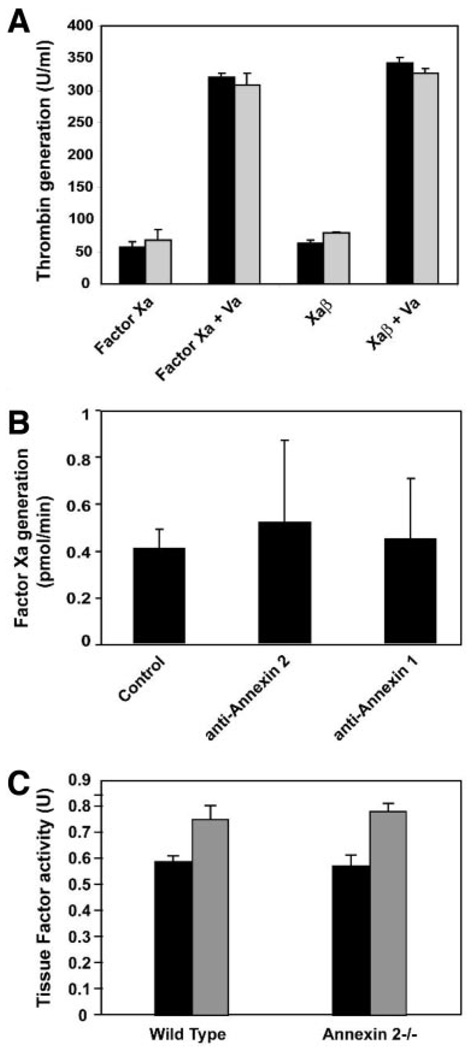
To test whether annexin 2 is selectively important for factor Xa coagulation or signaling events, factor Xa–dependent prothrombin generation was analyzed in the presence and absence of anti–annexin 2 antibody. No effect on thrombin generation was observed (Figure 4A). As expected, TF-dependent factor Xa generation was also not influenced by anti–annexin 2 antibody (Figure 4B), and no difference in procoagulant function was detected between macrophages isolated from wild-type and annexin 2−/− mice (Figure 4C). Cells lysed with n-octyl-β-d-glucopyranoside yielded similar results to the intact cells (data not shown).
Figure 4.
Annexin 2 has no effect on coagulant function of factor Xa. A, Thrombin generation was performed on HUVECs alone (black) and in the presence of anti–annexin 2 (gray). B, HUVECs were assayed for TF-dependent factor Xa generation/substrate activity in the presence of anti–annexin 2 and anti–annexin 1. C, Thioglycollate elicited macrophages were isolated from wild-type (n=3) and annexin 2−/− (n=3) mice and assayed for TF procoagulant activity using the 1-stage plasma clotting assay. Untreated (black) and lipopolysaccharide-treated cells (gray) were measured.
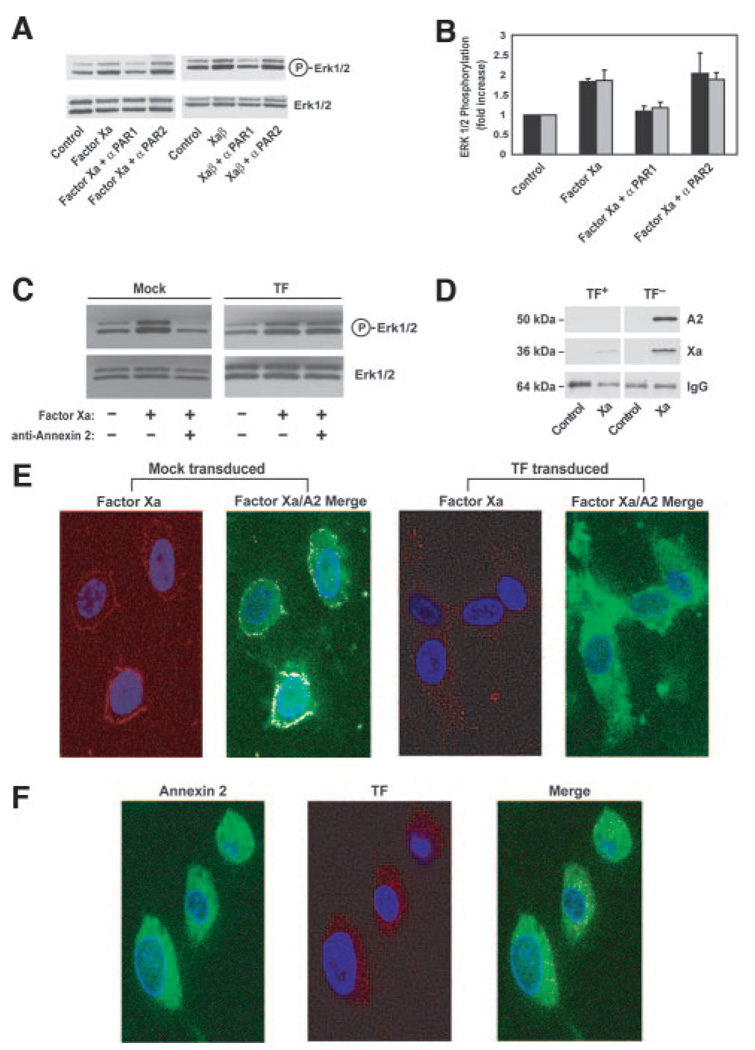
It has been shown previously that factor Xa–induced ERK1/2 phosphorylation is mediated by activation of the protease-activated receptors PAR1 or PAR2. Factor Xa– and Xaβ–induced ERK1/2 phosphorylation was dependent on PAR1 and not blocked by an anti-PAR2 antibody that efficiently inhibits TF:VIIa-mediated PAR2 cleavage (Figure 5A and 5B).19,29,35 Factor Xa signaling was also inhibited by pretreatment of cells with annexin 2 antibody (Figure 5C). Factor Xa can also induce ERK1/2 phosphorylation as part of the TF:VIIa:factor Xa ternary complex. However, overexpression of TF, but not mock transduction with control adenovirus, abrogates annexin 2 dependence on factor Xa signaling (Figure 5C), indicating that annexin 2–mediated regulation of factor Xa signaling does not extend to the ternary complex.
Figure 5.
