Abstract
Objective
The aims of this study were to evaluate the spatial distribution of cartilage structure in controls and patients, and to quantitatively assess the cartilage overlying bone marrow edema-like lesion (BMEL) and within defined cartilage compartments in knees with anterior cruciate ligament (ACL) tears using T1ρ mapping technique at 3 T magnetic resonance imaging.
Materials and Methods
The knee joints of 15 healthy controls (4 women, 11 men, mean age = 30.1 year) and 16 patients with ACL tear (5 women, 11 men, mean age = 32.5 years) who showed BMEL was studied using a 3 T GEMR scanner and a quadrature knee coil. The imaging protocol included sequences for cartilage morphology and 3D quantitative T1ρ mapping. Lateral femoral condyle and medial femoral condyles compartments were partitioned into anterior and posterior nonweight-bearing (ant-nwb and postnwb) portions and weight-bearing (wb) portions in all subjects. In patients only, cartilage overlying BMEL and surrounding cartilage portions were also defined. T1ρ values were quantified in cartilage overlying BMEL and surrounding compartments and in each defined compartment of the ACL-injured knees, and compared with controls.
Results
Significantly elevated T1ρ values were found in the femoral nonweight-bearing (nwb) portions when compared with weight-bearing (wb) portions both in patients and controls. Significantly increased T1ρ values were found in cartilage overlying BMEL when compared with surrounding cartilage at the lateral tibia (LT), but no difference was found in the lateral femoral condyle.
Conclusion
T1ρ mapping technique provides tools to quantitatively evaluate the cartilage matrix overlying BMEL in patients with ACL injuries. Cartilage abnormalities are already present following initial ACL injuries over the lateral tibia. Quantitative MRI can allow critical evaluation of medical and surgical treatments for ligament and degenerative conditions of the knee.
Keywords: bone marrow edema-like lesion, T1ρ, magnetic resonance imaging, anterior cruciate ligament, cartilage
The anterior cruciate ligament (ACL) is the most commonly torn ligament in the knee, with more than 80,000 injuries occurring annually in the United States alone.1 The rupture of the ACL frequently occurs in young active individuals. The injury is usually a result of a valgus and internal rotational torque to the knee. During the injury, the knee can sublux after ACL rupture and the lateral femoral condyle (LFC) can impact the posterior aspect of the lateral tibia, resulting in the classic bone marrow edema-like lesions (BMELs) or osteochondral lesions within the lateral compartment, also known as the “kissing lesions.”2
The prevalence of BMEL in patients with ACL injuries has been reported to be up to 70% to 80%.3,4 The BMEL is usually located in the middle portion of the lateral femoral condyle and the posterior portion of the lateral tibial plateau. Despite the high prevalence of these BMEL with ACL ruptures, little is known about the clinical consequences of these findings or about their relationship with local and global cartilage degeneration.
Magnetic resonance imaging (MRI) is a useful diagnostic tool for ACL tears and related injuries.5 BMEL detected with MRI has been described previously as focally signal hyperintensity in the bone marrow on fat-suppressed T2-weighted MR images or in short inversion time inversion-recovery images due to multiple factors such as abnormal trabeculae, bone marrow necrosis, marrow hemorrhage, and marrow edema.6
The formation of bone bruises has been shown to cause substantial damage to the articular cartilage leading to cell death of a significant number of chondrocytes.7 Despite ACL reconstruction to correct instability, a significant proportion of patients develop clinical symptoms of osteoarthritis (OA) 5 to 10 years postinjury.8,9 These findings suggest that initial injury to the articular cartilage may predispose the joint to the development of OA. Therefore, a detailed topographic analysis of the articular cartilage is important to identify local cartilage degeneration after ACL injury.
