Abstract
The aims of this study were to further define the safety of sorafenib and erlotinib, given at their full approved monotherapy doses, and to correlate pharmacokinetic (PK) and pharmacodynamic (PD) markers with clinical outcome. In addition, a novel PD marker based on the real-time measurement of RAF signal transduction capacity (STC) is described. Sorafenib was administered alone for a one-week run-in period, and then both drugs were given together continuously. RAF STC was assessed in peripheral blood monocytes prior to erlotinib initiation. EGFR expression and K-RAS mutations were measured in archival tumor samples. Changes in pERK and CD31 were determined in fresh tumor biopsies obtained pre-treatment, prior to erlotinib dosing and during the administration of both drugs. In addition, PET-CT scans and PK assessments were performed. Eleven patients received a total of 57 cycles (median: 5, range: 1–10). Only 4 patients received full doses of both drugs for the entire study course, with elevation of liver enzymes being the main reason for dose reductions and delays. Among 10 patients evaluable for response, 8 experienced tumor stabilization of 4 or more cycles. PK analysis revealed no significant interaction of erlotinib with sorafenib. Sorafenib-induced decrease in RAF-STC showed statistically significant correlation with time-to-progression in 7 patients. Other PD markers did not correlate with clinical outcome. This drug combination resulted in promising clinical activity in solid tumor patients although significant toxicity warrants close monitoring. RAF-STC deserves further study as a predictive marker for sorafenib.
Keywords: sorafenib, erlotinib, phase I, pharmacodynamics, pharmacokinetics
Introduction
Sorafenib and erlotinib are oral kinase inhibitors with antiproliferative and antiangiogenic effects that target primarily RAF/VEGFR1-3/PDGFR-β and EGFR respectively [1–5]. Sorafenib has been approved for the treatment of advanced renal cell and hepatocellular carcinomas; erlotinib has been approved for the treatment of advanced pancreatic and non-small cell lung cancers [6–9]. The rationale for combining these two targeted agents has previously been reported; the dose escalation part of the phase I trial performed by our group suggested that both agents could be given at their full recommended phase II dose (RP2D) [10]. This combination therapy is currently being studied in multiple tumor types [11–15].
This manuscript describes the expansion cohort of a phase I trial, treated at the RP2D, aimed to further define the safety and tolerability of the combination, and to correlate pharmacokinetic (PK) and pharmacodynamic (PD) markers with clinical outcome. A common approach taken previously to define PD markers of kinase activity has been to use immunohistochemistry (IHC) to assess the phosphorylation status of the target kinase’s substrate in either tumor or surrogate tissues. However, this approach has yielded only modest results and no reproducible predictive test [16–28]. A major criticism of this strategy is that the dynamic nature of the signal transduction capacity (STC) of the target cannot be evaluated in dead and fixed tissues. As demonstrated by Nolan and colleagues, the stimulated status of a signalling network is more informative of the STC at a given moment than a static determination [29]. In this context, we have developed a quantitative and reproducible PD test - “phospho-shift” (pShift) - for sorafenib that is based on the measurement of the dynamic RAF-STC in freshly isolated peripheral blood mononuclear cells (PBMCs), with exploratory aims.
Patients and Methods
Study design and treatment regimen
For each patient, sorafenib alone at 400 mg p.o. bid was administered for a 1-week run-in period, followed thereafter by the addition of erlotinib at 150 mg p.o. daily. Both drugs were then given together continuously in 28-day cycles (Figure 1A). Toxicity was graded according to the Common Terminology Criteria for Adverse Events (v.3.0). The study was approved by the local institutional review board and was conducted in accordance with federal and institutional guidelines.
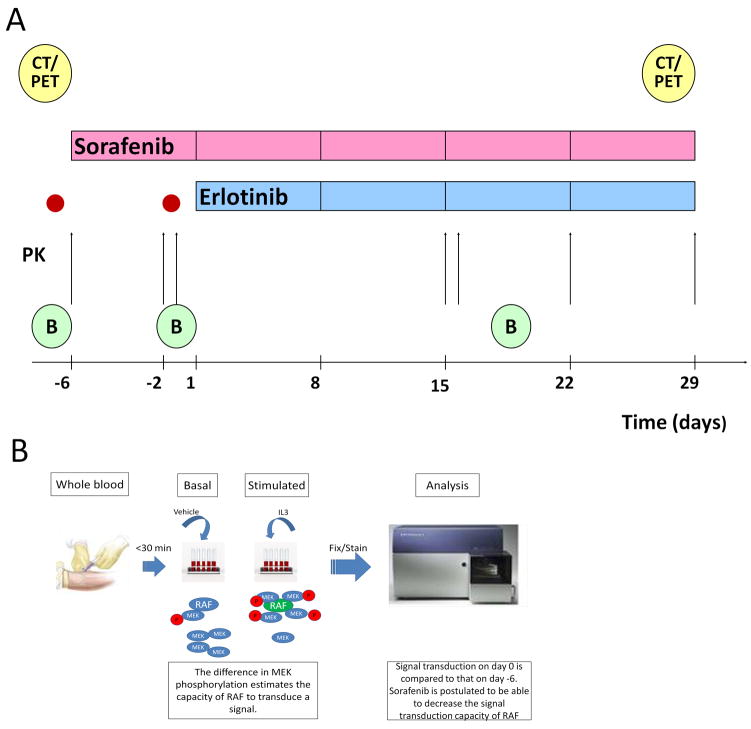
Figure 1. Treatment and correlative study schedule.
A) PK - arrows: pharmacokinetics sampling; B: tumor biopsy; CT/PET: CT scan and PET scan; red dots: MEK1/2 pShift assessment. B) Schematic representation of the pShift assessment: a blood sample is drawn and processed within 30 minutes. Whole blood is stimulated with vehicle or with IL3 which activates RAF in monocytes. RAF activation recruits and phosphorylates MEK. The difference between the degree of phosphorylation of MEK in the IL3-stimulated aliquots and the vehicle-stimulated aliquots is the pShift. As sorafenib is able to reduce the capacity of RAF to recruit and phosphorylate MEK, the changes in the pShift from day −6 to day 0 may estimate the PD effects of sorafenib on an individual basis.