Annexin 2 regulates factor Xa–mediated ERK1/2 phosphorylation through PAR1. A, Representative Western blots of factor Xa and Xaβ induced ERK1/2 phosphorylation in the presence of antibodies against PAR1 and PAR2. B, Densitometric quantification of inhibition of factor Xa (black) and Xaβ (gray) induced ERK1/2 phosphorylation by antibodies against PAR1 and PAR2. C, Mock-transduced (left) and TF-transduced (right) cells were left untreated or were treated with factor Xa alone or factor Xa in the presence of anti–annexin 2, followed by Western blotting using antibodies against phospho-ERK1/2 and ERK1/2. D, Mice were injected with thioglycollate 3 days before peritoneal lavage and macrophage harvest. Factor Xa/annexin 2 coprecipitation was performed as described above, followed by Western blotting with anti–annexin 2, anti-factor X/Xa, and IgG control. Cells were isolated from TFflox/floxLysM Cre− (TF+) (left gels; n=3) and TFflox/floxLysM Cre+ (TF−) (right gels; n=3) control mice. E, Immunofluorescence of mock-transduced and TF-transduced HUVECs with antibodies against factor Xa and annexin 2. F, TF-transduced HUVECs stained with anti–annexin 2 and anti-TF; images represent annexin 2 (left), TF (middle), and merge (right).
To determine whether the in vitro regulation of the annexin 2/factor Xa interaction by TF (Figure 4B) is replicated in primary mouse cells, we isolated macrophages from thioglycollate-injected TFflox/floxLysM Cre+ in which TF procoagulant activity is reduced by 93% in macrophages (data not shown) and TFflox/flox/LysM Cre− mice, which have normal levels of TF.26 We observe that the factor Xa/annexin 2 interaction is greatly enhanced in macrophages when TF levels are reduced (Figure 5D). In TF-transduced HUVECs, the typical colocalization of factor Xa with annexin 2 disappears without reduction in annexin 2 cell surface expression (Figure 5E). TF did not colocalize with annexin 2 (Figure 5F). These data support the conclusion that annexin 2 plays a selective and specific role in supporting TF-independent factor Xa signaling without affecting cell surface coagulant properties.