Quantitative MRI is a noninvasive technique for assessing acute knee injuries and can play an important role in determining treatment options and evaluating surgical interventions. In cartilage, the proteoglycan (PG) is largely responsible for the high elasticity and resilience of tissue. The PG content of cartilage can be probed using spin lattice relaxation in the rotating frame: T1ρ-weighted imaging.10 This particular quantitative method based on spin-locking technique reflects the slow motion interactions between motion-restricted water molecules and their local macromolecular environment. T1ρ mapping has recently been proposed as a potential of MRI to reflect changes in the biochemical composition of cartilage with early OA, such as PG loss.11–13 Several recent ex vivo14 and in vivo15 studies investigated this relaxation technique in human OA, suggesting that T1ρ relaxation time increases with the degree of OA. Our previous case report on quantitative MRI has demonstrated that cartilage injury was present overlying BMEL after ACL injuries.16 In that report, elevated T1ρ relaxation time was found over the lateral tibia confirmed with arthroscopic findings of cartilage injury. This study prompted our current clinical evaluation on determining the severity of cartilage injury over BMEL. The goal of this study was to assess cartilage overlying areas of BMEL versus surrounding cartilage and to analyze defined cartilage compartments using T1ρ quantification in patients with acute ACL tears. The hypothesis of the study is that the cartilage degeneration can occur during acute ACL injuries. We also hypothesized that there can be a different T1ρ quantification at various regions of the knee. In this study, we compared T1ρ quantification in patients with acute ACL injuries versus healthy individuals.
MATERIALS AND METHODS
Subjects
Imaging was performed using a 3 T GE MR scanner and a quadrature knee coil. Two groups of subjects were defined for this study; 15 healthy controls–4 women, 11 men, (mean age = 30.1 year), and body mass index (BMI) of 23.9 ± 2.2, without any clinical symptoms of OA or other knee injuries, and 16 consecutive ACL-injured patients— 5 women, 11 men, (mean age = 32.5 years), and BMI of 23.5 ± 1.9 who had BMEL. All ACL-injured patients were scanned within 2 months of the injury and before ACL reconstruction. The controls were physically active subjects, recruited to match the demographic data of the ACL-injured patients (gender, age, BMI). This study was performed in accordance with the rules and regulations of the Committee for Human Research at our institution. Informed consent was obtained from all of the subjects after the nature of the examinations had been fully explained.
MRI Protocol
MRI of the study knee of each subject was acquired using a 3 T GE Excite Signa MR scanner (General Electric, Milwaukee, WI) with a transmit/receive quadrature knee coil (Clinical MR Solutions, Brookfield, WI).
Morphologic Imaging
The imaging protocol included sagittal 2D T2-weighted fat-saturated fast spin-echo (FSE) images (TR/TE = 4300/51 milliseconds, FOV = 14 cm, matrix = 512 × 256, slice thickness = 2.5 mm, gap = 0.5 mm, echo train length = 9, bandwidth = 31.25 kHz, number of excitations (NEX) = 2) and sagittal 3D water excitation high-resolution spoiled gradient-echo (SPGR) imaging (TR/TE = 15/6.7 milliseconds, flip angle = 12, FOV = 14 cm, matrix = 512 × 512, slice thickness = 1 mm, bandwidth = 31.25 kHz, NEX = 0.75). The T2-weighted fat-saturated FSE images were used to visualize the presence of BMEL and to evaluate the cartilage signal and morphology, and the high-resolution SPGR images were used for cartilage segmentation.
T1ρ Relaxation Time Mapping
Sagittal 3D T1ρ-weighted images were acquired based on a spin-lock technique and 3D SPGR acquisition.18 The sagittal 3D T1ρ-weighted imaging sequence was composed of 2 parts: magnetization preparation for imparting T1ρ contrast, and an elliptical-centered segmented 3D SPGR acquisition immediately after T1ρ preparation during transient signal evolution. The duration of the spin-lock pulse was defined as time of spin-lock (TSL), and the strength of the spin-lock pulse was defined as spin-lock frequency. The number of pulses after each T1ρ magnetization preparation was defined as views per segment (VPS). There was a relatively long delay (time of recovery, Trec) between each magnetization preparation to allow enough and equal recovery of the magnetization before each T1ρ preparation. The main parameters of this T1ρ sequence were: FOV = 14 cm, matrix = 256 × 192, slice thickness = 3 mm, TR/TE = 9.3/3.7 milliseconds, BW = 31.25 kHz, VPS = 48, Trec = 1.5 seconds, TSL = 0/10/40/80 milliseconds, spin lock frequency = 500 Hz, with an acquisition time of approximately 13 minutes.
