Abstract
Background:
Little is known about the role of endothelial progenitor cells (EPCs) in atherosclerosis. Accordingly, we performed a series of assessments with hypercholesterolemic (ApoE−/−) and wild type (WT) mice to evaluate how cholesterol influences re-endothelialization, atherosclerosis, and EPC function after arterial injury.
Methods and Results:
Unexpectedly, re-endothelialization (assesed via resistance to Evans blue staining) and circulating EPC counts (EPC-culture assay) were greater in ApoE−/− mice than in WT mice, and transplantation of ApoE−/− bone marrow (BM) in WT mice accelerated endothelial recovery and increased recruitment of BM-derived EPCs to the neo-endothelium. Cholesterol concentration-dependently promoted the proliferation (MTS assay) of both ApoE−/− and WT EPCs, and the concentration dependence of EPC adhesion (to vitronectin-, collagen type I-, fibronectin-, and laminin-coated plates), migration (modified Boyden's-chamber assay), and anti-apoptotic (TUNEL staining) activity was biphasic. Cholesterol enhanced the mRNA expression (quantitative, real-time RT-PCR) of vascular endothelial growth factor and inhibited Notch1 mRNA expression in both ApoE−/− and WT EPCs; whereas eNOS mRNA expression increased in ApoE−/− EPCs and declined in WT EPCs after cholesterol exposure. EPC activity was greater in Notch1+/– EPCs than in WT EPCs, and transplantation of Notch1+/– BM accelerated endothelial recovery after arterial injury in WT mice.
Conclusions:
The results presented here provide novel insights into the role of EPCs during atherosclerosis and suggest that cholesterol and Notch1 may be involved in the regulation of EPC activity.
Keywords: Atherosclerosis, Endothelial progenitor cells, Hypercholesterolemia, Notch, Nitric oxide synthase
Introduction
Hypercholesterolemia has been repeatedly identified as a risk factor for atherosclerosis; however, its influence on re-endothelialization (i.e., the recovery of endothelial integrity) after arterial injury is uncertain. Animal studies and clinical reports provide conflicting evidence regarding the importance of cholesterol after angioplasty or stenting and on the relationship between cholesterol levels and the risk of restenosis.1-4 The loss of endothelial integrity that occurs during angioplasty/stenting appears to induce the accumulation of inflammatory cells and the proliferation and migration of vascular smooth-muscle cells, which can lead to neointimal thickening.5 Re-endothelialization inhibits neointimal thickening, thereby suppressing development of the substrate for lipid deposition and macrophage accumulation that is believed to induce the formation of atherosclerotic lesions and may contribute to restenosis. Drug-eluting stents (DESs) have significantly reduced the rate of restenosis; however, DESs also appear to delay re-endothelialization.6 This delay results in excessive rates of thrombosis, which could increase the occurrence of acute coronary syndromes.
Endogenous re-endothelialization occurs through the proliferation and migration of endothelial cells adjacent to the site of arterial injury and via the activity of endothelial progenitor cells (EPCs). Studies performed in our laboratory and others indicate that both exogenously infused EPCs and EPCs mobilized from the bone marrow (BM) are recruited to the sites of arterial injury, where they promote re-endothelialization. Mobilization occurs in response to ischemia,7,8 physical training,9 and the administration of statins,10,11 estrogen,12,13 and a variety of cytokines,14-16 then the mobilized cells are recruited to ischemic tissue, where they form a structural component of the new vasculature and promote the proliferation and migration of local endothelial cells by secreting signaling molecules and growth factors.17-20
Kwon, et al. have recently reported that a knockout mutation of the Notch1 receptor Jagged-1 inhibits EPC-mediated angiogenesis by reducing EPC differentiation and bioactivity.21 Blood-flow recovery and postnatal neovascularization after hindlimb ischemia are also impaired in haploinsufficient Notch1+/− mice22; however, similar results were not observed in mice lacking expression of another Notch1 receptor, Delta-like 1,21 and Notch1 activation in endothelial cells reduced the expression of vascular endothelial growth factor (VEGF)-receptor-2 and VEGF-mediated endothelial-cell proliferation.23,24 Thus, Notch signaling appears to modulate adult angiogenesis,23 but whether it acts as a positive or negative regulator is somewhat unclear. Mutations in Notch family members have also been linked to an adult-onset vascular dementia that is accompanied by an increased risk of coronary occlusion.25
The number and function of circulating EPCs is inversely correlated with many risk factors for atherosclerosis and cardiovascular disease26,27; however, the relationship between EPC function, gene expression, hypercholesterolemia, Notch1, and re-endothelialization after arterial injury has not been completely characterized. Accordingly, we performed a series of investigations with hypercholesterolemic (ApoE−/−) and wild type (WT) mice to evaluate 1) the contribution of EPCs to atherosclerotic lesion formation, 2) EPC-mediated re-endothelialization in the setting of hypercholesterolemia, 3) EPC function and gene expression under high cholesterol conditions, and 4) the impact of reduced Notch1 expression on EPC function.
Methods
Animals and surgical procedure
All procedures were approved by St. Elizabeth's Institutional Animal Care and Use Committee. Male ApoE−/− (B6.129P2-Apoetm1Unc/J), WT (C57BL/6J), and FVB-TgN(Tie2-LacZ)182Sato mice were obtained from The Jackson Laboratories (Bar Harbor, ME, USA). Tie2-LacZ mice were generated in our laboratory by back-crossing FVB-TgN(Tie2-LacZ)182Sato mice with C57BL/6J mice for more than 10 generations. Tie2-Cre Notch1+/− mice22 were provided by Dr. James K. Liao. Wire-induced carotid denudation was performed as described previously28 in animals aged 10-12 weeks. Mice were sacrificed for histological analyses via cervical dislocation.