Discussion
Using a combination of biochemical and proteomic analysis, we find that the coagulation protease factor Xa binds to annexin 2 on the endothelial cell surface. Annexin 2 interaction is required for signal transduction (Figure 5), whereas the procoagulant function of factor Xa is independently regulated (Figure 4). Only the Xaβ form of factor Xa binds to annexin 2, and this binding occurs independently of C-terminal lysine residues (Figure 3A and 3C). It appears that autoproteolysis is important for binding, because the factor Xa–specific inhibitor Nap5 or inactivation with a chloromethyl-ketone inhibitor reduced annexin 2 binding (Figure 1D and 3A). The interaction of factor Xa with annexin 2 is also specific, because annexin 1 does not bind factor Xa and factor X does not bind annexin 2 (Figure 1D). Annexin 2 has previously been found to function as a receptor for the fibrinolytic zymogen plasminogen (Plg).22 Recent studies also indicate that the anti-angiogenic factor angiostatin may bind annexin 2.36 Neither Plg nor angiostatin competes for factor Xa binding to annexin 2 (Figure 3A), and EACA does not inhibit annexin 2/factor Xa coprecipitation (Figure 3C), indicating that the factor Xa/annexin 2 interaction is distinct from that of other annexin 2 ligands. Interestingly, previous work has shown that autoproteolysis or plasmin-mediated cleavage of Xaα to Xaβ exposes a Plg binding site and inhibits coagulation. 7 This result, in combination with our studies here, indicates a possible regulatory mechanism in which Xaβ is the primary regulator of signal transduction when TF is not present.
In the presence of TF, factor Xa mediates signal transduction through the activation of PAR-1 and PAR-2.12,19 In the absence of TF, annexin 2 mediates factor Xa signaling (Figure 5A) predominantly through PAR-1. Interestingly, in the presence of TF, the overall level of factor Xa signaling does not change. However, the annexin 2–dependent components of factor Xa signaling are completely abolished. The coagulation-independent, signaling-specific regulation of factor Xa by annexin 2 that we observe in vitro is also replicated in primary mouse cells (Figure 4C and 5D). Whether this TF effect is attributable to the regulation of factor Xa autoproteolysis or by influencing factor Xa–Gla domain interactions with annexin 2, perhaps in some combination of PAR-1 and PAR-2, remains to be fully elucidated.
Recent in vitro and in vivo studies have demonstrated the importance of annexin 2 in the regulation of fibrinolysis and neoangiogenesis. Higher annexin 2 expression was observed in cells from acute promyelocytic leukemia patients when compared with other leukemic cells. This resulted in increased tissue plasminogen activator–dependent plasmin generation and may account for the increased hemorrhagic complications associated with acute promyelocytic leukemia. 37 Recombinant annexin 2 restored plasmin generation in HUVECs impaired by plasminogen activator inhibitor-1 or homocysteine, and injection of annexin 2 enhanced the patency of thrombosed arteries in a rat carotid artery thrombosis model.38 Increased annexin 2 on the surface of aortic endothelial cells is associated with increased plasmin activity in diabetic rat aortas.39
In vivo studies have demonstrated that annexin 2–knockout mice displayed enhanced fibrin deposition in the microvasculature and improper clearance of thrombi as well as inefficient tissue plasminogen activator–dependent plasmin generation.25 In 3 different angiogenesis models, fibroblast growth factor–stimulated cornea, oxygen-primed neonatal retina, and aortic ring explants, annexin 2–null mice demonstrated significantly reduced neovascularization and capillary sprouting, respectively.25 The decrease in aortic ring sprouting was attributable to impaired matrix metalloproteinase (MMP)-9 and MMP-13 activation.25
Factor Xa has also been shown to play a role in the activation of MMPs, including conversion of pro–MMP-2 to MMP-2 in vascular smooth muscle cells40 and promotion of MMP-1 release in gingival fibroblasts.41 Although only a small amount of factor Xa is necessary to initiate coagulation, 42,43 studies have shown that higher levels of factor Xa can be generated under certain pathologic circumstances,44,45 in addition to the physiologic conversion of factor X to factor Xa that occurs under TF-independent circumstances via the intrinsic pathway. These observations indicate a potential physiological relevance for the results we observe in vitro regarding factor Xa binding to annexin 2 in the absence of TF. Previous work indicates that annexin 2 plays a predominant role in cell invasion, fibrin homeostasis, and fibrin degradation, but not in blood coagulation.25,37 The coagulation-independent regulation of factor Xa signaling that we demonstrate for annexin 2 is consistent with these observations. IL-6, IL-8, and MCP-1 expression have been shown to be increased by factor Xa in HUVECs,46 and PAR-1 has been demonstrated to upregulate cyclooxygenase-2 in HUVECs.47 To determine the role of annexin 2 in regulating these downstream signaling functions, we will further characterize factor Xa signaling in TF expressing and nonexpressing cells isolated from annexin 2 knockout mice.