Generally, radio-frequency (RF) power deposition in a T1ρ sequence would be higher because of the long RF spin-lock pulse cluster when compared with conventional imaging pulse sequences. The amount of RF power deposited–specific absorption rate (SAR)–depends on several factors, such as the static field strength, amplitude, TSL, duty cycle (ie, number of RF pulses applied within each TR), and the conductivity of the body part being imaged. The distribution of the SAR is influenced strongly by the geometry of the imaged tissue, especially as the static magnetic field becomes stronger. The RF power deposited causes an increase in the local tissue temperature. Because of this, the tissue SAR has specific limitations. The Food and Drug Administration (FDA) and the International Electrotechnical Commission (IEC) recommend specific limits for SAR for 1 g of tissue of different body parts. Because of this, the distribution of local SAR levels has to be known to make sure that the limits are not exceeded. Because the deposited radio frequency (RF) power is proportional to the square of the static field strength (B0), SAR becomes an even more important issue to be considered during the design of a pulse sequence at 3.0 T. Previous work17,18 demonstrated the feasibility of implementing 3D-T1ρ-relaxation mapping at a 3.0 T clinical scanner without exceeding the SAR limits. SAR simulations performed by Collins et al19 were used to determine the sequence parameters that would ensure the satisfaction of SAR limits mandated by Food and Drug Administration (FDA). The T1ρ imaging sequence employed here was previously developed and validated18 and can acquire a certain number [ie, user-defined views per segmentation (VPS)] of phase encoding lines after each T1ρ preparation, and therefore is less SAR-intensive and more time efficient. The RF power deposition resulting from the 3D T1ρ sequence with the imaging parameters employed in this study is well within the SAR guidelines of FDA.
MR Images Postprocessing
All images were transferred to a Sun Workstation (Sun Microsystems, Mountain View, CA) for off-line data processing. Semi-automatic cartilage segmentation was performed on the sagittal SPGR images using an in-house software21 developed with Matlab (Mathworks, Natick, MA) based on Bezier splines and edge detection.
T1ρ maps were reconstructed using a Levenberg-Marquardt mono-exponential in-house developed fitting algorithm. T1ρ-weighted images obtained with different TSLs were fitted pixel-by-pixel to the following equation: S(TSL) ∝ exp(−TSL/T1ρ). Next, the reconstructed T1ρ maps were rigidly registered to the previously acquired high-resolution T1-weighted SPGR images using the VTK CISG Registration Toolkit.22
Four main compartments were defined for cartilage analysis in the femoral-tibial joint: LFC and MFC, and lateral and medial tibia (LT and MT). Subsequently, LFC and MFC were partitioned into anterior and posterior nonweight-bearing (ant-nwb and postnwb) portions and weight-bearing (wb) portions (Fig. 1A), automatically using an in-house developed program, similar to one previously reported.23 This algorithm consisted of 3 steps. First, the longitudinal axis of the femur was computed. Second, the intersection angle between the longitudinal femur axis and the angle of the normal vector to each point of the Bezier splines generated during the segmentation process was computed. Third, each point of the Bezier splines was assigned to a region– points belonging to the weight-bearing region were those with an absolute value of the intersection angle of ≤30-degree (as computed in the previous step); the remaining points belong to the nonweight-bearing region. The thick vertical line parallels the longitudinal femur axis, as shown in Figure 1A. The defined weight-bearing regions correspond roughly to the anterior and posterior rims of the menisci with the knee in full extension.
FIGURE 1.
Postprocessing illustration: LFC and MFC were partitioned into ant-nwb and postnwb and wb portions (A); BMEL localization on fat-saturated FSE T2-weighted images (B), and cartilage overlying BMEL and surrounding cartilage portions defined in high-resolution SPGR images (C).
For determining the overlying BMEL and surrounding cartilage portions, the T2-weighted fat-saturated FSE images from which BMEL was identified (Fig. 1-B), were aligned to the 3D cartilage contour generated from SPGR images. Then the overlying BMEL and surrounding cartilage portions were determined manually, as shown in Figure 1C.
Statistical Analysis
Mean and standard deviation (SD) of T1ρ values were calculated in each of the cartilage compartments for all the subjects. Paired t tests were employed to compare the mean intragroup T1ρ relaxation time values within all defined portions. Standard t tests were used to compare the mean of T1ρ relaxation time values intergroup within all defined portions.