Inclusion criteria included: histologically confirmed incurable solid tumor, age ≥18 years; Eastern Cooperative Oncology Group performance status ≤ 2; adequate hematologic, hepatic, and renal functions (absolute neutrophil count (ANC) ≥ 1.5 × 109/L, platelets ≥ 100 × 109/L, bilirubin ≤ upper limit of normal (ULN), aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ≤ 2.5 x ULN, and creatinine ≤ ULN or creatinine clearance ≥ 60 mL/min). Exclusion criteria included: prior treatment with any agents targeting EGFR or RAF or VEGF/VEGFR; systolic blood pressure > 140 mmHg and/or diastolic pressure > 90 mmHg despite appropriate medical treatment; brain/meningeal metastases; and concurrent use of antiepileptic drugs or CYP3A4 inducers.
Patients were required to meet the following criteria before starting each cycle: ANC ≥ 1.0 × 109/L, platelets ≥ 100 × 109/L and non-hematologic toxicity recovered to tolerable grade ≤ 2. Sequential dose reductions for sorafenib were 200 mg bid and 200 mg daily; and for erlotinib, 100 mg daily and 75 mg daily. Patients in whom one study drug was held or discontinued could continue to receive the other. Objective responses were independently reviewed and assessed by RECIST criteria [30] every other cycle.
Pharmacodynamic analysis
MEK1/2 pShift assay
The STC of RAF before starting sorafenib and before the addition of erlotinib, was assessed by measuring MEK1/2 pShift. The assay is based on the following principles: 1) standard sorafenib dose yields different toxicity/efficacy effects among different patients; 2) when RAF is stimulated, its STC increases to recruit and phosphorylate MEK1/2, resulting in an increase in phospho-MEK1/2; 3) the difference between basal and stimulated phospho-MEK1/2 levels constitutes pShift which estimates RAF STC; 4) sorafenib inhibits RAF kinase activity, such that a greater inhibition results in a smaller MEK1/2 pShift; 5) the comparison of pShift before and after sorafenib exposure, reflects its PD effect on RAF STC. A schematic representation of the procedure is shown in Figure 1B.
Approximately 7.5 ml of peripheral blood was obtained and aliquotted into 6 × 200 μl samples, one triplicate each for measuring basal and stimulated MEK1/2 phosphorylation respectively. Samples were equilibrated at 37°C (15 min). One triplicate was stimulated with 200 ng/ml of interleukin-3 (IL3) for 5 min at 37°C and the other with vehicle. Samples were fixed and red blood cells lysed simultaneously with BD-Fix-Lysis buffer (BD Biosciences, Franklin Lakes, NJ), washed, permeabilized with BD-Perm buffer III for 30 min at 4°C, and washed again. The remaining leukocyte pellet was re-suspended in 100 μl of phosphate buffered saline (PBS) containing blocking antibodies (1 μg of anti-CD16/32, Cederlane labs, Burlington, ON) for 10 min at room temperature to diminish background staining. Cells were stained with mouse-anti-CD45-PERCP and rabbit-anti-pMEK (BD and Cell Signaling Technology, Danvers, MA) for 30 min at 4°C, washed and incubated with anti-rabbit-Alexa488 (Invitrogen, Carlsbad, CA) for 45 min at 4°C to visualize pMEK1/2. Upon a final washing, cells were re-suspended in PBS, sorted on a digital FACS-Canto machine equipped with FACS Diva software V.5.0 (BD) and analyzed using FlowJo V.7.5.5 (Tree Star Inc., Ashland, OR).
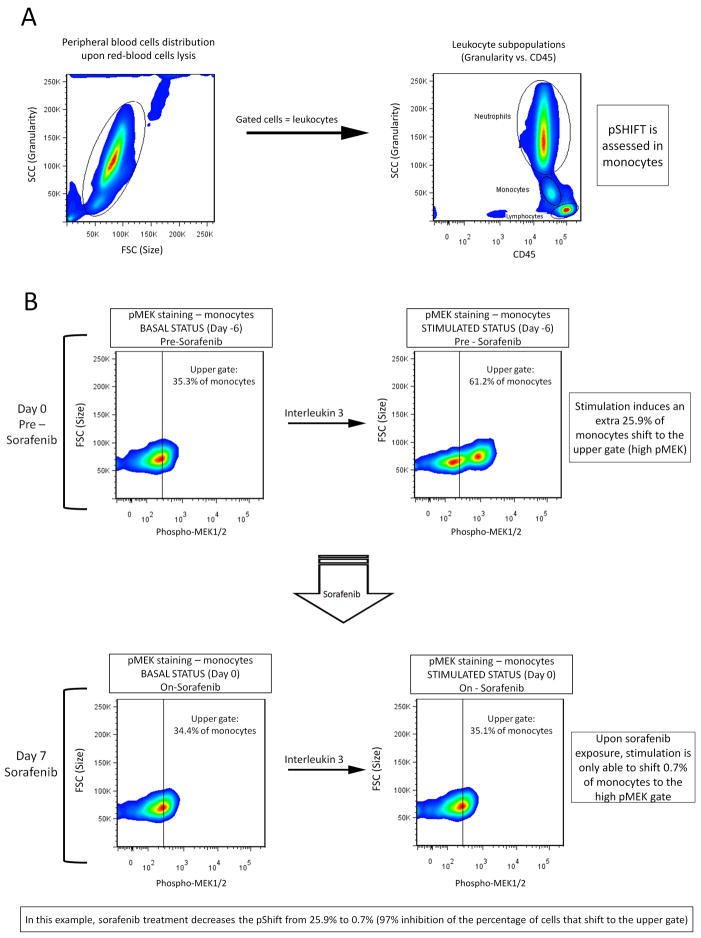
Preliminary evaluation of the assay in healthy volunteers is offered in Appendix A1. The evaluation of MEK1/2 pShift is depicted by flow cytometric charts in Figures 2A-B. The target cell population for MEK1/2 pShift assessment is constituted by the monocytes. Figure 2A (left panel) shows a typical forward scatter/side scatter (FSC/SSC) distribution of cell size versus granularity of peripheral blood cells obtained upon red blood cells lysis from whole blood. Viable leukocytes are gated. The right panel of Figure 2A shows the typical three leukocyte subpopulations: neutrophils (upper gate), monocytes (mid gate) and lympnhocytes (lower gate) by plotting the viable leukocytes from the right panel in a chart of CD45 versus granularity. Monocytes are then plotted in charts (Figure 2B) where the X-axis represents pMEK1/2 fluorescence staining intensity. The analysis of the samples from healthy volunteers revealed that a geometric mean of fluorescence intensity value of 281 segregates the 1/3 of monocytes with highest pMEK1/2 fluorescence into the “upper gate”. Cells with increased pMEK1/2 staining intensity after stimulation with IL3 are shifted to the upper gate. pShift is calculated by substracting the average percentage of monocyte shifts in vehicle-stimulated triplicates from that in IL3-stimulated triplicates. Figure 2B represents an example of one patient’s monocytes before and after sorafenib treatment. Prior to sorafenib treatment (Figure 2B upper panels), ex vivo stimulation of the patient’s monocytes with IL3 resulted in an additional 25.9% of cells shifting to the upper gate, compared to stimulation with vehicle. After 7 days of sorafenib treatment (Figure 2B lower panels), only an additional 0.7% of monocytes shifted to the upper gate when stimulated ex vivo with IL3 versus vehicle. The percent of pShift suppression as a result of sorafenib treatment for an individual patient is determined by (1-(Y/X))*100%, where X is the pre-sorafenib pShift value and Y is the on-sorafenib pShift value. For standardization purpose, percentages were chosen as the units for quantitation of pShift suppression rather than a median fluorescence intensity value for the cell population. The individual shift values for each patient and sample are available in supplementary Table 1.