Factor Xa–mediated signal transduction via PAR-1 and PAR-2 has been shown to be cell type–dependent using endothelial cells and fibroblasts from PAR-1 and PAR-2 knockout mice.12 PAR-2 was demonstrated to be the primary mediator of factor Xa–induced phosphoinositide hydrolysis and ERK1/2 phosphorylation in the murine endothelial cells, whereas in murine fibroblasts the majority of signaling was regulated by PAR-1.12 PAR-2 activation has also been shown to play a role in P-selectin–dependent leukocyte rolling resulting in microvascular inflammation.48 Whether the interaction of factor Xa and annexin 2 is relevant to some of the aforementioned hemostatic, angiogenic, and inflammatory functions remains to be elucidated. Future mechanistic studies are required to determine the differences in factor Xa signaling via PAR-1 and PAR-2 and how annexin 2 binding affects these processes. In conclusion, our work proposes a novel role for annexin 2 as an endothelial cell surface receptor for factor Xa that regulates factor Xa–mediated signal transduction via PAR-1 in a coagulation-independent manner.
Acknowledgments
We thank Dr Katherine Hajjar (Cornell University, New York) for providing us with annexin 2−/− mice; Elizabeth Boyd and Cheryl Johnson for assistance in manuscript preparation; and Pabs Tejada, Jennifer Royce, David Revak, Cindi Biazak, and Paul Kuo for technical assistance.
Sources of Funding
Supported by NIH grant P01 HL-16411 (to T.S.E.).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Edgington TS, Mackman N, Brand K, Ruf W. The structural biology of expression and function of tissue factor. Thromb Haemost. 1991;66:67–79. [PubMed] [Google Scholar]
- 2.Ahamed J, Belting M, Ruf W. Regulation of tissue factor-induced signaling by endogenous and recombinant tissue factor pathway inhibitor 1. Blood. 2005;105:2384–2391. doi: 10.1182/blood-2004-09-3422. [DOI] [PubMed] [Google Scholar]
- 3.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 4.Fujikawa K, Titani K, Davie EW. Activation of bovine factor X (Stuart factor): conversion of factor Xaalpha to factor Xabeta. Proc Natl Acad Sci U S A. 1975;72:3359–3363. doi: 10.1073/pnas.72.9.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jesty J, Spencer AK, Nemerson Y. The mechanism of activation of factor X. Kinetic control of alternative pathways leading to the formation of activated factor X. J Biol Chem. 1974;249:5614–5622. [PubMed] [Google Scholar]
- 6.Pryzdial ELG, Kessler GE. Kinetics of blood coagulation factor Xa alpha autoproteolytic conversion to factor Xa beta. Effect on inhibition by antithrombin, prothrombinase assembly, and enzyme activity. J Biol Chem. 1996;271:16621–16626. doi: 10.1074/jbc.271.28.16621. [DOI] [PubMed] [Google Scholar]
- 7.Pryzdial ELG, Kessler GE. Autoproteolysis or plasmin-mediated cleavage of factor Xa alpha exposes a plasminogen binding site and inhibits coagulation. J Biol Chem. 1996;271:16614–16620. doi: 10.1074/jbc.271.28.16614. [DOI] [PubMed] [Google Scholar]
- 8.Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci U S A. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riewald M, Ruf W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc Natl Acad Sci U S A. 2001;98:7742–7747. doi: 10.1073/pnas.141126698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruf W, Dorfleutner A, Riewald M. Specificity of coagulation factor signaling. J Thromb Haemost. 2003;1:1495–1503. doi: 10.1046/j.1538-7836.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 11.Ahamed J, Ruf W. Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J Biol Chem. 2004;279:23038–23044. doi: 10.1074/jbc.M401376200. [DOI] [PubMed] [Google Scholar]
- 12.Camerer E, Kataoka H, Kahn M, Lease K, Coughlin SR. Genetic evidence that protease-activated receptors mediate factor Xa signaling in endothelial cells. J Biol Chem. 2002;277:16081–16087. doi: 10.1074/jbc.M108555200. [DOI] [PubMed] [Google Scholar]