RESULTS
Control Subjects Data
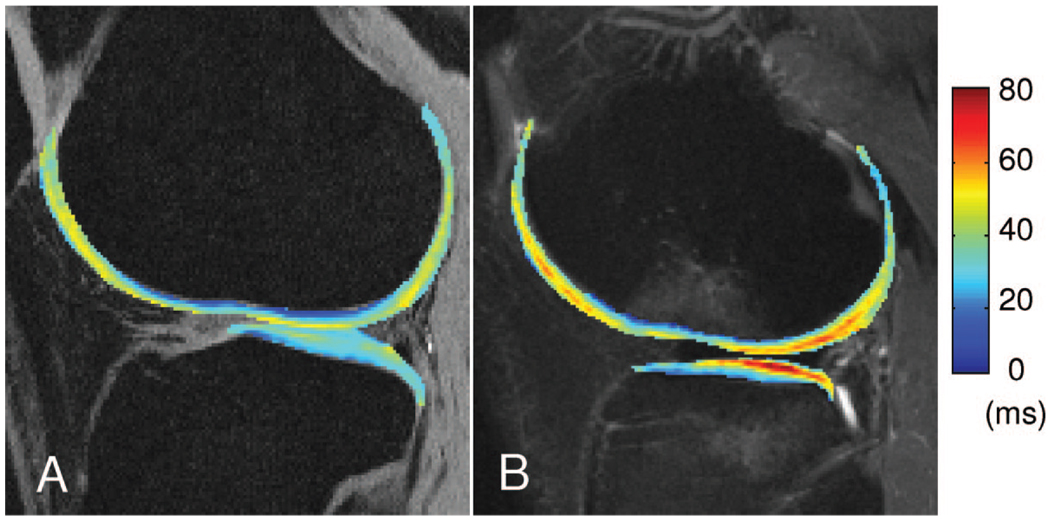
The nwb and wb portion data for the control subjects are presented in Table 1. Mean and SD of T1ρ were significantly higher (P < 0.05) in nwb portions compared with wb portions at the LFC compartment and at the MFC compartment. No significant difference was found between the lateral side and the medial side over all compartments for both femur and tibia. Figure 2A shows a representative T1ρ color-coded map from the lateral side of a healthy control subject’s knee.
TABLE 1.
T1ρ (ms) in Healthy Controls and ACL-Injured Patients
| LFC ant-nwb | LFC Postnwb | LFC wb | MFC ant-nwb | MFC Postnwb | MFC wb | LT | MT | |
|---|---|---|---|---|---|---|---|---|
| T1ρ (ms) in Healthy Controls | ||||||||
| Mean | 44.97* | 41.96* | 38.73 | 40.38* | 40.21* | 37.26 | 36.36 | 34.97 |
| SD | 3.59 | 3.72 | 2.90 | 7.03 | 3.31 | 3.13 | 2.65 | 3.51 |
| T1ρ (ms) in ACL-injured patients | ||||||||
| Mean | 45.90* | 42.40* | 39.62 | 43.71* | 40.31* | 37.88 | 36.78* | 33.72 |
| SD | 6.49 | 5.48 | 7.09 | 10.68 | 5.01 | 8.35 | 7.28 | 5.95 |
T1ρ analysis in nwb and wb portions: in femur, significantly increased T1[ρ] values (*P < 0.05) were found for nwb portions compared with wb portions both for healthy controls and ACL-injured patients. However, T1ρ values were slightly elevated for patients compared with controls; moreover, a significant elevation (*P < 0.05) was found at the LT compared with MT.
FIGURE 2.
Representative T1ρ (A) color-coded map from the lateral side of a healthy control knee (the map is overlaid on SPGR images) and representative T1ρ (B) color-coded map from the lateral side of an ACL-injured patient knee; BMEL is present at the LFC and LT (the map is overlaid on FSE-T2 images).
ACL Injured Patients Data
A radiologist examined the MR images and performed a clinical assessment of the 16 ACL-injured patients based on the T2-weighted FSE images. Only 15 out of the total of 16 patients required ACL reconstruction because of complete ACL tears. No direct correlation with arthroscopy was performed in this study. BMELs were present within all the ACL injured patients. Among them, 13 (81%) had BMEL located at the LT, 9 (56%) patients had BMEL located at the LFC, and 7 (44%) patients had BMEL both at the LT and LFC. The meniscus was clinically evaluated using WORMS scoring.24 Within 12 (75%) patients, there were 6 meniscal tears of grade I and 4 of grade II in the lateral meniscus, whereas 7 meniscal tears of grade I and only 1 of grade II, all located at the posterior horn only. No meniscal tears were found at the anterior horn of both lateral and medial menisci. Three of 12 patients required lateral meniscus debridement, whereas only 1 patient required meniscus repair, using all-inside technique. The remaining lateral meniscus tears were either stable or healed at the time of surgery.