EPC counts
The number of EPCs in the BM and circulation of ApoE−/− and WT mice was determined via the EPC culture assay as described previously.14,29
Bone-marrow transplantation (BMT)
Donor mice were strain- and age-matched to recipient mice aged 6-8 weeks. The BMT procedure was performed as described previously14,30 and as summarized in the Supplemental Methods.
Incorporation of BM-derived EPCs
The incorporation of BM-derived EPCs was evaluated in ApoE−/− and WT mice transplanted with BM from Tie2-LacZ donor mice, which express LacZ from the endothelial-specific Tie2 promoter. Carotid arteries were harvested from mice sacrificed 14 days after carotid denudation for en face histological assessments. BM-derived endothelial cells were identified and quantified via X-gal staining as described previously.10,12,30
Incorporation of infused EPCs
The incorporation of systemically infused ApoE−/− and WT EPCs was evaluated in both ApoE−/− mice and WT mice. The infused EPCs were cultured from mouse BM,14,30 and EPC transfusion was performed as described previously28 and as summarized in the Supplemental Methods. Carotid arteries were harvested 14 days after carotid denudation for en face histological assessments; transfused EPCs were visualized with fluorescent microscopy as described previously.28
Re-endothelialization
Re-endothelialization was evaluated as described previously31,32 and as summarized in the Supplemental Methods; assessments were performed in 1) ApoE−/− and WT mice, 2) ApoE−/− mice transplanted with BM from ApoE−/− or WT donor mice, 3) WT mice transplanted with BM from ApoE−/− or WT donor mice, and 4) WT mice transplanted with BM from Tie2-Cre Notch1+/− or Tie2-Cre donor mice.
Cellular contributions to atherosclerotic lesion formation
The formation of atherosclerotic lesions was evaluated in ApoE−/− and WT mice transplanted with BM from Tie2-LacZ donor mice. Assessments of lesion size, apoptosis, β-galactosidase (β-gal) expression (signaling the presence of BM-derived endothelial cells), and CD68 expression (signaling the presence of monocytes/macrophages) were performed as summarized in the Supplemental Methods.
EPC function and apoptosis
Proliferation, adhesion, migration, and apoptosis were assessed in EPCs isolated from the BM of ApoE−/− mice, WT mice, Tie2-Cre mice, and Tie2-Cre Notch1+/− mice. The effect of cholesterol on EPC function was determined by repeating the assays in the presence of 0, 50, 150, 300, and/or 500 mg/dL cholesterol (Sigma-Aldrich Co.). Assays were performed as summarized in the Supplemental Methods.
Quantitative real-time RT-PCR
mRNA expression was evaluated in EPCs isolated from the BM of ApoE−/− and WT mice as summarized in the Supplemental Methods (primer and probe sequences are also provided). Relative mRNA expression was calculated with the comparative CT method (relative expression=2ΔCT) and normalized to the expression of the endogenous 18S gene.
Statistical analysis
All values were expressed as mean±SEM. Statistical analyses were performed with commercially available software (StatviewTM, Abacus Concepts, Berkeley, CA, USA). Comparisons between two groups were tested for significance with the Student t-test; comparisons between multiple groups were tested via analysis of variance (ANOVA) followed by post-hoc testing with the Tukey procedure; measurements obtained at multiple time points were evaluated via repeat measure analysis. A P value less than 0.05 was considered significant.
Results
Circulating EPCs are more prevalent in ApoE−/− mice
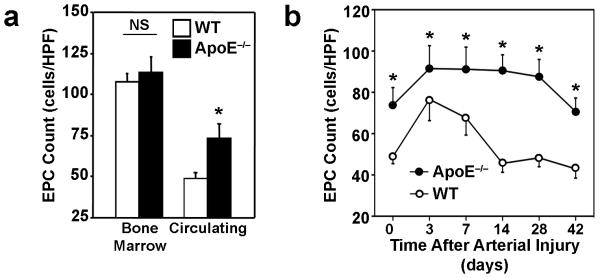
Before carotid artery injury, the number of EPCs in the BM of ApoE−/− and WT mice was similar (ApoE−/−: 113.3±9.3 cells/HPF, WT: 106.3±4.7 cells/HPF; n=5 in each group) (Figure 1a), but the number of circulating EPCs was significantly greater in ApoE−/− mice than in WT mice before injury (ApoE−/− mice: 73.9±8.4 cells/HPF; WT: 48.9±3.4 cells/HPF; P<0.05, n=10 in each group) and at all subsequent time points (Figure 1b). Circulating EPC counts increased after injury in both groups; however, ApoE−/− mice maintained this increase for 4 weeks after carotid injury, whereas counts in WT mice declined to pre-injury levels by Day 14. Serum cholesterol levels did not change significantly in either mouse strain during the course of the experiment (Supplemental Table).
Figure 1. EPC mobilization.
The numbers of EPCs present in (a) the BM (NS, not significant, n=5) and peripheral circulation (*P<0.05, n=10) of ApoE−/− and WT mice before arterial injury and (b) in the peripheral circulation before arterial injury and 3-42 days afterward (*P<0.0001, n=5 in each group) were determined via the EPC culture assay.