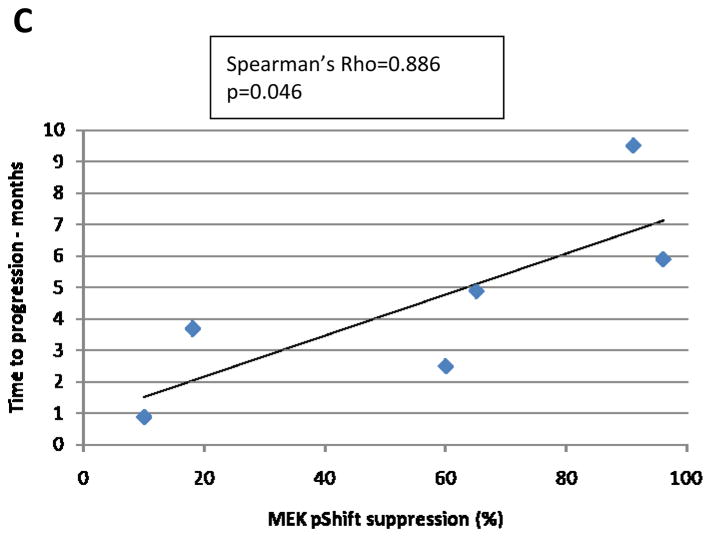
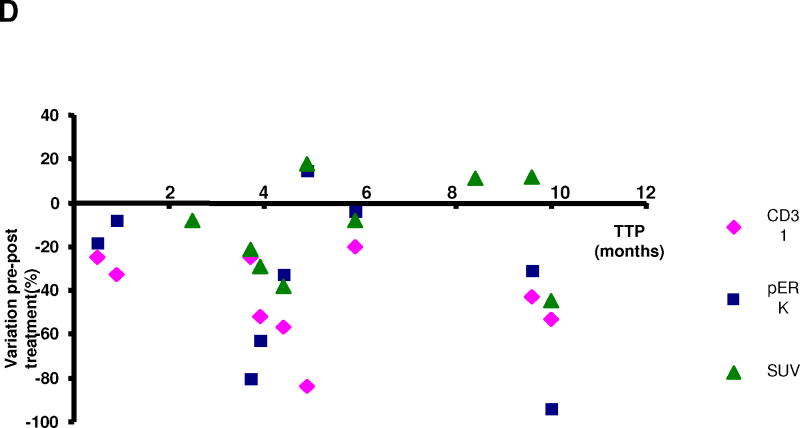
Figure 2. Pharmacodynamic correlates.
A) Gating pathway for identifying monocytes among PBMCs: the left chart plots the cells according to their size and granularity. Gated cells in this chart represent leukocytes. In the right chart, leukocytes are plotted according to their granularity and CD45 staining, defining 3 subpopulations: neutrophils, monocytes and lymphocytes (gated on the chart) B) Example of quantitation of sorafenib-induced MEK1/2 pShift inhibition (samples from patient 5). Monocytes are distributed according to their intracellular phospho-MEK1/2 staining; the “upper gate” is established with a fluorescence value of 281. This value serves as a cutoff value that limits 1/3 of monocytes with the highest pMEK staining in basal conditions in our healthy volunteers. The upper panels show the monocytes response to IL3 stimulation before sorafenib exposure; a large number of monocytes (25.9%) shift to the upper gate. After 7 days of sorafenib treatment (lower panels), the monocytes distribution according to pMEK staining in basal conditions (lower left panel) remains virtually unchanged compared to pre-treatment (upper left panel). However, only an extra 0.7% monocytes are able to shift to the upper gate (lower right panel) upon stimulation. This represents a 97% inhibition of the response to the stimulation or pShift (the average of the three samples for this patient yielded 96%) C) plot showing the correlation between MEK1/2 pShift inhibition and TTP. D) diagram plotting the treatment-induced changes in CD31, pERK and SUV versus TTP.
Other PD markers
Three fresh tumor biopsies were obtained on time points as shown in Figure 1. Biopsies were immediately immersed in Tissue-Tek optimal cutting temperature (OCT) embedding medium, snapped frozen in liquid nitrogen and stored in −80°C freezers. Phospho-ERK1/2 (#4376, Cell Signaling Technology, Danvers, MA) and CD31 clone JC70A (# M0823, DAKO, Glostrup, Denmark) were measured by immunofluorescence and quantified as described previously by our group [16, 31].
The relative abundance of phospho-ERK1/2 was represented by the product of mean integrated optical density (IOD) and labeled region fraction. The relative abundance of blood vessels was measured by vessel count and vessel size in tumor area. Archival samples were analyzed for K-RAS mutations by polymerase chain reaction and sequencing of exon 2, and for EGFR expression by IHC, with methods previously utilized by our group [32, 33]. EGFR staining intensity was graded as follows: 0=none; 1=weak; 2=weak-to-strong; 3=strong; the proportion score is a continuous variable from 0% to 100% cells staining positive. A hybrid-score (H-score) was calculated as the product of the EGFR intensity score and the proportion score, with a possible range of results from 0–300.