The cartilage overlying BMEL versus surrounding cartilage analysis was performed separately with respect to the BMEL location–LFC versus LT–as shown in Figure 3. At the LT, significantly increased T1ρ values (P = 0.002) were found in cartilage overlying BMEL (47.15 ± 12.96 milliseconds) when compared with surrounding cartilage (36.93 ± 7.10 milliseconds). At the LFC, no significant difference (P = 0.077) was found in cartilage overlying BMEL (36.12 ± 4.71 milliseconds) when compared with surrounding cartilage (40.52 ± 6.18 milliseconds), as shown in Figure 3.
FIGURE 3.
Cartilage overlying BMEL versus surrounding cartilage analysis for T1ρ relaxation time in lateral tibia and lateral femur.
The nwb and wb portion data for the ACL injured patients are presented in Table 1. Mean and SD of T1ρ were significantly higher (P < 0.05) in nwb portions when compared with wb portions at the LFC and MFC compartment. No significant difference was found between LFC and MFC over all compartments. A significant elevation (P = 0.002) in T1ρ values was found at the LT compared with MT. Figure 2B shows a representative T1ρ color-coded map from the lateral side of an ACL injured patient knee. On average, no significant difference was found between patients and controls within all femoral and tibial compartments.
DISCUSSION
The T1ρ parameter reflects the slow motion interactions between motion-restricted water molecules and their local macromolecular environment. The extracellular matrix (ECM) in articular cartilage provides a motion-restricted environment for water molecules, so changes to the ECM, therefore, may be reflected in measurements of T1ρ. T1ρ relaxation rate (1/T1ρ) has been shown to decrease linearly with decreasing PG content in ex vivo bovine patellae12 and in trypsinized cartilage.13 In vivo studies have also shown increased cartilage T1ρ values for patients with OA.25,26
Delayed contrast-enhanced MRI of cartilage (dGEMRIC) represents another technique that can also be used to study cartilage glycosaminoglycan (GAG) content and distribution in the knee.27–29 In diseased cartilage, the contrast agent is easily absorbed because of the lack of GAG. However, compared with T1ρ technique, the dGEMRIC is invasive in the imaging protocol as suggested by Burstein et al;27 the contrast agent being injected intravenously, the subject exercises for approximately 10 minutes, and imaging is performed after about 2 to 3 hours.
Several studies ex vivo14 and in vivo15 investigated the relationship between T1ρ and T2 relaxation times in human OA, suggesting that both T1ρ and T2 increase with the degree of OA, but that T1ρ has a higher dynamic range for detecting early cartilage degeneration and, in consequence, is more sensitive than T2. Li et al30 reported significantly increased T1ρ values in OA patients compared with controls, whereas the increase in T2 was not significant, demonstrating that patients with similar average T2 may have different T1ρ, or vice versa. They suggested that the average T1ρ may be used to distinguish OA cartilage from healthy cartilage, whereas T2 may not be able to.
Therefore, in this study, T1ρ mapping technique was performed to examine patients with acute ACL tears within 2 months of injury and to quantitatively assess the articular cartilage overlying and surrounding the BMEL. We also studied the regional T1ρ variations of the normal and ACL-injured knees.