EPC recruitment to the site of carotid artery injury is enhanced in ApoE−/− mice
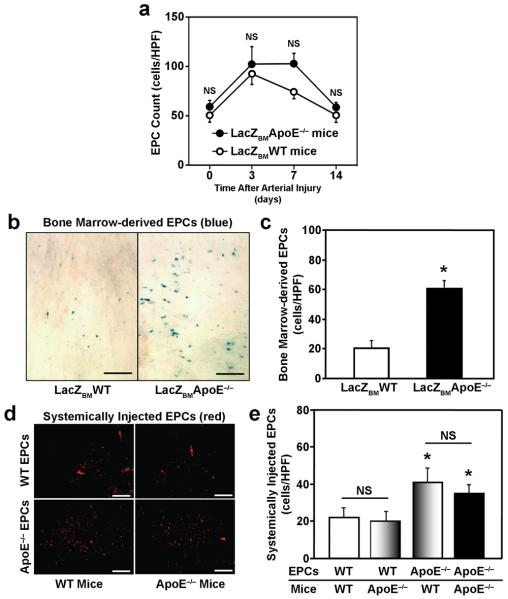
Circulating EPC counts were higher after carotid artery injury in ApoE−/− mice transplanted with BM from Tie2-LacZ WT mice (LacZBMApoE−/− mice) (102.8±10.6 cells/HPF; n=5) than in WT mice transplanted with WT Tie2-LacZ BM (LacZBMWT mice) (74.0±6.6 cells/HPF; n=5) (Figure 2a), but the difference did not reach statistical significance, and circulating EPC levels in LacZBMApoE−/− mice declined to pre-injury levels by Day 14. Nevertheless, significantly more (P<0.001, n=5 in each group) X-gal positive cells were observed on the luminal surface of carotid arteries from LacZBMApoE−/− mice (60.4±5.4 cells/HPF) than in arteries from LacZBMWT mice (20.2±4.1 cells/HPF) (Figures 2b, c). When DiI-labeled ApoE−/− or WT EPCs were administered systemically, significantly more (P<0.001, n=5 in each group) ApoE−/− EPCs than WT EPCs were observed in the neo-endothelium of either mouse strain (ApoE−/− mice: 35.0±3.5 ApoE−/− EPCs/HPF versus 20.4±3.3 WT EPCs/HPF; WT mice: 41.4±4.9 ApoE−/− EPCs/HPF versus 22.0±3.9 WT EPCs/HPF) (Figures 2d, e); however, ApoE−/− EPC recruitment in ApoE−/− and WT mice was similar, as was WT EPC recruitment. Collectively, these observations suggest that the enhanced EPC mobilization observed in ApoE−/− mice (Figure 1b) could be largely dependent on the genotype of the BM cells, whereas both the genotype of the circulating ApoE−/− EPCs and the physiological environment associated with the ApoE−/− mutation (e.g., hypercholesterolemia) may increase EPC biopotency.
Figure 2. EPC recruitment after carotid artery injury.
(a) Circulating EPC counts in ApoE−/− mice transplanted with BM from Tie2-LacZ donor mice (LacZBMApoE−/− mice) and in WT mice transplanted with BM from Tie2-LacZ donor mice (LacZBMWT mice) were determined via the EPC culture assay before carotid artery injury and 3, 7, and 14 days afterward (NS, not significant; n=5 in each group). (b-c) Carotid arteries were harvested 14 days after carotid-artery injury in LacZBMWT mice and in LacZBMApoE−/− mice. (b) Representative X-gal-stained images of injured carotid arteries; cells derived from transplanted BM are stained blue (bar=100 μm). (c) Quantification of incorporated BM-derived EPCs (*P<0.01, n=5 in each group). (d-e) Carotid arteries were harvested 14 days after carotid artery injury in WT and ApoE−/− mice infused with DiI-acLDL-labeled WT or ApoE−/− EPCs. (d) Representative fluorescent images of injured carotid arteries; infused EPCs fluoresce red (bar=100 μm). (e) Quantification of incorporated, systemically administered EPCs (NS, not significant; *P<0.001 versus the same mouse strain transplanted with WT EPCs, n=5 in each group).
Re-endothelialization is accelerated in ApoE−/− mice and is dependent on EPC genotype
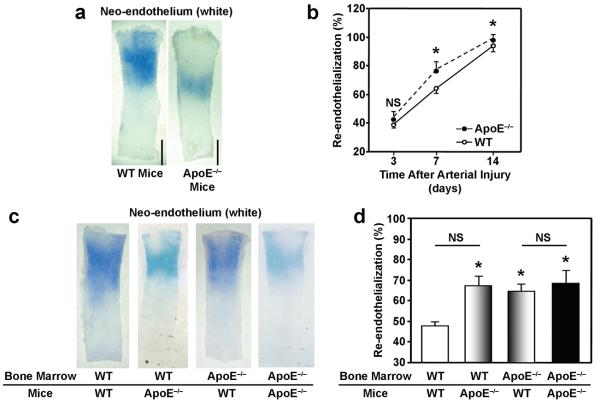
Evans-blue staining in whole-mount carotid arteries indicated that re-endothelialization was slightly, but significantly (P<0.05, n=5 in each group), elevated in ApoE−/− mice on Day 7 (ApoE−/− mice: 77.7±4.1%, WT mice: 63.4±4.7%) and Day 14 (ApoE−/− mice: 99.3±1.6%, WT mice: 94.8±5.3%) after carotid artery injury (Figures 3a, b). The difference between groups was more apparent in mice that had undergone BM transplantation surgery; re-endothelialization was significantly greater (P<0.001, n=5 in each group) in ApoE−/− mice transplanted with ApoE−/− or WT BM (69.5±3.6% and 67.1±2.2%, respectively) and in WT mice transplanted with ApoE−/− BM (64.3±1.9%) than in WT mice transplanted with WT BM (48.6±0.6%) (Figures 3c, d). Thus, both the physiological environment in ApoE−/− mice and the ApoE−/− BM genotype appear to improve re-endothelialization.
Figure 3. Re-endothelialization after carotid artery injury.