PET-CT scan
Positron emission tomography-computed tomography (PET-CT) scans were performed as listed in Figure 1. Standardized uptake values (SUV) of target lesions were summed for each PET scan.
Pharmacokinetic analysis
Blood samples for sorafenib PK analysis were collected during cycle 1 on day −6 before morning dosing and days −2 and +15 before morning dose and at 1, 2, 4, 6, 8, 12, and 24 hours post-dose. PK analysis for sorafenib was performed by Bayer HealthCare Pharmaceuticals using a validated liquid chromatography-mass spectrometry method [34]. Lower limit of quantification for sorafenib was 0.1 μg/mL. A non-compartmental method was used to compute pharmacokinetic variables. Minimum steady-state plasma concentrations (Css,min) for erlotinib and its metabolite OSI-420 were measured on cycle 1 days +15, 16, 22, and 29 using a high-performance liquid chromatography assay [35], with lower limits of detection of 12.5 ng/ml and 5 ng/ml respectively.
Statistical considerations
Spearman correlation was used to examine if the percentage change in on-treatment PD end points (pERK, SUV, pShift and EGFR) relative to pre-treatment was correlated with time to progression (TTP). For all PK variables of Sorafenib, including AUC, Cmax, Tmax and elimination half-life, the Wilcoxon signed rank test was carried out to compare values in the presence or absence of erlotinib (day −2 vs day +15). Spearman correlation was also conducted to examine if the median sorafenib AUC value could predict the percentage change in pShift on-treatment compared with pre-treatment.
Results
Between July 2006 to November 2007, eleven patients were enrolled in the expansion cohort of the sorafenib and erlotinib targeted combination phase I trial. Table 1 lists the patients’ pre-treatment characteristics.
Table 1.
Baseline characteristics of patients enrolled in the expansion cohort
| No. of patients | 11 |
| Age (years), median [range] | 51 [38–69] |
| Gender (male/female) | 10/1 |
| ECOG performance status (0/1) | 8/3 |
| Smoker/non-smoker | 3/8 |
| Tumor type: | |
| - cholangiocarcinoma | 5 |
| - hepatocellular cancer | 2 |
| - pancreatic cancer | 1 |
| - neuroendocrine tumor | 1 |
| - germ cell tumor | 1 |
| - adenoid cystic carcinoma | 1 |
| Prior treatment: | |
| - adjuvant chemotherapy | 1 |
| - palliative chemotherapy | 7 |
| - radiotherapy | 1 |
| No. of prior chemotherapy regimen (0/1/2/3) | 3/4/3/1 |
ECOG: Eastern Cooperative Oncology Group
Dose delivery
In total, 11 patients received 57 cycles of treatment (median = 5 cycles, range = 1–10 cycles). Only 4 patients could receive the treatment regimen without dose reductions for the entire study course (2 for only 1 cycle; Table 2). Specifically, throughout the study, sorafenib doses were delivered in full in 4 patients and reduced in 7 patients, whereas erlotinib doses were delivered in full in 6 patients, reduced in 2 patients and discontinued in 3 patients. Nine patients needed at least 1 dose delay with sorafenib dosing and 7 needed at least 1 dose delay with erlotinib dosing.
Table 2.
Study course: dose delays, reductions, clinical outcome and pharmacodynamic correlate.
| Tumor type | No. of cycles | Any delay (yes/no) | Reason of delay | Length of delay€ | No. of dose reduction | Timing of dose reduction | TTP (Days) | MEK pSHIFT suppression | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | E | S | E | S | E | ||||||
| Cholangiocarcinoma | 4 | No | n/a | n/a | n/a | - | - | n/a | n/a | 117 | - |
| Neuroendocrine | 9 | Yes | LFT | 6 | 3.5 | 1 | 2 | C2 | C2+C3* | 252 | - |
| Tumor | HFR | ||||||||||
| Cholangiocarcinoma | 5 | No | n/a | n/a | n/a | - | - | n/a | n/a | 132 | - |
| Adenoid Cystic | 10 | Yes | Hyponatremia | 4 | 4 | 1 | 2 | C2 | C2+C3* | 300 | - |
| Carcinoma | Chest pain Fatigue |
||||||||||
| Cholangiocarcinoma | 6 | Yes | LFT | 3.5 | 4.5 | 1 | 2 | C2 | C2+C3* | 176 | 96% |
| Cholangiocarcinoma | 5 | Yes | HFR | 1 | n/a | 2 | - | C2+C4 | n/a | 147 | 65% |
| Hepatocellular | 4 | Yes | LFT HFR |
2 | n/a | 2 | - | C1+C4 | n/a | 75 | 60% |
| Hepatocellular | 1 | Yes | LFT | 0.5 | 0.5 | - | - | n/a | n/a | 15 | 79%¥ |
| Carcinoma | |||||||||||
| Germ Cell Tumor | 1 | Yes | Fatigue | 2 | 2 | - | - | n/a | n/a | 28 | 10% |
| Cholangiocarcinoma | 2 | Yes | LFT | 4 | 6 | 1 | 1 | C1 | C1 | 112 | 18% |
| Pancreatic Cancer | 10 | Yes | Diarrhea HFR Hypophosphatemia |
2 | 2 | 2 | 2 | C1+C3 | C1+C2 | 287 | 91% |
S: sorafenib ; E: erlotinib ; n/a: not applicable ;
drug stopped; C: cycle ; HFR: hand-foot reaction ; LFT: liver function test abnormalities; TTP: Time to progression.
Time expressed in weeks.
This patient received sorafenib alone from day −6 to 0. He had a history of chronic hepatitis B infection. On day 0 he presented with a grade 4 ALT elevation and treatment was stopped. He was diagnosed with possible hepatitis B flare and never started the experimental combination. The subsequent clinical deterioration led to the diagnosis of clinical PD. As the analyzed outcome was TTP under the experimental combination treatment, and this patient never received it, he was excluded from the pharmacodynamic analysis.
Safety
Table 3 reports adverse events (AE) which were at least possibly related to study drugs, out of 57 treatment cycles. The most commonly observed AEs (possibly related) were fatigue (11 patients) and diarrhea (8 patients). Diarrhea was usually grade 1–2 and manageable with loperamide; but it led to dose reduction in 2 patients and dose delay in 1 patient, respectively. Alterations in liver enzymes were common, with 10, 9 and 6 patients, respectively experiencing some degree of AST/ALT/bilirubin increase during treatment course.