The results of our study are consistent with previous studies where BMELs were found predominantly on the lateral side of the joint, commonly identified in T2-weighted fat-saturated FSE images.4,31–33 For the BMEL located at the LT only, significant elevated T1ρ values were found in cartilage overlying BMEL when compared with surrounding cartilage. The significant elevation of T1ρ within the cartilage overlying BMEL at the LT suggests that early cartilage breakdown may have taken place at the time of injury. For the BMEL located in LFC only, no significant differences in T1ρ values were found between cartilage overlying BMEL and surrounding cartilage. Different hypotheses are discussed to explain this aspect. The lateral femoral notch sign described as an indirect sign of ACL tear might play an important role to answer these questions. It is known that, during the acute ACL injury, the cartilage is highly compressed, and this causes a pattern of injuries known as “kissing contusions.” These contusions are caused by a “pivot-shift” mechanism of injury whereby excessive valgus stress tears the ACL, resulting in anterior tibial translation with relative external rotation of the femur, allowing the LFC to impact the posterolateral tibial plateau. As a consequent, the cartilage becomes compressed and its thickness at the central site of the lateral femur decreases. The GAGs have a high propensity to attract and hold water (about 75% of weight) which, when the cartilage is load bearing as on the bone surface of joints, can be squeezed out into the joint space to assist synovial fluid in lubrication (weeping lubrication). Different studies reported regional tissue response to physiologic joint loading in the human knee, suggesting that a change in superficial collagen fiber orientation is likely the mechanism for the observed T2 shortening.34 Also, previous T1ρ studies have shown that the relaxation times were reduced in the presence of mechanical loading of cartilage.35 This might explain the fact that there were no significant increased T1ρ values within the cartilage overlying BMEL at the LFC.
In a previous study, Johnson et al7 performed biopsies in patients with acute ACL rupture and have demonstrated histologic changes (chondrocyte and matrix degeneration) and biochemical variations in the cartilage overlying BMEL. Another study reported histologic and immunostaining assessment for cartilage oligomeric matrix protein–an abundant noncollagenous ECM protein in cartilage–performed in cartilage biopsies overlying MRI-detected BMEL associated with acute ACL tears.36 The authors demonstrated scattered chondrocyte necrosis or apoptosis and altered distribution of PG in cartilage tissue adjacent to the cartilage overlying BMEL. A more recent study33 investigated GAG content using dGEMRIC in ACL-injured patients. The authors found significant loss of GAG in the LFC of patients with isolated BMEL when compared with controls. The findings of the recent study are consistent with those of previous studies demonstrating that cartilage overlying BMEL has already undergone degeneration after acute injury.
The weight-bearing (wb) and nonweight-bearing (nwb) portions of LFC and MFC were also investigated using T1ρ relaxation time techniques in both healthy controls and ACL-injured patients. Significantly elevated T1ρ values were found in ant-nwb and postnwb portions of the LFC and MFC when compared with wb portions of either condyle. For ACL-injured patients, the results demonstrated a similar distribution and no significant differences were found when comparing with the control data. Our data suggested that in wb portions there is higher concentration of PG when compared with the nwb portions. This finding is consistent with a previous ex vivo37 study that demonstrated, using spectrophotometric measurements, significantly increased concentration in GAG and, therefore, PG in wb portion compared with nwb portion from human cartilage specimens. Also, a very interesting point were the significant increased T1ρ values in LT compared with MT for the ACL-injured patients (Table 1), reinforcing the fact that in the acute stage of ACL injuries, the major changes occur at the lateral side of the knee, especially at the posterior location of the LT, as emphasized recently.38
Currently, the natural history of the ACL-injured knee is unknown. A few prospective studies have assessed the long-term outcome of the condition.4,39,40 Further studies evaluating the longitudinal course of BMEL are needed. It has been speculated that BMEL present on MRI scans might represent irreversible injury to the articular cartilage,32,41 and may continue to deteriorate despite ligament reconstruction and develop clinical symptoms of OA.7 Therefore, better understanding of cartilage and bone injuries are extremely important for the future noninvasive development with regard to cartilage repair tissue evaluation, as reported recently.42 This current cohort of ACL-injured patients will be followed up to examine the longitudinal cartilage changes in these compartments postsurgery. We expect that cartilage overlying BMEL in the lateral compartment will show more significant abnormalities than the medial compartment if no cartilage repair is present. However, the medial compartment is more susceptible to kinematic abnormalities and may develop cartilage degeneration sooner despite less initial injury.43
In conclusion, quantitative T1ρ assessment of cartilage overlying BMEL compared with surrounding cartilage and wb portions compared with nonwb portions demonstrated pathologic changes related to ACL injuries. The ability to correlate MRI appearance of BMEL with pathologic findings on cartilage would ideally offer clinicians the opportunity to monitor the early course of cartilage degeneration and to predict the clinical outcome of different treatments. Quantitative MRI can be a more valuable and direct tool to study nonoperative and operative interventions to prevent the development of OA.
ACKNOWLEDGMENTS
The authors thank Dr. Eric Han from Applied Science Lab (ASL), GE Healthcare, for helping with sequence development, and Dr. Marc Safran for referring a part of the patients involved in this study.