Carotid arteries were harvested 3, 7, and 14 days after carotid artery injury in WT and ApoE−/− mice; mice were injected with Evans blue dye 10 minutes before sacrifice. (a) Representative Evans blue–stained arteries from mice sacrificed 7 days after carotid artery injury, re-endothelialized regions are resistant to the dye and appear white (bar=1 mm). (b) Quantification of re-endothelialization (NS, not significant; *P<0.05, n=5 in each group). (c-d) Carotid arteries were harvested 7 days after carotid artery injury in WT and ApoE−/− mice transplanted with BM from WT or ApoE−/− mice; mice were injected with Evans blue dye 10 minutes before sacrifice. (c) Representative Evans blue–stained arteries, re-endothelialized regions are resistant to the dye and appear white (bar=1 mm). (d) Quantification of re-endothelialization (NS, not significant; *P<0.001, n=5 in each group).
Bone marrow–derived EPCs are present in atherosclerotic lesions
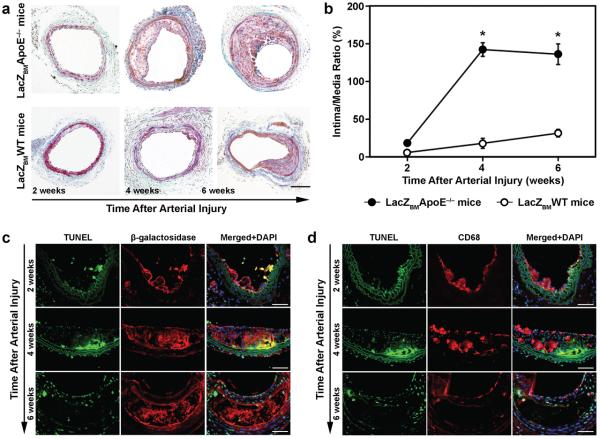
Advanced atherosclerotic lesions were observed in Masson's trichrome–stained carotid artery sections from ApoE−/− mice transplanted with WT Tie2-LacZ BM (Figure 4a), and the lesions were significantly larger than those observed in WT mice transplanted with WT Tie2-LacZ BM (Week 4: LacZBMApoE−/− mice, 143.5±9.0%; LacZBMWT mice, 18.1±6.7%. Week 6: LacZBMApoE−/− mice, 136.3±13.7%; LacZBMWT mice, 31.5±5.0%. P<0.001 at both time points, n=4 in each group) (Figure 4b) Two weeks after carotid artery injury, double-immunostained sections identified apoptotic (i.e., TUNEL-stained cells) and BM-derived (i.e., cells stained positively for Tie2-β-gal expression) cells on both the internal elastic lamina and the luminal surface (Figure 4c), suggesting that the plaque was surrounded by BM-derived EPCs shortly after the onset of re-endothelialization. Evidence of apoptosis and EPC accumulation persisted through Week 4, but the plaque core contained only amorphous β-gal protein, and no viable EPCs, at Week 6. Similar staining patterns were observed in sections double-immunostained for evidence of apoptosis and monocytes/macrophages (i.e., cells stained positively for CD68 expression); macrophages accumulated through Week 4 but were not present in the plaque core by Week 6 (Figure 4d). Thus, both EPCs and inflammatory cells contributed to the formation of the atherosclerotic lesion.
Figure 4. Formation of atherosclerotic lesions after carotid artery injury.
Carotid arteries were harvested 2, 4, and 6 weeks after carotid artery injury in WT mice transplanted with BM from Tie2-LacZ donor mice (LacZBMWT mice) and in ApoE−/− mice transplanted with BM from Tie2-LacZ donor mice (LacZBMApoE−/− mice). (a) Representative Masson's trichrome–stained sections of carotid arteries (bar=200 μm). (b) Quantification of lesion development; the intima/media ratio was calculated and expressed as a percentage (*P<0.001, n=4 in each group). (c) Representative sections of carotid arteries double immunofluorescently stained for evidence of apoptosis (left panels, green TUNEL staining), BM descent (middle panels, red immunofluorescent staining for β-galactosidase expression), or both (right panels, stained with DAPI to identify nuclei); areas stained positively for both apoptosis and BM descent appear yellow (bar=100 μm). (d) Representative sections of carotid arteries double immunofluorescently stained for evidence of apoptosis (left panels, green TUNEL staining), monocyte/macrophage lineage (middle panels, red immunofluorescent staining for CD68 expression), or both (right panels, stained with DAPI to identify nuclei); areas stained positively for apoptosis and monocyte/macrophage lineage appear yellow (bar=100 μm).
Cholesterol modulates EPC function
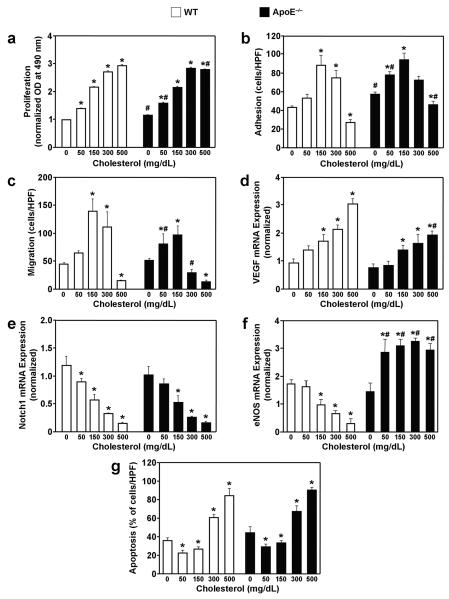
In the absence of cholesterol, proliferation (ApoE−/− EPCs: 1.15±0.02 OD, WT EPCs: 1.00±0.00 OD; P<0.01) and adhesion (ApoE−/− EPCs: 57.4±2.4 cells/HPF, WT EPCs: 41.3±3.2 cells/HPF; P<0.05) were significantly greater in ApoE−/− EPCs than in WT EPCs (Figures 5a, b), but the migration of ApoE−/− and WT EPCs (51.3±1.5 and 47.0±3.1 cells/HPF, respectively) was similar (Figure 5c). Cholesterol enhanced the proliferation of both ApoE−/− EPCs and WT EPCs in a concentration-dependent manner (Figure 5a), whereas the effect of progressively greater cholesterol concentrations on EPC adhesion and migration was biphasic (Figures 5b, c). Peak adhesion and migration was observed at 150 mg/dL, and measurements were significantly lower (P<0.05) at 500 mg/dL than in the absence of cholesterol.