- 13.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 14.Kataoka H, Hamilton JR, McKemy DD, Camerer E, Zheng YW, Cheng A, Griffin C, Coughlin SR. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood. 2003;102:3224–3231. doi: 10.1182/blood-2003-04-1130. [DOI] [PubMed] [Google Scholar]
- 15.Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV, Jr, Tam C, Coughlin SR. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- 17.Fan L, Yotov WV, Zhu T, Esmailzadeh L, Joyal JS, Sennlaub F, Heveker N, Chemtob S, Rivard GE. Tissue factor enhances protease-activated receptor-2-mediated factor VIIa cell proliferative properties. J Thromb Haemost. 2005;3:1056–1063. doi: 10.1111/j.1538-7836.2005.01250.x. [DOI] [PubMed] [Google Scholar]
- 18.Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res. 2006;66:307–314. doi: 10.1158/0008-5472.CAN-05-1735. [DOI] [PubMed] [Google Scholar]
- 19.Riewald M, Kravchenko VV, Petrovan RJ, O’Brien PJ, Brass LF, Ulevitch RJ, Ruf W. Gene induction by coagulation factor Xa is mediated by activation of protease-activated receptor 1. Blood. 2001;97:3109–3116. doi: 10.1182/blood.v97.10.3109. [DOI] [PubMed] [Google Scholar]
- 20.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 21.Glenney JR, Jr, Boudreau M, Galyean R, Hunter T, Tack B. Association of the S-100-related calpactin I light chain with the NH2-terminal tail of the 36-kDa heavy chain. J Biol Chem. 1986;261:10485–10488. [PubMed] [Google Scholar]
- 22.Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. J Biol Chem. 1994;269:21191–21197. [PubMed] [Google Scholar]
- 23.MacLeod TJ, Kwon M, Filipenko NR, Waisman DM. Phospholipid-associated annexin A2–S100A10 heterotetramer and its subunits: characterization of the interaction with tissue plasminogen activator, plasminogen, and plasmin. J Biol Chem. 2003;278:25577–25584. doi: 10.1074/jbc.M301017200. [DOI] [PubMed] [Google Scholar]
- 24.Kassam G, Le BH, Choi KS, Kang HM, Fitzpatrick SL, Louie P, Waisman DM. The p11 subunit of the annexin II tetramer plays a key role in the stimulation of t-PA-dependent plasminogen activation. Biochemistry. 1998;37:16958–16966. doi: 10.1021/bi981713l. [DOI] [PubMed] [Google Scholar]
- 25.Ling Q, Jacovina AT, Deora A, Febbraio M, Simantov R, Silverstein RL, Hempstead B, Mark WH, Hajjar KA. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J Clin Invest. 2004;113:38–48. doi: 10.1172/JCI200419684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawlinski R, Tencati M, Holscher T, Pedersen B, Voet T, Tilley RE, Marynen P, Mackman N. Role of cardiac myocyte tissue factor in heart hemostasis. J Thromb Haemost. 2007;5:1693–1700. doi: 10.1111/j.1538-7836.2007.02649.x. [DOI] [PubMed] [Google Scholar]
- 27.Fair DS, Revak DJ, Hubbard JG, Girolami A. Isolation and characterization of the factor X Friuli variant. Blood. 1989;73:2108–2116. [PubMed] [Google Scholar]
- 28.Deutsch DG, Mertz ET. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970;170:1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- 29.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C, Bhattacharjee G, Boisvert W, Dilley R, Edgington T. In vivo interrogation of the molecular display of atherosclerotic lesion surfaces. Am J Pathol. 2003;163:1859–1871. doi: 10.1016/S0002-9440(10)63545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorfleutner A, Ruf W. Regulation of tissue factor cytoplasmic domain phosphorylation by palmitoylation. Blood. 2003;102:3998–4005. doi: 10.1182/blood-2003-04-1149. [DOI] [PubMed] [Google Scholar]
- 32.Ruf W, Edgington TS. Two sites in the tissue factor extracellular domain mediate the recognition of the ligand factor VIIa. Proc Natl Acad Sci U S A. 1991;88:8430–8434. doi: 10.1073/pnas.88.19.8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semple JE, Levy OE, Minami NK, Owens TD, Siev DV. Novel, potent and selective chimeric FXa inhibitors featuring hydrophobic P1-ketoamide moieties. Bioorg Med Chem Lett. 2000;10:2305–2309. doi: 10.1016/s0960-894x(00)00458-3. [DOI] [PubMed] [Google Scholar]