This work was supported by Aircast Foundation, NIH K25 AR053633 and R01 AR46905.
REFERENCES
- 1.Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Fowler PJ. Bone injuries associated with anterior cruciate ligament disruption. Arthroscopy. 1994;10:453–460. doi: 10.1016/s0749-8063(05)80198-7. [DOI] [PubMed] [Google Scholar]
- 3.Bretlau T, Tuxoe J, Larsen L, et al. Bone bruise in the acutely injured knee. Knee Surg Sports Traumatol Arthrosc. 2002;10:96–101. doi: 10.1007/s00167-001-0272-9. [DOI] [PubMed] [Google Scholar]
- 4.Costa-Paz M, Muscolo DL, Ayerza M, et al. Magnetic resonance imaging follow-up study of bone bruises associated with anterior cruciate ligament ruptures. Arthroscopy. 2001;17:445–449. doi: 10.1053/jars.2001.23581. [DOI] [PubMed] [Google Scholar]
- 5.Ayerza M, Costa-Paz M, Musculo L, et al. Arthroscopic anterior cruciate ligament reconstruction: Magnetic resonance imaging findings and knee stability. Arthroscopy. 1998;14 suppl 21:S26. abstract. [Google Scholar]
- 6.Zanetti M, Bruder E, Romero J, et al. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215:835–840. doi: 10.1148/radiology.215.3.r00jn05835. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DL, Urban WP, Jr, Caborn DN, et al. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998;26:409–414. doi: 10.1177/03635465980260031101. [DOI] [PubMed] [Google Scholar]
- 8.Daniel DM, Stone ML, Dobson BE, et al. A prospective outcome study. Fate of the ACL-injured patient. Am J Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 9.Friederich NF, O’Brien WR. Gonarthrosis after injury of the anterior cruciate ligament: a multicenter, long-term study. Z Unfallchir Versicherungsmed. 1993;86:81–89. [PubMed] [Google Scholar]
- 10.Redfield AG. Nuclear spin thermodynamics in the rotating frame. Science. 1969;164:1015–1023. doi: 10.1126/science.164.3883.1015. [DOI] [PubMed] [Google Scholar]
- 11.Akella SV, Regatte RR, Gougoutas AJ, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46:419–423. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 12.Duvvuri U, Reddy R, Patel SD, et al. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 13.Regatte RR, Akella SV, Borthakur A, et al. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9:1388–1394. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 14.Regatte RR, Akella SV, Lonner JH, et al. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23:547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Benjamin Ma C, Link TM, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lozano J, Li X, Link TM, et al. Detection of posttraumatic cartilage injury using quantitative T1rho magnetic resonance imaging. A report of two cases with arthroscopic findings. J Bone Joint Surg Am. 2006;88:1349–1352. doi: 10.2106/JBJS.E.01051. [DOI] [PubMed] [Google Scholar]
- 17.Pakin SK, Schweitzer ME, Regatte RR. 3D-T1rho quantitation of patellar cartilage at 3. 0T. J Magn Reson Imaging. 2006;24:1357–1363. doi: 10.1002/jmri.20733. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Han ET, Busse RF, et al. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59:298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins CM, Li S, Smith MB. SAR and B1 field distributions in a heterogeneous human head model within a birdcage coil. Specific energy absorption rate. Magn Reson Med. 1998;40:847–856. doi: 10.1002/mrm.1910400610. [DOI] [PubMed] [Google Scholar]
- 20.Han ET, Busse RF, Li X, et al. 3D Segmented Elliptic-Centric Spoiled Gradient Echo Imaging for the In Vivo Quantitation of Cartilage T1rho. Proceedings of the 13th Annual Meeting of ISMRM; Miami Beach; Florida. 2005. Abstract 473. [Google Scholar]
- 21.Carballido-Gamio J, Bauer J, Lee KY, et al. Combined image processing techniques for characterization of MRI cartilage of the knee; Conf Proc IEEE Eng Med Biol Soc; 2005. pp. 3043–3046. [DOI] [PubMed] [Google Scholar]