Figure 5. Cellular functions of EPCs in the presence of cholesterol.
EPCs were isolated from WT and ApoE−/− mice then treated with 0, 50, 150, 300, and 500 mg/dL cholesterol for 48 hours (a, g), 24 hours (d-f), 18 hours (c), or 1 hour (b); the following evaluations were performed immediately after treatment. (a) Proliferation was assessed with optical density (OD) measurements obtained via the colorimetric MTS assay and normalized to the mean OD of WT EPCs in the absence of cholesterol. (b) Adhesion was assessed by incubating EPCs for 1 hour on plates coated with the extracellular matrix protein fibronectin, then washing away the nonadherent cells with phosphate-buffered saline and counting the adherent cells. (c) Migration of isolated EPCs was assessed with a modified Boyden's chamber assay; cell suspensions were placed in the upper chamber, the lower chamber was filled with 50 ng/mL recombinant mouse VEGF protein, and the EPCs that had migrated to the lower chamber were counted 16 hours later. (d-f) The expression of VEGF, Notch1, and eNOS mRNA was determined via real-time RT-PCR and normalized to 18S expression. (g) Apoptosis was assessed as the number of TUNEL-positive cells observed after 48 hours in culture. *P<0.05 versus the same strain in the absence of cholesterol; #P<0.05 versus WT at the same cholesterol concentration.
Cholesterol influences the expression of VEGF, eNOS, and Notch1 mRNA in EPCs and EPC apoptosis
The influence of cholesterol on the mRNA expression of VEGF and eNOS, which promote angiogenesis and EPC activity, and Notch1, which determines cell fate (including apoptosis), was assessed via real-time RT-PCR. In the absence of cholesterol, there was no significant difference between ApoE−/− and WT EPCs in VEGF, eNOS, or Notch1 expression (Figures 5d-f). Cholesterol concentration-dependently increased VEGF expression (Figure 5d) and decreased Notch1 expression (Figure 5e) in both ApoE−/− EPCs and WT EPCs, whereas eNOS expression was significantly higher (P<0.05) in ApoE−/− EPCs but declined in WT EPCs after exposure to cholesterol (Figure 5f).
The effect of progressively greater cholesterol concentrations on EPC apoptosis was biphasic (Figure 5g). Apoptotic cells were significantly less frequent (P<0.05) in 50 mg/dL cholesterol (29.0±2.9% of ApoE−/− EPCs and 22.3±3.4% of WT EPCs) and 150 mg/dL cholesterol (33.1±2.8% of ApoE−/− EPCs and 25.9±1.9% of WT EPCs) than in the absence of cholesterol (44.5±8.1% of ApoE−/− EPCs and 35.8±2.7% of WT EPCs); however, apoptosis was significantly higher (P<0.0001) in 300 mg/dL (67.2±6.4% of ApoE−/− EPCs and 60.4±3.7% of WT EPCs) or 500 mg/dL (90.3±3.1% of ApoE−/− EPCs and 84.2±8.1% of WT EPCs) cholesterol than in 0 mg/dL cholesterol.
A moderate reduction of Notch1 expression enhances EPC function, reduces EPC apoptosis, and accelerates re-endothelialization
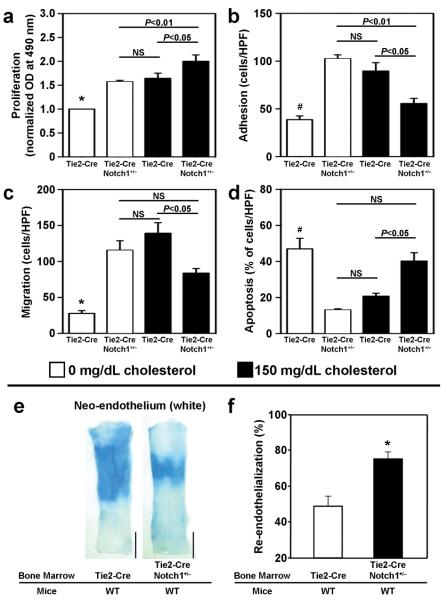
The cholesterol-independent effects of Notch1 were investigated by comparing EPCs obtained from Tie2-Cre Notch1+/− mice, in which Notch1 expression is reduced by approximately 50%,22 with EPCs from Tie2-Cre (i.e., Notch1+/+) mice. All EPC functional measures were significantly greater (P<0.05) in Tie2-Cre Notch1+/− EPCs than in Tie2-Cre EPCs (Proliferation: Tie2-Cre Notch1+/−, 1.57±0.12 OD; Tie2-Cre, 1.0±0.0 OD. Adhesion: Tie2-Cre Notch1+/−, 102.6±4.0 cells/HPF; Tie2-Cre, 38.8±3.9 cells/HPF. Migration: Tie2-Cre Notch1+/−, 115.6±13.2 cells/HPF; Tie2-Cre, 27.8±3.9 cells/HPF.), and Tie2-Cre Notch1+/− EPCs also exhibited less apoptosis (Tie2-Cre Notch1+/−: 13.1±0.7%, Tie2-Cre: 47.0±5.9%; P<0.05) (Figures 6a-d). Furthermore, exposure to 150 mg/dL cholesterol, which decreased Notch1 expression by approximately 50% (Figure 5e), increased Tie2-Cre EPC function (proliferation: 1.65±0.43 OD, adhesion: 89.6±8.8 cells/HPF, migration: 139.3±22.5 cells/HPF) and decreased Tie2-Cre EPC apoptosis (18.4±2.0%) to levels similar to those measured in Tie2-Cre Notch1+/− EPCs. Treatment of Tie2-Cre Notch1+/− EPCs with 150 mg/dL cholesterol enhanced proliferation, impaired adhesion and migration, and increased apoptosis, which is consistent with the results obtained when Notch1 expression declined to less than 50% of baseline levels in WT and ApoE−/− EPCs treated with 300-500 mg/dL cholesterol (Figures 5a-c, g).