- 34.Stenflo J. Contributions of Gla and EGF-like domains to the function of vitamin K-dependent coagulation factors. Crit Rev Eukaryot Gene Expr. 1999;9:59–88. [PubMed] [Google Scholar]
- 35.O’Brien PJ, Molino M, Kahn M, Brass LF. Protease activated receptors: theme and variations. Oncogene. 2001;20:1570–1581. doi: 10.1038/sj.onc.1204194. [DOI] [PubMed] [Google Scholar]
- 36.Tuszynski GP, Sharma MR, Rothman VL, Sharma MC. Angiostatin binds to tyrosine kinase substrate annexin II through the lysine-binding domain in endothelial cells. Microvasc Res. 2002;64:448–462. doi: 10.1006/mvre.2002.2444. [DOI] [PubMed] [Google Scholar]
- 37.Menell JS, Cesarman GM, Jacovina AT, McLaughlin MA, Lev EA, Hajjar KA. Annexin II and bleeding in acute promyelocytic leukemia. N Engl J Med. 1999;340:994–1004. doi: 10.1056/NEJM199904013401303. [DOI] [PubMed] [Google Scholar]
- 38.Ishii H, Yoshida M, Hiraoka M, Hajjar KA, Tanaka A, Yasukochi Y, Numano F. Recombinant annexin II modulates impaired fibrinolytic activity in vitro and in rat carotid artery. Circ Res. 2001;89:1240–1245. doi: 10.1161/hh2401.101066. [DOI] [PubMed] [Google Scholar]
- 39.Lei H, Romeo G, Kazlauskas A. Heat shock protein 90alpha-dependent translocation of annexin II to the surface of endothelial cells modulates plasmin activity in the diabetic rat aorta. Circ Res. 2004;94:902–909. doi: 10.1161/01.RES.0000124979.46214.E3. [DOI] [PubMed] [Google Scholar]
- 40.Rauch BH, Bretschneider E, Braun M, Schror K. Factor Xa releases matrix metalloproteinase-2 (MMP-2) from human vascular smooth muscle cells and stimulates the conversion of pro-MMP-2 to MMP-2: role of MMP-2 in factor Xa-induced DNA synthesis and matrix invasion. Circ Res. 2002;90:1122–1127. doi: 10.1161/01.res.0000019240.72809.76. [DOI] [PubMed] [Google Scholar]
- 41.Matsushita K, Imamura T, Tomikawa M, Tancharoen S, Tatsuyama S, Maruyama I. DX-9065a inhibits proinflammatory events induced by gingipains and factor Xa. J Periodontal Res. 2006;41:148–156. doi: 10.1111/j.1600-0765.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad SS, Rawala-Sheikh R, Walsh PN. Components and assembly of the factor X activating complex. Semin Thromb Hemost. 1992;18:311–323. doi: 10.1055/s-2007-1002570. [DOI] [PubMed] [Google Scholar]
- 43.Butenas S, van ‘t Veer C, Mann KG. Evaluation of the initiation phase of blood coagulation using ultrasensitive assays for serine proteases. J Biol Chem. 1997;272:21527–21533. doi: 10.1074/jbc.272.34.21527. [DOI] [PubMed] [Google Scholar]
- 44.Bauer KA, Goodman TL, Kass BL, Rosenberg RD. Elevated factor Xa activity in the blood of asymptomatic patients with congenital antithrombin deficiency. J Clin Invest. 1985;76:826–836. doi: 10.1172/JCI112040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iversen N, Lindahl AK, Abildgaard U. Elevated plasma levels of the factor Xa-TFPI complex in cancer patients. Thromb Res. 2002;105:33–36. doi: 10.1016/s0049-3848(01)00404-2. [DOI] [PubMed] [Google Scholar]
- 46.Senden NH, Jeunhomme TM, Heemskerk JW, Wagenvoord R, van’t Veer C, Hemker HC, Buurman WA. Factor Xa induces cytokine production and expression of adhesion molecules by human umbilical vein endothelial cells. J Immunol. 1998;161:4318–4324. [PubMed] [Google Scholar]
- 47.Houliston RA, Keogh RJ, Sugden D, Dudhia J, Carter TD, Wheeler-Jones CP. Protease-activated receptors upregulate cyclooxygenase-2 expression in human endothelial cells. Thromb Haemost. 2002;88:321–328. [PubMed] [Google Scholar]
- 48.Lindner JR, Kahn ML, Coughlin SR, Sambrano GR, Schauble E, Bernstein D, Foy D, Hafezi-Moghadam A, Ley K. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol. 2000;165:6504–6510. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]