- 22.Rueckert D, Sonoda LI, Hayes C, et al. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 23.Stahl R, Blumenkrantz G, Carballido-Gamio J, et al. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthr Cartil. 2007;15:1225–1234. doi: 10.1016/j.joca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Regatte RR, Akella SV, Wheaton AJ, et al. 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004;11:741–749. doi: 10.1016/j.acra.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 26.Duvvuri U, Kudchodkar S, Reddy R, et al. T(1rho) relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthr Cartil. 2002;10:838–844. doi: 10.1053/joca.2002.0826. [DOI] [PubMed] [Google Scholar]
- 27.Burstein D, Velyvis J, Scott KT, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45:36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.McKenzie CA, Williams A, Prasad PV, et al. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) at 1.5 T and 3.0 T. J Magn Reson Imaging. 2006;24:928–933. doi: 10.1002/jmri.20689. [DOI] [PubMed] [Google Scholar]
- 29.Tiderius C, Hori M, Williams A, et al. dGEMRIC as a function of BMI. Osteoarthr Cartil. 2006;14:1091–1097. doi: 10.1016/j.joca.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Han ET, Ma CB, et al. In Vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med. 2005;54:929–936. doi: 10.1002/mrm.20609. [DOI] [PubMed] [Google Scholar]
- 31.Murphy BJ, Smith RL, Uribe JW, et al. Bone signal abnormalities in the posterolateral tibia and lateral femoral condyle in complete tears of the anterior cruciate ligament: a specific sign? Radiology. 1992;182:221–224. doi: 10.1148/radiology.182.1.1727286. [DOI] [PubMed] [Google Scholar]
- 32.Rosen MA, Jackson DW, Berger PE. Occult osseous lesions documented by magnetic resonance imaging associated with anterior cruciate ligament ruptures. Arthroscopy. 1991;7:45–51. doi: 10.1016/0749-8063(91)90077-b. [DOI] [PubMed] [Google Scholar]
- 33.Tiderius CJ, Olsson LE, Nyquist F, et al. Cartilage glycosaminoglycan loss in the acute phase after an anterior cruciate ligament injury: delayed gadolinium-enhanced magnetic resonance imaging of cartilage and synovial fluid analysis. Arthritis Rheum. 2005;52:120–127. doi: 10.1002/art.20795. [DOI] [PubMed] [Google Scholar]
- 34.Mosher TJ, Smith HE, Collins C, et al. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology. 2005;234:245–249. doi: 10.1148/radiol.2341040041. [DOI] [PubMed] [Google Scholar]
- 35.Regatte RR, Kaufman JH, Noyszewski EA, et al. Sodium and proton MR properties of cartilage during compression. J Magn Reson Imaging. 1999;10:961–967. doi: 10.1002/(sici)1522-2586(199912)10:6<961::aid-jmri8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 36.Fang C, Johnson D, Leslie MP, et al. Tissue distribution and measurement of cartilage oligomeric matrix protein in patients with magnetic resonance imaging-detected bone bruises after acute anterior cruciate ligament tears. J Orthop Res. 2001;19:634–641. doi: 10.1016/S0736-0266(00)00039-5. [DOI] [PubMed] [Google Scholar]
- 37.Rogers BA, Murphy CL, Cannon SR, et al. Topographical variation in glycosaminoglycan content in human articular cartilage. J Bone Joint Surg Br. 2006;88:1670–1674. doi: 10.1302/0301-620X.88B12.18132. [DOI] [PubMed] [Google Scholar]
- 38.Nishimori M, Deie M, Adachi N, et al. Articular cartilage injury of the posterior lateral tibial plateau associated with acute anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2008;16:270–274. doi: 10.1007/s00167-007-0458-x. [DOI] [PubMed] [Google Scholar]
- 39.Faber KJ, Dill JR, Amendola A, et al. Six-year magnetic resonance imaging follow-up study. Occult osteochondral lesions after anterior cruciate ligament rupture. Am J Sports Med. 1999;27:489–494. doi: 10.1177/03635465990270041301. [DOI] [PubMed] [Google Scholar]
- 40.Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–336. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 41.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–466. [PubMed] [Google Scholar]
- 42.Trattnig S, Mamisch TC, Welsch GH, et al. Quantitative T2 mapping of matrix-associated autologous chondrocyte transplantation at 3 Tesla: an in vivo cross-sectional study. Invest Radiol. 2007;42:442–448. doi: 10.1097/01.rli.0000262088.67368.49. [DOI] [PubMed] [Google Scholar]
- 43.Andriacchi TP, Briant PL, Bevill SL, et al. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]