Figure 6. The influence of Notch1 expression on EPC function, EPC apoptosis, and re-endothelialization.
(a-d) Assessments of EPC function and apoptosis in the presence and absence of 150 mg/dL cholesterol were performed with EPCs obtained from Tie2-Cre mice and from Tie2-Cre Notch1+/− mice, in which Notch1 expression is reduced by approximately 50%. (a) Proliferation was assessed with optical density (OD) measurements obtained via the colorimetric MTS assay and normalized to the mean OD of Tie2-Cre EPCs in the absence of cholesterol. (b) Adhesion was assessed by incubating EPCs for 1 hour on plates coated with the extracellular matrix protein fibronectin, then washing away the nonadherent cells with phosphate-buffered saline and counting the adherent cells. (c) Migration was assessed with a modified Boyden's chamber assay; cell suspensions were placed in the upper chamber, the lower chamber was filled with 50 ng/mL recombinant mouse VEGF protein, and the EPCs that had migrated to the lower chamber were counted 16 hours later. (d) Apoptosis was assessed as the number of TUNEL-positive cells observed after 48 hours in culture. (e-f) Carotid arteries were harvested 7 days after carotid artery injury in WT mice transplanted with BM from Tie2-Cre or Tie2-Cre Notch1+/− mice; mice were injected with Evans blue dye 10 minutes before sacrifice. (e) Representative Evans blue–stained arteries, re-endothelialized regions are resistant to the dye and appear white (bar=1 mm). (f) Quantification of re-endothelialization (n=4 in each group). *P<0.05 versus all other groups; #P<0.05 versus Tie2-Cre Notch1+/−, 0 mg/dL cholesterol and versus Tie2-Cre, 150 mg/dL cholesterol.
The potential contribution of Notch1 expression to endothelial recovery was evaluated in WT mice transplanted with BM from Tie2-Cre Notch1+/− or Tie2-Cre mice. Evans-blue staining of whole-mount carotid arteries revealed significantly greater (P<0.01, n=4 in each group) evidence of re-endothelialization on Day 7 after carotid artery injury in mice transplanted with Tie2-Cre Notch1+/− BM (75.2±3.2%) than in mice transplanted with Tie2-Cre BM (48.8±5.5%) (Figures 6e, f), suggesting that a moderate reduction of Notch1 expression in BM-derived cells promotes endothelial recovery.
Discussion
Results from the series of experiments presented here indicate that the number of circulating EPCs and their recruitment to the site of arterial injury is greater in ApoE−/− mice than in WT mice, and that the enhanced recruitment likely evolves from the properties of the EPCs themselves as well as the physiological environment. ApoE−/− mice also displayed greater evidence of re-endothelialization than WT mice, which was somewhat surprising, because ApoE−/− mice are prone to atherosclerosis, and accelerated re-endothelialization is commonly believed to inhibit the formation of atherosclerotic lesions.33-35 In general, ApoE−/− and WT EPCs responded similarly to the presence of cholesterol in vitro: progressively greater cholesterol concentrations enhanced VEGF expression and EPC proliferation, whereas Notch1 expression declined and the relationship between cholesterol concentration and EPC adhesion, migration, and apoptosis was biphasic. However, eNOS expression increased with cholesterol exposure in ApoE−/− EPCs and declined in WT EPCs. In vivo, the recruitment of WT EPCs derived from transplanted BM was higher in ApoE−/− mice than in WT mice, whereas the recruitment of systemically administered WT EPCs was similar in both strains, perhaps because the duration of exposure to high serum cholesterol levels (i.e., the length of time between BM transplantation or EPC injection and the evaluation of recruitment) was 6-8 weeks for the transplanted cells compared to just 2 weeks for the injected cells.
The concentration-dependent enhancement of VEGF expression by cholesterol could explain the greater EPC proliferation observed at high cholesterol levels. The secretion of VEGF and other growth factors by EPCs is believed to enhance angiogenesis in ischemic tissues by promoting the proliferation and migration of local endothelial cells,17-20 and this paracrine mechanism could contribute to re-endothelialization in response to arterial injury. However, VEGF has also been linked to lesion formation and the progression of atherosclerosis.36-38
Although mild impairment of Notch1 expression was accompanied by declines in apoptosis both in Tie2-Cre Notch1+/− EPCs and in EPCs exposed to low cholesterol levels, excessive impairment of Notch1 expression by high cholesterol levels increased apoptosis. This potential biphasic relationship between Notch1 expression and apoptosis has been implied by results from previous investigations indicating that both Notch1 deletion39 and overexpression40 increase apoptosis in endothelial cells. Notch1 may also regulate EPC activity in the presence of cholesterol, because moderate, cholesterol-induced reductions in Notch1 expression were associated with greater EPC adhesion and migration; however, confirmation of this potential regulatory mechanism requires additional investigation.