Table 3.
Adverse events at least possibly related to study drugs
| Cycles, n (%) Total = 57 cycles |
|||
|---|---|---|---|
| Adverse events | Grade 1/2 | Grade 3 | Grade 4 |
| Constitutional symptoms | |||
| Fatigue | 41 (72%) | 3 (5%) | 0 |
| Gastro-intestinal | |||
| Diarrhea | 30 (53%) | 1 (2%) | 0 |
| Dermatologic | |||
| Hand foot reaction | 29 (51%) | 3 (5%) | 0 |
| Rash desquamating | 10 (18%) | 1 (2%) | 0 |
| Dry skin | 38 (67%) | 0 | 0 |
| Acneiform rash | 17 (30%) | 0 | 0 |
| Nail disorders | 31 (54%) | 0 | 0 |
| Metabolic | |||
| ALT increase | 26 (46%) | 4 (7%) | 1 (2%) |
| AST increase | 34 (60%) | 4 (7%) | 0 |
| Hyperbilirubinemia | 14 (25%) | 2 (4%) | 0 |
| ALP increase | 21 (37%) | 1 (2%) | 0 |
| Hypophosphatemia | 35 (61%) | 14 (25%) | 0 |
| Hyponatremia | 5 (9%) | 1 (2%) | 0 |
ALT: alanine aminotransferase ; AST: aspartate aminotransferase ; ALP: alkaline phosphatase
Clinically meaningful grade ≥3 AEs at least possibly related to the study drugs included AST/ALT/bilirubin increase (in 4/4/2 patients respectively), hand-foot reaction (3), fatigue (2), diarrhea, hypertension and uveitis (1 each). Hematologic side effects were uncommon (grade 3 lymphopenia in 2 patients). There were no toxic deaths on study.
Efficacy
Tumor response was assessed in 10 patients. One patient was considered non-evaluable for response due to possible flare of hepatitis B after 1 week of sorafenib treatment. Nine of 10 patients evaluable for response experienced stable disease as best response, one remaining patient had progressive disease during cycle 1. Median time-to-progression (TTP) was 4.8 months (95% confidence interval [CI]: 3.8 months – not reached); 6-months progression-free rate was 33% (95%CI: 13%–84%). Three patients remained on study treatment for 9, 10 and 10 cycles, respectively (Table 2).
Pharmacodynamic analysis
Table 2 depicts the MEK1/2 pShift suppression induced by sorafenib treatment in the 7 patients assayed. Although all patients received the same sorafenib dose within the run-in period when this parameter was measured, large inter-patient variations were noted. The correlation between MEK pShift suppression and TTP is plotted on Figure 2C; these two variables showed a direct correlation (Spearman’s rho=0.886) that reached statistical significance (p=0.046).
EGFR H-score on archival tumor specimens was evaluated in 10 patients and ranged from 0–140 (median: 10). No correlation between EGFR H-score and TTP was found. No K-RAS mutations were identified. CD31 and pERK were assessed pre- and post-treatment in 9 patients. pERK values decreased in 8/9 (88%) patients, ranging from −4% to −94% (median: −32%), while CD31 decreased in all patients, ranging from −20% to −84% (median: −43%). Nine patients underwent PET scans before and after treatment. Six patients had a decrease in SUV uptake while 3 patients had an increase, ranging from −8% to −45% (median: −25%) and from +11% and +18% (median: +12%), respectively. There was no correlation between variations of pERK, CD31 and SUV with TTP (Figure 2D). None of these parameters showed correlation with MEK1/2 pShift results.
Pharmacokinetic analysis
All patients were eligible for PK analysis. Mean AUC0–12, Tmax, and Cmax values of sorafenib on day −2 and day +15 showed significant intra-patient and inter-patient variability (Table 4). No statistically significant differences in any PK variables of sorafenib were detected in the presence or absence of erlotinib, suggesting a lack of effect of erlotinib on the PK profile of sorafenib. The availability of only trough erlotinib levels precludes any conclusion of the effects of sorafenib on erlotinib. The average Css,min values of erlotinib and its main metabolite OSI-420 also revealed wide inter-individual variability, with mean trough erlotinib concentration of around 500 ng/ml (Appendix A2). No significant effect of smoking status on the PK profile of sorafenib or erlotinib was detected (data not shown). Mean sorafenib AUC did not demonstrate any correlation with TTP or any of the pre- or post-treatment PD endpoints, including MEK1/2 pShift (data not shown).
Table 4.
Sorafenib pharmacokinetic variables
| Day −2 Mean [range] | Day 15 Mean [range] | P | |
|---|---|---|---|
| n | 11 | 6 | |
| AUC (μg.h/mL) | 70.9 [29.7–149.8] | 53.2 [28.3–71.6] | 0.89 |
| Cmax (μg/mL) | 8.6 [3.1–16.7] | 8.6 [4.4–13.8] | 0.13 |
| Tmax (h) | 4.2 [0–12] | 4 [0–12] | 0.88 |
Discussion
Our previously reported dose escalation part suggested that sorafenib and erlotinib can be combined at their full single agent RP2D and also demonstrated preliminary antitumor activity [10]. We report here the detailed safety, PK and PD results of the expansion cohort of patients treated with this targeted combination at the RP2D. Even though over the short term this combination is safe at the RP2D, patients must be monitored carefully due to the potential for cumulative toxicity. Except for 1 patient who had possible flare of hepatitis B after 1 week of sorafenib treatment, all other patients tolerated the first cycle at full doses of both drugs. However, two-third of the patients required dose reductions after 1–4 cycles, mainly due to liver function test abnormalities, but also as a result of fatigue, gastrointestinal or skin toxicity. This outcome is not surprising as these are overlapping toxicities of both drugs [34, 36–39]. In addition, 82% of the patients required at least 1 dose delay during the study course. The toxicity profile encountered in this expansion cohort of patients was similar to that reported in the dose finding part [10]. Similar targeted combinations, such as sorafenib plus gefitinib [40], and sunitinib plus erlotinib [41], have displayed comparable adverse event profiles but a lower frequency of toxicities mandating dose delays and reductions. The lower frequency of toxicities encountered with these other targeted combinations may be explained by their evaluation in selected, less-pretreated, tumor-specific patient populations. Also, in the case of the sorafenib plus gefitinib combination, gefitinib was administered at a lowered dose of 250 mg/day instead of 500 mg/day [41–43]. A recent phase I/II trial evaluated the identical doublet of sorafenib and erlotinib in patients with recurrent glioblastoma who were not on enzyme-inducing antiepileptic agents [15]. The RP2D was determined as sorafenib 200 mg BID and erlotinib 100 mg daily, which was lower than the RP2D reported in our phase I study [10]. This discrepancy may be partly explained by the poorer performance status of patients with glioblastoma. Preliminary efficacy results of the sorafenib plus erlotinib doublet in this trial were disappointing with the phase II portion failing to meet the pre-set criteria to proceed to the second stage. The efficacy data encountered in our current report are encouraging, with prolonged SD of 4 cycles or longer observed in 8/10 patients evaluable for response, among different types of solid malignancies. However, due to the small sample size and the heterogeneity of patients in our current report, these efficacy results should be considered exploratory. Further efficacy data of this targeted combination from other tumor-specific studies are eagerly awaited.