Cholesterol increased the expression of eNOS in ApoE−/− EPCs and decreased eNOS expression in WT EPCs. eNOS is essential for the mobilization of stem and progenitor cells from the BM,41 and re-endothelialization after carotid artery injury is retarded in both eNOS knockout mice12 and diabetic mice,28 in which eNOS expression is attenuated. Thus, the higher circulating EPC counts and accelerated re-endothelialization observed in ApoE−/− mice might be attributable to enhanced eNOS expression under hypercholesterolemic conditions. However, the recruitment of transplanted WT (Tie2-LacZ) EPCs was greater in the hypercholesterolemic ApoE−/− mice than in WT mice, despite the cholesterol-induced inhibition of eNOS expression, so the potential relationship between cholesterol, eNOS expression, and EPC recruitment remains uncertain.
Transplanted, BM-derived WT (Tie2-LacZ) EPCs were found at the border of atherosclerotic lesions in ApoE−/− mice and were accompanied by ample evidence of apoptosis. Although these results could imply that EPCs participate in the formation of atherosclerotic lesions, the mechanism and consequences associated with the cholesterol-mediated modulation of EPC activity have yet to be determined. Our in vitro results indicate that hypercholesterolemia likely enhances EPC adhesion, migration, and VEGF expression, but these effects might be offset (at least partially) by cholesterol-induced EPC apoptosis, perhaps caused by very high local cholesterol concentrations at the luminal surface. Furthermore, EPCs comprise a heterogeneous cell population, and individual subpopulations of EPCs may function differently during recovery from vascular injury.
In conclusion, this investigation provided novel insights into the role of EPCs during recovery from arterial injury and identified the potential importance of cholesterol and Notch1 for regulation of EPC activity. EPCs appear to participate in re-endothelialization, which is enhanced in hypercholesterolemic mice, and BM-derived EPCs and monocyte/macrophages may also contribute to the formation of atherosclerotic lesions. Both cholesterol and Notch1 influence EPC function and viability; however, the influence of cholesterol on many EPC functions is biphasic, so the consequences of EPC activity under hypercholesterolemic conditions warrant further investigation.
Clinical Summary.
Abundant evidence has linked hypercholesterolemia with the advent and progression of atherosclerosis. In order to facilitate the study of the mechanisms by which hypercholesterolemia leads to atherosclerosis, animal models have been developed. Most notably, the ApoE-null and LDL-receptor–null mice have been used extensively in attempts to gain a better understanding of the effects of high lipid levels on atheroma formation. In recent years, traditional cardiovascular risk factors have also been associated with decreased numbers of circulating endothelial progenitor cells (EPCs) which have been suggested to participate in endothelial repair and maintenance. Ample experimental evidence suggests that EPCs are mobilized from the bone marrow to the peripheral circulation after arterial injury (e.g., percutaneous transluminal coronary angioplasty or stent implantation) and promote re-endothelialization at the injury site, thereby speeding endothelial recovery and reducing the risk of restenosis and atherosclerotic plaque formation. Here, we compared EPC recruitment, re-endothelialization, and plaque formation after carotid artery injury in wild-type mice and ApoE−/− mice. Our findings indicate that EPC recruitment was higher in ApoE−/− mice than in wild-type mice, and that the enhanced recruitment likely evolves through a moderate decline in Notch expression. Re-endothelialization was also greater in ApoE−/− mice, which was somewhat surprising, because ApoE−/− mice are prone to atherosclerosis. Furthermore, transplanted, bone-marrow–derived EPCs were found at the border of the atherosclerotic lesions, which could suggest that EPCs contribute to the early stage of plaque formation. However, the clinical implications of these observations must be interpreted with caution, particularly since circulating EPC levels were higher in ApoE−/− mice than in wild-type mice, whereas hypercholesterolemia, hypertension, and many other risk factors for cardiovascular disease are associated with declines in human EPC levels. Collectively, our findings emphasize the need for additional experiments in other hypercholestrolemic animal models and underscore the potential for genetic mouse models of human disease to yield data that do not necessarily reflect clinical reality. To clarify the paradoxical observation regarding the direct effect of cholesterol on EPC contribution to re-endothelialization versus plaque formation in Apo E KO mice following arterial injury, further investigations using different types of hypercholestrolemic animals and, importantly, additional clinical correlation, is required.
Supplementary Material
Acknowledgements
We thank W. Kevin Meisner, PhD, ELS, for editorial support and M. Neely for administrative assistance.
Funding Sources This study was supported in part by NIH grants HL-53354, HL-57516, HL-77428, HL-95874, HL-80137, PO1HL-66957 (to DL), and HL-052233 (to JL).
Footnotes
Disclosures There are no significant relationships to report that are relevant to this study.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson HV, McNatt J, Clubb FJ, Herman M, Maffrand JP, DeClerck F, Ahn C, Buja LM, Willerson JT. Platelet inhibition reduces cyclic flow variations and neointimal proliferation in normal and hypercholesterolemic-atherosclerotic canine coronary arteries. Circulation. 2001;104:2331–2337. doi: 10.1161/hc4401.098434. [DOI] [PubMed] [Google Scholar]
- 2.Benchimol D, Benchimol H, Bonnet J, Dartigues JF, Couffinhal T, Bricaud H. Risk factors for progression of atherosclerosis six months after balloon angioplasty of coronary stenosis. Am J Cardiol. 1990;65:980–985. doi: 10.1016/0002-9149(90)91000-v. [DOI] [PubMed] [Google Scholar]
- 3.Napoli C, Cirino G, Del Soldato P, Sorrentino R, Sica V, Condorelli M, Pinto A, Ignarro LJ. Effects of nitric oxide-releasing aspirin versus aspirin on restenosis in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 2001;98:2860–2864. doi: 10.1073/pnas.041602898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Violaris AG, Melkert R, Serruys PW. Influence of serum cholesterol and cholesterol subfractions on restenosis after successful coronary angioplasty. A quantitative angiographic analysis of 3336 lesions. Circulation. 1994;90:2267–2279. doi: 10.1161/01.cir.90.5.2267. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–805. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 6.Liistro F, Colombo A. Late acute thrombosis after paclitaxel eluting stent implantation. Heart. 2001;86:262–264. doi: 10.1136/heart.86.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asahara T, Isner JM. Endothelial progenitor cells for vascular regeneration. J. Hematother. Stem Cell Res. 2002;11:171–178. doi: 10.1089/152581602753658385. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 9.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 10.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 11.Werner N, Priller J, Laufs U, Endres M, Bohm M, Dirnagl U, Nickenig G. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 12.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 13.Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, Laufs K, Ghaeni L, Milosevic M, Bohm M, Nickenig G. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107:3059–3065. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- 14.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. Embo J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho HJ, Kim HS, Lee MM, Kim DH, Yang HJ, Hur J, Hwang KK, Oh S, Choi YJ, Chae IH, Oh BH, Choi YS, Walsh K, Park YB. Mobilized endothelial progenitor cells by granulocyte-macrophage colony-stimulating factor accelerate reendothelialization and reduce vascular inflammation after intravascular radiation. Circulation. 2003;108:2918–2925. doi: 10.1161/01.CIR.0000097001.79750.78. [DOI] [PubMed] [Google Scholar]
- 16.Kong D, Melo LG, Gnecchi M, Zhang L, Mostoslavsky G, Liew CC, Pratt RE, Dzau VJ. Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation. 2004;110:2039–2046. doi: 10.1161/01.CIR.0000143161.01901.BD. [DOI] [PubMed] [Google Scholar]
- 17.Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, Losordo DW. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 18.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 19.Murasawa S, Llevadot J, Silver M, Isner JM, Losordo DW, Asahara T. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation. 2002;106:1133–1139. doi: 10.1161/01.cir.0000027584.85865.b4. [DOI] [PubMed] [Google Scholar]
- 20.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Kwon SM, Eguchi M, Wada M, Iwami Y, Hozumi K, Iwaguro H, Masuda H, Kawamoto A, Asahara T. Specific Jagged-1 signal from bone marrow microenvironment is required for endothelial progenitor cell development for neovascularization. Circulation. 2008;118:157–165. doi: 10.1161/CIRCULATIONAHA.107.754978. [DOI] [PubMed] [Google Scholar]
- 22.Takeshita K, Satoh M, Ii M, Silver M, Limbourg FP, Mukai Y, Rikitake Y, Radtke F, Gridley T, Losordo DW, Liao JK. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res. 2007;100:70–78. doi: 10.1161/01.RES.0000254788.47304.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bicknell R, Harris AL. Novel angiogenic signaling pathways and vascular targets. Annu Rev Pharmacol Toxicol. 2004;44:219–238. doi: 10.1146/annurev.pharmtox.44.101802.121650. [DOI] [PubMed] [Google Scholar]
- 24.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG. Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 26.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 27.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 28.Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 29.Mallat Z, Silvestre JS, Le Ricousse-Roussanne S, Lecomte-Raclet L, Corbaz A, Clergue M, Duriez M, Barateau V, Akira S, Tedgui A, Tobelem G, Chvatchko Y, Levy BI. Interleukin-18/interleukin-18 binding protein signaling modulates ischemia-induced neovascularization in mice hindlimb. Circ Res. 2002;91:441–448. doi: 10.1161/01.res.0000033592.11674.d8. [DOI] [PubMed] [Google Scholar]
- 30.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 31.Goukassian DA, Kishore R, Krasinski K, Dolan C, Luedemann C, Yoon YS, Kearney M, Hanley A, Ma H, Asahara T, Isner JM, Losordo DW. Engineering the response to vascular injury: divergent effects of deregulated E2F1 expression on vascular smooth muscle cells and endothelial cells result in endothelial recovery and inhibition of neointimal growth. Circ Res. 2003;93:162–169. doi: 10.1161/01.RES.0000082980.94211.3A. [DOI] [PubMed] [Google Scholar]
- 32.Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993;73:792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- 33.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 34.Van Eck M, Herijgers N, Vidgeon-Hart M, Pearce NJ, Hoogerbrugge PM, Groot PH, VanBerkel TJ. Accelerated atherosclerosis in C57Bl/6 mice transplanted with ApoE-deficient bone marrow. Atherosclerosis. 2000;150:71–80. doi: 10.1016/s0021-9150(99)00372-x. [DOI] [PubMed] [Google Scholar]
- 35.Veniant MM, Withycombe S, Young SG. Lipoprotein size and atherosclerosis susceptibility in Apoe(−/−) and Ldlr(−/−) mice. Arterioscler Thromb Vasc Biol. 2001;21:1567–1570. doi: 10.1161/hq1001.097780. [DOI] [PubMed] [Google Scholar]
- 36.Celletti FL, Hilfiker PR, Ghafouri P, Dake MD. Effect of human recombinant vascular endothelial growth factor165 on progression of atherosclerotic plaque. J Am Coll Cardiol. 2001;37:2126–2130. doi: 10.1016/s0735-1097(01)01301-8. [DOI] [PubMed] [Google Scholar]
- 37.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med. 2001;7:425–429. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- 38.Inoue M, Itoh H, Ueda M, Naruko T, Kojima A, Komatsu R, Doi K, Ogawa Y, Tamura N, Takaya K, Igaki T, Yamashita J, Chun TH, Masatsugu K, Becker AE, Nakao K. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998;98:2108–2116. doi: 10.1161/01.cir.98.20.2108. [DOI] [PubMed] [Google Scholar]
- 39.Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK. Essential role of endothelial Notch1 in angiogenesis. Circulation. 2005;111:1826–1832. doi: 10.1161/01.CIR.0000160870.93058.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi R, An H, Yu Y, Zhang M, Liu S, Xu H, Guo Z, Cheng T, Cao X. Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis. Cancer Res. 2003;63:8323–8329. [PubMed] [Google Scholar]
- 41.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.