We implemented in this study a novel, exploratory laboratory technique that proposes to measure the effect of a kinase inhibitor over its target kinase activity in real time as a potential PD marker. In order to assess STC in an otherwise quiescent pathway in a surrogate tissue (RAF-MEK-ERK in monocytes), stimulation with a specific agent (IL3) is required and the assay is designed to be performed in whole blood within 15 minutes of sampling. This avoids manipulations that may affect the drug or its surrounding physiological conditions, rendering the assay as close to in vivo testing as current techniques allow. The PD effects measured by this technique reflect exclusively sorafenib activity over RAF, as the change in STC of RAF is assayed during the sorafenib run-in period and prior to the initiation of erlotinib. The inability to additionally account for the PD effects of erlotinib is a limitation of this correlative study. There were large inter-individual variations in the MEK1/2 pShift suppression in the 7 patients assayed, and even though these results appear to correlate with TTP, the small sample size, and the random effects of multiple comparisons, preclude definitive conclusions. The lack of correlation with sorafenib PK parameters would suggest that the underlying molecular mechanisms are not entirely based on drug concentrations or exposures, but likely due to biological interactions of sorafenib with its cellular targets. Finally, the finding that non-dynamic PD assessments in tumor samples (e.g. pERK) did not show a concordant behavior as MEK1/2 pShift inhibition would support the proposition that these markers are likely capturing different biological profiles. In other studies, the evaluation of pERK in tumor tissues to predict for response to erlotinib has led to conflicting results [16, 42, 44, 45]. In two phase II clinical trials of sorafenib (head and neck cancer and thyroid cancer), pERK has been shown to decrease in a limited number of paired tumor biopsies but did not correlate with clinical outcome [31, 46]. In a third phase II clinical trial with only pre-treatment tissue available (hepatocellular carcinoma), the tumor staining intensity of pERK correlated positively with a longer TTP [47]. Our dynamic PD assay, while yielded interesting results, must be further validated in tumor types where sorafenib has known antitumor activity. In fact, to date, different PD effects explored using variable surrogate tissues with targeted drugs have seldom been validated in a consistent manner, with a few exceptions such as the development of skin rash with EGFR inhibitors [48–50]. Accordingly, further evaluations to measure predictive effects of the MEK pShift in independent trials of sorafenib are mandatory prior to drawing definitive conclusions. Finally, the ideal platform for PD effects assessment would involve the utilization of tumor tissues. However, the challenges in using tumor tissues include dissociation techniques to obtain single cell suspension and washing steps which may alter cell response to drug stimulation. In addition, the presence of cell types from several different lineages present in a solid tumor sample makes it difficult to assess pShift response as compared to a single cell population in sorted PBMCs.
We explored whether changes in CD31 (an endothelial cell marker) in tumor could constitute a predictive marker for sorafenib. Despite showing a constant decrease in CD31 staining (Figure 2D), the lack of correlation with TTP may be due to the analysis of a limited and non-representative tissue section in each patient, or that this marker reflects pharmacodyamic activities rather than being predictive of outcome. Reports in the literature of the predictive value of CD31 with different antiangiogenic agents have been inconsistent [31, 51, 52]. Finally, in contrast to other studies involving agents that target VEGFR or EGFR pathways [53–57], we did not find a relationship between decrease in SUV and clinical outcome. Given the small sample size and the heterogeneity of our patient population, these results may have limited power to detect subtle associations even if they exist.
The PK parameters of sorafenib were similar to those obtained in single-agent trials [34–37], and did not vary significantly in the presence or absence of erlotinib. Even though the effect of sorafenib on erlotinib could not be assessed with our trial design, in comparison to historical PK data of erlotinib alone, the steady-state concentration of erlotinib and OSI-420 appeared to be reduced in the presence of sorafenib. However, the small number of patients sampled renders the conclusion difficult to interpret [38, 45, 56]. Data from other trials of similar combinations corroborate with our findings. For instance, the phase I/II trial of sorafenib and erlotinib in glioblastoma showed a lack of accumulation of erlotinib over time, and erlotinib did not significantly affect sorafenib concentrations [15, 58]. Similarly, in the phase I trial of sorafenib plus gefitinib, no PK effect of gefitinib on sorafenib was found, whereas the presence of sorafenib led to a decrease in gefitinib Cmax and AUC [40]. As well, a slight decrease in Cmax, AUC and steady-state-concentration of erlotinib was found when it was combined with bevacizumab [59]. Although the definitive mechanism has not yet been elucidated, it has been proposed that sorafenib binding may increase the maximal velocity of CYP3A4 metabolism of erlotinib or gefitinib [58]. These findings are consistent with our observations that sorafenib had to be dose reduced in more patients than erlotinib.
In summary, this targeted combination has demonstrated promising biological activity in different tumor types, but at the expense of a high incidence of some side effects such as fatigue, gastrointestinal toxicity and rise in liver enzymes. Our exploratory PD marker for RAF inhibition deserves further study in larger patient series. Sorafenib may lead to sub-therapeutic erlotinib levels when given in combination; detailed analysis of this PK interaction should be undertaken.
Supplementary Material
Acknowledgments
We thank Mary Saunders, PhD, for critical review of the manuscript.
Grant support: NCI-CTEP grant NOI-CM-62203 and the Translational Research Initiative grant P7178/22XS108-Task order 17. Christophe Le Tourneau is in part supported by a grant of the Fondation de France. Tak Mak is recipient of OCRN 2006 grant number 05NOV00203. Miguel Quintela-Fandino is recipient of the “Spanish Society of Medical Oncology – Young Investigators Grant 2005” and of the “Rafael Hervada Biomedical Research Award” in November-2008 in La Coruna, Spain for this work.
Abbreviations list
- PK
pharmacokinetic
- PD
pharmacodynamic
- STC
signal transduction capacity
- RP2D
recommended phase II dose
- IHC
immunohistochemistry
- PBMCs
peripheral blood mononuclear cells
- ANC
absolute neutrophil count
- ULN
upper normal limit
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- IL3
interleukin-3
- PBS
phosphate buffered saline
- FSC/SSC
forward scatter/side scatter
- IOD
integrated optical density
- H-score
hybrid score
- PET
positron emission tomography
- SUV
standardized uptake value
- TTP
time to progression
- AE
adverse event
Footnotes
Prior presentation in abstract format:
This study was presented in part in the 20th European Organization for Research and Treatment of Cancer-National Cancer Institute-American Association for Cancer Research (EORTC-NCI-AACR) Symposium on Molecular Targets and Cancer Therapeutics, October 2008, Geneva, Switzerland, and at the 100th AACR meeting, April 2009, Denver, Colorado.
Conflicts of interest:
Miguel Quintela-Fandino: has conducted research funded by Bayer Pharmaceuticals.
Eric X. Chen: has received honoraria from and conducted research funded by Roche Pharmaceuticals.
Ming Tsao: has exerted compensated advisory role for and received honoraria from Roche Pharmaceuticals.
Lillian L. Siu: has conducted research funded by Bayer Pharmaceuticals.
References
- 1.Moyer JD, Barbacci EG, Iwata KK, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838–4848. [PubMed] [Google Scholar]
- 2.Pollack VA, Savage DM, Baker DA, et al. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther. 1999;291:739–748. [PubMed] [Google Scholar]
- 3.Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar((R))), a Dual-Action Inhibitor That Targets RAF/MEK/ERK Pathway in Tumor Cells and Tyrosine Kinases VEGFR/PDGFR in Tumor Vasculature. Methods Enzymol. 2005;407:597–612. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- 4.Mangiameli DP, Blansfield JA, Kachala S, et al. Combination therapy targeting the tumor microenvironment is effective in a model of human ocular melanoma. J Transl Med. 2007;5:38. doi: 10.1186/1479-5876-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 6.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 10.Duran I, Hotte SJ, Hirte H, et al. Phase I targeted combination trial of sorafenib and erlotinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13:4849–4857. doi: 10.1158/1078-0432.CCR-07-0382. [DOI] [PubMed] [Google Scholar]
- 11.http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=587665&version=HealthProfessional&protocolsearchid=6179449
- 12.http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=614959&version=HealthProfessional&protocolsearchid=6179449
- 13.http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=616195&version=HealthProfessional&protocolsearchid=6179449
- 14.http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=618003&version=HealthProfessional&protocolsearchid=6179449
- 15.Prados M, Gilbert M, Kuhn M, et al. Phase I/II study of Sorafenib and Erlotinib for patients with recurrent glioblastoma (GBM) (NABTC 05-02) Proc of Am Soc Clin Oncol. 2009 abstract 2005. [Google Scholar]
- 16.Agulnik M, da Cunha Santos G, Hedley D, et al. Predictive and pharmacodynamic biomarker studies in tumor and skin tissue samples of patients with recurrent or metastatic squamous cell carcinoma of the head and neck treated with erlotinib. J Clin Oncol. 2007;25:2184–2190. doi: 10.1200/JCO.2006.07.6554. [DOI] [PubMed] [Google Scholar]
- 17.Baselga J, Albanell J, Ruiz A, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol. 2005;23:5323–5333. doi: 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 18.Booth CM, Calvert AH, Giaccone G, et al. Endpoints and other considerations in phase I studies of targeted anticancer therapy: recommendations from the task force on Methodology for the Development of Innovative Cancer Therapies (MDICT) Eur J Cancer. 2008;44:19–24. doi: 10.1016/j.ejca.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 19.Dowlati A, Haaga J, Remick SC, et al. Sequential tumor biopsies in early phase clinical trials of anticancer agents for pharmacodynamic evaluation. Clin Cancer Res. 2001;7:2971–2976. [PubMed] [Google Scholar]
- 20.Dowlati A, Robertson K, Radivoyevitch T, et al. Novel Phase I dose de-escalation design trial to determine the biological modulatory dose of the antiangiogenic agent SU5416. Clin Cancer Res. 2005;11:7938–7944. doi: 10.1158/1078-0432.CCR-04-2538. [DOI] [PubMed] [Google Scholar]
- 21.Duran I, Kortmansky J, Singh D, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95:1148–1154. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duran I, Nicklee T, Ho J, et al. Feasibility of successfully obtaining paired, evaluable research tumor biopsies (bx) for correlative studies. Ann Oncol. 2006;17:407p. [Google Scholar]
- 23.Haas-Kogan DA, Prados MD, Lamborn KR, et al. Biomarkers to predict response to epidermal growth factor receptor inhibitors. Cell Cycle. 2005;4:1369–1372. doi: 10.4161/cc.4.10.2105. [DOI] [PubMed] [Google Scholar]
- 24.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 25.Jimeno A, Rudek MA, Kulesza P, et al. Pharmacodynamic-guided, modified continuous reassessment method-based, dose finding study of rapamycin in adult patients with solid tumors. J Clin Oncol. 2008;26:4172–4179. doi: 10.1200/JCO.2008.16.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan AR, Steinberg SM, Parr AL, et al. Markers in the epidermal growth factor receptor pathway and skin toxicity during erlotinib treatment. Ann Oncol. 2008;19:185–190. doi: 10.1093/annonc/mdm444. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka C, O’Reilly T, Kovarik JM, et al. Identifying Optimal Biologic Doses of Everolimus (RAD001) in Patients With Cancer Based on the Modeling of Preclinical and Clinical Pharmacokinetic and Pharmacodynamic Data. J Clin Oncol. 2008 doi: 10.1200/JCO.2007.14.1127. [DOI] [PubMed] [Google Scholar]
- 28.Tong FK, Chow S, Hedley D. Pharmacodynamic monitoring of BAY 43-9006 (Sorafenib) in phase I clinical trials involving solid tumor and AML/MDS patients, using flow cytometry to monitor activation of the ERK pathway in peripheral blood cells. Cytometry B Clin Cytom. 2006;70:107–114. doi: 10.1002/cyto.b.20092. [DOI] [PubMed] [Google Scholar]
- 29.Irish JM, Hovland R, Krutzik PO, et al. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 2004;118:217–228. doi: 10.1016/j.cell.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 31.Elser C, Siu LL, Winquist E, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25:3766–3773. doi: 10.1200/JCO.2006.10.2871. [DOI] [PubMed] [Google Scholar]
- 32.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 33.Zhu CQ, da Cunha Santos G, Ding K, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR. 21. J Clin Oncol. 2008;26:4268–4275. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- 34.Moore M, Hirte HW, Siu L, et al. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol. 2005;16:1688–1694. doi: 10.1093/annonc/mdi310. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Siu LL, Moore MJ, Chen EX. Simultaneous determination of OSI-774 and its major metabolite OSI-420 in human plasma by using HPLC with UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;814:143–147. doi: 10.1016/j.jchromb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Awada A, Hendlisz A, Gil T, et al. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92:1855–1861. doi: 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark JW, Eder JP, Ryan D, et al. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–5480. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 38.Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–3279. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 39.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 40.Adjei AA, Molina JR, Mandrekar SJ, et al. Phase I trial of sorafenib in combination with gefitinib in patients with refractory or recurrent non-small cell lung cancer. Clin Cancer Res. 2007;13:2684–2691. doi: 10.1158/1078-0432.CCR-06-2889. [DOI] [PubMed] [Google Scholar]
- 41.Ryan CW, Curti CB, Patee KJ, et al. A dose-escalation phase II study of sunitinib (s) plus erlotinib (e) in advanced renal cell carcinoma (RCC) Proc of Am Soc Clin Oncol (Genitourinary Cancers Symposium) 2008 abstract 361. [Google Scholar]
- 42.Calvo E, Malik SN, Siu LL, et al. Assessment of erlotinib pharmacodynamics in tumors and skin of patients with head and neck cancer. Ann Oncol. 2007;18:761–767. doi: 10.1093/annonc/mdl495. [DOI] [PubMed] [Google Scholar]
- 43.Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib 250 compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1864–1871. doi: 10.1200/JCO.2008.17.0530. [DOI] [PubMed] [Google Scholar]
- 44.Garland LL, Rankin C, Gandara DR, et al. Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. J Clin Oncol. 2007;25:2406–2413. doi: 10.1200/JCO.2006.09.7634. [DOI] [PubMed] [Google Scholar]
- 45.Townsley CA, Major P, Siu LL, et al. Phase II study of erlotinib (OSI-774) in patients with metastatic colorectal cancer. Br J Cancer. 2006;94:1136–1143. doi: 10.1038/sj.bjc.6603055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 48.Saif MW, Merikas I, Tsimboukis S, Syrigos K. Erlotinib-induced skin rash. Pathogenesis, clinical significance and management in pancreatic cancer patients. Jop. 2008;9:267–274. [PubMed] [Google Scholar]
- 49.Tsimboukis S, Merikas I, Karapanagiotou EM, et al. Erlotinib-induced skin rash in patients with non-small-cell lung cancer: pathogenesis, clinical significance, and management. Clin Lung Cancer. 2009;10:106–111. doi: 10.3816/CLC.2009.n.013. [DOI] [PubMed] [Google Scholar]
- 50.Patel DK. Clinical use of anti-epidermal growth factor receptor monoclonal antibodies in metastatic colorectal cancer. Pharmacotherapy. 2008;28:31S–41S. doi: 10.1592/phco.28.11-supp.31S. [DOI] [PubMed] [Google Scholar]
- 51.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26:271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang SX, Steinberg SM, Nguyen D, et al. Gene expression profile and angiogenic marker correlates with response to neoadjuvant bevacizumab followed by bevacizumab plus chemotherapy in breast cancer. Clin Cancer Res. 2008;14:5893–5899. doi: 10.1158/1078-0432.CCR-07-4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prior JO, Montemurro M, Orcurto MV, et al. Early prediction of response to sunitinib after imatinib failure by 18F-fluorodeoxyglucose positron emission tomography in patients with gastrointestinal stromal tumor. J Clin Oncol. 2009;27:439–445. doi: 10.1200/JCO.2008.17.2742. [DOI] [PubMed] [Google Scholar]
- 54.Siemerink EJ, Mulder NH, Brouwers AH, Hospers GA. 18F-Fluorodeoxyglucose positron emission tomography for monitoring response to sorafenib treatment in patients with hepatocellular carcinoma. Oncologist. 2008;13:734–735. doi: 10.1634/theoncologist.2008-0063. author reply 736–737. [DOI] [PubMed] [Google Scholar]
- 55.Sunaga N, Oriuchi N, Kaira K, et al. Usefulness of FDG-PET for early prediction of the response to gefitinib in non-small cell lung cancer. Lung Cancer. 2008;59:203–210. doi: 10.1016/j.lungcan.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Tan AR, Yang X, Hewitt SM, et al. Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Clin Oncol. 2004;22:3080–3090. doi: 10.1200/JCO.2004.08.189. [DOI] [PubMed] [Google Scholar]
- 57.van den Boogaart V dLA, Groen HJ, et al. A phase II study of erlotinib (E) and bevacizumab (B) in chemo naive patients (pts) with locally advanced or metastatic non small cell lung cancer (NSCLC): Predictive value of molecular imaging. Proc Am Soc Clin Oncol. 2008 abstract 8055. [Google Scholar]
- 58.Kuhn JGGM, Wen P, et al. Interaction between sorafenib and erlotinib. Proc of Am Soc Clin Oncol. 2009 abstract 2500. [Google Scholar]
- 59.Herbst RS, Johnson DH, Mininberg E, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:2544–2555. doi: 10.1200/JCO.2005.02.477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.